Abstract
Neuropsychiatric and muscular symptoms can develop as part of hypothyroidism. However, frank psychosis or rhabdomyolysis due to hypothyroidism are uncommon and have been reported rarely as the first presenting features of hypothyroidism. We report a case of a 44-year-old man who presented with a 2-week history of delusions, hallucinations and mild bilateral leg pain, without apparent signs of myxedema. Investigations revealed raised thyroid stimulation hormone >100 mIU/L and high creatine kinase >21 000 U/L. Diagnosis of hypothyroidism-induced psychosis and rhabdomyolysis was made. He received thyroxine, olanzapine and a short course of steroids. His symptoms improved after 2 weeks of treatment and he remained free of symptoms at 6 months of follow-up. To the best of our knowledge, this is the first case of concomitant psychosis and rhabdomyolysis leading to hypothyroidism diagnosis. This case highlights the importance of hypothyroidism screening when faced with unexplained psychosis or rhabdomyolysis, especially if combined.
Keywords: psychotic disorders (incl schizophrenia), thyroid disease, psychiatry, endocrinology, endocrine system
Background
Hypothyroidism is a common disease, with an estimated prevalence of 3.6%;1 associated neuropsychiatric features,2 and elevated muscle enzymes are known and reported.3 However, it is unusual that hypothyroidism leads to frank psychosis or clinical rhabdomyolysis. Moreover, the first presentation of hypothyroidism as psychosis or clinical rhabdomyolysis is rather rare and has been reported only a few times in literature.4 5 Here we report a case of a middle-aged man presenting with myxedema psychosis and rhabdomyolysis leading to hypothyroidism diagnosis. This combination is extremely rare and, to our knowledge, has not been reported as the first presenting feature of hypothyroidism. The diagnosis can be simply overlooked if not accounted for when faced with unexplained psychosis or rhabdomyolysis.
Case presentation
We report a case of a 44-year-old man, previously healthy, brought by ambulance to the emergency department after an episode of abnormal behaviour. He was praying at a mosque when he started acting strangely and repeating words, as per his friends. Shortly after presenting to the emergency, he started biting his left index finger, stating that the devil had instructed him to do so and otherwise, he would die. According to his friends, he had been isolating himself for around 2 weeks prior to the presentation. When questioned, he stated that he heard strange voices and saw strange people and was concerned that one of his friends was trying to harm him (auditory and visual hallucinations with persecutory delusions). Upon reviewing other systems, he mentioned that he had been feeling a bit cold for the past 1–2 months, had generalized body pain, mostly in the lower limbs, decreased bowel movement and depressed mood. He denied any history of suicidal tendencies, headache, fever, neck stiffness, joint pains, previous similar complaints, loss of consciousness, voice change or weight change. He also denied alcohol intake or illicit drug use.
During examination, he was initially agitated, not answering questions and trying to bite his finger. He calmed down after receiving a benzodiazepine injection. When assessed later, he was conscious, oriented to time, place, and person and was responding properly to questions. His blood pressure measured 136/81 mm Hg, heart rate 98 bpm, with normal temperature and oxygen saturation. His physical examination was unremarkable except for the bite wound on his left index finger, and mild muscle tenderness and weakness in lower limbs. However, he had no clinical signs of myxedema or thyromegaly and his cardiovascular, chest and abdominal examination were insignificant.
Investigations
Laboratory investigations (table 1) revealed low serum sodium, raised creatinine, markedly elevated thyroid stimulating hormone (TSH) >100 mIU/L (0.3–4.2 mIU/L), anti-thyroid peroxidase (TPO) initially negative at 31 and repeat level of 38 IU/ml, just above the reference range (<36 IU/mL) with negative anti-thyroglobulin antibody. Creatine kinase (CK) was strikingly high at >21 000 U/L (39–308 U/L) and urine dipstick was positive for blood (suggestive of myoglobinuria). Alanine aminotransferase (ALT) was 45 U/L (0–41 U/L) and aspartate aminotransferase 81 U/L (0–40 U/L). He had dyslipidemia with elevated low-density lipoprotein (LDL) 5.2 mmol/L (3.3 mmol/L). Antinuclear antibody and antismooth muscle antibody were negative, lumbar puncture was performed with difficulty in the sitting position, cerebrospinal fluid (CSF) analysis was remarkable for protein level of of 0.76 gm/L (0–0.45 g/L) and red blood cell level of 175 cells/µL (0–2 cells/µL) which was likely traumatic, and otherwise, normal white blood cells of 1cells/µL (0–5cells/µL). Brain MRI and electroencephalogram (EEG) were unremarkable.
