Abstract
Autism spectrum disorder (ASD) is a clinically heterogeneous neurodevelopmental disorder that is caused by gene‐environment interactions. To improve its diagnosis and treatment, numerous efforts have been undertaken to identify reliable biomarkers for autism. None of them have delivered the holy grail that represents a reproducible, quantifiable, and sensitive biomarker. Though blood platelets are mainly known to prevent bleeding, they also play pivotal roles in cancer, inflammation, and neurological disorders. Platelets could serve as a peripheral biomarker or cellular model for autism as they share common biological and molecular characteristics with neurons. In particular, platelet‐dense granules contain neurotransmitters such as serotonin and gamma‐aminobutyric acid. Molecular players controlling granule formation and secretion are similarly regulated in platelets and neurons. The major platelet integrin receptor αIIbβ3 has recently been linked to ASD as a regulator of serotonin transport. Though many studies revealed associations between platelet markers and ASD, there is an important knowledge gap in linking these markers with autism and explaining the altered platelet phenotypes detected in autism patients. The present review enumerates studies of different biomarkers detected in ASD using platelets and highlights the future needs to bring this research to the next level and advance our understanding of this complex disorder.
Keywords: autism spectrum disorders, blood platelets, gamma‐aminobutyric acid, integrin αIIbβ3, melatonin, neurotransmitter agents, platelet dense granules, serotonin
Essentials.
Autism spectrum disorder (ASD) is a heterogeneous and complex neurodevelopmental disorder.
Easily accessible, quantifiable, and reproducible biomarkers have not been found in ASD research.
Blood platelets and neurons contain similar molecular interactors and neurotransmitters.
Platelet‐dense granule morphology, content, and function can be useful biomarkers for ASD.
1. INTRODUCTION TO BIOMARKER RESEARCH RELATED TO AUTISM
Autism spectrum disorder (ASD) comprises a group of neurodevelopmental disorders that include autism, Asperger syndrome, childhood disintegrative disorder, and pervasive developmental disorder. ASD is characterized by difficulty in social interaction, communication, and repetitive behavior patterns.1 Normally, most of the symptoms start presenting during the first 2 years of life. The prevalence of ASD has been steadily growing, and according to recent studies, it is as high as 1 in 59 children under the age of 8 in the United States 2 and around 1 in 100 in the European population.3 This increasing prevalence is most probably due to improved diagnostic methods. Because early intervention can help manage symptoms and improve the quality of life, identification of physiological biomarkers aiding in the early diagnosis of ASD is very relevant. Due to the high phenotypic heterogeneity and frequent comorbidities, ASD is often classified into 2 types: syndromic ASD, in which autism occurs as a comorbidity with known clinical syndromes; and idiopathic ASD, in which the patient exhibits only classical ASD features.4, 5 Numerous studies have focused on the identification of biomarkers that would aid diagnosis and predict disease severity or evaluate treatment efficacy. A recent detailed overview of neuroimaging abnormalities and diverse metabolic and genetic biomarkers points out that though some possible candidates have been put forward, not a single highly predictive biomarker to diagnose and follow up ASD has yet been found.6 Many biomarker discovery studies try to focus on finding genetic variations linked to or causing the disorder, as it is well known that there is a high heritability in ASD, inferred from twin and family studies. These genetic studies suggest many possible contributing loci and different biological pathways underlying ASD, such as chromatin remodeling, synaptic plasticity, and neuronal connectivity.7, 8, 9
Biomarker research in ASD is complicated by the wide variability in clinical presentation within core symptoms and variables such as language, cognition, and comorbid psychiatric or other symptoms. Due to the complex nature of the disease, any causative pathway or biomarker search would inevitably require a large sample size and more in‐depth analysis of the genome. Therefore, ASD research, especially for idiopathic ASD cases, is recently more focused on using next‐generation sequencing approaches.10, 11 But with the discovery of more candidate gene variants, the genetic complexity of ASD has increased even more, and there is a scarcity of patient‐derived material or relevant human cell models to study these genes and the associated defective molecular pathways. It is impossible to obtain neurons from the central nervous system (CNS) of living subjects for ex vivo research. Gene expression and imaging studies have been performed on postmortem brains, but there are many known limitations of this method, such as post‐mortem interval, cause of death, storage procedures and biomaterial integrity.12 This has hindered research on candidate gene pathways, diagnostic biomarkers, and possible therapies for ASD. To overcome these challenges, it is still important to use alternative patient‐derived cells (blood, fibroblasts, and stem cells) or material (cerebral spinal fluid, urine, feces, serum, and plasma) to gain more insights into pathophysiology and to discover biomarkers. This review focuses on studies that have used blood platelets as a patient‐derived cell model to study ASD.
2. PARALLELS BETWEEN PLATELETS AND NEURONS
Platelets are small anucleate cell fragments circulating in the blood, originally derived from megakaryocytes in the bone marrow. While the relevance of platelet activation for blood clot formation after vascular injury is well established, more exploratory research has shown that platelets contribute to the (patho)physiological processes important for cancer, inflammation, infection, and neurological diseases.13, 14, 15, 16 The parallel between platelets and serotonergic neurons was drawn in the early 1970s.17, 18 The link with neurological disorders still mostly relates to the similarities between the platelet release reaction of stored agonists following stimulation and the neurotransmitter release following an action potential in a neuron (Figure 1).19 Platelets and neurons have a strikingly similar calcium‐dependent activation and secretion mechanism, secretory vesicles (containing neurotransmitters and activating molecules such as serotonin or 5‐hydroxytryptamine [5‐HT], dopamine, epinephrine, glutamate, gamma‐aminobutyric acid [GABA], calcium, and ADP and ATP that are secreted upon activation), 5‐HT transporters (serotonin transporter [SERT] and vesicular monoamine transporter 2 [VMAT2]), and cell surface receptors such as receptors for the listed neurotransmitters, integrin β3, ephrin, and many more.19, 20, 21 Surprisingly, certain neuron‐specific markers are expressed in platelets such as reelin and amyloid precursor protein.22 Following endocytosis of different small molecules and proteins, multivesicular bodies are involved in the formation and sorting of granules in both neurons and platelets. Mutations disrupting these cargo sorting pathways have been known to cause disorders with bleeding and neurological defects.23 Even though there are many studies reporting similarities between these cell types, existing literature knowledge cannot explain why it is so. In fact, they have different embryonic signatures, as neurons have an ectoderm origin, while platelets are formed by megakaryocytes that originate from the mesoderm.20, 24
Figure 1.
