Proliferating Cell Nuclear Antigen (PCNA) is a DNA polymerase clamp conserved in all eukaryotes that coordinates many activities at the replication fork and at sites of DNA damage. Alleles of PCNA, encoded by POL30 in Saccharomyces cerevisiae, disrupt transcriptional...
Keywords: POL30, transcriptional silencing, recombination, nucleosome assembly, intragenic complementation
Abstract
In Saccharomyces cerevisiae, transcriptional silencing at HML and HMR maintains mating-type identity. The repressive chromatin structure at these loci is replicated every cell cycle and must be re-established quickly to prevent transcription of the genes at these loci. Mutations in a component of the replisome, the proliferating cell nuclear antigen (PCNA), encoded by POL30, cause a loss of transcriptional silencing at HMR. We used an assay that captures transient losses of silencing at HML and HMR to perform extended genetic analyses of the pol30-6, pol30-8, and pol30-79 alleles. All three alleles destabilized silencing only transiently and only in cycling cells. Whereas pol30-8 caused loss of silencing by disrupting the function of Chromatin Assembly Factor 1, pol30-6 and pol30-79 acted through a separate genetic pathway, but one still dependent on histone chaperones. Surprisingly, the silencing-loss phenotypes of pol30-6 and pol30-79 depended on ploidy, but not on POL30 dosage or mating-type identity. Separately from silencing loss, the pol30-6 and pol30-79 alleles also displayed high levels of mitotic recombination in diploids. These results established that histone trafficking involving PCNA at replication forks is crucial to the maintenance of chromatin state and genome stability during DNA replication. They also raised the possibility that increased ploidy may protect chromatin states when the replisome is perturbed.
EUKARYOTIC genomes include tightly packaged and transcriptionally repressed domains referred to as heterochromatin. The nucleosomes in heterochromatin are enriched for particular chromatin marks made by specialized chromatin-modifying enzymes. The marks left by these enzymes are recognized by other proteins that silence gene transcription. Although the exact histone modifications and heterochromatin proteins differ from organism to organism, there are unifying characteristics of heterochromatin, including independence from underlying DNA sequence, replication late in S phase, and structural compaction.
To maintain the repression of genes within heterochromatin, histone modifications and chromatin-binding proteins must be faithfully replicated onto both daughter chromatids during DNA replication. The process that is required for inheritance of chromatin state through DNA replication is unclear, but requires the interaction of chromatin regulators with various factors in the eukaryotic replisome [reviewed in Alabert et al. (2017)].
Proliferating cell nuclear antigen (PCNA) is a DNA polymerase processivity clamp conserved from yeast to human [reviewed in Moldovan et al. (2017)]. PCNA is a homotrimer that assembles around individual DNA molecules and, through protein-protein interactions, coordinates many activities at the DNA replication fork, including the processivity of DNA polymerase, Okazaki fragment processing, and chromatin assembly and remodeling. PCNA is also required for many different DNA repair pathways. Many chromatin modifiers and remodelers are recruited to replication forks through direct and indirect interactions with PCNA.
PCNA has a direct role in the stability of heterochromatin. In mice, Heterochromatin Protein 1 (HP1) is recruited to replication forks through direct interaction with the histone chaperone complex Chromatin Assembly Factor 1 (CAF-1) (Murzina et al. 1999), which itself is recruited to replication forks through direct interaction with PCNA (Shibahara and Stillman 1999; Zhang et al. 2000; Ben-Shahar et al. 2009). PCNA, in concert with CAF-1, is also required for the asymmetric specification of cell fate in the Caenorhabditis elegans nervous system, an epigenetic process (Nakano et al. 2011). Additionally, the maintenance of transcriptional silencing requires functional and stable DNA-bound PCNA in Saccharomyces cerevisiae (Zhang et al. 2000; Miller et al. 2008; Janke et al. 2018) These results suggest an important role for PCNA and CAF-1 in the inheritance of chromatin states through DNA replication.
Circumstantial evidence for the importance of PCNA in the assembly of heterochromatin is also found in humans and Drosophila melanogaster. In humans, Histone Deacetylase 1 (HDAC1), which is associated with transcriptional repression, interacts with PCNA in vitro and colocalizes with PCNA at replication forks in vivo (Milutinovic et al. 2002). In D. melanogaster, Polycomb Group proteins, required for the establishment and maintenance of facultative heterochromatin, transiently associate with PCNA and CAF-1 during DNA replication (Petruk et al. 2012).
S. cerevisiae contains well-characterized heterochromatin domains that we used here to study the role of PCNA in epigenetic inheritance through DNA replication. Two of these loci, HML and HMR, share characteristics of heterochromatin in other organisms. Silencing of HML and HMR requires the activity of the Silent Information Regulator (SIR) complex, composed of Sir2, Sir3, and Sir4. The Sir proteins are recruited first to the E and I silencers, nucleation sites flanking HML and HMR, and subsequently bind to nucleosomes that span the entire 3–4 kb region between the silencers. Through the histone deacetylation activity of Sir2 and nucleosome-bridging ability of Sir3, the SIR complex creates a hypoacetylated, compact chromatin structure [reviewed in Gartenberg and Smith (2016)].
In S. cerevisiae, alleles of PCNA, encoded by POL30, have been isolated that disrupt transcriptional silencing of reporter genes at telomeres and the silent mating-type locus HMR. (Zhang et al. 2000). These alleles (pol30-6, pol30-8, and pol30-79) differ in phenotype and in the degree of silencing loss they cause. Using the ADE2 reporter at HMR, the pol30-8 allele results in sectored colonies, suggesting the existence of two heritable states of gene expression: heritable silencing (ADE2 expression off, resulting in red sectors) and heritable expression (ADE2 expression on, resulting in white sectors). In contrast, colonies containing pol30-6 or pol30-79 are pink, suggesting a partial reduction of silencing in all cells (Zhang et al. 2000).
In combination with a deletion of CAC1, which encodes the large subunit of the histone chaperone CAF-1, the pol30-6 and pol30-79 alleles synergistically reduce silencing of URA3 at telomere VII-L and of ADE2 at HMR. However, the combination of cac1∆ and pol30-8 result in similarly sectored ADE2 colonies as pol30-8 alone and no further decrease in telomeric silencing than pol30-8 alone. These two results suggest that PCNA may contribute to heritable silencing through at least two different mechanisms, one of which is through the histone chaperone activity of CAF-1 (Zhang et al. 2000).
Although reporter genes have a long history of successful use in genetic studies, the reliability of the ADE2 and URA3 reporters has been called into question, especially for situations involving DNA metabolism (Rossmann et al. 2011; Takahashi et al. 2011). Using a silencing-reporter assay that more sensitively captures loss-of-silencing events, better maintains the gene structure of HML and HMR, and is free of the complications of nucleotide metabolism, we re-evaluated earlier claims about the silencing phenotypes of pol30-6, pol30-8, and pol30-79, extended the analyses substantially, and provided new interpretations of published observations.
Materials and Methods
Yeast strains
All strains in this study were derived from W303 and are listed in Supplemental Material, Table S1. Plasmids used in this study are listed in Table S2. Gene deletions (except for bar1∆) were created by one-step integration of PCR-amplified disruption cassettes (Goldstein and McCusker 1999; Gueldener et al. 2002), using primers listed in Table S3. The pol30-8 (R61A, D63A) allele, mcm2-3A (Y79A, Y82A, Y91A) allele, and bar1∆ were introduced using Cas9 technology. Guide RNAs targeting POL30, MCM2, and BAR1 are listed in Table S3. The single guide RNA dropout-Cas9 expression plasmid (pJR3428) was assembled using a toolkit from Lee et al. (2015). The guide RNA target and nontarget strands were integrated into pJR3428 by Golden Gate cloning, using the restriction enzyme BsmBI as described in Lee et al. (2015). The repair templates were made by annealing oligos in Table S3 and extending the 3′ ends using Phusion Polymerase (New England Biolabs, Beverly, MA). The pol30-6 (D41A, D42A) and pol30-79 (L126A, I128A) alleles were created by integrating gene blocks containing each allele along with the selectable marker URA3, found in Table S3. A detailed description of this method is in File S1. Double mutants were created by genetic crosses and were confirmed by tetrad analysis, using selectable markers for gene disruptions and mutant-specific PCR and/or sequencing for pol30-6, pol30-8, pol30-79, and mcm2-3A. Creation of POL30 hemizygotes and the tetraploid strain (JRY12026) used plasmid shuffles with pBL230-0 [CEN/ARS TRP1 POL30] (Ayyagari et al. 1995; Zhang et al. 2000), described in detail in File S1.
Colony growth and imaging
Strains were grown on YPD and grown overnight. Cre-reported altered states of heterochromatin (CRASH) strains were first patched onto selective medium plates to select for cells expressing hphMX, and thus had not lost silencing and excised the RFP-hphMX cassette (Figure 1A): YPD containing 200 μg/ml G418 (Geneticin; Life Technologies) for strains carrying the kanMX cassette or YPD containing 300 μg/ml Hygromycin B (MilliporeSigma) for strains carrying the hphMX cassette. Cells were then resuspended in water and plated onto complete supplement mixture (CSM) or CSM–Trp (Sunrise Science Products) plus 1.5% agar, at a density of ∼30 cells per plate. Colonies were imaged after 5–6 days of growth at 30°. At least 10 colonies per genotype were imaged using a Leica M205FA fluorescence stereomicroscope, a Leica DFC3000G CCD camera, and a Plan Apo ×0.63 objective. All colonies were imaged at a magnification of 10X. Image analysis and assembly was performed using Fiji software (Schindelin et al. 2012).
