Abstract
Background
Immune tolerance induction (ITI) therapy is currently unaffordable in China. Management of hemophilia A children with high‐titer inhibitor is therefore a challenge.
Aim
To describe the ITI strategy using plasma‐derived factor VIII/von Willebrand factor concentrate (pdFVIII/VWF) +/− immunosuppression and to report its efficacy in children with hemophilia A having poor‐risk status for ITI success.
Methods
A prospective pilot study on children with hemophilia A having poor‐risk status (all with at least inhibitor titer > 10 BU pre‐ITI initiation). Patients received ~50 IU/kg FVIII every other day using domestic intermediate purity pdFVIII/VWF products, either alone or in combination with rituximab +/− prednisone.
Results
Sixteen patients with median age 2.9 (range, 2.2‐13.2) years and median pre‐ITI inhibitor titer 30.7 (range, 10.4‐128) BU were enrolled. Analysis at median 14.7 (range, 12.4‐22.6) months’ follow‐up showed a total response rate of 87.5%. This included success (achieving inhibitor < 0.6 BU) in 13 patients (81.3%) in a median of 8.8 (range, 3.2‐11.8) months, and partial success (achieving inhibitor < 5 BU but > 0.6BU) in 1 (6.3%). Compared to the pre‐ITI period, the mean bleeds/month during ITI was 0.51 (64.0% reduction), and joint bleeds/month was 0.34 (64.3% reduction). This low‐dose ITI strategy cost less by 70% to 87% than that for the high‐dose FVIII regimen. No severe adverse events were observed.
Conclusion
This low‐dose ITI strategy of pdFVIII/VWF +/− immunosuppression achieved relatively satisfactory outcomes in children with hemophilia A inhibitor having poor‐risk status. This low‐dose regimen showed economic advantages and is therefore suitable for using in China. However, further study in a larger cohort with a longer follow‐up time is needed.
Keywords: child, hemophilia A, immune tolerance induction, immunosuppression, pilot projects, rituximab
Essentials.
High‐dose immune tolerance induction (ITI) is expensive. Management of boys with hemophilia A with inhibitors is a challenge in China.
We describe a low‐dose ITI strategy using plasma‐derived factor VIII/von Willebrand factor concentrate +/− immunosuppression in poor‐risk patients.
Inhibitors disappeared in 81.3% patients in 8.8 months and cost was 80% less than high‐dose ITI.
This ITI strategy was cost‐effective and enabled ITI to be carried out in a developing country.
1. INTRODUCTION
The development of alloantibodies (inhibitor) that neutralize coagulant activity of factor VIII (FVIII) is the most serious complication related to the treatment of severe hemophilia A, occurring in 20% to 30% of these patients. About two‐thirds of FVIII inhibitors developed in these patients with severe hemophilia A are high ‐titer, which increases the risk for uncontrollable bleeding and morbidity.1 According to a previous longitudinal study, the incidence of inhibitors in China is similar to that reported worldwide.2
Immune tolerance induction (ITI) therapy is the most established treatment to eradicate inhibitors. Guidelines suggest that the ITI regimen should be stratified based on pre‐ITI Bethesda titer using an escalating dose of FVIII (50 IU/kg every other day [QOD] to 100 IU/kg twice daily).3, 4, 5 Although low‐dose ITI regimen (FVIII 50 IU/kg thrice weekly or QOD) is recommended only for low‐titer inhibitors, this regimen of relatively lower cost is the only widely acceptable therapy in China with economic constraint.
There are now several reports showing that using plasma‐derived FVIII containing von Willebrand factor (pdFVIII/VWF),6, 7 either alone or in combination with immunosuppression agents, particularly rituximab, could achieve better results in patients who failed first‐line ITI or in those with high‐titer inhibitors and poor‐risk status for ITI success.8, 9, 10 We report our experience with this low‐dose ITI with an immunosuppression strategy in children with hemophilia A with high‐titer inhibitors and poor‐risk status.
2. METHODS
This open‐label, pilot, prospective cohort study was registered at ClinicalTrials.gov (NCT03598725) and was approved by the Ethics Committee of Beijing Children's Hospital, Capital Medical University, Beijing, China. We consecutively enrolled 16 patients at the study center from September 2016 to July 2017. Data were collected and analyzed at the end of July 2018, with a median 14.7 (range, 12.4‐22.6) months follow‐up time. Each child enrolled provided written informed consent from a parent or a legal guardian.
2.1. Inclusion and exclusion criteria
Inclusion criteria include (1) boys from 1 to 14 years old with severe or moderate hemophilia A (FVIII < 5 IU/dL based on the initial FVIII level before inhibitor development as tested in the local laboratories); (2) having ≥ 1 poor‐risk factors including at least FVIII inhibitor titer ≥ 10 BU before ITI; and (3) ability to follow the study protocol. Exclusion criteria include (1) having congenital or acquired bleeding defects other than hemophilia A; (2) concomitant immunological disease; (3) receiving other immunosuppressive agent(s) (except for rituximab and prednisone used in this study); and (4) inability to follow the study protocol.