Table 1.
Patient’s significant laboratory tests
| Laboratory values | Day 0 | Day 7 | Day 13 | Day 25 | 6 months |
| Haemoglobin (130-170 g/L) |
134 | 145 | NA | NA | 137 |
| Creatinine (62–106 umol/L) |
128 | 91 | 87 | 88 | 86 |
| Sodium (136–145 mmol/L) |
131 | 136 | 141 | 140 | 141 |
| Total cholesterol (<5.2 mmol/L) | 6.7 | NA | 5.4 | NA | 4.5 |
| Low-density lipoprotein (<3.36 mmol/L) |
5.2 | NA | 2.8 | NA | 2.6 |
| Thyroid stimulating hormone (0.3–4.2 mIU/L) |
>100 | NA | NA | 4.46 | 2.36 |
| Tetraiodothyronine (11.6–21.9 pmol/L) |
1.6 | NA | NA | 16.9 | 15.8 |
| Creatine kinase * (39–308 U/L) |
18 963 | 10 763 | 1877 | NA | NA |
| Anti-thyroid peroxidase (positive >35 IU/mL) | 31 | 38 | NA | NA | NA |
| Anti-thyroglobulin Ab (positive>60 IU/mL) | NA | 29 | NA | NA | NA |
| Cerebrospinal fluid (CSF) analysis shown below: | |||||
| White blood count (0–5 cells/uL) | 1 | NA | NA | NA | NA |
| Red blood cell count (0–2 cells /uL) | 175 | NA | NA | NA | NA |
| Glucose (2.22–3.89 mmol/L) |
4.81 | NA | NA | NA | NA |
| Protein (0.15–0.45 gm/L) |
0.76 | NA | NA | NA | NA |
| Tuberculosis PCR | Negative | NA | NA | NA | NA |
| Cerebrospinal fluid culture | Negative | NA | NA | NA | NA |
| Viral PCR† | Negative | NA | NA | NA | NA |
*Highest value of creatine kinase was obtained on the second day and was 21 356 U/L.
†Viruses tested by PCR: adenovirus, EBV, HSV1, HSV2, VZV, mumps, parecho, enterovirus.
NA, not applicable; the test was not done or not repeated.
Differential diagnosis
Even though the case was a diagnostic challenge, hypothyroid-related psychosis remained the most probable diagnosis, given the onset of psychosis coexisting with other mild symptoms of hypothyroidism. Also the presence of active hypothyroidism was evidenced by very high TSH, and associated rhabdomyolysis. Moreover, equivocal levels of anti-TPO, and the absence of significant MRI, EEG or CSF changes. Another differential was a primary psychiatric illness which could be ascertained only by follow-up in case of relapse on discontinuing antipsychotics in the presence of normal thyroid hormones. Hashimoto’s encephalopathy (HE), an entity that should be considered in the differential diagnosis of patients with autoimmune hypothyroidism and neuropsychiatric features, is usually associated with high TPO antibodies titre and euthyroid status with EEG abnormalities; so HE was deemed less likely.
Treatment
We started the patient on thyroxine, 300 mcg once daily (later decreased to 100 mcg once daily), augmentin for bite wound and intravenous fluids for rhabdomyolysis. The patient was improving on initial treatment (thyroxine); however, he unexpectedly absconded on day five of hospitalisation. Fortunately, we readmitted him the next day. The on-call team decided to start him on intravenous steroids (hydrocortisone 100 mg 6 hourly, for 2 days). The endocrine team decided to add a 2-week course of prednisolone (40 mg) followed by a taper to treat a possible HE. He was also started on olanzapine 10 mg and escitalopram 10 mg by the psychiatry team. We transferred him to the mental care hospital for further monitoring.
