Abstract
The Target of Rapamycin (TOR or mTOR) is a serine/threonine kinase that regulates growth, development, and behaviors by modulating protein synthesis, autophagy, and multiple other cellular processes in response to changes in nutrients and other cues. Over recent years, TOR has been studied intensively in mammalian cell culture and genetic systems because of its importance in growth, metabolism, cancer, and aging. Through its advantages for unbiased, and high-throughput, genetic and in vivo studies, Caenorhabditis elegans has made major contributions to our understanding of TOR biology. Genetic analyses in the worm have revealed unexpected aspects of TOR functions and regulation, and have the potential to further expand our understanding of how growth and metabolic regulation influence development. In the aging field, C. elegans has played a leading role in revealing the promise of TOR inhibition as a strategy for extending life span, and identifying mechanisms that function upstream and downstream of TOR to influence aging. Here, we review the state of the TOR field in C. elegans, and focus on what we have learned about its functions in development, metabolism, and aging. We discuss knowledge gaps, including the potential pitfalls in translating findings back and forth across organisms, but also describe how TOR is important for C. elegans biology, and how C. elegans work has developed paradigms of great importance for the broader TOR field.
Keywords: WormBook, Caenorhabditis elegans development, metabolism, aging, TOR, TORC1, TORC2, nutrient signaling, growth regulation, Raptor, Rictor, DAF-15, Rheb, Rheb-1, RagA, RAGA-1, RagC, RSKS-1, S6 kinase, Nprl2, NPRL-2, Nprl3, NPRL-3, sphingolipid
TOR is a serine/threonine kinase that was first discovered as a Target Of Rapamycin in yeast, and the mammalian TOR homolog was identified soon after in studies using cultured cells (Kunz et al. 1993; Blenis 2017; Sabatini 2017) . TOR is also commonly referred to as mTOR (mammalian, or mechanistic, target of rapamycin). Extensive work in yeast and mammalian cell culture led to the identification of two mutually exclusive TOR-binding proteins, Raptor (Regulatory Associated Protein of mTOR) and Rictor (Rapamycin-Insensitive Companion of mTOR) (Hara et al. 2002; Kim et al. 2002; Loewith et al. 2002; Sarbassov et al. 2004). The association of TOR with each of these binding proteins defined the two TOR complexes: TOR Complex 1 (TORC1, containing TOR and Raptor) and TOR Complex 2 (TORC2, containing TOR and Rictor), each of which have distinct functions and signaling activities (Saxton and Sabatini 2017) (Figure 1).
Figure 1.
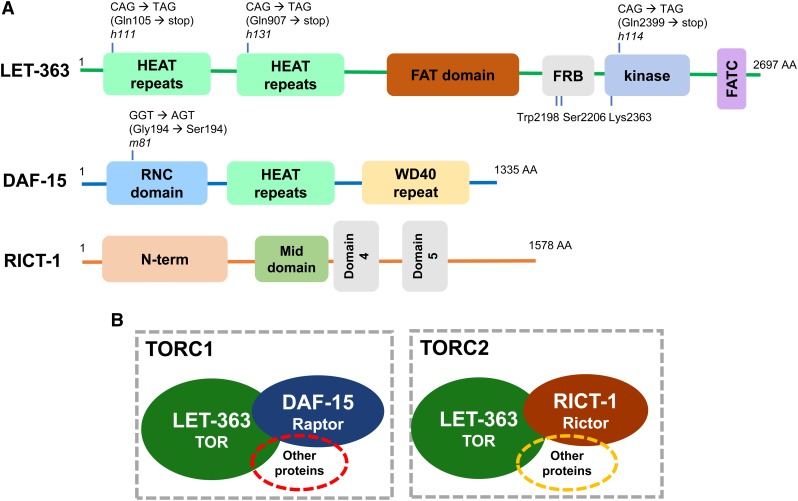
Core components of TOR signaling in C. elegans. (A) Cartoon diagram of the protein structures and domains in LET-363/TOR, DAF-15/Raptor, and RICT-1/Rictor [adapted from Long et al. (2002) and Jia et al. (2004)]. Mutant alleles (h111, h131, h114, and m81) and key conserved residues (Trp2198, Ser2206, and Lys2363) are indicated. HEAT repeats are named for four proteins (Huntingtin, EF3, PP2A, and TOR1) that contain this repeat structure. The RICT-1 domains are less characterized (domain identities taken from InterPro/European Molecular Biology Laboratory-European Bioinformatics Institute). (B) TORC1 is defined as the complex containing LET-363/TOR and DAF-15/Raptor. TORC2 is defined as the complex containing LET-363/TOR and RICT-1/Rictor. It is expected that other proteins are found in these complexes and required for TOR signaling. Please see the text for more discussion. AA, amino acid; FAT, focal adhesion-targeting domain; FATC, focal adhesion-targeting C-terminal domain; FRB, FKBP-Rapamycin-Binding domain (where FKBP stands for FK506-binding protein); RNC, Raptor N-terminal CASPase-like domain; TOR, Target of Rapamycin; TORC, TOR Complex; WD40 repeat, ∼40AA motif that terminates in W-D.
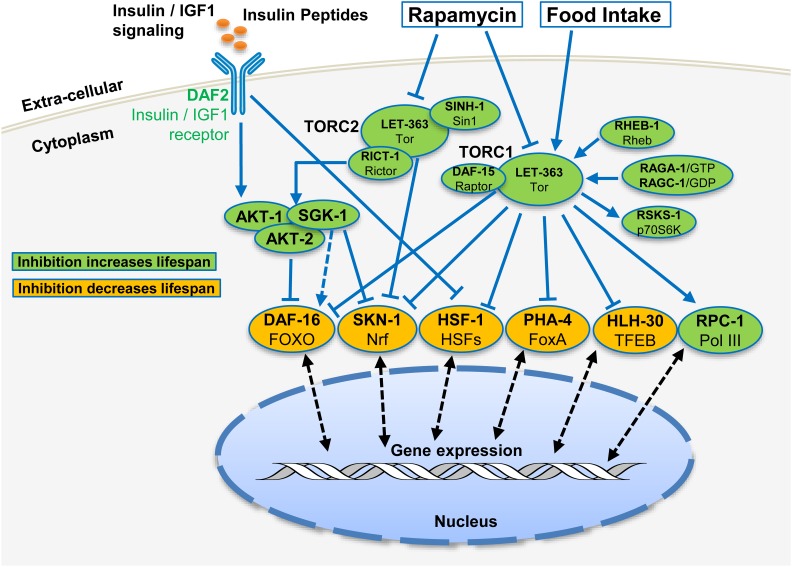
TOR complexes are widely described as signaling systems that sense the levels of various nutrients, energy, and growth factors, and instruct changes in downstream activities involved in development, reproduction, metabolism, behavior, stress responses, and aging (Menon and Manning 2013; Saxton and Sabatini 2017) (Figure 2). In essence, the TOR complexes are critical because they drive growth, development, and anabolic metabolism, but reductions in TOR activity can also have profound consequences by leading to mobilization of mechanisms that protect cells and organisms from stress. Understanding how TOR complexes function is therefore of high significance in our pursuit of mechanisms that underlie aging and various human diseases. While mechanistic studies using yeast and mammalian tissue culture have made much progress in understanding the fundamental cellular functions and molecular mechanisms of TOR signaling, studies using model organisms have increasingly become important to study functions of TOR complexes in vivo under specific physiological conditions. The genetically amenable organism Caenorhabditis elegans has been an outstanding model system for new discoveries in animal development, metabolic regulation, aging, and neuronal functions. Since the connections between nutrient availability and these physiological functions present many unresolved disease-related biological problems that involve TOR functions, the worm system provides unique opportunities to learn about the physiological functions of TOR complexes. In particular, research in C. elegans pioneered the study of how metazoan life span can be increased by decreasing TOR activity, and C. elegans has continued to be a major contributor in understanding the mechanisms involved (Figure 2).
Figure 2.
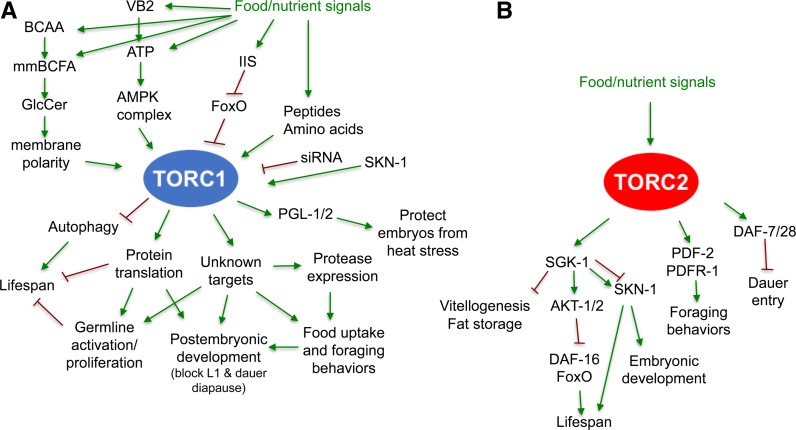
Abbreviated illustration of pathways, or cellular processes, identified to act upstream or downstream of TORC1 (A) and TORC2 (B) in C. elegans. These models are based mainly upon genetic analyses but also incorporate mechanistic findings from other systems. In many cases, C. elegans researchers analyzed the TORC1 or TORC2 function under starvation or dietary restriction conditions, when food/nutrient signals were absent or reduced. Arrows indicate the positive regulation or input, whereas T-bars indicate negative regulation, with neither necessarily indicating direct regulation. Please see the text for more discussion on these pathways and downstream physiological functions. TOR functions are notably complex and wide ranging, extending beyond the canonical functions that have been identified in other organisms. For example, the stress response transcription factor SKN-1 has three different roles regarding the two TOR complexes: it promotes transcription of several TORC1 components, its target genes are activated when TORC1 or translation is inhibited, and it is regulated downstream of TORC2 (see sections Roles of TORC2 in regulating development and behaviors and Life span extension and increased stress resistance from TORC1 inhibition). BCAA, branched-chain AAs; GlcCer, glycosylceramide; IIS, insulin/IGF signaling; mmBCFA, monomethyl branched-chain fatty acid; TOR, Target of Rapamycin; TORC, TOR Complex; VB2, vitamin B2.
TOR Signaling Complexes are Conserved in C. elegans
C. elegans has orthologs of TOR, Raptor, Rictor, and many other conserved regulators of TORC1 and TORC2 activities (Table 1). Note that in many cases the null phenotype of these genes is not yet known.
Table 1. Major players in TOR signaling.
| Gene | Sequence name | Mammalian homolog | References | ||
|---|---|---|---|---|---|
| Complex components | |||||
| let-363 | B0261.2 | TOR | Long et al. (2002) | ||
| daf-15 | C10C5.6 | RAPTOR | Hara et al. (2002) | ||
| rict-1 | F29C12.3 | RICTOR | Jones et al. (2009), Soukas et al. (2009) | ||
| mlst-8 | C10H11.8 | mLST8 | Jones et al. (2009) | ||
| sinh-1 | Y57A10A.20 | mSIN1 | Soukas et al. (2009) | ||
| No homolog identified | DEPTOR | ||||
| No homolog identified | PRAS40 | ||||
| Interactors | |||||
| raga-1 | T24F1.1 | RAGA/RAGB | Schreiber et al. (2010) | ||
| ragc-1 | Y24F12A.2 | RAGC/RAGD | Fukuyama et al. (2012), Robida-Stubbs et al. (2012) | ||
| rheb-1 | F54C8.5 | RHEB | Honjoh et al. (2009) | ||
| No homolog identified | LAMTOR1 | ||||
| lmtr-2 | Y97E10AR.7 | LAMTOR2 | Kim et al. (2018) | ||
| lmtr-3 | C06H2.6 | LAMTOR3 | Shaye and Greenwald (2011), Kim and Guan (2019) | ||
| T08A11.1 | T08A11.1 | DEPDC5 | Kim et al. (2018) | ||
| nprl-2 | F49E8.1 | NPRL2 | Zhu et al. (2013) | ||
| nprl-3 | F35H10.7 | NPRL3 | Zhu et al. (2013) | ||
| F39C12.1 | F39C12.1 | MIOS | |||
| Y32H12A.8 | Y32H12A.8 | WDR24 | Kim et al. (2018) | ||
| No homolog identified | WDR59 | ||||
| npp-18 | Y43F4B.4 | SEH1L | Kim et al. (2018) | ||
| npp-20 | Y77E11A.13 | SEC13 | Galy et al. (2003) | ||
| F13H10.3 | F13H10.3 | SLC38A9 | Kim et al. (2018) | ||
| sesn-1 | Y74C9A.5 | SESN2 | Yang et al. (2013) | ||
| No homolog identified | CASTOR1 | ||||
| No homolog identified | CASTOR2 | ||||
| F54B3.1 | F54B3.1 | SZT2 | Kim et al. (2018) | ||
| No homolog identified | KPTN | ||||
| No homolog identified | ITFG2 | ||||
| No homolog identified | C12orf66 | ||||
| Substrates | |||||
| pgl-1 | ZK381.4 | none | Zhang et al. (2018) | ||
| pgl-3 | C18G1.4 | none | Zhang et al. (2018) | ||
| rsks-1 | Y47D3A.16 | S6K | Long et al. (2002) | ||
| ifet-1a | F56F3.1 | 4E-BP | Li et al. (2009), Nukazuka et al. (2011) | ||
| atg-13 | D2007.5 | ATG13 | Tian et al. (2010) | ||
| sgk-1 | W10G6.2 | SGK1 | Hertweck et al. (2004) | ||
| pkc-2 | E01H11.1 | PKC(βδζ) | Islas-Trejo et al. (1997) | ||
| akt-1 | C12D8.10 | AKT | Paradis and Ruvkun (1998) | ||
| akt-2 | F28H6.1 | AKT | Paradis and Ruvkun (1998) | ||
| Regulators | |||||
| aak-1 | PAR2.3 | PRKAA(1,2),AMPK | Fukuyama et al. (2012) | ||
| aak-2 | T01C8.1 | PRKAA(1,2),AMPK | Fukuyama et al. (2012) | ||
| daf-18 | T07A9.6 | PTEN | Fukuyama et al. (2012) | ||
| daf-16 | R13H8.1 | FoxO | Jia et al. (2004) | ||
| No homolog identified | TSC1 | ||||
| No homolog identified | TSC2 | ||||
This table includes the C. elegans homologs of genes that encode proteins identified as components, interactors, substrates, or regulators of mTORC1 or mTORC2. They are organized by their putative roles in TOR signaling, although many need further characterization to determine function. “No homolog identified” means no apparent homolog based on ortholog prediction programs (Kim et al. 2018), but it is possible that there is a functional ortholog that is too diverged to identify in this manner. The references listed correspond to the related C. elegans studies. TOR, Target of Rapamycin; TORC, TOR Complex.
ifet-1 has also been known as spn-2.
Identification of key components
LET-363/TOR:
The ortholog of TOR in C. elegans, LET-363 (Figure 1), was named based upon its lethal mutant phenotype (Howell et al. 1987), and later identified as a TOR protein in a sequence homology search for phosphatidylinositol kinase-related proteins (Long et al. 2002). Strong conservation was found in all key domains including the kinase domain, HEAT repeats [named for four proteins (Huntingtin, EF3, PP2A, and TOR1)], and the FKBP-rapamycin-binding (FRB), FAT (focal adhesion-targeting), phosphatidylinositol kinase homology, and FAT C-terminal domains [Figure 1; see Long et al. (2002) for more details]. The expression of a GFP reporter driven by a let-363 promoter began in the comma-stage embryo, and was subsequently seen at all stages and in all major tissues (and perhaps all cells). The let-363 gene is clearly essential for animal development as multiple different null mutants (obtained from heterozygous mothers) arrested at the L3 larval stage (Long et al. 2002). The mutants also displayed increased refractile and autofluorescent intestinal granules, decreased intestinal cytoplasmic volume with increased gut lumen size, increased fat storage, as well as compromised digestion. These phenotypes were also seen in worms with the let-363 gene knocked down by bacteria feeding-mediated RNA interference (RNAi) (Long et al. 2002). A stronger embryonic lethal phenotype was reported after maternal injection of let-363 double-stranded RNA [Sönnichsen et al. (2005) and WormBase], suggesting there could be additional maternally provided functions of let-363/Tor in embryos.
DAF-15/Raptor:
In response to food deprivation, C. elegans enter an alternative developmental pathway in which they become specialized dauer larvae (rather than L3 larvae) after the second larval molt (Hu 2007) (see section Roles of TORC1 in regulating development and behaviors for more description). daf-15 was named based upon its loss-of-function mutant phenotype, which is constitutive abnormal dauer formation (Albert and Riddle 1988). daf-15(m81) missense mutants segregated from heterozygotes arrested postembryonic development at the L2 molt with some dauer-like morphological features. However, unlike typical dauer-constitutive mutants, daf-15(m81) mutants displayed sporadic feeding and increased body size (the potential connection between TOR and dauer formation is discussed further in section Roles of TORC1 in regulating development and behaviors.). Structural similarity between DAF-15 and mammalian Raptor was later recognized in one of the studies that first identified mammalian Raptor (Hara et al. 2002) (Figure 1). daf-15 RNAi resulted in L3 larval arrest with additional phenotypes including increased refractile and autofluorescent granules in the intestine, decreased intestinal cell cytoplasm with increased intestinal lumen, increased fat storage, and increased hypodermal granules (Hara et al. 2002; Jia et al. 2004). Since essentially all of these phenotypes are common between daf-15(RNAi) and let-363(RNAi), these findings were consistent with the predicted roles of let-363/Tor and daf-15/raptor in C. elegans TORC1.
RICT-1/Rictor:
The C. elegans putative rictor ortholog, RICT-1 (Figure 1), was identified in two forward genetic screens for genes that impact fat storage (Jones et al. 2009; Soukas et al. 2009). Loss-of-function alleles of rict-1, independently isolated by the two laboratories, displayed increased body fat, developmental delay (slow growth), and decreased body size. rict-1(RNAi) phenocopied the high-fat phenotype of the mutants and did not further enhance the phenotype of rict-1(lf) mutants. These rict-1 alleles can have varied effects on life span, depending upon diet and other conditions (see Role of TOR signaling in aging and stress response). Notably, unlike loss of let-363/Tor or daf-15/raptor, these alleles of rict-1 did not result in larval arrest, suggesting that TORC2 is not essential for larval development. rict-1 promoter-driven GFP reporters were expressed in the intestine, hypodermis, and neurons (Jones et al. 2009; Soukas et al. 2009). rict-1 expression driven by intestinal specific promoters in the form of extrachromosomal arrays was sufficient to rescue the fat-storage phenotype in rict-1(-) mutants (Soukas et al. 2009), suggesting that rict-1 may act in the gut to regulate fat storage. While mammals have three genes that encode Rictor proteins, C. elegans appears to have only one protein that shares this structural and functional similarity (Figure 1).
Biochemical analysis of the two TOR complexes:
The worm field is still in an early stage of characterizing the TOR complexes by biochemical methods. Targeted co-immunoprecipitations using transgenes expressing tagged proteins have been encouraging, successfully pulling down LET-363::FLAG with DAF-15::Myc or RICT-1::HA (Nukazuka et al. 2011), which supported the prediction that C. elegans has both canonical TOR complexes, as seen in mammals (Figure 1). RICT-1 and DAF-15 were also among proteins recently identified as LET-363 interactors in C. elegans embryos in an IP experiment using FLAG::LET-363, confirming protein binding with endogenous proteins of the TORC1 and TORC2 complexes (Zhang et al. 2018). This work also demonstrated for the first time the kinase activity of LET-363 with known targets in C. elegans. By immunoprecipitation of FLAG::LET-363, followed by a radiolabeled kinase assay, PGL-1 and PGL-3, two RGG-domain P granule assembly proteins with RNA endonuclease activities (Kawasaki et al. 1998; Aoki et al. 2016), were shown to be phosphorylation targets of LET-363.
Other conserved TOR components and key interactors:
The proteins discussed below have been identified as members of the mTOR complexes, or key interacting proteins, in mammalian and/or yeast studies (Table 1). However, in most cases functional conservation has not been fully demonstrated in C. elegans.
MLST-8:
The LST8 (aka GβL) protein is present in both TORC1 and TORC2 complexes in mammals (Kim et al. 2003), and genetic evidence suggests that C. elegans MLST-8 (MTor-associated protein, LST8 homolog) also functions in both complexes. Knockdown of mlst-8 by RNAi produced many of the same phenotypes seen with rict-1(-) alleles (Jones et al. 2009), but not the larval arrest that resulted from reduced let-363 or daf-15 (although null alleles remain to be characterized). Biochemical assays using transgenic proteins have shown that knockdown of mlst-8 eliminated binding of LET-363::FLAG to RICT-1::HA and increased binding of LET-363::FLAG to DAF-15::Myc (Nukazuka et al. 2011), and phenotypes suppressed by rict-1 RNAi were also suppressed by mlst-8 RNAi (Ruf et al. 2013). These findings suggested that MLST-8 is required for the proper formation of TORC2, which is consistent with a role of LST8 in mTORC2-related functions reported in mammals (Guertin et al. 2006). However, mlst-8(RNAi) phenocopied loss of other TORC1-related genes in suppressing accumulation of germline PGL (P GranuLe) protein-containing granules, suggesting that MLST-8 plays a role in TORC1 function in the embryo (Zhang et al. 2018). This is consistent with the association of LST8 with mammalian TORC1, making it interesting to further investigate the possible role MLST-8 plays in C. elegans TORC1 formation or activity.
SINH-1:
SINH-1/mSin1, named for mammalian stress-activated protein kinase (SAPK)-interacting protein, is an mTORC2 component that is required for mTORC2 assembly and function (e.g., Yang et al. 2006). C. elegans sinh-1 (Sin-1 homolog) was identified in an RNAi screen, with reduced sinh-1 extending mean life span, increasing thermotolerance and stress resistance, and enhancing dauer formation (Hansen et al. 2005). Phenotypes suppressed by rict-1 RNAi were also partially suppressed by sinh-1 RNAi (Ruf et al. 2013). sinh-1(pe420) null mutants are viable, unlike let-653/Tor or daf-15/raptor mutants, and share several other phenotypes with rict-1 mutants, including viability, increased fat storage, and similar performance in associative learning assays (Sakai et al. 2017). Tissue-specific transgenes were used to show that expression of sinh-1 in the intestine or neurons was sufficient to rescue the learning defect of the mutant (Sakai et al. 2017), suggesting that sinh-1 and rict-1 can function in some of the same tissues. All of these findings are consistent with a role for SINH-1 in TORC2.
