Abstract
Online patient registries are used to collect data on clinical conditions with rare occurrence or unclear diagnostic and management practices. The success of these registries depends on clear definition of goals, correct identification of patient population/inclusion criteria, availability of appropriate setup and maintenance tools, and the quality of dissemination. The Scientific and Standardization Committee (SSC) for Women's Health Issues in Thrombosis and Hemostasis, one of 20 committees of the International Society on Thrombosis and Haemostasis (ISTH) has developed 6 registries for women's bleeding and thrombotic conditions over the past 2 years and are currently in various stages of progress. Here, we provide information about these registries, including rationale, objectives, and methods for data collection. The aim is to enhance worldwide participation and thus promote the success of these registries. We used ISTH REDCap, a mature and secure Web application for building and managing online surveys and databases, and the ISTH advertising platform to maximize participation. Registries (links and project details available on ISTH and Women's SSC Web sites) include: (1) WiTEAM, project on thrombophilia and placenta‐mediated obstetric complications; (2) a registry for disseminated intravascular coagulation in pregnancy; (3) severe congenital protein C deficiency—an obstetric study; (4) obstetric and gynecologic outcomes of women with platelet function disorders; (5) thrombolysis and invasive treatments for massive pregnancy‐related pulmonary embolism; (6) pregnancy and exposure to direct oral anticoagulants. The ISTH promotes online registries on women's issues to enhance understanding of current practices, identify knowledge gaps, promote research, and ultimately improve patient safety and quality of life.
Keywords: blood platelets, disseminated intravascular coagulation, pregnancy, protein C deficiency, pulmonary embolism, registries, thrombophilia
Essentials.
Online patient registries are large data collection tools for conditions with rare occurrence or unclear diagnostic and management practices.
The Women’s Scientific and Standardization Committee (SSC) for Health Issues in Thrombosis and Haemostasis of the International Society on Thrombosis and Haemostasis developed 6 registries for women’s bleeding and thrombotic conditions, using the ISTH REDCap secure web application.
The Women’s SSC invites clinicians from the international community to participate in these online registries to enhance the understanding of current practices.
This data shall help identify knowledge gaps, promote research and collaborations, and ultimately improve patient safety and quality of life.
1. INTRODUCTION
Patient online registries are fundamental for enhancing data collection, understanding clinical/laboratory features of rare diseases, identifying knowledge gaps in clinical practice, promoting research, and ultimately improving patient safety and quality of life. Key elements to consider are defining clearly the goal(s) of the registry, correct identification of the patient population and inclusion criteria, sound methodology, and study design.1 Securing tools and funds to set up and maintain the registries together with clear communication and wide dissemination are essential for the success of any international registry.
To support registries in the field of thrombosis and hemostasis, the International Society on Thrombosis and Haemostasis (ISTH) is offering a mature and secure Web application for building and managing online surveys and databases (REDCap). Our goal here is to inform on the ongoing efforts of ISTH member projects and the Scientific and Standardization Committee's (SSC) projects on Women's Health Issues in Thrombosis and Hemostasis, using this platform.
Improving gestational morbidity and mortality is one of the highest priorities of the World Health Organization.2 The SSC for Women's Health Issues in Thrombosis and Hemostasis has effectively used the ISTH REDCap platform to develop registries that serve patient care. We have launched 6 registries (Figure 1) for different conditions pertaining to women's bleeding and thrombosis, which are listed at the ISTH Web site (https://www.isth.org/page/redcap). We provide information about these registries, including rationale, objectives, and type for data collection (Table 1). We invite worldwide participation to enrich data collection and promote outcomes. Local ethics committees at the project site have approved the registries. Participants or their representatives are expected to secure appropriate approvals as per their local ethics regulations. All collected patient‐related data are securely protected and are nonidentifiable.
Figure 1.
