Abstract
Background
A prolonged activated partial thromboplastin time (APTT) of unknown cause is one of the most frequent reasons why outpatients are referred for hemostasis consultation. Nevertheless, very few data are available on the relative contribution of individual causes of this common clinical scenario. Here, we present a systematic evaluation of all causes of APTT prolongation in a consecutive population of outpatients referred for specialized hemostasis consultation during a 14‐year period.
Methods
All cases referred to an academic specialized hemostasis outpatient unit due to APTT prolongation of unknown etiology whose prolonged APTT was confirmed in the first visit were included in the study. Data were obtained from the electronic medical records.
Results
Among 187 consecutive patients, the most frequent causes were antiphospholipid antibodies in 22.6%, contact pathway factor deficiencies in 17.4%, other coagulation factor deficiencies in 11.6%, and vitamin K deficiency/liver disease in 11.6%. A definite cause was not identified in 22.1% of patients. Presence of antiphospholipid antibodies, and absence of bleeding symptoms were both associated with significantly longer APTT values compared to other categories/clinical scenarios. The investigation of each case required a mean of 18.2 additional tests per patient, with estimated costs ranging from US$191.60 to US$1055.60.
Conclusions
Our results describe the main causes of APTT prolongation in outpatients, as well as estimates of resource use required to investigate this condition, thus providing evidence supporting the importance of measures to minimize the indiscriminate use of this assay.
Keywords: blood coagulation, clinical laboratory techniques, health care costs, hemostasis, partial thromboplastin time
Essentials.
Prolonged activated partial thromboplastin time (APTT) is one of the most common reasons for hematology consultation.
In this scenario, the main causes of prolonged APTT are not associated with increased bleeding risk.
Antiphopholipid antibodies represent the main cause of APTT prolongation in outpatients.
In conclusion, our data reinforce the importance of rational use of this common screening assay.
1. INTRODUCTION
The past 3 decades have witnessed tremendous improvements in our understanding about hemostasis and in our technical capability to scale up the measurement of coagulation and platelet function in clinical laboratories. However, the pace of these technical developments frequently outruns our capacity to generate data about the clinical and financial impact of the incorporation of these technologies in patient care. The activated partial thromboplastin time (APTT) and prothrombin time (PT) are screening assays indicated for the initial assessment of disorders of hemostasis. Although these assays have been described for several decades, recent improvements of coagulation automated analyzers now available in most clinical laboratories led to their widespread use, even in conditions in which their indication is not clear.1 The APTT is a coagulometric assay in which platelet‐poor plasma is stimulated by a reagent containing phospholipids, an activator (eg, ellagic acid, silica), and calcium chloride. The time to fibrin formation, measured by optical or mechanical methods is dependent on the concentration and/or function of coagulation factors of the intrinsic and final pathways of the coagulation cascade, as well as on the presence of specific (ie, against discrete coagulation factors) or nonspecific (ie, against antiphospholipids) inhibitors to components of the reaction. In addition to preanalytical variables, which are known to strongly influence all plasma‐based assays of hemostasis, differences in APTT sensitivity dependent on the phospholipid composition and the type of activator used in different APTT reagents are also a cause of significant imprecision of this assay,2, 3 a fact that supports the recommendation that laboratories should establish reagent‐specific reference ranges.4 Accordingly, the potential impact of these variables (ie, preanalytical and reagent‐specific sensitivity) should be acknowledged when results are interpreted in the clinical setting.
Although the APTT was developed as a tool to aid in the diagnosis of hemophilia,5 it is also indicated to evaluate or monitor patients with other disorders of hemostasis (affecting the intrinsic and common final pathways of the coagulation cascade) and in the monitoring of therapy with unfractionated heparin.6 However, this assay is also used for less clear‐cut indications such as the investigation of consumptive coagulopathies7 and in preoperative screening.8 As mentioned above, it has recently been demonstrated that a high proportion of APTT requests are not supported by evidence, even in academic medical centers.1
Despite the use of the APTT for several decades in nearly all medical services, to our knowledge only 1 study systematically addressed the relative contribution of different conditions known to prolong this test in a population of outpatients referred to hemostasis consultation, which represents a common clinical problem faced by hematologists. Herein, we consecutively evaluated all cases of APTT prolongation in an outpatient hemostasis laboratory during a 14‐year period, thereby providing data on the relative contribution of different conditions for APTT prolongations. We also describe the resources used to investigate these results and discuss the financial impact thereof.
2. METHODS
2.1. Study population
The study was designed as a retrospective cohort study performed in the outpatient hemostasis clinic of the University of Campinas, which is the only service that provides specialized consultation in hemostasis for an area of around 6 million inhabitants in southeastern Brazil. Patients attending this clinic are referred from primary care services or from other medical specialties, including hematologists from our region. The clinic is also a reference hemostasis service for hematologists from other areas of the country on a more sporadic basis. The study was performed in accordance with the Declaration of Helsinki and approved by the local Institutional Review Board.