Outcome and follow-up
After 2 weeks of his initial presentation, the patient’s symptoms completely improved and he denied residual delusions, hallucinations, constipation or muscle pain. The following laboratory values showed improvement: CK (figure 1), lipid profile, liver enzymes, creatinine, calcium and sodium levels. As a result, his olanzapine dose was reduced to 5 mg, he was observed in the mental care hospital for 10 more days after recovery, and discharged safely with no signs of psychosis or mood disturbance. The patient was asked to discontinue olanzapine on follow-up. The patient travelled to his home country where he continued olanzapine 5 mg daily, thyroxine 100 mcg and escitalopram 10 mg. He returned after 5 months and visited the psychiatry clinic where olanzapine was stopped given that he had recovered fully, with a plan of tapering escitalopram later on. We saw the patient 1 month after the psychiatric appointment. He was still free of symptoms and not on antipsychotics. Medication adherence was instructed. The patient will continue to follow-up with psychiatry and endocrinology clinics.
Figure 1.
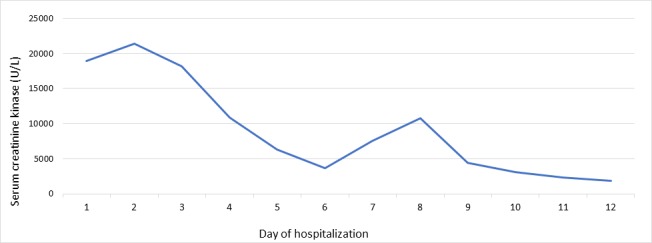
Serum creatine kinase (CK) level during the patient’s hospitalisation. *The patient absconded on day five and did not take his thyroid treatment, which may explain the rise in CK in the following days, followed by a drop. *CK level was measured until day 12 of hospitalisation, and no further values could be obtained.
Discussion
Prof Asher first described myxedema psychosis, also known as myxedema madness, in 1949 in BMJ.6 He described 14 cases presenting with psychotic changes that he diagnosed as cases of hypothyroidism and after treatment, nine of them showed complete recovery. Prof Asher’s cases had morphological evidence of myxedema. He stated that persecutory delusions,6 as the case with our patient, were common in this population of patients. We do not know whether any of those patients had HE as, at that time, this entity was not known and the diagnostic tests were not available. This, in part, may explain the partial or non-response of the remaining patients. Other possible causes for non-response to thyroid treatment are coexisting primary psychiatric disease or a long-standing disease where chronic metabolic changes have led to irreversible brain damage; Azzopardi et al also described the latter.7
The mechanism of hypothyroid-induced neuropsychiatric manifestations is unclear in humans. However, we learn from studies performed on rats that triiodothyronine (T3) receptors concentrate heavily in the amygdala and the hippocampus.8 The amygdala plays a crucial role in emotional and behavioural integration.9 Also, there is a significant increase in the transformation rate of thyroxine to T3 in cases of hypothyroidism.10 Moreover, hypothyroidism is associated with increased cerebral neurotransmitter dopamine and adrenal catecholamine activities11; all these may be responsible for the neuropsychiatric changes associated with thyroid hormone hemostasis abnormalities.
Neuropsychiatric manifestations may be the first presenting features of hypothyroidism as highlighted by our case,4 12–16 and the diagnosis may be simply overlooked. Hockings CA et al also described the case of an adolescent who presented with psychiatric manifestations as the first presentation of hypothyroidism12. The author suggested having a low threshold for testing thyroid function, not only in adults but also in children and adolescents presenting with unexplained psychosis.