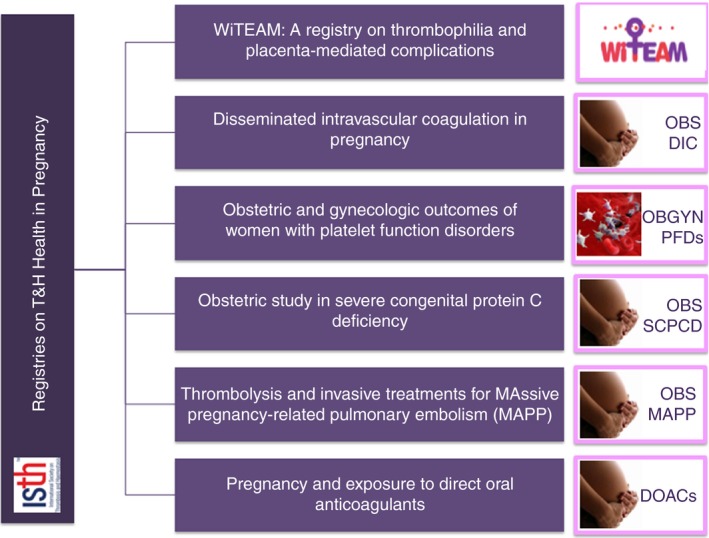
Snapshot of current ISTH registries on thrombosis and hemostasis health in pregnancy. DOAC, direct oral anticoagulants; OBS DIC, obstetric disseminated intravascular coagulopathy; PFDs, platelet function disorders; SCPCD, severe congenital protein C deficiency; WiTEAM, Women International Team
Table 1.
Summary of ISTH registries on Women's Health Issues in Thrombosis and Hemostasis
| Registry title | Disease condition | Link | Value | Project lead and contact |
|---|---|---|---|---|
| WiTEAM: A registry on patients with thrombophilia and placenta‐mediated complications | Thrombophilia together with placenta‐mediated complications or recurrent pregnancy loss | https://redcap.isth.org/surveys/?s=AY8H7WXXJR |
|
Amparo Santamaría Maria Cerdá Spain asantamaria@vhio.net |
| A registry on DIC in pregnancy | DIC diagnosed during pregnancy or postpartum period | https://redcap.isth.org/surveys/?s=KFC8RN8XWC |
|
Offer Erezl Israel offererez@gmail.com |
| Obstetric and gynecologic outcomes of women with inherited PFDs | Pregnancy associated with diagnosis of PFD | https://redcap.isth.org/surveys/?s=HTHE4TAMTM |
|
Deborah Obeng‐Tuudah Rezan Abdul‐Kadir UK d.obeng‐tuudah@nhs.net |
| Obstetric study in severe congenital protein C deficiency | Families who have had children with severe congenital protein C deficiency | https://redcap.isth.org/surveys/?s=CRDP3MXN4J |
|
Adrian Minford Rezan Kadir UK adrian.minford@sky.com |
| The registry for thrombolysis and invasive treatments for MAPP | Massive PE diagnosed in pregnancy or postpartum period | https://redcap.isth.org/surveys/?s=FLRDCEY4WT |
|
Marc Blondon Switzerland marc.blondon@hcuge.ch |
| DOAC exposure during pregnancy | Confirmed pregnancy while on DOACs | https://redcap.isth.org/surveys/?s=P99ARFCM3J |
|
Saskia Middeldorp and Ingrid Bistervels The Netherland i.m.bistervels@amsterdamumc.nl |
Abbreviations: DIC, disseminated intravascular coagulation; DOAC, direct oral anticoagulant; ECMO, extracorporeal membrane oxygenation; MAPP, massive pregnancy‐related pulmonary embolism; PE, pulmonary embolism; PFD, platelet function disorder; PMCs, placenta‐mediated complications; SSC, Scientific and Standardization Committee.
