Blood platelets and resident neurons are fundamentally different cells, with peculiar features in embryological origin, function, and localization. However, they share common characteristics in subcellular organization and in protein composition.1 One of the most intriguing observations is that several proteins are typically expressed in both neurons and circulating platelets. In the latter, their concentrations are unusually high, and they are found to regulate processes such as platelet activation, hemostasis, and thrombosis.
Four excellent examples are:
The neuronal protein reelin, which regulates cell migration and synaptic plasticity, is present in plasma and in blood platelets where it regulates arterial thrombosis.2, 3
Amyloid Aβ peptides, which accumulate in senile plaques in Alzheimer disease, and the amyloid precursor protein (APP) are expressed in megakaryocytes, are stored in platelet α‐granules, and are released upon platelet activation. APP controls coagulation and thrombosis,4, 5 whereas amyloid Aβ peptides stimulate platelet aggregation.6
Brain‐derived neurotrophic factor, which is implicated in the pathophysiology of depression, is synthesized in megakaryocytes and accumulates in platelets, where it modulates thrombosis.7, 8
The neurotransmitter serotonin, which has important roles in controlling behavior and sociality, is stored in platelet‐dense granules where it is released upon activation to act as a weak agonist.9
Because of these and other parallelisms, circulating platelets have been proposed as an alternative model to investigate neuronal dysfunctions and as an accessible peripheral biomarker to monitor the onset and the progression of neurological disorders. In this context, the majority of platelet studies have so far been focused mostly on depression and Alzheimer disease. However, investigations on schizophrenia, autism, and Parkinson disease have recently become popular. Conversely, since platelets reflect some features of neurons in term of protein expression, it has been suggested that changes in neuronal metabolism may also be reflected in dysfunctions in platelet activation or coagulation.
A recent review article by Padmakumar and colleagues, published in RPTH, 10 provides a timely summary of the literature on autism spectrum disorders (ASDs) and platelet studies since the early 1970s.
ASDs are complex neurological disorders with different grades of severity that include autism, Asperger syndrome, and variable degrees of childhood disintegrative and pervasive disorders. The pathology of ASD is unknown, but underlying gene‐environment interactions have been suggested.11
Platelets do not synthesize serotonin, but they adsorb it from plasma through the serotonin transporter SERT and pack it into dense granules; 99.9% of plasma serotonin (corresponding to about 250 ng/mL) is stored in platelets. Several works have been published in the past 50 years describing platelet alterations in ASD patients. This review critically analyzes the principal findings obtained from different studies, comparing cohorts of patients and experimental results, with useful summary tables. Indeed, most studies reveal high levels of serotonin in platelets of autism patients. This hyperserotonemia correlates with age, ethnicity, sex, and genetic variants in genes that are known to be correlated with serotonin metabolism and transport. More interestingly, genetic variations in the platelet fibrinogen receptor integrin αIIbβ3 correlate with ASD. The expression, but not the affinity of the serotonin transporter SERT, is increased on the surface of ASD platelets, and integrin αIIbβ3 directly interacts with SERT. Also, metabolism of the serotonin derivatives N‐acetyl serotonin and melatonin is altered in ASD platelets. Finally, platelet alterations in dense granule contents and in platelet function have been observed in ASD patients.10
The critical analysis of previous results and the discussion presented in this review article strongly support the use of platelets as reliable biomarkers for ASD. In addition, new perspectives are open in the diagnosis of ASD with functional platelet tests, dense granule ultrastructural analysis, and next‐generation sequencing that are shown to complement the measurement of platelet serotonin levels.
Most importantly, these studies support the notion that platelets can be rightfully considered as circulating mirrors of neurons (see Figure 1). In the near future, the analysis of platelet morphology, functionality, metabolism, and protein and lipid composition may advance the study of neuron abnormalities. In this context, platelets may become the perfect peripheral biomarkers for diagnosis of neuronal dysfunctions.
Figure 1.