RAGA-1 and RAGC-1:
The Rag GTPases (RAS-related GTP-binding protein) are well characterized in mammalian cells as positive regulators of the TORC1 kinase, through which it is activated by amino acid (AA) availability signals (Jewell et al. 2013). In mammals, the Rag proteins function as obligate heterodimers that are made up of a member from each of two protein families, RagA/RagB and RagC/RagD, but in C. elegans only one homolog from each family is present (RAGA-1 and RAGC-1). RAGA-1/RagA was identified in an RNAi screen for improved locomotion in aged C. elegans, and studies using null mutations and transgenes containing putative dominant negative and gain-of-function (gf) mutations suggested that RAGA-1/RagA activity negatively impacts life span (Schreiber et al. 2010). The raga-1(gf) transgene was also used to putatively “hyperactivate” TORC1 to suppress the larval arrest caused by a loss-of-function mutation in a fatty acid elongase elo-5 (Zhu et al. 2013). RNAi knockdown of raga-1 and ragc-1 revealed that a reduction in RagA/C function resulted in increased autophagy, decreased mRNA translation, and increased life span and stress tolerance, as observed with reduced TORC1 activity (Robida-Stubbs et al. 2012). Loss or reduction of the ragc-1, raga-1, and rheb-1 gene activities all suppressed the ectopic germline proliferation seen in animals with deletion mutations in both of the AMPK genes (aak-1 and aak-2), or in a daf-18/PTEN deletion mutant (Fukuyama et al. 2012), which is consistent with TORC1 having functions downstream of these factors. RAGA-1 and RAGC-1 were identified as weak binding partners with FLAG:LET-363, and loss of raga-1 and ragc-1 phenocopied loss of other TORC1-related genes in suppressing accumulation of PGL granules, consistent with these proteins being positive regulators of TORC1 in the embryo (Zhang et al. 2018).
The developmental defects produced by knocking down raga-1 or ragc-1 (slowed development, and modestly reduced body size and reproduction) are not as severe as those seen with knockdown of let-363/TOR or daf-15/raptor, suggesting that TORC1 may have residual activities that do not depend upon RAG-mediated signaling. Consistent with this idea, a deletion mutation in raga-1 slows but does not block larval development, and extends adult life span (Schreiber et al. 2010). However, it is not clear whether RAGA-1/RagA and RAGC-1/RagC form an obligate heterodimer in C. elegans as in mammals, so potential redundancy between the two Rag proteins cannot be excluded.
RHEB-1:
Rheb is another member of the Ras superfamily of small GTPases that is a critical positive upstream regulator of TORC1 in multiple organisms (Jewell et al. 2013; Saxton and Sabatini 2017). rheb-1 encodes the C. elegans ortholog of Rheb (Reiner and Lundquist 2018). A Prheb-1::RHEB-1::GFP reporter was expressed at all stages and in all cells (Honjoh et al. 2009). rheb-1(RNAi) resulted in mild developmental phenotypes (Honjoh et al. 2009), and a putative rheb-1(gf) mutant transgene appeared to promote TORC1 activation (Zhu et al. 2013). The effects of rheb-1 on aging and life span extension are discussed in a later section (Importance of TORC1 regulation in life span extension mechanisms). rheb-1(RNAi) has also been shown to phenocopy knockdown of other TORC1-related genes in suppressing accumulation of PGL granules, suggesting that RHEB-1 functions with TORC1 in the embryo (Zhang et al. 2018).
TSC proteins (or lack thereof):
In mammals and Drosophila, the TSC1 and TSC2 (Tuberous sclerosis) proteins indirectly inhibit TORC1, and mediate its functional interaction with growth factor signaling pathways (Dibble and Cantley 2015). C. elegans lacks orthologs of both TSC1 and TSC2 (Table 1), which is highly intriguing given the conservation of signaling pathways connecting to these proteins. TSC1 and TSC2 form a complex and act as GTPase-activating proteins (GAPs) for the Rheb GTPase. Structural similarities between the TSC1-TSC2 complex and RalGAPα-RalGAPβ propelled one study that indicated a regulatory role of RalGAP proteins (and Ral GTPase) on TORC1 activity, in both mammalian cells and C. elegans (Martin et al. 2014). Furthermore, analysis of sequence alignments between C. elegans, Drosophila, and mammalian Rheb-family proteins has led to the argument that C. elegans RHEB-1 has converged toward RAL-1, while the Drosophila and mammalian Rheb proteins have not converged toward their Ral proteins (Reiner and Lundquist 2018). This observation was used to support the model that C. elegans RHEB-1 and RAL-1 are jointly repressed by the RalGAPs HGAP-1 and HGAP-2, which might be a reasonable explanation for the loss of the TSC proteins in nematodes. It would be interesting to test whether HGAP-1 and HGAP-2 have TSC-like activity on the RHEB-1 GTPase in addition to their roles on RAL-1 in C. elegans. Additionally, RNAi knockdown of ral-1 extends life span (Kim and Sun 2007; Martin et al. 2014), suggesting that both RAL-1 and RHEB-1 might promote TORC1 activity in C. elegans.
NPRL-2/3:
NPRL-2 and NPRL-3 are orthologs of the NPR2 and NPR3 proteins, respectively, which were first identified as negative regulators of TORC1 in yeast (Neklesa and Davis 2009). In C. elegans, nprl-3 was identified in an unbiased genetic screen as a suppressor of the growth-arrest phenotype caused by depleting monomethyl branched-chain fatty acids (mmBCFAs) (see Upstream inputs to TOR signaling), with nprl-2 having a similar suppressor role (Zhu et al. 2013) (Figure 3A). Additional assays using RNAi or hyperactivation of TORC1 supported the idea that NPRL-2/3 are negative regulators of TORC1 in the C. elegans intestine. Consistent with these findings in yeast and C. elegans, a parallel study initiated by a biochemical approach identified mammalian NPRL2 and NPRL3 (named as part of the GATOR1 complex), as negative regulators of TORC1 (Bar-Peled et al. 2013). More specifically, in the mammalian study GATOR1 was found to be the GAP for RagA/C GTPases. Therefore, disruption of GATOR1 results in activation of RagA/C, which in turn activates TORC1 (Saxton and Sabatini 2017). While such a GAP function has not yet been demonstrated for C. elegans NPRL-2/3, the negative regulatory nature of the NPRL-2/3 complex rendered it an excellent tool in analyzing TORC1-related functions in C. elegans (Zhu et al. 2013; B. Qi et al. 2017; Zhang et al. 2018).
Figure 3.
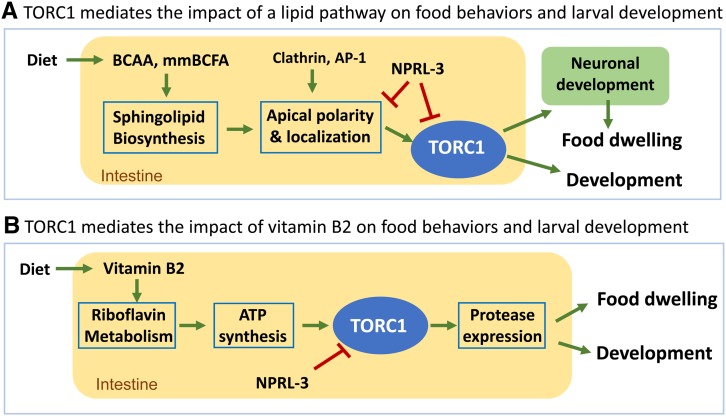
Proposed role of intestinal TORC1 in mediating the impact of lipids (A) and vitamin B2 (B) on animal development, and food behavior. Neuronal function related to food behaviors has been linked to the axis in (A) but … expression in (B). BCAA, branched-chain amino acids. mmBCFA, monomethyl branched-chain fatty acids. mmBCFA are derived from BCAA and both can be obtained from diet. BCAA, branched-chain AAs; mmBCFA, monomethyl branched-chain fatty acid; TORC, Target of Rapamycin Complex.
Rapamycin
Rapamycin is an antifungal metabolite produced by Streptomyces hygroscopicus that is well known for its inhibitory effects on mTOR in mammalian, Drosophila, and yeast cells (Huang et al. 2003; Li et al. 2014; Kennedy and Lamming 2016). Rapamycin is widely used as an immunosuppressant in humans, and is of great interest as a paradigm for an antiaging drug (see Life span extension and increased stress resistance from TORC1 inhibition). In mammals, a complex between rapamycin and the cellular protein FKBP12 (FK506-binding protein 12) binds to the FRB domain of TOR, thereby inhibiting the TORC1 kinase in a manner that affects some substrates more severely than others (Huang et al. 2003). C. elegans LET-363 is highly related to TOR proteins from other species, with conservation that extends to the FRB domain (Figure 1A) (Long et al. 2002). However, binding of rapamycin to an FKB protein remains to be demonstrated in C. elegans, and an unambiguous FKBP12 ortholog has not yet been designated. Yet, a number of FKBP family members are present in C. elegans, of which FKB-2 is more similar to human FKBP12 than is the FKBP12 ortholog in Saccharomyces cerevisiae (FPR1) (Pemberton and Kay 2005), in which genetic analyses initially revealed TOR to be the biological target of FPR1/rapamycin action (Kunz et al. 1993; Blenis 2017). Therefore, it appears likely that the mechanisms through which rapamycin inhibits TORC1 in other species are conserved in C. elegans.
While the evolutionary conservation of TORC1 and its sensitivity to rapamycin suggested that rapamycin would be likely to reduce TORC1 activity in C. elegans, the impact of rapamycin on C. elegans growth is limited, and initial efforts to elicit a phenotype related to let-363(-) (e.g., larval arrest) by several methods and concentrations failed (Long et al. 2002). A later study revealed a stage-dependent, dose-dependent rapamycin effect in adult worms, caused by treatment with a high dose (100 µM) of rapamycin, resulted in upregulation of genes that are activated by genetic TORC1 inhibition, increase in life span, reduction in translation, and resemblance to reducing TORC1/2 activities in genetic interaction tests (see Life span extension and increased stress resistance from TORC1 inhibition) (Robida-Stubbs et al. 2012). The high dosage used in this study (much higher than that used in mammalian cell culture) suggested that in C. elegans the bioavailability of rapamycin is poor, as is typical for many compounds. While C. elegans has been a useful system for studying how rapamycin acts as an antiaging drug (see Life span extension and increased stress resistance from TORC1 inhibition), given the lack of a developmental phenotype and its high cost, the value of rapamycin for studying TOR functions in this organism is limited, particularly compared to the many genetic tools that are available. To date, no analyses of other chemical inhibitors of TOR have been reported in C. elegans.
Upstream inputs to TOR signaling
In mammals and other organisms, the TOR complexes have been characterized as nutrient-sensing centers that respond to changes in various nutrients, energy, and growth factors (Menon and Manning 2013; Saxton and Sabatini 2017). Here, we review studies in C. elegans that aim to connect upstream nutrient status to in vivo physiological functions (Figure 2).
AAs and peptides:
A plethora of literature in the TOR field is devoted to understanding how TOR senses the availability of AAs (Jewell et al. 2013). The prevailing model is that AAs impact the localization of mTORC1, whereby high AA levels induce a relocation of mTORC1 from the cytosol to the lysosome, thereby activating the complex. This relocation is mediated by the Rag family of small GTPases (see Other conserved TOR components and key interactors), which can bind Raptor to recruit mTORC1 to the lysosome (Jewell et al. 2013; Saxton and Sabatini 2017).
As a powerful genetic model organism, C. elegans is potentially an excellent system to study the physiological role of AA sensing by TORC1. However, at this point, the specific connection between AA availability and TORC1 signaling in C. elegans is not yet well established. This may partly be due to the difficulty of establishing a good synthetic culturing medium where, unlike with live bacterial food, the AA level may be reduced. Instead, the upstream connections to AAs or proteins have been mostly indirect, and were first analyzed with the investigation of the oligopeptide transporter PEP-2/OPT-2/PEPT-1. One study showed that pept-1 encodes a functional homolog of mammalian PEPT1/SLC15A1 by demonstrating that a pept-1(-) mutation causes loss of di- and tripeptide uptake, and developmental defects (Meissner et al. 2004). When loss of peptide uptake was combined with partial knockdown of let-363, an increase in the severity of the developmental retardation and intestinal phenotypes was observed. This result is consistent with pept-1 acting upstream or downstream of, or in parallel with, TOR. The upstream model was supported by quantitative proteome analysis and transcriptome profiling that showed that pept-1(-) animals have reduced AA levels, which lead to reduced ribosome biogenesis and protein translation downstream of TOR (Geillinger et al. 2014). However, interestingly, the same group had suggested earlier that PEPT-1 acts downstream of the TOR complexes, based upon how RNAi knockdown of key TOR complex components reduced PEPT-1 protein levels and peptide uptake (Benner et al. 2011). These studies on PEPT-1 perhaps pointed out the complex interplay between diet, nutrient sensing, and protein expression and function, where feedback loops can complicate interpretation of epistasis experiments, especially when such experiments utilize RNAi or nonnull mutants.
While depleting AAs from the diet is difficult in C. elegans, raising the AA level by dietary supplementation, or genetic mutations, in the AA catabolic pathway can generate significant information regarding the functional relationships between AAs and TOR for specific physiological functions. In one study, AA supplementation was part of a series of tests leading to the model that TORC1 mediates the impact of AA availability to promote hypodermal P and M blast cell release from the quiescent state (Fukuyama et al. 2015) (Figure 4A) (also see Roles of TORC1 in regulating development and behaviors). Elevation of AA levels was also employed in two other studies to analyze the role of TOR signaling in aging, with very different conclusions about the relationship between AAs and TOR. In one study, the authors provided evidence that elevation of branched-chain AAs (BCAAs) in specific neurons caused by mutating a key BCAA catabolic gene (bcat-1) lead to increased life span in a let-363/TOR-dependent manner (Mansfeld et al. 2015). Interestingly, whereas activation of TOR by AAs fits the with current models, this role of TOR signaling in promoting life span is in contrast to the numerous other studies indicating that TORC1 opposes longevity (see Life span extension and increased stress resistance from TORC1 inhibition). In another study, supplementation of heat-killed Escherichia coli with most L-AAs in liquid culture was also shown to extend life span (Edwards et al. 2015). However, circumstantial evidence led to the suggestion that AA supplementation may inhibit TOR signaling, which is in contrast to the consensus in the field. Such a result done with heat-killed bacteria could be subject to alternative explanations (B. Qi et al. 2017; Qi and Han 2018). It may be worth noting that excess AAs may induce complex metabolic responses in animals, including the activities in AA catabolic pathways and AA transport systems that are known to have profound impacts on various physiology (e.g., Tărlungeanu et al. 2016).
Figure 4.
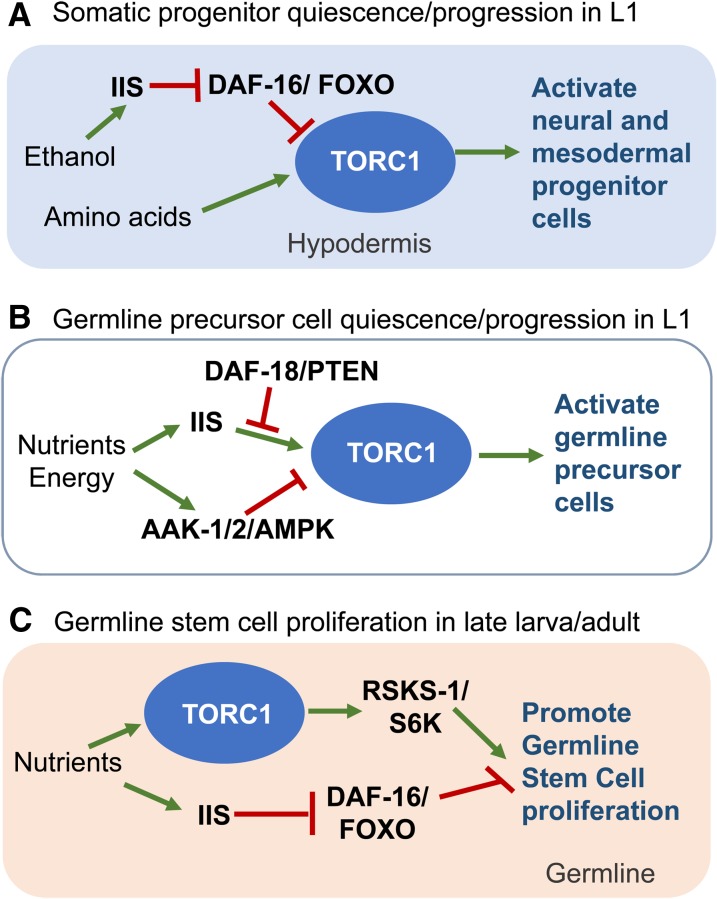
Proposed roles of TORC1 in three specific developmental events. Available experimental evidence supports a site of action for TORC1 in the hypodermis for (A) and in the germline for (B) and (C). IIS, insulin/IGF signaling; TORC, Target of Rapamycin Complex.
The studies of TOR sensing of AAs in C. elegans have been wisely focused mainly on specific physiological functions such as development and aging. However, the potential to identify new insights regarding the mechanistic aspects of AA sensing has been somewhat limited by the fact that direct readouts of TOR activity have not been well established (discussed more below). In addition, the study of AA sensing by TORC1 in C. elegans has not yet addressed localization changes of the complex in specific tissues and under specific physiological conditions, even though the live animal system may permit new insights beyond what has been found in cultured cells.
AMPK/ATP:
In other species, the AMP-activated kinase (AMPK) has been shown to negatively regulate mTORC1 in response to the AMP:ATP energy ratio, primarily by phosphorylation and activation of TSC2, and TSC-independent phosphorylation and inhibition of Raptor [reviewed by Garcia and Shaw (2017)]. Since unambiguous TSC orthologs have not yet been identified in C. elegans [see TSC proteins (or lack thereof)], the mechanism by which TORC1 senses AMPK activity is unclear. In C. elegans, the homologs of the mammalian AMPK catalytic α subunit (aak-1 and aak-2), the regulatory β subunit (aakb-1 and aakb-2), and the γ subunit (aakg-1, aakg-2, aakg-3, aakg-4, and aakg-5) are conserved [reviewed by Ahmadi and Roy (2016)]. Work in C. elegans has explored the AMPK-TOR connection through the study of several specific developmental events. Specifically, one study observed a critical role for AMPK in germline quiescence, in L1 larvae under starvation-induced diapause, as mutating both aak-1 and aak-2 increased germline cell number in these developmentally arrested animals (Fukuyama et al. 2012) (Figure 4B). Knocking down let-363 or other TORC1 components suppressed the germline defects of aak-1/2 double mutants, consistent with the model that AMPK inhibits germline proliferation (promotes germline quiescence) by repressing TORC1 activity in L1 diapause induced by food deprivation. Similarly, two other studies provided genetic data that suggest that the AMPK-TORC1 axis regulates gonadogenesis and aging (Yuan et al. 2013; Ishii et al. 2016). However, other genetic data point to a TORC1-independent role of AMPK as the lethality of aak-1/2 double mutants was not suppressed by reducing TORC1 activity (Fukuyama et al. 2012). Other studies have addressed the impact of ATP on TORC1 activity, with a focus on life span and nutrient deprivation-induced behavioral changes (Chin et al. 2014; Fu et al. 2015; B. Qi et al. 2017) (discussed in TOR signaling plays pivotal roles in regulating development and behaviors and Role of TOR signaling in aging and stress responses).
Insulin/IGF signaling pathway:
Studies in mammalian cells have revealed a complex functional relationship between the insulin/IGF signaling (IIS) pathway and TOR complexes (Dibble and Cantley 2015; Saxton and Sabatini 2017). The IIS pathway has been shown to act upstream of mTORC1 activity through a succession of negative regulatory events in which the Akt kinase of the IIS pathway inhibits TSC, which in turn inhibits Rheb activation (binding to GTP) and hence inhibits TORC1. In addition, other studies indicate an inhibitory feedback mechanism, where the IIS pathway is downregulated by mTORC1 (through phosphorylation of Grb10 that blocks insulin signaling) in regulation of glucose homeostasis and insulin resistance (Hsu et al. 2011; Yu et al. 2011). In addition, Akt has also been shown to be a downstream target of mTORC2 under certain conditions (Sarbassov et al. 2005). Thus, it is important that the study of the functional relationship between these two pathways is linked to specific physiological functions.
The major components of the IIS pathway are conserved in C. elegans [DAF-2/IGFR (IGF receptor), DAF-16/FoxO, etc.) (Murphy and Hu 2013). Although the IIS and TOR pathways have been investigated for common roles in nutrient sensing in C. elegans, regulation of one by the other has not been clearly demonstrated [discussed in a WormBook chapter by Murphy and Hu (2013)]. It has been shown that DAF-16/FoxO negatively regulates the transcriptional expression of daf-15, although the study could not conclude if this regulation was direct or indirect (Jia et al. 2004). DAF-16-dependent regulation of daf-15 was also shown to play a role in germline tumorigenesis (W. Qi et al. 2017). The genetic data in the study by Fukuyama et al. (2015) also suggested that the IIS pathway regulates somatic progenitor cell quiescence, partly through modulating TORC1 activity (Figure 4A). However, as we discuss below (see Importance of TORC1 regulation in life span extension mechanisms), a number of aging-related studies in C. elegans have indicated that the TORC1 and IIS pathways also function independently in important ways.
mmBCFA/GlcCer-dependent apical polarity:
Monomethyl BCFAs (mmBCFAs), derived from BCAAs, are conserved fatty acids present in C. elegans and humans, but their physiological functions were essentially unknown before genetic work done in C. elegans showed that they are required for postembryonic development (Kniazeva et al. 2004, 2012; Watts and Ristow 2017). Mutants for the fatty acyl elongase gene elo-5 lacked mmBCFAs and arrested as early L1 larvae, but could be rescued by exogenous supplementation with mmBCFAs. Further analysis indicated that this role of mmBCFAs on early larval development is mediated by mmBCFA-derived glycosylceramide (d17iso-GlcCer), which in turn promotes intestinal TORC1 activity (Zhu et al. 2013) (Figure 3A). One piece of critical evidence is that the L1 arrest phenotype of either or both elo-5(-) and reducing the ceramide glucosyltransferase activity [cgt-1(-) and cgt-3(RNAi)] is suppressed by activation of TORC1 (see Other conserved TOR components and key interactors). Such an impact of lipids on TORC1 activity in the intestine appeared to be mediated by apical membrane polarity that in turn affected the localization of TORC1 regulators (e.g., V-ATPase) at the apical membrane (Zhu et al. 2015) (Figure 3A). The authors have proposed that the d17iso-GlcCer biosynthesis pathway, along with the downstream membrane polarity and subsequent TORC1 activity, may serve as a mechanism to connect the availability of certain nutrients or metabolites to development and behaviors (also see Roles of TORC1 in regulating development and behaviors).