2. WOMEN INTERNATIONAL TEAM: A REGISTRY ON THROMBOPHILIA AND PLACENTA‐MEDIATED OBSTETRIC COMPLICATIONS
2.1. Aim and rationale
Women International TEAM (WiTEAM) is an observational, prospective, international, and noninterventional phase IV registry‐based study. The goal of this registry is to examine and document prospectively the worldwide practices regarding the management of women with thrombophilia and previous placenta‐mediated complications. Those complications, such as preeclampsia, placental abruption, and birth of a small‐for‐gestational age neonate due to placental insufficiency and fetal death, remain leading causes of fetal‐maternal morbidity and mortality and are risk factors for maternal cardiovascular diseases.3 Effective preventive strategies and assessment of risk of recurrence do not currently exist.4, 5, 6, 7, 8 Controversy remains around the decision to perform thrombophilia testing or prescribe low‐molecular‐weight heparin. This is mostly based on the opinion of the consulting expert and sometimes the patient's preference.
2.2. Inclusion criteria and methods
The registry aims to collect data on women aged >18 years with a placenta‐mediated complication or recurrent pregnancy loss. Placenta‐mediated complications include early‐onset or severe preeclampsia, small‐for‐gestational age neonates under the 5th percentile, placental abruption, fetal or neonatal death at >10 weeks’ gestational age and ≤28 days postpartum, with normal fetal morphology having been documented by ultrasound scan or direct examination of the fetus. Recurrent pregnancy loss is defined as 3 unexplained consecutive miscarriages before the 10th week of gestation, not caused by maternal anatomic or hormonal abnormalities or by paternal and maternal chromosomal causes.
Clinicians who care for patients with placenta‐mediated complications or recurrent pregnancy loss will recruit patients prospectively in the first pregnancy visit and evaluate them at 3 follow‐up visits during pregnancy as well as 3 months after delivery. This follow‐up includes routine clinical and management practice, incidence and type of thrombophilia, anticoagulation, the safety of therapeutic management during pregnancy and fetal outcomes. Our goal is to include 2000 cases over 3 years.
2.3. Implications
Gathering data on the worldwide practice around placenta‐mediated complications will inform about the routine management practice of women with these pregnancy complications and known thrombophilia, and help assess the safety of therapeutic management during pregnancy, which will help generate SSC guidance in this regard.
2.4. Link to registry
The registry is available at https://redcap.isth.org/surveys/?s=AY8H7WXXJR
3. A REGISTRY ON DISSEMINATED INTRAVASCULAR COAGULATION IN PREGNANCY
3.1. Aim and rationale
The aim of this registry is to improve the current understanding of the definitions and perspectives on the diagnosis and management of disseminated intravascular coagulation (DIC) in obstetrics.
DIC is a pathological maladaptive activation of the coagulation system that arises as a complication of different disease states such as trauma and sepsis.9 In pregnancy, DIC has been described in association with fetal demise, placental abruption, amniotic fluid embolism, HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count), and septic abortion.10, 11 The incidence of DIC during pregnancy is not well defined and ranges from 0.03%12 to 0.35%.13 The leading etiologies include placental abruption, especially when associated with a stillbirth12 in developed countries, and preeclampsia and retained stillbirth in developing countries.14, 15
The definition of DIC in obstetrics remains unclear and is often based on the clinical impression of the attending physician and loosely on some laboratory tests. Preeclampsia, preterm labor, preterm rupture of membranes, fetal demise, and fetal growth restriction are associated with increased maternal thrombin generation,16, 17, 18, 19 platelet activation,20, 21 and abnormal fibrinolysis.22 Placenta microthrombi, infarcts, and fibrin deposition have been documented in these conditions.23 These pathologies are in line with the DIC definition.24
A considerable controversy exists between obstetricians and hematologists regarding what should be defined as obstetric or postpartum hemorrhage with associated coagulation factor consumption and which of these hemorrhagic complications should be defined as a “true” DIC. A guidance document on the management of coagulopathy associated with postpartum hemorrhage was published by the SSC of the ISTH in 2016.25 Scoring systems with diagnostic value for DIC were developed in Japan, Israel, and the United States. However, there remains a need for further guidance and standardization with regard to DIC in pregnancy.
3.2. Inclusion criteria and methods
The registry aims to collect data on the epidemiologic characteristics, clinical conditions leading to a consideration of DIC, how DIC was diagnosed (clinical and lab features) and treated, and the subsequent maternal and fetal outcomes.
Hematologists or obstetricians who have diagnosed patients with DIC during pregnancy or the postpartum period can participate.