2.2. Definitions of poor‐risk status and outcomes
Poor‐risk status was defined as ≥1 of the following: peak historical inhibitor titer ≥ 200 BU, pre‐ITI inhibitor titer ≥ 10 BU, peak inhibitor titer during ITI > 100 BU, age at ITI initiation ≥ 8 years, time since inhibitor diagnosis to ITI initiation ≥ 5 years, and history of ITI failure.11
ITI outcomes were defined as success—achieving negative inhibitor titer (<0.6 BU); partial success—inhibitor titer continued to be positive but < 5 BU; or failure—partial success not yet achieved at the time of data analysis or ITI discontinued prematurely.12 Total response represents the combined rate of success and partial success.
2.3. ITI strategy and management
The patients with inhibitor came to our center and were started on ITI as soon as possible. All patients received domestic pdFγVIII/VWF ~50 FVIII IU/kg QOD as low‐dose ITI. Immunosuppression would be added according to the following criteria: (1) if inhibitor titer was ≥ 40 BU before or during ITI, rituximab 375 mg/m2 weekly × 4 weeks (maximum 600 mg) and prednisone 2 mg/kg daily × 1 month (maximum 60 mg, tapering over 3 months) would be added immediately; (2) if the inhibitor titer was < 40 BU before or during ITI but with no downward trend (of at least 15% decline over a 3 months ITI period), prednisone alone (same dose schedule as above) would be added. For the patients receiving rituximab, intravenous immunoglobulin 200 mg/kg was administered every 2 weeks for 6 consecutive months to decrease the risk of infections.13, 14
Breakthrough bleeding would be treated with bypassing agents when inhibitor titer was > 2 BU. Bypassing agents that could be used include domestic prothrombin complex concentrate (PCC) at 40 to 50 IU/kg every 12‐24 hours for 1 to 3 doses (similar to the dosage used for activated prothrombin complex concentrate [aPCC]) or recombinant factor VIIa (rFVIIa; Novo Nordisk) 90 μg/kg every 2 to 4 hours for 1 to 3 doses. 15, 16 If the inhibitor titer was ≤ 2 BU, pdFVIII/VWF 50 IU/kg would be used every 12 to 24 hours for 1 to 3 doses. For patient having frequent or life‐threatening bleeding during ITI, prophylaxis would be instituted using a domestic PCC at 40 to 50 IU/kg 2 or 3 times per week.
2.4. Clinic visiting and inhibitor monitoring
Inhibitor titer was tested in the laboratory of the study center by the Bethesda assay (Nijmegen modification)17 before and 1 to 2 times weekly during ITI until a steady decline was observed. Testing was then performed 1 to 3 times monthly. In vivo FVIII recovery was performed once the inhibitor titer was negative for 2 consecutive months, by measuring FVIII level before and 15 to 30 minutes after pdFVIII/VWF infusion at 50 FVIII IU/kg without a washout period. After the in vivo FVIII recovery was > 66%, monitoring frequency was reduced to every 3 months.
The inhibitor tests before referral to the study center were performed at the local laboratories.
pdFVIII/VWF was administered at the local referral hospital or by home infusion, while rituximab was administered at the study center. Central venous access devices were not used because of the difficulties in their care.
2.5. Cost analysis
The cost of this low‐dose ITI strategy was calculated based on the domestic price of each treatment product in China from the start of ITI to the disappearance of inhibitors, and was compared with that of the high‐dose regimen reported by Hay et al,18 also based on cost in China.
2.6. Statistical analysis
Statistical analyses were performed with SPSS version 22.0 (IBM Corp). The type I error probability was 0.05. Kaplan‐Meier survival curves were used to estimate probabilities of inhibitor disappearance over time.
3. RESULTS
3.1. Patient characteristics
Sixteen boys with hemophilia A with median age 2.9 (range, 2.2‐13.2) years, 13 with severe and 3 with moderate disease, were enrolled in this pilot study. The median follow‐up period was 14.7 (range, 12.4‐22.6) months. Expressed in median (range), their estimated exposure days at inhibitor development were 18.0 (8.0‐75.0), time since inhibitor diagnosis to ITI initiation was 7.0 (0‐75.0) months, peak historical inhibitor titer was 33.7 (15.2‐256.0) BU, inhibitor titer in pre‐ITI was 30.7 (10.4‐128) BU, and peak inhibitor titer during ITI was 25.6 (6.5‐281.6) BU.
At ITI initiation, all 16 patients had pre‐ITI inhibitor titer ≥ 10 BU, 1 (6.3%) patient had peak historical titer ≥ 200 BU, 3 (18.8%) were ≥ 8 years of age, 1 (6.3%) was ≥ 5 years from inhibitor diagnosis to ITI initiation, and 1 (6.3%) failed ITI 5 months previously. During ITI, 4 patients (25%) had a peak inhibitor titer > 100 BU. Overall, all the patients had at least 1 poor‐risk factor (pre‐ITI inhibitor titer ≥ 10 BU) and 43.7% (7 of 16 patients) had 2 or more.
