Abstract
Background
Direct oral anticoagulants (DOACs), namely rivaroxaban, apixaban, dabigatran, and edoxaban, are now included together with warfarin as standards of care for the primary treatment of venous thromboembolism (VTE). The extent to which the DOACs have been adopted since receiving US Food and Drug Administration (FDA) approval is unknown.
Objective
To document temporal trends in oral anticoagulant (OAC) prescriptions among anticoagulant‐naïve patients initiating OACs for VTE primary treatment in the United States and to report participant characteristics by OAC prescribed for the year 2017.
Methods
MarketScan databases for years 2012 through 2017 were used to identify VTE cases and comorbidities using International Classification of Diseases codes and prescriptions for OACs via outpatient pharmaceutical claims data.
Results
The 137 203 VTE cases were on average (± standard deviation) 56.7 ± 16.0 years old and 49.9% female. Warfarin was prescribed to 98.7% of VTE patients receiving an OAC in quarter 1 (January through March) of 2012. By quarter 4 (October through December) of 2017, warfarin was prescribed to 17.5%, while rivaroxaban was prescribed to 42.7%, apixaban to 38.6%, dabigatran to 1.3%, and edoxaban to <0.1%. In 2017, the comorbidity burden was highest among patients prescribed warfarin, intermediate among patients prescribed apixaban, and lowest among patients prescribed rivaroxaban.
Conclusions
Rivaroxaban and apixaban use to treat VTE has increased dramatically since receiving FDA approval, whereas warfarin use has plummeted. Dabigatran and edoxaban are infrequently prescribed. Given widespread usage of rivaroxaban and apixaban, there is a need for continued monitoring of the comparative effectiveness of these OAC therapies in real‐world settings.
Keywords: apixaban, direct oral anticoagulants, prescription trends, rivaroxaban, venous thromboembolism, warfarin
Essentials.
Trends in adoption of direct oral anticoagulants for venous thromboembolism (VTE) treatment are not documented.
VTE cases were identified from a large US administrative data source for the years 2012 to 2017.
Warfarin was prescribed to 98.7% in quarter 1 of 2012 but only 17.5% in quarter 4 of 2017.
By quarter 4 of 2017, 42.7% of VTE patients were prescribed rivaroxaban and 38.6% apixaban.
1. INTRODUCTION
Over 1 million Americans annually experience venous thromboembolism (VTE), which consists of both deep vein thrombosis and pulmonary embolism. After decades in which vitamin K antagonists (warfarin in the United States) were the mainstay for primary treatment of VTE, options have expanded with the advent and regulatory approval of several direct oral anticoagulants (DOACs) to treat VTE.1, 2, 3, 4, 5, 6 Rivaroxaban received US Food and Drug Administration (FDA) approval for VTE primary treatment on November 2, 2012; dabigatran on April 7, 2014; apixaban on August 21, 2014; and edoxaban on January 8, 2015. Guidelines from the American College of Chest Physicians (ACCP) suggest the initiation of DOACs over vitamin K antagonists for the primary treatment (first 3‐6 months after initial event) of VTE in patients without cancer or kidney disease.7
It is widely assumed that DOAC use has increased dramatically, given advantages of DOACs over warfarin (eg, no need for routine laboratory monitoring, fewer food and drug interactions, no need for parenteral heparin bridging with apixaban and rivaroxaban) and their endorsement by the ACCP. However, this trend has not yet been documented in the US population. Herein, we describe temporal trends for the time period from 2012 to 2017, in oral anticoagulant (OAC) prescriptions to anticoagulant‐naïve patients initiating OACs for VTE primary treatment. We also describe, for the year 2017, VTE patient characteristics according to OAC prescribed.