Downstream targets
LET-363/TOR is a serine/threonine kinase, but it is important to note that for almost all of the TOR-regulated processes in C. elegans, no protein has yet been conclusively demonstrated to be a direct phosphorylation target of either TORC1 or TORC2. In the sections below, we will review the genetic and functional evidence that does, or does not, support particular mechanisms and proteins being regulated by TOR in the worm (Figure 2).
S6K/RSKS-1:
S6K is the common name given to the ribosomal protein S6 kinase (also known as p70S6K). In vitro analyses in mammalian cell culture and other model organisms revealed S6K to be a direct phosphorylation target of mTORC1, and measurement of this phosphorylation has become the primary assay for mTORC1 activity (Ma and Blenis 2009; Magnuson et al. 2012). It was natural for C. elegans researchers to test this connection genetically. An early study identified Y47D3A.16 (later named rsks-1) as the worm homolog of p70S6K (Long et al. 2002). As would be predicted (Figure 2A), a null rsks-1 mutation does not generate all of the phenotypes associated with loss of let-363/TOR (Long et al. 2002). However, the sharing of certain specific functions (such as life span extension, translation regulation, and germline proliferation) and genetic interactions with other factors (such pha-4), along with the established kinase–target relationship in other organisms, has led to the model that RSKS-1 acts downstream of TORC1 for these functions (e.g., Hansen et al. 2007; Pan et al. 2007; Sheaffer et al. 2008; Nukazuka et al. 2011; Korta et al. 2012). Also consistent with this idea, a genetic interaction in developing larvae, in which a rsks-1/S6K null mutation suppressed loss-of-function in the transcriptional regulator heat-shock factor (hsf-1), was phenocopied by RNAi against TORC1 components (daf-15/raptor and ragc-1/RagC), but not inhibition of translation (Chisnell et al. 2018). This suggests that RSKS-1/S6K and TORC1 act on a common downstream mechanism that is distinct from translation regulation. However, genetic epistasis analysis between TORC1 and RSKS-1 using a mutation that constitutively activates either kinase has not been performed.
Given the extensive literature in other species, it is tempting to assume that RSKS-1 must be a direct phosphorylation target of TORC1 in C. elegans (Lin et al. 2014; Nakamura et al. 2016; Chen et al. 2017). However, this idea is not yet supported by direct biochemical evidence, and is still largely based upon conserved sequence homology, genetic analyses, and related mutant phenotypes that are consistent with findings in other species (see also Germline development and Life span extension and increased stress resistance from TORC1 inhibition). Some studies have used anti-S6K antibodies to detect changes in RSKS-1 phosphorylation as evidence of TORC1 activity. However, sequence alignment and phosphoproteomic data mining indicate that the relevant RSKS-1 residues are either not phosphorylated in C. elegans (Homo sapiens T389/C. elegans T404) (Bodenmiller et al. 2008; Zielinska et al. 2009), or not mTORC1-dependent phosphorylation targets (H. sapiens S411/C. elegans S439) (Magnuson et al. 2012). These findings should be carefully considered if RSKS-1 phosphorylation is used as a readout for TORC1 activity in C. elegans. Direct biochemical tests of TORC1 activity in C. elegans, such as in vitro kinase assays or TORC1-dependent phosphoproteomic profiling, may be necessary for a definitive conclusion on this point.
4E-BP1/ eukaryotic initiation factor 4E:
4E-BP1, named for eukaryotic initiation factor 4E (eIF4E)-binding protein, is a translational repressor that has been shown to be a phosphorylation target of mTORC1 in mammalian cell culture (Ma and Blenis 2009; Magnuson et al. 2012). Although it was first thought that C. elegans lacked a homolog of 4E-BP1 (Long et al. 2002), a later study identified IFET-1 (aka SPN-2) as the worm 4E-BP1-like protein (Li et al. 2009). The sequence homology is very limited (which is likely why it was not identified earlier) and IFET-1/SPN-2 lacks the consensus eIF4E-binding motif, but the authors went further to demonstrate that IFET-1/SPN-2 can bind several C. elegans eIF4E homologs in vitro, demonstrating conservation of the function. Another group expressed a human 4E-BP1 protein, h4EBP1, in C. elegans, and observed significant reduction of its phosphorylation in let-363(RNAi) and daf-15(RNAi) worms, as well as expected phenotypes from ifet-1(RNAi) (Nukazuka et al. 2011). These data indicated conservation of the specificity of TOR kinase and supported a likely role of IFET-1/SPN-2 as 4E-BP1. However, these studies are not yet sufficient to make a firm conclusion, as phosphorylation of IFET-1/SPN-2 by TORC1 has not been demonstrated and the interpretation of the genetic phenotype was made with the assumption that IFET-1/SPN-2 is the worm 4E-BP1. An alternative possibility is that IFET-1/SPN-2 is not a direct TORC1 target, and that TORC1 directly phosphorylates and inhibits one of several eIF4E isoforms, such as IFE-2, that have been implicated in longevity in C. elegans (Jankowska-Anyszka et al. 1998; Syntichaki et al. 2007).
Protein translation:
Promoting protein translation has been well established in multiple organisms as a central downstream function of TORC1, with multiple direct phosphorylation targets being involved in translation (Ma and Blenis 2009; Magnuson et al. 2012). In addition to those related to S6K and 4EBP, genetic data also support TORC1 regulation of translation in C. elegans. Loss-of-function analysis of several other homologs of TOR effectors identified in other organisms, such as initiation factors M110.4/ifg-1, Y37E3.10/eif-2α, and K04G2.1/eif-2β produced most of the let-363 loss-of-function phenotypes (Long et al. 2002). Knocking down ifg-1 and eif-1 also resembles raga-1 knockdown in increase of life span (Robida-Stubbs et al. 2012) (see Life span extension and increased stress resistance from TORC1 inhibition). Other evidence includes inhibition of translation by rapamycin treatment or ragc-1 knockdown, and promotion of rRNA maturation in nucleoli (which is expected to affect protein translation) by let-363 (Sheaffer et al. 2008; Robida-Stubbs et al. 2012). Sheaffer et al. (2008) also introduced the localization of FIB-1, a box C/D small nucleolar ribonucleoprotein, in nucleoli as a useful indirect readout of TORC1 activity. This was significant for the C. elegans field, in which a direct phosphorylation target of TORC1 has not yet been defined (Zhu et al. 2013).
Autophagy:
Extensive studies in multiple organisms including C. elegans have indicated clearly that TORC1 inhibits autophagy (Toth et al. 2007; Hansen et al. 2008; Meléndez and Levine 2009). While the TORC1–autophagy axis has been extensively studied in aging regulation (see Role of TOR signaling in aging and stress response), it could also potentially influence other cellular events. In several cases, a GFP reporter of LGG-1 (a worm ATG8 protein) (a plgg-1::GFP::LGG-1 translational fusion transgene), which is commonly used as an autophagy marker (Meléndez et al. 2003; Tian et al. 2010), has been a helpful yet indirect way to evaluate TORC1 activity (e.g., Meléndez et al. 2003; Hansen et al. 2008; Tian et al. 2010; Robida-Stubbs et al. 2012; Chin et al. 2014; B. Qi et al. 2017). Unlike in yeast and mammals, where TOR has been shown to directly phosphorylate several ATG proteins to regulate autophagy (ATG13, Ulk1/ATG1, and AGT14) (Jung et al. 2009, 2010; Russell et al. 2013, 2014), phosphorylation of corresponding proteins in C. elegans by TOR has not been biochemically demonstrated. In addition, consistent with studies in mammalian cells, regulation of the mRNA levels of lgg-1 and other autophagy genes by TORC1 is at least partly mediated by the transcription factor HLH-30/TFEB in C. elegans (Lapierre et al. 2013; Settembre et al. 2013; Nakamura et al. 2016). Phosphorylation of TFEB by TORC1 regulates its subcellular localization and activity in mammals, but this has not yet been demonstrated in C. elegans (Napolitano and Ballabio 2016).
SGK-1:
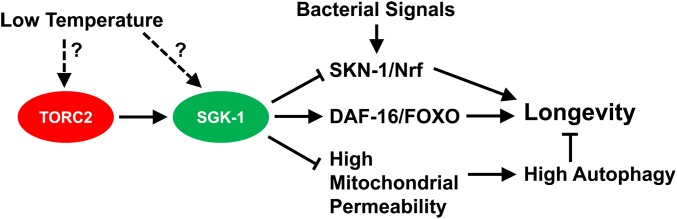
The C. elegans homolog of serum and glucocorticoid-induced kinase 1, sgk-1, was first characterized for a role in stress response and life span (Hertweck et al. 2004). Later independent studies in two laboratories showed that sgk-1(-) phenocopied rict-1(-) in fat accumulation and smaller body size (Jones et al. 2009; Soukas et al. 2009). These studies provided two lines of evidence to support that SGK-1 acts downstream of RICT-1 in the same pathway: loss-of-function alleles in sgk-1 did not enhance the defects in rict-1 mutants, and gain-of-function or overexpression of sgk-1 suppressed the defects in rict-1 mutants. This RICT-1-SGK-1 pathway appears to act in the intestine to regulate fat metabolism independently of DAF-16/FoxO. Several additional studies have presented data to support the idea that SGK-1 is a downstream factor of TORC2, even though no one has yet demonstrated direct regulation by phosphorylation in C. elegans. For example, one study linked TORC2 and SGK-1 in regulating mesendodermal embryonic development (Ruf et al. 2013), and another presented genetic data to support the idea that SGK-1 acts downstream of TORC2 in the intestine to regulate vitellogenesis and fat mobilization for oogenesis (Dowen et al. 2016). Finally, two studies that are discussed below (see Roles of TORC2 in aging and stress responses) indicate that SGK-1 acts downstream of RICT-1 in determining life span (Mizunuma et al. 2014; Zhou et al. 2019).
P granule proteins:
The C. elegans P granules are germline-specific granules containing a group of perinuclear RNAs and their binding proteins (Wang and Seydoux 2014). PGL-1 and PGL-3 are RGG-domain P granule components, which have recently been found to be direct targets of TORC1 in C. elegans embryos through immunoprecipitation and in vitro kinase assays (Zhang et al. 2018) (also see Roles of TORC1 in regulating development and behaviors).
TOR Signaling Plays Pivotal Roles in Regulating Development and Behaviors
Theoretically, there may be two types of developmental regulation by the TOR complexes. One type would be directly linked to their well-known roles as major “nutrient sensors,” under which TORC1/2 may promote developmental events under conditions with sufficient nutrients, whereas TOR inhibition may arrest or alter developmental events when specific or overall nutrients are deprived. Studies in C. elegans have offered a few excellent examples [e.g., TORC1 regulating postembryonic development and food behaviors in response to vitamin B2 (VB2) availability (B. Qi et al. 2017)]. The other type of developmental regulation would involve TOR complexes functioning as built-in machinery in a regulatory network that controls cell differentiation and developmental pattern formation without a direct link to nutrient availability. For example, the role of TORC2 in mesendodermal development in the embryo does not seem to have an obvious connection to nutrient availability (Ruf et al. 2013). However, these two types of functions may not be as distinct as they appear. First, “nutrient sensing” may often be indirectly mediated by metabolic pathways or cellular events that modulate the activity of TOR complexes, which is exemplified by the role of glucosylceramide and apical membrane polarity in mediating the effect of fatty acid availability on TORC1 and postembryonic development (Zhu et al. 2015). Second, the connections to nutrient components in some cases are yet to be identified (e.g., regulation of vitellogenesis by TORC2; Dowen et al. 2016). Given that the connection between nutrient availability and development is a relatively new and exciting research frontier, TOR functions in development will continue to be an attractive research topic in the C. elegans field.
Through its well-documented anabolic functions in mammalian and Drosophila cells, TORC1 regulates cell/organ size independently of the cell cycle so that flies with reduced TOR activity are smaller because they have smaller rather than fewer cells (Tumaneng et al. 2012; Lloyd 2013). Many studies have observed that TORC1 is required for completion of C. elegans larval growth and development, but comparatively little has been done to investigate its possible effects on cell or body size. It has been noted that distal germ cell size is smaller in rsks-1 mutants, though not in daf-2/IIS mutants, which also slow the mitotic germ cell cycle (Korta et al. 2012). Mutation of raga-1 modestly reduces adult body size, an effect that appears to derive from the importance of RAGA-1 for larval development (Schreiber et al. 2010). One speculative possibility is that cellular growth regulation might be less plastic in C. elegans than in more complex organisms, possibly because of constraints imposed by the worm cuticle or specific aspects of C. elegans body-size regulation, a process that is not well understood.
Roles of TORC1 in regulating development and behaviors
Germline development:
C. elegans L1 larvae hatch with two primordial germ cells, Z2 and Z3, which then begin to proliferate mitotically as long as conditions are favorable (Kimble and Crittenden 2005). When C. elegans hatch in the absence of food, they enter an L1 diapause or arrest state, and suspend growth until food is available (Baugh 2013). During this L1 diapause, primordial germ cells arrest in G2 phase, with arrest dependent upon DAF-18/PTEN (a negative regulator of the IIS pathway) and on aak-1/2/AMPK, but not dependent on DAF-16/FoxO (Fukuyama et al. 2006, 2012). Loss-of-function mutations in aak-1/2 or daf-18 resulted in ectopic germline proliferation in the absence of food, and this ectopic proliferation was partially suppressed by RNAi targeting components of TORC1. Indeed, AMPK and PTEN have been shown to negatively regulate mTORC1 in mammals (Feng et al. 2007). The C. elegans studies suggest that TORC1 activity may be suppressed by AMPK to maintain germline quiescence during L1 diapause (Figure 4B). Whether TORC1 acts cell autonomously in the germline for this function has not yet been firmly addressed by experiments (Fukuyama et al. 2012).
Beginning in the L3 stage, and continuing into adulthood, some germ cells exit mitosis and enter meiosis, and eventually give rise to sperm in the L4 stage or oocytes in the adult (Hubbard and Greenstein 2005). GLP-1/Notch pathway signaling is required to prevent differentiation of germline progenitors. TGF-β and MAPK pathways also act to prevent differentiation (e.g., Lee et al. 2007; Dalfó et al. 2012). In addition, the IIS pathway and TORC1 act in the germline to positively regulate cell cycle progression in the larval germline, though independently (Michaelson et al. 2010; Korta et al. 2012) (Figure 4C).
Based on the marked reduction of the larval germline progenitor pool in rsks-1(−) and the viability of the null mutant, the specific role of TORC1 and of this putative TORC1 target was further investigated. Among many defects, L3/L4-stage rsks-1(−) worms displayed a reduced number of germline progenitors, and this defect was rescued by germline-specific expression of rsks-1. The authors found that let-363/Tor(RNAi) and daf-15/raptor(RNAi) also reduced progenitor number, but less severely than rsks-1(−). However, the phenotype of a double-mutant combination of rsks-1(−) and ife-1(-), an eIF4E ortholog that acts in germline progenitors, closely resembled the phenotype of let-363/Tor or daf-15/raptor RNAi, suggesting the possibility that TORC1 acts primarily via 4E-BP and S6K in this context. Further tests demonstrated that nutrients impact the germline progenitors in a rsks-1-dependent manner. When the conserved, putative TORC1 phosphorylation site of RSKS-1 was mutated in a rsks-1(+) transgene (T404A) (Schalm and Blenis 2002) it no longer rescued the larval germline progenitor accumulation phenotype (Korta et al. 2012). Although this result seems to support the idea that RSKS-1 is a phosphorylation target of TORC1, as mentioned earlier (see S6K/RSKS-1), biochemical analyses have not indicated that this T404 residue is a TORC1 phosphorylation site in C. elegans. In addition to a role in promoting germline cell cycle progression, RSKS-1 also promotes germline stem cell (GSC) maintenance in conjunction with GLP-1/Notch, a role that does not appear to be shared by TORC1 (Korta et al. 2012). This specific role is also germline autonomous and is dependent on residue T404 (Roy et al. 2018).
C. elegans germline tumor formation has become a significant model for the study of tumorigenesis (Singh and Hansen 2017). The IIS signaling target DAF-16/FoxO plays both cell autonomous and nonautonomous roles in germline proliferation, and tumor formation. DAF-16/FoxO activity inhibits germ cell proliferation in gld-1(lf) mutants (Pinkston et al. 2006), whereas DAF-16/FOXO activity from a transgene expressed in the hypodermis can induce the germline tumor phenotype (Qi et al. 2012). W. Qi et al. (2017) screened for kinases involved in this latter DAF-16/FOXO-dependent tumor formation in L3 staged larvae, and found that reducing rsks-1 and genes for TORC1 components moderately suppressed the tumorous phenotype, which is consistent with the known roles of mTORC1 in cell growth and cancer in humans (W. Qi et al. 2017). Based on the genetic data, W. Qi et al. (2017) suggested a model where DAF-16/FOXO activity (along with a TGF-β pathway) in the hypodermis may promote germline proliferation in part by upregulating the transcription of TORC1 pathway components daf-15, rsks-1, and rheb-1 (W. Qi et al. 2017). This functional relationship between DAF-16 and TORC1 is consistent with earlier findings on several somatic developmental events (see Upstream inputs to TOR signaling).
Through a different approach, one study made an interesting finding regarding the role of germline small RNAs in regulating TORC1 (Barberán-Soler et al. 2014). PRG-1 and CSR-1 are two germline-specific Argonaute proteins that are required for proper germline development; prg-1(-) mutants displayed a partial sterility phenotype that progressively worsened over multiple generations. Via RNA-sequencing profiling of prg-1 mutants, Barberán-Soler et al. (2014) discovered abnormal splicing products of let-363/Tor and an abnormal presence of a male germline-specific endo-siRNA produced from the antisense strand of a let-363 intron. RNAi against the intron containing this siRNA in prg-1(-) animals restored normal let-363 splicing and expression, and thereby reversed the sterility phenotype in prg-1(-) mutants. Additional genetic tests led to the model that PRG-1 and CSR-1 regulate germline development by antagonistically regulating the splicing, and expression, of let-363 through modulating the activity of this siRNA. Whether such regulation is conserved in mammals would be an interesting question to address.
Embryonic development:
C. elegans with a null allele in let-363/TOR arrest development at the third postembryonic stage (L3) (Long et al. 2002) but let-363/TOR(RNAi) by injection caused embryonic lethality (Sönnichsen et al. 2005; WormBase), suggesting that certain embryonic functions of TOR are masked by maternal rescue in the null mutants. Additional roles could also be masked by genetic redundancy, under which the effect of knocking down single genes may only be observed when another contributing gene is also compromised. As discussed below (see Roles of TORC2 in regulating development and behaviors), a critical role of TORC2 in embryogenesis was identified in a sensitive suppressor assay. Therefore, additional roles of TORC1 during embryogenesis may yet to be identified by employing genetic suppressor or enhancer screens.
TORC1 requirements could also depend on environmental conditions. For example, a recent study identified two P granule proteins as TOR phosphorylation targets and indicated a role of TORC1 in protecting embryogenesis from stress (Zhang et al. 2018). Specifically, under heat stress, TORC1-dependent phosphorylation of P granule components PGL-1 and PGL-3 led to the formation of P granules that are resistant to autophagy, and increased embryo viability.
Postembryonic development and behavior:
The L3 arrest of let-363(lf) and daf-15(lf) mutants indicates roles of TORC1 in promoting larval growth and development (see Identification of key components). The observation that let-363(RNAi) suppresses L1 larval lethality associated with a pha-4/FOXA mutation also suggests a role of TOR in early larval development (Sheaffer et al. 2008). Whether and how TORC1 perceives the availability of specific nutrients to instruct postembryonic development are challenging questions, which are well suited for C. elegans researchers to address.
Dauer formation:
In response to food deprivation, C. elegans arrest their development and form specialized dauer larvae after the second molt to extend their survival until they encounter new food (Hu 2007). Dauer larvae are morphologically and behaviorally distinct from typical L3 larvae, and are highly resistant to stress; they can survive for months until conditions improve, whereupon they reenter the reproductive life cycle. More than 40 daf genes, including many acting in the IIS and TGF-β pathways, were defined based on either constitutive dauer formation under abundant food (Daf-c phenotype) or failure to form dauer under food deprivation (Daf-d phenotype). The mutant phenotypes of daf-15/raptor and daf-9 (encoding cytochrome P450 family protein) were distinct from other daf-c mutants in that the “dauer-like” arrested worms appeared to be in an intermediate state, with only a subset of dauer characteristics (Albert and Riddle 1988; Jia et al. 2004). daf-15(RNAi) generated larval arrest phenotypes resembling those caused by let-363/TOR(RNAi) (Hara et al. 2002; Long et al. 2002), which, along with other data presented below, support an essential role of TORC1 in regulating postembryonic larval growth. However, an additional role of TORC1 in dauer formation is also suggested by genetic data beyond just the “dauer-like” morphology of daf-15(lf) mutants: mutation or RNAi knockdown of let-363 appears to enhance the dauer-constitutive phenotype of a daf-2/IGFR mutant, and daf-15 expression is regulated by daf-16/FOXO (Vellai et al. 2003; Jia et al. 2004). Further studies may be necessary to confirm these interactions, which have important implications. A potential role of TORC1 in inhibiting dauer entry would raise the questions of whether TORC1 directly responds to food cues in this capacity, and what target(s) TORC1 might act on to influence the L3 vs. dauer decision.
Food deprivation-induced L1 diapause:
Besides dauer formation, food deprivation-induced L1 diapause is also an excellent system to study how the postembryonic developmental program is regulated by nutrient/food availability (Baugh 2013). While extensive early studies uncovered the critical role of the IIS pathway in regulating L1 diapause (Baugh and Sternberg 2006; Zhang et al. 2011; Baugh 2013), more recent work has indicated the role of TORC1 in somatic cells. Specifically, newly hatched L1 larvae halted development in the absence of food (M9 solution), but supplementing the M9 solution with AAs and ethanol was sufficient to reactivate the quiescent somatic progenitor cells (the P, M, and Z1/Z4 cell lineages) (Fukuyama et al. 2015). It was further shown that ectopic expression of a putative activated raga-1 transgene in the hypodermis (and not the intestine or neurons) was sufficient to stimulate M and P cell progression, and these effects were suppressed by RNAi against let-363/TOR or daf-15/raptor. This study also provided evidence that TORC1 may act downstream of IIS and DAF-16/FOXO to mediate the ethanol effect on the activation of somatic progenitor cells. These and additional results led to an interesting model where AA levels are monitored by TORC1 in the hypodermis to regulate somatic progenitor cell progression (Fukuyama et al. 2015) (Figure 4A).