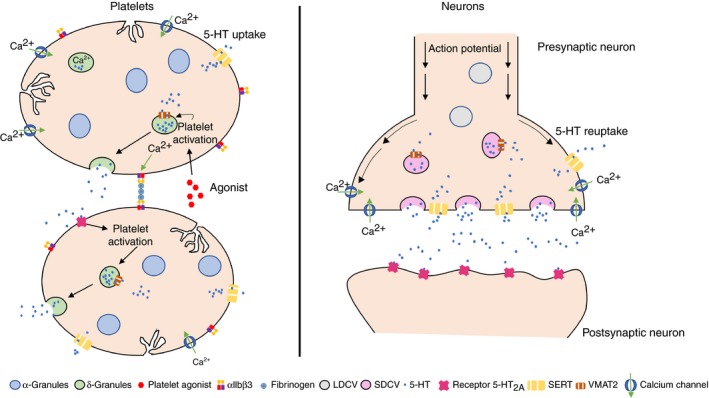
Platelets and neurons share similar features related to granule activation and secretion. Left panel shows platelets taking up 5‐HT through SERT and storing it in dense granules via VMAT2. Platelets that are activated via diverse agonists and calcium signaling, release their dense granules including 5‐HT, which is detected by 5‐HT receptors for the amplification of platelet activation. The integrin receptor αIIbβ3 becomes activated after initial platelet activation to initiate platelet‐platelet interactions via coupling with fibrinogen. Right panel shows neurons activated via calcium signaling and the arrival of the action potential, triggering release of synaptic vesicles containing neurotransmitters such as 5‐HT, which bind to the 5‐HT receptors of the postsynaptic neurons to complete neurotransmission. The released 5‐HT is reabsorbed by the presynaptic neuron via SERT activity and transported back into synaptic vesicles through VMAT2. 5HT, 5‐hydroxytryptamine; LDCV, large dense‐core vesicle; SDCV, small dense‐core vesicleSERT, serotonin transporter; VMAT2, vesicular monoamine transporter 2
5‐HT mediates a wide range of neuropsychological processes. A very interesting recent review provides detailed insights into the 5‐HT metabolism in and outside the CNS.25 Mammals have 2 major sites of 5‐HT production, the brain and the gastrointestinal system, where 95% of total body 5‐HT is found in the enterochromaffin cells of the gut, from where it can be released. After its release, 5‐HT reuptake is coordinated by SERT into epithelial cells, where it is enzymatically degraded, or it enters the bloodstream, where it is transported into platelets and stored for future use. The blood‐brain barrier is considered impermeable to 5‐HT, though it is expected that peripheral 5‐HT from the gastrointestinal system is released into the blood circulation, also flowing through the brain in the vicinity of neurons.25 However, there is no evidence that peripheral 5‐HT that remains mostly stored in platelets plays any role in the brain.26, 27 Platelets do not synthesize 5‐HT, but they take it up from the circulating blood via SERT (or SLC6A4)28 to package into their dense granules via VMAT2 (or SLC18A2)29 (Figure 1). Quantification of 5‐HT levels in whole blood samples is similar to the values obtained in platelets (200‐350 μg/L), while 5‐HT plasma levels are estimated to be about 1000 times lower.30 This means that a large part of the peripheral 5‐HT, once released into the blood, will be stored in platelet‐dense granules. One of the first biomarkers for ASD was elevated blood serotonin, as discussed further in a separate section in more detail.
Many studies have shown that platelets are useful cells to gain insights in neurological diseases such as Parkinson disease,31, 32 Alzheimer disease,33, 34 schizophrenia,24, 33 and ASD.16, 35 A recent review by Pellerin et al16 illustrates in detail how platelets were used to study Fragile X syndrome. Despite the fact that platelets are easily accessible blood cells and have diverse structural and functional similarities with neurons, studies using platelets in ASD research are rather limited (except for the numerous studies that performed 5‐HT measurements). This is probably due to the specialized nature of the in vitro tests used to evaluate platelet function, as typically performed in clinical hemostasis labs for patients with bleeding disorders. Their use is limited for patients who do not have an obvious bleeding tendency, and the expertise required is not available in most ASD research groups. For this review providing an overview of platelet studies performed for ASD, a PubMed search was performed using following search parameters: “platelet OR platelets AND autism NOT PECAM NOT Platelet Factor 4.” This resulted in 165 publications until the end of 2018 (Figure 2). By removing PECAM and platelet factor 4 in the search terms, only 4 publications were excluded, and they did not contain data in support of the hypothesis that platelets can mimic the neuronal defects causing ASD. On the basis of these selected publications, this review provides an overview of our current knowledge related to platelets and autism. We also discuss the perspectives of using this cell type as a biomarker for ASD.
Figure 2.
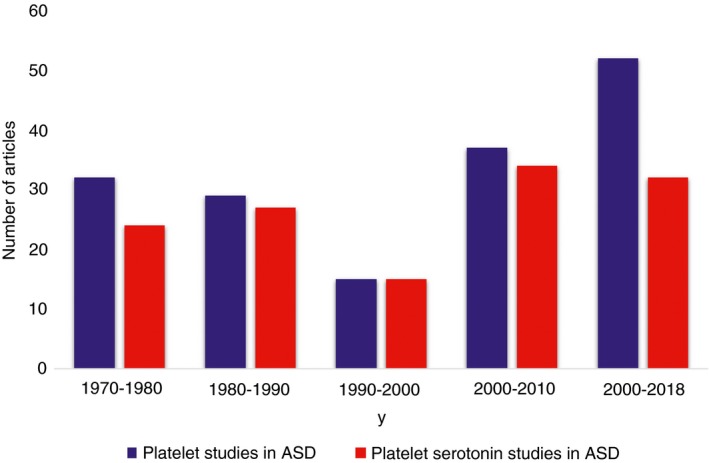
PubMed search data for platelet studies related to autism. The number of studies in an interval of 10 years were plotted from 1970 to 2018. All platelet studies in autism were retrieved using the keywords “platelet OR platelets AND autism NOT PECAM NOT Platelet Factor 4” are shown in blue. All platelet studies related to the serotonin metabolism were found using the keywords “Platelet OR Platelets AND Autism AND serotonin” are shown in red. About 80% of all platelet studies have focused on the serotonin metabolism, while only in the most recent 8 years, studies appeared that focused on the many other platelet characteristics
3. PLATELET‐DENSE GRANULES AND AUTISM
Platelets contain 2 types of specialized granules, alpha granules and dense granules, in addition to lysosomes (Figure 1). The more abundant alpha granules (40‐80 per platelet) are the largest (200‐500 nm) and comprise several important proteins such as fibrinogen, P‐selectin, von Willebrand factor, growth factors, and other small polypeptides.36 The smaller (~250 nm), scarcely populated (3‐9 per platelet) dense granules contain signaling molecules (ATP, ADP, calcium) and neurotransmitters, of which some are critical for secondary platelet activation.20 These dense granules and their secretion pathway have been compared to the regulated secretion in neurons.20, 21 In short, the neuronal secretion process uses mainly 2 types of secretory granules, the large dense‐core vesicles (LDCVs) and the small synaptic vesicles (SSVs) (Figure 1). The LDCVs contain a variety of proteins, including slow‐acting neuropeptides, growth factors, amines, and hormones and could be compared to alpha granules in platelets. However, the content of LDCVs in neurons and alpha granules in platelets is quite different, and no striking homology in their function or regulation has been found. A subgroup of the SSVs, the small dense‐core vesicles, are comparable to dense granules in platelets because their cores appear similarly dense in electron microscopy images and they both contain, at least in part, the same small molecules and neurotransmitters.20, 21 Therefore, it is not surprising that mainly platelet‐dense granule contents or their formation or function seem to be perturbed in ASD cases, as discussed in the next section.
4. PLATELET‐DENSE GRANULE MORPHOLOGY AND AUTISM
Alpha and dense granules are formed in megakaryocytes in the bone marrow prior to their transport into forming platelets.37 In platelets, granules can release their content following platelet activation. It is known that the BEACH (named after Beige and Chediak‐Higashi) domain‐containing proteins are important for platelet granule formation and secretion. For instance, genetic variants in NBEAL2 cause gray platelet syndrome, a bleeding disorder characterized by absence of alpha granules,38 while variants in LYST cause Chediak‐Higashi syndrome, an immune disorder associated with a bleeding tendency characterized by a reduced number of dense granules.39 Together with the other BEACH domain–containing family members, these proteins are often described as playing a role in membrane dynamics and/or intracellular trafficking of endosome‐ or lysosome‐related proteins and vesicles.40 However, exactly how these roles are executed by each of these family members remains largely obscure.
