Summary:
Manganese(III)-promoted addition of various 1,3-dicarbonyl compounds to enol ethers or terminal enol esters, followed by hydrolysis of the resulting adducts and base catalyzed aldol cyclization provides an effective process for the synthesis of a wide range of fused and spiro 2-cyclopentenones.
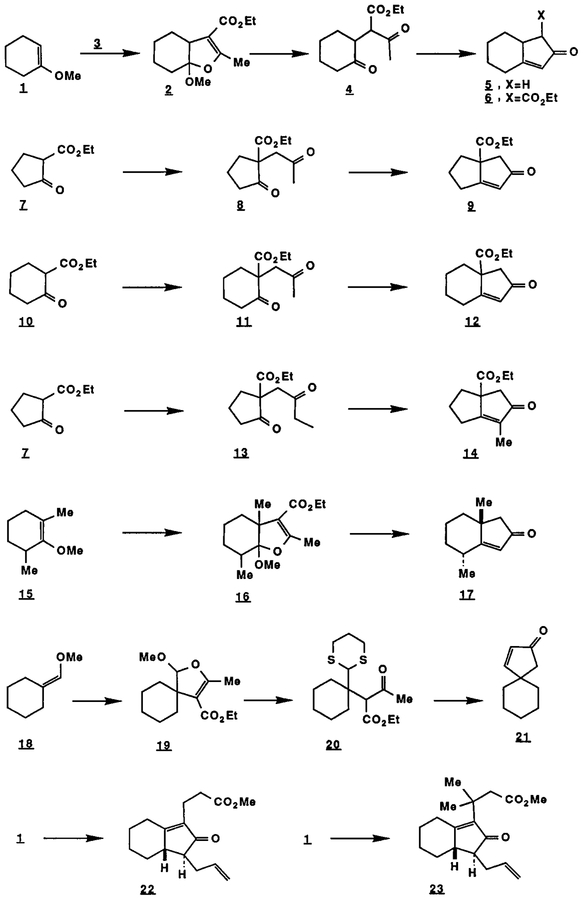
The reaction of enol ethers, enolizable β-dicarbonyl compounds, and Mn30(0Ac)7 (Mn(IΠ) acetate) leads to l-alkoxy-l,2-dihydrofurans in 70–95% yields.1,2 For example, dihydrofuran 2 was formed in 86% yield from 1-methoxycyclohexene (1), ethyl acetoacetate (3) and Mn3O(OAc)73 in acetic acid at 23°C for 10 min.4 This reaction makes possible a useful annulation process leading to the construction of a 2-cyclopentenone unit either fused or spiro to an existing ring. Thus, dihydrofuran 2 upon stirring with 5% aqueous sulfuric acidtetrahydrofuran (THF) (20:1) at 23°C for 4 h was converted into diketone 4 (91%) which was transformed into the cyclopentenone 5 (68%) by treatment with 10% aqueous potassium hydroxide-methanol (1:1) at 70°C for 6 h. Upon exposure to 1.1 equiv of sodium ethoxide in ethanol at 23°C for 48 h diketone 4 gave keto ester 6 (mixture of diastereomers) in 63% yield5.
Reaction of 2-carbethoxy cyclopentanone (7) with 4 equiv of isopropenyl acetate and 1.3 equiv of Mn(III) acetate in acetic acid at 40°C for 30 min afforded directly diketone 8 (64%) which upon heating at reflux in toluene for 4 h with sodium hydride produced bicylic enone 9 (78% yield).6 Similarly 2-carboethoxy cyclohexanone (10) was converted to diketone 11 (71%, 30 min reaction time at 40°C) and subsequently to bicyclic enone 12 (76%). Further, 2-carboethoxy cyclopentanone and 2-t-butyldimethylsilyloxy-l-butene (from 2-butanone, lithium diisopropylamide and t-butyldimethylsilyl chloride in THF containing hexamethylphosphoric triamide at −78°C) underwent Mn(III) promoted coupling to yield diketone 13 (46%) which gave upon heating with sodium hydride in toluene bicyclic enone 14 (81%).
Enol ether 15, ethyl acetoacetate and Mn(IΠ) acetate afforded after reaction in acetic acid at 60°C for 30 min the dihydrofuran 16 which was transformed by exposure first to 5% aqueous sulfuric acid-THF (20:1) and then to 10% aqueous potassium hydroxide-methanol (1:1) into bicyclic enone 17 (73%).