Lipid deficiency-induced developmental arrest and foraging behavior change:
When C. elegans embryos are deficient for mmBCFAs, they uniformly arrest postembryonic development after hatching, a state resembling food deprivation-induced L1 diapause, with the exception that the lipid deficiency also dramatically altered C. elegans food-seeking behavior (Kniazeva et al. 2008, 2015). The lipid deficiency also impairs survival of the arrested animals (Cui et al. 2017). Extensive further studies, including the isolation of a suppressor mutation in nprl-3, suggested that this growth arrest is due to a lack of d17iso-GlcCer and insufficient TORC1 activity (Kniazeva et al. 2008; Zhu et al. 2013) (also see Upstream inputs to TOR signaling) (Figure 3A).
Behavioral changes in mmBCFA- or d17iso-GlcCer-deficient L1 larvae also could be attributed to insufficient TORC1 activity (Kniazeva et al. 2015). Larvae provided with a normal diet typically spend more time dwelling near the food and less time roaming away from the food, and neuronal circuits controlling such foraging behavior have been extensively analyzed (e.g., Ben Arous et al. 2009; Milward et al. 2011; Flavell et al. 2013). In contrast, mmBCFA-deficient L1 larvae failed to respond to the bacterial food (reduced dwelling) unless mmBCFAs were added, or unless TORC1 activity was otherwise elevated via genetic manipulations (Kniazeva et al. 2015). Since behavioral defects are often the consequence of defects in neuronal development, regulation of behavior by TORC1 may be closely linked to its role in postembryonic development. Indeed, Kniazeva et al. (2015) found evidence that mmBCFA deficiency reduced the expression of a known regulator of neuronal differentiation (ceh-36), which partially contributes to the change in food behavior. Therefore, the behavior defect is at least in part due to a defect in neuronal development and TORC1 plays a critical role to mediate the impact of mmBCFAs on these behaviors (Figure 3A).
VB2 deficiency-induced developmental arrest and foraging behavior change:
Dietary VB2 is essential for the normal developmental progression of C. elegans (B. Qi et al. 2017). It was shown that heat-killed bacteria lack sufficient VB2 to support worm growth, and that worms stop eating such food and change their foraging behavior to search rather than dwell (B. Qi et al. 2017). Furthermore, VB2-deficient worms showed reduced intestinal expression of several specific proteases, suggesting that they may not be able to properly digest food. Providing exogenous VB2 (or its derivative flavin adenine dinucleotide) could partially restore worm growth, behavior, and protease expression, but this effect was dependent on functional TORC1, as it was eliminated by daf-15/raptor(RNAi) or ragc-1(RNAi). Genetic manipulations thought to increase TORC1 activity also restored worm growth, behavior, and protease expression. Based on these and other data, B. Qi et al. (2017) suggested that VB2-derived ATP stimulates TORC1 activity to upregulate intestinal protease expression (Figure 3B). Further work is needed to show just how the expression of these proteases is regulated by TORC1 and translated into a signal to change neuronal development or functions.
Vulva development:
Signaling by LIN-3/EGF and the Ras-ERK pathway promotes development of the vulva, an epithelial tube used for egg laying (Sundaram 2013). In certain vulvaless mutants with reduced signaling, starvation has been shown to restore vulval fates (Euling and Ambros 1996). A recent study demonstrated that loss of pept-1 was equivalent to starvation in suppressing the vulvaless phenotype caused by a partial loss-of-function allele in the lin-3/EGF gene (Grimbert et al. 2018) (see Upstream inputs to TOR signaling for discussion on pept-1). Moreover, let-363(RNAi) or rsks-1(−) also displayed significant suppression, albeit not as strong as that by pept-1(-). This study suggested a potential role of TORC1 in repressing EGF signaling under nonstarved conditions, although the mechanism underlying such a role and its physiological significance remain to be investigated.
Roles of TORC2 in regulating development and behaviors
Fat mobilization to the germline during larval development:
TORC2 has been shown to regulate reproductive development by acting in a somatic tissue (intestine). Lipid transportation from the intestine to germ cells by vitellogenins is a critical step for reproductive development and this process, including vitellogenesis, is coordinated with postembryonic development (Lemieux and Ashrafi 2016; Watts and Ristow 2017). The IIS pathway was first identified to play an important role in the process (DePina et al. 2011). Through a tandem genetic screen, one study found that several factors that regulate developmental timing, including the microRNAs let-7 and lin-29, act in the hypodermis to promote lipid mobilization in the intestine (Dowen et al. 2016). In the intestine, this signal is conveyed by TORC2, not TORC1, with SGK-1 and the transcription factor PQM-1 acting downstream of TORC2 to promote the expression of genes involved in vitellogenesis, and other activities needed for fat mobilization. Specific signals generated by the hypodermis that remain unidentified must therefore trigger activation of TORC2 nonautonomously in the gut, thereby tightly coordinating lipid mobilization for reproduction with development (Dowen et al. 2016; Weaver et al. 2016).
Embryonic development:
A role for TORC2 in embryonic development was revealed through its genetic interactions with skn-1 (Ruf et al. 2013). SKN-1 is a transcription factor that has been well characterized for its role in promoting mesodermal and endodermal cell fates, stress resistance, and life span (Blackwell et al. 2015). skn-1(RNAi) causes cell fate transformations and consequent embryonic lethality, but such lethality is partly suppressed by a rict-1 loss-of-function mutation (Ruf et al. 2013). These genetic data suggested that TORC2 acts downstream of (or in parallel to) SKN-1 to repress a gene expression network necessary for both mesodermal and endodermal fates. SGK-1 appears to mediate many TORC2 functions, but in this case the connection with SGK-1, as well as the connection between SKN-1 and TORC2, remain to be explored.
Postembryonic development and foraging behavior:
Loss of rict-1 confers many phenotypes, including developmental delay and small body size. A targeted RNAi suppressor screen identified dpy-21 as a suppressor of the rict-1(-) slow-growth phenotype (Webster et al. 2013). Further tests showed that additional members of the dosage compensation complex also suppressed the rict-1 phenotypes, leading to the conclusion that the dosage compensation complex acts downstream of RICT-1/TORC2 to negatively regulate development. Since not all rict-1(lf) phenotypes were suppressed by this pathway, it was proposed that the roles of TORC2 in impacting life span and body size are carried out by independent mechanisms.
A recent study discovered a fascinating role of Rictor/TORC2 in the intestine in regulating dauer formation and foraging behavior (O’Donnell et al. 2018). Entry into dauer usually occurs in response to high temperatures and/or crowded conditions (Hu 2007). Loss-of-function mutations in rict-1 or the likely TORC2 downstream target sgk-1 drastically increased dauer formation at 27°, and this phenotype was rescued by intestinal expression of the corresponding gene. Further tests have indicated that TORC2 promotes the expression of DAF-7/TGF-β and an insulin-like peptide (DAF-28) to negatively regulate heat-induced dauer entry. Moreover, the study showed that RICT-1 is required for food-induced dwelling behavior, and provided genetic data to support a model in which Rictor inhibits foraging by promoting the signaling activity of neuropeptides PDF-1 and PDF-2 (O’Donnell et al. 2018). This study also raises interesting questions regarding how TORC2 perceives the levels of specific nutrients, and how intestinal TORC2 and SGK-1 regulate gene expression and activities in neurons. Interestingly, these data, along with the studies discussed above (see Roles of TORC1 in regulating development and behaviors), demonstrated roles of both intestinal TORC1 and TORC2 in regulating postembryonic developmental events (albeit distinct events) and foraging behaviors, raising fascinating questions regarding the differences between TORC1 and TORC2, and the physiological significance and mechanism for each system. In addition, another recent study showed that loss of several TOR-related proteins results in changes in “taste-associated learning,” suggesting a potentially critical role of TORC2 in a sensory neuron’s ability to respond to salt-level changes (Sakai et al. 2017).
In a study of the role of Rho GTPases in axon guidance, CDC-42-induced neuronal protrusions were found to be dependent on rict-1, but not daf-15 nor daf-16, implicating TORC2 in these processes (Alan et al. 2013). Neuron-specific RNAi experiments lead to the conclusion that these are cell-autonomous activities in the PDE neuron.
Fat storage:
Studies in mammals have indicated that both mTORC1 and mTORC2 promote lipogenesis and adipogenesis (Lamming and Sabatini 2013; Caron et al. 2015), but studies in C. elegans suggest that TORC2 instead inhibits fat storage. An apparent role of TOR in lipid metabolism in C. elegans was first revealed by observations that let-363(RNAi) led to a significant increase in Nile Red staining (Vellai et al. 2003). Several years later, two independent forward genetic screens identified a fat storage-increase phenotype associated with loss of rict-1 activity (by staining with Nile Red, Oil Red O, and boron-dipyrromethene (BODIPY)-labeled fatty acid dye) (Jones et al. 2009; Soukas et al. 2009). In addition, rict-1 was also found to be required for roles of several genes in regulating Nile Red accumulation and autofluorescence of lysosome-related organelles (Soukas et al. 2013). Consistently, mutations in the TORC2 component sinh-1 also caused an increase in body fat (Sakai et al. 2017). Both Jones et al. (2009) and Soukas et al. (2009) indicated that TORC2 acts mainly through SGK-1, although the two studies have different conclusions about the role of the AKT pathway, which is thought to be a major downstream target in mammals (Jones et al. 2009; Soukas et al. 2009; Saxton and Sabatini 2017). These studies may have revealed a unique aspect of TORC2 regulation of fat metabolism that is yet to be uncovered in mammals. Such a function may be related to the role of TORC2 in stress responses, including coping with changes in nutrient availability, which may warrant future investigation.
Role of TOR Signaling in Aging and Stress Responses
For the last several years, TOR has been a prominent topic in the aging field in C. elegans, and beyond. One reason is that rapamycin represents the current “gold standard” for an antiaging drug. Rapamycin extends life span in mice, even when administered in later life (Harrison et al. 2009; Kennedy and Lamming 2016), and provides a model for elucidating how a drug that acts on a single defined target can extend life span. Mounting evidence indicates that rapamycin treatment or genetic TOR inhibition also improves multiple health-related parameters in mice, and even dogs (Wu et al. 2013; Kennedy and Lamming 2016; Urfer et al. 2017). TOR is also of great interest because it has been linked to aging in organisms ranging from single-cell eukaryotes to mice, and plays an important role in life span extension by other genetic or pharmacological interventions that promote longevity (Johnson et al. 2013; Antikainen et al. 2017). Of particular importance, there is general agreement in the aging field that reduced TOR activity is a major mediator of the beneficial effects of dietary restriction (DR) (Figure 5) (Kenyon 2010; Johnson et al. 2013). DR, defined as a reduction in nutrient intake that does not induce malnutrition, can extend life span robustly and confers metabolic benefits in essentially all eukaryotes. For many reasons, DR is not practical as an antiaging strategy to be adopted by humans, making it important to identify DR-related mechanisms that could be more realistic strategies for intervention. Given the importance of elucidating specific protective mechanisms acted upon by either rapamycin or DR, it is not surprising that TOR is very much in the spotlight.
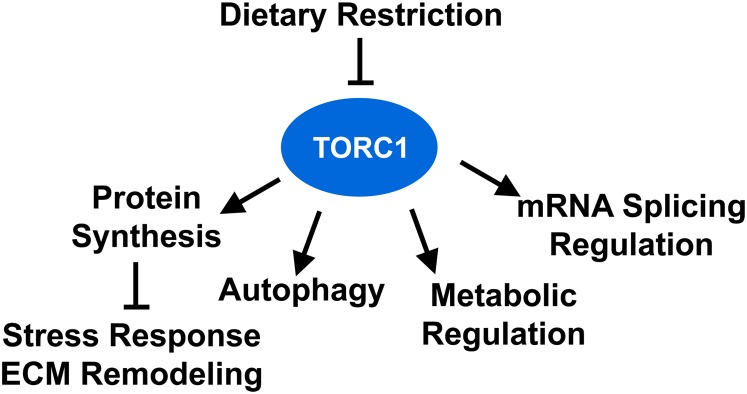
Figure 5.
Processes through which TORC1 affects C. elegans life span. A partial list of major biological functions through which TORC1 modulates life span is shown. The critical role of TORC1 in dietary restriction is indicated, but TORC1 is also involved in other pathways that promote longevity. Please see the text for a more thorough discussion. ECM, extracellular matrix; TORC, Target of Rapamycin Complex.
The notion that life span can be altered by a single genetic mutation, and hence by intervention in a specific pathway, was shown to be true over two decades ago with the groundbreaking finding that C. elegans life span can be extended by reductions in IIS activity (Friedman and Johnson 1988; Kenyon et al. 1993; Dorman et al. 1995; Morris et al. 1996; Kimura et al. 1997; Lin et al. 1997; Ogg et al. 1997; Kenyon 2010; Shore and Ruvkun 2013). Arguably, C. elegans remains the premier model organism for genetic, metabolic, and pharmacological analyses of how aging can be slowed. It was in C. elegans that it was first demonstrated that life span can be extended when TOR is reduced (Vellai et al. 2003), and subsequent C. elegans studies have made many major contributions to our understanding of how TOR affects aging. Given its short life span and genetic tractability, the worm has been especially valuable for elucidating mechanisms through which reduced TOR signaling promotes longevity and stress resistance. In particular, novel insights into TOR functions that have been obtained in C. elegans include identification of mechanisms that function downstream of TOR to extend life span, and teasing apart the effects of TORC1 and TORC2 on life span.
Life span extension and increased stress resistance from TORC1 inhibition
A good starting point for discussing how TORC1 influences C. elegans aging is to consider the effects of reducing or eliminating its activity, and treatment with rapamycin. The initial link between the TOR pathway and aging had been made in 2001 in S. cerevisiae, when it was shown that deletion of the S6 kinase ortholog Sch9 doubled the survival of cells in stationary phase, but at the time it was thought that Sch9 corresponded to AKT rather than S6K (Fabrizio et al. 2001). While TORC1 is required for C. elegans larval development and reproduction (see Identification of key components), it is possible to study its effects on C. elegans life span by examining animals in which its activity is disrupted partially, or by RNAi knockdown after development is complete. C. elegans thereby provided the very first evidence in any organism that TOR signaling affects aging, with the observation that RNAi against let-363/TOR or loss of one daf-15/raptor gene copy extended life span (Vellai et al. 2003; Jia et al. 2004). Subsequent work showed that in C. elegans, yeast, Drosophila, and mice, life span could be extended by reducing the activity of several TORC1 signaling components or by treatment with rapamycin (Johnson et al. 2013; Antikainen et al. 2017). Across the many C. elegans studies referenced in this article, genetic ablation of TORC1 pathway components typically extends mean life span by ∼18–25%. While this degree of life span extension is considerably lower than is typically associated with DR or rIIS (> 50%) (Kenyon 2010; Moroz et al. 2014), a comparable increase in healthy human life span would generate considerable excitement! Ablation or inhibition of TORC1 signaling also improves C. elegans “healthspan,” the length of time that the animal fails to show defined signs of aging or exhibits behaviors associated with youthfulness. For example, Rag GTPase knockdown or mutation dramatically delays the aging-related decline in various metrics of activity (Schreiber et al. 2010; Robida-Stubbs et al. 2012).
Less protein synthesis, reduced stress, and longer life:
Across species, the biological process that is most solidly established as being regulated by TORC1 is mRNA translation (Figure 5) (Johnson et al. 2013; Kennedy and Lamming 2016; Saxton and Sabatini 2017). This appears to be true in C. elegans, since functional studies of IFET-1/SPN-2 (Li et al. 2009) indicate that it is an eIF4E-binding protein. In the worm, the rate of protein synthesis, as indicated by 35S incorporation, is reduced by adulthood RNAi knockdown of let-363/TOR, ragc-1/RagC, or rsks-1/S6K and by rapamycin treatment (Hansen et al. 2007; Robida-Stubbs et al. 2012). Although it has not been demonstrated conclusively that TORC1 directly phosphorylates RSKS-1/S6K in C. elegans (see Downstream targets), in this section we will consider it to be a putative TORC1 target because of the conservation of S6K being directly regulated by TORC1 in organisms as diverse as yeast and humans (Saxton and Sabatini 2017), the genetic evidence cited above (see Downstream targets), and the evolutionarily predicted role of RSKS-1/S6K in promoting ribosome function and protein synthesis (a function of TORC1 in other eukaryotes that is conserved in C. elegans).
The intense level of interest in the TORC1 pathway, together with the identification of translation-related proteins in C. elegans genetic screens for life span extension or stress response activation, led a number of laboratories to investigate whether C. elegans life span can be increased simply by reducing the rates of protein synthesis (Henderson et al. 2006; Chen et al. 2007; Curran and Ruvkun 2007; Hansen et al. 2007; Pan et al. 2007; Syntichaki et al. 2007; Tohyama et al. 2008; Wang et al. 2010). These studies revealed that C. elegans life span and stress resistance are increased by mutations that decrease rates of translation initiation, or when translation is inhibited in adults by RNAi against ribosomal proteins or several different translation initiation factors. In agreement with these findings in the worm, genetic mutations that reduce translation also increase life span in yeast, Drosophila, and mice, suggesting that the relationship between protein synthesis and life span is evolutionarily conserved (Johnson et al. 2013).
The reduced rates of translation that result from TORC1 inhibition could influence aging in a number of ways. A reduction in protein synthesis lessens the burden of damaged or misfolded proteins, an important factor in aging (Kenyon 2010; López-Otin et al. 2013; Labbadia and Morimoto 2015; Solis et al. 2018). This might also result in a beneficial decrease in energy or resource demand. However, several studies indicate that the picture is more complex. Across species, a reduction in protein synthesis rates results in preferential translation of a subset of mRNAs that have particular structural features (Johnson et al. 2013; Steffen and Dillin 2016; Kapahi et al. 2017). These genes tend to be heavily weighted toward stress response proteins, providing a mechanism for defending the organism in times of resource limitation. This model is supported by work in C. elegans, in which inhibition of the translation initiation factor IFG-1/eIF4G resulted in preferential synthesis of many proteins that protect against stress (Rogers et al. 2011). This translational preference correlated with greater mRNA transcript length, suggesting a mechanism that may mediate part of this effect. In another potentially beneficial mechanism, in mammals TORC1 inhibition leads to derepression of translation elongation factor 2 kinase, a regulator that slows translational elongation, enhances translation accuracy, and in C. elegans contributes to life span (Xie et al. 2019).
Other studies have shown that transcription is altered when translation is reduced. Life span extensions arising from knockdown of some translation initiation factors are partially or fully dependent upon the transcription factor DAF-16/FOXO (Henderson et al. 2006; Hansen et al. 2007; Tohyama et al. 2008; Wang et al. 2010), and associated with increased DAF-16/FOXO transcriptional activity (Henderson et al. 2006). DAF-16/FOXO regulates many processes implicated in life span extension, including stress resistance, proteostasis, metabolism, and immunity (Kenyon 2010; Shore and Ruvkun 2013). DAF-16/FOXO also represents an important benchmark in the longevity field: it is inhibited by IIS in an evolutionarily conserved manner, and is fully required for reduced IIS and several other conditions to increase C. elegans life span (Figure 6), and in Drosophila and humans its FOXO orthologs are associated with longevity (Kenyon 2010; Shore and Ruvkun 2013; Fontana and Partridge 2015). Life span extension from reduced translation was also found to require the transcription factor SKN-1/Nrf, or overlapping functions of SKN-1/Nrf and DAF-16/FOXO (Wang et al. 2010). Alternatively spliced SKN-1/Nrf isoforms are orthologous to the mammalian transcription factors Nrf1 and Nrf2 (NF-E2-related factor), which mediate conserved responses to proteasomal and xenobiotic/oxidative stress, respectively (Blackwell et al. 2015; Lehrbach and Ruvkun 2016). SKN-1/Nrf is important in many contexts of C. elegans life span extension, including rIIS (Figure 6), and Nrf2 has been implicated in life span extension in Drosophila and mice (Blackwell et al. 2015). SKN-1/Nrf target genes are involved not only in stress resistance, but also in metabolism and immunity (Blackwell et al. 2015), and in C. elegans many are activated when translation is inhibited (Wang et al. 2010; Li et al. 2011). It is unknown how reduced translation causes these effects on transcription, although other transcription factors that defend against stress are known to be preferentially translated when translation rates are low (Harding et al. 2000; Johnson et al. 2013; Shpilka and Haynes 2018).
Figure 6.
Regulatory mechanisms through which TOR signaling affects C. elegans life span. A partial list of transcription regulators through which TOR signaling affects life span is shown. Note the potential cross talk between TOR signaling and IIS that has been suggested by genetic analyses in C. elegans, and mechanistic studies in other species. Please see the text for a more thorough discussion. Pol, polymerase; TOR, Target of Rapamycin; TORC, TOR Complex.
If TORC1 inhibition increases C. elegans life span in part by reducing translation (Figure 5), knockdown of TORC1-specific pathway components would be expected to phenocopy the effects described above. Accordingly, daf-16/FOXO mutation prevented life span extension from loss of one daf-15/raptor copy (Jia et al. 2004), and both DAF-16/FOXO and SKN-1/Nrf were required for longevity from knockdown of raga-1/RagA, ragc-1/RagC, daf-15/raptor, or rheb-1 specifically during adulthood (Robida-Stubbs et al. 2012). RNAi against these TORC1 components activated SKN-1/Nrf and DAF-16/FOXO target gene transcription without increasing overall SKN-1/Nrf nuclear occupancy, as occurs when translation initiation is inhibited (Robida-Stubbs et al. 2012). These effects on gene expression were distinct from those that occur in response to reduced IIS. By several criteria, the effects of TORC1 inhibition on life span, stress resistance, and transcription were mimicked by inactivation of CGEF-1, a guanine nucleotide exchange factor that physically interacts with RHEB-1 in cell culture assays, and thus may be a new player in TORC1 signaling (Li et al. 2018). Together, the data suggest that SKN-1/Nrf- and DAF-16/FOXO-mediated transcriptional programs are critical for life span extension from reduced TORC1 activity, and are induced in response to reduced translation. These effects may be evolutionarily conserved, as Nrf2 targets are upregulated in livers from rapamycin-treated mice (Robida-Stubbs et al. 2012). Interestingly, when TORC1 activity is inhibited in C. elegans by ragc-1 knockdown or rapamycin treatment, SKN-1 directly upregulates expression of multiple TORC1 pathway genes (raga-1/RagA, daf-15/raptor, and rsks-1/S6K), forming a feedback loop that might compensate by enhancing TORC1 activity (Robida-Stubbs et al. 2012).
