Abstract
Atrial fibrillation (AF) is a frequent comorbid condition in patients with end‐stage renal disease on hemodialysis (HD) with a prevalence of up to 27%. The incidence rate of stroke in AF patients on HD is approximately 5%. The AF‐associated risk of stroke is a major clinical challenge because current evidence for anticoagulation in HD patients with AF is based on observational data. Results from these observational studies is largely contradictory because they do not show a clear benefit of vitamin K antagonists over no treatment in terms of stroke prevention, and they show an increased risk of hemorrhage associated with anticoagulation treatment in HD patients. HD patients were not included in randomized trials of the direct oral anticoagulants (DOACs), and therefore there is no evidence to support efficacy and safety of DOACs compared to vitamin K antagonists in HD patients. The pharmacological characteristics of DOACs are of particular interest in the HD setting. The factor Xa inhibitors rivaroxaban, apixaban, and edoxaban are not predominantly eliminated via the kidneys. The thrombin inhibitor dabigatran is 80% eliminated via the kidneys but is dialyzable due to its low protein binding. In this narrative review, we examine the current state of evidence regarding the prevalence of AF in patients on HD, the associated risk of stroke, and the efficacy and safety of anticoagulation for stroke prevention in the HD setting. Further, based on the pharmacokinetic properties of DOACs, we discuss their potential use in patients on HD and ongoing randomized trials.
Keywords: anticoagulation, atrial fibrillation, bleeding, chronic stroke, factor Xa inhibitors, kidney failure, renal dialysis
1. INTRODUCTION
Atrial fibrillation (AF) is associated with increased risk of stroke and systemic thromboembolism.1 Long‐term oral anticoagulation is the treatment of choice for prevention of stroke and thromboembolism and superior to any other antithrombotic treatment.2, 3 For many decades vitamin K antagonists were the only orally available anticoagulation agents.4 The drug class of anticoagulants have been introduced in several indications for the treatment and/or prevention of thromboembolic disorders. These include prevention and treatment of deep vein thrombosis and pulmonary embolism, and also stroke prevention in AF.4 Patients with end‐stage renal disease (ESRD) have increased risk of AF,5 but a definitive indication for anticoagulation treatment in ESRD patients with AF was never established,6, 7 because the risk‐benefit profile of anticoagulation in patients with ESRD is unclear.8, 9 The population of patients with ESRD on hemodialysis (HD) treatment were not included in any trials on stroke prevention and treatment of venous thromboembolism and have therefore not profited from the introduction of direct oral anticoagulants (DOACs).10 These direct anticoagulation agents are small molecules and act via direct factor inhibition,11, 12 and are therefore classified as DOACs. The members of the DOAC drug class differ with respect to their pharmacokinetic properties. Differences between DOACs include renal elimination, oral bioavailability, protein binding, and plasma half‐life.
The aim of this narrative review is to examine and discuss current evidence on AF, stroke occurrence, and prevention in patients with ESRD on HD as well as the opportunities of DOAC use in this clinically challenging setting.
2. LITERATURE SEARCH STRATEGY
Combinations of key words related to ESRD (eg, renal failure, dialysis, chronic kidney disease), atrial fibrillation (eg, arrhythmia, af, afib), and anticoagulation (eg, oral anticoagulation, DOAC, NOAC, antithrombotics) were used to search the MEDLINE database. The last search was performed in March 2019. The retrieved literature was carefully checked, focusing on primary data on epidemiology of AF in the HD population, risk of stroke associated with AF in patients with HD, and data on the efficacy and safety of anticoagulation in patients with HD with AF. The authors selected the most relevant articles to give a narrative review of the literature.
3. ATRIAL FIBRILLATION IN PATIENTS ON HEMODIALYSIS AND RISK OF STROKE AND THROMBOEMBOLISM
The risk of cardiovascular diseases increases with decreasing kidney function, reaching its peak in patients with ESRD on HD.13 Among cardiovascular diseases in HD patients, the risk of ischemic stroke in particular is increased,14 and AF represents a well‐known risk factor for ischemic stroke.1 The incidence rate of stroke in AF patients on HD is reported to be between 4.8 and 5.6 per 100 person‐years.15, 16The incidence of cardioembolic strokes in patients with AF in the non‐ESRD population can be significantly reduced with anticoagulation treatment, as established and confirmed by randomized controlled trials comparing warfarin, a vitamin K antagonist, to antiplatelet agents such as aspirin or placebo.2, 3, 17, 18 In these pivotal, practice‐changing studies, patients with ESRD were not included. Therefore, no hard evidence for the use of anticoagulation agents exists in patients with ESRD on maintenance HD treatment. The 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society guideline for the management of patients with AF recommends the use of warfarin with target international normalized ratio (INR) of 2.0 to 3.0 for patients with nonvalvular AF with a CHA2DS2‐VASc score of ≥2,6 while the 2016 European Society of Cardiology/European Society for Cardio‐Thoracic Surgery guidelines for the management of AF refrain from giving a recommendation.7
4. PREVALENCE OF ATRIAL FIBRILLATION IN PATIENTS ON HEMODIALYSIS
The epidemiologic evidence in fact indicates a high prevalence of AF in HD patients that has been underestimated for quite some time. In the multinational Dialysis Outcomes and Practice Patterns Study (DOPPS) of 17 513 randomly sampled HD patients, 2188 had a prevalent diagnosis of AF.19 The overall prevalence in DOPPS was 12.5%, but varied from 5.6% in Japan to 24.7% in Belgium. The large variability of AF prevalence across different countries observed in the DOPPS registry may be attributed to ethnic differences, but methodology of diagnosis confirmation may have also played a role.19, 20 Among the highest prevalences of AF in observational studies are one prospective cohort study with 27% in a province of northern Italy21 and results from our own research project, a population‐based cross‐sectional cohort from Vienna, Austria, with a prevalence of 26.5%.22 The results of these 2 studies confirm that the prevalence of AF may be underestimated in large registries, which obtained the AF diagnosis from national or health care provider databases.21, 22 The prevalence of AF in patients on HD also appears to be increasing over time. In the United States Renal Data System registry from 1989 to 2006, including 2 483 199 patients over a 15‐year time period, the prevalence of AF increased more than 3‐fold, from 3.5% to 10.7%.5 The high AF prevalence in patients with ESRD on HD should, however, also be regarded with respect to detection bias due to increased exposure of this patient population to health care.23
5. THE ASSOCIATION OF ATRIAL FIBRILLATION AND RISK OF STROKE IN PATIENTS ON HEMODIALYSIS