3.3. Implications
The data from this registry will assist in understanding the perspectives and current practices in the diagnosis and management of DIC in pregnancy and also identifying the key areas of variance internationally among health care professionals worldwide. This will contribute to standardization of definitions and improving diagnosis and management. Additionally, the data will help modify existing DIC scoring systems to fit in the pregnant state for better diagnosis and management of DIC in obstetrics.
3.4. Link to registry
The registry is available at https://redcap.isth.org/surveys/?s=KFC8RN8XWC
4. REGISTRY ON OBSTETRIC AND GYNECOLOGIC OUTCOMES OF WOMEN WITH PLATELET FUNCTION DISORDERS
4.1. Aim and rationale
This registry aims to capture information in women with the different types of platelet function disorders, syndromic and nonsyndromic, to aid the development of a holistic guidance document regarding management. The primary emphasis is on their gynecologic and obstetric presentations, management course, and outcomes.
The presentations and management of women with platelet function disorders or platelet dysfunction in the obstetrics and gynecology settings can be variable. There is limited clinical experience in this area due to the rarity and the diversity of these disorders. Normal events such as menstruation, ovulation, labor, and delivery all pose significant bleeding risks in these women. In certain instances, fetal/neonatal bleeding risks are implicated as well. In the literature, most information is scattered in case reports and small case series. There are systematic reviews of 2 common syndromic platelet function disorders, Glanzmann thrombasthenia and Bernard‐Soulier in pregnancy, with management guidance.26, 27 Detailed clinical guidance is needed for managing women with this heterogeneous group of diseases.
4.2. Inclusion criteria and methods
Women diagnosed with platelet function disorders from the age of menarche onwards can be included. The diagnosis and characterization of the inherited platelet function disorder need to be confirmed by well‐established diagnostic criteria28 before including patients in the registry.
Clinicians (obstetricians or hematologists with special interest in hemostasis and pregnancy) who care for female patients diagnosed with platelet function disorders who present with obstetric/gynecologic complaints or concerns can participate. Patients'clinical presentations, management, and outcomes need to be recorded.
4.3. Implications
The registry will strengthen a current UK pilot project by increasing the total number of patients with these rare disorders and make interpretation of the data applicable worldwide.
4.4. Link to registry
The registry is available at https://redcap.isth.org/surveys/?s=HTHE4TAMTM
5. RETROSPECTIVE OBSTETRIC STUDY IN SEVERE CONGENITAL PROTEIN C DEFICIENCY
5.1. Aim and rationale
The study aims to investigate the obstetric history of mothers who have given birth to children with severe congenital protein C deficiency to determine the incidence of fetal loss during pregnancy and previous neonatal death that might be due to undiagnosed severe congenital protein C deficiency.
Patients with this condition present in the neonatal period with purpura fulminans, often accompanied by intracranial and retinal vessel thrombosis. It is an autosomal recessive disorder with an estimated prevalence of 2.25/million based on a carrier rate of 0.3% in the general population. There should be 135 cases in the UK alone, but there are currently only 11 known cases. This discrepancy could be due to high loss of affected fetuses or early neonatal death without a severe congenital protein C deficiency diagnosis.29, 30
5.2. Inclusion criteria and methods
The registry will collect data from families who have had children with severe congenital protein C deficiency (protein C levels <1 IU/dL or levels 1‐20 IU/dL with a typical clinical presentation and heterozygous parents). Cases of acquired protein C deficiency are not included. Subjects will be asked to provide informed written consent for data collection and processing.
Clinicians worldwide who care for families who have had children with this condition are invited to participate.
Clinicians who have managed and reported such cases have been identified from the literature and by networking at scientific meetings and from Shire Pharmaceuticals (manufacturer of Ceprotin, used for treatment of severe congenital protein C deficiency) database and invited to participate.
5.3. Implications
Information from the study may support targeted screening (eg, consanguinity with a history of excess fetal loss) for at‐risk couples, with beneficial clinical implications, offering antenatal diagnosis if the genetic mutation is known to identify affected fetuses and offer the option of termination of pregnancy; or early (32‐34 weeks) elective delivery to avoid blindness and neurological handicap, as fetal retinal vessel and cerebral thrombosis often occur in the late stages of pregnancy.