Interestingly, NBEA, the gene for the BEACH domain–containing protein neurobeachin (NBEA), was located on the autism‐susceptible region of chromosome 13q, as identified by linkage studies.41, 42, 43 A de novo chromosomal translocation disrupting NBEA was later found in an ASD patient.44 Eight years later, Castermans et al45 showed defects in stimulated secretion of vesicles from mouse β‐TC3 cells that were depleted of NBEA. In addition, defects in platelet‐dense granule morphology were found in the ASD patient with the NBEA disruption.45 Electron microscopy showed platelets with smaller, more irregular, and differently localized dense granule cores when compared to control platelets.45, 46 This study suggested the involvement of NBEA in ASD via its function in vesicle trafficking, morphology, and secretion. Heterozygous NBEA knockout mice suggested NBEA involvement in neurotransmitter release and synaptic functioning46 and these mice also presented with defects in social behavior, conditioned fear response, spatial learning, and memory.43 Platelets from these NBEA knockout mice were smaller than controls and had abnormal dense‐core halos.47 A proteomics study further revealed that these platelets had a reduction in actin‐interacting elements, which could contribute to the cytoskeletal changes observed in the platelets.47 These studies highlight a promising avenue of using platelets to study ASD patients. Nonetheless, more studies on ASD subjects need to be performed to completely understand the role of NBEA in platelet‐dense granule secretion and function. A recent study validated NBEA as a neurodevelopmental disorder gene, but patients presented with a broader phenotypic spectrum than ASD, including early generalized epilepsy.48 Platelet studies were not performed for this cohort.
5. HYPERSEROTONEMIA IN AUTISM PLATELETS
In the search for a biomarker for ASD using platelets, the first studies appeared in the 1970s, when it was noted that platelet 5‐HT levels were higher in patients with early‐onset autism compared to controls.17, 18 By refining our PubMed search for studies focusing on the 5‐HT metabolism, it is clear that over the past 50 years, 80% of all the platelet‐based research for ASD has focused on this topic (Figure 2). To inform the readers about this field, we selected research‐based articles that each added a unique finding on top of measuring 5‐HT levels in whole blood, plasma, or platelets, and this in cohorts ranging from 17 to 292 ASD cases. These studies are introduced in Table 1, together with their main conclusion. Most of these studies actually confirmed the initial discovery and hyperserotonemia was detected in about 17% to 40% of the ASD cases using whole blood or platelets. Studies that found normal or even lower 5‐HT levels in ASD cases used small cohorts (<25 cases) and plasma samples, therefore excluding platelet‐derived 5‐HT. Hyperserotonemia was found to be correlated to age; ethnicity; sex; genetic variants in genes regulating 5‐HT uptake (SLC6A4), synthesis (TPH1), or degradation (MAOA and MAOB); autoimmunity; gastrointestinal system, cognitive ability; and genetic variation in ITGB3, the gene coding for part of the important platelet activation receptor αIIbβ3 (Table 1). A recent meta‐analysis by Gabriele et al,49 comparing 22 previously established studies involving a total of 739 autism subjects and 868 controls, revealed significantly elevated 5‐HT levels in ASD cases compared to controls, and this for both whole blood (~23%) and platelet‐rich plasma (~29%) data sets. According to Gabriele et al,49 the overall sensitivity of high platelet 5‐HT in ASD is quite high (28% in whole blood and 22% in platelet‐rich plasma when compared across various studies). In another study, a combination of 5‐HT, N‐acetyl serotonin (NAS) and melatonin together, resulted in a sensitivity of 80% and specificity of 85%, when distinguishing ASD cases from controls.50 There are different methods to quantify 5‐HT. The most widely used are high‐performance liquid chromatography electrochemical detection (HPLC‐ECD) and ELISA.51, 52 Both methods are sensitive, time consuming, and expensive and give comparable results, though ELISA can be slightly more sensitive but less specific compared to HPLC‐ECD.51, 53 Therefore, most studies seem to support the hypothesis that blood and/or platelet hyperserotonemia could serve as an ASD biomarker, though more larger‐cohort studies are required.
Table 1.
Literature review on serotonin quantifications using blood or blood‐derived substances from patients with autism
| Serotonin levels | Number of ASD samples | Blood or blood‐derived substances | Study outcome | References | |
|---|---|---|---|---|---|
| 1 | Normal | 6 | Platelets | Endogenous 5‐HT was slightly diminished but significant increase in platelet/mL plasma; 2‐fold higher efflux of radioactive 5‐HT from ASD patient platelets, which could indicate defective 5‐HT turnover in the brain | Boullin et al18 |
| 2 | Hyperserotonemia — Normal | 24 | Whole blood | 5‐HT level and platelet count are higher in ASD, while 5‐HT level corrected for platelet count was similar between ASD and controls | Ritvo et al17 |
| 3 | Hyperserotonemia | 77 | Whole blood | Higher 5‐HT levels in ASD while normal in mentally retarded or cognitively impaired cases. Study points to the importance of matching for age and ethnicity | McBride et al60 |
| 4 | Hyposerotonemia | 10 | Plasma | Lower 5‐HT levels in adults with ASD and inversely correlated with Overt Aggression Scale score | Spivak et al90 |
| 5 | Hyposerotonemia | 17 | Plasma | Lower 5‐HT levels in mothers of ASD cases supporting the hypothesis that maternal 5‐HT would be a risk factor for ASD through effects on fetal brain development | Connors et al91 |
| 6 | Hyperserotonemia | 53 | Platelets | Higher 5‐HT levels in 32% of ASD cases and a negative correlation with their speech development | Hranilovic et al92 |
| 7 | Hyperserotonemia | 109 | Platelets | Higher 5‐HT levels in ASD cases and this in association with common SLC6A4 and ITGB3 haplotypes, each separately but also via a significant interaction between those genetic markers | Coutinho et al93 |
| 8 | Hyperserotonemia | 23 | Platelets | Higher 5‐HT levels in 17% of PDD cases without an elevation in intestinal permeability measured by sugar absorption | Kemperman et al94 |
| 9 | Hyperserotonemia | 63 | Platelets | Association between high 5‐HT in ASD and common variants in genes regulating 5‐HT synthesis (TPH1) and degradation (MAOA). | Hranilović et al95 |
| 10 | Hyperserotonemia | 50 | Serum | Higher 5‐HT and autoimmunity marker anti‐myelin‐basic protein (anti‐MBP) levels in ASD but no correction between both markers | Mostafa et al96 |
| 11 | Normal | 23 | Plasma | Normal 5‐HT levels in plasma point out that hyperserotonemia in ASD platelets results from the platelet's handling of 5‐HT and not from their increased exposure to 5‐HT | Anderson et al97 |
| 12 | Hyperserotonemia | 279 | Whole blood | Higher whole blood 5‐HT levels in 40%, lower plasma melatonin in 51%, and higher platelet NAS in 47% of ASD cases. This study points to a disruption of the 5‐HT/NAS/melatonin pathway in ASD | Pagan et al50 |
| 13 | Hyperserotonemia | 20 | Plasma | Higher 5‐HT levels in ASD patients and their unaffected siblings, suggesting heritability of this trait | Bijl et al59 |
| 14 | Hyperserotonemia | 203 | Platelets | Association between high 5‐HT in male ASD cases and common variants in MAOB gene regulating 5‐HT degradation | Chakraborti et al98 |
| 15 | Hyperserotonemia | 82 | Whole blood | Correlation between high 5‐HT in ASD and lower gastrointestinal symptoms | Marler et al99 |
| 16 | Hyperserotonemia | 292 | Whole blood | The largest study performed to date showing higher 5‐HT levels but only in prepubertal ASD patients (42%) and this more likely in males | Shuffrey et al100 |
| 17 | Hyperserotonemia | 213 | Whole blood | Platelet NAS has higher heritability than hyperserotonemia and lowered melatonin in ASD | Benabou et al77 |
| 18 | Hyperserotonemia | 181 | Whole blood | Negative correlation between maternal 5‐HT levels and cognitive abilities in ASD | Montgomery et al101 |
| 19 | Hyperserotonemia | 176 | Platelet rich plasma | A promotor SNP in ITGB3 that results in enhanced promoter activity during megakaryocyte differentiation is associated with higher integrin β3 protein expression and higher platelet 5‐HT levels in ASD patients. ITGB3 is known to support SERT trafficking to the platelet membrane allowing enhanced 5‐HT uptake in platelets | Gabriele et al63 |
Abbreviations: 5‐HT, 5‐hydroxytryptamine; ASD, autism spectrum disorder; NAS, N‐acetyl serotonin; PDD, pervasive developmental disorder (part of ASD).
