Abstract
Background
Thrombin generation (TG) assays evaluate the balance between pro‐ and anticoagulant forces, to better assess bleeding and thrombotic risks. Although TG readouts obtained with the calibrated automated TG have been investigated in multiple clinical conditions, TG still needs standardization and clinical validation. The automated TG instrument ST Genesia® (STG, Stago, Asnières‐sur‐Seine, France) provides a normalization of TG parameters based on a reference plasma aiming to reduce the interlaboratory variability and the variability between different measurement runs.
Objectives
To evaluate STG in a group of healthy adults.
Methods
Reference intervals in healthy adults and variability of the new standardized reagents for bleeding (BleedScreen) and thrombophilic (ThromboScreen) conditions were determined using STG.
Results
TG was measured in platelet‐free plasma (PFP) samples of 123 healthy adults. Reference intervals were determined for TG parameters. Intra‐ and interassay coefficients of variation were calculated on quality controls and PFP samples from healthy adults. Oral contraception (OC) possibly influenced TG parameters, resulting in a higher median and a broader reference interval for peak height and endogenous thrombin potential (ETP) in women aged 20 to 49 years than in all other sex and age categories. Therefore, we propose the following reference interval categories: men, women aged <50 years not using OC, women aged <50 years using OC, and women aged ≥50 years. Normalization was effective to reduce the interassay variability of quality controls for ETP (BleedScreen assay), and peak height and ETP (ThromboScreen assay without thrombomodulin), but had little impact on PFP sample variability.
Conclusion
STG appears suitable for accurate measurement of TG in healthy adults.
Keywords: blood coagulation factors, clinical laboratory tests, quality control, reference ranges, thrombin generation
Essentials.
ST Genesia® (STG) is a new automated instrument to measure thrombin generation (TG) parameters.
Reference intervals were determined for TG parameters measured with STG in healthy adults.
Oral contraception possibly influenced TG parameters.
STG appears to be suitable for the accurate measurement of TG in healthy adults.
1. INTRODUCTION
Predicting the risk of bleeding and thromboembolic disorders remains a major health issue. Through the formation of an insufficient or an exaggerated amount of thrombin, the dysfunctional coagulation system is responsible for these pathologies. Therefore, several global coagulation assays have been developed during the past 50 years to assess the overall coagulation potential. Among them, thrombin generation (TG) testing investigates TG itself and the entire process of thrombin inhibition as a whole, to get insight into the complicated balance between pro‐ and anticoagulant forces and to identify potential hemostatic impairments.1, 2, 3, 4 In platelet‐poor plasma (PPP), TG testing can detect all coagulation factor deficiencies except factor XIII deficiency.5, 6, 7 It is sensitive to anticoagulant drugs8, 9 and to procoagulant states such as deficiency in antithrombin,10 protein C, or protein S11 as well as to hyperprothrombinemia12 and resistance to activated protein C.13, 14
The method of TG testing developed by Hemker et al,1, 15 the so‐called calibrated automated thrombography (CAT) has been used and studied in numerous conditions during the past 15 years. The use of a calibrator allows an accurate measurement of the amount and activity of the generated thrombin. In addition, the detection of fluorescence allows the measurement in cellular milieu such as platelet‐rich plasma.16
However, although numerous data have been published with CAT assay consistent with high added value for clinical practice, this methodology still misses standardization and clinical validation. This is due to the lack of a standard experimental protocol, reagents, and analyzer, responsible for a large interlaboratory variability, and to the absence of large clinical studies in which the treatment decision is based on TG testing results.17, 18, 19, 20, 21, 22, 23 Recently, a larger study on lab‐to‐lab variability has been published showing an improvement by normalization of the data using a single reference plasma.24
Lately, a new TG analyzer (ST Genesia®, Stago) has been released as the first fully automated TG analyzer for clinical routine laboratories together with a set of reagents balanced for sensitivity to procoagulant and anticoagulant protein deficiencies (https://www.stago.com/products‐services/new‐products/detail/article/thrombin‐generation‐now‐available‐in‐laboratories/). In comparison to the CAT assay, ST Genesia® provides a normalization of each TG parameter based on a reference plasma for each test aiming to reduce the interlaboratory variability as well as the variability between different measurement runs (https://www.stago.com/products-services/new-products/detail/article/thrombin-generation-now-available-in-laboratories/).
In the present study, the ST Genesia® Thrombin Generation System was evaluated in a healthy adult sample. Our intent was not only to test the inter‐ and intra‐assay variability of the new analyzer and reagents but also to establish accurate normal reference intervals for all TG parameters to provide a clear view on the interindividual TG variability. Furthermore, we investigated the effect of sex, age, and oral contraception (OC) on the different TG parameters and determined which plasma pro‐ and anticoagulant proteins influence TG parameters.
2. METHODS
2.1. Cohort sample, blood collection, and processing
Blood samples were collected from healthy adult individuals, aged 20 to 80 years, current or former employees of the hospital or acquaintances of the hospital employees or former employees. An advertisement for the study was posted on the hospital intranet and posters were placed in hospitals buildings. Demographic data as well as information on medication, OC consumption, and hormone replacement therapy (HRT) were obtained by a questionnaire and completed by an interview. Inclusion criteria were age 20 to 80 years and not known to suffer any significant illness, in particular, no cardiovascular disease including thrombotic disorders (except arterial hypertension under therapy) or bleeding disorder, diabetes, cancer, or infectious disease. Exclusion criteria were age <20 years or >80 years, chronic medication except pantoprazole and medication for arterial hypertension or depression, and pregnancy. The ethics committee at our institution approved the study, and written informed consent was obtained from all participants. No subject was directed to fast before collection.
Venous blood was obtained through antecubital venipuncture and collected into 3.2% sodium citrate (10 mL, S‐Monovette, Sarstedt, Nümbrecht, Germany) using a 21‐gauge needle (0.80 × 19 mm, Terumo Surflo Winged Infusion Set, Terumo, Spreitenbach, Switzerland). Samples for TG were processed by double centrifugation according to the guidelines recommendation of the subcommittee of the Scientific and Standardization Committee of the ISTH.19 Briefly, whole blood samples (10 mL) were centrifuged at 2500 g for 15 minutes at room temperature and 3 mL of PPP was collected in the middle of the tube, thus avoiding the platelets at the top surface and those at the buffy coat. The collected plasma was recentrifuged at 2500 g for 15 minutes at room temperature, and 2.7 mL of supernatant (platelet‐free plasma [PFP]) was collected. For routine coagulation analysis, 10 mL of venous blood was centrifuged at 1500 g for 20 minutes at room temperature to obtain approximately 4 mL of PPP. The resulting PPP was stored in aliquots at −80°C. All the samples were collected between January and May 2018. Measurements were all completed at the end of May 2018.