2. METHODS
The present analysis used IBM MarketScan Commercial Claims and Encounter and Medicare Supplemental and Coordination of Benefits databases from 2012 through 2017. The databases contain individual‐level, deidentified, Health Insurance Portability and Accountability Act–compliant health care claims information from US employers, health plans, hospitals, and Medicare programs. In 2016, MarketScan databases contained health care data for >43.6 million covered individuals.8 Data on all enrollment records and inpatient, outpatient, ancillary, and drug claims are collected and are linked via individual‐level identifiers. Missing from this database are individuals with no insurance and those with only Medicare Part D. Individuals working at small companies are also underrepresented. Given the deidentified nature of the MarketScan data, this analysis was deemed exempt from review by the University of Minnesota Institutional Review Board.
2.1. Identification of VTE cases
The present analysis includes individuals aged 18 to 99 with incident VTE, at least 1 prescription for an OAC within the 31 days before or after their first VTE claim (due to nuances of dates in claims‐based data), and ≥3 months of continuous enrollment prior to their first OAC prescription. As in previous work,9 we defined VTE as having at least 1 inpatient claim for VTE or 2 outpatient claims for VTE, which were 7 to 185 days apart, in any position, based on International Classification of Diseases (ICD) 9th and 10th Revision codes. The positive predictive value (PPV) of this definition was 91% in a recent validation study that employed a definition similar to that used in this analysis, which was inclusive of both inpatient and outpatient encounters and additionally required treatment.10
The initial sample included 472 757 VTE patients aged 18 to 99 years. The analytic sample was 367 886 once restricted to individuals ever prescribed an OAC between January 1, 2011, and December 31, 2017; 231 189 after requiring the first OAC prescription be ±31 days of the VTE date; 189 076 after requiring ≥3 months of continuous enrollment before the first OAC prescription and 137 203 after excluding individuals who used low‐molecular‐weight heparin as their sole anticoagulant prescription or who had evidence of cancer as different recommendations for treatment exist for this population.7 See the analysis flowchart (Figure 1).
Figure 1.
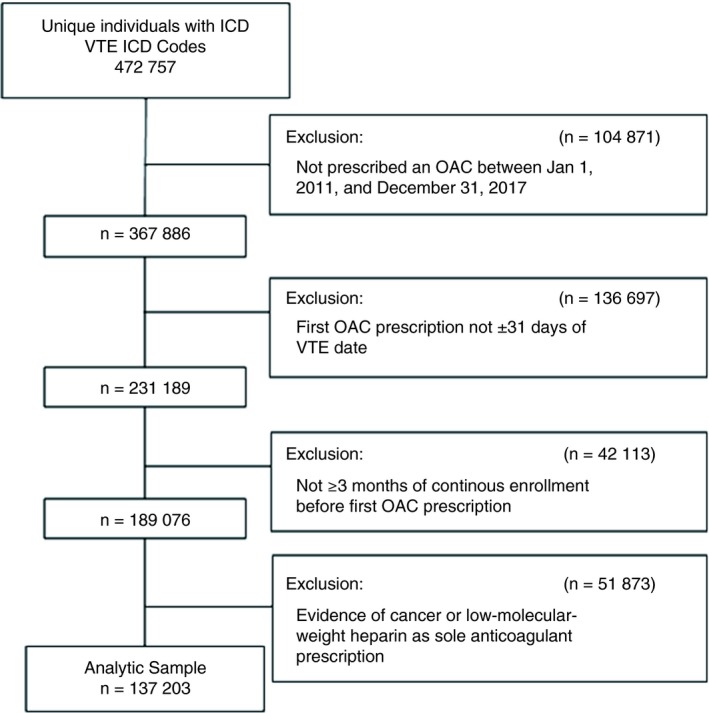
Analysis flowchart. ICD, International Classification of Diseases; OAC, oral anticoagulant; VTE, venous thromboembolism.
2.2. Anticoagulant use
Prescriptions for DOACs and warfarin were identified using outpatient pharmaceutical claims data, which include information on the National Drug Code, the prescription fill date, and the number of days supplied. Validity of warfarin claims in administrative databases is excellent (sensitivity, 94%; PPV, 99%).11 Validation studies of DOAC claims have not yet been conducted. For the present analysis, which focuses on VTE primary treatment, we considered only the first OAC prescribed. Several of the DOACs received approval for atrial fibrillation management prior to being granted approval for the indication of VTE. As such, via off‐label prescriptions, it is possible for VTE patients to have been prescribed these DOACs prior to their FDA approval for the indication of VTE.