Different studies have tried to explain the mechanism behind platelet hyperserotonemia detected in ASD. Recognizing the importance of SERT in both platelets and CNS, studies were performed to assess its potential role in ASD hyperserotonemia. It was found that ASD patients express higher levels of SERT on platelet membranes while the affinity of SERT for 5‐HT remains the same, resulting in increased 5‐HT internalization.54 Coding and noncoding variants in the SERT gene SLC6A4 have been identified, which are associated with elevated 5‐HT levels in ASD.55 Interestingly, transgenic mice overexpressing the coding gain‐of‐function (GOF) variant Ala56 in SERT presented with behavioral impairment and hyperserotonemia.56 Therefore, enhanced SERT on platelet membranes can account for hyperserotonemia in at least some ASD cases, but other contributing factors have also been detected. Common variants in ITGB3, which encodes the β‐chain of the platelet activation receptor integrin αIIbβ3 (glycoprotein GPIIbIIIa), have been linked to increased SERT activity and elevated blood 5‐HT levels, as discussed in more detail in the next section.57, 58 Another reason for whole blood hyperserotonemia could be the mildly increased platelet counts noted in some ASD studies,17, 59, 60 though this cannot explain the hyperserotonemia detected in most studies.
6. PLATELET ACTIVATION RECEPTOR αIIbβ3 AND AUTISM
Platelet‐platelet interaction during their activation is regulated via fibrinogen that binds to its membrane receptor integrin αIIbβ3. Following mild platelet activation, αIIbβ3 integrins undergo a conformational change to expose their binding site for fibrinogen (Figure 1). Numerous platelets are eventually linked by such fibrinogen bridges, resulting in a platelet plug.61, 62 As mentioned above, the β‐chain of the αIIbβ3 receptor is encoded by ITGB3 and variants for this gene have been associated with elevated blood 5‐HT levels in ASD.57, 63, 64 The β3‐subunit associates with αIIb to form the integrin αIIbβ3 in platelets, and with αv to form the integrin αvβ3 in neurons.65 In platelets, activated integrin αIIbβ3 interacts directly with the C‐terminus of SERT and enhances the externalization and function of SERT.66 Similarly, in the midbrain and cortical neurons, integrin αvβ3 was also observed to be involved in the regulation of SERT.67 Preliminary data from Carter et al68 showed that Itgb3 knockout mice were found to have altered social and repetitive behavior patterns, and they did not show interest toward social novelty. Such behavioral defect has not been validated in patients with Glanzmann thrombasthenia with inactivating variants in ITGB3, according to the literature.61, 69 These patients have a recessive bleeding disorder with absent platelet aggregation responses to most agonists, but ASD phenotypes have never been reported for these patients.61 Severe psychomotor retardation was reported in only a single patient with Glanzmann thrombasthenia in combination with tuberous sclerosis, which is a multisystemic disorder, sometimes presenting with ASD.70 Interestingly, a recent study showed that a variant in the ITGB3 gene promoter is associated with enhanced promoter activity in megakaryocytes, higher β3‐subunit expression in platelets, enhanced expression of SERT on the plasma membrane, and elevated 5‐HT levels in blood from ASD cases.63 This would mean that ASD is not associated with inactivating but rather GOF variants in ITGB3, probably explaining the absence of obvious autism phenotypes in patients with Glanzmann thrombasthenia. However, a case report with a GOF variant in ITGB3 was reported with bleeding symptoms and mild thrombocytopenia, but a neurological phenotype was not reported.71 Platelets from this patient showed enhanced fibrinogen binding but also low αIIbβ3 expression levels, and this probably will not result in enhanced SERT expression on the platelet membrane, though it was not studied. Further studies are needed that focus on the role of this important platelet activation receptor in neuropathology.
7. PLATELET FUNCTION, COUNT, AND SIZE STUDIES IN AUTISM
Using a light‐transmission aggregometer, platelets are activated by adding an agonist such as ADP, collagen, thrombin, epinephrine or thromboxane A2 analog (U46619) that boosts platelet‐platelet interactions and the release of their granules to achieve a full activation response20, 72 (Figure 1). Table 2 presents the few studies that have performed platelet activation studies for ASD. In 1988, platelet aggregations were performed for children with infantile autism, and a consistent, though not significant, trend of lower platelet responses was detected after activation with collagen and ADP.73 About 20 years later, a study showed a borderline significant decrease in ADP‐induced platelet aggregation for ASD cases with normal but not increased platelet 5‐HT levels.74 Recently, detailed platelet function studies were performed for a larger cohort of 159 idiopathic ASD cases and their first‐degree relatives.59 Though the maximal platelet aggregation response to epinephrine was not lower for ASD cases, its secondary wave response, which is dependent on dense granule secretion, was more frequently delayed or absent in ASD cases compared to controls. In addition, stimulated release of ATP from dense granules was reduced in ASD cases and their first‐degree relatives compared to unrelated controls following activation with ADP and collagen.59 These findings suggest that at least for a subgroup of ASD cases, platelet granule secretion and subsequent activation is impaired, but it was not clear if this observation is linked to defects in 5‐HT uptake or release. Again, larger cohort studies are required to validate platelet functional parameters. Other platelet parameters such as their size (mean platelet volume) and count are not widely studied. Bijl et al59 reported only a mild increase in platelet count for ASD cases and their siblings.
Table 2.
Literature review on platelet function studies in ASD patients
| Functional platelet test | Number of ASD samples | Agonists | Study outcome | References |
|---|---|---|---|---|
| Aggregation | 14 | ADP, collagen | Reduced platelet aggregation with both agonists but did not reach significance due to low sample size | Safai‐Kutti et al73 |
| Aggregation | 7 | ADP and 5‐HT | Reduced 5‐HT amplified aggregation indicating a defect in platelet 5‐HT2 receptor complex | McBridge et al102 |
| Aggregation | 17 | ADP | Marginally significant reduction in aggregation in ASD patients with normal platelet 5‐HT levels indicating that the platelet functional defect could be independent of the serotonergic system | Hranilović et al74 |
| ATP secretion | 159 | ADP, collagen | Reduced stimulated release of ATP from platelets of ASD patients and first‐degree relatives indicating potentially heritable dense granule secretion defect | Bijl et al59 |
Abbreviations: 5‐HT, 5‐hydroxytryptamine; ASD, autism spectrum disorder.