5.4. Link to registry
The registry is available at https://redcap.isth.org/surveys/?s=CRDP3MXN4J
6. REGISTRY ON THROMBOLYSIS AND INVASIVE TREATMENTS FOR MASSIVE PREGNANCY‐RELATED PULMONARY EMBOLISM (MAPP REGISTRY)
6.1. Aim and rationale
The global aim of the registry is to better inform treatment decisions in patients with massive pulmonary embolism (PE) in pregnancy. The primary objective is to explore the maternal effectiveness and maternal/obstetric/neonatal safety of thrombolysis, mechanical thrombectomy, or extracorporeal membrane oxygenation (ECMO) for massive PE during pregnancy and the 6 weeks postpartum.
PE occurs in about 1 in 1000‐3000 pregnancies.31 Its most severe manifestation, a hemodynamically unstable PE, also called high‐risk or “massive” PE, is a common cause of maternal mortality in Europe and North America,32 but its treatment options are guided by scarce data. A recent systematic review identified 127 cases of massive and submassive PE during pregnancy and the postpartum period, published between 1967 and 2016.33 With thrombolysis (n = 83), maternal and fetal survivals were high (94% and 88%); major bleeding occurred in 17% in the antepartum but was frequent (58%) in the postpartum period. However, the inference from such data is tempered by the heterogeneity of cases and the risk of publication bias, with a greater likelihood of publication of positive cases with good outcomes. Further, there were few cases of pregnancy‐related massive PE treated with ECMO and with percutaneous thrombectomy. Given this lack of strong evidence, current guidelines consider pregnancy as a relative contraindication to the use of thrombolytics34 but advise to administer thrombolytic therapy for life‐threatening pregnancy‐related PE.35, 36
6.2. Inclusion criteria and methods
Patients with an objective diagnosis of PE during pregnancy or during the 6‐week postpartum period and at least 1 criterion of massive PE (sustained hypotension, acute drop in blood pressure, need for inotropic support, or cardiac arrest) can be included.
Clinicians are invited to report cases of objectively diagnosed massive pregnancy‐related PE MAPP) in this online registry. The collected data include the administered treatments (thrombolysis, thrombectomy, and/or ECMO) and maternal, obstetric, and neonatal outcomes. We are anticipating a sample size of 80 cases for the 5‐ to 8‐year registry duration.
6.3. Implications
Data from this registry will inform the clinical management of MAPP through its data on safety and effectiveness of thrombolysis, thrombectomy, or ECMO for massive PE during pregnancy and the 6 weeks postpartum.
6.4. Link to registry
The registry is available at https://redcap.isth.org/surveys/?s=FLRDCEY4WT
7. REGISTRY ON PREGNANCY IN PATIENTS EXPOSED TO DIRECT ORAL ANTICOAGULANTS (DOACS)
7.1. Aim and rationale
The goal of this registry is to register all pregnant women who have received anticoagulant treatment with a direct oral anticoagulant (DOAC) while pregnant and to assess the effects of exposure to DOACs in utero on the fetus. This is a multicenter, international, observational cohort study. The registry is designed to collect both retrospective and prospective data.
Many patients receiving DOACs for venous thromboembolism are of reproductive age, but there are limited data about the safety of DOACs in pregnancy. Both animal and human placental models have demonstrated that all DOACs cross the placenta,37, 38, 39 and the risk of reproductive toxicity at therapeutic to toxic dosages was documented in animals. Therefore, the ISTH guidance statement advises against the use of DOACs during pregnancy and in women planning pregnancy.40 However, unintended pregnancy while on DOACs does occur in clinical practice. Data from case series suggest that the risk of embryopathy due to DOAC exposure is small,41 but an unfavorable effect on the fetus cannot be excluded and more data are needed.