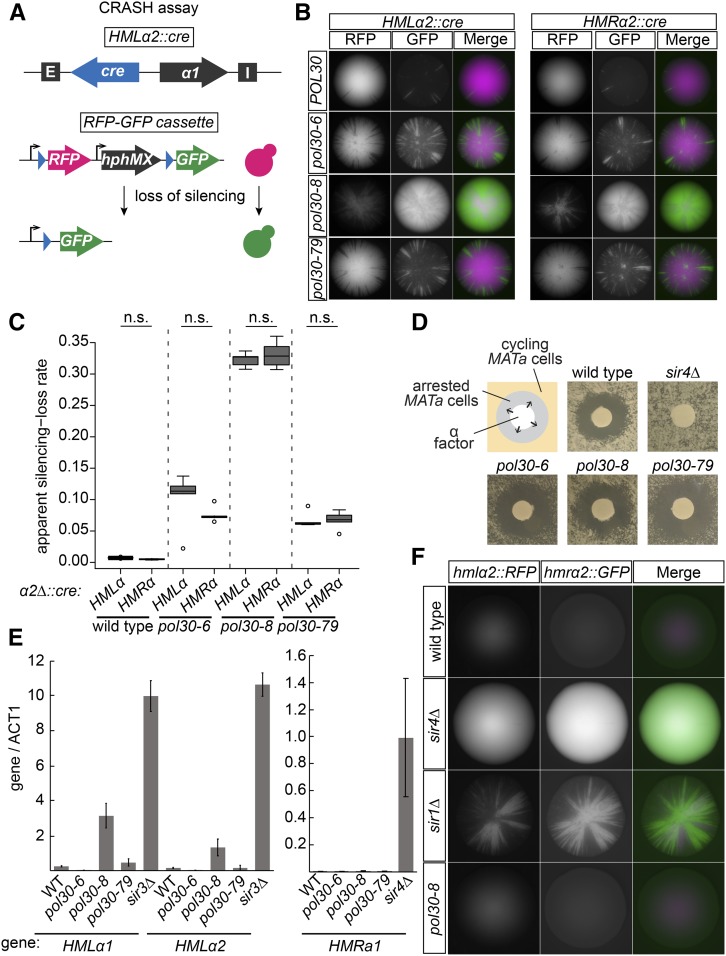
Figure 1.
Mutants of POL30 caused transient loss of silencing. (A) Schematic of the CRASH loss-of-silencing assay. Expression of cre from HMLα2::cre occurs when transcriptional silencing is disturbed. In cells that lose silencing even transiently, Cre causes a permanent switch from expressing RFP to expressing GFP. In a similar strain, cre is expressed from HMRα2 to detect loss-of-silencing events at HMR. (B) Colonies of HMLα2::cre (left panel) and HMRα2::cre (right panel) strains for each POL30 allele. Each green sector represents a loss-of-silencing event. Wild-type strains (JRY10790, left and JRY10710, right) had few sectors. Strains containing pol30-6 (JRY11137, left and JRY11186, right), pol30-8 (JRY11188, left and JRY11187, right), or pol30-79 (JRY11141, left and JRY11608, right) had elevated sectoring compared to wild type. (C) The apparent silencing-loss rates for each of the strains in B were quantified by flow cytometry as described in Materials and Methods and in Janke et al. (2018). Significance (Nonsignificant difference = n.s.) was determined by one-way ANOVA and Tukey’s honestly significant difference post hoc test. The center line of each box plot represents the median of at least five biological replicates. The boxes represent the 25th and 75th percentiles. Whiskers represent the range of values within 1.5× the interquartile range. Values extending past 1.5× the interquartile range are marked as outliers (circles). (D) α-Factor halo assay. Filter papers soaked in the mating pheromone α-factor (200 μM in 100 mM sodium acetate) were placed onto a freshly spread lawn of MATa cells of each indicated genotype. MATa cells that maintain silencing at HMLα will arrest in G1 phase around the filter paper, creating a “halo.” Cells that heritably lose silencing at HMLα do not arrest in response to α-factor. Representative images of wild type (JRY4012), sir4Δ (JRY4577), pol30-6 (JRY11645), pol30-8 (JRY11647), and pol30-79 (JRY11649) are shown. (E) Quantitative RT-PCR of α 1 and α2 transcripts from HMLα and a1 from HMRa. Quantification was performed using a standard curve for each set of primers and normalized to ACT1 transcript levels. Error bars represent SD. Bars represent the normalized average of three technical replicates of each indicated strain: WT (JRY11699 matΔ), sir3Δ (JRY9624, matΔhmrΔ) sir4Δ (JRY12174 MATα), pol30-6 (JRY11700 mat Δ), pol30-8 (JRY11701 matΔ), and pol30-79 (JRY11702 matΔ). (F) Genes encoding fluorescence reporters were placed at HMLα2 (RFP) and HMRα2 (GFP) to report on transcription from the two loci. Shown are representative images of colonies from each strain: WT (JRY11129), sir4Δ (JRY11131), sir1Δ (JRY11130) pol30-8 (JRY11132). WT, wild type.
Quantification of silencing loss by flow cytometry
For each strain, three to five single colonies were inoculated in 1 ml of selective media to select for cells that had not lost silencing. These 1 ml cultures were grown in deep 96-well plates (VWR International) at 30° overnight to saturation. Overnight cultures were diluted into 1 ml of fresh, nonselective YPD to ∼105 cells/ml in a deep 96-well plate, and grown for 5–6 hr before flow cytometry. For each culture, a minimum of 15,000 events were collected using a BD High-Throughput Sampler on a BD LSR Fortessa X20 Cell Analyzer. Gating and quantification were performed as previously described (Janke et al. 2018).
α-Factor halo assay
MATa strains were scraped from a YPD plate with a toothpick, resuspended in water, and ∼150,000 cells were freshly spread onto CSM plates (Sunrise Science Products). Hole-punched Whatman filter papers were soaked for ∼5 sec in 200 μg/μl α-factor in 100 mM sodium acetate and then placed onto the plates. Three soaked, filter-paper circles were placed on each plate. Plates were incubated at 30° for 36–48 hr before imaging.
RNA preparation for quantitative PCR
Cells were grown to midlog phase in YPD, and RNA was extracted using hot acidic phenol and chloroform (Collart and Oliviero 2001). Samples were treated with DNase I (New England Biolabs) and subsequently purified using the Qiagen RNeasy Mini Kit. Complementary DNA was synthesized using the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA) and oligo (dT) primers. Quantitative PCR of complementary DNA was performed using the DyNAmo HS SYBR Green kit (Thermo Fisher Scientific) on an Mx3000P machine (Stratagene, La Jolla, CA) using the primers listed in Table S3. Standard curves were generated using the sir3∆ mat∆ hmr∆ (JRY9624) or sir4∆ strain (JRY12714).
Live-cell imaging
All single-cell microscopy images were collected on a Zeiss Axio Observer Z1 inverted microscope equipped with a Plan-Apochromat 63× oil-immersion objective (Zeiss, Thornwood, NY) and the Definite Focus System for maintenance of focus over time. yEGFP was excited with the 420–500 nm spectrum range at 20% intensity, and yEmRFP was excited with the 500–755 nm spectrum range at 20% intensity with a CoolLED pE-300 ultra, and collected with the Multiband Semrock Filter (LF405/488/594-A-ZHE). Images were acquired with a Teledyne Photometrics Prime 95B sCMOS camera. For time-lapse experiments, images were collected every 5 or 7 min, using an exposure time of 20 msec for brightfield, 50 msec for yEGFP, and 200 msec for yEmRFP. At each time point, multiple stage positions were collected using an ASI MS-2000 XYZ piezo stage. The microscope, camera, and stage were controlled with the Micro-Manager software (Edelstein et al. 2014). Image analysis and assembly was performed using Fiji software (Schindelin et al. 2012).
For the CRASH time-course setup, each strain was inoculated in 5 ml of selective media to select for cells that had not lost silencing. Cultures were grown to saturation overnight at 30°. Overnight cultures were diluted back to 106 cells/ml in 10 ml of YPD containing G418 or Hygromycin B, and grown to early-log phase (∼4 × 106 cells/ml). The culture was then split into 5 ml of YPD containing G418 or Hygromycin B and 40 nM α-factor in 100 mM sodium acetate, and 5 ml of YPD containing G418 or Hygromycin B. The cultures were grown for another 90 min (∼1 doubling). The cells were then harvested and resuspended in water. The resuspended cells were diluted to ∼2 × 107 cells/ml, and 5 μl were pipetted onto a 1 × 1 cm2 of CSM–Trp plus 2% agar with or without 40 nM α-factor in 100 mM sodium acetate. Both agar pads were placed into a 27 mm glass dish (Thermo Scientific) and mounted in a Pecon Incubator XL with Heating Unit XL S (Zeiss) controlled by TempModule S (Zeiss) and kept at 30° for the duration of the experiment. To calculate the number of switches per 10,000 cells in the arrested condition, the number of cells that were RFP-expressing at time zero were counted. The total number of switches (RFP-to-GFP) were counted for all of those cells and divided by the total number of RFP-expressing cells at time zero. To calculate the number of switches per 10,000 divisions in the cycling condition, the number of switches over the entire time course was divided by the calculated total number of divisions. The number of divisions was calculated using the following formula:
Where t is the total number of minutes in the time course (480 min for an 8 hr time course), n0 is the number of RFP-expressing cells at time zero, and f is the division rate (per minute). To determine f, the time from small bud to the next small bud for five cells at the beginning of the time course, five cells at the end of the time course in the center of a microcolony, and five cells at the end of the time course at the edge of a microcolony was averaged to get the time for one division.
Protein isolation and immunoblotting
Each strain was inoculated in 5 ml of YPD and grown overnight to saturation. Overnight cultures were diluted to ∼2 × 105 cells/ml in fresh YPD and grown to midlog phase, and ∼108 cells were harvested and pelleted. Pellets were resuspended in 1 ml of 5% trichloroacetic acid and incubated at 4° for 90 min. The precipitates were pelleted, washed twice with 1 ml of 100% acetone, and air-dried. Dried pellets were resuspended in 100 μl of protein breakage buffer (50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 3 mM DTT) and an equal volume of 0.5 mm zirconium ceramic beads (BioSpec Products) followed by five cycles of vortexing at 1 min bursts with 1 min of incubation on ice between each cycle. 50 μl of 3× SDS sample buffer (188 mM Tris-HCl, pH 6.8, 30% glycerol, 150 mM DTT, 6% SDS, 0.03% bromophenol blue, 2% β-mercaptoethanol) was added to each sample and incubated at 95° for 5 min. Insoluble material was pelleted by centrifugation and an equal volume of the soluble fraction from each sample was run on an SDS-polyacrylamide gel (Mini-PROTEAN TGX Any kD precast gel; BioRad, Hercules, CA) and transferred to a nitrocellulose membrane using a TransBlot Turbo Transfer Pack (BioRad) on the Mixed MW setting of a TransBlot Turbo machine (BioRad). The membrane was cut horizontally in half between the 50 and 37 kDa markers and separated, to blot for Hxk2 on the top half and PCNA on the bottom half. The membranes were blocked in Odyssey Blocking Buffer (LI-COR Biosciences), and the following primary antibodies and dilutions were used for detection: PCNA (ab221196, 1:1000; Abcam), Hxk2 (#100-4159, 1:10,000; Rockland Immunochemicals). The secondary antibody used was IRDyeCW800 goat anti-rabbit (1:20,000; LI-COR Biosciences), and the membrane was imaged on a LI-COR Odyssey Imager. All washing steps were performed with PBS + 0.1% Tween-20. Quantitative analysis was performed using Fiji software (Schindelin et al. 2012): The area under the intensity peak above background for each band was used for normalization to Hxk2 followed by comparison between lanes.