8. SEROTONIN DERIVATIVES N‐ACETYL SEROTONIN AND MELATONIN IN AUTISM
The biosynthesis of melatonin occurs following 5‐HT acetylation by the enzyme arylalkylamine N‐acetyltransferase (AANAT) to NAS, which is then methylated by acetylserotonin O‐methyltransferase (ASMT) to melatonin.75, 76 It has been shown in the literature that there is a reduction in the plasma level of melatonin in ASD cases.50 NAS is an important intermediate in the pathway of melatonin biosynthesis from 5‐HT, and it has been found to be transported to platelet‐dense granules, along with 5‐HT.75 Recent studies found increased NAS levels in ASD platelets and that this observation was more heritable in ASD families than hyperserotonemia and decreased melatonin (Table 1).50, 75, 77 Increased NAS, together with decreased melatonin, probably points to a defect in the melatonin synthesis pathway. Indeed, the activities of AANAT and ASMT, the 2 key enzymes contributing to melatonin synthesis, were reduced in postmortem samples of the pineal gland, gut, and platelets of ASD cases.75 Furthermore, it was noted that the heritability of reduction in ASMT activity was also higher than hyperserotonemia but similar to NAS.77 Melatonin has been well studied due to its use as a sleep inducer, but more recent studies have shown that NAS can regulate mood.78, 79 It has also been shown to bind to the tropomyosin receptor kinase B receptor similar to the brain‐derived neurotrophic factor, suggesting a role in neuronal growth and proliferation.75, 79 This was confirmed in hippocampal neurons in sleep‐deprived mice.78, 79 Therefore, increased platelet NAS could echo its levels in the CNS, which in turn supports an abnormal growth acceleration in neural precursor cells from ASD cases as observed in a study by Schafer et al.80 Platelet NAS should be measured in larger ASD cohorts in addition to 5‐HT to evaluate its potential as an ASD biomarker.
9. OTHER NEUROTRANSMITTERS STORED IN PLATELETS AND STUDIED IN AUTISM
There is very limited evidence of other neurotransmitter changes in platelets of ASD patients. In one of the earliest studies focused on peripheral biomarkers for autism, Lake et al81 discovered an increased level of plasma norepinephrine, which they attributed to the low activity of the enzyme dopamine β‐hydroxylase, which is responsible for the conversion of dopamine to norepinephrine. Ten years later, this observation was confirmed by another study that also detected a significant increase of epinephrine in plasma, together with a decrease in platelet epinephrine, norepinephrine, and dopamine in ASD subjects.82 Such data suggest a broader bioamine metabolism defect in ASD. Following these initial studies (performed in only 22 ASD cases), no substantial progress has been made regarding the status of these neurotransmitters in larger ASD cohorts.
Other platelet neurotransmitters studied in ASD are the major inhibitory neurotransmitter GABA and the major excitatory neurotransmitter glutamate. The imbalance in the excitation/inhibition pathways of these neurotransmitters has been reported in ASD.83 Platelets contain glutamate in their dense granules, which is released exclusively by activated platelets and this can enhance platelet activation.84, 85 On the other hand, platelet aggregation is influenced by GABA, which is only 30% less abundant in platelets compared to cultured neurons.86 Though these neurotransmitters are actively studied in ASD cases, studies investigating their expression or function in autism platelets, are very limited. While early studies suggested decreased levels of both GABA and glutamate in ASD platelets,87 subsequent studies have shown an elevation of plasma GABA and glutamate levels in ASD.83, 88, 89
10. FUTURE PERSPECTIVES USING PLATELETS FOR ASD RESEARCH
The exact pathophysiology of ASD has remained elusive over the years even though diagnostic and monitoring strategies have improved over time. This is in part due to ASD essentially being a spectrum that encompasses several disorders that seem to converge when it comes to the ASD core symptoms. Biomarkers, endophenotypes, and patient‐derived peripheral cell models can prove very useful in understanding ASD, thereby providing better stratification along the spectrum. To this end, platelet studies have shed more light on the ASD, defined by platelet hyperserotonemia. It would be interesting to determine 5‐HT and its related markers, NAS and melatonin, in very large cohort studies to study associations with diverse autism characteristics that are now all grouped under the term ASD. However, though hyperserotonemia in blood and platelets from ASD cases is a consistent observation that is over half a century old, the exact underlying mechanism deserves further molecular studies. Perhaps the answer lies in the basic biochemical properties shared by platelets and neurons, the reason behind which has never been explored in the literature. The small steps forward in this field are probably the result of the fact that blood and especially platelets are relatively foreign systems to most neuroscientists. Some initial studies have been performed to explain hyperserotonemia that have focused on genetic factors that modify SERT expression or activity to modify 5‐HT uptake by platelets as described in this review. The main question remains as to where the extra 5‐HT detected in ASD cases originates. As 95% of the total body 5‐HT is produced by the enterochromaffin cells of the gut, alterations in this metabolism could result in altered 5‐HT uptake in the blood and platelets. It would be interesting to study this in Itgb3 knockout mice. Future studies combining microbiome and platelet studies in ASD cases could also provide novel insights.
Furthermore, studies that focus on platelet functional tests and platelet‐dense granule formation and morphology, should be undertaken for larger ASD cohorts and in animal models for known ASD genes. It would also be very interesting to know if the defects of platelet‐dense granules and hyperserotonemia in ASD are related. Due to the complex heritability of ASD, the ultimate goal would be to split all ASD cases into subgroups with a more specific endophenotype, such as having ASD with a platelet‐dense granule secretion defect, to assist the analysis of next‐generation sequencing studies for ASD gene discovery, by the selection of candidate genes related to granule biology.
RELATIONSHIP DISCLOSURE
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors contributed to writing and reviewing the article.
Padmakumar M, Van Raes E, Van Geet C, Freson K. Blood platelet research in autism spectrum disorders: In search of biomarkers. Res Pract Thromb Haemost. 2019;3:566–577. 10.1002/rth2.12239;
Contributor Information
Manisha Padmakumar, @ManishaPadmaku1.
Kathleen Freson, Email: kathleen.freson@med.kuleuven.be, @kathleenfreson.