7.2. Inclusion criteria and methods
Clinicians who have a patient with a confirmed pregnancy test during DOAC use can participate. Currently, 53 cases have been reported, of which 35 case report forms are completed. Cases are from Austria, Canada, Chile, France, Germany, Israel, Italy, The Netherlands, the UK, Poland, Portugal, New Zealand, Spain, and Switzerland.
7.3. Implication
The data will help determine the level of safety of the use of DOACs in pregnancy.
7.4. Link to registry
The registry is available at https://redcap.isth.org/surveys/?s=P99ARFCM3J
RELATIONSHIP DISCLOSURE
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
MO developed the idea and wrote the full manuscript. MO oversees the progress of all 6 projects, and facilitates knowledge dissemination; she is the current chairman of the Women's SSC. AS and MC lead the WiTEAM project, wrote the section related to the project, and reviewed the full manuscript. OE leads the DIC project, wrote the section related to the project, and reviewed the full manuscript. AM leads the protein C project, wrote the section related to the project, and reviewed the full manuscript. DO‐T leads the platelet function disorders project, wrote the section related to the project, and reviewed the full manuscript. MB leads the PE project, wrote the section related to the project, and reviewed the full manuscript. IB and SM lead the DOACs in pregnancy project, wrote the section related to the project, and reviewed the full manuscript. RA‐K is the supervisor of the protein C and platelet function disorders projects, wrote the section related to these projects, and reviewed the full manuscript; she is a former chairman of the Women's SSC.
ACKNOWLEDGMENT
The authors acknowledge Professor Peter Verhamme (UZ Leuven) and Professor Jan Beyer‐Westendorf (University Hospital Dresden) for their support to the DOAC‐ related registry. We also acknowledge Sue Pavord (John Radcliffe Hospital Oxford UK) for her support to the protein C–related registry.
Othman M, Santamaría Ortiz A, Cerdá M, et al. Thrombosis and hemostasis health in pregnancy: Registries from the International Society on Thrombosis and Haemostasis. Res Pract Thromb Haemost. 2019;3:607–614. 10.1002/rth2.12243
Contributor Information
Maha Othman, @MahaOthman.
Amparo Santamaría Ortiz, @santamariaparo.
María Cerdá, @mariacsabater.
Marc Blondon, @MarcBlondon.
Saskia Middeldorp, @MiddeldorpS.
REFERENCES
- 1. Hoque DME, Kumari V, Hoque M, Ruseckaite R, Romero L, Evans SM. Impact of clinical registries on quality of patient care and clinical outcomes: a systematic review. PLoS ONE. 2017;12:e0183667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Betrán AP, Wojdyla D, Posner SF, Gülmezoglu AM. National estimates for maternal mortality: an analysis based on the WHO systematic review of maternal mortality and morbidity. BMC Public Health. 2005;5:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang J, Elam‐Evans LD, Berg CJ, Herndon J, Flowers L, Seed KA, et al. Pregnancy‐related mortality surveillance United States 1991‐1999. MMWR Surveill Summ. 2003;52:1–8. [PubMed] [Google Scholar]
- 4. Rodger M, Gris JC, de Vries J, Martinelli I, Rey É, Schleussner E, et al. Low‐molecular‐weight heparin and recurrent placenta‐mediated pregnancy complications: a meta‐analysis of individual patient data from randomized controlled trials. Lancet. 2016;388:2629–41. [DOI] [PubMed] [Google Scholar]
- 5. Rodger MA, Betancourt MT, Clark P, Lindqvist PG, Dizon‐Townson D, Said J, et al. The association of factor V Leiden and prothrombin gene mutation and placenta‐mediated pregnancy complications: a systematic review and meta‐analysis of prospective cohort studies. PLoS Med. 2010;7:e1000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bouvier S, Cochery‐Nouvellon E, Lavigne‐Lissalde G, Mercier E, Fabbro‐Peray P, Balducchi JP, et al. Comparative incidence of pregnancy outcomes in thrombophilia‐positive women from the NOH‐APS observational study. Blood. 2014;123:414–21. [DOI] [PubMed] [Google Scholar]