The study evaluated all cases of prolonged APTT, with or without bleeding symptoms, referred to specialized consultation between September 2003 and April 2017. Cases were identified by a stepwise evaluation of our electronic database and medical records. An APTT ratio >1.30 was used as a threshold to initially screen for cases from our laboratory database. Because the aim of our study was to describe the distribution of conditions causing a prolonged APTT in this specific outpatient setting (ie, patients referred for diagnostic evaluation of a prolonged APTT), only cases in which the APTT prolongation was confirmed in the first visit were included. In addition, all cases in which the diagnosis of a hereditary bleeding disorder was established before patient referral (eg, patients with a confirmed diagnosis of factor VIII [FVIII] or factor IX [FIX] deficiencies referred to our hemophilia unit) or patients referred due to a diagnosis of conditions known to be associated with prolonged APTT (eg, patients referred for unfractionated heparin monitoring) were also excluded from the analysis.
2.2. Data collection and investigation protocols
Clinical and laboratory data were obtained from the electronic medical records of our institution. In the first visit, the APTT was repeated in our laboratory, along with a PT. Mixing studies were performed in the same sample in all cases with a prolonged APTT, and these results were available for the medical evaluation. A detailed clinical history and physical examination focused on bleeding and thrombotic events was then obtained. Standardized bleeding scores9 were used at the attending physician's discretion until 2012 and systematically thereafter. No other significant change was introduced in patient management during the study period, except for improvements in the quality of laboratory reagents. Additional laboratory assays were requested according to the clinical presentation until a confirmatory diagnosis was obtained.
2.3. Laboratory measurements
All assays were performed in the hemostasis laboratory of our institution, which participates in the National External Quality Assessment Scheme in hemostasis. Only commercial reagents and control material were used. Assays were performed in accordance with guidelines from the Brazilian Ministry of Health10 and of the World Federation of Hemophilia.11 During the study period, different reagents were used in the assays always preceded by standardized validation protocols. All assays were performed in automated coagulometers (Siemens XP system, Siemens Healthcare, Munich, Germany; and ACLTop 550‐IL, Instrumentation Laboratory, Devens, MA). For the APTT, the following reagents were used during the study period: Pathromtin SL (Siemens Healthcare) from 2003 to 2013,and HemosIL APTT‐SP liquid (Instrumentation Laboratory) from 2013 to 2017, both based on silica. Lupus anticoagulants (LAs) were detected following recommendations available.12, 13 Two different assays, consisting of a dilute Russell's viper venom time (dRVVT; first choice) and a second LA‐sensitive assay, were always used. The dRVVT reagents were LA1 Screening and LA2 Confirmation (Siemens Healthcare) from 2003 to 2015, and dRVVT screen and dRVVT confirm (Instrumentation Laboratory) from 2015 to 2017. Of note, from 2013 on, cutoff values for the dRVVT interpretation began to be locally established using the 99th percentile of the distribution of a local pool of blood donors instead of values provided by the manufacturer. The second LA‐sensitive assay was an in‐house kaolin clotting time (only in 2003), which was substituted for an LA‐sensitive APTT from 2004 to 2016 (APTT Actin FSL, Siemens Healthcare) and for the Silica Clotting Time (Instrumental Laboratory) from 2016 to 2017.
2.4. Financial impact analysis
We next evaluated the use of additional laboratory resources in the investigation of prolonged APTT. The number of specific assays was individually counted per patient. The cost in US dollars was estimated using 2 Brazilian national reimbursement tables: (1) the national reference table with suggested reimbursement costs for private health insurance14; and (2) costs from a reference private hemostasis laboratory that provides services for the whole country.15
2.5. Statistical analyses
Data are presented as means, medians, standard deviation, and range, as detailed in each table or figure. Means were compared using Student's t‐test or Mann Whitney test, according to variable distribution. A P value <0.05 was considered as statistically significant. All analyses and graphs were performed using Prism 7.0 (GraphPad Software, La Jolla, CA).
3. RESULTS
Between September 2003 and April 2017, a total of 7983 prolonged APTTs were released by the hemostasis laboratory of the University of Campinas. After exclusion of repetitions from the same patient, a total of 2468 results were identified. Of these, 941 results were from 941 patients in the first visit to our hemostasis outpatient clinic, with the remaining results corresponding to patients who were already followed in our center with a known diagnosis. Of these, 754 patients were excluded because they were referred with a definitive diagnosis associated with APTT prolongation such as hemophilia or unfractionated heparin use. In total, 187 patients who were specifically referred to our clinic to investigate a confirmed prolongation of APTT on unknown etiology were included in our study. A detailed flowchart is shown in Figure 1.
Figure 1.