Tetrad analysis
Diploid cells were sporulated on 1% potassium acetate, 2% agar, 0.25× CSM plates for 2–3 days at room temperature. Tetrads were dissected onto YPD plates using a micromanipulator and grown for 2 days before replica plating and scoring.
Patch mating assay
All strains were patched on YPD and grown at 30° for 3 days. A small sample of yeast was scraped from the center of each patch and patched onto a fresh YPD plate and grown overnight at 30°. The following day, the YPD plate was replica plated onto a YPD plate with a fresh lawn of MATa haploid testers and a YPD plate with a fresh lawn of MATα haploid testers. The replica plates were grown overnight and then replica plated onto solid minimum media the next day. Minimal medium plates were grown for 2 days at 30° and then imaged.
Data availability
All data necessary for confirming the conclusions presented in the article are represented fully within the article. Supplemental material has been uploaded to the FigShare public repository. File S1 contains supplemental materials and methods. Table S1 contains details about the yeast strains used in this study. Table S2 contains the plasmids used in this study. Table S3 contains oligonucleotides used in this study. Figure S1 contains quantitative RT-PCR results for CRE transcript levels in POL30 mutants. Supplemental material available at FigShare: https://doi.org/10.25386/genetics.9343232.
Results
Mutants of POL30 caused transient loss of silencing
We introduced alleles of POL30 implicated in heterochromatic silencing, pol30-6, pol30-8, and pol30-79 (Zhang et al. 2000), into a strain we previously constructed that allows sensitive detection of losses of heterochromatin silencing (Figure 1A, Dodson and Rine 2015). In this strain, the α2 coding sequence at HMLα or HMRα is replaced with the coding sequence of Cre recombinase. The URA3 locus on chromosome V is replaced by loxP sites flanking the RFP gene and the selectable marker hphMX downstream of the strong TDH3 promoter. Downstream of loxP-RFP-hphMX-loxP is a promoterless GFP gene. Upon loss of silencing at HMLα or HMRα, Cre recombinase is expressed and excises the RFP and hphMX sequences, resulting in a permanent switch from RFP expression to GFP expression (Figure 1A). Within a colony, a sector of green cells represents a loss-of-silencing event in a cell born at the vertex with the sector representing growth of the descendants following the loss event. This assay is referred to as the CRASH assay (Dodson and Rine 2015).
Each of the pol30 mutants resulted in increased sectoring compared to wild-type POL30 at both HMLα and HMRα (Figure 1B). We also quantified loss-of-silencing events in these strains using flow cytometry. The apparent silencing-loss rate was calculated as the number of yellow cells (cells that had recently excised the RFP gene) divided by the sum of all yellow cells and red cells. The loss rates from flow cytometry experiments mirrored qualitative assessments of loss rates from colony sectoring (Figure 1C). pol30-8 cells had the most unstable silencing, followed by pol30-6 and then pol30-79 (Figure 1, B and C). There was no significant difference between silencing instability at HML and HMR for each of the mutants (Figure 1C).
The CRASH assay reveals how unstable transcriptional silencing is in a given strain, but because it is a permanent switch, the assay is unable to capture the heritability of the de-repressed state at HML and HMR. To determine how heritable the loss-of-silencing events were in strains with the pol30-6, pol30-8, and pol30-79 alleles, we first performed an α-factor halo assay (Figure 1D). When MATa cells are exposed to the mating pheromone α-factor, they arrest in G1. On a lawn of MATa cells, this results in a halo of arrested cells surrounding the source of α-factor (Figure 1D, wild type) However, if MATa cells lose silencing at HMLα, they no longer arrest in response to α-factor (Figure 1D, sir4∆). If the loss-of-silencing events created by the pol30 mutants were heritable, colonies would grow within the halo, as noted for the sir4∆ strain. However, for all three alleles, we observed no cell growth within the halos (Figure 1D). In agreement with the α-factor halo results, strains containing pol30-6, pol30-8, or pol30-79 also showed only low levels of HMLα1, HMLα2, and HMRa1 transcripts by quantitative RT-PCR (Figure 1E). Analysis of cre transcripts from CRASH strains with the POL30 alleles also revealed only low levels of transcription (Figure S1).
The absence of a notable increase in transcripts from HML and HMR was particularly surprising for pol30-8, which had an extremely high CRASH sectoring rate (Figure 1, B and C) and was previously suggested to have bistable epigenetic states based upon the HMR::ADE2 reporter (Zhang et al. 2000; Miller et al. 2010). Therefore, we also placed this allele in another reporter strain that encodes GFP at HMRα2 and RFP at HMLα2. In sir4∆ colonies, every cell expresses both RFP and GFP, whereas sir1∆ colonies have GFP and RFP sectors (Figure 1F), representing the bistable epigenetic states characteristic of this deletion (Pillus and Rine 1989). If the pol30-8 allele resulted in a population of cells with stable expression from HML or HMR, we would expect fluorescent sectors, just like sir1∆ (Figure 1F). Instead, pol30-8 colonies were not sectored, meaning that any transcription occurring from the locus following a loss-of-silencing event was transient (Figure 1F).
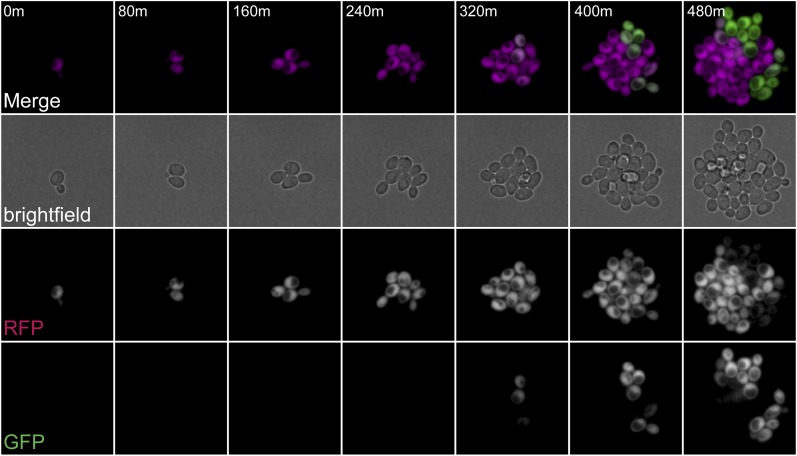
Loss-of-silencing events in strains with defective POL30 alleles occurred predominantly in cycling cells
Given the major role of PCNA in DNA replication, we considered that the loss-of-silencing events may occur only during S phase. Alternatively, because PCNA is involved in replication-independent roles such as DNA repair, it was possible that heterochromatin assembled in the pol30 mutants might be unstable at any point in the cell cycle. We performed time-lapse microscopy using CRASH strains to compare the rate that silencing was lost in G1-arrested cells to the rate in cycling cells. As an example, in cycling pol30-8 cells, switches were readily visible over the time course of 8 hr (Figure 2). In wild-type cells, the low rate of switching was about the same for arrested vs. cycling cells. However, in cells containing each of the pol30 mutants, losses of silencing predominantly occurred in cycling cells. Arrested pol30 mutants exhibited a low frequency of silencing loss comparable to that seen in wild type (Table 1). These results suggested that the pol30-6, pol30-8, and pol30-79 alleles caused only transient losses of silencing in actively cycling cells, with quick re-establishment of the silent state.
Figure 2.
Loss-of-silencing events in strains with defective POL30 alleles occurred predominantly in cycling cells. Representative images of two loss-of-silencing events in one micro-clone of cycling cells in the HMLα2::cre CRASH assay containing the pol30-8 allele (JRY11635, bar1Δ). See Table 1 for calculation of switching rates for all POL30 alleles.
Table 1. Loss-of-silencing events in strains with defective POL30 alleles occurred predominantly in cycling cells.
| Strain | Allele | Arrested (switches/10,000 cells) | No. of cells counted | Cycling (switches/10,000 divisions | No. of divisions calculated |
|---|---|---|---|---|---|
| JRY11597 | POL30 | 8 | 6070 | 5 | 115,832 |
| JRY11682 | pol30-6 | <10a | 1035 | 60 | 24,000 |
| JRY11635 | pol30-8 | 17 | 1870 | 179 | 16,319 |
| JRY11599 | pol30-79 | <9a | 1323 | 73 | 14,923 |
Quantification of CRASH switches in arrested and cycling cells for strains containing different POL30 alleles. Time courses for each strain were done in α-factor–arrested (40 nM) and cycling conditions over an 8-hr time frame with images taken every 5 or 7 min. Quantification was performed as described in Materials and Methods and took into account differences in generation time of each strain. The time course was performed once for POL30 and twice for pol30-6, pol30-8, and pol30-79, with results being combined from each experiment for the mutants.
No switches were observed during the 8-hr time course, so we reported the upper limit of loss rates.