Myxedema psychosis can respond as quickly as within days to a few weeks to thyroxine replacement.6 12–14 16–18 Some physicians use anti-psychotic treatment for a short period to control the psychosis symptoms at the beginning of the illness; these medications can be safely discontinued on follow-up after ensuring that there is no recurrence of symptoms.17
HE is another entity that is difficult to distinguish from myxedema psychosis; it is presumed to be an autoimmune phenomenon that is not directly related to the thyroid hormone level.19Patients with HE are usually euthyroid, having CSF, EEG and in some cases, head imaging changes. Also, patients with HE tend to have high titres of anti-TPO, depicting autoimmunity of the condition.19 20 So, the rapid response to treatment (even before starting steroids), the absence of significant EEG and MRI changes, a low titre of anti-thyroid peroxidase antibodies and the presence of active hypothyroidism as evident from the TSH level and rhabdomyolysis, makes HE less likely as a diagnosis in our patient; however, it cannot be excluded. We would reconsider HE if our patient had a recurrence of his psychosis, especially with normal thyroid hormone level.
Another exciting feature of our case is the presence of concomitant psychosis and rhabdomyolysis, with a high level of CK reaching up to 21 365 U/L and a mild acute kidney injury, both improved on treatment. Only a few cases reported where hypothyroidism alone caused rhabdomyolysis without other inciting factors. The level of CK varied in these cases but mostly at around 10 000 U/L or less; CK level was <6000 U/L in six of the cases, <11 000U/L in another two cases, only one case had a strikingly high CK level of 80 730U/L, and that patient had significant concomitant myoedema.5 21–29
The mechanism of hypothyroid-induced myopathy is thought to be due to the following changes associated with hypothyroidism: impaired muscle metabolism,30 decreased fast-twitching type 2 muscle fibres, reduced adenosine triphosphatase activity,31 low muscle carnitine level,32 and the recently found lower level of irisin in hypothyroid patients and its inverse correlation with TSH and CK levels.33 All these changes may explain in part the myopathic changes, but do not precisely define the mechanism of muscle lysis; the definite cause is yet to be defined by studies.
To our knowledge, this is the first case where the first presenting features of hypothyroidism are psychosis coupled with significant rhabdomyolysis, and a very high CK level; these two entities are uncommon even alone. We stress that physicians should screen for hypothyroidism when faced with the first episode of psychosis agreeing with current guidance,34 and also when dealing with unexplained rhabdomyolysis or myopathy. The combination of psychosis and rhabdomyolysis warrants hypothyroid screening even if no other features of hypothyroidism prevail.
Patient’s perspective.
I remember seeing all of the doctors at the hospital, thanks for your care. I don’t recall much from what happened, but what I know is that I am fine now. I have spent the past 4 months with the family at my home country, and I used to take the medications as prescribed and followed with a physician over there.
When instructed about the importance of adherence to thyroid hormone replacement and follow-up he responded saying I did not know that thyroid gland problem can cause so many troubles, I will take the medications, and I will not stop them, and I will follow with the doctors surely, thanks for everything.
Learning points.
Hypothyroidism can present with psychotic features, and/or rhabdomyolysis; the diagnosis can be simply overlooked especially when there is no prior history of hypothyroidism.
The condition is treatable and largely reversible, especially when the treatment is initiated early; hence, screening is important.
Physicians should screen for hypothyroidism when faced with first time psychosis even in the absence of hypothyroidism history or other clinical evidence of hypothyroidism.
The treatment is appropriate thyroid hormone replacement with or without antipsychotics in the initial period until psychotic symptoms abate and thyroid hormones normalise.
Rhabdomyolysis with no apparent reason should also prompt screening for thyroid dysfunction, especially when it is coupled with psychotic symptoms.