2.2. Thrombin generation assays
Thrombin generation measurements were performed with the ST Genesia® Thrombin Generation System (Stago, Asnières‐sur‐Seine, France). Two types of reagents were used: the STG®‐BleedScreen (STG‐BLS) and the STG®‐ThromboScreen (STG‐TS). TG with the STG‐BLS assay was initiated by a mixture of procoagulant phospholipids and low picomolar level of human tissue factor (TF), balanced for sensitivity to procoagulant factor deficiencies while minimizing contact activation. The assay contained 2 quality controls for low and normal TG potential, respectively, and a reference plasma for parameter normalization. TG with the STG‐TS assay was initiated by a mixture of procoagulant phospholipids and medium picomolar level of human TF in the presence or absence of thrombomodulin (TM), balanced for sensitivity to deficiencies in natural anticoagulants, without interfering contact activation. The assay contained 3 quality controls for low, normal, and high TM resistance and a reference plasma for parameter normalization. In the presence of both reagents, TG was triggered by dispensing a fluorogenic substrate and CaCl2. For the entire study, the same lots of STG‐BLS (Lot: 202639, RUO label) and STG‐TS reagents (LOT number: 202640, RUO label) were used. The following parameters were measured: lag time, peak height, time to peak, endogenous thrombin potential (ETP), ETP inhibition, and velocity index.
A comparison with CAT (Stago) was performed in a small subgroup of samples (n = 12), using 2 experimental settings similar to STG‐BLS and STG‐TS conditions. Briefly, in the first setting, 74 μL of PFP was added to 20 μL of a mixture of 1 pmol/L TF and 4 μmol/L phospholipids (PPP Reagent LOW, Stago)25 and 6 μL of HN‐buffer (HEPES 20 mmol/L, NaCl 140 mmol/L, pH 7.4 + 5 mg/mL bovine serum albumin [BSA]), in a 96‐well round‐bottom microtiter plate (Immulon2HB, Thermo Fisher Scientific, Reinach, Switzerland). For the second setting, 74 μL PFP was added to 20 μL of a mixture of 5 pmol/L TF and 4 μmol/L phospolipids (PPP Reagent)25 and 6 μL of recombinant human TM (4 nmol/L, Sekisui Alveo AG, Lucern, Switzerland) or HN‐buffer (HEPES 20 mmol/L, NaCl 140 mmol/L, pH 7.4 + 5 mg/mL BSA), in a 96‐well round‐bottom microtiter plate (Immulon2HB). The reaction was initiated with 20 μl of a mixture of fluorogenic substrate and CaCl2 (Fluobuffer, Stago) and fluorescence measured using a Fluoroskan Ascent reader (Thermo Labsystems). All experiments were carried out in duplicate at 37°C. Thrombin generation curves were generated using Thrombinoscope software, version 5.0.0.742 (Thrombinoscope BV, Maastricht, The Netherlands). Lag time, peak height, time to peak, and ETP were calculated.
2.3. Coagulation assays
Prothrombin time (Dade Innovin, Siemens Healthcare, Munich, Germany); activated partial thromboplastin time (APTT; SL Pathrombin, Siemens); fibrinogen (Clauss Method, Dade Thrombin, Siemens); antithrombin (Innovance Antithrombin, Siemens); prothrombin; and factors V, VII, and X (FII, FVII, FV, and FX Deficient Plasmas, Siemens) were measured using a Sysmex CS‐5100 coagulometer (Siemens). Free protein S (Free PS Ag Innovance, Siemens); protein C (chromogenic: Berichrom, Siemens; coagulant: PC Reagent, Siemens); activated protein C resistance (Coatest APC Resistance V, Endotell), and factors VIII, IX, and XI were determined using a BCS‐XP analyzer (Siemens).
2.4. Statistical analysis
Continuous variables were expressed as median and interquartile range (25%‐75%). Interindividual variability (coefficient of variation [CV]) was calculated for each variable as 100 × (standard deviation/mean). Normal reference intervals for STG‐TGA parameters were calculated on normalized values by using nonparametric tests, in agreement with Clinical and Laboratory Standards Institute guidelines.26 The lower reference interval limit was estimated as the 2.5th percentile and the upper limit as the 97.5th percentile of the distribution. Data were checked for normality. Groups were compared by using the Mann–Whitney U‐test for independent samples and Tukey's multiple comparisons test. The Pearson correlation was calculated to verify the association between different parameters. Statistical analysis was performed using Prism 7 software (GraphPad Software, San Diego, CA).
3. RESULTS
3.1. Characteristics of the study group
Of 124 healthy adults aged 20 to 80, one was excluded because of pregnancy at week 13 of gestation, leaving a study sample of 123. Characteristics of the studied sample are listed in Table 1. Overall, 61 subjects were men (49.6%) and the median age was 47 (interquartile range [IQR], 24‐80) for men and 49.5 (IQR 21‐77) for women. All subjects, men and women, were almost equally distributed in 2 age categories: 20 to 49 and 50 to 80. Ninety‐five percent of the men and 92% of the women were Caucasian. Eight women took OC (24% of the women aged 20‐49), indicating that, regarding OC, the studied sample is representative of the Swiss population, of which 25% of the women aged 15 to 49 take OC.27 Five women used HRT (16% of the women aged 50 to 80). Twenty‐four healthy adults took medication only once during the last 7 days before the blood draw: 11 of them took nonsteroidal antirheumatic drugs (8 aged 20‐49 and 3 aged 50‐80), 2 took aspirin (1 aged 20‐49 and 1 aged 50‐80), 1 aged 20‐49 took metamizole, 9 took acetaminophen (5 aged 20‐49 and 4 aged 50‐80), and 1 took a painkiller (not otherwise specified). Several healthy adults were on the following medication: nonoral contraceptive (1 woman aged 20‐49), pantoprazole (2 healthy adults), duloxetine,1 fluoxetine,1 blood pressure medication (6, mainly angiotensin‐converting enzyme inhibitors; among them, 2 were in the 20‐49 age group and 4 were in the 50‐80 age category).
Table 1.
Characteristics of the studied sample
| Characteristic | Men (n) | Women (n) |
|---|---|---|
| Number | 61 | 62 |
| Age categories, y | ||
| 20‐49 | 34 | 31 |
| 50‐80 | 27 | 31 |
| Hormonal therapy | ||
| Oral contraceptiona | — | 8 |
| Hormone replacement therapy | — | 5 |
| Race/Ethnicity | ||
| Asian | 1 (2%) | 1 (1.5%) |
| Black | 2 (3%) | 1 (1.5%) |
| Caucasian | 58 (95%) | 60 (97%) |
Twenty‐four percent of women aged 20‐49 use hormonal contraception.
Coagulation parameters, including pro‐ and anticoagulants, measured in the studied population are shown in Table S1. Some differences were found between sexes (Table S1A). Men aged 20 to 49 displayed lower fibrinogen and factor VIII levels, and higher free protein S levels than women belonging to the same age category. Men aged 50 to 80 had lower factor V and antithrombin levels but higher factor IX than women aged 50 to 80. Differences were also found between age categories of adults of the same sex (Table S1B). Women and men aged 50 to 80 displayed a shorter prothrombin time as well as higher levels of fibrinogen, factor V, factor VII, and factor IX than women and men aged 20 to 49. Factor XI, antithrombin, protein C, and free protein S were higher in women aged 50 to 80 than in women aged 20 to 49. Factor VIII was higher in men aged 50 to 80 than in men aged 20 to 49, whereas antithrombin level was higher in men aged 20 to 49 than in men aged 50 to 80. There were also differences regarding coagulation parameters between women using and not using OC (Table S2). Indeed, women taking OC displayed a shorter APTT as well as higher factors II, X, and IX. Although there was a trend for a lower free protein S level in women taking OC in comparison to women not taking OC, the difference was not statistically significant.