3.2. ITI outcomes
Table 1 summarizes the outcomes of ITI.
Table 1.
ITI outcomes
| Group | ITI‐alone | ITI‐ immunosuppression | |||
|---|---|---|---|---|---|
| Rituximab and prednisone | Prednisone | Total | |||
| Median (rangea) | >40 BU before ITI | >40 BU during ITI | |||
| N (%) | 7 (43.7) | 3 (33.3) | 4 (44.4) | 2 (22.2) | 9 (56.3) |
| Severity, N (%) | |||||
| Severe | 7 (100) | 2 (66.6) | 3 (75.0) | 1 (50.0) | 6 (66.7) |
| Moderate | 0 | 1 (33.3) | 1 (25.0) | 1 (50.0) | 3 (33.3) |
| Age at inhibitor development, y | 2.3 (0.6‐5.5) | 1.2 (1.1‐4.8) | 2.2 (1.2‐2.5) | 7.5 (6.9‐8.0) | 2.4 (1.1‐8.0) |
| Age at start of ITI, y | 2.8 (2.4‐8.8) | 5.7 (2.9‐6.8) | 2.8 (2.2‐2.9) | 10.8 (8.4, 13.2) | 2.9 (2.2‐13.2) |
| Time from inhibitor diagnosis to ITI started, mo | 8 (0‐39) | 25.0 (22.0‐50.0) | 2.5 (0‐4.0) | 40.5 (6.0‐75.0) | 6.0 (0‐75) |
| Peak historical inhibitor titer, BU | 23.0 (15.2‐54.0) | 96.0 (93.4‐256.0) | 49.5 (23.0‐70.0) | 29.7 (28.0‐31.4) | 64.0 (23.0‐256.0) |
| Pre‐ITI inhibitor titer, BU | 14.7 (10.4‐32.3) | 120.0 (100.0‐128.0) | 32.7 (30.0‐35.0) | 21.1 (10.8‐31.4) | 33.3 (10.8‐128.0) |
| Peak inhibitor titer during ITI, BU | 10.3 (6.5‐23.4) | 130.5 (83.2‐281.6) | 58.9 (40.0‐128.0) | 23.0 (18.1‐27.8) | 64.0 (18.1‐281.6) |
| Success, N (%) | 7 (100) | 1 (33.3) | 4 (100) | 1 (50) | 6 (66.7) |
| Time to success, mo | 8.5 (3.2‐11.8) | 4.6 | 10.2 (5.1‐11.3) | 5.1 | 10.2 (4.6‐11.3) |
| In vivo FVIII recovery > 66%, N (%) | 5 (71.4) | 1 (33.3) | 3 (75.0) | 1 (50) | 5 (55.6) |
| Time to in vivo FVIII recovery > 66%, N (%) | 10.6 (5.8‐12.7) | 13.2 | 13.1 (6.9‐13.6) | 6.5 | 13.2 (6.5‐13.6) |
| FVIII recovery, % | 81.1 (64.6‐93.9) | 74.0 | 69.7 (62.3‐76.0) | 91.0 | 72.5 (62.3‐91.0) |
| Partial Success, N (%) | 0 | 1 (33.3) | 0 | 0 | 1 (11.1) |
| Failure, N (%) | 0 | 1 (33.3) | 0 | 1 (50) | 2 (22.2) |
ITI, immune tolerance induction; FVIII, factor VIII.
Range = minimum‐maximum.
3.2.1. All patients
Of the 16 patients, 7 (43.7%) received pdFVIII/VWF alone (ITI‐alone group), and 9 (56.3%) received pdFVIII/VWF in combination with rituximab and/or prednisone (ITI‐immunosuppression group). The total response rate was 87.5% (14 of 16 patients), with success achieved in 81.3% (13 of 16 patients) in a median of 8.8 (range, 3.2‐11.8) months and partial success achieved in 6.3% (1 of 16 patients). Failure occurred in 12.5% (2 of 16 patients). Of the 13 patients who achieve success, 10 had in vivo FVIII recovery > 66% (median 75.5% [range, 66.7%‐93.9%]) in median 12.7 (range, 5.8‐13.6) months.
3.2.2. ITI‐alone group
All 7 patients (100%) achieved success in a median of 8.5 months, and 71.4% (5 of 7 patients) reached in vivo FVIII recovery > 66% (median, 81.1%) in a median of 10.6 months. Of the remaining 2 patients, 1 patient still had recovery < 66%, while another had not yet been tested at the time of data analysis.