Silencing loss caused by POL30 alleles was dependent on ploidy
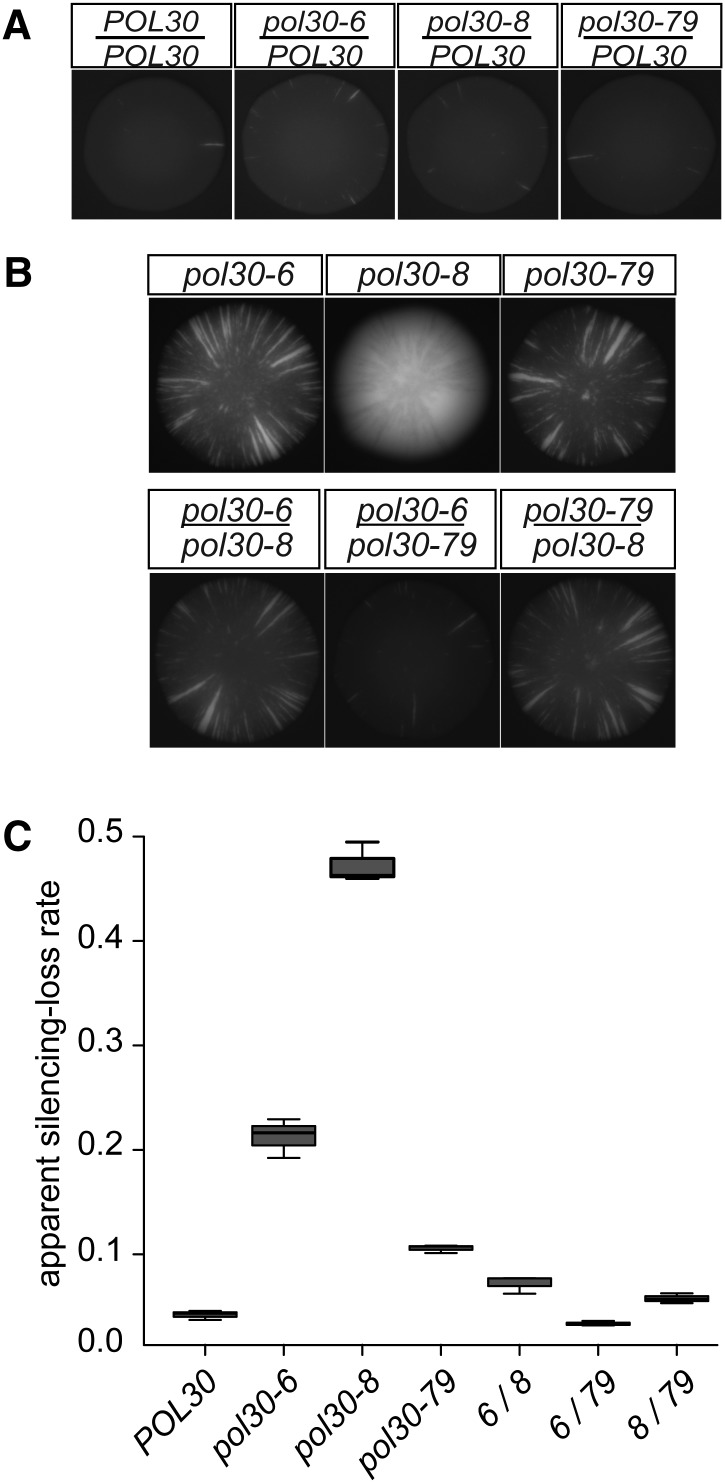
To determine whether each of the pol30 mutants disrupted silencing in the CRASH assay through the same mechanism, we performed pairwise complementation testing among the three alleles. As a necessary prerequisite, we tested each allele in a diploid in combination with wild-type POL30. pol30-6, pol30-8, and pol30-79 were all recessive to POL30 by this assay (Figure 3A).
Figure 3.
POL30 alleles complemented in diploids. (A) Each pol30 allele was recessive to wild-type POL30 in the CRASH assay POL30/POL30 (JRY11159), pol30-6/POL30 (JRY11160), pol30-8/POL30 (JRY11169), and pol30-79/POL30 (JRY11161). Only the GFP channel is shown. These diploid strains contained only one HMLα2::cre and one RFP-hphMX-GFP cassette. (B) Complementation of pol30-6, pol30-8, and pol30-79 in the CRASH assay. Only the GFP channel is shown. The top row shows representative haploid colonies containing the indicated allele: pol30-6 (JRY11137), pol30-8 (JRY11188), and pol30-79 (JRY11141). The bottom row shows representative diploid colonies containing a combination of the indicated alleles: pol30-6/pol30-8 (JRY11656), pol30-6/pol30-79 (JRY11657), and pol30-8/pol30-79 (JRY11658). Diploid strains contained only one HMLα 2::cre and one RFP-hphMX-GFP cassette. (C) The apparent silencing-loss rates for each of the strains in B and POL30 (JRY10790) were quantified by flow cytometry as described in Figure 1C.
If two recessive pol30 mutants disrupt heterochromatin through different mechanisms, then the combination of those two alleles in the diploid should complement, decreasing the frequency of RFP-to-GFP switches compared to each allele alone. All three combinations of pol30 mutants in heteroallelic diploids decreased sectoring relative to haploids with each allele individually, most dramatically evident in the pol30-6/pol30-8 diploid (Figure 3, B and C). Because the sectoring phenotype of pol30-79 was weak on its own, its effect in combination with the other alleles was not as striking but still noticeable in combination with both pol30-6 and pol30-8 (Figure 3, B and C).
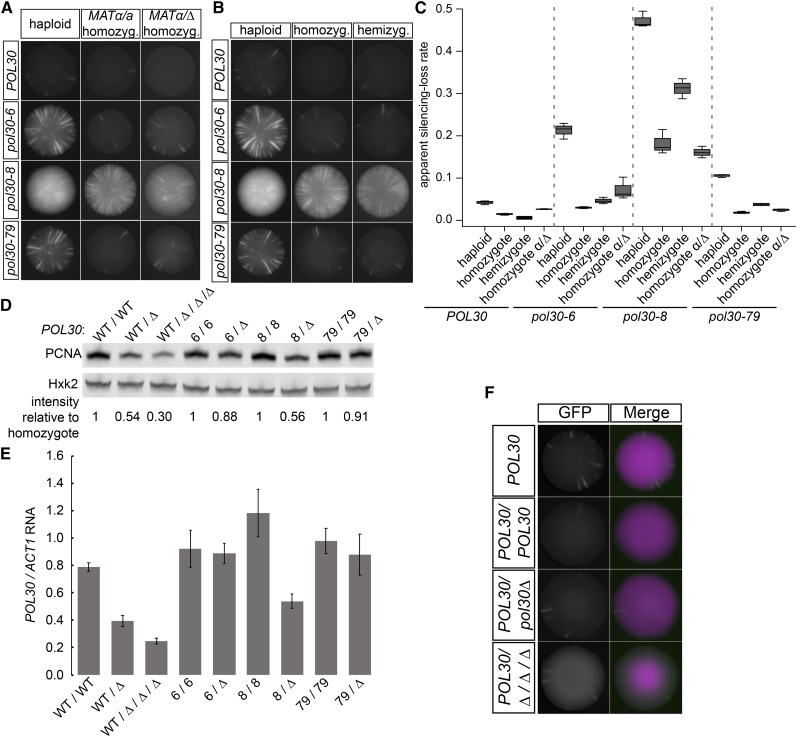
In the simplest manifestation of the complementation test in yeast genetics, the phenotype of haploids containing each mutant of interest is compared to the phenotype of diploids containing both mutations, often ignoring potential complications of ploidy in assessing whether the mutations complement. Surprisingly, homozygosity of each allele at least partially suppressed the loss-of-silencing phenotype as measured by the CRASH assay (Figure 4, A–C).
Figure 4.
The effect of POL30 mutants on silencing was dependent on ploidy. (A) Representative images of haploids, MATα/MATa homozygotes, and MATα/matΔ homozygotes for each indicated POL30 allele in the CRASH assay. Homozygous diploid strains contained two copies of each indicated allele. Only the GFP channel is shown. Diploid strains contained only one HMLα 2::cre and one RFP-hphMX-GFP cassette. POL30 row: JRY10790, JRY11159, and JRY11718. pol30-6 row: JRY11137, JRY11686, and JRY11719. pol30-8 row: JRY11188, JRY11687, and JRY11744. pol30-79 row: JRY11141, JRY11688, and JRY11720. (B) Representative images of haploids, homozygotes, and hemizygotes for each indicated POL30 allele in the CRASH assay. Homozygotes are diploid strains containing two copies of each indicated allele. Hemizygotes are diploid strains containing one copy of the indicated allele over a deletion of POL30 (pol30Δ). Diploid strains contained only one HMLα2::cre and one RFP-hphMX-GFP cassette. POL30 row: JRY10790, JRY11159, and JRY11745. pol30-6 row: JRY11137, JRY11686, and JRY11822. pol30-8 row: JRY11188, JRY11687, and JRY11749. pol30-79 row: JRY11141, JRY11688, and JRY11823. (C) The apparent silencing-loss rates for each of the strains in A and B were quantified by flow cytometry as described in Figure 1C. (D) Immunoblot analysis of PCNA protein levels in homozygotes and hemizygotes of each allele (same strains as B) as well as a tetraploid containing just one copy of wild-type POL30 (WT/Δ/Δ/Δ, JRY12026). The tetraploid contained two copies of HMLα2::cre and the RFP-hphMX-GFP cassette. Hxk2 levels served as a loading control. POL30 allele nomenclature was abbreviated. Each PCNA band intensity was normalized to Hxk2 intensity. After normalization to Hxk2, the relative intensity of each lane to its corresponding POL30, pol30-6, pol30-8, or pol30-79 homozygote was calculated and displayed. (E) Quantitative RT-PCR analysis of POL30 RNA levels in homozygotes and hemizygotes of each allele and a tetraploid with one copy of wild-type POL30 (same strains as B and D). Quantification was performed as in Figure 1E. (F) Representative images of a wild-type haploid (POL30 JRY10790), homozygote (POL30/POL30 JRY11159), hemizygote (POL30/Δ JRY11745), and tetraploid with one copy of POL30 (POL30/Δ/Δ/Δ JRY12026). The haploids and diploids contained only one HMLα2::cre and one RFP-hphMX-GFP cassette. The tetraploid contained two copies of HMLα2::cre and the RFP-hphMX-GFP cassette. The increased background in the GFP channel of the tetraploid was due to loop-out of one RFP-hphMX cassette, leaving just one RFP-hphMX-GFP cassette able to switch. WT, wild type.
To test whether the phenotypic suppression of pol30 mutations reflected mating-type differences between haploids and diploids, we created MATα/mat∆ diploids homozygous for each POL30 allele. These cells, although diploid, express only α-specific genes and therefore behave as MATα haploids. If mating-type were the cause of sectoring suppression in the pol30-6, pol30-8, and pol30-79 diploids, then MATα/mat∆ diploids would be expected to increase the sectoring rate back to the same level as the haploids. For all three alleles, changing the mating type had little or no effect on the reduced sectoring phenotype of diploids (Figure 4, A and C). Therefore, mating type was not responsible for the difference between haploid and diploid pol30 mutants.