3.2. Thrombin generation parameters measured by ST Genesia® using the STG‐BLS
Normalized peak height values are presented in Figure 1A and normalized ETP values, in Figure 1B. Men aged 20 to 49 years showed significantly lower normalized peak height and ETP values than women belonging to the same age category (P < 0.0001). This difference might be possibly due to the consumption of OC by some of the women of this age group (P < 0.0001; Figure 1A, B). Indeed, there was no difference regarding the normalized peak height and ETP values between men aged 20 to 49 and women aged 20 to 49 not taking OC (Figure 1A, B). Normalized peak height and ETP values, however, did not differ significantly in men and women aged 50 to 80 (Figure 1A, B). In addition, both parameters statistically differed for women aged 20 to 49 and 50 to 80 (P < 0.0001). There was no difference between men aged 20 to 49 and 50 to 80. We then established reference intervals for all thrombin generation parameters in all sex and age categories (Table 2). Based on these results, we propose to use the 4 following reference interval categories for the routine hemostasis laboratory: men, women aged <50 years not using OC, women aged <50 years using OC, and women ≥50 years (Table 2).
Figure 1.
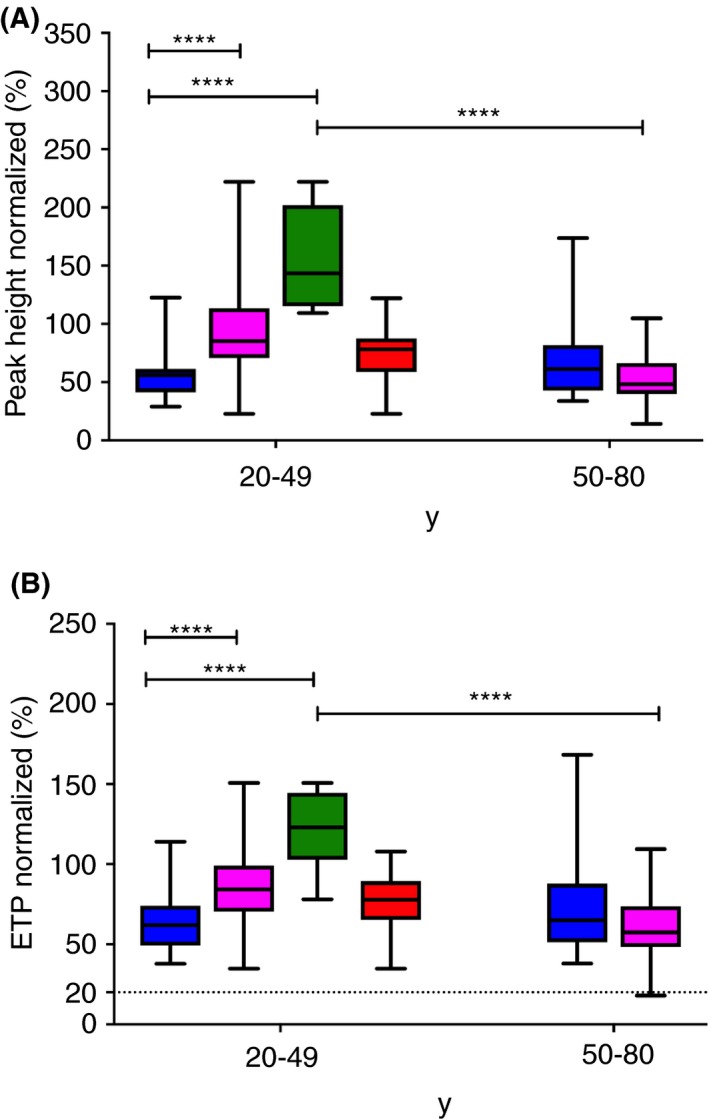
ST Genesia BleedScreen parameters in the age categories 20‐49 years and 50‐80 years, respectively. (A) Peak height normalized. (B) Endogenous thrombin potential (ETP) normalized. Data from all men are shown by blue boxes. Data from all women are shown by pink boxes. Data from women aged 20‐49 are divided in 2 groups: women using oral contraception (green boxes) and women not using oral contraception (red boxes). Median and interquartile range are indicated. Groups were compared using the Mann‐Whitney U‐test. The dotted lines show the threshold proposed by Stago. ns, not significant; ****P < 0.0001
Table 2.
Interindividual variability and reference intervals of thrombin generation parameters measured by ST Genesia in healthy adults using the BleedScreen assay
| Age categories (y) | Lag time normalized (ratio) | Peak normalized (%) | Time to peak normalized (ratio) | ETP normalized (%) | Velocity index normalized (%) |
|---|---|---|---|---|---|
| Men | |||||
| 20‐49 | 1.1 (1.0‐1.2) | 56 (41‐61) | 1.1 (1.0‐1.2) | 62 (49‐74) | 56 (44‐71) |
| 50‐80 | 1.2 (1.1‐1.4) | 61 (43‐82) | 1.1 (1.1‐1.3) | 66 (51‐92) | 58 (42‐90) |
| All men (20‐80) | 1.1 (1.0‐1.3) | 57 (42‐72) | 1.1 (1.0‐1.2) | 64 (51‐79) | 58 (44‐76) |
| Women | |||||
| 20‐49 | 1.0 (0.9‐1.1) | 85 (71‐114) | 1.0 (0.9‐1.0) | 84 (70‐99) | 95 (73‐141) |
| 50‐80 | 1.2 (1.0‐1.3) | 48 (40‐67) | 1.2 (1.0‐1.3) | 57 (48‐74) | 47 (35‐67) |
| Women | |||||
| 20‐49 using OC | 1.0 (0.9‐1.0) | 143 (115‐202) | 0.9 (0.8‐1.0) | 123 (103‐145) | 174 (144‐322) |
| 20‐49 not using OC | 1.0 (0.9‐1.1) | 78 (59‐88) | 1.0 (0.9‐1.1) | 78 (65‐90) | 82 (68‐105) |
Data are expressed as median and interquartile range (25th‐75th percentile). ETP, endogenous thrombin potential; OC, oral contraception.