3.2.3. ITI‐immunosuppression group
Nine patients received immunosuppression, of whom 7 received rituximab and prednisone, and 2 received prednisone alone. Success was achieved in 66.7% (6 of 9 patients) in a median of 10.2 months, partial success in 11.1% (1 of 9 patients), failure in 22.2% (2 of 9 patients). Of the 6 successes, 5 achieved FVIII recovery > 66% in a median of 13.2 months. The single partial success case reached an inhibitor titer < 5 BU in 3.9 months from 100 BU but remained partially tolerized for the next 10.2 months at the time of follow‐up. The ITI‐immunosuppression group could be further divided into 3 subgroups as indicated in the flowchart (Figure 1).
Figure 1.
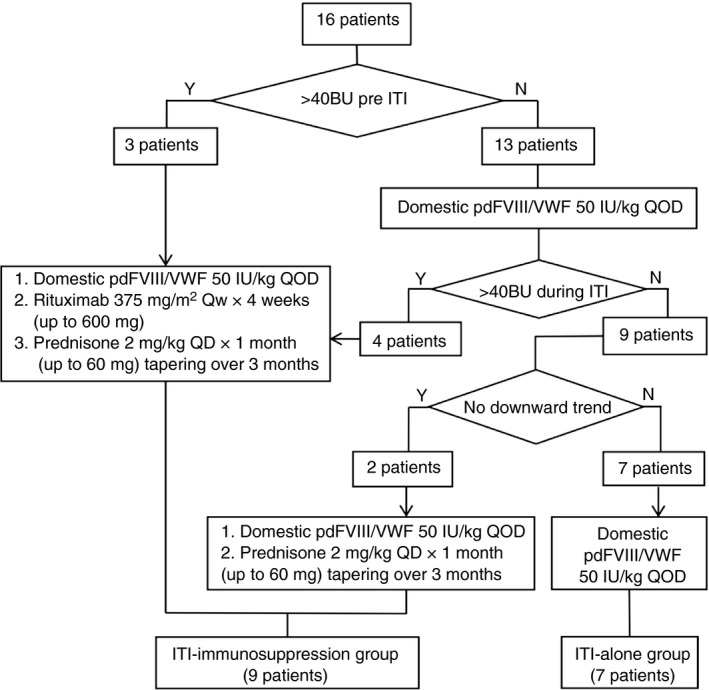
Flowchart of the cohort. ITI, immune tolerance induction; pdFVIII/VWF, plasma‐derived factor VIII/von Willebrand factor concentrate.
Subgroup 1 (pre‐ITI inhibitor titer > 40BU): Each of the 3 patients in this subgroup received rituximab and prednisone initially. Among them, 1 of 3 achieved success, 1 of 3 partial success, and 1 of 3 failure.
Subgroup 2 (pre‐ITI inhibitor titer < 40 BU but > 40BU during ITI): Four patients progressed into this subgroup from the ITI‐alone regimen. Rituximab and prednisone were added when the inhibitor titer was increased to > 40 BU during ITI. All 4 patients achieved success.
Thus, in total, of the 7 patients who received rituximab and prednisone, 5 (71.4%) achieved success in a median of 10.2 (range 4.6‐11.3) months, 1 (14.3%) achieved partial success, and 1 (14.3%) failed. Of the 5 successes, 4 achieved FVIII recovery > 66% in a median of 13.2 (range, 6.9‐13.6) months.
Subgroup 3 (inhibitors < 40BU but with no downward trend during ITI): There were 2 patients in this subgroup, and both of them received added prednisone alone. Of these, 1 achieved success and 1 failed.
Failure: In all, 2 patients failed ITI. Case 1 was a boy with severe hemophilia A whose inhibitor titer at the start of ITI was 281 BU. This gradually declined to 105 BU over a 6‐month period on ITI with added rituximab and prednisone. ITI was stopped when the inhibitor titer increased to 217 BU at the sixth month. Case 2 was a boy with moderate hemophilia A with a pre‐ITI titer of 31.4 BU. He was initially on an ITI‐alone regimen, and the inhibitors declined to 2.2 BU steadily in the first 4 weeks. The inhibitor titer then increased to 27.8 BU over the next 7 weeks. Prednisone was then added, and the inhibitor again declined and stabilized at < 2 BU. Unfortunately, the titer increased to > 5 BU at 14.3 months, and rituximab was then added. The inhibitor titer decreased but was still > 5 BU at the time of data analysis.
3.3. Bleeding control
In total, 72 bleeding episodes were reported in 14 of the 16 (87.5%) patients during the ITI period. The majority of bleeding episodes occurred in joints (50 of 72; 69.4%) before the disappearance of inhibitors. The overall bleeds/month did reduce markedly by 64.0% compared with the pre‐ITI period (mean, 1.6; median, 1.0 [range, 0.4‐5.3] vs. mean, 0.5; median, 0.4 [range, 0‐2.0]; P = 0.06; d = 0.90). Joint bleeds/month were also reduced by 64.3% (mean, 0.8; median, 0.4 [range, 0‐3.3] vs. mean, 0.3; median, 0.2 [range, 0‐1.4]; P = 0.19; d = 0.60). Bleeding episodes declined more dramatically in the first 3 months (P = 0.07; d = 0.84) than in the fourth month to time of negative inhibitor (P = 0.20; d = 0.29). Patients in the ITI‐alone and ITI‐immunosuppression groups had statistically similar overall bleeds/month (median, 0.1 [range, 0‐0.8] vs. median, 0.4 [range, 0.1‐2.0]; P = 0.20; d = –0.74) and joint bleeds/month (median, 0.1 [range, 0‐0.8] vs. median, 0.2 [range, 0‐1.4]; P = .52; d = –0.35).