Additional evidence supports the idea that reduced protein synthesis is a major mechanism through which TORC1 inhibition increases life span. In general, conditions that increase C. elegans life span decrease ribosome biogenesis and the size of nucleoli, where ribosomal components are synthesized and assembled, and small nucleoli are predictive of longevity across diverse eukaryotes (Tiku et al. 2017). The putative TORC1 target RSKS-1/S6K promotes ribosomal function and protein synthesis, and reducing its activity extends life span in yeast, C. elegans, Drosophila, and female mice (Selman et al. 2009; Fontana et al. 2010; Johnson et al. 2013). C. elegans, Drosophila, and yeast (chronological) life spans can be also extended by genetic inactivation of RNA Polymerase III (Pol III), which transcribes tRNA and 5S rRNA, and is required for wild-type (WT) levels of protein synthesis (Filer et al. 2017). C. elegans life span is extended when Pol III is inactivated in an apparently gut-specific manner (Filer et al. 2017), as is also true for ragc-1/RagC (Robida-Stubbs et al. 2012). Pol III transcription is increased by TORC1 acting directly at target gene loci, and in Drosophila life span extensions from Pol III inactivation and rapamycin are not additive, placing Pol III within the TORC1 pathway (Filer et al. 2017). As Pol III is a potentially druggable target, these results suggest a TORC1-based mechanism for extending life span that could be more specific than rapamycin (see below).
TORC1 and TORC2 affect longevity differently, with both inhibited by rapamycin in vivo:
Many analyses of TOR and longevity in C. elegans, particularly earlier studies, developed models based upon knockdown of the TOR kinase (LET-363) itself. However, it is crucial to remember that let-363/TOR RNAi eliminates both TORC1 and TORC2, which have distinct functions (Figure 2 and Figure 6). The effects of TORC2 on life span, which are complex and will be discussed in detail below (see Roles of TORC2 in Aging and stress responses), can be seen in let-363(RNAi) animals. Adulthood RNAi against the TORC2 component rict-1/Rictor increases SKN-1/Nrf nuclear occupancy and extends life span independently of DAF-16/FOXO (Robida-Stubbs et al. 2012; Mizunuma et al. 2014). In striking contrast to the effects of inhibiting TORC1 alone, these events also occur with let-363/TOR RNAi (Vellai et al. 2003; Jia et al. 2004; Robida-Stubbs et al. 2012), as would be expected with both TORC1 and TORC2 being impaired (Robida-Stubbs et al. 2012). Going forward, it will be important to remember that to draw conclusions about TORC1 functions from genetic studies, it is necessary to take advantage of the well-characterized genetic tools that are specific to the TORC1 pathway (e.g., daf-15/raptor, raga-1/RagA, and ragc-1/RagC).
Epistasis analyses of TORC1 and TORC2 have been informative for understanding how the TOR inhibitor rapamycin acts in vivo. Rapamycin has been of great interest because of its effectiveness as a mammalian antiaging drug. It became possible to study rapamycin effects in C. elegans once it was found that very high concentrations were needed to achieve bioavailability, as indicated by the SKN-1 target gene activation that occurs when TORC1 is blocked (Robida-Stubbs et al. 2012). Importantly, skn-1/Nrf but not daf-16/FOXO was required for rapamycin to increase C. elegans life span, as was seen with let-363/TOR RNAi, consistent with the idea that both TORC1 and TORC2 are inhibited in vivo (Robida-Stubbs et al. 2012; Calvert et al. 2016). Moreover, like rict-1/Rictor RNAi, rapamycin induces SKN-1 nuclear accumulation when C. elegans is provided with particular food sources (see Importance of TORC1 regulation in life span extension mechanisms) (Robida-Stubbs et al. 2012). Why would rapamycin affect both TOR complexes in the worm? While rapamycin directly inhibits TORC1, in mammalian cells continued rapamycin treatment disrupts TORC2 complexes through its interactions with the TOR kinase, and in mice rapamycin thereby inactivates TORC2 along with TORC1 to an extent that varies among tissues (Lamming et al. 2012; Kennedy and Lamming 2016). Thus, impairment of both TOR complexes in vivo appears to be the rule rather than the exception for rapamycin and related compounds. Unraveling the biological effects of rapamycin and other TOR inhibitors on TORC1 and TORC2 is currently a topic of active investigation in the mammalian aging field (Kennedy and Lamming 2016). However, while the worm has proven valuable for examining effects of rapamycin in vivo, most C. elegans investigations of TORC1 per se use genetic approaches given the relative ease of genetics in the animal, the technical difficulty of administering rapamycin, and the understanding that rapamycin affects both TOR complexes (see Rapamycin).
Autophagy is critical:
Genetic studies indicate that a functioning autophagy system is required for C. elegans life span to be extended by essentially any intervention, including rIIS and DR (Figure 5) (Hansen et al. 2018). Autophagy has similarly been implicated as broadly essential for life span extension in yeast and Drosophila, suggesting that its importance for longevity is evolutionarily conserved (Shpilka and Haynes 2018). In the setting of aging, autophagy may be critical for eliminating and recycling damaged proteins and organelles, and maintaining energy reserves. Autophagy also can have salutary effects on metabolism; for example, when reproduction is inhibited the resulting increase in autophagy upregulates expression of LIPL-4, a lysosomal acid lipase that promotes longevity through lipid-mediated signaling (Lapierre et al. 2011; Folick et al. 2015).
The role of autophagy in life span extension from reduced TORC1 activity has been of interest because TORC1 inhibits autophagy across eukaryotes, including C. elegans (see Downstream targets) (Hansen et al. 2008; Robida-Stubbs et al. 2012; Johnson et al. 2013). As would be predicted, genetic ablation of the autophagy-regulatory transcription factor HLH-30/TFEB (Figure 6) or of key autophagy proteins abrogates life span extension from knockdown or mutation of let-363/TOR, daf-15/raptor, or rsks-1/S6K (Hansen et al. 2008; Lapierre et al. 2013). Life span extension from let-363 or rsks-1/S6K RNAi also requires the transcription factor PHA-4/FOXA (Figure 6), a regulator of autophagy that genetic analysis indicates is antagonized by TORC1 signaling (Sheaffer et al. 2008). Activation of PHA-4/FOXA by let-363/TOR knockdown has been linked to the kinase GCN-2, which regulates translation, thereby intertwining regulation of translation and autophagy by TOR (Rousakis et al. 2013). Additionally, life span extension from knockdown of daf-15/raptor or let-363/TOR depends upon the conserved kinase HPK-1, which is also required for autophagy to be increased when TORC1 signaling is reduced (Das et al. 2017). Finally, spermidine induces chromatin modifications that increase life span in yeast, C. elegans, Drosophila, and mice by upregulating autophagy (Eisenberg et al. 2009), and in C. elegans overexpression of HLH-30/TFEB on its own extends life span (Lapierre et al. 2013).
Together, the data cited above show that derepression and upregulation of autophagy is a major factor in life span extension from TORC1 inhibition. However, two recent C. elegans studies indicate that in some biological contexts, autophagy activation can be taken to an extreme. First, after reproduction ceases, yolk continues to be produced through autophagic breakdown of intestinal tissue, and prevention of this process appears to enhance both longevity and health (Ezcurra et al. 2018). In addition, as we describe below in “Roles of TORC2 in aging and stress responses,” when TORC2 and SGK-1 activity are ablated genetically, mitochondrial permeability is increased, resulting in elevated autophagic activity that is deleterious (Zhou et al. 2019). This effect is a major contributor to the complex effects of TORC2 on C. elegans life span. Thus, while the bulk of evidence indicates that autophagy is an important driver of life span extension (Hansen et al. 2018), under some circumstances too much of a good thing can be harmful, suggesting that it could be advantageous to understand how to optimize activity of the various autophagic activities. It will be interesting to delve deeper into how the increase in autophagy that results from TORC1 inhibition affects specific tissues and parameters associated with pathology and health.
Other mechanisms through which TORC1 limits life span:
TORC1 also limits C. elegans life span through mechanisms besides direct regulation of protein synthesis and autophagy (Figure 5). Adulthood RNAi knockdown of TORC1 pathway components (daf-15/raptor or ragc-1/RagC, and rsks-1/S6K) or rapamycin treatment induces HSF-1 (Figure 5) to activate genes that are important for cytoplasmic proteostasis, and are required along with HSF-1 for the resulting life span extension (Seo et al. 2013). It remains to be determined how TORC1 inhibition activates HSF-1. The predicted TORC1 target RSKS-1/S6K limits longevity through two pathways that seem to be independent of protein synthesis regulation. First, like mammalian S6K, RSKS-1/S6K inhibits the energy sensor AMPK (see Upstream inputs to TOR signaling), which promotes C. elegans longevity, and is required for rsks-1/S6K mutation and some DR conditions to extend life span (Selman et al. 2009; D. Chen et al. 2013; Burkewitz et al. 2014; McQuary et al. 2016). When RSKS-1/S6K is inactivated, a conserved arginine kinase (ARGK-1) becomes expressed in a subset of glial cells, resulting in AMPK activation and delayed aging of the organism, presumably through tissue nonautonomous signaling (McQuary et al. 2016). Mutation of rsks-1/S6K impairs reproduction and growth independently of AMPK (Selman et al. 2009; McQuary et al. 2016), suggesting that RSKS-1/S6K may regulate translation independently of the latter kinase. In addition, genetic analysis suggests that TORC1 acts through RSKS-1/S6K to limit production of monounsaturated fatty acids that promote C. elegans longevity (Han et al. 2017). This intriguing study suggests that TORC1 might regulate production of signals that coordinate metabolism and aging across tissues. Finally, in mammals, TORC1 modulates several metabolic pathways and affects the phosphorylation status of hundreds of proteins (Hsu et al. 2011; Yu et al. 2011; Kennedy and Lamming 2016; Saxton and Sabatini 2017), suggesting that we have much to learn about how it acts in C. elegans. Therefore, it would be very surprising if TORC1 did not affect C. elegans metabolism in several additional ways that influence aging.
While most work in the aging field has focused on mechanisms that protect or repair cellular structures and functions, TORC1 inhibition also appears to enhance the functions of extracellular matrices (ECMs), which maintain tissue architecture, and are also critical for cell–cell communication and other functions (Figure 5). In C. elegans, diverse interventions that promote longevity, including DR and rapamycin treatment, delay a decline in adulthood expression of particular collagens and other ECM genes, and thus appear to promote ECM remodeling and cuticle strengthening that is important for life span extension (Ewald et al. 2015). It is not understood how these gene expression effects are mediated, but SKN-1/Nrf plays an important role. In mammals, various ECM parameters decline with age, most visibly represented by skin integrity, and in mice rapamycin treatment preserves tendon strength and elasticity (Wilkinson et al. 2012). This area of aging research is still in its infancy, but it appears that enhancement of extracellular structures is an important factor in the antiaging benefits of TORC1 inhibition and other interventions.
One intriguing theory posits that an important cause of aging is “run-on” activity of processes that are advantageous for development or reproduction, but later unneeded and possibly harmful (Williams 1957; Demidenko et al. 2009). While most of the field sees the relevant goal to be understanding how lower TORC1 activity leads to mobilization of processes that benefit the organism during aging, according to this idea it could be beneficial simply to reduce TORC1 activity below levels that might be maladaptive after growth and reproduction have been completed. These two views are not mutually exclusive, and the long list of mechanisms enumerated in this section illustrates how much we still have to learn, both about the causes of aging and how modulating TORC1 signaling can interfere with this process.
Importance of TORC1 regulation in life span extension mechanisms
DR:
Under DR conditions, TORC1 is presumably inhibited because nutrient availability is reduced, making it a logical model that decreased TORC1 activity is an important mediator of DR life span extension (Figure 5) (Johnson et al. 2013; Fontana and Partridge 2015; Kapahi et al. 2017). Consistent with this idea, let-363/TOR knockdown failed to extend life span further in long-lived eat-2 animals, in which an impairment of pharyngeal pumping may induce a DR-like state (Hansen et al. 2007). Genetic epistasis studies in yeast and Drosophila have yielded similar results (Kenyon 2010; Johnson et al. 2013). The transcription regulator hypoxia-inducible factor-1, which is regulated by TORC1 in mammals, modulates C. elegans life span downstream of rsks-1/S6K and is important in DR (Chen et al. 2009). DR longevity also partially depends upon DRR-2, a translation initiation factor that appears to function downstream of TORC1 (Ching et al. 2010). Other evidence implicating lower TORC1 activity in C. elegans DR came from a study that profiled mRNA levels over time when DR was imposed by food limitation (Hou et al. 2016). In a systems analysis of these gene expression data, many of the effects of DR could be mimicked by the expected combined effects of lower TORC1 and IIS activity, along with AMPK activation. Additionally, targeting these three regulatory “nodes” together resulted in an extremely long life span extension that was refractory to a further increase from DR (Hou et al. 2016). Cross talk among TORC1, IIS, and AMPK signaling has been demonstrated in mammals (Johnson et al. 2013; Kennedy and Lamming 2016; Sabatini 2017; Saxton and Sabatini 2017), and in C. elegans genetic evidence indicates that TORC1 signaling and IIS interact to process nutritional cues during larval development (Jia et al. 2004; Fukuyama et al. 2015) (Figure 4A and Figure 6). In addition, AMPK is important for synergistic life span extensions that result from double mutations in rsks-1/S6K and daf-2/IGFR (A. Chen et al. 2013b).
Given the evidence cited above, it appears likely that DR life span extension results from the effects of reduced TORC1 activity acting in parallel to rIIS, AMPK, and almost certainly additional mechanisms. However, inactivation of translation factors or raga-1/RagA can extend life span additively with some C. elegans DR conditions (Hansen et al. 2007; Schreiber et al. 2010), seemingly arguing against the idea that TORC1 is a key player in DR. These last discrepancies may arise from differences among DR protocols and the essential impossibility of performing true epistasis experiments with a dietary intervention, and, in many cases, RNAi. Despite these last caveats, the idea that reduced TORC1 activity is critical in DR has been largely accepted (Johnson et al. 2013; Fontana and Partridge 2015; Kapahi et al. 2017).
Modulation of TORC1 also appears to be important for benefits of DR besides longevity. Inhibiting its activity improves healthspan parameters in C. elegans and mammals (see Life span extension and increased stress resistance from TORC1 inhibition) (Johnson et al. 2013; Kennedy and Lamming 2016), suggesting that TORC1 might be a major modulator of aging-associated disease in the setting of DR. In C. elegans, TORC1 inhibition and DR also dramatically improve function in an associative learning paradigm, through a neuronal signaling pathway that involves downregulation of a specific neuroinhibitory metabolite and is not required for life span extension (Vohra et al. 2017). TORC1 may thus be an important modulator of cognitive function independently of its effects on aging per se.
While it is clear that longevity and health can be enhanced by reducing TORC1 activity, some studies have revealed a positive role for TORC1 components during aging. In C. elegans and other species, DR can be imposed by intermittent fasting (IF), the repetitive deprivation of food for limited periods (Honjoh et al. 2009; Fontana and Partridge 2015; Kapahi et al. 2017). Life span extension from IF is suppressed by mutation of rheb-1, an effect that does not seem to be mediated through TORC1 regulation of translation (Honjoh et al. 2009). Perhaps analogously, TORC1 signaling in the intestine is required for a transcriptional response through which transient hypoxia extends C. elegans life span (Schieber and Chandel 2014).
It is unknown why inadequate TORC1 signaling limits life span extension in the above scenarios, but a recent C. elegans study identified a specific mechanistic parameter of “youthfulness” that is dependent upon TORC1. The relative representation of many alternatively spliced mRNAs changes during aging but DR delays this effect, resulting in the persistence of youthful splicing patterns (Heintz et al. 2017). This enhancement of mRNA splicing function appears to promote longevity, in keeping with the idea that longevity interventions in general may preserve mechanisms that maintain the fidelity of gene expression. Paradoxically, while it is generally accepted that DR reduces TORC1 activity, genetic inactivation of raga-1 prevents DR from delaying the age-related decline in mRNA splicing. Thus, TORC1 signaling is essential for maintenance of this particular parameter of youth. Consistent with these findings, a recent study in mammalian cells revealed that S6 kinase promotes functional alternative splicing at key lipid metabolism genes by phosphorylating and regulating SRPK2, a kinase that modulates some mRNA splicing events (Lee et al. 2017). It will be interesting to elucidate how broadly TORC1 influences mRNA splicing, how this occurs, and how this contributes to the functions of TORC1 in growth, development, and aging. The idea that TORC1 is required for some mechanisms that promote longevity emphasizes the potential importance of determining the levels and tissue distribution of TORC1 activity most compatible with life span extension, and whether it might be possible to inhibit specific activities downstream of TORC1 in a way that promotes longevity and health without interfering with beneficial functions.
Reduced GSC number:
TORC1 signaling has also been implicated in one of the most complicated mechanisms through which C. elegans life span can be extended, GSC ablation. When precursors to the germline are ablated with a laser or GSCs are genetically prevented from proliferating [conditions referred to here as GSC(-)], germ cells do not form, life span is increased by 25–40%, and resistance to multiple stresses is increased (Kenyon 2010; Lemieux and Ashrafi 2016; Hansen et al. 2018). This life span extension involves nuclear receptor and lipid signaling, and somatic activation of a network of numerous transcription factors that have been implicated in longevity or lipid metabolism: DAF-16/FOXO, DAF-12/FXR, PHA-4/FOXA, NHR-80/HNF4, NHR-49/PPARα, SKN-1/Nrf, HLH-30/TFEB, MML-1/Mondo, and MXL-2/Max (Hsin and Kenyon 1999; Lin et al. 2001; Goudeau et al. 2011; Lapierre et al. 2011; O’Rourke and Ruvkun 2013; Ratnappan et al. 2014; Steinbaugh et al. 2015). This complex response may have evolved to protect the animal under adverse conditions or to coordinate life span with reproduction, so that it can survive to reproduce when conditions are favorable (Kenyon 2010). Another possibility that is not mutually exclusive is that this response to GSC absence represents a metabolic defense against a glut of lipids and other resources that would normally have been allocated to reproduction (Steinbaugh et al. 2015; Lemieux and Ashrafi 2016). Evidence for an inverse relationship between GSC formation and life span has been also obtained in Drosophila and human males (Flatt et al. 2008; Min et al. 2012; Steinbaugh et al. 2015), suggesting that aspects of this relationship might be conserved.
Given that GSC(-) life span extension depends upon activation of PHA-4/FOXA, HLH-30/TFEB, and autophagy, it is not surprising that TORC1 signaling would be involved (Lapierre et al. 2011, 2013; Nakamura et al. 2016). Heterodimeric MML-1/Mondo:MXL-2/Max complexes regulate numerous genes involved in longevity and stress resistance, and are important in various contexts of life span extension (Johnson et al. 2014; Nakamura et al. 2016). In GSC(-) animals, MML-1/Mondo:MXL-2/Max increases HLH-30/TFEB nuclear accumulation and autophagy activity by inhibiting TORC1, as detected by phosphorylation of the predicted TORC1 target RSKS-1/S6K (Nakamura et al. 2016). This inhibition is mediated through MML-1/Mondo:MXL-2/Max transcriptionally repressing expression of leucyl tRNA synthase-1, a protein that activates TORC1 signaling (Nakamura et al. 2016).
While TORC1 is unequivocally central to the effects of GSC ablation on autophagy, it is unclear whether TORC1 signaling might be a more general mediator of GSC(-) life span extension. Consistent with this model, the life span of GSC(-) animals was not increased further by let-363/TOR knockdown (Lapierre et al. 2011). However, knockdown of raga-1/RagA or translation initiation factors extended life span additively with GSC loss, arguing against this idea (Robida-Stubbs et al. 2012). It is still unknown whether RSKS-1/S6K phosphorylation or other predicted markers of TORC1 signaling are actually reduced in GSC(-) animals. GSC loss decreases LET-363/TOR mRNA and protein levels compared to WT (Lapierre et al. 2011), but also eliminates around two-thirds of the cell bodies in the animal, making it difficult to draw clear conclusions from reduced expression of genes like let-363/TOR that are active in GCs (Steinbaugh et al. 2015). Germ cell number and total let-363/TOR mRNA levels are both decreased by DR in a genetically linked manner (Thondamal et al. 2014), consistent with the possibility that a major proportion of let-363/TOR mRNA expression derives from GCs. Finally, nuclear occupancy of SKN-1/Nrf and certain DAF-16/FOXO isoforms is induced by GSC loss, but not TORC1 inhibition (Lin et al. 2001; Robida-Stubbs et al. 2012; Steinbaugh et al. 2015; Wei and Kenyon 2016). Further work will be required to tease out the functions of TOR signaling in the complex network of overlapping gene expression responses that have been implicated in this very intriguing pathway.
TORC1 and IIS:
In other organisms, the TORC1 and IIS pathways are directly integrated (see Upstream inputs to TOR signaling and Figure 6). While there is some evidence of integration between TORC1 and IIS pathways in C. elegans (see Upstream inputs to TOR signaling and Figure 6), these pathways also appear to act independently in some of their roles during C. elegans development (e.g., Korta et al. 2012). Similarly, the TORC1 and IIS pathways affect various aging-related parameters independently. As noted above, genetic and expression profiling evidence suggests that these pathways function largely in parallel during DR (Hou et al. 2016), an idea consistent with the synergistic life span increase seen with the combination of rsks-1/S6K and daf-2/IGFR loss-of-function mutations (D. Chen et al. 2013). Earlier studies observed that daf-2/IGFR mutant life span was not increased by RNAi against let-363/TOR (Vellai et al. 2003) or rsks-1/S6K (Hansen et al. 2007), but this lack of synergy might have reflected incomplete RNAi penetrance or different conditions. IIS and TORC1 each oppose the activities of DAF-16/FOXO and SKN-1/Nrf, but appear to do so through different mechanisms (Robida-Stubbs et al. 2012). Additionally, rIIS modulates activity of a key innate immunity pathway by allowing DAF-16/FOXO to suppress food intake, but this does not occur when TORC1 activity is reduced by RNAi knockdown of let-363/TOR, Rag proteins, or rsks-1/S6K (Wu et al. 2019). A better understanding of differences and similarities between how life span is increased by rIIS and TORC1 inhibition may shed light on why the life span increases from rIIS are typically more robust (see Life span extension and increased stress resistance from TORC1 inhibition), and on fundamental mechanisms that are likely to be relevant to aging in more complex organisms.