Alternatively, the reduced sectoring in diploids could reflect a difference in gene dosage of POL30 between haploids and diploids. We therefore created hemizygotes for each allele in which diploids contained only one copy of the allele instead of two. Although hemizygosity did not increase the sectoring rate to the same level as the haploid, the pol30-8 hemizygote had a statistically significant increase in the loss-of-silencing rate compared to the homozygote (Figure 4C). In contrast to pol30-8, the pol30-6 and pol30-79 hemizygotes had only minor, statistically insignificant increases in sectoring (Figure 4, B and C). Immunoblotting of Pol30 protein levels and quantitative RT-PCR of POL30 RNA levels in the various mutants revealed that pol30-6 and pol30-79 expression was comparable in hemizygotes and homozygotes, whereas wild-type POL30 and pol30-8 expression decreased by half at both the protein and RNA level (Figure 4, D and E).
The effect of pol30 mutants on heterochromatin stability was different in haploids and diploids, and this difference was independent of mating type and largely independent of gene dosage. Moreover, silencing in tetraploid cells with just one copy of POL30 was as stable as in haploids and homozygous or hemizygous diploids (Figure 4F), even with a quarter the expression of POL30 relative to the amount of chromatin the cell (Figure 4, D and E).
pol30-6 and pol30-79 caused high rates of mitotic recombination and gene conversion in diploids
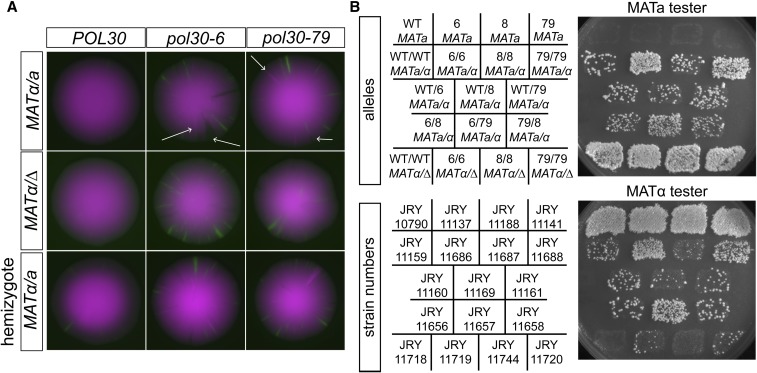
In the course of characterizing the POL30 mutants, we found that pol30-6 and pol30-79 homozygous diploids had high rates of mitotic recombination/gene conversion in diploids that was dependent on mating type but not on POL30 gene dosage. CRASH colonies of pol30-6 and pol30-79 homozygotes revealed mitotic recombination of the RFP-GFP cassette through the existence of sectors that were twice as bright and sectors that were nonfluorescent, suggesting duplication and loss of the cassette, respectively (Figure 5A). pol30-8 homozygotes did not display mitotic recombination (data not shown). In mat∆/MATα diploids homozygous for pol30-6 and pol30-79, this phenotype was suppressed (Figure 5A). Hemizygosity for pol30-6 or pol30-79 did not suppress the high levels of mitotic recombination seen in homozygous diploids (Figure 5A).
Figure 5.
pol30-6 and pol30-79 caused high rates of mitotic recombination and gene conversion in diploids. (A) Representative CRASH colonies of each indicated genotype. The pol30-6 homozygote MATa/MATα diploid (JRY11686) and the pol30-79 homozygote MATa/MATα diploid (JRY11688) both had extrabright sectors and nonfluorescent sectors (examples illustrated by arrows). No, or very few, sectors were observed in POL30 MATa/MATα (JRY11159), POL30 matΔ/ MATα (JRY11718), pol30-6 matΔ/MATα (JRY11719), or pol30-79 matΔ/MATα (JRY11720). Hemizygosity of POL30 (JRY11745), pol30-6 (JRY11822), or pol30-79 (JRY11823) in MATa/ MATα diploids had no effect on the mitotic recombination phenotype. (B) Patch-mating assay. Each indicated strain was patched onto complete medium plates seeded with a freshly plated lawn of either MATa or MATα haploid cells with complementary auxotrophies. After ∼18 hr, mating patches were replica plated onto minimal medium plates. Growth occurs within the patch only if the indicated strains mated with the mating tester lawn. WT, wild type.
Both homozygotes and hemizygotes of pol30-6 and pol30-79 had higher rates of spore inviability than expected, whereas wild type and pol30-8 did not (Table 2). The pol30-6 and pol30-79 alleles complemented one another by this assay, and their combination with pol30-8 also reduced the high levels of spore inviability (Table 2). The consistency between spore inviability and recombination of the GFP cassette in pol30-6 and pol30-79 diploids suggested that the increased spore death might be a result of high levels of unequal or intrachromosomal crossing over, since well-aligned reciprocal recombination would not be expected to cause inviability.
Table 2. pol30-6 and pol30-79 cause high rates of mitotic recombination and gene conversion in diploids.
| % Dead:alive tetrads | ||||||||
|---|---|---|---|---|---|---|---|---|
| Alleles | 0:4 | 1:3 | 2:2 | 3:1 | 4:0 | % Inviable spores | Total tetrads | Strain numbers |
| POL30/POL30 | 85 | 5 | 5 | 5 | 0 | 8 | 20 | JRY11159 |
| POL30/pol30∆ | 0 | 0 | 93 | 5 | 3 | 52 | 40 | JRY11745, JRY11746 |
| pol30-6/pol30-6 | 30 | 20 | 36 | 11 | 4 | 34 | 56 | JRY11686, JRY11714, JRY11715 |
| pol30-6/pol30∆ | 0 | 0 | 50 | 20 | 30 | 63 | 40 | JRY11747, JRY11748 |
| pol30-8/pol30-8 | 90 | 10 | 0 | 0 | 0 | 3 | 20 | JRY11687 |
| pol30-8/pol30∆ | 0 | 0 | 80 | 20 | 0 | 55 | 40 | JRY11749, JRY11750 |
| pol30-79/pol30-79 | 20 | 11 | 36 | 20 | 14 | 46 | 56 | JRY11688, JRY11716, JRY11717 |
| pol30-79/pol30∆ | 0 | 0 | 40 | 45 | 16 | 65 | 38 | JRY11751, JRY11752 |
| pol30-6/pol30-79 | 88 | 12 | 0 | 0 | 0 | 3 | 42 | JRY11657, JRY11685 |
| pol30-6/pol30-8 | 61 | 22 | 11 | 5 | 0 | 15 | 18 | JRY11656 |
| pol30-79/pol30-8 | 75 | 5 | 15 | 5 | 0 | 13 | 20 | JRY11658 |
Spore inviability measurements. At least one, and up to three, independent diploids were sporulated and their tetrads dissected from each of the indicated genotypes. After 2–3 days of growth, tetrads were scored for inviability. The first five columns are the percentages of each combination of dead:alive spores per tetrad.
Further evidence of genome instability of pol30-6 and pol30-79 mutants came from mating-type testing of diploids homozygous for these alleles. Diploid cells express both MATa and MATα information, which prevents them from mating. However, if they undergo mitotic recombination between the centromere and MAT or gene conversion event at the MAT locus, they could become MATa/MATa or MATα/MATα, resulting in some cells in a patch of cells gaining the ability to mate with MATα or MATa tester lawns, respectively. We patched each strain onto normal growth medium with a lawn of either MATa or MATα haploids with complementary auxotrophies. After allowing time for mating, we replica plated these patches onto minimal medium, selecting for diploid cells by the complementation of auxotrophic markers.
As expected, haploid MATa strains mated only with the MATα tester (Figure 5B, top row). Additionally, MATα/mat∆ diploids mated robustly with the MATa tester (Figure 5B, bottom row). There is some mitotic recombination/gene conversion that occurs in wild-type diploids, allowing them to mate inefficiently with MATa and MATα cells (Figure 5B, WT/WT MATa/α). However, pol30-6 and pol30-79 homozygous diploids had much higher levels of mitotic recombination/gene conversion, demonstrated by the greater density of colonies in those patches (Figure 5B, row 2).
Combination of pol30-6 or pol30-79 with a wild-type POL30 allele or the pol30-8 allele reduced the mating efficiency back to wild-type levels (Figure 5B, rows 3 and 4). Although still elevated compared to wild-type, MATα/mat∆ diploids of pol30-6 and pol30-79 had lower amounts of mitotic recombination/gene conversion (Figure 5B, bottom row). Mutations that elevate the rate of chromosome loss would be expected to have a similar phenotype, but the involvement of mating type was suggestive of recombination events rather than chromosome losses being elevated in the homozygous diploids.
In contrast to the spore inviability results, where pol30-6 and pol30-79 complemented one another, there was no detectable complementation by the mating assay. The diploids with both pol30-6 with pol30-79 still had increased ability to mate as a diploid (Figure 5B, row 4) compared to the wild-type diploid.
Coordination of histone chaperones at replication forks by PCNA was required for full transcriptional silencing
Because the pol30 mutants appeared to have separable defects in heterochromatic silencing, we combined each of the alleles with known mutants affecting histone chaperone events at the replication fork: cac1∆, dpb3∆, and mcm2-3A. We made each combination in a CRASH assay strain and compared the sectoring phenotype of the double mutants with the corresponding single mutants.