3.4. Safety
Among the 7 patients who received rituximab, 1 experienced an allergic reaction with nausea and headache. The symptoms resolved with an antihistamine drug and rituximab could be resumed without further allergic reaction. Additionally, 6 upper respiratory tract infections and 1 case of diarrhea were reported during the first 6 months in 5 of 7 patients after rituximab injection. Severe adverse events were not observed.
3.5. Cost and consumption analysis (Table 2)
Table 2.
Cost of various ITI protocols (per kilogram of body weight) from ITI initiation to success (disappearance of inhibitors)
| Low‐dose ITI‐alone or Low‐dose ITI + prednisonea | Low‐dose ITI‐ immunosuppression (rituximab ± prednisone)a | High‐dose ITI18 (pdFVIII/VWF)b | High‐dose ITI18 (rFVIII) | |
|---|---|---|---|---|
| ITI regimen (FVIII IU/kg) | 50 QOD | 50 QOD | 100 Q12 h | 100 Q12 h |
| Median time to disappearance of inhibitors, mo | 8.5 | 10.2 | 4.6 | 4.6 |
| Cost of FVIII concentrate per ITI | ¥17 451 (US$2548) | ¥20 942 (US$3058) | ¥75 555 (US$11 031) | ¥137 118 (US$20 019) |
| Mean bleeds/month | 0.31 | 0.66 | 0.28 | 0.28 |
| PCC dose (IU/kg) × N doses per bleed | 50.0 × 2 doses | 50.0 × 2 doses | 85.0 × 2 dosesc , 30 | 85.0 × 2 dosesc , 30 |
| Cost of PCC per ITI | ¥343 (US$50) | ¥875 (US$128) | ¥285 (US$42) | ¥285 (US$42) |
| Cost of immunosuppression per ITI | ‐ | ¥1050 (US$153) | ‐ | ‐ |
| Total cost per kilogram per ITI | ¥17 794 (US$2598) | ¥22 867 (US$3338) | ¥75 840 (US$11 073) | ¥137 403 (US$20 061) |
aPCC, activated prothrombin complex concentrate; FVIII, factor VIII; ITI, immune tolerance induction; PCC, prothrombin complex concentrate; pdFVIII/VWF, plasma‐derived factor VIII/von Willebrand factor concentrate; rFVIII, recombinant factor VIII. Currency conversion rate: Chinese RMB, ¥100 = US $14.6; cost calculation based on Chinese domestic price of therapeutic agents: pdFVIII/VWF, ¥2.7/IU; rFVIII, ¥4.9/IU; PCC, ¥1.3/IU; rituximab, ¥20.0/mg; prednisone, ¥0.003/mg. Cost calculation per ITI course to success (inhibitor titer < 0.6 BU) = Median number of ITI days(n) × Unit or milligram therapeutic agent(s) cost × Units or milligram per kilogram per dose × Number of doses over the ITI period.
aFor ITI‐immunosuppression, only cost of ITI adding rituximab throughout the ITI course is shown, as the contribution cost of prednisone (¥0.003/mg) was negligible. Thus, the cost of ITI in combination with rituximab and prednisone would be similar to the cost of ITI with rituximab, and the cost of ITI with prednisone will be similar to the cost of ITI‐alone.
bOriginal protocol of Hay et al18 allowed the use of either pdFVIII/VWF or rFVIII – calculation here are performed separately for pdFVIII/VWF and for rFVIII.
cDose based on aPCC, price based on Chinese domestic PCC.
Cost was calculated on the basis of the domestic price of all treatment products in terms of consumption per kilogram of body weight during the ITI period from initiation to success. The average cost (per kilogram of body weight) was ¥17 794 (US$2598) for the ITI‐alone group, and ¥22 867 (US$3338) for the ITI‐ immunosuppression group. Among the expenditure, pdFVIII/VWF accounted for 91.5% to 98.1%, rituximab and prednisone accounted for 4.6%, and PCC bypassing treatment for bleeds accounted for 1.9%‐3.8%. No patients used rFVIIa in this study.
We also compared the cost of our low‐dose ITI/immunosuppression strategy with a high‐dose regimen (200 IU/kg/day) according to the data presented in the international ITI study by Hay et al.18 The expenditure using our regimen was lower by 70% (based on domestic pdFVIII/VWF usage) and by 87% (based on rFVIII usage in China).