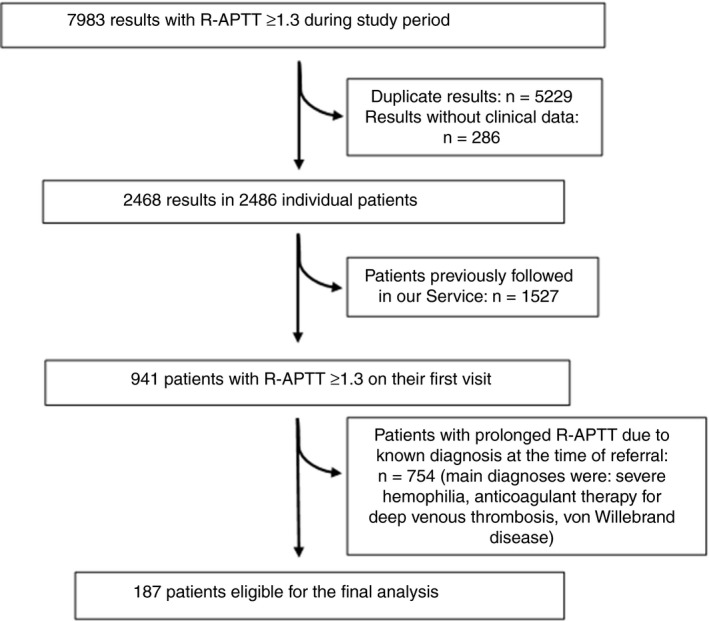
Flowchart of the study population. R‐APTT, activated thromboplastin time ratio
The main clinical and demographic characteristics of study patients are shown in Table 1. Of note, approximately half of the patients reported the presence of at least 1 bleeding symptom at the first evaluation, although the median bleeding score9 was low in the majority of patients.
Table 1.
Demographic and clinical characteristics of the study population
| Patient characteristics | n = 187 |
|---|---|
| Age, median (IQR) | 22 (8‐46) |
| Sex (male:female) | (1.27:1) |
| Reason for referral | |
| Isolated prolongation of APTT, n (%) | 123 (65.8) |
| Combined prolongation of APTT + PT, n (%) | 64 (34.2) |
| Presence of bleeding symptoms, n (%) | 97 (51.8) |
| Bleeding score,a median (IQR) | 1 (0‐5) |
| Family history of abnormal bleeding, n (%) | 16 (8.6) |
APTT, activated partial thromboplastin time; IQR, interquartile range; PT, prothrombin time.
Data available for 36 of 97 with bleeding symptoms.
The distribution of the APTT ratio (R‐APTT) ranged from 1.3 to 8.0 as shown in Figure 2A.
Figure 2.
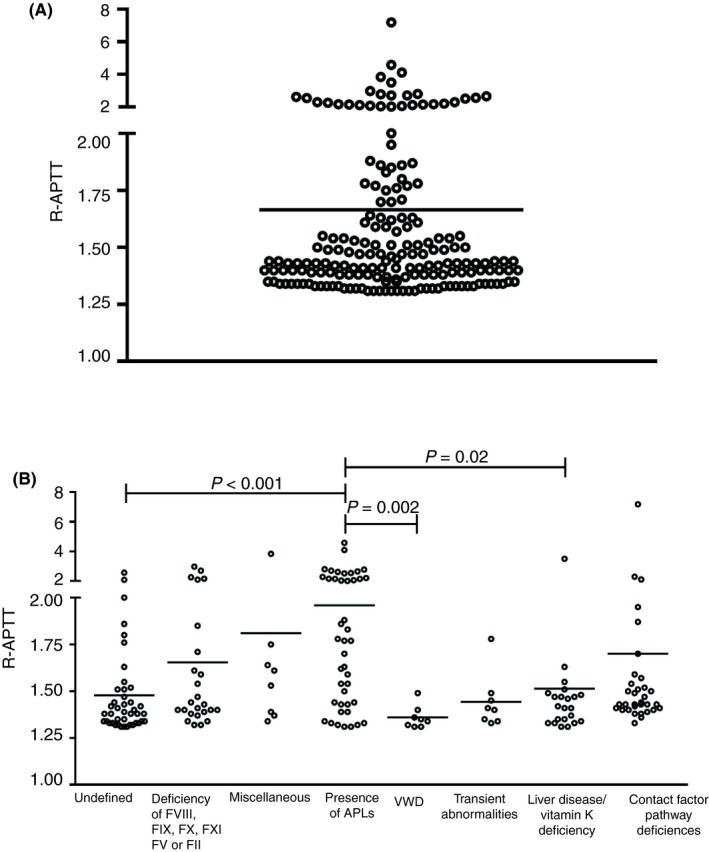
(A) Dot plot of the activated partial thromboplastin time ratio (R‐APTT) of the study population. Horizontal bar indicates the median. (B) R‐APTT values for each diagnostic category are shown. Patients with APLs had a significantly higher R‐APTT than other categories (Kruskal‐Wallis test). APLs, antiphospholipid antibodies; F, factor; VWD, von Willebrand disease
A specific diagnosis for the prolonged APTT was defined in 77.9% of patients. To facilitate interpretation of these results, we grouped all causes into 8 categories: presence of antiphospholipid antibodies (APLs); deficiencies of a factor of the contact pathway (factor XII [FXII], high‐molecular‐weight kininogen, prekallikrein); deficiencies of factors of the intrinsic and common pathways (factors VIII, IX, X, XI, V, II); von Willebrand disease (VWD); liver disease/vitamin K deficiency; transient APTT prolongation (refers to cases in which the APTT normalized in the course of the investigation); miscellaneous causes (hypofibrinogenemia, disseminated intravascular coagulation, and supercoumarin intoxication); and undefined causes. Of note, the APL category included all patients who tested positive for an LA in 2 independent samples, and VWD was diagnosed when low FVIII levels were associated with von Willebrand factor and/or ristocetin cofactor activity <30 IU/dL. The relative distribution of these causes and their relative distribution according to age and the presence of bleeding symptoms are shown in Table 2.