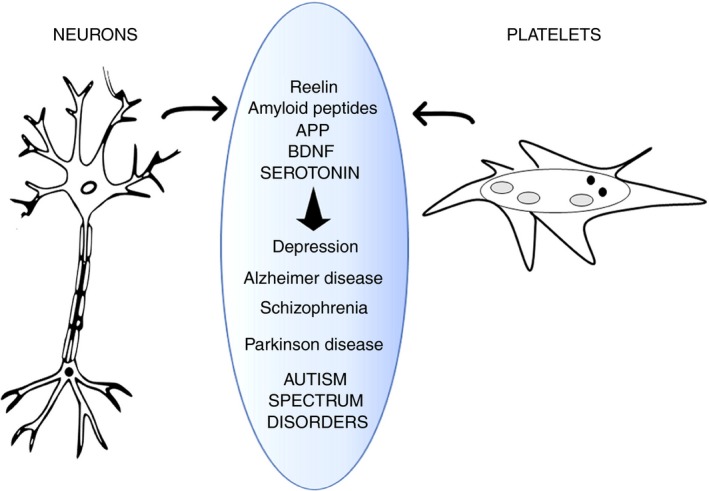
Schematic representation of platelets mirroring neurons. Neurons and platelets share common proteins (reelin, amyloid peptides, APP, BDNF, serotonin, and others). Alterations in metabolism of these proteins are related to neurological disorders and may be reflected in abnormalities in circulating platelets. APP, amyloid precursor protein; BDNF, brain‐derived neurotrophic factor
RELATIONSHIP DISCLOSURE
The author has nothing to declare.
This is a commentary on Padmakumar et al [2019]: https://doi.org/10.1002/rth2.12239
REFERENCES
- 1. Canobbio I, Guidetti GF, Torti M. Platelets in neurological disorders. In: Gresele P, Kleiman NS, Lopez JA, Page CP, editors. Platelets in thrombotic and non‐thrombotic disorders. Cham, Switzerland: Springer International; 2017.. p. 513–30. [Google Scholar]
- 2. Tseng WL, Chen TH, Huang CC, Huang YH, Yeh CF, Tsai HJ, et al. Impaired thrombin generation in reelin‐deficient mice: a potential role of plasma Reelin in hemostasis. J Thromb Haemost. 2014;12:2054–64. [DOI] [PubMed] [Google Scholar]
- 3. Gowert NS, Krüger I, Klier M, Donner L, Kipkeew F, Gliem M, et al. Loss of reelin protects mice against arterial thrombosis by impairing integrin activation and thrombus formation under high shear conditions. Cell Signal. 2017;40:210–21. [DOI] [PubMed] [Google Scholar]
- 4. Van Nostrand WE, Schmaier AH, Farrow JS, Cunningham DD. Protease nexin‐II (amyloid beta‐protein precursor): a platelet alpha‐granule protein. Science. 1990;248:745–8. [DOI] [PubMed] [Google Scholar]
- 5. Canobbio I, Visconte C, Momi S, Guidetti GF, Zarà M, Canino J, et al. Platelet amyloid precursor protein is a modulator of venous thromboembolism in mice. Blood. 2017;130:527–36. [DOI] [PubMed] [Google Scholar]
- 6. Canobbio I, Guidetti GF, Oliviero B, Manganaro D, Vara D, Torti M, et al. Amyloid β‐peptide‐dependent activation of human platelets: essential role for Ca2+ and ADP in aggregation and thrombus formation. Biochem J. 2014;462:513–23. [DOI] [PubMed] [Google Scholar]
- 7. Chacón‐Fernández P, Säuberli K, Colzani M, Moreau T, Ghevaert C, Barde YA. Brain‐derived neurotrophic factor in megakaryocytes. J Biol Chem. 2016;291:9872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amadio P, Porro B, Sandrini L, Fiorelli S, Bonomi A, Cavalca V, et al. Patho‐ physiological role of BDNF in fibrin clotting. Sci Rep. 2019;9:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gabriele S, Sacco R, Persico AM. Blood serotonin levels in autism spectrum disorder: a systematic review and meta‐analysis. Eur Neuropsychopharmacol. 2014;24:919–29. [DOI] [PubMed] [Google Scholar]
- 10. Padmakumar M, Van Raes E, Van Geet C, Freson K. Blood platelet reserach in autism spectrum disorders: in search of biomarkers. Res Pract Thromb Haemost. 2019;3:566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bjørklund G, Meguid NA, El‐Ansary A, El‐Bana MA, Dadar M, Aaseth J, et al. Diagnostic and severity‐tracking biomarkers for autism spectrum disorder. J Mol Neurosci. 2018;6:492–511. [DOI] [PubMed] [Google Scholar]