4. DISCUSSION
This study reports the use of a low‐dose ITI (pdFVIII/VWF) +/− immunosuppression strategy to eliminate high‐titer inhibitors in patients with hemophilia A having poor‐risk status. This strategy achieved a success rate of 81.3%, which is in line with the reported success ranges of 60% to 90% for patients with high‐titer inhibitors in the literature.19
The randomized dose comparison of the international ITI study18 concluded that success rates were similar between low‐dose and high‐dose regimens. However, low‐dose ITI took longer and had a significantly higher bleeding rate, and as a result the study was stopped early. In this study, all patients had inhibitor titer < 10 BU at ITI initiation, although their peak historical inhibitor titer could be between ≥ 5 and 200 BU. Using our regimen, our results appear to be in keeping with those low‐dose arm in the international ITI study with a similar time to negative inhibitor (median, 8.8 vs. 9.2 months) and similar monthly bleeding rate (mean, 0.5 vs. 0.6 times). Our findings suggested that this strategy was effective in patients with poor‐risk status. However, compared to the international ITI study high‐dose regimen, 18 the bleeding rate was higher (mean, 0.5 vs. 0.3), and the time taken for our patients to achieve complete tolerance was longer (median, 8.8 vs. 4.6 months); therefore, our low‐dose regimen is expected to have experienced more breakthrough bleeds.
There are studies in the literature using a low‐dose regimen without immunosuppression in patients having poor‐risk status. A Turkish study20 showed only 26% achieving success (half‐life > 6 hours). Early Egyptian study results21 suggested that patients with pre‐ITI inhibitor titer < 30 BU usually had a good response. The success rate in patients with pre‐ITI inhibitor titer > 40 BU was lower, at 66%. A Netherlands study22 also showed poorer response in patients with inhibitor titer > 40 BU, succeeding in only 1 of 4 patients. These results suggest that regimens with low‐dose ITI alone without immunosuppression would not perform as well in those patients having poor‐risk status. Without additional immunosuppression, these patients will need the higher‐dose strategy for better success.
The potential approaches to improve success rates include increasing FVIII dose, changing to pdFVIII/VWF product, adding immunosuppression or use of rFVIIIFc.3, 5, 23, 24 In China, pdFVIII/VWF and immunosuppression are the better choice for economic reasons. An earlier study showed that negative inhibitor could be achieved in 71.4% in poor‐risk status patient undergoing ITI using pdFVIII/VWF alone.25 Among immunosuppressive agents, rituximab has previously been shown to give a beneficial response rate of 58% in patients who failed first‐line ITI,8 and prednisone is inexpensive and readily accessible.26, 27 Our patients clearly benefited from the ITI regimen with pdFVIII/VWF ± immunosuppression using prednisone alone or in combination with rituximab depending on inhibitor titer parameters, with almost 80% having their inhibitors eliminated overall and a decrease in bleeding rate. In addition to rituximab and prednisone, use of other immunosuppressive agents such as intravenous immunoglobulin and mycophenolate mofetil5 had been reported. Other new drugs targeting T cells (rapamycin) and plasma cells (bortezomib) apparently showed effectiveness in preventing inhibitors formation.28 Ultimately, how rituximab and prednisone will compare in effectiveness with other immunosuppressive agent(s) and what the best agent(s) is to prevent inhibitors formation, and to eliminate them once formed, will need further studies. On the other hand, there are studies suggesting that remissions achieved through the use of immunosuppressants may have a higher likelihood to recur.8, 29 The follow‐up period in our study was too short to allow determination of recurrences likelihood. A much longer follow‐up time is needed.
High‐dose ITI is expensive and is not readily affordable for the majority of patients in China, who have limited health insurance in the current health care system. In comparison, our low‐dose ITI with immunosuppression strategy is much more affordable and enables ITI therapy to be carried out, as its cost is reduced by 70% to 87%. The monthly bleeding rate of our low‐dose ITI strategy is higher than that using high‐dose FVIII,18 together with a longer time period to success (disappearance of inhibitors); thus, the number of breakthrough bleeding will be higher before tolerance in our patients with poor‐risk status compared to patients using high‐dose ITI according to Hay et al.18 Our regimen of low‐dose ITI using pdFVIII/VWF with added immunosuppression based on inhibitor titer parameters may also be useful for other regions with economic constraint.
4.1. Limitations
There are a number of limitations in this study. We defined success as achieving a negative inhibitor titer (<0.6 BU), but the inhibitor assay was performed without a washout period, so a low inhibitor titer could have been missed. The patients were mostly referred from other centers and had no knowledge of their FVIII pharmacokinetics before inhibitor development. During ITI, in vivo FVIII recovery results obtained were based on recovery of what was infused and could not be translated into percentage of the original recovery (before inhibitor development) for the individual patient. Also, our inability to carry out FVIII half‐life determination regularly also put us at a disadvantage, with inability to use laboratory data to precisely determine the time of complete tolerance. Bias could also have been introduced given that the original diagnosis of inhibitor in most of our patients were made elsewhere in the referring center. We had to depend on what information was made available to us or on recalls of patient families. As a pilot study, the cohort size was relatively small and the follow‐up period was short. A longer follow‐up time is needed to allow the determination of final response and to document recurrences.