A modification of the above described annulation sequence was necessary for the synthesis of spiro 2- cyclopentenones as illustrated for the substrate 18. Reaction of 18 with ethyl acetocetate and Mn(III) acetate occurred at 23°C in 25 min to form adduct 19 (86% yield). Hydrolysis of 19 to the corresponding keto aldehyde could not be effected directly, but 19 could be converted to the keto thioacetal 20 (96%) by reaction with propane-1,3-dithiol, BF3*Et2O in methylene chloride at O°C for 30 min. The transformation of 20 to cyclopentenone 21 was accomplished in good yield by the sequence: (1) ester hydrolysis-decarboxylation by exposure to 1:2.5 3N lithium hydroxide-dimethoxyethane at 40°C for 12 h (84%), (2) thioacetal hydrolysis with 1.4 equiv of mercuric perchlorate in aqueous acetonitrile (93%), and (3) cyclization with methanolic potassium hydroxide to 21 (91%). The 5,5-spiro lower homolog of 21 was prepared by this same process starting with methoxymethylene cyclopentane in 58% overall yield.
More complex annulations can be carried out using the Mn(ΠI)-based methodology outlined above by utilizing the intermediate 1,4-dicarbonyl compounds in other ways prior to aldol ring closure. Thus, 1- methoxycyclohexene (1) was converted to the bicyclic enone 22 by the sequence: (1) Mn(ΠI)-promoted addition of cyclohexan-l,3-dione to 1 to give the corresponding dihydrofuran adduct (76%), (2) hydrolysis with aqueous acid to form a triketone, (3) alkylation with allyl iodide and potassium carbonate in acetone, and (4) 1,3- dicarbonyl cleavage and aldol cyclization by heating with potassium methoxide in methanol at 65°C for 2 h to give 22 (66% overall from the dihydrofuran). Similarly 23 was synthesized starting from 1 and dimedone in 66% overall yield.
It is clear from the above results that the Mn(III) acetate-promoted conversion of enol ethers to dihydrofurans provides a practical entry for the synthesis of a wide variety of 2-cyclopentenones.
The following procedures illustrate the experimental aspects of the annulation described herein.7
Ethvl 2. 4. 5. 6. 7. 8-hexahvdroindene-2-one-8-carhoxvlate (12).
Anhydrous manganese (III) acetate (815 mg, 1.9 mmol) was dissolved in glacial acetic acid (3 ml) at 40°C under argon. The resulting solution was cooled1,3 to 23°C and treated with 255 mg (1.5 mmol) of 2-carbethoxycyclohexanone (10) and 600 mg (6 mmol) of isopropenyl acetate. The reaction mixture was stirred at 23°C for 5 min and then at 40°C for 30 min at which time the dark brown color of manganese (ΠI) disappeared. The solvent was removed under reduced pressure and the residue was diluted with saturated sodium bicarbonate solution (2 ml) and extracted with ethyl acetate (2 × 5 ml). The combined organic extracts were dried over anhydrous sodium sulfate and concentrated in vacuo. The crude product was purified by silica gel chromatography [hexane-ethyl acetate, 3:1 (v/v)] to afford 241 mg (71%) of diketone 11 as a colorless oil.
To a stirred suspension of 58 mg (2.4 mmol) of sodium hydride in dry toluene (5 ml) under nitrogen was added 150 mg (0.6 mmol) of diketone 11 in toluene (1 ml). The resulting mixture was heated at reflux for 18 h, cooled to 0°C and carefully acidified with 10% hydrochloric acid (1 ml). The layers were separated and the aqueous layer was extracted with ethyl acetate (2 × 5 ml). The combined organic extracts were washed with brine and dried over anhydrous sodium sulfate. Removal of solvent in vacuo gave a light yellow oily residue which was purfied by silica gel chromatography [hexane-ethyl acetate-triethylamine, 3:1:0.1 (v/v)] to yield 105 mg (76%) of enone 12 as a colorless oil. 1H NMR (CDCl3, 270MHz): δ 6.0 (s, 1H), 4.2 (q, 2H, J = 7.3Hz), 2.8 (m, 2H), 2.65 (d, 1H, J = 18.5Hz), 2.4 (m, 1H), 2.3 (d, 1H, J = 18.5Hz), 2.0 – 1.4 (m, 5H), 1.3 (t, 3H, J = 7.3Hz). IR (thin film): 2920, 2850, 1730, 1690, 1630, 1440, 1280 crn−1; MS (70 ev): 208 (M+), 193, 179, 135. UV (λ max): 232nm(EtOH).
Spiro [4.51 dec-3-en-2-one (21).
Anhydrous manganese (III) acetate (1.63 g, 3.9 mmol) was dissolved in glacial acetic acid (8 ml) at 40°C under an argon atmosphere. The resulting solution was cooled to 23°C and treated with 650 mg (5 mmol) of ethyl acetoacetate (3) and 378 mg (3 mmole) of enol ether 18. The reaction mixture was stirred at 23°C for 25 min at which time the dark brown color of manganese (ΠI) disappeared. After isolation by the procedure described above for 12, 655 mg of dihydrofuran 19 (86%) was obtained as a colorless oil.