- 7. Greer IA, Brenner B, Gris JC. Antithrombotic treatment for pregnancy complications: which path for the journey to precision medicine? Br J Haematol. 2014;165:585–99. [DOI] [PubMed] [Google Scholar]
- 8. Rey E, Kahn SR, David M, Shrier I. Thrombophilic disorders and fetal loss: a meta‐analysis. Lancet. 2003;361:901–8. [DOI] [PubMed] [Google Scholar]
- 9. Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83:536–45. [DOI] [PubMed] [Google Scholar]
- 10. Erez O, Mastrolia SA, Thachil J. Disseminated intravascular coagulation in pregnancy: insights in pathophysiology, diagnosis and management. Am J Obstet Gynecol. 2015;213:452–63. [DOI] [PubMed] [Google Scholar]
- 11. Thachil J, Toh CH. Disseminated intravascular coagulation in obstetric disorders and its acute haematological management. Blood Rev. 2009;23:167–76. [DOI] [PubMed] [Google Scholar]
- 12. Rattray DD, O'Connell CM, Baskett TF. Acute disseminated intravascular coagulation in obstetrics: a tertiary centre population review (1980 to 2009). J Obstet Gynaecol Can. 2012;34:341–7. [DOI] [PubMed] [Google Scholar]
- 13. Erez O, Novack L, Beer‐Weisel R, Dukler D, Press F, Zlotnik A, et al. DIC score in pregnant women–a population based modification of the International Society on Thrombosis and Hemostasis score. PLoS ONE. 2014;9:e93240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kor‐anantakul O, Lekhakula A. Overt disseminated intravascular coagulation in obstetric patients. J Med Assoc Thai. 2007;90:857–64. [PubMed] [Google Scholar]
- 15. Naz H, Fawad A, Islam A, Shahid H, Abbasi AU. Disseminated intravascular coagulation. J Ayub Med Coll Abbottabad. 2011;23:111–3. [PubMed] [Google Scholar]
- 16. Chaiworapongsa T, Espinoza J, Yoshimatsu J, Kim YM, Bujold E, Edwin S, et al. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002;11:368–73. [DOI] [PubMed] [Google Scholar]
- 17. Chaiworapongsa T, Yoshimatsu J, Espinoza J, Kim YM, Berman S, Edwin S, et al. Evidence of in vivo generation of thrombin in patients with small‐for‐gestational‐age fetuses and pre‐eclampsia. J Matern Fetal Neonatal Med. 2002;11:362–7. [DOI] [PubMed] [Google Scholar]
- 18. Erez O, Gotsch F, Mazaki‐Tovi S, Vaisbuch E, Kusanovic JP, Kim CJ, et al. Evidence of maternal platelet activation, excessive thrombin generation, and high amniotic fluid tissue factor immunoreactivity and functional activity in patients with fetal death. J Matern Fetal Neonatal Med. 2009;22:672–87. [DOI] [PubMed] [Google Scholar]
- 19. Erez O, Romero R, Vaisbuch E, Kusanovic JP, Mazaki‐Tovi S, Chaiworapongsa T, et al. The pattern and magnitude of “in vivo thrombin generation” differ in women with preeclampsia and in those with SGA fetuses without preeclampsia. J Matern Fetal Neonatal Med. 2017;31:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gardiner C, Vatish M. Impact of haemostatic mechanisms on pathophysiology of preeclampsia. Thromb Res. 2017;151(Suppl 1):S48–s52. 10.1016/s0049-3848(17)30067-1. [DOI] [PubMed] [Google Scholar]
- 21. Tannetta DS, Hunt K, Jones CI, Davidson N, Coxon CH, Ferguson D, et al. Syncytiotrophoblast extracellular vesicles from pre‐eclampsia placentas differentially affect platelet function. PLoS ONE. 2015;10:e0142538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopez‐Llera M, de la Luz Espinosa M, Diaz de Leon M, Linares GR. Abnormal coagulation and fibrinolysis in eclampsia. A clinical and laboratory correlation study. Am J Obstet Gynecol. 1976;124:681–7. [DOI] [PubMed] [Google Scholar]