5. CONCLUSION
This low‐dose ITI strategy using pdFVIII/VWF +/− immunosuppression achieved a relatively satisfactory success rate in boys with hemophilia A having poor‐risk status for ITI success. The regimen costs less compared to that of high‐dose ITI and therefore enabled ITI therapy to be carried out in a developing country. However, further evaluations in larger cohorts with longer follow‐up time is needed, especially to clarify the role of immunosuppression.
6. RELATIONSHIP DISCLOSURE
The authors report nothing to disclose.
ACKNOWLEDGMENTS
RW initiated the study and wrote the manuscript. ZLI performed the research, analyzed the data, and wrote the manuscript. ZC, XC, XW, GL, and YZ contributed to the study performance. SC provided data analysis. M‐CP provided input on study design and critical review. This work was supported by grants from Capital Health Development Research Project (No. Capital Development 2018‐2‐2094), Beijing Natural Science Foundation of China (No. 7162059), Beijing Municipal Science and Technology Commission (code Z181100001718182), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding (code ZY201404).
Li Z, Chen Z, Cheng X, et al. Low‐dose immune tolerance induction for children with hemophilia A with poor‐risk high‐titer inhibitors: A pilot study in China. Res Pract Thromb Haemost. 2019;3:741–748. 10.1002/rth2.12248
REFERENCES
- 1. Astermark J, Morado M, Rocino A, Van den Berg HM, Von Depka M, Gringeri A, et al. Current European practice in immune tolerance induction therapy in patients with haemophilia and inhibitors. Haemophilia. 2006;12:363–71. [DOI] [PubMed] [Google Scholar]
- 2. Wei QQ, Li G, Tang L, Chen ZP, Zhen YZ, Wu XY, et al. A cross‐sectional survey of coagulation factor VIII inhibitor in children with hemophilia A. Chin J Pediatr. 2014;52:99–102. [PubMed] [Google Scholar]
- 3. Collins PW, Chalmers E, Hart DP, Liesner R, Rangarajan S, Talks K, et al. Doctors UKHC. Diagnosis and treatment of factor VIII and IX inhibitors in congenital haemophilia: UK Haemophilia Centre Doctors Organization. Br J Haematol. 2013;160:153–70. [DOI] [PubMed] [Google Scholar]
- 4. Collins P, Chalmers E, Alamelu J, Hay C, Liesner R, Makris M, et al. First‐line immune tolerance induction for children with severe haemophilia A: a protocol from the UK Haemophilia Centre Doctors’ Organisation Inhibitor and Paediatric Working Parties. Haemophilia. 2017;23:654–9. [DOI] [PubMed] [Google Scholar]
- 5. Valentino LA, Kempton CL, Kruse‐Jarres R, Mathew P, Meeks SL, Reiss UM, et al. US Guidelines for immune tolerance induction in patients with haemophilia A and inhibitors. Haemophilia. 2015;21:559–67. [DOI] [PubMed] [Google Scholar]
- 6. Gringeri A. VWF/FVIII concentrates in high‐risk immunotolerance: the RESIST study. Haemophilia. 2007;13(Suppl 5):73–7. [DOI] [PubMed] [Google Scholar]
- 7. Kurth MA, Dimichele D, Sexauer C, Sanders JM, Torres M, Zappa SC, et al. Immune tolerance therapy utilizing factor VIII/von Willebrand factor concentrate in haemophilia A patients with high titre factor VIII inhibitors. Haemophilia. 2008;14:50–5. [DOI] [PubMed] [Google Scholar]
- 8. Collins PW, Mathias M, Hanley J, Keeling D, Keenan R, Laffan M, et al. Liesner R, Organisation UKHCD. Rituximab and immune tolerance in severe hemophilia A: a consecutive national cohort. J Thromb Haemost. 2009;7:787–94. [DOI] [PubMed] [Google Scholar]
- 9. Fox RA, Neufeld EJ, Bennett CM. Rituximab for adolescents with haemophilia and high titre inhibitors. Haemophilia. 2006;12:218–22. [DOI] [PubMed] [Google Scholar]
- 10. Moschovi M, Aronis S, Trimis G, Platokouki H, Salavoura K, Tzortzatou‐Stathopoulou F. Rituximab in the treatment of high responding inhibitors in severe haemophilia A. Haemophilia. 2006;12:95–9. [DOI] [PubMed] [Google Scholar]
- 11. Leissinger CA. Advances in the clinical management of inhibitors in hemophilia A and B. Semin Hematol. 2016;53:20–7. [DOI] [PubMed] [Google Scholar]
- 12. Nakar C, Manco‐Johnson MJ, Lail A, Donfield S, Maahs J, Chong Y, et al. Prompt immune tolerance induction at inhibitor diagnosis regardless of titre may increase overall success in haemophilia A complicated by inhibitors: experience of two US centres. Haemophilia. 2015;21:365–73. [DOI] [PubMed] [Google Scholar]
- 13. Kaplan B, Kopyltsova Y, Khokhar A, Lam F, Bonagura V. Rituximab and immune deficiency: case series and review of the literature. J Allergy Clin Immunol Pract. 2014;2:594–600. [DOI] [PubMed] [Google Scholar]
- 14. Barmettler S, Ong MS, Farmer JR, Choi H, Walter J. Association of immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA Netw Open. 2018;1:e184169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cao H, Lin F, Li C, Shi X, Qin L, Yuan J. Comparison of several human prothrombin complex products. The 7th Blood Transfusion Conference of the 2014 China Blood Transfusion Association. Wu Han, China, 2014, 287‐96.