To a stirred solution of 584 mg (2.3 mmol) of above dihydrofuran 19 in dichloromethane (10 ml) under argon at 0°C was added 324 mg (3 mmol) of l,3·propanedithiol and 423 mg (3.0 mmol) of boron trifluoride etherate. After 30 min the reaction mixture was poured into saturated aqueous sodium bicarbonate solution (10 ml) and extracted thoroughly with dichloromethane (2 × 5 ml). The combined organic extracts were dried over anhydrous sodium sulfate and the solvent was removed in vacuo to give an oil. Purification by silica gel chromatography [hexane-ethyl acetate, 3:1 (v/v)] gave 729 mg (96%) of thioacetal 20 as a colorless oil.
Aqueous lithium hydroxide solution (3N, 2 ml) was added to 581 mg (1.76 mmol) of thioacetal 20 in dimethoxyethane (5 ml) at room temperature. The resulting mixture was stirred at 45°C for 12 h. The solvent was removed in vacuo and the residue was acidified with IN hydrochloric acid to pH 3. The mixture was extracted with ethyl acetate (2 × 5 ml); the combined extracts were dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product was purified by chromatography to afford 384 mg (84%) of decarboxylated methyl ketone.
To a stirred solution of 384 mg (1.5 mmol) of the above thioacetal methyl ketone in acetonitrile (3 ml) at room temperature was treated with mercuric perchlorate (2M, 2 ml) dropwise over a 10 min period. A white precipitate formed during the addition. The resulting mixture was stirred at 23°C for 30 min, the precipitate was removed by filtration, and the filtrate was concentrated under reduced pressure. The residue was extracted with chloroform, the combined extracts were washed successively with aqueous sodium bicarbonate and brine, and dried over anhydrous sodium sulfate. Removal of solvent and purification by chromatography gave 233 mg (93%) of aldehyde-methyl ketone as a colorless oil.
To a stirred solution of 230 mg (1.37 mmol) of the above ketoaldehyde in methanol (4 ml) at 0°C under argon was added a solution of 10% potassium hydroxide in methanol (2 ml). The mixture was stirred at 0°C for 5 h and the solvent was removed under reduced pressure. The resultant mixture was treated with brine and ether and the combined organic extracts were dried over anhydrous sodium sulfate. Evaporation of solvent gave an oily residue which was purified by silica gel chromatography [hexane-ethyl acetate-triethylamine, 3:1:0.1 (v/v)] to afford 187 mg (91%) of enone (21) as a colorless oil. 1H NMR (CDC13, 270MHz): 7.35 (d, 1H, J = 6.0Hz), 6.02 (d, 1H, J = 6.0Hz), 2.2 (s, 2H), 1.3 – 1.7 (m, 10H). IR (thin film): 2950, 1700, 1580, 1420 cm-1; MS (70 ev): 150 (M+), 122, 107. UV (λ max): 237 nm (EtOH).
References and Notes
- 1.Corey EJ and Ghosh AK, Chemistry Letters, in press. [Google Scholar]
- 2.The corresponding reaction with simple olefins either fails (e.g. with cyclohexene) or proceeds in mediocre yield (e.g. with styrene); see,; Dessau RM and Heiba EI, J. Org. Chem, 39, 3456 (1974). [Google Scholar]
- 3.Chrétien A and Varga G, Bull. Soc. Chim. France, [5], 3, 2387 (1936). [Google Scholar]
- 4.For other recent applications of the Mn(III) reagent in synthesis see; (a) Heiba EI, Dessau RM, Rodewald PG, J. Am. Chem. Soc, 96, 7977 (1974); [Google Scholar]; (b) Corey EJ and Kang M, J. Am. Chem. Soc, 106, 5384 (1984); [Google Scholar]; (c) Corey EJ and Gross AW, Tetrahedron Letters, 26, 4291 (1985); [Google Scholar]; (d) Snider BB, Mohan R, and Kates SA, J. Org. Chem, 50, 3659 (1985). [Google Scholar]
- 5.Satisfactory infrared, 1H NMR and mass spectral data were obtained for each product.
- 6.The use of enol acetate or enol silyl ethers in this process is restricted to those in which the olefinic linkage is terminal. The enol silyl ethers or esters of cyclohexanone, for example, were found not to participate in Mn(ΠI)-promoted reaction with 1,3-dicarbonyl compounds.
- 7.This research was assisted financially by a grant from the National Science Foundation.