REFERENCES
- 1. Fakhoury M. Autistic spectrum disorders: A review of clinical features, theories and diagnosis. Int J Dev Neurosci. 2015;43:70–7. [DOI] [PubMed] [Google Scholar]
- 2. Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. 2018;67:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saemundsen E, Magnússon P, Georgsdóttir I, Egilsson E, Rafnsson V. Prevalence of autism spectrum disorders in an Icelandic birth cohort. BMJ Open. 2013;3:e002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caglayan AO. Genetic causes of syndromic and non‐syndromic autism. Dev Med Child Neurol. 2010;52:130–8. [DOI] [PubMed] [Google Scholar]
- 5. Lintas C, Persico AM. Autistic phenotypes and genetic testing: state‐of‐the‐art for the clinical geneticist. J Med Genet. 2009;46:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bjørklund G, Meguid NA, El‐Ansary A, El‐Bana MA, Dadar M, Aaseth J, et al. Diagnostic and severity‐tracking biomarkers for autism spectrum disorder. J Mol Neurosci. 2018;66:492–511. [DOI] [PubMed] [Google Scholar]
- 7. Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci. 2015;16:551–63. [DOI] [PubMed] [Google Scholar]
- 8. Gai X, Xie HM, Perin JC, Takahashi N, Murphy K, Wenocur AS, et al. Rare structural variation of synapse and neurotransmission genes in autism. Mol Psychiatry. 2012;17:402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pinto D, Delaby E, Merico D, Barbosa M, Merikangas A, Klei L, et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet. 2014;94:677–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Turner TN, Coe BP, Dickel DE, Hoekzema K, Nelson BJ, Zody MC, et al. Genomic patterns of de novo mutation in simplex autism. Cell. 2017;171:710–722.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanders SJ. Next‐generation sequencing in autism spectrum disorder. Cold Spring Harb Perspect Med. 2018; pii:a026872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stan AD, Ghose S, Gao X‐M, Roberts RC, Lewis‐Amezcua K, Hatanpaa KJ, et al. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marx S, Xiao Y, Baschin M, Splittstöhser M, Altmann R, Moritz E, et al. The role of platelets in cancer pathophysiology: focus on malignant glioma. Cancers (Basel). 2019;11:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Speth C, Löffler J, Krappmann S, Lass‐Flörl C, Rambach G. Platelets as immune cells in infectious diseases. Future Microbiol. 2013;8:1431–51. [DOI] [PubMed] [Google Scholar]
- 15. Olumuyiwa‐Akeredolu O, Page MJ, Soma P, Pretorius E. Platelets: emerging facilitators of cellular crosstalk in rheumatoid arthritis. Nat Rev Rheumatol. 2019;15:237–48. [DOI] [PubMed] [Google Scholar]
- 16. Pellerin D, Lortie A, Corbin F. Platelets as a surrogate disease model of neurodevelopmental disorders: insights from Fragile X Syndrome. Platelets. 2018;29:113–24. [DOI] [PubMed] [Google Scholar]
- 17. Ritvo ER, Yuwiler A, Geller E, Ornitz EM, Saeger K, Plotkin S. Increased blood serotonin and platelets in early infantile autism. Arch Gen Psychiatry. 1970;23:566–72. [DOI] [PubMed] [Google Scholar]
- 18. Boullin DJ, Coleman M, O'Brien RA. Abnormalities in platelet 5‐hydroxytryptamine efflux in patients with infantile autism. Nature. 1970;226:371–2. [DOI] [PubMed] [Google Scholar]
- 19. Fresh Ponomarev ED. Evidence for platelets as neuronal and innate immune cells: their role in the activation, differentiation, and deactivation of Th1, Th17, and Tregs during tissue inflammation. Front Immunol. 2018;9:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reed GL, Fitzgerald ML, Polgár J. Molecular mechanisms of platelet exocytosis: insights into the “secret” life of thrombocytes. Blood. 2000;96:3334–42. [PubMed] [Google Scholar]
- 21. Goubau C, Buyse GM, Van Geet C, Freson K, Geet CVAN, Freson K. The contribution of platelet studies to the understanding of disease mechanisms in complex and monogenetic neurological disorders. Dev Med Child Neurol. 2014;56(8):724–31. [DOI] [PubMed] [Google Scholar]
- 22. Canobbio I, Guidetti GF, Torti M. Platelets in Neurological Disorders. Platelets in Thrombotic and Non‐Thrombotic Disorders. Cham: Springer International Publishing; 2017: pp. 513–30. [Google Scholar]
- 23. Gissen P, Maher ER. Cargos and genes: insights into vesicular transport from inherited human disease. J Med Genet. 2007;44:545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asor E, Ben‐Shachar D. Platelets: a possible glance into brain biological processes in schizophrenia. World J Psychiatry. 2012;2(6):124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Janušonis S. Serotonin dynamics in and around the central nervous system: is autism solvable without fundamental insights? Int J Dev Neurosci. 2014;39:9–15. [DOI] [PubMed] [Google Scholar]
- 26. Namkung J, Kim H, Park S. Peripheral serotonin: a new player in systemic energy homeostasis. Mol Cells. 2015;38:1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lv J, Liu F. The role of serotonin beyond the central nervous system during embryogenesis. Front Cell Neurosci. 2017;11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lesch KP, Wolozin BL, Murphy DL, Reiderer P. Primary structure of the human platelet serotonin uptake site: identity with the brain serotonin transporter. J Neurochem. 1993;60:2319–22. [DOI] [PubMed] [Google Scholar]
- 29. Höltje M, Winter S, Walther D, Pahner I, Hörtnagl H, Ottersen OP, et al. The vesicular monoamine content regulates VMAT2 activity through Galphaq in mouse platelets. Evidence for autoregulation of vesicular transmitter uptake. J Biol Chem. 2003;278:15850–8. [DOI] [PubMed] [Google Scholar]
- 30. Janusonis S. Origin of the blood hyperserotonemia of autism. Theor Biol Med Model. 2008;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boullin DJ, O'Brien RA. Accumulation of dopamine by blood platelets from normal subjects and parkinsonian patients under treatment with L‐DOPA. Br J Pharmacol. 1970;39:779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Behari M, Shrivastava M. Role of platelets in neurodegenerative diseases: a universal pathophysiology. Int J Neurosci. 2013;123:287–99. [DOI] [PubMed] [Google Scholar]
- 33. Ehrlich D, Humpel C. Platelets in psychiatric disorders. World J Psychiatry. 2012;2:91–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pluta R, Ułamek‐Kozioł M, Januszewski S, Czuczwar SJ. Platelets, lymphocytes and erythrocytes from Alzheimer's disease patients: the quest for blood cell‐based biomarkers. Folia Neuropathol. 2018;56:14–20. [DOI] [PubMed] [Google Scholar]
- 35. Piven J, Tsai G, Nehme E, Coyle JT, Chase GA, Folstein SE. Platelet serotonin, a possible marker for familial autism. J Autism Dev Disord. 1991;21:51–9. [DOI] [PubMed] [Google Scholar]
- 36. Blair P, Flaumenhaft R. Platelet α‐granules: basic biology and clinical correlates. Blood Rev. 2009;23:177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sharda A, Flaumenhaft R. The life cycle of platelet granules. F1000Res. 2018;7:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Albers CA, Cvejic A, Favier R, Bouwmans EE, Alessi M‐C, Bertone P, et al. Exome sequencing identifies NBEAL2 as the causative gene for gray platelet syndrome. Nat Genet. 2011;43:735–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huizing M, Anikster Y, Gahl WA. Hermansky‐Pudlak syndrome and Chediak‐Higashi syndrome: disorders of vesicle formation and trafficking. Thromb Haemost. 2001;86:233–45. [PubMed] [Google Scholar]