3.3. Thrombin generation parameters measured by ST Genesia® using the STG‐TS
Normalized peak height and normalized ETP values obtained in the absence of TM are presented in Figure 2A and 2B, respectively. Men aged 20 to 49 years showed significantly lower normalized peak height and ETP values than women belonging to the same age category (P < 0.0001). This difference was possibly due to the OC consumption by some of the women of this age group (Figure 2A, B; P < 0.00). Indeed, there was no difference regarding the normalized peak height and ETP values between men aged 20 to 49 and women aged 20 to 49 not taking OC (Figure 2A, B). Normalized peak height and ETP values, however, did not differ significantly in men and women aged 50 to 80. However, these 2 parameters statistically differed for women aged 20 to 49 and 50 to 80 (P < 0.00; Figure 2A, B). There was a difference in normalized peak height but not in normalized ETP between women aged 20 to 49 not using OC and women aged 50 to 80 (Figure 2A, B). In presence of soluble TM, we observed a significantly higher ETP inhibition in men aged 20 to 49 years as compared to women belonging to the same age category (Figure 2C). This difference was possibly due to the consumption of OC by some of the women of this age group (Figure 2C). Indeed, there was no difference regarding ETP inhibition between men aged 20 to 49 and women aged 20 to 49 not using OC (Figure 2C). This difference might be due to OC consumption in the female group aged 20 to 49. ETP inhibition, however, did not differ significantly in men and women aged 50 to 80. However, ETP inhibition differed for women aged 20 to 49 and 50 to 80 (P < 0.0001; Figure 2C). We then established reference intervals for all thrombin generation parameters in all sex and age categories (Table 3). Based on these results, we propose for the routine laboratory to use the 4 following reference interval categories: men, women aged <50 years not using OC, women aged <50 years using OC, and women aged ≥50 years (Table 3).
Figure 2.
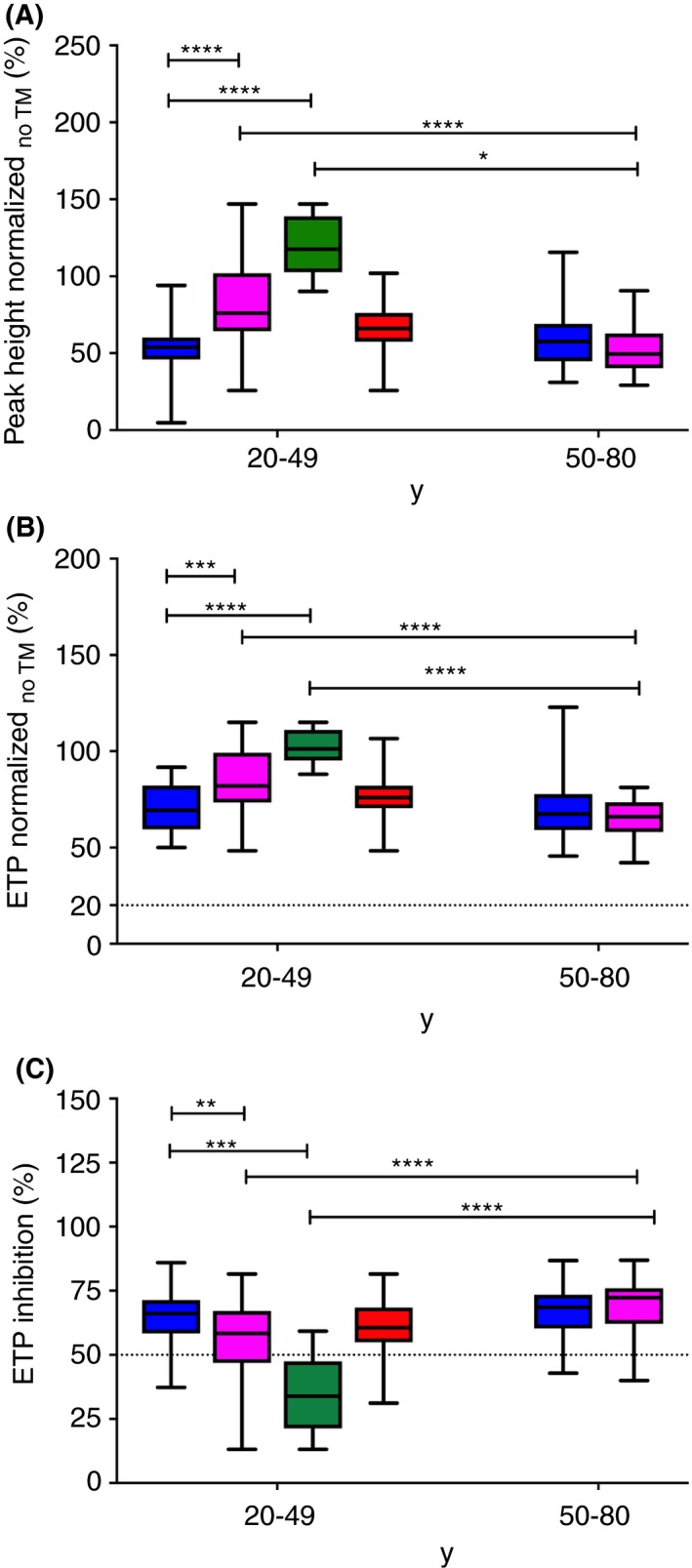
ST Genesia ThromboScreen parameters in the age categories 20‐49 and 50‐80, respectively. (A) Peak height normalized. (B) Endogenous thrombin potential (ETP) obtained in absence of thrombomodulin normalized. (C) Endogenous thrombin potential (ETP) obtained in presence of thrombomodulin normalized. Data from all men are shown by blue boxes. Data from all women are shown by pink boxes. Data from women aged 20‐49 are divided in 2 groups: women using oral contraception (green boxes) and women not using oral contraception (red boxes). Medians and interquartile ranges are indicated. Groups were compared using the Mann‐Whitney U‐test. The dotted lines show the threshold proposed by STAGO. TM, thrombomodulin; ns, not significant; **P < 0.01; ***P < 0.001; ****P < 0.0001
Table 3.
Interindividual variability and reference intervals of thrombin generation parameters measured by ST Genesia in healthy adults using the ThromboScreen assay
| Age categories (y) | Lag time normalized (ratio) | Peak normalized (%) | Time to peak normalized (ratio) | ETP normalized (%) | Velocity index normalized (%) | ETP inhibition (%) |
|---|---|---|---|---|---|---|
| Men | ||||||
| 20‐49 | 1.1 (1.1‐1.3) | 54 (46‐60) | 1.2 (1.1‐1.4) | 69 (59‐82) | 46 (34‐54) | 66 (58‐71) |
| 50‐80 | 1.3 (1.2‐1.3) | 58 (45‐69) | 1.2 (1.2‐1.3) | 67 (59‐78) | 52 (37‐62) | 69 (60‐73) |
| All men (20‐80) | 1.2 (1.1‐1.3) | 55 (45‐66) | 1.2 (1.2‐1.3) | 68 (59‐80) | 49 (36‐57) | 67 (60‐73) |
| Women | ||||||
| 20‐49 | 1.0 (1.0‐1.1) | 76 (64‐102) | 1.1 (1.0‐1.2) | 82 (73‐99) | 69 (54‐112) | 58 (47‐67) |
| 50‐80 | 1.3 (1.1‐1.4) | 49 (40‐63) | 1.4 (1.2‐1.5) | 66 (58‐74) | 40 (31‐50) | 72 (62‐76) |
| Women | ||||||
| 20‐49 not using OC | 1.1 (1.0‐1.1) | 66 (58‐76) | 1.2 (1.0‐1.2) | 76 (70‐82) | 61 (53‐77) | 61 (55‐68) |
| 20‐49 using OC | 0.9 (0.9‐1.1) | 118 (103‐139) | 0.9 (0.8‐1.1) | 101 (95‐111) | 149 (95‐214) | 33 (21‐48) |
Data are expressed as median and interquartile range (25th‐75th percentile). Normalized lag time, peak, time to peak, and ETP values have been obtained using ThromboScreen assay in the absence of thrombomodulin. The ETP inhibition parameter has been calculated from ETP values obtained in presence and in absence of thrombomodulin. ETP, endogenous thrombin potential; OC, oral contraception.