Table 2.
Causes of prolonged APTT in the study population and their distribution according to age and bleeding symptoms
| Cause of prolonged APTT | n (%) | Children n (%) | Adolescent/adult n (%) | Bleeding (+) | Bleeding (−) |
|---|---|---|---|---|---|
| Presence of APLs | 43 (22.6) | 9 (13.8) | 34 (27.4) | 18 (18.5) | 25 (28.4) |
| Contact factor pathway deficiencies | 32 (17.4) | 20 (30.7) | 13 (10.4) | 16 (16.4) | 17 (19.3) |
| Deficiency of factor VIII, IX, X, XI, V, or II | 26 (13.7) | 5 (7.6) | 20 (16.1) | 14 (14.4) | 10 (11.3) |
| von Willebrand disease | 8 (4.2) | 5 (7.6) | 3 (2.4) | 2 (2) | 5 (5.6) |
| Liver disease/vitamin K deficiency | 22 (11.6) | 2 (3) | 20 (16.1) | 9 (9.2) | 11 (12.5) |
| Transient abnormalitiesa | 8 (4.2) | 2 (3) | 6 (4.8) | 3 (3) | 5 (5.6) |
| Miscellaneous b | 8 (4.2) | 5 (7.6) | 3 (2.4) | 8 (8.2) | 0 (0) |
| Undefined | 42 (22.1) | 17 (26.1) | 25 (20.1) | 27 (27.8) | 15 (17) |
| Total | 189c (100) | 65 (100) | 124 (100) | 97 (100) | 88 (100) |
APLs, antiphospholipid antibodies (defined as the presence of a lupus anticoagulant in 2 independent samples); APTT, activated partial thromboplastin time.
Transient abnormalities (refers to cases in which the APTT normalized in the course of the investigation).
Miscellanous included hypofibrinogenemia, disseminated intravascular coagulation, and supercoumarin intoxication.
Two patients had 2 different diagnoses, which results in 189 diagnoses.
We also investigated whether this pattern of causes remained stable throughout the whole study, which was divided into 3 periods for this analysis. As shown in Figure 3, except for a clear increase in the proportion of cases attributed to the presence of APLs, other causes remained stable through time.
Figure 3.
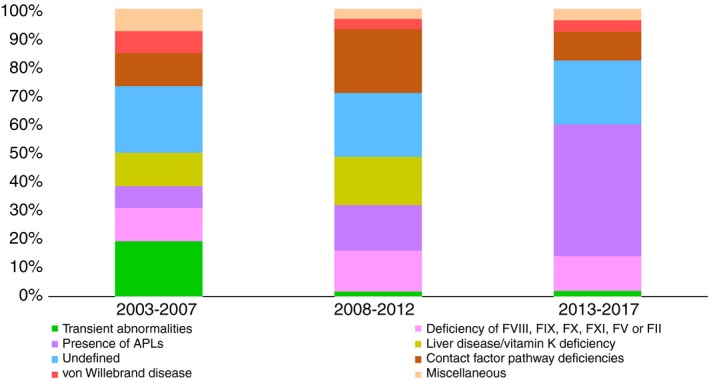
The 3 bars indicate the contribution of each cause for APTT prolongation in 3 consecutive time periods. Except for an increase in the diagnosis of antiphospholipid antibodies (APLs), and a reduction of transient abnormalities toward more recent periods of the study, the contribution of other causes seemed to be stable during the whole study period. APTT, activated partial thromboplastin time; F, factor
We next compared the magnitude of APTT prolongation between children (ages 0‐12; n = 63) and adolescents/adults (age >12; n = 124), as well as between patients with or without bleeding symptoms at the time of their first evaluation. Of note, the median R‐APTT in patients without bleeding symptoms (1.41; interquartile range [IQR], 1.35‐1.58; n = 88) was significantly higher than in patients who reported any bleeding symptom (1.48; IQR, 1.38‐1.77; n = 96; P = 0.04). In addition, the R‐APTT was significantly longer in patients in whom a definitive cause could be established (1.47; IQR, 1.39‐1.77; n = 42) compared to patients with undefined causes (1.38; IQR, 1.33‐1.51; n = 147; P = .0015) (Figure 4).