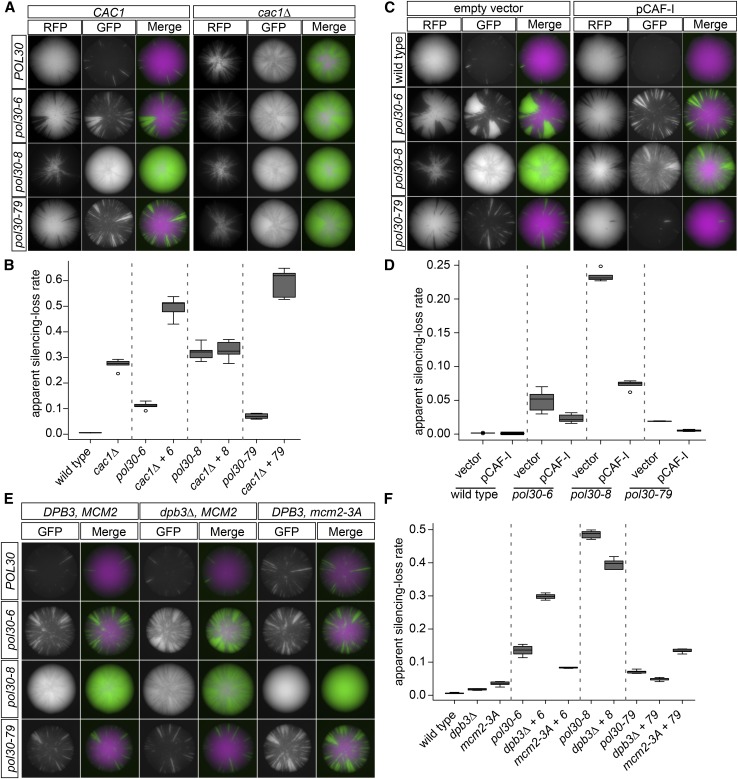
CAC1 is a subunit of the histone chaperone complex CAF-1. CAF-1 deposits newly synthesized H3/H4 tetramers on daughter chromatids of DNA during replication (Smith and Stillman 1989; Serra-Cardona and Zhang 2018). Previous double-mutant analyses using a different silencing assay concluded that pol30-8 results in loss of silencing through a defect in CAF-1 activity, but that pol30-6 and pol30-79 act through a different mechanism (Zhang et al. 2000). In contrast to previous reports of a weak silencing defect for cac1∆ (Zhang et al. 2000; Huang et al. 2005), it had a severe sectoring phenotype in the CRASH assay, comparable to that of pol30-8 alone (Figure 6, A and B). The combination of cac1∆ with pol30-8 was similar in phenotype to the single mutants, in agreement with previous results and the hypothesis that pol30-8 and cac1∆ decrease silencing stability through the same mechanism (Figure 6, A and B). Also, in agreement with previous results, the combination of cac1∆ with pol30-6 or pol30-79 worsened their phenotype significantly, suggesting that pol30-6 and pol30-79 had defects distinct from cac1∆ (Figure 6, A and B).
Figure 6.
Coordination of histone chaperones by PCNA was required for transcriptional silencing. (A) Double-mutant analysis of POL30 alleles with cac1Δ. Representative images of CRASH colonies. The left panel shows colonies with each of the POL30 alleles with wild-type CAC1 strain: POL30 (JRY10790), pol30-6 (JRY11137), pol30-8 (JRY11188), and pol30-79 (JRY11141). The right panel shows colonies with each of the POL30 alleles in combination with deletion of CAC1 (cac1 Δ): POL30 cac1Δ (JRY11193), pol30-6 cac1Δ (JRY11192), pol30-8 cac1Δ (JRY11189), and pol30-79 cac1Δ (JRY11163). (B) The apparent silencing-loss rates for each of the strains in A were quantified by flow cytometry as described in Figure 1C. (C) Overexpression of the CAF-1 complex in combination with POL30 alleles. Representative images of CRASH colonies. The left panel shows colonies with each of the POL30 alleles in combination with a 2μ vector (pRS425): POL30 (JRY11175), pol30-6 (JRY11176), pol30-8 (JRY11177), and pol30-79 (JRY11178). The right panel shows colonies with each of the POL30 alleles in combination with a 2μ plasmid expressing all three subunits of the CAF-1 complex, CAC1, CAC2, and CAC3 (pJR3418): POL30 pCAF-1 (JRY11165), pol30-6 pCAF-1 (JRY11166), pol30-8 pCAF-1 (JRY11167), and pol30-79 pCAF-1 (JRY11168). (D) The apparent silencing-loss rates for each of the strains in C were quantified by flow cytometry as described in Figure 1C. (E) Double-mutant analysis of POL30 alleles with dpb3Δ and mcm2-3A alleles. Representative images of CRASH colonies. In the left panel are each of the POL30 alleles in a wild-type strain: POL30 (JRY10790), pol30-6 (JRY11137), pol30-8 (JRY11188), and pol30-79 (JRY11141). In the middle panel are each of the POL30 alleles in combination with deletion of DPB3 (dpb3Δ): POL30 dpb3Δ (JRY11760), pol30-6 dpb3Δ (JRY11806), pol30-8 dpb3Δ (JRY11808), and pol30-79 dpb3Δ (JRY11810). In the right panel are each of the POL30 alleles in combination with the mcm2-3A allele: POL30 mcm2-3A (JRY11812), pol30-6 mcm2-3A (JRY11987), pol30-8 mcm2-3A (JRY11989), and pol30-79 mcm2-3A (JRY11991). (F) The apparent silencing-loss rates for each of the strains in E were quantified by flow cytometry as described in Figure 1C. The silencing-loss rate for pol30-8 mcm2-3A double mutant could not be quantified because it uniformly expressed GFP.
To further test each allele’s dependence on CAF-1 for their silencing phenotype, we overexpressed the three subunits of CAF-1, CAC1, CAC2, and CAC3, from a 2μ plasmid (pCAF-1, pJR3418; Janke et al. 2018). If overexpression of CAF-1 could suppress or rescue the phenotype of a pol30 mutant, it would be strong evidence that the pol30 mutant weakened silencing through reduced CAF-1 activity. Compared to an empty vector control, overexpression of CAF-1 strongly suppressed the phenotype of pol30-8 (Figure 6, C and D), in agreement with the conclusion from the double-mutant analysis between pol30-8 and cac1∆ (Figure 6, A and B). In contrast, overexpression of CAF-1 in strains harboring pol30-6 and pol30-79 had no statistically significant effect on their silencing defects (Figure 6, C and D).
Although CAF-1 chaperones newly synthesized histones, recent evidence implicates both Dpb3 and Mcm2 as having a role in chaperoning parental histones at the replication fork. Dpb3 and Dpb4 are part of DNA polymerase ε, the leading strand polymerase, and redeposit parental (old) histones onto the leading strand during DNA replication (He et al. 2017; Bellelli et al. 2018). Mcm2 is a subunit of the replicative helicase MCM2-7 and is responsible for redeposition of parental histones onto the lagging strand during DNA replication (Gan et al. 2018; Petryk et al. 2018). Because MCM2 is an essential gene in yeast, we used the mcm2-3A allele, which has a defect in its histone chaperone activity, but not in its helicase activity (Foltman et al. 2013).
In the CRASH assay, dpb3∆ and mcm2-3A on their own had minor but statistically insignificant sectoring phenotypes (Figure 6, E and F). Their combination with different pol30 mutants showed an interesting pattern: if combination of an allele with dpb3∆ enhanced the sectoring phenotype, then combination with mcm2-3A weakened it, and vice versa (Figure 6F). Except for the pol30-6 dpb3∆ combination, the differences in phenotype between the single and double mutants are modest, suggesting that these effects might be indirect.
Discussion
The pol30-8 allele did not cause epigenetically bistable states
Previous studies of transcriptional silencing in strains carrying pol30-8 used the ADE2 gene within the HMR locus as a reporter of gene silencing. When transcriptional silencing is disrupted, cells express ADE2 and colonies are white instead of red. pol30-8 colonies have red sectors and white sectors, previously thought to represent two stable populations of cells: silencing ADE2 or expressing ADE2. Surprisingly, although pol30-8 had highly unstable silencing in the CRASH assay, we found no evidence of bistable populations in strains carrying the pol30-8 allele. The CRASH assay relies on alleles of HML and HMR that more closely resemble the native structure of those loci; it uses the native α2 promoter and leaves the 5′ and 3′ UTRs and the α1 gene intact (Dodson and Rine 2015), none of which is true for the previously used HMR::ADE2 reporter. Moreover, the α-factor halo assay failed to reveal any clonally stable populations of cells expressing HML in any of the pol30 mutants, nor did the HMLα2::RFP, HMRα2::GFP reporter. Therefore, the ADE2 sectors were likely an artifact of the metabolically responsive ADE2 reporter in this context (Rossman et al. 2011) as we found no evidence of bistability among pol30 mutants by two independent and metabolically neutral assays.
Defective assembly vs. maintenance of silenced chromatin POL30 mutants
Although the expression of PCNA is highest in S phase and its major role in the cell occurs at replication forks, it is still present at lower levels in all stages of the cell cycle, functioning in DNA damage signaling and repair (Bauer and Burgers 1990). By monitoring loss-of-silencing events in unsynchronized cycling cells and cells arrested in G1 over time, we found that all three of the pol30 mutants increased loss-of-silencing rates compared to wild-type POL30 only in cycling cells.
As the replication fork progresses, nucleosomes in front of the fork are disassembled and reassembled onto daughter strands and newly synthesized nucleosomes are also deposited onto daughter strands. Sir proteins must reassemble on nucleosomes to re-set the heterochromatin state. Heterochromatin is then maintained through G2, M, and G1 until it is assembled again in the next round of replication. Since pol30-6, pol30-8, and pol30-79 only caused loss of silencing in actively cycling cells, the predominant role of PCNA in silencing stability is most likely through heterochromatin assembly during S phase.
Histone chaperones ensure the stability of heterochromatin through DNA replication
Previous results and supporting results in the CRASH assay shown here suggest that the unstable silencing caused by pol30-8 is due to a defect in new histone deposition by the replication-coupled histone chaperone complex CAF-1 (Zhang et al. 2000). Genetic evidence also suggests an interaction between two other histone chaperones, Hir1 and Asf1, and the pol30-6 and pol30-79 alleles (Sharp et al. 2001). If the pol30 mutants caused slower or defective histone deposition during DNA replication, with fewer nucleosomes to bind, the SIR complex would be less able to properly block transcription at HML and HMR until the chromatin state was restored.
We explored the possibility that the pol30 mutants might affect histone recycling from the mother DNA duplex to daughter chromatids by performing double-mutant analysis with dpb3∆ and mcm2-3A. The effects on CRASH sectoring phenotypes were minor but displayed an intriguing pattern: mcm2-3A and dpb3∆ had opposite effects for each given pol30 mutant. These results could be interpreted as a leading strand or lagging strand bias for each allele. Biochemical studies of pol30-79 show that it has a defect in binding to DNA polymerase δ (DNA Pol δ), the lagging strand polymerase, but not DNA polymerase ε (DNA Pol ε), the leading strand polymerase (Eissenberg et al. 1997). In contrast to pol30-79, pol30-6 is completely unable to bind DNA Pol ε, with only reduced binding to DNA Pol δ (Ayyagari et al. 1995). Its CRASH phenotype in combination with mcm2-3A and dpb3∆ also mirror pol30-79 analyses. Binding studies between the DNA polymerases and pol30-8 have not been done, but the phenotype of the double-mutant analyses, interpreted in the light of a strand-bias model, suggested that pol30-8 may exhibit weakened binding to DNA Pol δ but not DNA Pol ε.