- 40. Cullinane AR, Schäffer AA, Huizing M. The BEACH is hot: a LYST of emerging roles for BEACH‐domain containing proteins in human disease. Traffic. 2013;14:749–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith M, Woodroffe A, Smith R, Holguin S, Martinez J, Filipek PA, et al. Molecular genetic delineation of a deletion of chromosome 13q12→q13 in a patient with autism and auditory processing deficits. Cytogenet Genome Res. 2002;98:233–9. [DOI] [PubMed] [Google Scholar]
- 42. Barrett S, Beck JC, Bernier R, Bisson E, Braun TA, Casavant TL, et al. An autosomal genomic screen for autism. Collaborative linkage study of autism. Am J Med Genet. 1999;88:609–15. [DOI] [PubMed] [Google Scholar]
- 43. Nuytens K, Gantois I, Stijnen P, Iscru E, Laeremans A, Serneels L, et al. Haploinsufficiency of the autism candidate gene Neurobeachin induces autism‐like behaviors and affects cellular and molecular processes of synaptic plasticity in mice. Neurobiol Dis. 2013;51:144–51. [DOI] [PubMed] [Google Scholar]
- 44. Castermans D, Wilquet V, Parthoens E, Huysmans C, Steyaert J, Swinnen L, et al. The neurobeachin gene is disrupted by a translocation in a patient with idiopathic autism. J Med Genet. 2003;40:352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Castermans D, Volders K, Crepel A, Backx L, De Vos R, Freson K, et al. SCAMP5, NBEA and AMISYN: three candidate genes for autism involved in secretion of large dense‐core vesicles. Hum Mol Genet. 2010;19:1368–78. [DOI] [PubMed] [Google Scholar]
- 46. Volders K, Nuytens K, Creemers JW. The autism candidate gene neurobeachin encodes a scaffolding protein implicated in membrane trafficking and signaling. Curr Mol Med. 2011;11:204–17. [DOI] [PubMed] [Google Scholar]
- 47. Nuytens K, Tuand K, Di Michele M, Boonen K, Waelkens E, Freson K, et al. Platelets of mice heterozygous for neurobeachin, a candidate gene for autism spectrum disorder, display protein changes related to aberrant protein kinase A activity. Mol Autism. 2013;4:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mulhern MS, Stumpel C, Stong N, Brunner HG, Bier L, Lippa N; CAUSES study , Lopez‐Rangel E, Houcinat N, Barth M, den Hollander N, Hoffer MJV, et al. NBEA: developmental disease gene with early generalized epilepsy phenotypes. Ann Neurol. 2018;84:788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gabriele S, Sacco R, Persico AM. Blood serotonin levels in autism spectrum disorder : a systematic review and meta‐analysis. Eur Neuropsychopharmacol. 2014;24:919–29. [DOI] [PubMed] [Google Scholar]
- 50. Pagan C, Delorme R, Callebert J, Goubran‐Botros H, Amsellem F, Drouot X, et al. The serotonin‐N‐acetylserotonin–melatonin pathway as a biomarker for autism spectrum disorders. Transl Psychiatry. 2014;4:e479–e479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee GS, Simpson C, Sun B‐H, Yao C, Foer D, Sullivan B, et al. Measurement of plasma, serum, and platelet serotonin in individuals with high bone mass and mutations in LRP5. J Bone Miner Res. 2014;29:976–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maurer‐Spurej E, Pittendreigh C, Misri S. Platelet serotonin levels support depression scores for women with postpartum depression. J Psychiatry Neurosci. 2007;32:23–9. [PMC free article] [PubMed] [Google Scholar]
- 53. Fouassier M, Bourgerette E, Libert F, Pouplard C, Marques‐Verdier A. Determination of serotonin release from platelets by HPLC and ELISA in the diagnosis of heparin‐induced thrombocytopenia: comparison with reference method by [14C]‐serotonin release assay. J Thromb Haemost. 2006;4:1136–9. [DOI] [PubMed] [Google Scholar]
- 54. Marazziti D, Muratori F, Cesari A, Masala I, Baroni S, Giannaccini G, et al. Increased density of the platelet serotonin transporter in autism. Pharmacopsychiatry. 2000;33:165–8. [DOI] [PubMed] [Google Scholar]
- 55. Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, et al. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid‐compulsive behaviors. Am J Hum Genet. 2005;77:265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Veenstra‐VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, et al. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci U S A. 2012;109:5469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Napolioni V, Lombardi F, Sacco R, Curatolo P, Manzi B, Alessandrelli R, et al. Family‐based association study of ITGB3 in autism spectrum disorder and its endophenotypes. Eur J Hum Genet. 2011;19:353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dohn MR, Kooker CG, Bastarache L, Jessen T, Rinaldi C, Varney S, et al. The gain‐of‐function integrin β3 Pro33 variant alters the serotonin system in the mouse. Brain. 2017;37:11271–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bijl N, Thys C, Wittevrongel C, De la Marche W, Devriendt K, Peeters H, et al. Platelet studies in autism spectrum disorder patients and first‐degree relatives. Mol Autism. 2015;6:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McBride PA, Anderson GM, Hertzig ME, Snow ME, Thompson SM, Khait VD, et al. Effects of diagnosis, race, and puberty on platelet serotonin levels in autism and mental retardation. J Am Acad Child Adolesc Psychiatry. 1998;37:767–76. [DOI] [PubMed] [Google Scholar]
- 61. Nurden AT. Should studies on Glanzmann thrombasthenia not be telling us more about cardiovascular disease and other major illnesses? Blood Rev. 2017;31:287–99. [DOI] [PubMed] [Google Scholar]
- 62. Thomas SG. The structure of resting and activated platelets. Platelets. 2019;47–77. [Google Scholar]
- 63. Gabriele S, Canali M, Lintas C, Sacco R, Tirindelli MC, Ricciardello A, et al. Evidence that ITGB3 promoter variants increase serotonin blood levels by regulating platelet serotonin transporter trafficking. Hum Mol Genet. 2019;28:1153–61. [DOI] [PubMed] [Google Scholar]
- 64. Schuch JB, Muller D, Endres RG, Bosa CA, Longo D, Schuler‐Faccini L, et al. The role of β3 integrin gene variants in autism spectrum disorders — diagnosis and symptomatology. Gene. 2014;553:24–30. [DOI] [PubMed] [Google Scholar]
- 65. Yip PM, Zhao X, Montgomery AM, Siu CH. The Arg‐Gly‐Asp motif in the cell adhesion molecule L1 promotes neurite outgrowth via interaction with the alphavbeta3 integrin. Mol Biol Cell. 1998;9:277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Carneiro AMD, Cook EH, Murphy DL, Blakely RD. Interactions between integrin αIIbβ3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest. 2008;118:1544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Whyte A, Jessen T, Varney S, Carneiro AMD. Serotonin transporter and integrin beta 3 genes interact to modulate serotonin uptake in mouse brain. Neurochem Int. 2014;73:122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Carter MD, Shah CR, Muller CL, Crawley JN, Carneiro AMD, Veenstra‐VanderWeele J. Absence of preference for social novelty and increased grooming in integrin β3 knockout mice: initial studies and future directions. Autism Res. 2011;4:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nurden AT, Fiore M, Nurden P, Pillois X. Glanzmann thrombasthenia: a review of ITGA2B and ITGB3 defects with emphasis on variants, phenotypic variability, and mouse models. Blood. 2011;118:5996–6005. [DOI] [PubMed] [Google Scholar]
- 70. Ruan J, Schmugge M, Clemetson KJ, Cazes E, Combrie R, Bourre F, et al. Homozygous Cys542Arg substitution in GPIIIa in a Swiss patient with type I Glanzmann's thrombasthenia. Br J Haematol. 1999;105:523–31. [PubMed] [Google Scholar]
