Summary:
Ginkgolide A (1) has been synthesized from the trilactone 3 and also from ginkgolide B (2).
Recently we have described the first total syntheses of racemic1a and natural formslb of ginkgolide B (2), a potent antagonist of platelet activating factor, which shows promise as a therapeutic agent. Ginkgolide A (1), another member of the ginkgolide family, possesses insect antifeedant activity2. Herein we report the total synthesis of (±) ginkgolide A from an intermediate (3) used previously for the total synthesis of (±) ginkgolide B1 and also the conversion of natural ginkgolide B to ginkgolide A.
Treatment of bislactone 3 with 2.5 equiv of N,N’-thiocarbonyldiimidazole3 in 2 : 1 toluene-pyridine at 45° for 12 h gave selectively the thiocarbonylimidazole derivative 4 (84%) after silica gel (SG) chromatography (Rf = 0.2, 1 : 1 EtOAc : hexane). Reduction of 4 with tri-n-butyltin hydride was effected by drop wise addition of a solution of 4 in dioxane over 3 h to 5 equiv of tri-n-butyltin hydride in anhydrous dioxane at reflux to give the deoxygenated4 product 5 (90%). The 1H NMR (500Hz) spectrum of 5 confirmed the presence of two C(l) protons (Hα, δ 2.23; dd, J = 7.0Hz, 14.6Hz; Hβ, δ 2.02, dd, J = 8.5, 14.6Hz). Reaction of dihydrofuran 5 with osmium tetroxide in pyridine (60°C for 24 h) and workup with aqueous sodium bisulfite gave a diol which was oxidized directly using excess iodine in 10 : 1 CH3OH-H2O in the presence of calcium carbonate (23°, 12 h) to provide 10-epi(±) ginkgolide A (6) 48% from (5). The C(10) hydroxy group of 6 was epimerized to form (±)-ginkgolide A (61% overall) by the sequence: (1) oxidation with 3 equiv of benzeneselenic anhydride5 and 5 equiv of pyridine in anhydrous chlorobenzene at 80°C for 2 h (TLC Rf 0.5; chromatographed over silicAR CC-7 with 1 : 1 EtOAc-hexane as eluent), and (2) reduction of the resulting α-ketolactone with 5 equiv of sodium borohydride in ethanol at −45° for 15 min. Synthetic (±)-l thus obtained was identical with’natural ginkgolide A by 500 MHz 1H NMR, FT-IR, SG-TLC, and FAB mass spectral comparison.
Ginkgolide B (2) has also been converted to ginkgolide A (1) as follows. Ginkgolide B was treated with 5 equiv of chloromethyl methyl ether and 5 equiv of diisopropylethylamine in dry acetonitrile at 23° for 12 h to form 7 and the isomeric C(l) ether (ratio of 3 : 1, 89%). The isomer 7 was separated (Rf = 0.65, EtOAc-hexane) by SG chromatography and subjected to xanthate formation by treatment with 5 equiv of potassium hydride in dry THF at 23° for 1 h, followed by stirring with an excess of carbon disulfide (23° for 15 min and 45°C for 30 min), and finally reaction with 5 equiv of benzyl bromide at 23° for 12 h to give 9 (71%). When methyl iodide was used in place of benzylbromide for the alkylation of the thiolate anion, a 75% yield of 8 was obtained. Reduction of 9 with tri-n-butyltin hydride in dioxane at reflux afforded the deoxygenated product 10 (71%). Deprotection of 10 with BF3• Et2O in CH2Cl2 in the presence of thiophenol at −10°C for 1 h provided ginkgolide A (l).7
Figure 1.
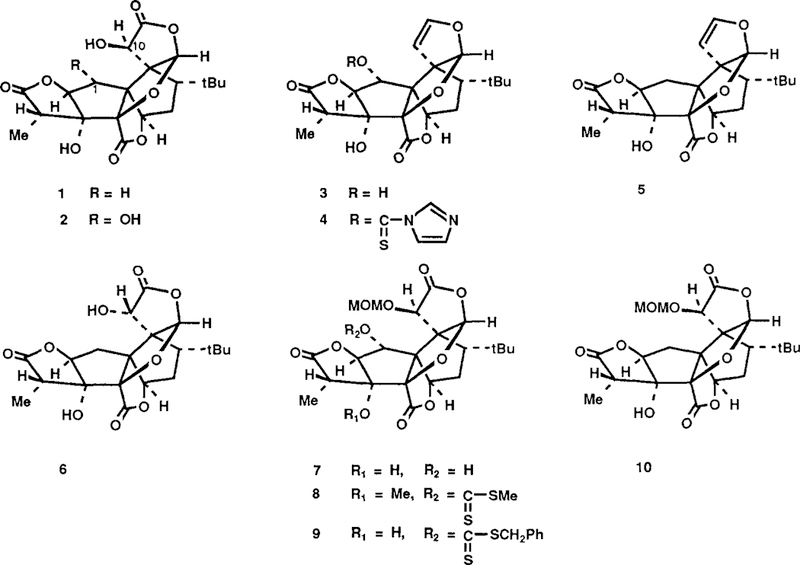
Synthesis of Ginkgolide A.
REFERENCES AND NOTES
- 1.(a) Corey EJ; Kang M-C; Desai MC; Ghosh AK; Houpis IN J. Am. Chem. Soc. 1988,110, 649–651; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Corey EJ; Gavai AV Tetrahedron Lett., preceding paper. [Google Scholar]
- 2.(a) Matsumoto T; Sei T Agric. Biol. Chem. 1987,51, 249–250; [Google Scholar]; (b) Major RT. Science 1967,157, 1270. [DOI] [PubMed] [Google Scholar]
- 3.Pullukat TJ; Urray G Tetrahedron Lett. 1967, 1953–1954. [Google Scholar]
- 4.(a) Barton DHR; McCombie SWJ Chem. Soc., Perkin Trans 11975, 1574–1585; [Google Scholar]; (b) Barton DHR; Motherwell WB Pure Appl. Chem. 1981,53, 15–31. [Google Scholar]
- 5.Barton DHR; Brewster AG; Hui RAHF; Lester DJ; Ley SV; Back TG J. Chem. Soc. Chem. Commun. 1978, 952. [Google Scholar]
- 6.Satisfactory 1H NMR, infrared and mass spectral data were obtained for each isolated intermediate described herein. Reactions involving air-sensitive reagents or products were conducted under argon atmosphere. [Google Scholar]
- 7.This research was assisted financially by a grant from the National Science Foundation. We are indebted to Dr. Pierre Braquet and Prof. S. Yamada for gifts of ginkgolides A and B.