3.4. Correlation between hemostasis/thrombophilia parameters and thrombin generation parameters measured with ST Genesia®
Correlation (coefficients) of the linear regression model (r) and P values between hemostasis/thrombophilia parameters and thrombin generation parameters were calculated in the study group (Table S3).
In STG‐BLS (low picomolar TF concentration), peak height and ETP values positively correlated with factors VIII and X, and negatively with APTT, factor XI, antithrombin, and free protein S (Figure 3A). Protein C (both coagulant and chromogenic values) correlated negatively only with ETP.
Figure 3.
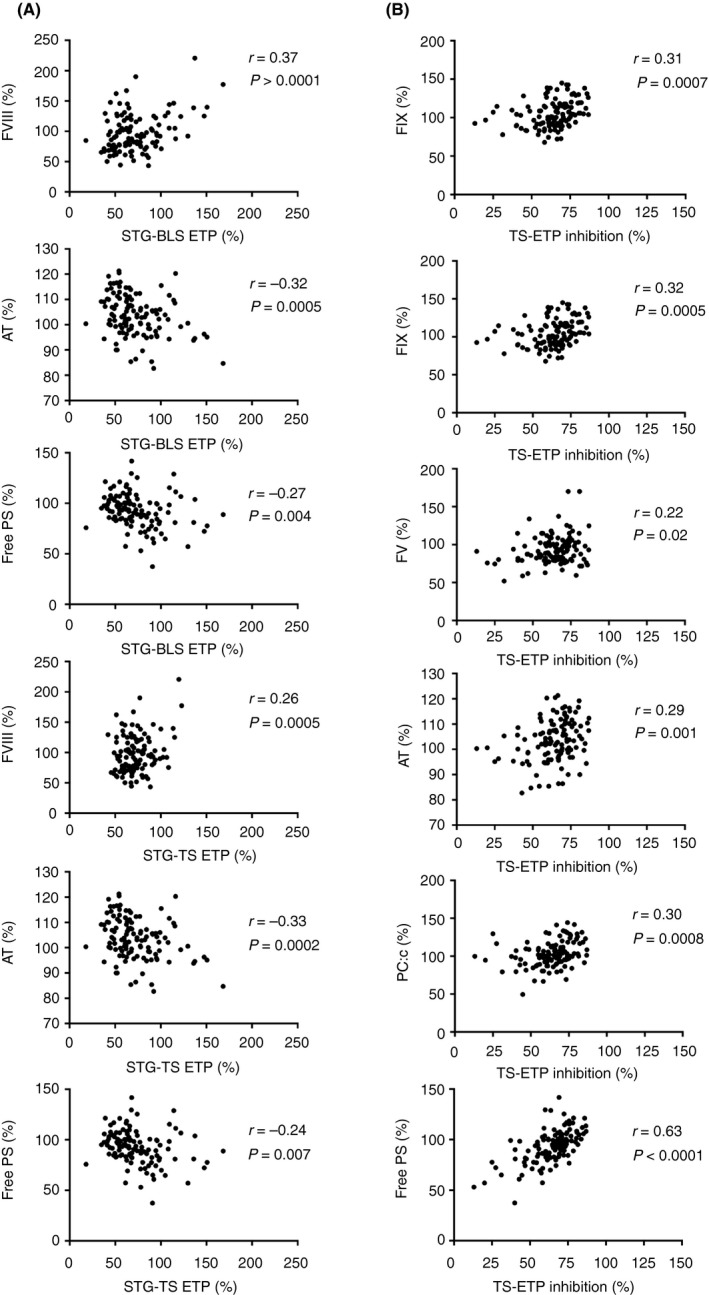
Correlations between some hemostasis/thrombophilia parameters and ETP values in the study group. (A) ETP was measured using the BleedScreen assay and the ThromboScreen assay. (B) Endogenous thrombin potential (ETP) inhibition was measured using the ThromboScreen (TS) assay in presence/absence of thrombomodulin. AT, antithrombin; ETP, endogenous thrombin potential; FIX, factor IX; free PS, free protein S; FV, factor V; FVIII, factor VIII; PC:c, protein C coagulant; r, Pearson coefficient; STG‐BLS, BleedScreen; STG‐TS, ThromboScreen
In STG‐TS (medium picomolar TF concentration), peak height positively correlated with factors VIII, IX, and X, while negative correlations were found for APTT, factor V, antithrombin, and free protein S. ETP without TM positively correlated with factor VIII and negatively with factor IX, antithrombin, and free protein S (Figure 3A).
STG‐TS ET‐inhibition parameters positively correlated with factors V, IX, and XI; antithrombin; protein C (both coagulant and chromogenic); and free protein S (Figure 3B).
3.5. Inter‐ and intra‐assay variability of thrombin generation parameters measured by ST Genesia®
We calculated the interassay variability of ST Genesia® quality controls (QCs) and reference plasma for both STG‐BLS and STG‐TS (with and without TM) assays (Tables S4 and S5). For the STG‐BLS assay (n = 33 tests), the CVs of all the absolute and normalized STG parameters (lag time, peak, time to peak, ETP, velocity index) ranged between 2.1% and 13% for the QCs (Table S4) and between 2.6% and 13%, for the reference plasma (Table S5). Similarly, for the STG‐TS without TM assay (n = 45 tests), the CVs of STG parameters ranged between 2% and 12% for all the QCs (Table S4) and reference plasma (Table S5). For the TS with TM assay (n = 45 tests), the CVs of STG parameters ranged between 2.0% and 13%.
Moreover, we calculated the inter‐ and intra‐assay variability of the TG parameters measured by the ST Genesia system in 4 healthy adults (n = 4; 2 men and 2 women) to evaluate the intraindividual variability of BLS and TS assays on frozen PFP samples (Tables 4, 5, S6, and S7).
Table 4.