The risk of stroke in patients on HD is increased compared to the general population.14, 24 The overall high frequencies of comorbidities such as hypertension, diabetes, and vascular disease may increase the risk of stroke significantly without AF being of significance. In fact, a retrospective study on hemodialysis patients from Austria showed that there was no significant difference in the incidence of strokes between patients with AF and those without the arrhythmia (1.0/100 patient‐years in AF vs. 2.8/100 patient‐years in non‐AF; P = 0.220).25 It is noteworthy that this was a retrospective cohort with 22% of patients on antithrombotic therapy, but the end point stroke included both ischemic and hemorrhagic strokes.25 In patients with AF not receiving anticoagulation, another study indicated that the risk of stroke is not elevated compared to patients without AF on HD.26 One prospective cohort study also did not report significantly increased risk of stroke for AF patients.27 Therefore, it may be necessary to take a closer look at the pattern of AF present in patients with HD, that is, first‐diagnosis, paroxysmal, persistent, long‐standing persistent, and permanent.7 A large epidemiologic study, powered to calculate the association of permanent AF on the risk of stroke, found a significantly increased risk of stroke compared to HD patients without permanent AF.28 In the general population, patients with AF of different patterns are similarly treated regarding anticoagulation,7 but the same level of evidence does not exist for patients with ESRD on HD. Paroxysmal AF in patients with HD may therefore not have the same risk of stroke in HD patients as it has in the general population. Another discussion‐worthy issue is whether patients with prevalent AF at beginning of HD treatment are at the same risk of stroke as patients who develop incidental AF after HD treatment commencement. A well‐conducted analysis of the Taiwanese National Health Insurance Research Database showed that incidental AF did not significantly increase the risk of stroke compared to absence of AF in patients with HD.29 Incidental AF may be an epiphenomenon that occurs during an HD session as a result of fluid and electrolyte shifts30 and may not be associated with increased risk of stroke. Recent results indicate the opposite. In a large US registry of patients with ESRD, patients with incidental AF after initiating HD treatment had a 2‐fold increased risk of ischemic stroke (hazard ratio [HR], 2.1; 95% confidence interval [CI], 1.6‐2.7) during the first 30 days after AF diagnosis.31 In the authors’ opinion, the risk of stroke in patients with HD should ideally be assessed with a score that is specific to the HD population taking into account the pattern of AF, comorbidities, and prior history of stroke or thromboembolism. Such an HD‐specific score is currently not available.
6. STROKE PREVENTION IN PATIENTS WITH ATRIAL FIBRILLATION ON HEMODIALYSIS
There are currently no results from randomized trials on the efficacy and safety of anticoagulation for stroke prevention in patients with AF with ESRD on HD treatment.8 In the absence of hard evidence, large registries and observational studies were used to compare the efficacy and safety of anticoagulation agents. An overview of selected studies is provided in Table 1. The majority of studies were retrospective cohort studies comparing the occurrence of the clinical outcomes stroke, bleeding, or death in warfarin users to nonusers. Among these, Chan and colleagues15 were the first to publish findings on their investigation of the use of warfarin in incident HD patients with prevalent AF (N = 1671) in a large data set from an HD provider in North America. They found a significantly increased risk for stroke in warfarin users compared to nonusers (HR, 1.93; 95% CI, 1.29‐2.90). This surprising finding was most notably contradicted by 3 retrospective cohort studies by Olesen et al,16 Carrero et al,32 and Shen et al,33 who all showed reduced risk of ischemic stroke in patients with AF on warfarin treatment and at the same time did not find increased risk of bleeding in warfarin users compared to nonusers.
Table 1.
Overview of observational studies investigating the use of anticoagulation in patients with ESRD with AF
| First author, journal, publication year | Cohort | Study design | Comparison | Efficacy | Safety | Death |
|---|---|---|---|---|---|---|
| Voskamp, NDT, 2018 | 1718 incident dialysis patients (not exclusively patients with AF) | Prospective cohort study | 244 patients on vitamin K antagonists, 1474 patients without vitamin K antagonists | Not provided | Not provided | Increased risk of all‐cause death in vitamin K antagonist users |
| Siontis, Circulation, 2018 | 25 523 patients with ESRD and AF on dialysis (HD and PD) | Retrospective cohort study | 2351 patients on apixaban and 23 172 patients on warfarin | No significant difference | Reduced risk of major bleeding in apixaban users | Borderline reduced risk of death in apixaban users |
| Yoon, Stroke, 2017 | 9974 HD patients with AF | Retrospective, population‐based cohort study | Warfarin users versus nonusers | No significant difference | Significantly increased risk of hemorrhagic stroke in warfarin users; bleeding risk overall not provided | Not provided |
| Genovesi, Journal of Nephrology, 2017 | 290 HD patients with AF | Prospective observational cohort study | Warfarin users versus nonusers | Intention‐to‐treat: no difference As‐treated: nonsignificant decrease of thromboembolic events in warfarin users | Intention‐to‐treat: no difference As‐treated: nonsignificant increase in bleeding in warfarin users | Intention‐to‐treat: no difference As‐treated: significant reduction in the risk of total and cardiovascular mortality in warfarin users |
| Kai, Heart Rhythm, 2017 | 4286 patients with AF on HD | Retrospective, population‐based cohort study | Warfarin vs. no warfarin | Reduced risk of ischemic stroke in warfarin users | No significant difference in risk of hemorrhagic stroke or gastrointestinal bleeding | Decreased risk of all‐cause death in warfarin users |
| Sarrat, Annals of Pharmacotherapy, 2017 | 160 HD patients with AF or venous thromboembolism | Retrospective cohort study | 120 warfarin patients and 40 apixaban patients | Not provided | No significant difference | Not provided |
| Chan, Circulation, 2015 | 8064 HD patients on warfarin, 281 HD patients on dabigatran, 244 patients on rivaroxaban | Population based retrospective cohort study | Rivaroxaban vs. warfarin and dabigatran vs. warfarin | Adjusted analysis not provided, unadjusted no significant difference | Dabigatran and rivaroxaban associated with an increased risk of major bleeding | Not provided |
| Shen, AJKD, 2015 | 12 284 prevalent HD patients with newly diagnosed AF | Retrospective cohort study | Warfarin vs. no warfarin | Reduced risk of ischemic stroke in warfarin users | No significant difference | No significant difference |
| Carrero, JAMA, 2014 | 478 patients with AF and acute myocardial infarction and eGFR < 15 mL/min/1.73 m2 | Prospective observational cohort study | Warfarin vs. no warfarin | Reduced risk of composite end point (death, stroke, myocardial infarction, and bleeding) in warfarin users | No significant difference | Nonsignificant reduction in death in warfarin users |
| Shah, Circulation, 2014 | 1626 dialysis patients with AF | Retrospective population‐based cohort study | Warfarin vs. no warfarin | No significant difference | Increased risk of bleeding events in warfarin users | Not provided |
| Olesen, NEJM, 2012 | 901 patients with AF requiring renal‐replacement therapy | Retrospective population‐based cohort study | Warfarin versus no antithrombotic agent | Reduced risk of stroke or systemic embolism in warfarin users | No significant difference | Not provided |
| Chan, JASN, 2009 | 1671 incident hemodialysis patients with preexisting AF | Retrospective cohort study | Warfarin vs. no warfarin | Increased risk of stroke in warfarin users | Not provided | No significant difference |
AF, atrial fibrillation; e GFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; HD, hemodialysis; PD, peritoneal dialysis.