Footnotes
Contributors: MFHM, ABM, SS and A-NE were all involved in the patient’s care, MFHM viewed the importance of the case and formed the research team. SS and MFHM obtained consent from the patient and followed him up. MFHM wrote the initial manuscript. ABM, SS and A-NE reviewed the manuscript, helped in preparing the updated final version, and approved it before submission. ABM constructed the figure and table. MMFH submitted the final version of the case to BMJ.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Aoki Y, Belin RM, Clickner R, et al. . Serum TSH and Total T 4 in the United States Population and Their Association With Participant Characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002). Thyroid 2007;17:1211–23. 10.1089/thy.2006.0235 [DOI] [PubMed] [Google Scholar]
- 2. Constant EL, Adam S, Seron X, et al. . Anxiety and depression, attention, and executive functions in hypothyroidism. J Int Neuropsychol Soc 2005;11:535–44. 10.1017/S1355617705050642 [DOI] [PubMed] [Google Scholar]
- 3. Hekimsoy Z, Kavalali Oktem I. Serum Creatine Kinase Levels in Overt and Subclinical Hypothyroidism. Endocr Res 2005;31:171–5. 10.1080/07435800500371706 [DOI] [PubMed] [Google Scholar]
- 4. Pomeranze J, King EJ. Psychosis as first sign of thyroid dysfunction. Geriatrics 1966;21:211–2. [PubMed] [Google Scholar]
- 5. Rabhi M, Chaari J, Toloune F. Rhabdomyolysis disclosing hypothyroidism. Eur J Intern Med 2006;17:220 10.1016/j.ejim.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 6. Asher R. Myxoedematous Madness. BMJ 1949;2:555–62. 10.1136/bmj.2.4627.555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Azzopardi L, Murfin C, Sharda A, et al. . Myxoedema madness. BMJ Case Rep 2010;2010:bcr0320102841 10.1136/bcr.03.2010.2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ruel J, Faure R, Dussault JH, et al. . Regional distribution of nuclear T3 receptors in rat brain and evidence for preferential localization in neurons1. J Endocrinol Invest 1985;8:343–8. 10.1007/BF03348511 [DOI] [PubMed] [Google Scholar]
- 9. Rasia-Filho AA, Londero RG, Achaval M. Functional activities of the amygdala: an overview. J Psychiatry Neurosci 2000;25:14–23. [PMC free article] [PubMed] [Google Scholar]
- 10. Dratman MB, Crutchfield FL, Gordon JT, et al. . Iodothyronine homeostasis in rat brain during hypo- and hyperthyroidism. Am J Physiol 1983;245:E185–E193. 10.1152/ajpendo.1983.245.2.E185 [DOI] [PubMed] [Google Scholar]
- 11. Sato T, Imura E, Murata A, et al. . Thyroid hormone-catecholamine interrelationship during cold acclimation in rats. Compensatory role of catecholamine for altered thyroid states. Acta Endocrinol 1986;113:536–42. 10.1530/acta.0.1130536 [DOI] [PubMed] [Google Scholar]
- 12. Hockings CA, Cattaneo E, Sanka SK, et al. . Auditory hallucinations in a 15-year-old boy: an unusual presentation of hypothyroidism. Case Reports 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mavroson MM, Patel N, Akker E. Myxedema Psychosis in a Patient With Undiagnosed Hashimoto Thyroiditis. J Am Osteopath Assoc 2017;117:50 10.7556/jaoa.2017.007 [DOI] [PubMed] [Google Scholar]
- 14. Gopinathan MV, James E. Hypothyroidism Induced Myxedema Madness and Hyponatremia: A Case Report. J Appl Pharm Sci 2017;7:206–8. [Google Scholar]
- 15. Ueno S, Tsuboi S, Fujimaki M, et al. . Acute psychosis as an initial manifestation of hypothyroidism: a case report. J Med Case Rep 2015;9:264 10.1186/s13256-015-0744-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nazou M, Parlapani Ε, Nazlidou E-I, et al. . Psychotic episode due to Hashimoto’s thyroiditis. Psychiatriki 2016;27:144–7. 10.22365/jpsych.2016.272.144 [DOI] [PubMed] [Google Scholar]