Peak and ETP interassay variability in normal frozen platelet‐free plasma samples for BleedScreen and ThromboScreen assays
| ST Genesia (STG) assay | STG parameter | Value |
Healthy adult 1 Mean ± SD, CV (%) |
Healthy adult 2 Mean ± SD, CV (%) |
Healthy adult 3 Mean ± SD, CV (%) |
Healthy adult 4 Mean ± SD, CV (%) |
|---|---|---|---|---|---|---|
| BleedScreen | Peak | Absolute (nmol/L) | 174 ± 20, 12 | 114 ± 16, 14 | 94 ± 18, 19 | 87 ± 23, 27 |
| Normalized (%) | 130 ± 13, 9.7 | 85 ± 11, 13 | 70 ± 13, 19 | 64 ± 17, 26 | ||
| ETP | Absolute (nmol/L × min) | 1099 ± 100, 9.1 | 953 ± 125, 13 | 677 ± 113, 17 | 699 ± 134, 19 | |
| Normalized (%) | 110 ± 9, 8.1 | 95 ± 11, 12 | 68 ± 12, 17 | 70 ± 13, 19 | ||
| ThromboScreen without TM | Peak | Absolute (nmol/L) | 235 ± 15, 6.4 | 177 ± 11, 6.3 | 169 ± 12, 6.9 | 148 ± 14, 9.6 |
| Normalized (%) | 90 ± 8, 8.7 | 68 ± 5, 7.1 | 64 ± 5, 7.0 | 56 ± 5, 9.5 | ||
| ETP | Absolute (nmol/L × min) | 1140 ± 512, 4.5 | 1138 ± 48, 4.2 | 958 ± 48, 5.1 | 937 ± 45, 4.8 | |
| Normalized (%) | 83 ± 4, 5.3 | 83 ± 4,4.9 | 69 ± 4, 6.2 | 67 ± 4,6.0 | ||
| ThromboScreen with TM | Peak | Absolute (nmol/L) | 152 ± 21, 14 | 83 ± 7, 8.9 | 93 ± 9,9.4 | 95 ± 12, 13 |
| ETP | Absolute (nmol/L × min) | 574 ± 81, 14 | 346 ± 31, 8.9 | 349 ± 29, 8.3 | 408 ± 52, 13 | |
| ETP inhibition | Ratio (%) | 50 ± 6,12 | 70 ± 2, 3.2 | 65 ± 5, 7.2 | 56 ± 5, 8.9 |
Data are expressed as mean and standard deviation (SD). CV, coefficient of variation; ETP, endogenous thrombin potential; TM, thrombomodulin.
Table 5.
Peak and ETP intra‐assay variability in normal frozen platelet‐free plasma samples for BleedScreen and ThromboScreen assays
| ST Genesia (STG) assay | STG parameter | Value |
Healthy adult 1 Mean ± SD, CV (%) |
Healthy adult 2 Mean ± SD, CV (%) |
Healthy adult 3 Mean ± SD, CV (%) |
Healthy adult 4 Mean ± SD, CV (%) |
|---|---|---|---|---|---|---|
| BleedScreen | Peak | Absolute (nmol/L) | 157 ± 8.5, 5.5 | 105 ± 11, 10 | 109 ± 15, 14 | 117 ± 8.6, 7.4 |
| Normalized (%) | 127 ± 6.9, 5.4 | 77 ± 7.8, 10 | 87 ± 12, 14 | 93 ± 6.9, 7.4 | ||
| ETP | Absolute (nmol/L × min) | 1029 ± 37, 3.6 | 865 ± 61, 7.0 | 805 ± 106, 13 | 960 ± 57, 5.9 | |
| Normalized (%) | 109 ± 3.9, 3.5 | 88 ± 6.1, 7.0 | 84 ± 11, 13 | 100 ± 6.0, 5.9 | ||
| ThromboScreen without TM | Peak | Absolute (nmol/L) | 218 ± 11, 4.9 | 173 ± 6.4, 3.7 | 157 ± 16, 9.8 | 136 ± 4.6, 3.4 |
| Normalized (%) | 85 ± 4.1, 4.9 | 65 ± 2.4, 3.7 | 60 ± 5.9, 9.8 | 51 ± 1.7, 3.4 | ||
| ETP | Absolute (nmol/L × min) | 1086 ± 41, 3.7 | 1102 ± 34, 3.1 | 908 ± 58, 6.4 | 889 ± 22, 2.4 | |
| Normalized (%) | 81 ± 3.0, 3.7 | 79 ± 2.4, 3.1 | 64 ± 4.1, 6.4 | 62 ± 1.5, 2.4 | ||
| ThromboScreen with TM | Peak | Absolute (nmol/L) | 134 ± 16, 12 | 73 ± 3.2, 4.3 | 87 ± 8.2, 9.5 | 94 ± 2.9, 3.1 |
| ETP | Absolute (nmol/L × min) | 512 ± 64, 13 | 3032 ± 10, 3.3 | 330 ± 26, 7.9 | 406 ± 13, 3.1 | |
| ETP inhibition | Ratio (%) | 52 ± 6.1, 13 | 73 ± 0.9, 1.2 | 64 ± 1.5, 2.4 | 54 ± 1.3, 2.3 |
Data are expressed as mean and standard deviation (SD). CV, coefficient of variation; ETP, endogenous thrombin potential; TM, thrombomodulin.
For the interassay variation (Tables 4 and S6), each healthy adult frozen PFP sample was measured 5 times in the 5 independent assays (different calibration curve and QCs/reference plasma check). For the BLS assay, the range of the CVs of all absolute and normalized STG parameters was large to very large for all 4 healthy adults (healthy adult 1, 0.2%‐14%; healthy adult 2, 3.6%‐17%; healthy adult 3, 3.8%‐21%; healthy adult 4, 2.3%‐34%). For the TS without TM assay, the CVs of all absolute and normalized STG parameters ranged between 1.6% and 10% for healthy adult 1, between 2.2% and 8% for healthy adult 2, between 1.9% and 8% for healthy adult 3, and between 1.9% and 11% for healthy adult 4. For the TS with TM assay, the CVs of STG parameters and ETP inhibition ranged between 2.1% and 15% for healthy adult 1, between 3.2% and 9% for healthy adult 2, between 5.0% and 13% for healthy adult 3, and between 1.6% and 13% for healthy adult 4.
For the intra‐assay variability (Tables 5 and S7), each healthy adult frozen PFP sample was measured 5 times in the same assay (same calibration curve and QCs/reference plasma check). For the BLS assay, the CVs of all the absolute and normalized STG parameters (lag time, peak, time to peak, ETP, start tail, and velocity index) ranged between 1.3% and 10% for healthy adult 1, between 2.3% and 12% for healthy adult 2, between 1.9% and 15% for healthy adult 3, and between 0.7% and 11% for healthy adult 4. For the TS without TM assay, the CVs of all absolute and normalized STG parameters ranged between 1.6% and 12% for donor 1, between 1.1% and 7.5% for healthy adult 2, between 2.9% and 12% for healthy adult 3, and between 1.1% and 13% for healthy adult 4. For the TS with TM assay, the CVs of STG parameters and ETP inhibition ranged between 1.8% and 14% for healthy adult 1, between 0.8% and 7.9% for healthy adult 2, between 5.1% and 13% for healthy adult 3, and between 2.0% and 4.9% for healthy adult 4.
3.6. Correlation between ST Genesia® Thrombin Generation Measurement System and CAT assay
TG parameters measured by ST Genesia® and by CAT in a subset of the studied population (n = 12) are provided in Table S8. We calculated the correlation between peak height and ETP parameters (absolute values) measured by ST Genesia and those determined by the CAT assay. Peak height and the ETP showed a positive correlation between the STG‐BLS and PPP Reagent LOW assays (r, 0.75 and r, 0.62; P < 0.001, respectively).