A surprising effect of ploidy on silencing instability
Complementation tests revealed that any combination of pol30-6, pol30-8, or pol30-79 in diploids complemented, resulting in colonies with fewer sectors in the CRASH assay than the haploids with each allele alone. These results suggested that POL30 contributed at least two, and maybe three, separable roles in the assembly of stable silent chromatin. However, we also found that diploids carrying homozygous copies of pol30-6 or pol30-79 displayed no CRASH phenotype, and diploids homozygous for pol30-8 had fewer sectors than a pol30-8 haploid. Diploids homozygous for POL30 alleles and expressing only MATα mating-type information all displayed the same suppression, establishing that the phenotype was not an unexpected manifestation of mating-type regulation. Likewise, hemizygosity of each allele in diploids failed to restore their CRASH sectoring phenotype back to haploids levels. Thus ploidy, independently of mating type and dosage of PCNA, changed the sensitivity of diploid cells to defects in histone deposition caused by the pol30 mutant. To our knowledge, this wrinkle is unique among complementation tests, although we caution that most complementation tests are inadequately powered to detect the effect of ploidy. We note that none of the genes studied here are among the set shown to have ploidy-dependent effects on their expression (Storchová et al. 2006).
HML and HMR cluster at the nuclear periphery with a higher local concentration of Sir proteins in the cluster than in the rest of the nucleoplasm (Gotta et al. 1996; Bystricky et al. 2009; Miele et al. 2009; Kirkland and Kamakaka 2013). The two copies of HML and HMR and the SIR genes in diploids might increase the local concentration of Sir proteins (despite the larger volume associated with increases in ploidy) enough that it could overcome a brief disruption in histone deposition during DNA replication in pol30 mutants. Although tethering to the nuclear periphery is not required for silencing in an otherwise wild-type strain (Gartenberg et al. 2004), the pol30 mutants might create a sensitized background that is more dependent on clustering of the SIR complex with HML and HMR for maintenance of silencing through replication.
A note on PCNA expression in hemizygotes
Compared to expression in homozygotes, the expression of PCNA in pol30-6 and pol30-79 strains did not decrease by half, whereas PCNA levels in POL30 and pol30-8 strains did. The expression of POL30 is cell-cycle regulated (Bauer and Burgers 1990) and increases in response to DNA damage (Jelinsky and Samson 1999; Lee et al. 2007). Differences in cell-cycle distribution or levels of DNA damage in pol30-6 and pol30-79 hemizygotes compared to homozygotes would be compatible explanations for observations on PCNA levels produced by the various alleles.
High levels of DNA damage and defective repair in pol30-6 and pol30-79
The high rates of mitotic recombination and gene conversion we observed in pol30-6 and pol30-79 diploids presumably reflected higher levels of DNA damage in these mutants. Work from multiple laboratories shows that all three alleles, and especially pol30-6 and pol30-79, are more sensitive to DNA damaging agents (Ayyagari et al. 1995; Eissenberg et al. 1997; Zhang et al. 2000; Miller et al. 2008). Additionally, pol30-79 causes higher rates of substitutions and small insertions and deletions compared to wild-type cells (Eissenberg et al. 1997).
The published studies were done using haploid cells, where mitotic recombination is seldom detected. MATa/MATα diploids avoid nonhomologous end-joining because the a1-α2 transcription factor represses required genes NEJ1 and LIF1 (Aström et al. 1999; Lee et al. 1999; Frank-Vaillant and Marcand 2001; Kegel et al. 2001; Valencia et al. 2001). Deleting MATa in the pol30-6 and pol30-79 homozygous diploids suppressed the increase in mitotic recombination, suggesting that the higher rates of mitotic recombination and gene conversion might be caused by homology-directed repair in lieu of nonhomologous end-joining. We observed synthetic lethality in the haploid double mutants pol30-6 rad54∆, pol30-79 rad54∆, and pol30-6 rad9∆ and synthetic growth defects in the double mutants pol30-79 rad9∆, pol30-6 H3K56R, and pol30-79 H3K56R (data not shown). These synthetic phenotypes fit with a higher DNA damage load in strains carrying pol30-6 and pol30-79. Our work in diploids provided more evidence that the pol30-6 and pol30-79 alleles have DNA damage and repair defects. It is unlikely that the mitotic recombination and silencing phenotypes of the pol30-6 and pol30-8 alleles are directly related since one phenotype was dependent on mating type (mitotic recombination) and the other was not (silencing).
The mutants pol30-6 and pol30-79 reduce global levels of histone H3 lysine 56 (H3K56) acetylation (Recht et al. 2006; Miller et al. 2008), a histone modification that increases the affinity of CAF-1 for H3/H4 dimers (Masumoto et al. 2005; Recht et al. 2006; Li et al. 2008). Misregulation of H3K56 acetylation, both hypoacetylation and hyperacetylation, is associated with increased DNA damage and sensitivity to DNA-damaging agents (Hyland et al. 2005; Masumoto et al. 2005; Recht et al. 2006; Clemente-Ruiz et al. 2011; Wurtele et al. 2012). Additionally, many DNA repair factors contain PCNA-Interacting Protein (PIP) or PIP-like motifs (reviewed in Boehm and Washington (2016)). The pol30-6 and pol30-79 mutations disrupt the cleft of PCNA that bind PIP motifs (Kondratick et al. 2018), which could prevent the recruitment of DNA repair factors to sites of DNA damage. These results could explain the high levels of DNA damage and mitotic recombination in pol30-6 and pol30-79. Furthermore, the recessive nature of these alleles in all of our assays suggests that PCNA trimers containing both mutant and wild-type alleles are fully functional for DNA repair and transcriptional silencing.
Acknowledgments
We thank the Stillman and Burgers laboratories for plasmids carrying POL30 alleles. We thank members of the Rine and Koshland laboratories for helpful discussions and Hector Nolla at the Cancer Research Laboratory Flow Cytometry Facility for technical help with flow cytometry. This work was supported by an National Science Foundation Predoctoral Fellowship (to M.B.) and grants from the National Institutes of Health (GM031105 and GM120374 to J.R.).
Footnotes
Supplemental material available at FigShare: https://doi.org/10.25386/genetics.9343232.
Communicating editor: O. Rando
Literature Cited
- Alabert C., Jasencakova Z., and Groth A., 2017. Chromatin replication and histone dynamics. Adv. Exp. Med. Biol. 1042: 311–333. 10.1007/978-981-10-6955-0_15 [DOI] [PubMed] [Google Scholar]
- Aström S. U., Okamura S. M., and Rine J., 1999. Yeast cell-type regulation of DNA repair. Nature 397: 310 10.1038/16833 [DOI] [PubMed] [Google Scholar]
- Ayyagari R., Impellizzeri K. J., Yoder B. L., Gary S. L., and Burgers P. M., 1995. A mutational analysis of the yeast proliferating cell nuclear antigen indicates distinct roles in DNA replication and DNA repair. Mol. Cell. Biol. 15: 4420–4429. 10.1128/MCB.15.8.4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer G. A., and Burgers P. M., 1990. Molecular cloning, structure and expression of the yeast proliferating cell nuclear antigen gene. Nucleic Acids Res. 18: 261–265. 10.1093/nar/18.2.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellelli R., Belan O., Pye V. E., Clement C., Maslen S. L. et al. , 2018. POLE3-POLE4 is a histone H3–H4 chaperone that maintains chromatin integrity during DNA replication. Mol. Cell 72: 112–126.e5. 10.1016/j.molcel.2018.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar T. R., Castillo A. G., Osborne M. J., Borden K. L. B., Kornblatt J. et al. , 2009. Two fundamentally distinct PCNA interaction peptides contribute to chromatin assembly factor 1 function. Mol. Cell. Biol. 29: 6353–6365. 10.1128/MCB.01051-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm E. M., and Washington M. T., 2016. R.I.P. to the PIP: PCNA binding motif no longer considered specific. BioEssays 38: 1117–1122. 10.1002/bies.201600116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystricky K., Van Attikum H., Montiel M. D., Dion V., Gehlen L. et al. , 2009. Regulation of nuclear positioning and dynamics of the silent mating type loci by the yeast Ku70/Ku80 complex. Mol. Cell. Biol. 29: 835–848. 10.1128/MCB.01009-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente-Ruiz M., Gonzalez-Prieto R., and Prado F., 2011. Histone H3K56 acetylation, CAF1, and Rtt106 coordinate nucleosome assembly and stability of advancing replication forks. PLoS Genet. 7: e1002376 10.1371/journal.pgen.1002376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart M. A., and Oliviero S., 2001. Preparation of yeast RNA. Curr. Protoc. Mol. Biol. Chapter 13: Unit13.12 10.1002/0471142727.mb1312s23 [DOI] [PubMed] [Google Scholar]
- Dodson A. E., and Rine J., 2015. Heritable capture of heterochromatin dynamics in Saccharomyces cerevisiae. eLife 4: e05007 10.7554/eLife.05007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein A. D., Tsuchida M. A., Amodaj N., Pinkard H., Vale R. D. et al. , 2014. Advanced methods of microscope control using μManager software. J. Biol. Methods 1: e10. 10.14440/jbm.2014.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J. C., Ayyagari R., Gomes X. V., and Burgers P. M., 1997. Mutations in yeast proliferating cell nuclear antigen define distinct sites for interaction with NA polymerase delta and DNA polymerase epsilon. Mol. Cell. Biol. 17: 6367–6378. 10.1128/MCB.17.11.6367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltman M., Evrin C., De Piccoli G., Jones R. C., Edmondson R. D. et al. , 2013. Eukaryotic replisome components cooperatre to process histones during chromosome replication. Cell Rep. 3: 892–904. 10.1016/j.celrep.2013.02.028 [DOI] [PubMed] [Google Scholar]