The safety end point of bleeding occurrence is of special significance in the HD setting because bleeding complications are common in HD patients, and it is of little surprise, therefore, that findings by Shah et al34 and Yoon et al35 showed increased risk of bleeding in warfarin users compared to nonusers in the respective retrospective cohort studies. 34, 35
Ultimately, patients with ESRD have multiple comorbid conditions and a reduced life expectancy.36 With regard to the mortality outcome in warfarin users compared to nonusers, findings, where provided in observational studies, were not conclusive (Table 1).
The study design and data quality of these retrospective studies is very heterogeneous and therefore limitations have to be addressed. In the publication by Olesen and colleagues,16 the analysis of the data set from the Danish National Registry did distinguish patients with ESRD into ones receiving HD, peritoneal dialysis, and patients after kidney transplantation; thus, HD patients were not specifically addressed. In the comprehensive analysis of stroke outcomes in prevalent HD patients by Shen and colleagues, the finding of a reduced risk of ischemic stroke in warfarin users (HR, 0.68; 95% CI, 0.47‐0.99) may be limited by the high warfarin discontinuation rate of 70% within the first year of treatment.33
The only prospective observational cohort study investigating warfarin use and nonuse in patients with AF on HD, by Genovesi and colleagues,37 reported occurrence of stroke, bleeding, and mortality in a cohort of 290 patients. In multivariable regression analysis, there was no conclusive result regarding the use of warfarin on occurrence of the composite end point of stroke and pulmonary embolism (HR, 0.12; 95% CI, 0.00‐3.59; P = 0.2), partially due to low event rates. Patients on warfarin treatment, however, had increased risk of hemorrhage (HR, 3.96; 95% CI, 1.15‐13.68; P = 0.03).37
In light of these results from nonrandomized studies, the overall efficacy and safety of warfarin or other vitamin K antagonists for stroke prevention in AF in the HD setting is not confirmed.38, 39 The emergence of DOACs as new treatment options for stroke prevention in AF raised hope that new evidence in the setting of patients with ESRD with AF would become available. The hope that the trials of DOACs for stroke prevention in AF would include patients with ESRD was not fulfilled. The DOACs proved to be noninferior to vitamin K antagonists for several indications including stroke prevention in AF, treatment of venous thromboembolism, and prevention of venous thromboembolism after orthopedic surgery.40, 41, 42 In these trials, creatinine clearance was assessed during screening of potential trial patients and implemented as exclusion criteria according to different cutoffs (Table 2). Consequentially, DOACs were not approved for use in patients with ESRD on hemodialysis by the European Medicines Agency, but only to the criteria set forth in the exclusion criteria of the phase 3 trials. The US Food and Drug Administration (FDA), however, approved the use of apixaban for patients with estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73 m2. This surprising expansion of the apixaban licensing gave Siontis et al43 and Sarrat et al44 the opportunity to retrospectively analyze the risk of stroke and bleeding in apixaban users compared to warfarin users on HD. In a data set of 25 523 patients with ESRD and AF on dialysis, of whom 2351 patients were taking apixaban and 23 172 patients were taking warfarin, Siontis and colleagues43 found no significant difference in the risk of stroke between the 2 groups, a reduced risk of major bleeding and a reduced risk of death in apixaban users. Given that the patients were not randomized to either treatment greatly limits the power of interpretation due to selection bias.
Table 2.
Renal insufficiency criteria for DOAC dose reduction in phase 3 trials75, 76, 77, 78 and in ESC recommendation7
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |
|---|---|---|---|---|
| Trial dose reduction criteria | Dose reduction randomly assigned | If CrCl 30‐49 mL/min | If 2 criteria applied: 1) age ≥ 80, 2) body weight ≤ 60 kg, or 3) serum creatinine level ≥ 1.5 mg/dL | If any 1 criterion applied: CrCl 30‐50 mL/min, body weight ≤ 60 kg or use of verapamil/quinidine/dronedarone |
| Trial exclusion criteria | CrCl < 30 mL/min | CrCl < 30 mL/min | Serum creatinine level > 2.5 mg/dL or CrCl < 25 mL/min | CrCl < 30 mL/min |
| Dose reduction criteria in ESC recommendation | CrCl < 50 mL/min | CrCl < 50 mL/min | Same as trial dose reduction criteria | CrCl ≤ 50 mL/min |
CrCl, creatinine clearance according to the Cockcroft‐Gault formula; DOAC, direct oral anticoagulant; ESC, European Society of Cardiology.
Using a health care provider national registry of patients with ESRD, Chan and colleagues further provided outcome data on the off‐label use for rivaroxaban and dabigatran in HD patients in the United States.45 The use of dabigatran and rivaroxaban in HD patients increased as soon as the drugs were licensed for use in the non‐ESRD population. The authors found that dabigatran users (relative risk, 1.48; 95% CI, 1.21‐1.81; P = 0.0001) and rivaroxaban users had an increased risk of hospitalization or death from bleeding compared to warfarin users but no significant difference in stroke or thromboembolism outcome due to low event rates.45 The findings have to be regarded critically because they were obtained outside of the approved licensing for rivaroxaban and dabigatran and treatment allocation was not controlled, so the analysis may be biased in multiple ways.