2.3. Statistical analysis
This analysis cross‐sectionally evaluated prevalence of use of new OACs among anticoagulant‐naïve VTE patients according to time; as such, it can be considered a repeated cross‐sectional design. Proportions of OACs prescribed were calculated according to calendar year and quarter (quarter 1: January through March; quarter 2: April through June; quarter 3: July through September; quarter 4: October through December). For the year 2017 we calculated participant characteristics (comorbidities and medication usage) by OAC prescribed. Patient comorbidities were identified using established algorithms.10, 12 P values for differences in patient characteristics between OACs were calculated using t tests for continuous variables and chi‐square tests for dichotomous variables.
3. RESULTS AND DISCUSSION
Our sample included 137 203 VTE patients who were on average (standard deviation [SD]) 56.7 ± 16.3 years old and 49.9% female. Warfarin was prescribed to 98.7% of anticoagulant‐naïve VTE patients receiving an OAC in quarter 1 of 2012 (Figure 2). By quarter 4 of 2017, use of warfarin had decreased dramatically, being prescribed to only 17.5% of VTE patients. Rivaroxaban was prescribed to 42.7%, apixaban to 38.6%, dabigatran to 1.3% and edoxaban to <0.1%. Use of rivaroxaban has been somewhat stable since 2014 quarter 2 when it was prescribed to 40.8%. Apixaban has continued to gain market share in virtually every quarter since its FDA approval in 2014 quarter 3. It is unclear whether this pattern will continue or if it, too, will stabilize. How a physician and patient decide between rivaroxaban and apixaban is also not clear. Both have a similar mechanism of action (factor Xa inhibitors),13 but rivaroxaban is a once‐daily regimen, whereas apixaban is twice daily. In comparative effectiveness studies, we9 and others14 have recently shown that risk of major bleeding is lower among users of apixaban than users of rivaroxaban. The extremely low use of dabigatran and edoxaban may be explained, at least partially, by their need for initial parenteral anticoagulation or differences in reimbursement relative to other OAC options. Results were similar when we restricted our analysis to participants with no evidence of atrial fibrillation (data not shown). Similar to our findings, an analysis of the Danish Nationwide Cohort study showed dramatic shifts in OAC use between February 2012 and September 2016.15 By September 2016, 12% of Danish VTE patients were initially prescribed warfarin, 70% rivaroxaban, 16% apixaban, and 2% dabigatran.
Figure 2.
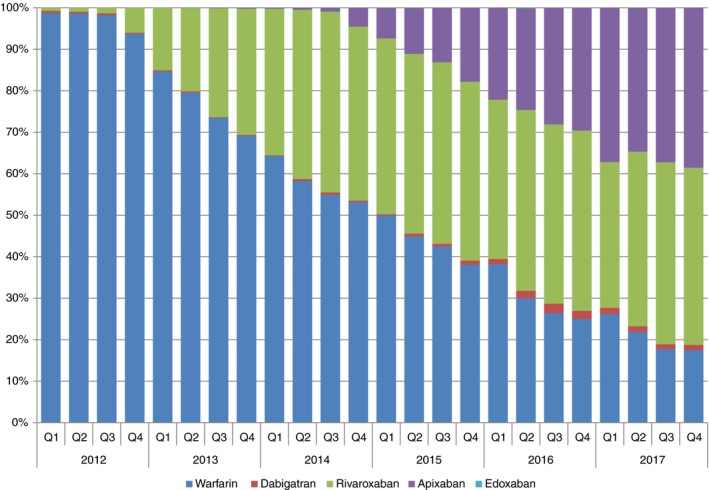
Temporal trends in oral anticoagulants prescribed for the primary treatment of venous thromboembolism from 2012 through 2017
Among patients initiating OAC therapy for VTE primary treatment in 2017, those prescribed warfarin were on average (±SD) 57.2 ± 16.4 years old and had the most comorbidities. Patients prescribed apixaban were similar in age to patients prescribed warfarin (56.8 ± 15.9) but had a slightly lower comorbidity burden, while patients prescribed rivaroxaban were the youngest (53.4 ± 14.8 years) and had the fewest comorbidities (Table 1). Keeping in mind that in 2017 (quarters 1‐4) 19.3% of patients were prescribed warfarin, 42.5% rivaroxaban, and 36.8% apixaban, these findings indicate that there is widespread usage of apixaban and rivaroxaban across patients with an array of comorbidities.