Antiaging compounds:
TORC1 has been implicated in C. elegans life span extension in response to a number of compounds besides rapamycin (Table 2). In many cases, the mechanisms involved are unknown, and the case that these compounds may inhibit TORC1 is limited to evidence that they do not extend life span further in genetic backgrounds in which TORC1 activity is reduced. A large-scale compound-testing project recently raised the bar for drawing mechanistic conclusions, by showing that effects on life span can vary widely among laboratories, strains, and conditions, and between C. elegans and the closely related nematode C. briggsae (Lucanic et al. 2017). Notwithstanding these caveats, the evidence for TORC1 involvement is particularly interesting and compelling for the diabetes drug metformin, and a set of natural metabolites that seem to mimic aspects of DR. We discuss those compounds below, and in Table 2 have listed them along with other compounds that seem to act through TORC1.
Table 2. Compounds proposed to increase C. elegans life span by reducing TORC1 signaling.
| Compound | Strain | Dose | Effect | Reference |
|---|---|---|---|---|
| Rapamycin (TOR inhibitor) | WT | 100 μM | Up to 19% life span extension | Robida-Stubbs et al. (2012), Seo et al. (2013), Ewald et al. (2015), Xie et al. (2019) |
| Rapamycin (TOR inhibitor) | WT | 10 μM | 18.9% life span extension | Calvert et al. (2016) |
| Rapamycin (TOR inhibitor) | WT | 100 μM | Taste-associative learning was disrupted | Sakai et al. (2017) |
| Sesamin (from sesame oil) | daf-15(+/−) | 5.75 μg per plate | Life span extension blocked in daf-15 heterozygotes | Nakatani et al. (2018) |
| 10-Hydroxy-2-decenoic acid (royal jelly component) | daf-15(+/−) | 25 μM | Life span extension blocked in daf-15 heterozygotes | Honda et al. (2015) |
| α-Ketoglutarate (metabolite) | let-363 RNAi | 8 mM | Failed to increase the life span of let-363 RNAi animals and inhibits TORC1 activity | Chin et al. (2014) |
| D-β-hydroxybutyrate (ketone body) | rsks-1(ok1255) | 20 mM | Less life span extension in rsks-1 mutants than in WT | Edwards et al. (2014) |
| LY-294002 (phosphoinositide 3-kinase inhibitor) | WT | 100 μM | 19% life span extension in WT, potential to target let-363 | Calvert et al. (2016) |
| 5-octanoyl salicylic acid (salicylic acid derivate) | WT | 100 μM | Increases life span through autophagy and reduces S6K phosphorylation in mammalian cells | Shamalnasab et al. (2018) |
| EPEA (endocannabinoid) | rsks-1(ok1255) | 50 μM | EPEA reduced life span in rsks-1 mutants, which have low EPEA levels | Lucanic et al. (2011) |
| Metformin (type 2 diabetes drug for > 60 yr) | WT | 50–100 mM | Life span extension; decreased Rag nuclear export and activity | Wu et al. (2016), Chen et al. (2017) |
EPEA, eicosapentaenoyl ethanolamide; RNAi, RNA interference; TOR, Target of Rapamycin; TORC, TOR Complex; WT, wild-type.
Metformin is a widely prescribed treatment for type 2 diabetes that increases sensitivity to insulin and may protect against some cancers [discussed in Castillo-Quan and Blackwell (2016), Wu et al. (2016), and Chen et al. (2017)]. It has been of great interest as a potential antiaging drug in part because it is well known to be safe. It is generally accepted that in humans, metformin alters mitochondrial function and increases AMPK activity, although its direct molecular target(s) remain unknown. In C. elegans, metformin seems to act as a DR mimetic, and extends life span and influences metabolism in part by altering metabolism of the bacterial food (Onken and Driscoll 2010; Cabreiro et al. 2013). Two recent C. elegans studies have looked more downstream, and elucidated mechanisms through which metformin regulates TORC1 along with AMPK. First, genetic screening identified the little-understood metabolic gene CeACAD10/F37H8.3, also known as bigr-1, as required for metformin action (Wu et al. 2016). Additional worm genetics, along with molecular analyses in mammalian cells, revealed the following pathway (Wu et al. 2016): by altering mitochondrial function, metformin impairs nuclear transport, thereby preventing RAGC-1/RagC from transiently passing through the nucleus. As a result, the Rag GTPase cannot become activated and TORC1 is inhibited, leading to CeACAD10 expression being induced in part by SKN-1/Nrf, which is known to be regulated by TORC1 (Robida-Stubbs et al. 2012). This model was particularly surprising because it was previously unknown that appearance in the nucleus was needed for Rag GTPase activity (Castillo-Quan and Blackwell 2016; Wu et al. 2016). Another study that blended C. elegans and mammalian cell experiments concluded that metformin acts directly at the lysosome, and the vacuolar ATPase, to activate AMPK and inhibit TORC1, and increases life span through this pathway (Chen et al. 2017). The metabolic benefits of metformin in humans contrast with the effects of rapamycin, which can increase insulin resistance in mammals by reducing TORC2 activity (see Importance of TORC1 regulation in life span extension mechanisms). These elegant worm genetic studies of metformin have thus provided further incentive for the development of TORC1 inhibitors that do not affect TORC2.
Various natural metabolites seem to influence life span through TORC1 signaling (Table 2). The tricarboxylic acid cycle intermediate α-ketoglutarate (α-KG) extends C. elegans life span, with epistasis analyses suggesting that it acts as a DR mimetic (Chin et al. 2014). Unexpectedly, α-KG binds and inhibits ATP synthase (mitochondrial complex V). As a result, ATP levels are reduced and TORC1 is inhibited, as indicated by reduced levels of TORC1 target phosphorylation in mammalian cells. Consistent with the idea that α-KG extends life span by reducing TORC1 activity, in C. elegans α-KG increases autophagy levels, and does not extend the life span of let-363/TOR(RNAi) or pha-4/FOXA mutant animals. This life span extension is partially dependent upon aak-2 (AMPK), a predicted TORC1 inhibitor (see Upstream inputs to TOR signaling). The oncometabolite 2-hydroxiyglutarate (2-HG) phenocopies these effects (Fu et al. 2015). D-β-hydroxybutyrate is synthesized in the liver during the fasting state (Chin et al. 2014). N-acylethanolamines (NAEs) are lipid signaling molecules that include endocannabinoids implicated in regulating energy balance (Lucanic et al. 2011). In C. elegans, DR and reduced TORC1 signaling each decrease NAE abundance, and administration of the NAE eicosapentaenoyl ethanolamide suppresses life span extension from rsks-1 mutation, suggesting that these compounds act downstream of TORC1 to accelerate aging (Lucanic et al. 2011). These results support the model that conditions that deplete energy (i.e., DR) or mimic this state extend life span at least partially through TORC1, and make it theoretically possible to promote longevity by developing strategies to alter their levels.
Roles of TORC2 in aging and stress responses
Compared to the progress made with TORC1, across eukaryotes less is understood about how TORC2 influences aging. This is an important question for the aging community beyond C. elegans, because all direct TORC1 inhibitors developed to date reduce TORC2 activity to some extent (Kennedy and Lamming 2016; Antikainen et al. 2017). By decreasing TORC2 activity, prolonged rapamycin treatment induces insulin resistance in mice, a side effect that could be particularly undesirable in humans (Lamming et al. 2012). Also in mice, rapamycin extends life span more effectively in females than males because male life span is reduced when TORC2 is blocked (Lamming et al. 2014).
Our understanding of how TORC2 influences aging and stress resistance has been developed most thoroughly in C. elegans, largely through genetic analyses of the TORC2-specific component RICT-1/Rictor and the TORC2 downstream phosphorylation target SGK-1 (see Downstream targets; Figure 2B and Figure 6). These studies indicate that the effects of TORC2 on life span are remarkably complex and involve at least three distinct mechanisms (Figure 7). First, RICT-1/Rictor and SGK-1 appear to function within a pathway in which a cold-sensitive TRP (transient receptor potential) channel (TRPA-1) acts in neurons and the intestine to promote life span by increasing the activity of DAF-16/FOXO, an apparent direct target of SGK-1 (Figure 6 and Figure 7) (Hertweck et al. 2004; A. Chen et al. 2013; Xiao et al. 2013). This pathway is particularly important at lower temperatures, and at 15 or 20°, rict-1 or sgk-1 loss-of-function mutations dramatically decrease life span (A. Chen et al. 2013; Xiao et al. 2013; Mizunuma et al. 2014). Second, RICT-1/Rictor acts through SGK-1 to inhibit opening of the mitochondrial permeability transition pore (mPTP) and prevent excessive mitochondrial permeability (Zhou et al. 2019) (Figure 7). When rict-1/Rictor or sgk-1 are ablated genetically, autophagy is elevated to levels that are harmful in this setting of increased mitochondrial permeability, apparently because mitophagy mechanisms that are supposed to clear damaged mitochondria are activated to excess. Third, and in contrast, TORC2/SGK-1 signaling limits life span and stress resistance by inhibiting nuclear localization of SKN-1/Nrf, also a direct phosphorylation target of SGK-1 (Figure 6 and Figure 7) (Tullet et al. 2008; Mizunuma et al. 2014). Interestingly, mutation of rict-1/Rictor or sgk-1 is sufficient to release repression of SKN-1 only when the animals are propagated on certain food sources, including the standard RNAi feeding bacteria HT115 (Robida-Stubbs et al. 2012; Mizunuma et al. 2014).
Figure 7.
Processes through which TORC2 affects C. elegans life span. Three distinct pathways have been described through which TORC2 and SGK-1 influence life span. Whether life span is increased or decreased depends upon the balance among these mechanisms. Please see the text for a more thorough discussion. TORC, Target of Rapamycin Complex.
The effects of TORC2/SGK-1 on life span therefore depend upon the balance among these three mechanisms (Figure 7), and are influenced not only by temperature, but also the bacterial food source (Soukas et al. 2009; Mizunuma et al. 2014; Xiao et al. 2015). Under most conditions, rict-1 and sgk-1 mutations decrease life span (Mizunuma et al. 2014; Zhou et al. 2019). However, at higher temperature (25°) the relative importance of the TRPA-1 and mPTP pathways seems to be decreased, and although rict-1/Rictor and sgk-1 mutations still shorten life span with propagation on the standard strain OP50, these mutations actually increase life span through SKN-1/Nrf when the animals are fed HT115 (Soukas et al. 2009; Mizunuma et al. 2014). With HT115 feeding, rict-1/Rictor or sgk-1 mutations also increase stress resistance in an SKN-1-dependent manner (Mizunuma et al. 2014). Similarly, although rict-1/Rictor or sgk-1 RNAi extends life span when animals consume the HT115 RNAi strain (Robida-Stubbs et al. 2012; Mizunuma et al. 2014), life span is decreased when these knockdowns are performed using RNAi-competent OP50 (Xiao et al. 2015). This SKN-1-dependent pathway seems to be particularly important in the intestine, where SKN-1/Nrf is expressed, and does not require DAF-16/FOXO for life span extension (Robida-Stubbs et al. 2012; Mizunuma et al. 2014). Accordingly, as noted above (see Life span extension and increased stress resistance from TORC1 inhibition), by inhibiting TORC2, rapamycin treatment extends C. elegans life span independently of DAF-16/FOXO (Robida-Stubbs et al. 2012).
It is unclear whether other activities of TORC2 or SGK-1 might also influence longevity. For example, loss of rict-1/Rictor or sgk-1 increases mitochondrial biogenesis, and affects the mitochondrial unfolded protein response (Gatsi et al. 2014), a stress defense mechanism implicated in aging (Shpilka and Haynes 2018). SKN-1/Nrf promotes both mitophagy and mitochondrial biogenesis (Palikaras et al. 2015), suggesting that SKN-1/Nrf might be involved in this effect. It also remains to be determined whether the influence of TORC2 on life span might also involve its regulation of fat metabolism, which is also mediated by SGK-1 (see Downstream targets) (Jones et al. 2009; Soukas et al. 2009; Dowen et al. 2016). SGK-1 appears to be activated directly not only by TORC2 but also by IIS (Figure 6) (Hertweck et al. 2004), although its role within the latter pathway has been controversial (A. Chen et al. 2013a). Interestingly, genetic analyses have revealed that TORC2/SGK-1 signaling interacts with the IIS pathway to regulate fat mobilization, mitochondrial function, and life span, suggesting that TORC2 signaling converges with IIS through its regulation of SGK-1 (Gatsi et al. 2014; Dowen et al. 2016). During development, RICT-1/Rictor inhibits dauer formation in part by signaling from the intestine to neurons to upregulate the insulin-like peptide DAF-28, providing an additional example of cross talk between TORC2 and IIS signaling (O’Donnell et al. 2018). However, although TORC2 signaling influences a number of aging-related signaling pathways, in contrast to TORC1 it does not appear to play a major role in DR longevity (Mizunuma et al. 2014). It seems unlikely that targeting TORC2 represents a promising strategy for promoting healthy human aging, but further analysis of this pathway in C. elegans should provide additional important models for how TORC2 might influence development, metabolism, and aging in higher organisms.
Perspectives
Over the past two decades or so, studies of the two TOR complexes have attracted numerous researchers working with yeast, cultured mammalian cells, and model organisms ranging from C. elegans to humans. In reviewing the literature, it is clear that a genetically amenable organism like C. elegans does not offer major advantages in uncovering the molecular or biochemical mechanisms underlying the formation, regulation, and activity of TOR complexes. However, C. elegans offers unique opportunities for scientists to analyze the regulation and functions of TOR complexes under physiological conditions, particularly in the contexts of development, aging, and responses to changes in nutrient availability and environmental conditions. For example, the life span-related studies in C. elegans led the way in establishing the connection between TOR and aging, and have made breakthroughs and major contributions to understanding the role of TOR signaling in various nutritional, genetic, and pharmacological interventions that promote longevity, and in identifying downstream mechanisms that delay aging. C. elegans TOR studies of development, metabolism, and behavior have also made unique contributions in understanding the physiological roles of TOR signaling. These contributions from C. elegans are likely to grow as we begin to understand more about the complexities of TOR functions in growth, development, and metabolism.
In the next few decades, we expect that C. elegans researchers will continue to focus on uncovering and understanding the regulation, and activities, of TORC1 and TORC2 regarding specific physiological functions in live animals. Another important frontier will be to tease apart how TOR acts in different tissues to exert its biological effects. However, to enhance the contribution of these studies to the TOR field overall, worm researchers also should put forth greater effort to increase the depth of the studies regarding molecular mechanisms. Specifically, as we alluded to in this review, the connections between upstream nutrients and environmental cues to the TOR complexes are not well understood at the molecular level in C. elegans. Similarly, although we have revealed many interesting physiological outcomes of TOR signaling, the mechanisms by which they are executed through TORC1/2 activities, including direct downstream targets, remain to be further investigated in many cases. One notable advantage of C. elegans is that by utilizing CRISPR and leveraging its relatively rapid lifecycle, it will be possible to develop and test specific mechanistic models rapidly in the context of physiological settings in vivo. Overall, we expect TOR-related problems to continue to be popular topics in the C. elegans field that should continue to make important contributions to the nutrient-response, development, and aging research fields.
Acknowledgments
We thank Collin Ewald for helpful suggestions, and the editors and reviewers for expending great effort in critiquing and helping us improve the manuscript. T.K.B. and Z.W. are supported by funding from the National Institutes of Health (NIH) (AG-054215 and GM-122610). M.H. and A.K.S. are supported by funding from the NIH (GM-047869) and the Howard Hughes Medical Institute.
Footnotes
Communicating editor: M. Sundaram
Literature Cited
- Ahmadi M., and Roy R., 2016. AMPK acts as a molecular trigger to coordinate glutamatergic signals and adaptive behaviours during acute starvation. Elife 5: e16349. 10.7554/eLife.16349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alan J. K., Struckhoff E. C., and Lundquist E. A., 2013. Multiple cytoskeletal pathways and PI3K signaling mediate CDC-42-induced neuronal protrusion in C. elegans. Small GTPases 4: 208–220. 10.4161/sgtp.26602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert P. S., and Riddle D. L., 1988. Mutants of Caenorhabditis elegans that form dauer-like larvae. Dev. Biol. 126: 270–293. 10.1016/0012-1606(88)90138-8 [DOI] [PubMed] [Google Scholar]
- Antikainen H., Driscoll M., Haspel G., and Dobrowolski R., 2017. TOR-mediated regulation of metabolism in aging. Aging Cell 16: 1219–1233. 10.1111/acel.12689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S. T., Kershner A. M., Bingman C. A., Wickens M., and Kimble J., 2016. PGL germ granule assembly protein is a base-specific, single-stranded RNase. Proc. Natl. Acad. Sci. USA 113: 1279–1284. 10.1073/pnas.1524400113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberán-Soler S., Fontrodona L., Ribó A., Lamm A. T., Iannone C. et al. , 2014. Co-option of the piRNA pathway for germline-specific alternative splicing of C. elegans TOR. Cell Rep. 8: 1609–1616. 10.1016/j.celrep.2014.08.016 [DOI] [PubMed] [Google Scholar]
- Bar-Peled L., Chantranupong L., Cherniack A. D., Chen W. W., Ottina K. A. et al. , 2013. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340: 1100–1106. 10.1126/science.1232044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh L. R., 2013. To grow or not to grow: nutritional control of development during Caenorhabditis elegans L1 arrest. Genetics 194: 539–555. 10.1534/genetics.113.150847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh L. R., and Sternberg P. W., 2006. DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr. Biol. 16: 780–785. 10.1016/j.cub.2006.03.021 [DOI] [PubMed] [Google Scholar]
- Ben Arous J., Laffont S., and Chatenay D., 2009. Molecular and sensory basis of a food related two-state behavior in C. elegans. PLoS One 4: e7584 10.1371/journal.pone.0007584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner J., Daniel H., and Spanier B., 2011. A Glutathione peroxidase, intracellular peptidases and the TOR complexes gegulate peptide transporter PEPT-1 in C. elegans. PLoS One 6: e25624 10.1371/journal.pone.0025624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Steinbaugh M. J., Hourihan J. M., Ewald C. Y., and Isik M., 2015. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 88: 290–301. 10.1016/j.freeradbiomed.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenis J., 2017. TOR, the gateway to cellular metabolism, cell growth, and disease. Cell 171: 10–13. 10.1016/j.cell.2017.08.019 [DOI] [PubMed] [Google Scholar]
- Bodenmiller B., Campbell D., Gerrits B., Lam H., Jovanovic M. et al. , 2008. PhosphoPep–a database of protein phosphorylation sites in model organisms. Nat. Biotechnol. 26: 1339–1340. 10.1038/nbt1208-1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkewitz K., Zhang Y., and Mair W. B., 2014. AMPK at the nexus of energetics and aging. Cell Metab. 20: 10–25. 10.1016/j.cmet.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro F., Au C., Leung K. Y., Vergara-Irigaray N., Cocheme H. M. et al. , 2013. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153: 228–239. 10.1016/j.cell.2013.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert S., Tacutu R., Sharifi S., Teixeira R., Ghosh P. et al. , 2016. A network pharmacology approach reveals new candidate caloric restriction mimetics in C. elegans. Aging Cell 15: 256–266. 10.1111/acel.12432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron A., Richard D., and Laplante M., 2015. The roles of mTOR complexes in lipid metabolism. Annu. Rev. Nutr. 35: 321–348. 10.1146/annurev-nutr-071714-034355 [DOI] [PubMed] [Google Scholar]
- Castillo-Quan J. I., and Blackwell T. K., 2016. Metformin: restraining nucleocytoplasmic shuttling to fight cancer and aging. Cell 167: 1670–1671. 10.1016/j.cell.2016.11.058 [DOI] [PubMed] [Google Scholar]
- Chen A. T., Guo C., Dumas K. J., Ashrafi K., and Hu P. J., 2013. Effects of Caenorhabditis elegans sgk-1 mutations on lifespan, stress resistance, and DAF-16/FoxO regulation. Aging Cell 12: 932–940. 10.1111/acel.12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Pan K. Z., Palter J. E., and Kapahi P., 2007. Longevity determined by developmental arrest genes in Caenorhabditis elegans. Aging Cell 6: 525–533. 10.1111/j.1474-9726.2007.00305.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Thomas E. L., and Kapahi P., 2009. HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet. 5: e1000486 10.1371/journal.pgen.1000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Li P. W., Goldstein B. A., Cai W., Thomas E. L. et al. , 2013. Germline signaling mediates the synergistically prolonged longevity produced by double mutations in daf-2 and rsks-1 in C. elegans. Cell Rep. 5: 1600–1610. 10.1016/j.celrep.2013.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Ou Y., Li Y., Hu S., Shao L. W. et al. , 2017. Metformin extends C. elegans lifespan through lysosomal pathway. Elife 6: e31268. 10.7554/eLife.31268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin R. M., Fu X., Pai M. Y., Vergnes L., Hwang H. et al. , 2014. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 510: 397–401. 10.1038/nature13264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. T., Paal A. B., Mehta A., Zhong L., and Hsu A. L., 2010. drr-2 encodes an eIF4H that acts downstream of TOR in diet-restriction-induced longevity of C. elegans. Aging Cell 9: 545–557. 10.1111/j.1474-9726.2010.00580.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisnell P., Parenteau T. R., Tank E., Ashrafi K., and Kenyon C., 2018. The mTOR target S6 kinase arrests development in Caenorhabditis elegans when the heat-shock transcription factor is impaired. Genetics 210: 999–1009. 10.1534/genetics.118.301533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M. X., Wang Y., Cavaleri J., Kelson T., Teng Y. D. et al. , 2017. Starvation-induced stress response is critically impacted by ceramide levels in Caenorhabditis elegans. Genetics 205: 775–785. 10.1534/genetics.116.194282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran S. P., and Ruvkun G., 2007. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet. 3: e56 10.1371/journal.pgen.0030056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalfó D., Michaelson D., and Hubbard E. J., 2012. Sensory regulation of the C. elegans germline through TGF-β-dependent signaling in the niche. Curr. Biol. 22: 712–719. 10.1016/j.cub.2012.02.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R., Melo J. A., Thondamal M., Morton E. A., Cornwell A. B. et al. , 2017. The homeodomain-interacting protein kinase HPK-1 preserves protein homeostasis and longevity through master regulatory control of the HSF-1 chaperone network and TORC1-restricted autophagy in Caenorhabditis elegans. PLoS Genet. 13: e1007038 10.1371/journal.pgen.1007038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidenko Z. N., Zubova S. G., Bukreeva E. I., Pospelov V. A., Pospelova T. V. et al. , 2009. Rapamycin decelerates cellular senescence. Cell Cycle 8: 1888–1895. 10.4161/cc.8.12.8606 [DOI] [PubMed] [Google Scholar]