Figure 4.
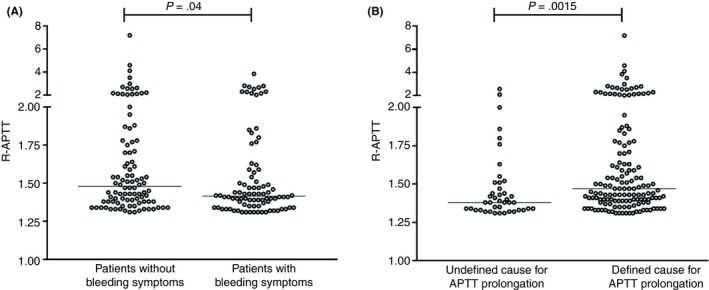
(A) APTT results are compared for patients with or without bleeding symptoms. A significantly higher R‐APTT was observed in patients without bleeding symptoms (Mann‐Whitney test). (B) We demonstrate that patients for whom a definitive cause for the prolongation of the APTT could be established had a significantly higher R‐APTT (Mann‐Whitney test). APTT, activated partial thromboplastin time; R‐APTT, activated partial thromboplastin time ratio
To investigate whether the magnitude of APTT prolongation was associated with any specific cause, we compared median R‐APTT values across all 8 different diagnostic categories. As shown in Figure 2B, patients with an APL had a significantly higher R‐APTT compared to other groups.
Finally, we evaluated resource used for each patient until a definite cause for APTT prolongation could be established. As shown in Table 3, each case required a mean of 16.8 (standard deviation, 11.2) additional assays during the etiological investigation.
Table 3.
Resources used to evaluate prolonged APTT
| Laboratory assays performed for the investigation | Total | Mean number of tests per patient (SD) |
|---|---|---|
| Hematology and hemostasis screening assaysa | 1868 | 9.8 (6.8) |
| Measurement of coagulation factor levels | 721 | 3.8 (3.3) |
| Additional hemostasis assaysb | 327 | 1.7 (2.9) |
| Inhibitor assaysc | 42 | 0.2 (0.6) |
| Antiphospholipid antibodies assaysd | 119 | 0.6 (0.9) |
| Liver function tests | 121 | 0.6 (1.9) |
| Total | 3198 | 16.8 (11.2) |
APTT, activated partial thromboplastin time; SD, standard deviation.Mean indicates the mean of the number of tests per each patient.
Hematology and hemostasis screening tests include complete blood counts, reticulocyte counts, repeated PT and APTT, thrombin time, and bleeding time.
Additional hemostasis assays include von Willebrand factor antigen assay, von Willebrand factor ristocetin cofactor assay, platelet aggregation test, fibrinogen assays, and euglobulin lysis time.
Inhibitor screening assays include mixing studies and Bethesda assays.
Antiphospholipid antibody assays: lupus anticoagulant tests; von Willebrand factor antigen, ristocetin cofactor activity.
In Table 4, the financial impact of the additional laboratory investigation for a prolonged APTT was estimated based on the number of assays required, according to the reference costs for 2 different categories of health care providers in Brazil: the cost suggested for reimbursement of laboratories by private health care insurance companies14 and costs charged by the laboratory by 1 reference private laboratory.15 Estimated costs with additional laboratory assays varied between US$157.40 and US$887.10.
Table 4.
Estimated financial impact of APTT investigation
| Tests | Costs according to health care provider category (in US$) | |
|---|---|---|
| Insurance | Private | |
| Hematology and hemostasis screening assays | 9.4 | 68.0 |
| Measurement of coagulation factor levels | 50.8 | 464.8 |
| Additional hemostasis assays | 61.7 | 181.7 |
| Inhibitor assays | 18.8 | 59.7 |
| Antiphospholipid antibody assays | 8.5 | 54.5 |
| Liver function tests | 8.2 | 58.4 |
| Total | 157.4 | 887.1 |
APTT, activated partial thromboplastin time.
Brazilian reais (R$) were converted into US dollars based on rates from September 2018. Costs were calculated by multiplying the average number of tests performed in each category by the individual value of each test.
4. DISCUSSION
Despite being one of the most widely used hemostasis screening assays, few data are available on the causes of prolonged APTT in patients referred for hematologic consultation. To our knowledge, only 1 study, published more than 30 years ago, described the relative contribution of different causes of APTT prolongation among 100 patients referred for hemostasis consultation.16 The main result of our study was a contemporary description of causes of prolonged APTT in 187 outpatients from an academic hemostasis clinic, complemented by an estimation of the financial impact of this investigation.