Regardless of the above discussion, DOACs may yet prove useful for stroke prevention in patients with ESRD to be viable candidates for use in ESRD patients. There are currently 3 ongoing randomized trials of stroke prevention in patients with AF on HD (Table 3): the AXADIA trial46 comparing apixaban 2.5 mg twice daily to phenprocoumon (vitamin K antagonist target INR, 2‐3), the AVKDIAL trial47 comparing vitamin K antagonists (target INR, 2‐3) to no treatment, and the RENAL‐AF trial48 comparing apixaban 5 mg twice daily to warfarin (target INR, 2‐3).
Table 3.
Overview of currently ongoing randomized trials investigating the use of anticoagulation for stroke prevention in HD patients with AF (taken from www.clinicaltrials.gov)
| Trial name and sponsor | Planned N | Study design | Intervention arm | Comparator arm | Primary outcome measure | Status |
|---|---|---|---|---|---|---|
| AXADIA, Atrial Fibrillation Network, Germany | 222 | Randomized open label | Apixaban 2.5 mg bid | Phenprocoumon (vitamin K antagonist) adjusted to target INR 2‐3 | Incidence of major and clinically relevant, nonmajor bleeding | Recruiting |
| AVKDIAL, University Hospital, Strasbourg, France | 855 | Randomized open label | No oral anticoagulation | Vitamin K antagonists adjusted to target INR 2‐3 | Cumulative incidence of severe bleedings and thrombosis | Recruiting |
| RENAL‐AF, Duke University, United States | 155 | Randomized open label | Apixaban 5 mg bid | Warfarin adjusted to target INR of 2‐3 | Time to occurrence of major or clinically relevant nonmajor bleeding | Active, not recruiting |
AF, atrial fibrillation; HD, hemodialysis; INR, international normalized ratio.
Given the contradictory evidence from nonrandomized studies regarding efficacy and safety in warfarin users compared to nonusers, the results of the AVKDIAL trial will be of great interest, especially as it is the only trial that appears to be powered for the net clinical benefit of anticoagulation treatment with a composite end point of thromboembolism and bleeding. Given the high bleeding complication rate in HD patients, it is of further note that apixaban was selected to be investigated in the AXADIA and RENAL‐AF trials, which are addressing safety as their primary end point. In the non‐ESRD population, apixaban had the best safety profile of the DOACs in stroke prevention in AF and has a lower percentage of renal elimination than dabigatran, rivaroxaban, and edoxaban (Table 4). Positive results of these trials, especially with regard to a net clinical benefit, may achieve a real practice change for stroke prevention among HD patients with AF. A closer look at the pharmacokinetic properties of anticoagulation agents in the HD setting is nevertheless warranted.
Table 4.
| Phenprocoumona | Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |
|---|---|---|---|---|---|
| Mechanism of action | Vitamin K antagonist | Direct FIIa inhibitor | Direct FXa inhibitor | Direct FXa inhibitor | Direct FXa inhibitor |
| Prodrug | No | Yes | No | No | No |
| Standard dose for stroke prevention in AF (reduced dose) | INR guided | 150 mg twice daily (110 mg twice daily) | 20 mg once daily (15 mg once daily) | 5 mg twice daily (2.5 mg twice daily) | 60 mg once daily (30 mg once daily) |
| Time to maximum plasma concentration | ~4 h | 0.5‐2 h | 2‐4 h | 3‐4 h | 1‐2 h |
| Oral bioavailability | ~99% | ~6.5% | 80%‐100% | ~50% | ~62% |
| Food interaction | Several dietary restrictions | No | Yes, uptake with food recommended | No | No |
| Renal elimination | <15% unchanged | 85% | ~33% unchanged | ~27% unchanged | 50% |
| Median plasma half‐life in non‐HD patients | 36‐42 h | 12‐14 h | 5‐9 h in young 11‐13 h in elderly | ~12 h | 10‐14 h |
| Known pharmacokinetic interactions | CYP2C9, 3A4 | P‐gp | CYP3A4, P‐gp | CYP3A4, P‐gp | P‐gp |
| Protein binding | 99% | 35% | 92%‐95% | 87% | 55% |
In comparison warfarin has a time to maximal plasma concentration of 90 min and a plasma half‐life of 36 to 42 h.
AF, atrial fibrillation; CYP, cytochrome P450; FIIa, factor IIa; FXa, factor Xa; HD, hemodialysis; INR, international normalized ratio; P‐gp, P‐glycoprotein.
7. COMPLICATIONS OF VITAMIN K ANTAGONIST USE IN HD PATIENTS
In patients with ESRD on HD treatment, management of vitamin K antagonist treatment is difficult because maintaining a stable INR is hindered by the downregulation of cytochrome P450 isoenzymes in chronic uremic conditions.49 Further, HD patients generally have several comorbid conditions and comedications with potential for drug interactions. In fact, with declining kidney function, the dose of vitamin K antagonists required to achieve and maintain therapeutic INR levels decreases.50
A further effect of vitamin K antagonists in patients with ESRD to be considered is the progression of vascular calcification. Patients with ESRD suffer from calcium overload, which in turn leads to loss of vascular smooth muscle cells.51 The result is vascular calcification, particularly of the arterial tunica media and preexisting intimal atherosclerotic plaques.51 Both types of calcification are associated with increased mortality in patients with ESRD.52 The physiologic antagonist of the vascular calcification process is the matrix Gla protein (MGP), which requires vitamin K for its carboxylation.53 Most HD patients are inherently vitamin K deficient,53 but conditions that further decrease vitamin K, such as in vitamin K antagonist treatment, may accelerate the process.54 Whether the progression of vascular calcification through vitamin K antagonist treatment outweighs the antithrombotic benefits remains to be seen. In the authors’ opinion, the risk of progression of vascular calcification has to be weighed against the benefits of antithrombotic treatment as part of an HD‐specific individualized treatment approach.
The most dreaded complication of vascular calcification is calciphylaxis. Although the pathomechanism of calciphylaxis is not entirely understood, vitamin K deficiency, vitamin K antagonist use, and uncarboxylated MGP are all associated with the disease.55 Fortunately, calciphylaxis is rare, but affected patients have a 1‐year survival rate of only 45%.56 This is why the mortality end point should be addressed in studies of anticoagulation agents in HD patients.