- DePina A. S., Iser W. B., Park S. S., Maudsley S., Wilson M. A. et al. , 2011. Regulation of Caenorhabditis elegans vitellogenesis by DAF-2/IIS through separable transcriptional and posttranscriptional mechanisms. BMC Physiol. 11: 11 10.1186/1472-6793-11-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibble C. C., and Cantley L. C., 2015. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 25: 545–555. 10.1016/j.tcb.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman J. B., Albinder B., Shroyer T., and Kenyon C., 1995. The age-1 and daf-2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans. Genetics 141: 1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowen R. H., Breen P. C., Tullius T., Conery A. L., and Ruvkun G., 2016. A microRNA program in the C. elegans hypodermis couples to intestinal mTORC2/PQM-1 signaling to modulate fat transport. Genes Dev. 30: 1515–1528. 10.1101/gad.283895.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C., Canfield J., Copes N., Rehan M., Lipps D. et al. , 2014. D-beta-hydroxybutyrate extends lifespan in C. elegans. Aging (Albany N.Y.) 6: 621–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C., Canfield J., Copes N., Brito A., Rehan M. et al. , 2015. Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans. BMC Genet. 16: 8 10.1186/s12863-015-0167-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg T., Knauer H., Schauer A., Buttner S., Ruckenstuhl C. et al. , 2009. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11: 1305–1314. 10.1038/ncb1975 [DOI] [PubMed] [Google Scholar]
- Euling S., and Ambros V., 1996. Reversal of cell fate determination in Caenorhabditis elegans vulval development. Development 122: 2507–2515. [DOI] [PubMed] [Google Scholar]
- Ewald C. Y., Landis J. N., Porter Abate J., Murphy C. T., and Blackwell T. K., 2015. Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity. Nature 519: 97–101. 10.1038/nature14021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezcurra M., Benedetto A., Sornda T., Gilliat A. F., Au C. et al. , 2018. C. elegans eats its own intestine to make yolk leading to multiple senescent pathologies. Curr. Biol. 28: 2544–2556 (erratum: Curr. Biol. 28: 3352). 10.1016/j.cub.2018.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P., Pozza F., Pletcher S. D., Gendron C. M., and Longo V. D., 2001. Regulation of longevity and stress resistance by Sch9 in yeast. Science 292: 288–290. 10.1126/science.1059497 [DOI] [PubMed] [Google Scholar]
- Feng Z. H., Hu W. W., de Stanchina E., Teresky A. K., Jin S. K. et al. , 2007. The regulation of AMPK beta 1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 67: 3043–3053. 10.1158/0008-5472.CAN-06-4149 [DOI] [PubMed] [Google Scholar]
- Filer D., Thompson M. A., Takhaveev V., Dobson A. J., Kotronaki I. et al. , 2017. RNA polymerase III limits longevity downstream of TORC1. Nature 552: 263–267. 10.1038/nature25007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt T., Min K. J., D’Alterio C., Villa-Cuesta E., Cumbers J. et al. , 2008. Drosophila germ-line modulation of insulin signaling and lifespan. Proc. Natl. Acad. Sci. USA 105: 6368–6373. 10.1073/pnas.0709128105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell S. W., Pokala N., Macosko E. Z., Albrecht D. R., Larsch J. et al. , 2013. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell 154: 1023–1035. 10.1016/j.cell.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folick A., Oakley H. D., Yu Y., Armstrong E. H., Kumari M. et al. , 2015. Aging. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans. Science 347: 83–86. 10.1126/science.1258857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L., and Partridge L., 2015. Promoting health and longevity through diet: from model organisms to humans. Cell 161: 106–118. 10.1016/j.cell.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L., Partridge L., and Longo V. D., 2010. Extending healthy life span–from yeast to humans. Science 328: 321–326. 10.1126/science.1172539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. B., and Johnson T. E., 1988. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 118: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X., Chin R. M., Vergnes L., Hwang H., Deng G. et al. , 2015. 2-Hydroxyglutarate Inhibits ATP synthase and mTOR signaling. Cell Metab. 22: 508–515. 10.1016/j.cmet.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama M., Rougvie A. E., and Rothman J. H., 2006. C. elegans DAF-18/PTEN mediates nutrient-dependent arrest of cell cycle and growth in the germline. Curr. Biol. 16: 773–779. 10.1016/j.cub.2006.02.073 [DOI] [PubMed] [Google Scholar]
- Fukuyama M., Sakuma K., Park R., Kasuga H., Nagaya R. et al. , 2012. C. elegans AMPKs promote survival and arrest germline development during nutrient stress. Biol. Open 1: 929–936. 10.1242/bio.2012836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama M., Kontani K., Katada T., and Rougvie A. E., 2015. The C. elegans hypodermis couples progenitor cell quiescence to the dietary state. Curr. Biol. 25: 1241–1248. 10.1016/j.cub.2015.03.016 [DOI] [PubMed] [Google Scholar]
- Galy V., Mattaj I. W., and Askjaer P., 2003. Caenorhabditis elegans nucleoporins Nup93 and Nup205 determine the limit of nuclear pore complex size exclusion in vivo. Mol. Biol. Cell 14: 5104–5115. 10.1091/mbc.e03-04-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D., and Shaw R. J., 2017. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell 66: 789–800. 10.1016/j.molcel.2017.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatsi R., Schulze B., Rodriguez-Palero M. J., Hernando-Rodriguez B., Baumeister R. et al. , 2014. Prohibitin-mediated lifespan and mitochondrial stress implicate SGK-1, insulin/IGF and mTORC2 in C. elegans. PLoS One 9: e107671 10.1371/journal.pone.0107671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geillinger K. E., Kuhlmann K., Eisenacher M., Giesbertz P., Meyer H. E. et al. , 2014. Intestinal amino acid availability via PEPT-1 affects TORC1/2 signaling and the unfolded protein response. J. Proteome Res. 13: 3685–3692. 10.1021/pr5002669 [DOI] [PubMed] [Google Scholar]
- Goudeau J., Bellemin S., Toselli-Mollereau E., Shamalnasab M., Chen Y. et al. , 2011. Fatty acid desaturation links germ cell loss to longevity through NHR-80/HNF4 in C. elegans. PLoS Biol. 9: e1000599 10.1371/journal.pbio.1000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbert S., Vargas Velazquez A. M., and Braendle C., 2018. Physiological starvation promotes Caenorhabditis elegans vulval induction. G3 (Bethesda) 8: 3069–3081. 10.1534/g3.118.200449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin D. A., Stevens D. M., Thoreen C. C., Burds A. A., Kalaany N. Y. et al. , 2006. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell 11: 859–871. 10.1016/j.devcel.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Han S., Schroeder E. A., Silva-Garcia C. G., Hebestreit K., Mair W. B. et al. , 2017. Mono-unsaturated fatty acids link H3K4me3 modifiers to C. elegans lifespan. Nature 544: 185–190. 10.1038/nature21686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M., Hsu A. L., Dillin A., and Kenyon C., 2005. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet. 1: 119–128. 10.1371/journal.pgen.0010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M., Taubert S., Crawford D., Libina N., Lee S. J. et al. , 2007. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell 6: 95–110. 10.1111/j.1474-9726.2006.00267.x [DOI] [PubMed] [Google Scholar]
- Hansen M., Chandra A., Mitic L. L., Onken B., Driscoll M. et al. , 2008. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 4: e24 10.1371/journal.pgen.0040024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M., Rubinsztein D. C., and Walker D. W., 2018. Autophagy as a promoter of longevity: insights from model organisms. Nat. Rev. Mol. Cell Biol. 19: 579–593 (erratum: Nat. Rev. Mol. Cell Biol. 19: 611). 10.1038/s41580-018-0033-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K., Maruki Y., Long X., Yoshino K., Oshiro N. et al. , 2002. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177–189. 10.1016/S0092-8674(02)00833-4 [DOI] [PubMed] [Google Scholar]
- Harding H. P., Novoa I., Zhang Y., Zeng H., Wek R. et al. , 2000. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6: 1099–1108. 10.1016/S1097-2765(00)00108-8 [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Strong R., Sharp Z. D., Nelson J. F., Astle C. M. et al. , 2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460: 392–395. 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz C., Doktor T. K., Lanjuin A., Escoubas C., Zhang Y. et al. , 2017. Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. elegans. Nature 541: 102–106 (erratum: Nature 541: 476). 10.1038/nature20789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S. T., Bonafe M., and Johnson T. E., 2006. daf-16 protects the nematode Caenorhabditis elegans during food deprivation. J. Gerontol. A Biol. Sci. Med. Sci. 61: 444–460. 10.1093/gerona/61.5.444 [DOI] [PubMed] [Google Scholar]
- Hertweck M., Gobel C., and Baumeister R., 2004. C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev. Cell 6: 577–588. 10.1016/S1534-5807(04)00095-4 [DOI] [PubMed] [Google Scholar]
- Honda Y., Araki Y., Hata T., Ichihara K., Ito M. et al. , 2015. 10-Hydroxy-2-decenoic acid, the major lipid component of royal jelly, extends the lifespan of Caenorhabditis elegans through dietary restriction and target of rapamycin signaling. J. Aging Res. 2015: 425261 10.1155/2015/425261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjoh S., Yamamoto T., Uno M., and Nishida E., 2009. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature 457: 726–730. 10.1038/nature07583 [DOI] [PubMed] [Google Scholar]
- Hou L., Wang D., Chen D., Liu Y., Zhang Y. et al. , 2016. A systems approach to reverse engineer lifespan extension by dietary restriction. Cell Metab. 23: 529–540. 10.1016/j.cmet.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A. M., Gilmour S. G., Mancebo R. A., and Rose A. M., 1987. Genetic-analysis of a large autosomal region in Caenorhabditis elegans by the use of a free duplication. Genet. Res. 49: 207–213. 10.1017/S0016672300027099 [DOI] [Google Scholar]
- Hsin H., and Kenyon C., 1999. Signals from the reproductive system regulate the lifespan of C. elegans. Nature 399: 362–366. 10.1038/20694 [DOI] [PubMed] [Google Scholar]
- Hsu P. P., Kang S. A., Rameseder J., Zhang Y., Ottina K. A. et al. , 2011. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 332: 1317–1322. 10.1126/science.1199498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, P. J., 2007 Dauer (August 8, 2007), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.144.1, http://www.wormbook.org.
- Huang S., Bjornsti M. A., and Houghton P. J., 2003. Rapamycins: mechanism of action and cellular resistance. Cancer Biol. Ther. 2: 222–232. 10.4161/cbt.2.3.360 [DOI] [PubMed] [Google Scholar]
- Hubbard E. J., and Greenstein D., 2005. Introduction to the germ line (September 1, 2005), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.18.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Funato Y., Hashizume O., Yamazaki D., Hirata Y. et al. , 2016. Mg2+ extrusion from intestinal epithelia by CNNM proteins is essential for gonadogenesis via AMPK-TORC1 signaling in Caenorhabditis elegans. PLoS Genet. 12: e1006276 10.1371/journal.pgen.1006276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islas-Trejo A., Land M., Tcherepanova I., Freedman J. H., and Rubin C. S., 1997. Structure and expression of the Caenorhabditis elegans protein kinase C2 gene. Origins and regulated expression of a family of Ca2+-activated protein kinase C isoforms. J. Biol. Chem. 272: 6629–6640. 10.1074/jbc.272.10.6629 [DOI] [PubMed] [Google Scholar]
- Jankowska-Anyszka M., Lamphear B. J., Aamodt E. J., Harrington T., Darzynkiewicz E. et al. , 1998. Multiple isoforms of eukaryotic protein synthesis initiation factor 4E in Caenorhabditis elegans can distinguish between mono- and trimethylated mRNA cap structures. J. Biol. Chem. 273: 10538–10542. 10.1074/jbc.273.17.10538 [DOI] [PubMed] [Google Scholar]
- Jewell J. L., Russell R. C., and Guan K. L., 2013. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 14: 133–139. 10.1038/nrm3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia K., Chen D., and Riddle D. L., 2004. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development 131: 3897–3906. 10.1242/dev.01255 [DOI] [PubMed] [Google Scholar]
- Johnson D. W., Llop J. R., Farrell S. F., Yuan J., Stolzenburg L. R. et al. , 2014. The Caenorhabditis elegans Myc-mondo/mad complexes integrate diverse longevity signals. PLoS Genet. 10: e1004278 10.1371/journal.pgen.1004278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. C., Rabinovitch P. S., and Kaeberlein M., 2013. mTOR is a key modulator of ageing and age-related disease. Nature 493: 338–345. 10.1038/nature11861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. T., Greer E. R., Pearce D., and Ashrafi K., 2009. Rictor/TORC2 regulates Caenorhabditis elegans fat storage, body size, and development through sgk-1. PLoS Biol. 7: e60 10.1371/journal.pbio.1000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C. H., Jun C. B., Ro S. H., Kim Y. M., Otto N. M. et al. , 2009. ULK-Atg13–FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol. Biol. Cell 20: 1992–2003. 10.1091/mbc.e08-12-1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C. H., Ro S. H., Cao J., Otto N. M., and Kim D. H., 2010. mTOR regulation of autophagy. FEBS Lett. 584: 1287–1295. 10.1016/j.febslet.2010.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P., Kaeberlein M., and Hansen M., 2017. Dietary restriction and lifespan: lessons from invertebrate models. Ageing Res. Rev. 39: 3–14. 10.1016/j.arr.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki I., Shim Y. H., Kirchner J., Kaminker J., Wood W. B. et al. , 1998. PGL-1, a predicted RNA-binding component of germ granules, is essential for fertility in C-elegans. Cell 94: 635–645. 10.1016/S0092-8674(00)81605-0 [DOI] [PubMed] [Google Scholar]
- Kennedy B. K., and Lamming D. W., 2016. The mechanistic target of rapamycin: the grand conducTOR of metabolism and aging. Cell Metab. 23: 990–1003. 10.1016/j.cmet.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C., Chang J., Gensch E., Rudner A., and Tabtiang R., 1993. A C. elegans mutant that lives twice as long as wild type. Nature 366: 461–464. 10.1038/366461a0 [DOI] [PubMed] [Google Scholar]
- Kenyon C. J., 2010. The genetics of ageing. Nature 464: 504–512 (erratum: Nature 467: 622). 10.1038/nature08980 [DOI] [PubMed] [Google Scholar]
- Kim D. H., Sarbassov D. D., Ali S. M., King J. E., Latek R. R. et al. , 2002. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163–175. 10.1016/S0092-8674(02)00808-5 [DOI] [PubMed] [Google Scholar]
- Kim D. H., Sarbassov D. D., Ali S. M., Latek R. R., Guntur K. V. P. et al. , 2003. G beta L, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol. Cell 11: 895–904. 10.1016/S1097-2765(03)00114-X [DOI] [PubMed] [Google Scholar]
- Kim J., and Guan K.-L., 2019. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol. 21: 63–71. 10.1038/s41556-018-0205-1 [DOI] [PubMed] [Google Scholar]
- Kim W., Underwood R. S., Greenwald I., and Shaye D. D., 2018. OrthoList 2: a new comparative genomic analysis of human and Caenorhabditis elegans genes. Genetics 210: 445–461. 10.1534/genetics.118.301307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., and Sun H., 2007. Functional genomic approach to identify novel genes involved in the regulation of oxidative stress resistance and animal lifespan. Aging Cell 6: 489–503. 10.1111/j.1474-9726.2007.00302.x [DOI] [PubMed] [Google Scholar]
- Kimble J., and Crittenden S. L., 2005. Germline proliferation and its control (August 15, 2005), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.13.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K. D., Tissenbaum H. A., Liu Y., and Ruvkun G., 1997. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277: 942–946. 10.1126/science.277.5328.942 [DOI] [PubMed] [Google Scholar]
- Kniazeva M., Crawford Q. T., Seiber M., Wang C. Y., and Han M., 2004. Monomethyl branched-chain fatty acids play an essential role in Caenorhabditis elegans development. PLoS Biol. 2: E257 10.1371/journal.pbio.0020257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeva M., Euler T., and Han M., 2008. A branched-chain fatty acid is involved in post-embryonic growth control in parallel to the insulin receptor pathway and its biosynthesis is feedback-regulated in C. elegans. Genes Dev. 22: 2102–2110. 10.1101/gad.1692008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeva M., Shen H., Euler T., Wang C., and Han M., 2012. Regulation of maternal phospholipid composition and IP(3)-dependent embryonic membrane dynamics by a specific fatty acid metabolic event in C. elegans. Genes Dev. 26: 554–566. 10.1101/gad.187054.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeva M., Zhu H., Sewell A. K., and Han M., 2015. A lipid-TORC1 pathway promotes neuronal development and foraging behavior under both fed and fasted conditions in C. elegans. Dev. Cell 33: 260–271. 10.1016/j.devcel.2015.02.015 [DOI] [PubMed] [Google Scholar]
- Korta D. Z., Tuck S., and Hubbard E. J., 2012. S6K links cell fate, cell cycle and nutrient response in C. elegans germline stem/progenitor cells. Development 139: 859–870. 10.1242/dev.074047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz J., Henriquez R., Schneider U., Deuter-Reinhard M., Movva N. R. et al. , 1993. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell 73: 585–596. 10.1016/0092-8674(93)90144-F [DOI] [PubMed] [Google Scholar]
- Labbadia J., and Morimoto R. I., 2015. Repression of the heat shock response is a programmed event at the onset of reproduction. Mol. Cell 59: 639–650. 10.1016/j.molcel.2015.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming D. W., and Sabatini D. M., 2013. A central role for mTOR in lipid homeostasis. Cell Metab. 18: 465–469. 10.1016/j.cmet.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming D. W., Ye L., Katajisto P., Goncalves M. D., Saitoh M. et al. , 2012. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335: 1638–1643. 10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming D. W., Mihaylova M. M., Katajisto P., Baar E. L., Yilmaz O. H. et al. , 2014. Depletion of rictor, an essential protein component of mTORC2, decreases male lifespan. Aging Cell 13: 911–917. 10.1111/acel.12256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre L. R., Gelino S., Meléndez A., and Hansen M., 2011. Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr. Biol. 21: 1507–1514. 10.1016/j.cub.2011.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre L. R., De Magalhaes Filho C. D., McQuary P. R., Chu C. C., Visvikis O. et al. , 2013. The TFEB orthologue HLH-30 regulates autophagy and modulates longevity in Caenorhabditis elegans. Nat. Commun. 4: 2267 10.1038/ncomms3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Zheng Y., Cho S., Jang C., England C. et al. , 2017. Post-transcriptional regulation of de novo lipogenesis by mTORC1–S6K1-SRPK2 signaling. Cell 171: 1545–1558.e18. 10.1016/j.cell.2017.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. H., Ohmachi M., Arur S., Nayak S., Francis R. et al. , 2007. Multiple functions and dynamic activation of MPK-1 extracellular signal-regulated kinase signaling in Caenorhabditis elegans germline development. Genetics 177: 2039–2062. 10.1534/genetics.107.081356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrbach N. J., and Ruvkun G., 2016. Proteasome dysfunction triggers activation of SKN-1A/Nrf1 by the aspartic protease DDI-1. Elife 5: e17721. 10.7554/eLife.17721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux G. A., and Ashrafi K., 2016. Investigating connections between metabolism, longevity, and behavior in Caenorhabditis elegans. Trends Endocrinol. Metab. 27: 586–596. 10.1016/j.tem.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Kim S. G., and Blenis J., 2014. Rapamycin: one drug, many effects. Cell Metab. 19: 373–379. 10.1016/j.cmet.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., DeBella L. R., Guven-Ozkan T., Lin R., and Rose L. S., 2009. An eIF4E-binding protein regulates katanin protein levels in C. elegans embryos. J. Cell Biol. 187: 33–42. 10.1083/jcb.200903003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Matilainen O., Jin C., Glover-Cutter K. M., Holmberg C. I. et al. , 2011. Specific SKN-1/Nrf stress responses to perturbations in translation elongation and proteasome activity. PLoS Genet. 7: e1002119 10.1371/journal.pgen.1002119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Finkbeiner S., Ganner A., Gerber J., Klein M. et al. , 2018. CGEF-1 regulates mTORC1 signaling during adult longevity and stress response in C. elegans. Oncotarget 9: 9581–9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K., Dorman J. B., Rodan A., and Kenyon C., 1997. daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278: 1319–1322. 10.1126/science.278.5341.1319 [DOI] [PubMed] [Google Scholar]
- Lin K., Hsin H., Libina N., and Kenyon C., 2001. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat. Genet. 28: 139–145. 10.1038/88850 [DOI] [PubMed] [Google Scholar]
- Lin Y. H., Chen Y. C., Kao T. Y., Lin Y. C., Hsu T. E. et al. , 2014. Diacylglycerol lipase regulates lifespan and oxidative stress response by inversely modulating TOR signaling in Drosophila and C. elegans. Aging Cell 13: 755–764. 10.1111/acel.12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A. C., 2013. The regulation of cell size. Cell 154: 1194–1205. 10.1016/j.cell.2013.08.053 [DOI] [PubMed] [Google Scholar]
- Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J. L. et al. , 2002. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10: 457–468. 10.1016/S1097-2765(02)00636-6 [DOI] [PubMed] [Google Scholar]
- Long X., Spycher C., Han Z. S., Rose A. M., Muller F. et al. , 2002. TOR deficiency in C. elegans causes developmental arrest and intestinal atrophy by inhibition of mRNA translation. Curr. Biol. 12: 1448–1461. 10.1016/S0960-9822(02)01091-6 [DOI] [PubMed] [Google Scholar]
- López-Otin C., Blasco M. A., Partridge L., Serrano M., and Kroemer G., 2013. The hallmarks of aging. Cell 153: 1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucanic M., Held J. M., Vantipalli M. C., Klang I. M., Graham J. B. et al. , 2011. N-acylethanolamine signalling mediates the effect of diet on lifespan in Caenorhabditis elegans. Nature 473: 226–229. 10.1038/nature10007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucanic M., Plummer W. T., Chen E., Harke J., Foulger A. C. et al. , 2017. Impact of genetic background and experimental reproducibility on identifying chemical compounds with robust longevity effects. Nat. Commun. 8: 14256 10.1038/ncomms14256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X. M., and Blenis J., 2009. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10: 307–318. 10.1038/nrm2672 [DOI] [PubMed] [Google Scholar]