- 71. Ruiz C, Liu CY, Sun QH, Sigaud‐Fiks M, Fressinaud E, Muller JY, et al. A point mutation in the cysteine‐rich domain of glycoprotein (GP) IIIa results in the expression of a GPIIb‐IIIa (alphaIIbbeta3) integrin receptor locked in a high‐affinity state and a Glanzmann thrombasthenia‐like phenotype. Blood. 2001;98:2432–41. [DOI] [PubMed] [Google Scholar]
- 72. Holinstat M. Normal platelet function. Cancer Metastasis Rev. 2017;36:195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Safai‐Kutti S, Denfors I, Kutti J, Wadenvik H. In vitro platelet function in infantile autism. Folia Haematol Int Mag Klin Morphol Blutforsch. 1988;115:897–901. [PubMed] [Google Scholar]
- 74. Hranilović D, Bujas‐Petković Z, Tomičić M, Bordukalo‐Nikšić T, Blažević S, Čičin‐Šain L. Hyperserotonemia in autism: activity of 5HT‐associated platelet proteins. J Neural Transm. 2009;116:493–501. [DOI] [PubMed] [Google Scholar]
- 75. Pagan C, Goubran‐Botros H, Delorme R, Benabou M, Lemière N, Murray K, et al. Disruption of melatonin synthesis is associated with impaired 14‐3‐3 and miR‐451 levels in patients with autism spectrum disorders. Sci Rep. 2017;7:2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Erren TC, Reiter RJ. Melatonin: a universal time messenger. Neuro Endocrinol Lett. 2015;36:187–92. [PubMed] [Google Scholar]
- 77. Benabou M, Rolland T, Leblond CS, Millot GA, Huguet G, Delorme R, et al. Heritability of the melatonin synthesis variability in autism spectrum disorders. Sci Rep. 2017;7:17746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sompol P, Liu X, Baba K, Paul KN, Tosini G, Iuvone PM, et al. N‐acetylserotonin promotes hippocampal neuroprogenitor cell proliferation in sleep‐deprived mice. Proc Natl Acad Sci U S A. 2011;108:8844–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Choudhury A, Singh S, Palit G, Shukla S, Ganguly S. Administration of N‐acetylserotonin and melatonin alleviate chronic ketamine‐induced behavioural phenotype accompanying BDNF‐independent and dependent converging cytoprotective mechanisms in the hippocampus. Behav Brain Res. 2016;297:204–12. [DOI] [PubMed] [Google Scholar]
- 80. Schafer ST, Paquola ACM, Stern S, Gosselin D, Ku M, Pena M, et al. Pathological priming causes developmental gene network heterochronicity in autistic subject‐derived neurons. Nat Neurosci. 2019;22:243–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lake CR, Ziegler MG, Murphy DL. Increased norepinephrine levels and decreased dopamine‐beta‐hydroxylase activity in primary autism. Arch Gen Psychiatry. 1977;34:553–6. [DOI] [PubMed] [Google Scholar]
- 82. Launay JM, Bursztejn C, Ferrari P, Dreux C, Braconnier A, Zarifian E, et al. Catecholamines metabolism in infantile autism: a controlled study of 22 autistic children. J Autism Dev Disord. 1987;17:333–47. [DOI] [PubMed] [Google Scholar]
- 83. Khalifa D, Shahin O, Salem D, Raafat O. Serum glutamate was elevated in children aged 3‐10 years with autism spectrum disorders when they were compared with controls. Acta Paediatr. 2019;108:295–9. [DOI] [PubMed] [Google Scholar]
- 84. Morrell CN, Sun H, Ikeda M, Beique J‐C, Swaim AM, Mason E, et al. Glutamate mediates platelet activation through the AMPA receptor. J Exp Med. 2008;205:575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kasatkina LA, Borisova TA. Glutamate release from platelets: exocytosis versus glutamate transporter reversal. Int J Biochem Cell Biol. 2013;45:2585–95. [DOI] [PubMed] [Google Scholar]
- 86. Kaneez FS, Saeed SA. Investigating GABA and its function in platelets as compared to neurons. Platelets. 2009;20:328–33. [DOI] [PubMed] [Google Scholar]
- 87. Rolf LH, Haarmann FY, Grotemeyer KH, Kehrer H. Serotonin and amino acid content in platelets of autistic children. Acta Psychiatr Scand. 1993;87:312–6. [DOI] [PubMed] [Google Scholar]
- 88. Shimmura C, Suda S, Tsuchiya KJ, Hashimoto K, Ohno K, Matsuzaki H, et al. Alteration of plasma glutamate and glutamine levels in children with high‐functioning autism. PLoS One. 2011;6:e25340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dhossche D, Applegate H, Abraham A, Maertens P, Bland L, Bencsath A, et al. Elevated plasma gamma‐aminobutyric acid (GABA) levels in autistic youngsters: stimulus for a GABA hypothesis of autism. Med Sci Monit. 2002;8:PR1‐6. [PubMed] [Google Scholar]
- 90. Spivak B, Golubchik P, Mozes T, Vered Y, Nechmad A, Weizman A, et al. Low platelet‐poor plasma levels of serotonin in adult autistic patients. Neuropsychobiology. 2004;50:157–60. [DOI] [PubMed] [Google Scholar]
- 91. Connors SL, Matteson KJ, Sega GA, Lozzio CB, Carroll RC, Zimmerman AW. Plasma serotonin in autism. Pediatr Neurol. 2006;35:182–6. [DOI] [PubMed] [Google Scholar]
- 92. Hranilovic D, Bujas‐Petkovic Z, Vragovic R, Vuk T, Hock K, Jernej B. Hyperserotonemia in adults with autistic disorder. J Autism Dev Disord. 2007;37:1934–40. [DOI] [PubMed] [Google Scholar]
- 93. Coutinho AM, Sousa I, Martins M, Correia C, Morgadinho T, Bento C, et al. Evidence for epistasis between SLC6A4 and ITGB3 in autism etiology and in the determination of platelet serotonin levels. Hum Genet. 2007;121:243–56. [DOI] [PubMed] [Google Scholar]
- 94. Kemperman RFJ, Muskiet FD, Boutier AI, Kema IP, Muskiet FAJ. Brief report: normal intestinal permeability at elevated platelet serotonin levels in a subgroup of children with pervasive developmental disorders in Curaçao (The Netherlands Antilles). J Autism Dev Disord. 2008;38:401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hranilović D, Novak R, Babić M, Novokmet M, Bujas‐Petković Z, Jernej B. Hyperserotonemia in autism: the potential role of 5HT‐related gene variants. Coll Antropol. 2008;32(Suppl 1):75–80. [PubMed] [Google Scholar]
- 96. Mostafa G, AL‐Ayadhi L. A lack of association between hyperserotonemia and the increased frequency of serum anti‐myelin basic protein auto‐antibodies in autistic children. J Neuroinflammation. 2011;8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Anderson GM, Hertzig ME, McBride PA. Brief report: platelet‐poor plasma serotonin in autism. J Autism Dev Disord. 2012;42:1510–4. [DOI] [PubMed] [Google Scholar]
- 98. Chakraborti B, Verma D, Karmakar A, Jaiswal P, Sanyal A, Paul D, et al. Genetic variants of MAOB affect serotonin level and specific behavioral attributes to increase autism spectrum disorder (ASD) susceptibility in males. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:123–36. [DOI] [PubMed] [Google Scholar]
- 99. Marler S, Ferguson BJ, Lee EB, Peters B, Williams KC, McDonnell E, et al. Brief report: whole blood serotonin levels and gastrointestinal symptoms in autism spectrum disorder. J Autism Dev Disord. 2016;46:1124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shuffrey LC, Guter SJ, Delaney S, Jacob S, Anderson GM, Sutcliffe JS, et al. Is there sexual dimorphism of hyperserotonemia in autism spectrum disorder? Autism Res. 2017;10:1417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Montgomery AK, Shuffrey LC, Guter SJ, Anderson GM, Jacob S, Mosconi MW, et al. Maternal serotonin levels are associated with cognitive ability and core symptoms in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2018;57:867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. McBride PA, Anderson GM, Hertzig ME, Sweeney JA, Kream J, Cohen DJ, et al. Serotonergic responsivity in male young adults with autistic disorder. Results of a pilot study. Arch Gen Psychiatry. 1989;46:213–21. [DOI] [PubMed] [Google Scholar]