- 23. Redline RW. Classification of placental lesions. Am J Obstet Gynecol. 2015;213:S21–8. [DOI] [PubMed] [Google Scholar]
- 24. Levi M. Disseminated intravascular coagulation. Crit Care Med. 2007;35:2191–5. [DOI] [PubMed] [Google Scholar]
- 25. Collins P, Abdul‐Kadir R, Thachil J. Management of coagulopathy associated with postpartum hemorrhage: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:205–10. [DOI] [PubMed] [Google Scholar]
- 26. Siddiq S, Clark A, Mumford A. A systematic review of the management and outcomes of pregnancy in Glanzmann thrombasthenia. Haemophilia. 2011;17(5):e858–69. [DOI] [PubMed] [Google Scholar]
- 27. Peitsidis P, Datta T, Pafilis I, et al. Bernard Soulier Syndrome in pregnancy: a systematic review. Haemophilia. 2010 Jul 1;16(4):584–91. [DOI] [PubMed] [Google Scholar]
- 28. Gresele P, Bury L, Bury L, Mezzasoma AM, Falcinelli E. Platelet function assays in diagnosis: an update. Expert Rev Hematol. 2019;12(1):29–46. [DOI] [PubMed] [Google Scholar]
- 29. Goldenberg NA, Manco‐Johnson MJ. Protein C deficiency. Haemophilia. 2008;14:1214–21. [DOI] [PubMed] [Google Scholar]
- 30. Marciniak E, Wilson HD, Marlar RA. Neonatal purpura fulminans: a genetic disorder related to the absence of protein C in blood. Blood. 1985;65:15–20. [PubMed] [Google Scholar]
- 31. Sultan AA, Tata LJ, West J, Fiaschi L, Fleming KM, Nelson‐Piercy C, et al. Risk factors for first venous thromboembolism around pregnancy: a population‐based cohort study from the United Kingdom. Blood. 2013;121:3953–61. [DOI] [PubMed] [Google Scholar]
- 32. Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy‐related mortality in the United States, 2011‐2013. Obstet Gynecol. 2017;130:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martillotti G, Boehlen F, Robert‐Ebadi H, Jastrow N, Righini M, Blondon M. Treatment options for severe pulmonary embolism during pregnancy and the postpartum period: a systematic review. J Thromb Haemost. 2017;15:1942–50. [DOI] [PubMed] [Google Scholar]
- 34. Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic Therapy for VTE Disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e419S–94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos A‐M, Vandvik PO; American College of Chest Physicians . VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;e691S–736S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bates SM, Rajasekhar A, Middeldorp S, McLintock C, Rodger MA, James AH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2:3317–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bapat P, Kedar R, Lubetsky A, Matlow JN, Aleksa K, Berger H, Koren G. Transfer of dabigatran and dabigatran etexilate mesylate across the dually perfused human placenta. Obstet Gynecol. 2014;123:1256–61. [DOI] [PubMed] [Google Scholar]
- 38. Bapat P, Pinto LS, Lubetsky A, Berger H, Koren G, et al. Rivaroxaban transfer across the dually perfused isolated human placental cotyledon. Am J Obstet Gynecol. 2015;213:710.e1–6. [DOI] [PubMed] [Google Scholar]
- 39. Bapat P, Pinto LS, Lubetsky A, Aleksa K, Berger H, Koren G, et al. Examining the transplacental passage of apixaban using the dually perfused human placenta. J Thromb Haemost. 2016;14:1436–41. [DOI] [PubMed] [Google Scholar]
- 40. Cohen H, Arachchillage DR, Middeldorp S, Beyer‐Westendorf J, Abdul‐Kadir R. Management of direct oral anticoagulants in women of childbearing potential: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1673–6. [DOI] [PubMed] [Google Scholar]
- 41. Beyer‐Westendorf J, Michalski F, Tittl L, Middeldorp S, Cohen H, Abdul‐Kadir R, et al. Pregnancy outcome in patients exposed to direct oral anticoagulants ‐ and the challenge of event reporting. Thromb Haemost. 2016;116:651–8. [DOI] [PubMed] [Google Scholar]