8. PHARMACOLOGY OF DOACS IN THE HD SETTING
Owing to the widespread success of DOACs in the prevention of stroke in patients with AF without ESRD, numerous investigations have dedicated significant efforts to explore the pharmacokinetics of the drug class with special regards to changes in renal function.
While vitamin K antagonists have a long half‐life of 36 to 42 hours,11 DOACs have a relatively short half‐life of 5 to 14 hours in renally healthy individuals.11 The half‐life increases with decreasing kidney function due to the renal elimination of the DOACs but greatly differs between DOAC substances (Table 4). The direct thrombin inhibitor dabigatran is eliminated renally to up to 80%, while the factor Xa inhibitors are renally eliminated 25% to 50%.57, 58, 59, 60 An immediate clinical consequence is the handling of perisurgical anticoagulation according to kidney function to reduce the risk of bleeding.61, 62 Another particularly important aspect of DOAC pharmacokinetic characteristics in the HD setting is the protein‐binding property. While rivaroxaban, apixaban, and edoxaban exhibit high protein binding, dabigatran is dialyzable due to its low protein‐binding characteristic.63, 64 The potential implications of the ability to dialyze dabigatran were explored for the emergency setting of dabigatran‐associated bleeding or overdose.65, 66, 67 Dedicated pharmacokinetic studies further explored the characteristic of dabigatran elimination during HD sessions. When dabigatran was administered in the 110‐mg dose at the beginning of the dialysis session, the maximum plasma concentration reached was significantly lower than in non‐HD patients, but the area under the curve (AUC) of dabigatran elimination during a 48‐hour interval was greater in the HD patients.68 By administering dabigatran at the beginning of the HD session, very high plasma concentrations and the associated risk of bleeding can possibly be avoided,69 while the patient maintains a low but steady dabigatran concentration during the 48 hours until the next HD session. The resultant plasma concentration curve was estimated based on the pharmacokinetic data and displayed in Figure 1. Further, the risk of long‐term accumulation is lower than in post‐HD administration.
Figure 1.
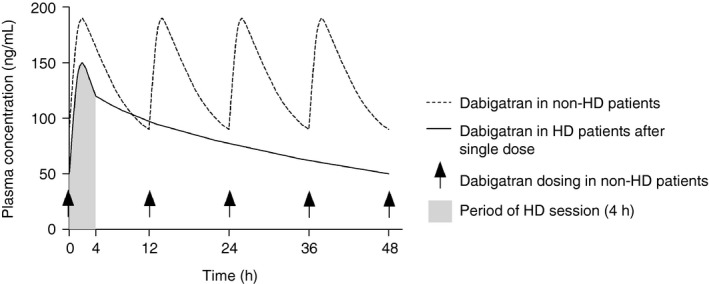
Hypothesized dosing regimen of dabigatran for stroke prevention in AF for patients on thrice‐weekly HD treatment based on data from dedicated pharmacokinetic studies on the use of dabigatran in HD patients68, 79
The properties of the factor Xa inhibitors in the HD setting may be different. A phase 1 trial of rivaroxaban showed a significant 56% increase in AUC but no significant difference between pre‐ and post‐HD administration after single dose of 15 mg.70 A 10‐mg dose of rivaroxaban in HD patients without residual kidney function resulted in drug exposure similar to findings published for 20 mg in healthy volunteers.71 The authors conclude that rivaroxaban is not significantly eliminated by dialysis.71
In addition, the clearance of the factor Xa inhibitor edoxaban did not differ significantly between the on‐ and off‐HD days in patients with ESRD.72 The reduced kidney function, however, does have an impact on the plasma concentration of factor Xa inhibitors. After 8 days of twice‐daily administration of apixaban 2.5 mg, the AUC of apixaban concentrations over time increased 2‐ to 5.4‐fold despite HD treatment.73 Although the renal elimination fraction of apixaban is stated as approximately 25%,59 there is a risk of accumulation over time in patients with ESRD.23 Inconsistent with these findings, the drug administration of apixaban 5 mg in one pharmacokinetic study significantly increased AUCs compared to healthy controls when given at the end of an HD session, and 6.7% of the apixaban dose was recovered in the dialysate.74 The FDA approval for apixaban 5 mg twice daily for stroke prevention in AF patients with ESRD is based on these findings. In the authors’ opinion, the license of apixaban for patients with ESRD based on limited pharmacokinetic and not data on efficacy and safety was premature and has to be regarded critically. Patients with ESRD are a population of patients with limited evidence regarding treatment with anticoagulants, but it is our firm conviction that evidence should not be generated on an ipso facto basis of giving apixaban and observing the outcomes in nonrandomized studies. Therefore, if DOACs were to be considered for stroke prevention in AF for HD patients in future trials, the appropriate dosage of DOACs for stroke prevention in AF in the HD setting would have to be evaluated in dedicated dose‐finding studies first. A dose adjustment of the factor Xa inhibitors may still be necessary to avoid accumulation and overdose. The thrombin inhibitor dabigatran, on the other hand, must be administered in relation to the HD procedure. Giving dabigatran at the beginning of the HD session would have the advantage of creating lower spikes in the plasma concentration and less chance of overdose.69 The elimination of dabigatran during the off‐HD period is slow enough to maintain sufficient concentration. The efficacy and safety of these administration regimens would have to be evaluated in trials (Figure 2).
Figure 2.