Peak height but not ETP measured in the absence of TM correlated between STG‐TS and PPP Reagent (r, 0.84, P < 0.00, and 0.28, P = 0.07, respectively). In presence of TM, the ETP and the ETP inhibition correlated in STG‐TS and PPP Reagent assays (r, 0.70, P = 0.00; and 0.85, P < 0.00, respectively).
4. DISCUSSION
We report the first study to date on TG measured by the automated ST Genesia® STG in a group of 123 healthy adults. STG is a new automated instrument to measure TG parameters. Reference intervals were determined for TG parameters measured with STG in healthy adults. We report here that OC possibly influenced TG parameters,1 and STG appears to be suitable for the accurate measurement of TG in healthy adults.2
As previously reported, we found differences in the levels of some coagulation factors and inhibitors between sex and age categories. In addition, women aged 20 to 49 using OC displayed a higher level of some coagulation factors and a trend for a lower free protein S level than women of the same age category who did not take OC. These results are consistent with previous publications.28, 29, 30, 31, 32
The investigation of the interindividual variability allowed us to define 4 different categories of reference interval for ST Genesia® TG parameters: men, women aged <50 years not using OC, women aged <50 years using OC, and women aged ≥50 years. The rationale for the establishment of these categories was the possible influence of OC on TG parameters, resulting in a higher median and a broader reference interval for peak height and ETP in women aged 20 to 49 years than in all other sex and age categories.
The most important determinants of the amount of thrombin formed, as quantified by peak height and/or ETP, were, for both BleedScreen and ThromboScreen assays, factor VIII, antithrombin, and free protein S. As expected, proteins C and S were determinants of the effect of TM. In the presence of medium picomolar concentration of TF and TM, the inhibition of the ETP was remarkably affected by factor IX and XI levels. Interestingly, fibrinogen as well as factor V and VII levels were not, in our study group, found to be relevant. There was no correlation between resistance to activated protein C and TG parameters in the studied population. Because previous studies demonstrated an increase of TG in subjects with APC resistance,13, 14 we did not expect, in the study group, to find no correlation between TG parameters and resistance to APC. However, this could be explained by the fact that none of the included healthy adults displayed APC resistance (Table S1).
Normalization was effective to reduce the inter‐assay variability of QCs for ETP (BleedScreen assay), and peak height and ETP (ThromboScreen assay without TM). As already reported,24 the velocity was the most variable parameter, the CVs being the highest of the series, and some of the values being even worse after normalization. For the ThromboScreen assay with TM, the values were not normalized and higher CVs were calculated (up to 13%).
Inter‐ and intra‐assay variability of normal plasma samples revealed very high CVs for the velocity index, even after normalization, particularly when measured with BleedScreen and for healthy adults 3 and 4. High CVs for the velocity index, the most variable TG parameter, were previously reported.24 In addition, the interassay variability study revealed extremely high CVs for the absolute peak height and ETP values when measured with the BleedScreen assay for healthy adults 3 and 4. Normalization had no or only little impact on variability. These results are in line with previous observations, indicating that variability of TG parameters remains important and is not always reduced by normalization.24 However, normalization might be useful to reduce lab‐to‐lab variability the ST Genesia® system,24 but this needs to be investigated.
Finally, we were able to show that TG parameters measured with ST Genesia® correlated well with most of the comparable parameters measured with the CAT assay. The exception is the ETP without TM (ThromboScreen) measurement that did not correlate with ETP obtained with the CAT assay with 5 pM TF. We are unable to comment on this result, as the concentration of TF and phospholipids used for the ST Genesia® ThromboScreen assay was not disclosed by Stago.
Our study has some limitations. First, the study included only 123 participants from a single center, with samples analyzed in 1 laboratory. Second, the study comprised a small number of healthy adults. Thus, confirmation on a larger cohort would be required, and a multicenter study would certainly be the most appropriate design. Third, only 1 batch of reagents was used for this study. Therefore, it would be necessary to verify that different batches of reagents do not affect the validity of the results. Previous data obtained with CAT showed that changes of reagent batches did not impact the results.25 However, this was not tested with the ST Genesia®. Fourth, the studied sample was mainly Caucasian, being representative of the Swiss population. Thus, it is indeed unclear whether the results of this study can be extrapolated to other race/ethnic groups. Additional studies should be undertaken to investigate whether the same range can be used for all race/ethnic groups. Fifth, we did not study within‐person variability. This is a gap that will need to be filled by future studies. Sixth, we do not provide data on freeze‐thaw effects. Future studies will have to comprise these data.
In conclusion, the ST Genesia® Thrombin Generation System with BleedScreen and ThromboScreen assays appears to be suitable for the accurate measurement of TG parameters in healthy adults. However, this study was not performed to demonstrate the clinical utility of this system. Future studies will comprise pathologic samples to determine how this system can be used in the management of patients with bleeding or thrombotic disorders. The DrugScreen assay still has to be evaluated. Finally, it will also be important to assess the external quality, as it was performed for the CAT assay.24
RELATIONSHIP DISCLOSURE
The authors report nothing to disclose.
AUTHOR CONTRIBUTIONS
SC, JB, and AA‐S designed the protocol and the analyses plan, organized data collection, conducted the analyses, and drafted the manuscript. SC, CQ, and LM performed the thrombin generation measurements. JB and EG performed the coagulation assays. SC, JB, and JJ performed the statistical analysis. SC, JB, and AA‐S interpreted the data. JB, RN, LCR, EG, MF, and MN organized data collection and intellectually reviewed the manuscript. All authors approved the final version of the manuscript.
Supporting information
ACKNOWLEDGMENTS
We thank Armando Lenz (Clinical Trials Unit, University of Bern, Bern, Switzerland) for his valuable advice on the statistical analysis. This work was supported by a grant of the Swiss National Science Foundation (314730_173127).
Calzavarini S, Brodard J, Quarroz C, et al. Thrombin generation measurement using the ST Genesia Thrombin Generation System in a cohort of healthy adults: Normal values and variability. Res Pract Thromb Haemost. 2019;3:758–768. 10.1002/rth2.12238
Sara Calzavarini and Justine Brodard contributed equally.