Causes of prolonged APTT have been previously described in 2 different scenarios. In a study that included 177 inpatients from a general hospital, the main cause of prolonged APTT was the presence of LA, detected in 53.1% of patients, followed by undetermined causes in 31.6%. Factor deficiencies were rare, with those associated with bleeding risk observed in only 4.5%.17 However, these results cannot be extrapolated to outpatients. In a study performed in a clinical scenario similar to ours, Kitchens16 described causes of APTT prolongation in 100 consecutive patients referred for hemostasis consultation. The most frequent cause was the presence of an LA (33% of patients), followed by liver disease/vitamin K deficiency (9%). Coagulation factor deficiencies associated with bleeding risk were observed in 50% of patients, and FXII deficiency in only 2%.18
In the 3 decades since this publication, the quality of equipment and reagents used in hemostasis laboratories has improved, and accessibility to hemostasis assays has increased, leading to gains in accuracy of these assays and to an increase in the number of patients for whom an APTT was ordered. We therefore hypothesized that the pattern of distribution of causes for prolonged APTT also could have changed. In addition, we wanted to estimate the financial impact of these investigations. In regard to the first hypothesis, although the presence of APL (which in our study included all patients with a positive LA in at least 2 independent samples) was still the main cause of prolonged APTT, contact pathway factor deficiencies emerged as the third most frequent cause, and a definitive explanation for the prolonged APTT could not be obtained in 22.1% of patients despite a comprehensive investigation. Because the former 2 conditions are not associated with bleeding symptoms, our results indicate that in at least 60% of cases, the clinical benefit of the extensive investigation that follows an index APTT prolongation is not clear. Previously undiagnosed liver disease was also an important cause of APTT prolongation (in nearly 10% of patients). Of note, although we included only patients in whom the APTT prolongation was confirmed in a second sample, normalization of the APTT still occurred in almost 5% of cases, highlighting the importance of repeating the assay in the course of the investigation.
As for the 22.1% of patients in whom a defined cause of APTT prolongation was not identified, most were <12 years old and asymptomatic. We list several potential reasons to explain these cases. First, limitations on currently available laboratory assays to fully characterize hemostasis alterations could justify the presence of so‐called blind spots in laboratory hemostasis assessment. It should be noted, however, that because these patients were mostly asymptomatic, most of these alterations possibly are not clinically relevant. Second, as the APTT is determined by levels of several coagulation factors, borderline yet normal values of multiple factors could lead to mild prolongations not explained by any single factor deficiency. Again, the clinical relevance of this observation seems limited based on the clinical presentation of these patients. Third, C‐reactive protein levels have been shown to prolong APTT,18 although none of our patients in the “undefined” group presented clinical evidence of ongoing inflammation. Fourth, based on the classical definition of reference ranges, one should expect that roughly 2.5% of any unselected population would have an abnormal APTT, despite having a normal hemostatic system.19 This proportion could be higher in a population of asymptomatic individuals with marginally prolonged APTT values such as ours, which could explain the persistence of a significant number of patients with an undefined diagnosis. Finally, difficulties in establishing what is normal in terms of APTT values, particularly for children who are normally not included in studies that determine reference ranges, could also explain part of these results. The relevance of this fact was highlighted by an elegant study that used big data of glycated hemoglobin levels to demonstrate that the proportion of healthy individuals flagged as abnormal could reach up to 27% depending on how reference ranges were determined.20 Accordingly, although marginally prolonged APTT could result from mild factor deficiencies that can be clinically relevant when hemostasis is challenged, hematologists responsible for the investigation of these patients, particularly those with no bleeding symptoms, should not overlook the possibility that their laboratory alterations could also represent variations of a normal distribution. Of note, we believe that this inherent imprecision of reference ranges for hemostasis assays could also explain the transient nature of some APTT prolongations, which could be a result of the fact that a borderline normal value is more likely to fall outside the normal range when measured repeatedly.
Because our study spanned a long period, we also investigated the effect of time on our results and observed an increase in the proportion of APLs as a cause for prolonged APTT. Although our laboratory followed the recommendations of the Clinical and Laboratory Standards Institute and the Scientific and Standardization Committee of ISTH21, 22 for the selection of reagents with relative insensitivity to the presence of APLs for general‐purpose APTT, the protocols for the identification of these antibodies were reviewed in 2013, leading to an improvement in the performance of these assays in our laboratory, according to external quality evaluations, and reinforcing APL as the main cause of prolonged APTT in this clinical scenario.
We also demonstrate that patients without bleeding symptoms and with a defined cause of APTT prolongation presented significantly higher APTT values than patients with bleeding symptoms and with undefined causes, respectively. This is probably attributed to the effect of APLs (and also of some cases with severe contact factor deficiencies), which are responsible for higher R‐APTT values in our population, as these patients tend to be asymptomatic and are less likely to remain undiagnosed. However, the overlap of R‐APT across all categories precludes inferences about etiologic diagnoses based on this assay.