- Magnuson B., Ekim B., and Fingar D. C., 2012. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 441: 1–21. 10.1042/BJ20110892 [DOI] [PubMed] [Google Scholar]
- Mansfeld J., Urban N., Priebe S., Groth M., Frahm C. et al. , 2015. Branched-chain amino acid catabolism is a conserved regulator of physiological ageing. Nat. Commun. 6: 10043 10.1038/ncomms10043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. D., Chen X. W., Kaplan R. E., Saltiel A. R., Walker C. L. et al. , 2014. Ral and Rheb GTPase activating proteins integrate mTOR and GTPase signaling in aging, autophagy, and tumor cell invasion. Mol. Cell 53: 209–220. 10.1016/j.molcel.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuary P. R., Liao C. Y., Chang J. T., Kumsta C., She X. et al. , 2016. C. elegans S6K mutants require a creatine-kinase-like effector for lifespan extension. Cell Rep. 14: 2059–2067. 10.1016/j.celrep.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner B., Boll M., Daniel H., and Baumeister R., 2004. Deletion of the intestinal peptide transporter affects insulin and TOR signaling in Caenorhabditis elegans. J. Biol. Chem. 279: 36739–36745. 10.1074/jbc.M403415200 [DOI] [PubMed] [Google Scholar]
- Meléndez A., and Levine B., 2009. Autophagy in C. elegans (August 24, 2009), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.147.1, http://www.wormbook.org. 10.1895/wormbook.1.147.1 [DOI] [Google Scholar]
- Meléndez A., Talloczy Z., Seaman M., Eskelinen E. L., Hall D. H. et al. , 2003. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 301: 1387–1391. 10.1126/science.1087782 [DOI] [PubMed] [Google Scholar]
- Menon S., and Manning B. D., 2013. Cell signalling: nutrient sensing lost in cancer. Nature 498: 444–445. 10.1038/498444a [DOI] [PubMed] [Google Scholar]
- Michaelson D., Korta D. Z., Capua Y., and Hubbard E. J., 2010. Insulin signaling promotes germline proliferation in C. elegans. Development 137: 671–680. 10.1242/dev.042523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milward K., Busch K. E., Murphy R. J., de Bono M., and Olofsson B., 2011. Neuronal and molecular substrates for optimal foraging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 108: 20672–20677. 10.1073/pnas.1106134109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min K. J., Lee C. K., and Park H. N., 2012. The lifespan of Korean eunuchs. Curr. Biol. 22: R792–R793. 10.1016/j.cub.2012.06.036 [DOI] [PubMed] [Google Scholar]
- Mizunuma M., Neumann-Haefelin E., Moroz N., Li Y., and Blackwell T. K., 2014. mTORC2-SGK-1 acts in two environmentally responsive pathways with opposing effects on longevity. Aging Cell 13: 869–878. 10.1111/acel.12248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz N., Carmona J. J., Anderson E., Hart A. C., Sinclair D. A. et al. , 2014. Dietary restriction involves NAD(+) -dependent mechanisms and a shift toward oxidative metabolism. Aging Cell 13: 1075–1085. 10.1111/acel.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. Z., Tissenbaum H. A., and Ruvkun G., 1996. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature 382: 536–539. 10.1038/382536a0 [DOI] [PubMed] [Google Scholar]
- Murphy C. T., and Hu P. J., 2013. Insulin/insulin-like growth factor signaling in C. elegans (December 26, 2013), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.164.1, http://www.wormbook.org. 10.1895/wormbook.1.164.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Karalay O., Jager P. S., Horikawa M., Klein C. et al. , 2016. Mondo complexes regulate TFEB via TOR inhibition to promote longevity in response to gonadal signals. Nat. Commun. 7: 10944 10.1038/ncomms10944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani Y., Yaguchi Y., Komura T., Nakadai M., Terao K. et al. , 2018. Sesamin extends lifespan through pathways related to dietary restriction in Caenorhabditis elegans. Eur. J. Nutr. 57: 1137–1146. 10.1007/s00394-017-1396-0 [DOI] [PubMed] [Google Scholar]
- Napolitano G., and Ballabio A., 2016. TFEB at a glance. J. Cell Sci. 129: 2475–2481. 10.1242/jcs.146365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neklesa T. K., and Davis R. W., 2009. A genome-wide screen for regulators of TORC1 in response to amino acid starvation reveals a conserved Npr2/3 complex. PLoS Genet. 5: e1000515 10.1371/journal.pgen.1000515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukazuka A., Tamaki S., Matsumoto K., Oda Y., Fujisawa H. et al. , 2011. A shift of the TOR adaptor from Rictor towards Raptor by semaphorin in C. elegans. Nat. Commun. 2: 484 10.1038/ncomms1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell M. P., Chao P. H., Kammenga J. E., and Sengupta P., 2018. Rictor/TORC2 mediates gut-to-brain signaling in the regulation of phenotypic plasticity in C. elegans. PLoS Genet. 14: e1007213 10.1371/journal.pgen.1007213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg S., Paradis S., Gottlieb S., Patterson G. I., Lee L. et al. , 1997. The fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389: 994–999. 10.1038/40194 [DOI] [PubMed] [Google Scholar]
- Onken B., and Driscoll M., 2010. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One 5: e8758 10.1371/journal.pone.0008758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke E. J., and Ruvkun G., 2013. MXL-3 and HLH-30 transcriptionally link lipolysis and autophagy to nutrient availability. Nat. Cell Biol. 15: 668–676 [corrigenda: Nat. Cell Biol. 17: 104 (2015)]. 10.1038/ncb2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palikaras K., Lionaki E., and Tavernarakis N., 2015. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 521: 525–528. 10.1038/nature14300 [DOI] [PubMed] [Google Scholar]
- Pan K. Z., Palter J. E., Rogers A. N., Olsen A., Chen D. et al. , 2007. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell 6: 111–119. 10.1111/j.1474-9726.2006.00266.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S., and Ruvkun G., 1998. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 12: 2488–2498. 10.1101/gad.12.16.2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton T. J., and Kay J. E., 2005. Identification and comparative analysis of the peptidyl-prolyl cis/trans isomerase repertoires of H. sapiens, D. melanogaster, C. elegans, S. cerevisiae and Sz. pombe. Comp. Funct. Genomics 6: 277–300. 10.1002/cfg.482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkston J. M., Garigan D., Hansen M., and Kenyon C., 2006. Mutations that increase the life span of C. elegans inhibit tumor growth. Science 313: 971–975. 10.1126/science.1121908 [DOI] [PubMed] [Google Scholar]
- Qi B., and Han M., 2018. Microbial siderophore enterobactin promotes mitochondrial iron uptake and development of the host via interaction with ATP synthase. Cell 175: 571–582.e11. 10.1016/j.cell.2018.07.032 [DOI] [PubMed] [Google Scholar]
- Qi B., Kniazeva M., and Han M., 2017. A vitamin-B2-sensing mechanism that regulates gut protease activity to impact animal’s food behavior and growth. Elife 6: e26243. 10.7554/eLife.26243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W., Huang X., Neumann-Haefelin E., Schulze E., and Baumeister R., 2012. Cell-nonautonomous signaling of FOXO/DAF-16 to the stem cells of Caenorhabditis elegans. PLoS Genet. 8: e1002836 10.1371/journal.pgen.1002836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W., Yan Y., Pfeifer D., Donner V. G. E., Wang Y. et al. , 2017. C. elegans DAF-16/FOXO interacts with TGF-β/BMP signaling to induce germline tumor formation via mTORC1 activation. PLoS Genet. 13: e1006801 10.1371/journal.pgen.1006801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnappan R., Amrit F. R., Chen S. W., Gill H., Holden K. et al. , 2014. Germline signals deploy NHR-49 to modulate fatty-acid beta-oxidation and desaturation in somatic tissues of C. elegans. PLoS Genet. 10: e1004829 10.1371/journal.pgen.1004829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner D. J., and Lundquist E. A., 2018. Small GTPases. WormBook 2018: 1–65. 10.1895/wormbook.1.67.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robida-Stubbs S., Glover-Cutter K., Lamming D. W., Mizunuma M., Narasimhan S. D. et al. , 2012. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 15: 713–724. 10.1016/j.cmet.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A. N., Chen D., McColl G., Czerwieniec G., Felkey K. et al. , 2011. Life span extension via eIF4G inhibition is mediated by posttranscriptional remodeling of stress response gene expression in C. elegans. Cell Metab. 14: 55–66. 10.1016/j.cmet.2011.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousakis A., Vlassis A., Vlanti A., Patera S., Thireos G. et al. , 2013. The general control nonderepressible-2 kinase mediates stress response and longevity induced by target of rapamycin inactivation in Caenorhabditis elegans. Aging Cell 12: 742–751. 10.1111/acel.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy D., Kahler D. J., Yun C., and Hubbard E. J. A., 2018. Functional interactions between rsks-1/S6K, glp-1/Notch, and regulators of Caenorhabditis elegans fertility and germline stem cell maintenance. G3 (Bethesda) 8: 3293–3309. 10.1534/g3.118.200511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf V., Holzem C., Peyman T., Walz G., Blackwell T. K. et al. , 2013. TORC2 signaling antagonizes SKN-1 to induce C. elegans mesendodermal embryonic development. Dev. Biol. 384: 214–227. 10.1016/j.ydbio.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. C., Tian Y., Yuan H., Park H. W., Chang Y. Y. et al. , 2013. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 15: 741–750. 10.1038/ncb2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. C., Yuan H. X., and Guan K. L., 2014. Autophagy regulation by nutrient signaling. Cell Res. 24: 42–57. 10.1038/cr.2013.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. M., 2017. Twenty-five years of mTOR: uncovering the link from nutrients to growth. Proc. Natl. Acad. Sci. USA 114: 11818–11825. 10.1073/pnas.1716173114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N., Ohno H., Tomioka M., and Iino Y., 2017. The intestinal TORC2 signaling pathway contributes to associative learning in Caenorhabditis elegans. PLoS One 12: e0177900 10.1371/journal.pone.0177900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov D. D., Ali S. M., Kim D. H., Guertin D. A., Latek R. R. et al. , 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14: 1296–1302. 10.1016/j.cub.2004.06.054 [DOI] [PubMed] [Google Scholar]
- Sarbassov D. D., Guertin D. A., Ali S. M., and Sabatini D. M., 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101. 10.1126/science.1106148 [DOI] [PubMed] [Google Scholar]
- Saxton R. A., and Sabatini D. M., 2017. mTOR signaling in growth, metabolism, and disease. Cell 168: 960–976 (erratum: Cell 169: 361–371). 10.1016/j.cell.2017.02.004 [DOI] [PubMed] [Google Scholar]
- Schalm S. S., and Blenis J., 2002. Identification of a conserved motif required for mTOR signaling. Curr. Biol. 12: 632–639. 10.1016/S0960-9822(02)00762-5 [DOI] [PubMed] [Google Scholar]
- Schieber M., and Chandel N. S., 2014. TOR signaling couples oxygen sensing to lifespan in C. elegans. Cell Rep. 9: 9–15. 10.1016/j.celrep.2014.08.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber M. A., Pierce-Shimomura J. T., Chan S., Parry D., and McIntire S. L., 2010. Manipulation of behavioral decline in Caenorhabditis elegans with the Rag GTPase raga-1. PLoS Genet. 6: e1000972 10.1371/journal.pgen.1000972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C., Tullet J. M., Wieser D., Irvine E., Lingard S. J. et al. , 2009. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326: 140–144. 10.1126/science.1177221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo K., Choi E., Lee D., Jeong D. E., Jang S. K. et al. , 2013. Heat shock factor 1 mediates the longevity conferred by inhibition of TOR and insulin/IGF-1 signaling pathways in C. elegans. Aging Cell 12: 1073–1081. 10.1111/acel.12140 [DOI] [PubMed] [Google Scholar]
- Settembre C., De Cegli R., Mansueto G., Saha P. K., Vetrini F. et al. , 2013. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat. Cell Biol. 15: 647–658 (erratum Nat. Cell Biol. 15: 1016). 10.1038/ncb2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamalnasab M., Gravel S. P., St-Pierre J., Breton L., Jäger S. et al. , 2018. A salicylic acid derivative extends the lifespan of Caenorhabditis elegans by activating autophagy and the mitochondrial unfolded protein response. Aging Cell 17: e12830 [corrigenda: Aging Cell 18: e12917 (2019)]. 10.1111/acel.12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaye D. D., and Greenwald I., 2011. OrthoList: a compendium of C. elegans genes with human orthologs. PLoS One 6: e20085 [corrigenda: PLoS One 9 (2014)]. 10.1371/journal.pone.0020085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaffer K. L., Updike D. L., and Mango S. E., 2008. The target of rapamycin pathway antagonizes pha-4/FoxA to control development and aging. Curr. Biol. 18: 1355–1364. 10.1016/j.cub.2008.07.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D. E., and Ruvkun G., 2013. A cytoprotective perspective on longevity regulation. Trends Cell Biol. 23: 409–420. 10.1016/j.tcb.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpilka T., and Haynes C. M., 2018. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat. Rev. Mol. Cell Biol. 19: 109–120. 10.1038/nrm.2017.110 [DOI] [PubMed] [Google Scholar]
- Singh R., and Hansen D., 2017. Regulation of the balance between proliferation and differentiation in germ line stem cells. Results Probl. Cell Differ. 59: 31–66. 10.1007/978-3-319-44820-6_2 [DOI] [PubMed] [Google Scholar]
- Solis G. M., Kardakaris R., Valentine E. R., Bar-Peled L., Chen A. L. et al. , 2018. Translation attenuation by minocycline enhances longevity and proteostasis in old post-stress-responsive organisms. Elife 7: e40314. 10.7554/eLife.40314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönnichsen B., Koski L. B., Walsh A., Marschall P., Neumann B. et al. , 2005. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature 434: 462–469. 10.1038/nature03353 [DOI] [PubMed] [Google Scholar]
- Soukas A. A., Kane E. A., Carr C. E., Melo J. A., and Ruvkun G., 2009. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 23: 496–511. 10.1101/gad.1775409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukas A. A., Carr C. E., and Ruvkun G., 2013. Genetic regulation of Caenorhabditis elegans lysosome related organelle function. PLoS Genet. 9: e1003908 10.1371/journal.pgen.1003908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen K. K., and Dillin A., 2016. A ribosomal perspective on proteostasis and aging. Cell Metab. 23: 1004–1012. 10.1016/j.cmet.2016.05.013 [DOI] [PubMed] [Google Scholar]
- Steinbaugh M. J., Narasimhan S. D., Robida-Stubbs S., Moronetti Mazzeo L. E., Dreyfuss J. M. et al. , 2015. Lipid-mediated regulation of SKN-1/Nrf in response to germ cell absence. Elife 4: e07836. 10.7554/eLife.07836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram M. V., 2013. Canonical RTK-Ras-ERK signaling and related alternative pathways, WormBook, ed. The C. elegans Research Community, WormBook. doi/10.1895/wormbook.1.80.2, http://www.wormbook.org. [Google Scholar]
- Syntichaki P., Troulinaki K., and Tavernarakis N., 2007. Protein synthesis is a novel determinant of aging in Caenorhabditis elegans. Ann. N. Y. Acad. Sci. 1119: 289–295. 10.1196/annals.1404.001 [DOI] [PubMed] [Google Scholar]
- Tărlungeanu D. C., Deliu E., Dotter C. P., Kara M., Janiesch P. C. et al. , 2016. Impaired amino acid transport at the blood brain barrier is a cause of autism spectrum disorder. Cell 167: 1481–1494.e18. 10.1016/j.cell.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thondamal M., Witting M., Schmitt-Kopplin P., and Aguilaniu H., 2014. Steroid hormone signalling links reproduction to lifespan in dietary-restricted Caenorhabditis elegans. Nat. Commun. 5: 4879 10.1038/ncomms5879 [DOI] [PubMed] [Google Scholar]
- Tian Y., Li Z., Hu W., Ren H., Tian E. et al. , 2010. C. elegans screen identifies autophagy genes specific to multicellular organisms. Cell 141: 1042–1055. 10.1016/j.cell.2010.04.034 [DOI] [PubMed] [Google Scholar]
- Tiku V., Jain C., Raz Y., Nakamura S., Heestand B. et al. , 2017. Small nucleoli are a cellular hallmark of longevity. Nat. Commun. 8: 16083 10.1038/ncomms16083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohyama D., Yamaguchi A., and Yamashita T., 2008. Inhibition of a eukaryotic initiation factor (eIF2Bdelta/F11A3.2) during adulthood extends lifespan in Caenorhabditis elegans. FASEB J. 22: 4327–4337. 10.1096/fj.08-112953 [DOI] [PubMed] [Google Scholar]
- Toth M. L., Simon P., Kovacs A. L., and Vellai T., 2007. Influence of autophagy genes on ion-channel-dependent neuronal degeneration in Caenorhabditis elegans. J. Cell Sci. 120: 1134–1141. 10.1242/jcs.03401 [DOI] [PubMed] [Google Scholar]
- Tullet J. M., Hertweck M., An J. H., Baker J., Hwang J. Y. et al. , 2008. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 132: 1025–1038. 10.1016/j.cell.2008.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumaneng K., Russell R. C., and Guan K. L., 2012. Organ size control by Hippo and TOR pathways. Curr. Biol. 22: R368–R379. 10.1016/j.cub.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urfer S. R., Kaeberlein T. L., Mailheau S., Bergman P. J., Creevy K. E. et al. , 2017. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. Geroscience 39: 117–127. 10.1007/s11357-017-9972-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T., Takacs-Vellai K., Zhang Y., Kovacs A. L., Orosz L. et al. , 2003. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 426: 620 10.1038/426620a [DOI] [PubMed] [Google Scholar]
- Vohra M., Lemieux G. A., Lin L., and Ashrafi K., 2017. The beneficial effects of dietary restriction on learning are distinct from its effects on longevity and mediated by depletion of a neuroinhibitory metabolite. PLoS Biol. 15: e2002032 10.1371/journal.pbio.2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Robida-Stubbs S., Tullet J. M., Rual J. F., Vidal M. et al. , 2010. RNAi screening implicates a SKN-1-dependent transcriptional response in stress resistance and longevity deriving from translation inhibition. PLoS Genet. 6: e1001048 10.1371/journal.pgen.1001048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. T., and Seydoux G., 2014. P granules. Curr. Biol. 24: R637–R638. 10.1016/j.cub.2014.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J. L., and Ristow M., 2017. Lipid and carbohydrate metabolism in Caenorhabditis elegans. Genetics 207: 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver B. P., Sewell A. K., and Han M., 2016. Time to move the fat. Genes Dev. 30: 1481–1482. 10.1101/gad.285460.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster C. M., Wu L., Douglas D., and Soukas A. A., 2013. A non-canonical role for the C. elegans dosage compensation complex in growth and metabolic regulation downstream of TOR complex 2. Development 140: 3601–3612. 10.1242/dev.094292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., and Kenyon C., 2016. Roles for ROS and hydrogen sulfide in the longevity response to germline loss in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 113: E2832–E2841. 10.1073/pnas.1524727113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J. E., Burmeister L., Brooks S. V., Chan C. C., Friedline S. et al. , 2012. Rapamycin slows aging in mice. Aging Cell 11: 675–682. 10.1111/j.1474-9726.2012.00832.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. C., 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11: 398–411. 10.1111/j.1558-5646.1957.tb02911.x [DOI] [Google Scholar]
- Wu J. J., Liu J., Chen E. B., Wang J. J., Cao L. et al. , 2013. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 4: 913–920. 10.1016/j.celrep.2013.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Zhou B., Oshiro-Rapley N., Li M., Paulo J. A. et al. , 2016. An ancient, unified mechanism for metformin growth inhibition in C. elegans and cancer. Cell 167: 1705–1718.e13. 10.1016/j.cell.2016.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Isik M., Moroz N., Steinbaugh M. J., Zhang P. et al. , 2019. Dietary restriction extends lifespan through metabolic regulation of innate immunity. Cell Metab. 29: 1192–1205.e8. 10.1016/j.cmet.2019.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R., Zhang B., Dong Y., Gong J., Xu T. et al. , 2013. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell 152: 806–817. 10.1016/j.cell.2013.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R., Chun L., Ronan E. A., Friedman D. I., Liu J. et al. , 2015. RNAi interrogation of dietary modulation of development, metabolism, behavior, and aging in C. elegans. Cell Rep. 11: 1123–1133. 10.1016/j.celrep.2015.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., de Souza Alves V., von der Haar T., O’Keefe L., Lenchine R. V. et al. , 2019. Regulation of the elongation phase of protein synthesis enhances translation accuracy and modulates lifespan. Curr. Biol. 29: 737–749.e5. 10.1016/j.cub.2019.01.029 [DOI] [PubMed] [Google Scholar]
- Yang Q., Inoki K., Ikenoue T., and Guan K. L., 2006. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 20: 2820–2832. 10.1101/gad.1461206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. L., Loh K. S., Liou B. Y., Chu I. H., Kuo C. J. et al. , 2013. SESN-1 is a positive regulator of lifespan in Caenorhabditis elegans. Exp. Gerontol. 48: 371–379. 10.1016/j.exger.2012.12.011 [DOI] [PubMed] [Google Scholar]
- Yu Y., Yoon S. O., Poulogiannis G., Yang Q., Ma X. M. et al. , 2011. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science 332: 1322–1326. 10.1126/science.1199484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H. X., Xiong Y., and Guan K. L., 2013. Nutrient sensing, metabolism, and cell growth control. Mol. Cell 49: 379–387. 10.1016/j.molcel.2013.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Wang Z., Du Z., and Zhang H., 2018. mTOR regulates phase separation of PGL granules to modulate their autophagic degradation. Cell 174: 1492–1506.e22. 10.1016/j.cell.2018.08.006 [DOI] [PubMed] [Google Scholar]
- Zhang X., Zabinsky R., Teng Y., Cui M., and Han M., 2011. microRNAs play critical roles in the survival and recovery of Caenorhabditis elegans from starvation-induced L1 diapause. Proc. Natl. Acad. Sci. USA 108: 17997–18002. 10.1073/pnas.1105982108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Kreuzer J., Kumsta C., Wu L., Kamer K. J. et al. , 2019. Mitochondrial permeability uncouples elevated autophagy and lifespan extension. Cell 177: 299–314.e16. 10.1016/j.cell.2019.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H. H., Shen H. L., Sewell A. K., Kniazeva M., and Han M., 2013. A novel sphingolipid-TORC1 pathway critically promotes postembryonic development in Caenorhabditis elegans. Elife 2: e00429 10.7554/eLife.00429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H. H., Sewell A. K., and Han M., 2015. Intestinal apical polarity mediates regulation of TORC1 by glucosylceramide in C. elegans. Genes Dev. 29: 1218–1223. 10.1101/gad.263483.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinska D. F., Gnad F., Jedrusik-Bode M., Wisniewski J. R., and Mann M., 2009. Caenorhabditis elegans has a phosphoproteome atypical for metazoans that is enriched in developmental and sex determination proteins. J. Proteome Res. 8: 4039–4049. 10.1021/pr900384k [DOI] [PubMed] [Google Scholar]