- Frank-Vaillant M., and Marcand S., 2001. NHEJ regulation by mating type is exercised through a novel protein, Lif2p, essential to the ligase IV pathway. Genes Dev. 15: 3005–3012. 10.1101/gad.206801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan H., Serra-Cardona A., Hua X., Zhou H., Labib K. et al. , 2018. The Mcm2-Ctf4-Polα axis facilitates parental histone H3–H4 transfer to lagging strands. Mol. Cell 72: 140–151.e3. 10.1016/j.molcel.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartenberg M. R., and Smith J. S., 2016. The nuts and bolts of transcriptionally silent chromatin in Saccharomyces cerevisiae. Genetics 203: 1563–1599. 10.1534/genetics.112.145243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartenberg M. R., Neumann F. R., Laroche T., Blaszczyk M., and Gasser S. M., 2004. Sir-mediated repression can occur independently of chromosomal and subnuclear contexts. Cell 119: 955–967. 10.1016/j.cell.2004.11.008 [DOI] [PubMed] [Google Scholar]
- Goldstein A. L., and McCusker J. H., 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15: 1541–1553. [DOI] [PubMed] [Google Scholar]
- Gotta M., Laroche T., Formenton A., Maillet L., Scherthan H. et al. , 1996. The clustering of telomeres and colocalization with Rap1, Sir3, and Sir4 proteins in wild-type Saccharomyces cerevisiae. J. Cell Biol. 134: 1349–1363. 10.1083/jcb.134.6.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueldener U., Heinisch J., Koehler G. J., Voss D., and Hegemann J. H., 2002. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res. 30: e23 10.1093/nar/30.6.e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Li Y., Dong Q., Chang A. Y., Gao F. et al. , 2017. Coordinated regulation of heterochromatin inheritance by Dpb3-Dpb4 complex. Proc. Natl. Acad. Sci. USA 114: 12524–12529. 10.1073/pnas.1712961114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Zhou H., Katzmann D., Hochstrasser M., Atanasova E. et al. , 2005. Rtt106p is a histone chaperone involved in heterochromatin-mediated silencing. Proc. Natl. Acad. Sci. USA 102: 13410–13415. 10.1073/pnas.0506176102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland E. M., Cosgrove M. S., Molina H., Wang D., Pandey A. et al. , 2005. Insights into the role of histone H3 and histone H4 core modifiable residues in Saccharomyces cerevisiae. Mol. Cell. Biol. 25: 10060–10070. 10.1128/MCB.25.22.10060-10070.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke R., King G. A., Kupiec M., and Rine J., 2018. Pivotal roles of PCNA loading and unloading in heterochromatin function. Proc. Natl. Acad. Sci. USA 115: E2030–E2039. 10.1073/pnas.1721573115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinsky S. A., and Samson L. D., 1999. Global response of Saccharomyces cerevisiae to an alkylating agent. Proc. Natl. Acad. Sci. USA 96: 1486–1491. 10.1073/pnas.96.4.1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegel A., Sjostrand J. O., and Astrom S. U., 2001. Nej1p, a cell-type specific regulator of nonhomologous end joining in yeast. Curr. Biol. 11: 1611–1617. 10.1016/S0960-9822(01)00488-2 [DOI] [PubMed] [Google Scholar]
- Kirkland J. G., and Kamakaka R. T., 2013. Long-range heterochromatin association is mediated by silencing and double-strand DNA break repair proteins. J. Cell Biol. 201: 809–826. 10.1083/jcb.201211105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratick C. M., Litman J. M., Shaffer K. V., Washington M. T., and Dieckman L. M., 2018. Crystal structures of PCNA mutant proteins defective in gene silencing suggest a novel interaction site on the front face of the PCNA ring. PLoS One 13: e0193333 10.1371/journal.pone.0193333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. E., DeLoache W. C., Cervantes B., and Dueber J. E., 2015. A highly characterized yeast toolkit for modular, multipart assembly. ACS Synth. Biol. 4: 975–986. 10.1021/sb500366v [DOI] [PubMed] [Google Scholar]
- Lee M. W., Kim B. J., Choi H. K., Ryu M. J., Kim S. B. et al. , 2007. Global protein expression profiling of budding yeast in response to DNA damage. Yeast 24: 145–154. 10.1002/yea.1446 [DOI] [PubMed] [Google Scholar]
- Lee S. E., Paques F., Sylvan J., and Haber J. E., 1999. Role of yeast SIR genes and mating type in directing DNA double-strand breaks to homologous and non-homologous repair paths. Curr. Biol. 9: 767–770. 10.1016/S0960-9822(99)80339-X [DOI] [PubMed] [Google Scholar]
- Li Q., Zhou H., Wurtele H., Davies B., Horazdovsky B. et al. , 2008. Acetylation of histone H3 lysine 56 regulated replication-coupled nucleosome assembly. Cell 134: 244–255. 10.1016/j.cell.2008.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto H., Hawke D., Kobayashi R., and Verreault A., 2005. A role for cell-cycle-regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature 436: 294–298. 10.1038/nature03714 [DOI] [PubMed] [Google Scholar]
- Miele A., Bystricky K., and Dekker J., 2009. Yeast silent mating type loci form heterochromatic clusters through silencer protein-dependent long-range interactions. PLoS Genet. 5: e1000478 10.1371/journal.pgen.1000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., Yang B., Foster T., and Kirchmaier A. L., 2008. Proliferating cell nuclear antigen and ASF1 modulate silent chromatin in Saccharomyces cerevisiae via lysine 56 on histone H3. Genetics 179: 793–809. 10.1534/genetics.107.084525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., Chen J., Takasuka T. E., Jacobi J. L., Kaufman P. D. et al. , 2010. Proliferating cell nuclear antigen (PCNA) is required for cell cycle-regulated silent chromatin on replicated and nonreplicated genes. J. Biol. Chem. 285: 35142–35154. 10.1074/jbc.M110.166918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milutinovic S., Ahuang Q., and Szyf M., 2002. Proliferating cell nuclear antigen associates with histone deacetylase activity, integrating DNA replication and chromatin modification. J. Biol. Chem. 277: 20974–20978. 10.1074/jbc.M202504200 [DOI] [PubMed] [Google Scholar]
- Moldovan G. L., Pfander B., and Jentsch S., 2007. PCNA, the maestro of the replication fork. Cell 129: 665–679. 10.1016/j.cell.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Murzina N., Verreault A., Laue E., and Stillman B., 1999. Heterochromatin dynamics in mouse cells: interaction between chromatin assembly factor 1 and HP1 proteins. Mol. Cell 4: 529–540. 10.1016/S1097-2765(00)80204-X [DOI] [PubMed] [Google Scholar]
- Nakano S., Stillman B., and Horvitz H. R., 2011. Replication-coupled chromatin assembly generates a neuronal bilateral asymmetry in C. elegans. Cell 147: 1525–1536. 10.1016/j.cell.2011.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruk S., Sedkov Y., Johnston D. M., Hodgson J. W., Black K. L. et al. , 2012. TrxG and PcG proteins but not methylated histones remain associated with DNA through replication. Cell 150: 922–933. 10.1016/j.cell.2012.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryk N., Dalby M., Wenger A., Strom C. B., Strandsby A. et al. , 2018. MCM2 promotes symmetric inheritance of modified histones during DNA replication. Science 361: 1389–1392. 10.1126/science.aau0294 [DOI] [PubMed] [Google Scholar]
- Pillus L., and Rine J., 1989. Epigenetic inheritance of transcriptional states in S. cerevisiae. Cell 59: 637–647. 10.1016/0092-8674(89)90009-3 [DOI] [PubMed] [Google Scholar]
- Recht J., Tsubota T., Tanny J. C., Diaz R. L., Berger J. M. et al. , 2006. Histone chaperone Asf1 is required for histone H3 lysine 56 acetylation, a modification associated with S phase in mitosis and meiosis. Proc. Natl. Acad. Sci. USA 103: 6988–6993. 10.1073/pnas.0601676103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. P., Luo W., Tsaponina O., Chabes A., and Stillman B., 2011. A common telomeric gene silencing assay is affected by nucleotide metabolism. Mol. Cell 42: 127–136. 10.1016/j.molcel.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M. et al. , 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9: 676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Cardona A., and Zhang Z., 2018. Replication-coupled nucleosome assembly in the passage of epigenetic information and cell identity. Trends Biochem. Sci. 43: 136–148. 10.1016/j.tibs.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J. A., Fouts E. T., Krawitz D. C., and Kaufman P. D., 2001. Yeast histone deposition protein Asf1p requires Hir proteins and PCNA for heterochromatic silencing. Curr. Biol. 11: 463–473. 10.1016/S0960-9822(01)00140-3 [DOI] [PubMed] [Google Scholar]
- Shibahara K., and Stillman B., 1999. Replication-dependent marking of DNA by PCNA facilitates CAF-1-coupled inheritance of chromatin. Cell 96: 575–585. 10.1016/S0092-8674(00)80661-3 [DOI] [PubMed] [Google Scholar]
- Smith S., and Stillman B., 1989. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell 58: 15–25. 10.1016/0092-8674(89)90398-X [DOI] [PubMed] [Google Scholar]
- Storchová Z., Breneman A., Cande J., Dunn J., Burbank K. et al. , 2006. Genome-wide genetic analysis of polyploidy in yeast. Nature 443: 541–547. 10.1038/nature05178 [DOI] [PubMed] [Google Scholar]
- Takahashi Y. H., Schulze J. M., Jackson J., Hentrich T., Seidel C. et al. , 2011. Dot1 and histone H3K79 methylation in natural telomeric and HM silencing. Mol. Cell 42: 118–126. 10.1016/j.molcel.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia M., Bentele M., Vaze M. B., Herrmann G., Kraus E. et al. , 2001. NEJ1 controls non-homologous end joining in Saccharomyces cerevisiae. Nature 414: 666–669. 10.1038/414666a [DOI] [PubMed] [Google Scholar]
- Wurtele H., Kaiser G. S., Bacal J., St-Hilaire E., Lee E. H. et al. , 2012. Histone H3 lysine 56 acetylation and the response to DNA replication fork damage. Mol. Cell. Biol. 32: 154–172. 10.1128/MCB.05415-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Shibahara K., and Stillman B., 2000. PCNA connects DNA replication to epigenetic inheritance in yeast. Nature 408: 221–225. 10.1038/35041601 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data necessary for confirming the conclusions presented in the article are represented fully within the article. Supplemental material has been uploaded to the FigShare public repository. File S1 contains supplemental materials and methods. Table S1 contains details about the yeast strains used in this study. Table S2 contains the plasmids used in this study. Table S3 contains oligonucleotides used in this study. Figure S1 contains quantitative RT-PCR results for CRE transcript levels in POL30 mutants. Supplemental material available at FigShare: https://doi.org/10.25386/genetics.9343232.