Finally, we report for the first time the estimated impact of the investigation of APTT prolongation in outpatients. A median (mean) of 20 (18) assays were required per patient, costing almost $1000 per patient, or approximately 1000 times the value of a single APTT in Brazil. While it would not be reasonable to advocate that these cases should not be properly investigated, as important diagnoses were made such as VWD, liver disease, and other coagulation factor deficiencies, our data are sufficient to support measures to avoid the indiscriminate use of the APTT, thus limiting the number of asymptomatic patients requesting this investigation. Regulation of the use of APTT in preoperative evaluations is probably one such effective measure, given the high proportion of inadequate requests in this setting, even in academic centers,1 coupled with the recent confirmation of the low accuracy of this assay in identifying patients with bleeding diseases.23 We also feel that all prolonged APTTs that are not associated with a clinical history of bleeding should be confirmed before pursuing further workup.
Our study has limitations that should be acknowledged. First, the retrospective design is associated with inherent limitations such as incomplete information and variations in patients’ care. However, the fact that our outpatient clinic is part of an academic medical center limits the impact of these biases, as investigation protocols are strictly followed by our medical staff. The availability of electronic medical records and the stepwise protocol used to identify cases also limit the possibility of missing information. Second, we included only patients with a confirmed APTT prolongation, which precluded the discussion on the proportion of cases in which the prolongation is caused by laboratory errors (preanalytical, analytical, and postanalytical). While of interest to most clinicians, the contribution of these errors is dependent mainly on the quality of clinical and laboratory care of referring centers, so that results from a single center are difficult to generalize. We therefore focused on the description of cases with sustained APTT prolongations, which represent a well‐defined cohort of patients, prevalent in most hemostasis services. Changes in laboratory equipment and reagents during the study are also worth considering, although as our study aimed to capture clinical practice, this limitation should also be considered acceptable. Of note, our laboratory has been part of international external quality systems throughout most of the study period and constantly updates its protocols in accordance with major international guidelines. Finally, it is important to acknowledge that since the standardized bleeding questionnaire was not introduced as a routine in our center until after 2012, the associations involving the presence of bleeding symptoms should be evaluated with caution.
In conclusion, our study provides contemporary data on the relative distribution of causes for APTT prolongation in one of the most common clinical scenarios of consultative hemostasis, confirming APLs as the main cause of this laboratory alteration, and highlighting the significance of a prolonged APTT in the absence of a specific disease of hemostasis. We also provide evidence supporting the importance of measures to minimize the indiscriminate use of these assays, to avoid the inadequate use of the finite resources available for our health systems.
RELATIONSHIP DISCLOSURE
The authors declare no relationship to disclose.
AUTHOR CONTRIBUTIONS
ACNB collaborated with study design; collected, processed, and analyzed the data; and drafted the manuscript. SALM, MPC, JMAB and MCO analyzed laboratory data. KGNB contributed to statistical analyses and computational techniques to summarize results. EVDP designed the study, analyzed the data, and drafted the manuscript. All authors revised the final version of the manuscript.
Supporting information
ACKNOWLEDGMENTS
This study was funded by Sao Paulo Research Foundation grant #2016/14172‐6 to EVDP; CNPq Brasil and FAEPEX‐Unicamp.