- 16. Astermark J, Donfield SM, DiMichele DM, Gringeri A, Gilbert SA, Waters J, et al. A randomized comparison of bypassing agents in hemophilia complicated by an inhibitor: the FEIBA NovoSeven Comparative (FENOC) Study. Blood. 2007;109:546–51. [DOI] [PubMed] [Google Scholar]
- 17. Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg M, Mauser‐Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII: C inhibitors: improved specificity and reliability. Thromb Haemost. 1995;73:247–51. [PubMed] [Google Scholar]
- 18. Hay CR, DiMichele DM. International Immune Tolerance S. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood. 2012;119:1335–44. [DOI] [PubMed] [Google Scholar]
- 19. Brackmann HH, White GC 2nd, Berntorp E, Andersen T, Escuriola‐Ettingshausen C. Immune tolerance induction: what have we learned over time? Haemophilia. 2018;24(Suppl 3):3–14. [DOI] [PubMed] [Google Scholar]
- 20. Unuvar A, Kavakli K, Baytan B, Kazanci E, Sayli T, Oren H, et al. Low‐dose immune tolerance induction for paediatric haemophilia patients with factor VIII inhibitors. Haemophilia. 2008;14:315–22. [DOI] [PubMed] [Google Scholar]
- 21. ElAlfy MS, Tantawy AA, Ahmed MH, Abdin IA. Frequency of inhibitor development in severe haemophilia A children treated with cryoprecipitate and low‐dose immune tolerance induction. Haemophilia. 2000;6:635–8. [DOI] [PubMed] [Google Scholar]
- 22. Ter Avest PC, Fischer K, Gouw SC, Van Dijk K, Mauser‐Bunschoten EP. Successful low dose immune tolerance induction in severe haemophilia A with inhibitors below 40 Bethesda units. Haemophilia. 2010;16:71–9. [DOI] [PubMed] [Google Scholar]
- 23. DiMichele DM, Hoots WK, Pipe SW, Rivard GE, Santagostino E. International workshop on immune tolerance induction: consensus recommendations. Haemophilia. 2007;13(Suppl 1):1–22. [DOI] [PubMed] [Google Scholar]
- 24. Carcao M, Shapiro A, Hwang N, Pipe S, Ahuja S, Lieuw K, et al. Real‐world data of immune tolerance induction using rFVIIIFc in subjects with severe hemophilia A with inhibitors at high risk for ITI failure. 60th American Society of Hematology Annual Meeting & Exposition. San Diego, CA; 2018.
- 25. Kreuz W, Escuriola EC, Vdovin V, Zozulya N, Plyushch O, Svirin P, et al. First prospective report on immune tolerance in poor risk haemophilia A inhibitor patients with a single factor VIII/von Willebrand factor concentrate in an observational immune tolerance induction study. Haemophilia. 2016;22:87–95. [DOI] [PubMed] [Google Scholar]
- 26. Aznar JA, Jorquera JI, Peiro A, Garcia I. The importance of corticoids added to continued treatment with factor VIII concentrates in the suppression of inhibitors in haemophilia A. Thromb Haemost. 1984;51:217–21. [PubMed] [Google Scholar]
- 27. Gruppo RA, Valdez LP, Stout RD. Induction of immune tolerance in patients with hemophilia A and inhibitors. Am J Pediatr Hematol Oncol. 1992;14:82–7. [DOI] [PubMed] [Google Scholar]
- 28. Schep SJ, Schutgens REG, Fischer K, Boes ML. Review of immune tolerance induction in hemophilia A. Blood Rev. 2018;32:326–38. [DOI] [PubMed] [Google Scholar]
- 29. Antun A, Monahan PE, Manco‐Johnson MJ, Callaghan MU, Kanin M, Knoll C, et al. Inhibitor recurrence after immune tolerance induction: a multicenter retrospective cohort study. J Thromb Haemost. 2015;13:1980–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kenet G, Oladapo A, Epstein JD, Thompson C, Novack A, Nugent DJ. Estimating the potential cost of a high dose immune tolerance induction (ITI) therapy relative to the cost of a combined therapy of a low dose ITI therapy with bypassing agent prophylaxis. Haemophilia. 2017;23:e394–402. [DOI] [PubMed] [Google Scholar]