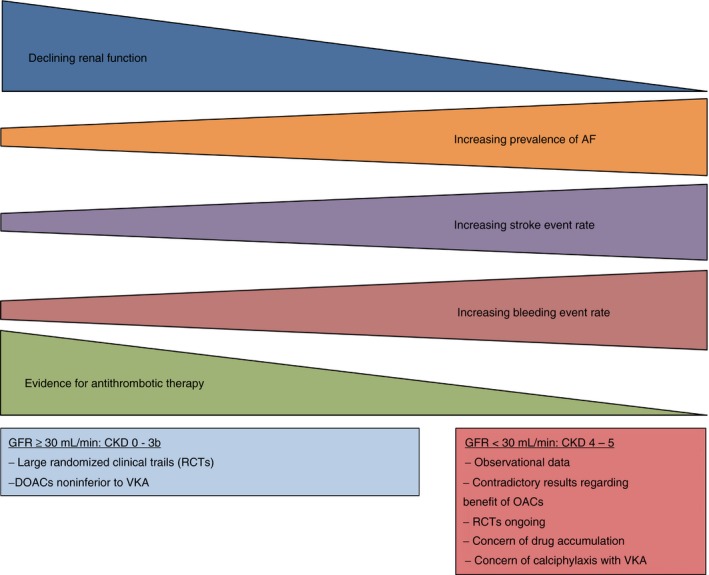
With declining renal function, the event rates of stroke and bleeding increase in patients with CKD. The evidence for antithrombotic therapy, however, decreases with the renal function. CKD, chronic kidney disease; DOACs, direct oral anticoagulants; GFR, glomerular filtration rate; VKA, vitamin K antagonist
9. CONCLUSION
Patients with ESRD on HD maintenance treatment are at increased risk of ischemic stroke and systemic thromboembolism. The high prevalence of AF among other cardiovascular risk factors may be a pivotal reason for the increased thromboembolic risk. While there is no hard evidence on the efficacy and safety of oral anticoagulation drugs in patients with AF on HD, observational evidence on the use of warfarin and other vitamin K antagonists indicates that we should be cautious because of the high risk of bleeding in HD patients and uncertain efficacy. The DOACs have not been tested in randomized trials including patients with ESRD on HD and are therefore not licensed for use in this setting outside of the United States. Specific pharmacokinetic properties of DOACs may make them viable candidates in patients with ESRD. Currently ongoing randomized trials in HD patients with AF may provide new evidence in this neglected population of patients.
RELATIONSHIP DISCLOSURE
The authors have no conflicts of interest or financial relationships to any commercial entities related to the subject of the paper to disclose.
AUTHOR CONTRIBUTIONS
OK and CA screened literature for relevant content, OK drafted the manuscript, and CA revised the manuscript and provided critical appraisal of intellectual content.
Supporting information
Königsbrügge O, Ay C. Atrial fibrillation in patients with end‐stage renal disease on hemodialysis: Magnitude of the problem and new approach to oral anticoagulation. Res Pract Thromb Haemost. 2019;3:578–588. 10.1002/rth2.12250
Contributor Information
Oliver Königsbrügge, Email: oliver.koenigsbruegge@meduniwien.ac.at, @ollyodawg.
Cihan Ay, @Cihan_Ay_MD.
REFERENCES
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22:983–8. [DOI] [PubMed] [Google Scholar]
- 2. Petersen P, Boysen G, Godtfredsen J, Andersen E, Andersen B. Placebo‐controlled, randomized trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK Study. Lancet. 1989;333:175–9. [DOI] [PubMed] [Google Scholar]
- 3. The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators . The effect of low‐dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med. 1990;323:1505–11. [DOI] [PubMed] [Google Scholar]
- 4. Steffel J, Braunwald E. Novel oral anticoagulants: focus on stroke prevention and treatment of venous thrombo‐embolism. Eur Heart J. 2011;32:1968–76. [DOI] [PubMed] [Google Scholar]
- 5. Winkelmayer WC, Patrick AR, Liu J, Brookhart MA, Setoguchi S. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol. 2011;22:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]
- 8. Winkelmayer WC. Cardiovascular disease: still unresolved: warfarin in ESRD with atrial fibrillation. Nat Rev Nephrol. 2014;10:244–5. [DOI] [PubMed] [Google Scholar]
- 9. Krüger T, Brandenburg V, Schlieper G, Marx N, Floege J. Sailing between Scylla and Charybdis: oral long‐term anticoagulation in dialysis patients. Nephrol Dial Transplant. 2013;28:534–41. [DOI] [PubMed] [Google Scholar]
- 10. Ng KP, Edwards NC, Lip GYH, Townend JN, Ferro CJ. Atrial fibrillation in CKD: balancing the risks and benefits of anticoagulation. Am J Kidney Dis. 2013;62:615–32. [DOI] [PubMed] [Google Scholar]
- 11. Riva N, Ageno W. Pros and cons of vitamin K antagonists and non–vitamin K antagonist oral anticoagulants. Semin Thromb Hemost. 2015;41:178–87. [DOI] [PubMed] [Google Scholar]
- 12. Ahrens I, Lip GYH, Peter K. New oral anticoagulant drugs in cardiovascular disease. Thromb Haemost. 2010;104:49–60. [DOI] [PubMed] [Google Scholar]
- 13. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. [DOI] [PubMed] [Google Scholar]
- 14. Seliger SL, Gillen DL, Longstreth WT, Kestenbaum B, Stehman‐Breen CO. Elevated risk of stroke among patients with end‐stage renal disease. Kidney Int. 2003;64:603–9. [DOI] [PubMed] [Google Scholar]
- 15. Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olesen JB, Lip GYH, Kamper A‐L, Hommel K, Køber L, Lane DA, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367:625–35. [DOI] [PubMed] [Google Scholar]
- 17. Stroke Prevention in Atrial Fibrillation Investigators . Stroke prevention in atrial fibrillation study. Circulation. 1991;84:527–39. [DOI] [PubMed] [Google Scholar]
- 18. Mant J, Hobbs FDR, Fletcher K, Roalfe A, Fitzmaurice D, Lip GYH, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. [DOI] [PubMed] [Google Scholar]
- 19. Wizemann V, Tong L, Satayathum S, Disney A, Akiba T, Fissell RB, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010;77:1098–106. [DOI] [PubMed] [Google Scholar]
- 20. Ohsawa M, Tanno K, Okamura T, Yonekura Y, Kato K, Fujishima Y, et al. Standardized prevalence ratios for atrial fibrillation in adult dialysis patients in Japan. J Epidemiol. 2016;26:272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Genovesi S, Pogliani D, Faini A, Valsecchi MG, Riva A, Stefani F, et al. Prevalence of atrial fibrillation and associated factors in a population of long‐term hemodialysis patients. Am J Kidney Dis. 2005;46:897–902. [DOI] [PubMed] [Google Scholar]
- 22. Königsbrügge O, Posch F, Antlanger M, Kovarik J, Klauser‐Braun R, Kletzmayr J, et al. Prevalence of atrial fibrillation and antithrombotic therapy in hemodialysis patients: cross‐sectional results of the Vienna InVestigation of AtriaL Fibrillation and Thromboembolism in Patients on HemoDIaLysis (VIVALDI). PLoS ONE. 2017;12:e0169400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Turakhia MP, Blankestijn PJ, Carrero J‐J, Clase CM, Deo R, Herzog CA, et al. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J. 2018;1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang HH, Hung SY, Sung JM, Hung KY, Wang JD. Risk of stroke in long‐term dialysis patients compared with the general population. Am J Kidney Dis. 2014;63:604–11. [DOI] [PubMed] [Google Scholar]