REFERENCES
- 1. Hemker HC, Beguin S. Phenotyping the clotting system. Thromb Haemost. 2000;84:747–51. [PubMed] [Google Scholar]
- 2. Macfarlane RG, Biggs R. A thrombin generation test; the application in haemophilia and thrombocytopenia. J Clin Pathol. 1953;6:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pitney WR, Dacie JV. A simple method of studying the generation of thrombin in recalcified plasma: application in the investigation of haemophilia. J Clin Pathol. 1953;6:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seegers WH. A personal perspective on hemostasis and thrombosis (1937‐1981). Semin Thromb Hemost. 1981;7:177–307. [PubMed] [Google Scholar]
- 5. Al Dieri R, Peyvandi F, Santagostino E, Giansily M, Mannucci PM, Schved JF, et al. The thrombogram in rare inherited coagulation disorders: its relation to clinical bleeding. Thromb Haemost. 2002;88:576–82. [PubMed] [Google Scholar]
- 6. Keularts IM, Hamulyak K, Hemker HC, Beguin S. The effect of DDAVP infusion on thrombin generation in platelet‐rich plasma of von Willebrand type 1 and in mild haemophilia A patients. Thromb Haemost. 2000;84:638–42. [PubMed] [Google Scholar]
- 7. Keularts IM, Zivelin A, Seligsohn U, Hemker HC, Beguin S. The role of factor XI in thrombin generation induced by low concentrations of tissue factor. Thromb Haemost. 2001;85:1060–5. [PubMed] [Google Scholar]
- 8. Freyburger G, Macouillard G, Labrouche S, Sztark F. Coagulation parameters in patients receiving dabigatran etexilate or rivaroxaban: two observational studies in patients undergoing total hip or total knee replacement. Thromb Res. 2011;127:457–65. [DOI] [PubMed] [Google Scholar]
- 9. Hacquard M, Perrin J, Lelievre N, Vigneron C, Lecompte T. Inter‐individual variability of effect of 7 low molecular weight antithrombin‐dependent anticoagulants studied in vitro with calibrated automated thrombography. Thromb Res. 2011;127:29–34. [DOI] [PubMed] [Google Scholar]
- 10. Wielders S, Mukherjee M, Michiels J, Rijkers DT, Cambus JP, Knebel RW, et al. The routine determination of the endogenous thrombin potential, first results in different forms of hyper‐ and hypocoagulability. Thromb Haemost. 1997;77:629–36. [PubMed] [Google Scholar]
- 11. Duchemin J, Pittet JL, Tartary M, Beguin S, Gaussem P, Alhenc‐Gelas M, et al. A new assay based on thrombin generation inhibition to detect both protein C and protein S deficiencies in plasma. Thromb Haemost. 1994;71:331–8. [PubMed] [Google Scholar]
- 12. Kyrle PA, Mannhalter C, Beguin S, Stumpflen A, Hirschl M, Weltermann A, et al. Clinical studies and thrombin generation in patients homozygous or heterozygous for the G20210A mutation in the prothrombin gene. Arterioscler Thromb Vasc Biol. 1998;18:1287–91. [DOI] [PubMed] [Google Scholar]
- 13. Martinelli I, Bottasso B, Duca F, Faioni E, Mannucci PM. Heightened thrombin generation in individuals with resistance to activated protein C. Thromb Haemost. 1996;75:703–5. [PubMed] [Google Scholar]
- 14. Ruhl H, Winterhagen FI, Berens C, Muller J, Oldenburg J, Potzsch B. In vivo thrombin generation and subsequent APC formation are increased in factor V Leiden carriers. Blood. 2018;131:1489–92. [DOI] [PubMed] [Google Scholar]
- 15. Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. [DOI] [PubMed] [Google Scholar]
- 16. Dargaud Y, Luddington R, Baglin T. Platelet‐dependent thrombography: a method for diagnostic laboratories. Br J Haematol. 2006;134:323–5. [DOI] [PubMed] [Google Scholar]
- 17. Dargaud Y, Luddington R, Gray E, Lecompte T, Siegemund T, Baglin T, et al. Standardisation of thrombin generation test–which reference plasma for TGT? An international multicentre study Thromb Res. 2010;125:353–6. [DOI] [PubMed] [Google Scholar]
- 18. Dargaud Y, Luddington R, Gray E, Negrier C, Lecompte T, Petros S, et al. Effect of standardization and normalization on imprecision of calibrated automated thrombography: an international multicentre study. Br J Haematol. 2007;139:303–9. [DOI] [PubMed] [Google Scholar]
- 19. Dargaud Y, Wolberg AS, Gray E, Negrier C, Hemker HC; Subcommittee on Factor VIII, Factor IX, and Rare Coagulation Disorders . Proposal for standardized preanalytical and analytical conditions for measuring thrombin generation in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2017;15:1704–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dargaud Y, Wolberg AS, Luddington R, Regnault V, Spronk H, Baglin T, et al. Evaluation of a standardized protocol for thrombin generation measurement using the calibrated automated thrombogram: an international multicentre study. Thromb Res. 2012;130:929–34. [DOI] [PubMed] [Google Scholar]
- 21. Loeffen R, Kleinegris MC, Loubele ST, Pluijmen PH, Fens D, van Oerle R, et al. Preanalytic variables of thrombin generation: towards a standard procedure and validation of the method. J Thromb Haemost. 2012;10(12):2544–54. [DOI] [PubMed] [Google Scholar]
- 22. Rodgers SE, Wong A, Gopal RD, Dale BJ, Duncan EM, McRae SJ. Evaluation of pre‐analytical variables in a commercial thrombin generation assay. Thromb Res. 2014;134:160–4. [DOI] [PubMed] [Google Scholar]
- 23. Spronk HM, Dielis AW, De Smedt E, van Oerle R, Fens D, Prins MH, et al. Assessment of thrombin generation II: validation of the calibrated automated thrombogram in platelet‐poor plasma in a clinical laboratory. Thromb Haemost. 2008;100:362–4. [PubMed] [Google Scholar]
- 24. Perrin J, Depasse F, Lecompte T. French‐speaking CATg, under the aegis of G, French‐speaking CATg, CAT group French‐speaking, CAT group all in France unless otherwise stated. Large external quality assessment survey on thrombin generation with CAT: further evidence for the usefulness of normalisation with an external reference plasma. Thromb Res. 2015;136:125–30. [DOI] [PubMed] [Google Scholar]
- 25. Bagot CN, Leishman E. Establishing a reference range for thrombin generation using a standard plasma significantly improves assay precision. Thromb Res. 2015;136:139–43. [DOI] [PubMed] [Google Scholar]
- 26. Wayne PA; CLSI . Defining, Establishing and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline. Clinical and Laboratory Standards Institute. CLSI document EP28‐A3c. 28. 3rd ed. 2008; pp 1–76.
- 27. Swiss Health Observatory. 2017. [Accessed 20 June, 2019]. Available from https://www.obsan.admin.ch/sites/default/files/publications/2017/obsan_dossier_59_4.pdf
- 28. Allen GA, Wolberg AS, Oliver JA, Hoffman M, Roberts HR, Monroe DM. Impact of procoagulant concentration on rate, peak and total thrombin generation in a model system. J Thromb Haemost. 2004;2:402–13. [DOI] [PubMed] [Google Scholar]
- 29. Brugge JM, Tans G, Rosing J, Castoldi E. Protein S levels modulate the activated protein C resistance phenotype induced by elevated prothrombin levels. Thromb Haemost. 2006;95:236–42. [DOI] [PubMed] [Google Scholar]
- 30. Dielis AW, Castoldi E, Spronk HM, van Oerle R, Hamulyak K, Ten Cate H, et al. Coagulation factors and the protein C system as determinants of thrombin generation in a normal population. J Thromb Haemost. 2008;6:125–31. [DOI] [PubMed] [Google Scholar]
- 31. Kluft C, Lansink M. Effect of oral contraceptives on haemostasis variables. Thromb Haemost. 1997;78:315–26. [PubMed] [Google Scholar]
- 32. Oral Contraceptive and Hemostasis Study Group . The effects of seven monophasic oral contraceptive regimens on hemostatic variables: conclusions from a large randomized multicenter study. Contraception. 2003;67:173–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