- 17. Morosán Allo YJ, Rosmarin M, Urrutia A, et al. . Myxedema madness complicating postoperative follow-up of thyroid cancer. Arch Endocrinol Metab 2015;59:359–64. 10.1590/2359-3997000000090 [DOI] [PubMed] [Google Scholar]
- 18. Larouche V, Snell L, Morris DV. Iatrogenic myxoedema madness following radioactive iodine ablation for Graves' disease, with a concurrent diagnosis of primary hyperaldosteronism. Endocrinol Diabetes Metab Case Rep 2015;2015 10.1530/EDM-15-0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kothbauer-Margreiter I, Sturzenegger M, Komor J, et al. . Encephalopathy associated with Hashimoto thyroiditis: diagnosis and treatment. J Neurol 1996;243:585–93. 10.1007/BF00900946 [DOI] [PubMed] [Google Scholar]
- 20. Shaw PJ, Walls TJ, Newman PK, et al. . Hashimoto’s encephalopathy: a steroid-responsive disorder associated with high anti-thyroid antibody titers-report of 5 cases. Neurology 1991;2(Pt 1):228–33. [DOI] [PubMed] [Google Scholar]
- 21. Katipoglu B, Ates I, Acehan F, et al. . Rhabdomyolysis case based on hypothyroidism. Endocrinol Diabetes Metab Case Rep 2016;2016 10.1530/EDM-16-0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nikolaidou C, Gouridou E, Ilonidis G, et al. . Acute renal dysfunction in a patient presenting with rhabdomyolysis due to Hypothyroidism attributed to Hashimoto’s Disease. Hippokratia 2010;14:281–3. [PMC free article] [PubMed] [Google Scholar]
- 23. Jobé J, Corman V, Fumal A, et al. . [Rhabdomyolysis and hypothyroidism]. Rev Med Liege 2007;62(7-8):484–6. [PubMed] [Google Scholar]
- 24. Altay M, Duranay M, Ceri M. Rhabdomyolysis due to hypothyroidism. Nephrology Dialysis Transplantation 2005;20:847–8. 10.1093/ndt/gfh745 [DOI] [PubMed] [Google Scholar]
- 25. Bhansali A. Acute myoedema: an unusual presenting manifestation of hypothyroid myopathy. Postgrad Med J 2000;76:99–100. 10.1136/pmj.76.892.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barahona MJ, Mauri A, Sucunza N, et al. . Hypothyroidism as a Cause of Rhabdomyolysis. Endocr J 2002;49:621–3. 10.1507/endocrj.49.621 [DOI] [PubMed] [Google Scholar]
- 27. Joint Meeting of British Endocrine Societies. Endocrine abstracts. BioScientifica 2001. [Google Scholar]
- 28. Swaminath D, Limsuwat C, Islam E. Acute Kidney Injury and Rhabdomyolysis as an Initial Presentation of Hashimoto’s Thyroiditis. The Southwest Respiratory and Critical Care Chronicles 2013;1:35–8. 10.12746/swrccc2013.0102.022 [DOI] [Google Scholar]
- 29. Salehi N, Agoston E, Munir I, et al. . Rhabdomyolysis in a Patient with Severe Hypothyroidism. Am J Case Rep 2017;18:912–8. 10.12659/AJCR.904691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Monzani F, Caraccio N, Siciliano G, et al. . Clinical and biochemical features of muscle dysfunction in subclinical hypothyroidism. J Clin Endocrinol Metab 1997;82:3315–8. 10.1210/jcem.82.10.4296 [DOI] [PubMed] [Google Scholar]
- 31. Wiles CM, Young A, Jones DA, et al. . Muscle Relaxation Rate, Fibre-Type Composition and Energy Turnover in Hyper- and Hypo-Thyroid Patients. Clin Sci 1979;57:375–84. 10.1042/cs0570375 [DOI] [PubMed] [Google Scholar]
- 32. Sinclair C, Gilchrist JM, Hennessey JV, et al. . Muscle carnitine in hypo- and hyperthyroidism. Muscle Nerve 2005;32:357–9. 10.1002/mus.20336 [DOI] [PubMed] [Google Scholar]
- 33. Ruchala M, Zybek A, Szczepanek-Parulska E. Serum irisin levels and thyroid function—Newly discovered association. Peptides 2014;60:51–5. 10.1016/j.peptides.2014.07.021 [DOI] [PubMed] [Google Scholar]
- 34. Galletly C, Castle D, Dark F, et al. . Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders [Internet]. Journal of Psychiatry 2016;50. [DOI] [PubMed] [Google Scholar]