Barbosa ACN, Montalvão SAL, Barbosa KGN, et al. Prolonged APTT of unknown etiology: A systematic evaluation of causes and laboratory resource use in an outpatient hemostasis academic unit. Res Pract Thromb Haemost. 2019;3:749–757. 10.1002/rth2.12252
REFERENCES
- 1. Capoor MN, Stonemetz JL, Baird JC, Ahmed FS, Awan A, Birkenmaier C, et al. Prothrombin time and activated partial thromboplastin time testing: a comparative effectiveness study in a million‐patient sample. PLoS ONE. 2015;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paula EV. Avaliação laboratorial da hemostasia. Possibilidades e limitações In: Zago MA, Falcão RP, Pasquini R, eds. Tratado de Hematologia. São Paulo: Atheneu; 2013:857–64. [Google Scholar]
- 3. Rogers GM, Lehman CM. Hemostasis screening assays In: Bennett ST, Lehman CM, Rodgers GM, eds. Laboratory Hemostasis: A Practical Guide for Pathologists. New York, NY: Springer; 2007:69–81. [Google Scholar]
- 4. Kamal AH, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc. 2007;82:864–73. [DOI] [PubMed] [Google Scholar]
- 5. Proctor RR, Rapaport SI. The partial thromboplastin time with kaolin: a simple screening test for first stage clotting factor deficiencies. Am J Clin Pathol. 1961;36:212–9. [DOI] [PubMed] [Google Scholar]
- 6. Basu D, Gallus A, Hirsh J, Cade J. A prospective study of the value of monitoring heparin treatment with the activated partial thromboplastin time. N Engl J Med. 1972;287:324–7. [DOI] [PubMed] [Google Scholar]
- 7. Mant MJ, Severe King EG. acute disseminated intravascular coagulation. A reappraisal of its pathophysiology, clinical significance and therapy based on 47 patients. Am J Med. 1979;67:557–63. [DOI] [PubMed] [Google Scholar]
- 8. Rapaport SI. Preoperative hemostatic evaluation: which tests, if any? Blood. 1983;61:229–31. [PubMed] [Google Scholar]
- 9. Rodeghiero F, Tosetto A, Abshire T, Arnold DM, Coller B, James P, et al., ISTH/SSC joint VWF and Perinatal/Pediatric Hemostasis Subcommittees Working Group . ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010;8:2063–5. [DOI] [PubMed] [Google Scholar]
- 10. Brazil. Ministry of health . Manual de Diagnóstico Laboratorial das Coagulopatias Hereditárias e Plaquetopatias. Brasília: Ministério da Saúde; 2016. http://bvsms.saude.gov.br/bvs/publicacoes/manual_diagnostico_coagulo patias _hereditarias_plaqueopatias.pdf. Accessed March 18, 2018. [Google Scholar]
- 11. Kitchen S, McCraw A, Echenagucia M, World Federation of Hemophilia . Diagnosis of Hemophilia and Other Bleeding Disorders: A Laboratory Manual, 2nd ed Quebec, QC: WFH; 2010:1–150 [Accessed January 20, 2018]. Available from: http://www1.wfh.org/publication /files/pdf‐1283.pdf. [Google Scholar]
- 12. Brandt JT, Triplett DA, Alving B, Scharrer I; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the ISTH. Criteria for the diagnosis of lupus anticoagulants: an update. Thromb Haemost. 1995;74:1185–90. [PubMed] [Google Scholar]
- 13. Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL, Galli M, et al. Update of the guidelines for lupus anticoagulant detection. J Thromb Haemost. 2009;7:1737–40. [DOI] [PubMed] [Google Scholar]
- 14. Unimed . Tabela de cobrança de coparticipação Unimed: teto de coparticipação [Accessed February 16, 2018]. Available from: http://www.unimed.coop.br/portal/conteudo/materias// 1492456554882Tabela%20Coparticipacao%202017.pdf.
- 15. Fleury SA. Diagnostic medicine center [Accessed January 27, 2015]. Available from: http://www.stf.jus.br/repositorio /cms/stfmed/stfmedprestador/anexo/tabela_fleury.pdf.
- 16. Kitchens CS. Prolonged activated partial thromboplastin time of unknown etiology: a prospective study of 100 consecutive cases referred for consultation. Am J Haematol. 1988;27:38–45. [DOI] [PubMed] [Google Scholar]
- 17. Chng WJ, Sum C, Kuperan P. Activated partial thromboplastin time in an acute care general hospital. Singapore Med H. 2005;46:450–6. [PubMed] [Google Scholar]
- 18. Van Rossum AP, Vlasveld LT, van den Hoven LJM, de Wit CWM, Castel A. False prolongation of the activated partial thromboplastin time (aPTT) in inflammatory patients: interference of C‐reactive protein. Br J Haematol. 2012;157:394–5. [DOI] [PubMed] [Google Scholar]
- 19. Siest G, Henny J, Grasbeck R, Wilding P, Petitclerc C, Queraltó JM, et al. The theory of reference values: an unfinished symphony. Clin Chem Lab Med 2013;51:47–64. [DOI] [PubMed] [Google Scholar]
- 20. Manrai AK, Patel CJ, Ioannidis JPA. In the era of precision medicine and big data, who is normal? JAMA. 2009;2018(319):1981–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ledford‐Kraemer M, Bottenus R, Brandt JT, Castellone DD, Daniele C, Groot PG, et al. CLSI Document H60‐A: Laboratory Testing for the Lupus Anticoagulant: Approved Guideline. Clinical and Laboratory Standards Institute; 2014. [Accessed January 5, 2018]. Available from: http://shop.clsi.org/hematology-documents /H60.html. [Google Scholar]
- 22. Devreese KMJ, Pierangeli SS, de Laat B, Tripodi A, Atsum T, Ortel TL; Subcommittee on Lupus Anticoagulant/Phospholipid/Dependent Antibodies. Testing for antiphospholipid antibodies with solid phase assays: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:792–5. [DOI] [PubMed] [Google Scholar]
- 23. Vries MJ, van der Meijden PE, Kuiper GJ, Wetzels RJ, van Oerle RG, Lancé MD, et al. Preoperative screening for bleeding disorders: a comprehensive laboratory assessment of clinical practice. Res Pract Thromb Haemost. 2018;2:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