- 25. Wiesholzer M, Harm F, Tomasec G, Barbieri G, Putz D, Balcke P. Incidence of stroke among chronic hemodialysis patients with nonrheumatic atrial fibrillation. Am J Nephrol. 2001;21:35–9. [DOI] [PubMed] [Google Scholar]
- 26. Mitsuma W, Matsubara T, Hatada K, Imai S, Tamura M. Atrial fibrillation had less impact on the risk of ischemic stroke in non‐anticoagulated patients undergoing hemodialysis: insight from the RAKUEN study. Intern Med. 2018;57:2295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Genovesi S, Vincenti A, Rossi E, Pogliani D, Acquistapace I, Stella A, et al. Atrial fibrillation and morbidity and mortality in a cohort of long‐term hemodialysis patients. Am J Kidney Dis. 2008;51:255–62. [DOI] [PubMed] [Google Scholar]
- 28. Wetmore JB, Ellerbeck EF, Mahnken JD, Phadnis MA, Rigler SK, Spertus JA, et al. Stroke and the “stroke belt” in dialysis: contribution of patient characteristics to ischemic stroke rate and its geographic variation. J Am Soc Nephrol. 2013;24:2053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shih C‐J, Ou S‐M, Chao P‐W, Kuo S‐C, Lee Y‐J, Yang C‐Y, et al. Risks of death and stroke in patients undergoing hemodialysis with new‐onset atrial fibrillation: a competing‐risk analysis of a nationwide cohort. Circulation. 2016;133:265–72. [DOI] [PubMed] [Google Scholar]
- 30. Buiten MS, de Bie MK, Rotmans JI, Gabreëls BA, van Dorp W, Wolterbeek R, et al. The dialysis procedure as a trigger for atrial fibrillation: new insights in the development of atrial fibrillation in dialysis patients. Heart. 2014;100:685–90. [DOI] [PubMed] [Google Scholar]
- 31. Airy M, Chang TI, Ding VY, Goldstein BA, Bansal N, Niu J, et al. Risk profiles for acute health events after incident atrial fibrillation in patients with end‐stage renal disease on hemodialysis. Nephrol Dial Transplant. 2018;33:1590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carrero JJ, Evans M, Szummer K, Spaak J, Lindhagen L, Edfors R, et al. Warfarin, kidney dysfunction, and outcomes following acute myocardial infarction in patients with atrial fibrillation. JAMA. 2014;311:919–28. [DOI] [PubMed] [Google Scholar]
- 33. Shen JI, Montez‐Rath ME, Lenihan CR, Turakhia MP, Chang TI, Winkelmayer WC. Outcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillation. Am J Kidney Dis. 2015;66:677–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shah M, Avgil Tsadok M, Jackevicius CA, Essebag V, Eisenberg MJ, Rahme E, et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation. 2014;129:1196–203. [DOI] [PubMed] [Google Scholar]
- 35. Yoon C‐Y, Noh J, Jhee JH, Chang TI, Kang EW, Kee YK, et al. Warfarin use in patients with atrial fibrillation undergoing hemodialysis. Stroke. 2017;48:2472–9. [DOI] [PubMed] [Google Scholar]
- 36. Plantinga LC, Fink NE, Levin NW, Jaar BG, Coresh J, Levey AS, et al. Early, intermediate, and long‐term risk factors for mortality in incident dialysis patients: the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. Am J Kidney Dis. 2007;49:831–40. [DOI] [PubMed] [Google Scholar]
- 37. Genovesi S, Rossi E, Gallieni M, Stella A, Badiali F, Conte F, et al. Warfarin use, mortality, bleeding and stroke in haemodialysis patients with atrial fibrillation. Nephrol Dial Transplant. 2015;30:491–8. [DOI] [PubMed] [Google Scholar]
- 38. Wong CX, Odutayo A, Emdin CA, Kinnear NJ, Sun MT. Meta‐analysis of anticoagulation use, stroke, thromboembolism, bleeding, and mortality in patients with atrial fibrillation on dialysis. Am J Cardiol. 2016;117:1934–41. [DOI] [PubMed] [Google Scholar]
- 39. Harel Z, Chertow GM, Shah PS, Harel S, Dorian P, Yan AT, et al. Warfarin and the risk of stroke and bleeding in patients with atrial fibrillation receiving dialysis: a systematic review and meta‐analysis. Can J Cardiol. 2017;33:737–46. [DOI] [PubMed] [Google Scholar]
- 40. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet 2014;383:2288–95. [DOI] [PubMed] [Google Scholar]
- 41. Van Es N, Coppens M, Schulman S, Middeldorp S, Harry RB, Van Es N, et al. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124:1968–76. [DOI] [PubMed] [Google Scholar]
- 42. Feng W, Wu K, Liu Z, Kong G, Deng Z, Chen S, et al. Oral direct factor Xa inhibitor versus enoxaparin for thromboprophylaxis after hip or knee arthroplasty: systemic review, traditional meta‐analysis, dose‐response meta‐analysis and network meta‐analysis. Thromb Res. 2015;136:1133–44. [DOI] [PubMed] [Google Scholar]
- 43. Siontis K, Zhang X, Eckard A, Bhave N, Schaubel D, He K, et al. Outcomes associated with apixaban use in patients with end‐stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;128:1519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sarratt SC, Nesbit R, Moye R. Safety outcomes of apixaban compared with warfarin in patients with end‐stage renal disease. Ann Pharmacother. 2017;51:445–50. [DOI] [PubMed] [Google Scholar]
- 45. Chan KE, Edelman ER, Wenger JB, Thadhani RI, Maddux FW. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation. 2015;972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT02933697. Compare Apixaban and Vitamin‐K Antagonists in Patients with Atrial Fibrillation (AF) and End‐Stage Kidney Disease (ESKD) (AXADIA). [Accessed 2016 October 14]. Available from: https://clinicaltrials.gov/ct2/show/NCT02933697.
- 47. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT02886962. Oral Anticoagulation in Haemodialysis Patients (AVKDIAL). [Accessed 2016 September 1] [cited 2019 Jul 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT02886962.