Table 1.
Characteristics of venous thromboembolism patients by anticoagulant initially prescribed, MarketScan databases, 2017
| Warfarin (N = 2172) | Rivaroxaban (N = 4773) | Apixaban (N = 4128) | P value | |||
|---|---|---|---|---|---|---|
| Rivaroxaban vs. warfarin | Apixaban vs. warfarin | Apixaban vs. rivaroxaban | ||||
| Age, y | 57.2 ± 16.4 | 53.4 ± 14.8 | 56.8 ± 15.9 | <0.00 | 0.42 | <0.00 |
| Female, % | 49.9 | 49.7 | 50.5 | 0.89 | 0.63 | 0.43 |
| Comorbidities, % | ||||||
| Hypertension | 60.5 | 47.4 | 59.1 | <0.00 | 0.29 | <0.00 |
| Diabetes mellitus | 24.3 | 16.9 | 22.1 | <0.00 | 0.05 | <0.00 |
| Myocardial infarction | 9.6 | 4.3 | 6.5 | <0.00 | <0.00 | <0.00 |
| Heart failure | 18.1 | 7.9 | 13.4 | <0.00 | <0.00 | <0.00 |
| Atrial fibrillation | 9.3 | 4.6 | 8.9 | <0.00 | 0.61 | <0.00 |
| Ischemic stroke | 3.9 | 2.0 | 3.3 | <0.00 | 0.28 | <0.00 |
| Peripheral artery disease | 15.3 | 8.0 | 12.7 | <0.00 | 0.004 | <0.00 |
| Dementia | 4.1 | 1.6 | 3.2 | <0.00 | 0.08 | <0.00 |
| Chronic pulmonary disease | 23.2 | 19.8 | 22.1 | 0.001 | 0.36 | 0.007 |
| Renal disease | 15.1 | 5.3 | 10.4 | <0.00 | <0.00 | <0.00 |
| Liver disease | 8.6 | 6.4 | 8.0 | 0.001 | 0.36 | 0.01 |
| Depression | 20.2 | 16.0 | 17.7 | <0.00 | 0.02 | 0.03 |
| Hematologic disorders | 15.2 | 8.7 | 10.2 | <0.00 | <0.00 | 0.02 |
| Alcohol abuse | 3.0 | 2.4 | 2.6 | 0.16 | 0.48 | 0.41 |
| Medications, % | ||||||
| Antiplatelets | 6.5 | 3.2 | 5.5 | <0.00 | 0.10 | <0.00 |
| ACE inhibitors | 21.1 | 16.7 | 21.4 | <0.00 | 0.81 | <0.00 |
| Angiotensin receptor blockers | 14.3 | 12.1 | 15.0 | 0.01 | 0.47 | <0.0001 |
| Beta‐blockers | 27.3 | 17.9 | 25.2 | <0.00 | 0.08 | <0.00 |
| Calcium channel blockers | 18.1 | 13.6 | 18.6 | <0.00 | 0.59 | <0.00 |
| Statins | 30.0 | 23.7 | 29.3 | <0.00 | 0.60 | <0.00 |
| Diabetes mellitus medications | 6.3 | 3.8 | 4.8 | <0.00 | 0.02 | 0.01 |
| SSRIs | 27.1 | 24.2 | 26.0 | 0.01 | 0.36 | 0.05 |
ACE, angiotensin‐converting enzyme; SSRI, selective serotonin reuptake inhibitors.
Values correspond to mean ± standard deviation or percentage.
Strengths of this study are the large sample of US insured individuals, information on comorbidities, and availability of data since the approval of DOACs for VTE primary treatment. Limitations are potential misclassification in the exposure status (because the validity of DOACs in administrative data have not been determined) and in the identification of individuals with incident VTE and various comorbidities. However, established algorithms were used.10, 12 We also lack information about what led to selection of a particular OAC; factors such as socioeconomic status, laboratory values, formulary preference, and provider's perception of patient compliance are unavailable.
Rivaroxaban and apixaban use have increased dramatically since receiving FDA approval, while warfarin use has plummeted. Given the extensiveness of their use, there is a continued need for postmarketing surveillance to (1) understand the comparative effectiveness of these agents in terms of VTE recurrence, bleeding, and mortality across diverse patient groups; and (2) monitor for unexpected adverse events. Additionally, as DOACs are now ubiquitous in the context of VTE primary treatment, there is a continued need to evaluate their comparative effectiveness in the context of VTE, and to understand how to optimize their management in high‐risk situations.
RELATIONSHIP DISCLOSURE
The authors report nothing to disclose.
AUTHOR CONTRIBUTIONS
PLL, AA, and NZ conceived the study; PLL, RWF, and RFM conducted the data analysis; all authors provided critical intellectual input on the manuscript draft.
Supporting information
ACKNOWLEDGMENTS
This work was supported by NIH National Heart Lung and Blood Institute grants R01‐HL131579 and R01‐HL122200.
Lutsey PL, Walker RF, MacLehose RF, Alonso A, Adam TJ, Zakai NA. Direct oral anticoagulants and warfarin for venous thromboembolism treatment: Trends from 2012 to 2017. Res Pract Thromb Haemost. 2019;3:668–673. 10.1002/rth2.12222
Contributor Information
Pamela L. Lutsey, Email: Lutsey@umn.edu, @PLutsey.
Alvaro Alonso, @alonso_epi.
REFERENCES
- 1. Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52. [DOI] [PubMed] [Google Scholar]
- 2. Einstein Investigators , Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363: 2499–510. [DOI] [PubMed] [Google Scholar]
- 3. Buller HR, Decousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–15. [DOI] [PubMed] [Google Scholar]
- 4. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. [DOI] [PubMed] [Google Scholar]
- 5. Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129:764–72. [DOI] [PubMed] [Google Scholar]
- 6. Einstein‐PE Investigators , Buller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–97. [DOI] [PubMed] [Google Scholar]
- 7. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 8. IWH Watson Health (TM) . IBM MarketScan Research Databases for Health Services Researchers (White Paper). Somers, NY: IBM Corporation; 2018. [Google Scholar]
- 9. Lutsey PL, Zakai NA, MacLehose RF, Norby FL, Walker RF, Roetker NS, et al. Risk of hospitalized bleeding in comparisons of oral anticoagulant options for the primary treatment of venous thromboembolism. Br J Hematol. 2019;185:903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sanfilippo KM, Wang T‐F, Gage BF, Liu W, Carson KR. Improving accuracy of International Classification of Diseases codes for venous thromboembolism in administrative data. Thromb Res. 2015;135:616–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garg RK, Glazer NL, Wiggins KL, Newton KM, Thacker EL, Smith NL, et al. Ascertainment of warfarin and aspirin use by medical record review compared with automated pharmacy data. Pharmacoepidemiol Drug Saf. 2011;20:313–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 13. Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet. 2014;53:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dawwas GK, Brown J, Dietrich E, Park H. Effectiveness and safety of apixaban versus rivaroxaban for prevention of recurrent venous thromboembolism and adverse bleeding events in patients with venous thromboembolism: a retrospective population‐based cohort analysis. Lancet Hematol. 2019;6:e20–8. [DOI] [PubMed] [Google Scholar]
- 15. Sindet‐Pedersen C, Pallisgaard JL, Staerk L, Berger JS, Lamberts M, Torp‐Pedersen C, et al. Temporal trends in initiation of VKA, rivaroxaban, apixaban and dabigatran for the treatment of venous thromboembolism – a Danish nationwide cohort study. Sci Rep. 2017;7:3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