- 48. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT 02942407. Trial to Evaluate Anticoagulation Therapy in Hemodialysis Patients With Atrial Fibrillation (RENAL‐AF). [Accessed 2016 October 24] [cited 2019 Jul 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT02942407.
- 49. Albrecht D, Turakhia MP, Ries D, Dillon D, Milner PG, Midei MG, et al. Pharmacokinetics of tecarfarin and warfarin in patients with severe chronic kidney disease. Thromb Haemost. 2017;117:2026–33. [DOI] [PubMed] [Google Scholar]
- 50. Limdi NA, Limdi MA, Cavallari L, Aaron M, Crowley MR, Baird MF, et al. Warfarin dosing in patients with impaired kidney function. Am J Kidney Dis. 2010;56:823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–57. [DOI] [PubMed] [Google Scholar]
- 52. London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H. Arterial media calcification in end‐stage renal disease: impact on all‐cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–40. [DOI] [PubMed] [Google Scholar]
- 53. Westenfeld R, Krüger T, Schlieper G, Cranenburg EC, Magdeleyens EJ, Heidenreich S, et al. Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: randomized trial. Am J Kidney Dis. 2012;59:186–95. [DOI] [PubMed] [Google Scholar]
- 54. Chatrou M, Winckers K, Hackeng T, Reutelingsperger C, Schurgers L. Vascular calcification: the price to pay for anticoagulation therapy with vitamin K‐antagonists. Blood Rev. 2012;26:155–66. [DOI] [PubMed] [Google Scholar]
- 55. Nigwekar S, Bloch D, Nazarian R, Vermeer C, Booth S, Xu D, et al. Vitamin K–dependent carboxylation of matrix Gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28:1717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mazhar AR, Johnson RJ, Gillen D, Stivelman JC, Ryan MJ, Davis CL, et al. Risk factors and mortality associated with calciphylaxis in end‐stage renal disease. Kidney Int. 2001;60:324–32. [DOI] [PubMed] [Google Scholar]
- 57. Boehringer Ingelheim International GmbH . Summary of product characteristics ‐ dabigatran. Latest version, April 6, 2017.
- 58. Bayer Pharma AG . Summary of product characteristics ‐ rivaroxaban. Latest version, October 14, 2016.
- 59. Bristol‐Myers Squibb/Pfizer . Summary of product characteristics ‐ apixaban. Latest version, February 22, 2017.
- 60. Daiichi‐Sankyo . Summary of product characteristics ‐ edoxaban. Latest version, September 23, 2016.
- 61. Spyropoulos AC, Al‐Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14:875–85. [DOI] [PubMed] [Google Scholar]
- 62. Beyer‐Westendorf J, Gelbricht V, Förster K, Ebertz F, Köhler C, Werth S, et al. Peri‐interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. Eur Heart J. 2014;35:1888–96. [DOI] [PubMed] [Google Scholar]
- 63. Huisman MV, Lip GYH, Diener H‐C, Brueckmann M, van Ryn J, Clemens A. Dabigatran etexilate for stroke prevention in patients with atrial fibrillation: resolving uncertainties in routine practice. Thromb Haemost. 2012;107:838–47. [DOI] [PubMed] [Google Scholar]
- 64. van Ryn J, Goss A, Hauel N, Wienen W, Priepke H, Nar H, et al. The discovery of dabigatran etexilate. Front Pharmacol. 2013;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Montaruli B, Erroi L, Vitale C, Berutti S, Cosseddu D, Sivera P, et al. Dabigatran overdose. Blood Coagul Fibrinolysis. 2015;26:225–9. [DOI] [PubMed] [Google Scholar]
- 66. Chai‐Adisaksopha C, Hillis C, Lim W, Boonyawat K, Moffat K, Crowther M. Hemodialysis for the treatment of dabigatran‐associated bleeding: a case report and systematic review. J Thromb Haemost. 2015;13:1790–8. [DOI] [PubMed] [Google Scholar]
- 67. Marino KK, Santiago RA, Dew RB, Berliner N, Connors JM, Connell NT, et al. Management of dabigatran‐associated bleeding with two doses of idarucizumab plus hemodialysis. Pharmacotherapy. 2016;36:e160–5. [DOI] [PubMed] [Google Scholar]
- 68. Wilson JAS, Goralski KB, Soroka SD, Morrison M, Mossop P, Sleno L, et al. An evaluation of oral dabigatran etexilate pharmacokinetics and pharmacodynamics in hemodialysis. J Clin Pharmacol. 2014;54:901–9. [DOI] [PubMed] [Google Scholar]
- 69. Liesenfeld KH, Clemens A, Kreuzer J, Brueckmann M, Friedrich F. Dabigatran treatment simulation in patients undergoing maintenance haemodialysis. Thromb Haemost. 2016;115:562–9. [DOI] [PubMed] [Google Scholar]
- 70. Dias C, Moore KT, Murphy J, Ariyawansa J, Smith W, Mills RM, et al. Pharmacokinetics, pharmacodynamics, and safety of single‐dose rivaroxaban in chronic hemodialysis. Am J Nephrol. 2016;43:229–36. [DOI] [PubMed] [Google Scholar]
- 71. De Vriese AS, Caluwe R, Van VB, De BD, Vandecasteele SJ, Emmerechts J. Dose‐finding study of rivaroxaban in hemodialysis patients. Am J Kidney Dis. 2015;66:91–8. [DOI] [PubMed] [Google Scholar]
- 72. Parasrampuria DA, Marbury T, Matsushima N, Chen S, Wickremasingha PK, He L, et al. Pharmacokinetics, safety, and tolerability of edoxaban in end‐stage renal disease subjects undergoing haemodialysis. Thromb Haemost. 2015;113:719–27. [DOI] [PubMed] [Google Scholar]
- 73. Mavrakanas TA, Samer CF, Nessim SJ, Frisch G, Lipman ML. Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol. 2017;28:2241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang X, Tirucherai G, Marbury TC, Wang J, Chang M, Zhang D, et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end‐stage renal disease on hemodialysis. J Clin Pharmacol. 2016;56:628–36. [DOI] [PubMed] [Google Scholar]
- 75. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 76. Patel M, Mahaffey K, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 77. Granger C, Alexander J, McMurray JJV, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 78. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- 79. van Ryn J, Stangier J, Haertter S, Liesenfeld K‐H, Wienen W, Feuring M, et al. Dabigatran etexilate–a novel, reversible, oral direct thrombin inhibitor: Interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


