Abstract
Re-myelination of CNS nerves after injury is ineffective. Here, Petersen et al. (2017) show that the blood clotting protein fibrinogen inhibits nerve repair by preventing oligodendrocyte progenitor cells from differentiating into myelinating oligodendrocytes. Targeting fibrinogen or its downstream BMP signaling pathway may help with CNS repair.
Fibrin, the Good Cop
When a tissue is damaged and blood vessels are broached, the remarkable blood clotting system springs into action. This system is an intricate cascade of proteases arranged in series; once activated, each enzyme then activates its downstream pro-enzyme target. This organization generates catalytic amplification, and a small initiating signal can eventually manifest as a large clot. In this way, the system can quickly provide a protein plug to staunch blood flow. Fibrin, the principle protein component of blood clots, is formed when thrombin cleaves two peptides from its precursor protein, fibrinogen. This cleavage exposes binding sites in fibrin that allow its polymerization. The fibrin fibers form a network that, along with platelets, constitutes the clot.
In addition to its immediate utility to prevent hemorrhage, the fibrin network has a more long-term function. The deposited fibrin recruits inflammatory cells to migrate into the damaged area to help clean up the mess. A fibrinolytic system is also activated, and gradually the clot disappears, the inflammation dies down, and the tissue is repaired. These functions of fibrin are beneficial in the correct context, amount, and time. The crucial function of fibrin is illustrated by analysis of patients who lack fibrinogen—in a disorder termed afibrinogenemia—who are viable but have severe bleeding complications.
Fibrin, the Bad Cop
When fibrin is deposited abnormally or cannot be degraded, there are deleterious consequences. Mice lacking plasminogen, the precursor of the main fibrinolytic enzyme plasmin, are viable but die at about 6 months of age. This morbidity is due to the accumulation of fibrin, since depleting fibrinogen in plasminogen-KO mice rescues the phenotype (Bugge et al., 1996). These experiments demonstrate that excess fibrin can be pathological in the whole animal.
But exactly how does fibrin exert its negative effects? One obvious way is that excess fibrin deposition in blood vessels can restrict or block blood flow, causing ischemia, which could lead to heart attacks and ischemic strokes. As a consequence of insufficient oxygen, blood vessels can become damaged and fragile and are thus more prone to rupture and hemorrhage.
A second and more insidious pathological pathway is via fibrin deposition in tissues after vascular damage or blood-brain barrier (BBB) disruption. Fibrin is a potent pro-inflammatory molecule and can interact with receptors on macrophages and microglia, neurons, and glial cells (Ryu et al., 2009). Persistent fibrin can thus cause chronic inflammation, which can kill cells and lead to severe pathology. Persistent fibrin can also impair wound healing, as shown in the plasminogen‒KO mice (Bugge et al., 1996)
Glia Beware
Previous work by Katerina Akassoglou and her colleagues, as well as others, has established pathways by which fibrin can lead to neuronal dysfunction. Peripheral nerves can repair themselves after injury, and mice deficient in tissue plasminogen activator (tPA), a protease that can launch a fibrinolytic pathway, have impaired regeneration (Akassoglou et al., 2000). The reason for this phenotype is that, in the absence of tPA, fibrin deposited around the nerve is not degraded and interacts with Schwann cells to pre vent re-myelination of the nerve (Akassoglou et al., 2002). Thus, in this case, the nervous system dysfunction—i.e., impaired regeneration—is not due to effects on the neuron but instead on the glial cells supporting the neuron.
Fibrin can also negatively affect nerve function in the central nervous system (CNS). It is a potent inhibitor of neurite outgrowth and can promote demyelination in mouse models of multiple sclerosis (MS) (Adams et al., 2007; Akassoglou et al., 2004). It may also inhibit axonal regeneration in CNS events such as traumatic brain injury and spinal cord injury. Petersen et al. (2017) have now investigated how fibrin might prevent CNS re-myelination after injury. Since re-myelination is critical for recovery after severe nerve injury and there are no therapeutic options currently available to promote regeneration, a better understanding of this system is needed.
Oligodendrocyte progenitor cells (OPCs)—a subtype of glial cells that can differentiate into oligodendrocytes, neurons, or astrocytes depending on the culture conditions—are often unable to differentiate into mature myelinating oligodendrocytes to aid in the repair of neurons in diseases such as MS, stroke, and neonatal white-matter injury. Petersen et al. (2017) hypothesized that fibrin, which is present in the brains of patients with these disorders, may be triggering an inhibitory signal to control differentiation of OPCs and their ability to induce re-myelination. Using various cell co-culture models and techniques, the authors show that exposing OPCs to fibrinogen in vitro prevents their differentiation into mature oligodendrocytes and their ability to myelinate while not affecting the cells’ ability to proliferate. In order to take a first stab at understanding how fibrinogen could be having such an effect on OPC differentiation, the authors used whole genome microarray and gene ontology analysis. They found that bone morphogenetic protein (BMP)-responsive genes were upregulated upon fibrinogen treatment of OPCs.
The group next investigated the precise effects of fibrinogen on the BMP-signaling pathway using cultured OPCs. Not only did the addition of fibrinogen activate BMP downstream signaling through Smad1/5 phosphorylation and Id1–3, but interestingly, it also induced the differentiation of OPCs into GFAP-positive astrocytes (which could in turn induce detrimental astrocytic scar formation). Using agents to block BMP signaling and also lineage analysis, the authors show that fibrinogen activates ACVR1 receptors in OPCs to induce downstream BMP signaling. This pathway induces the differentiation of OPCs into astrocytes instead of mature oligodendrocytes and prevents re-myelination of damaged nerve tissue.
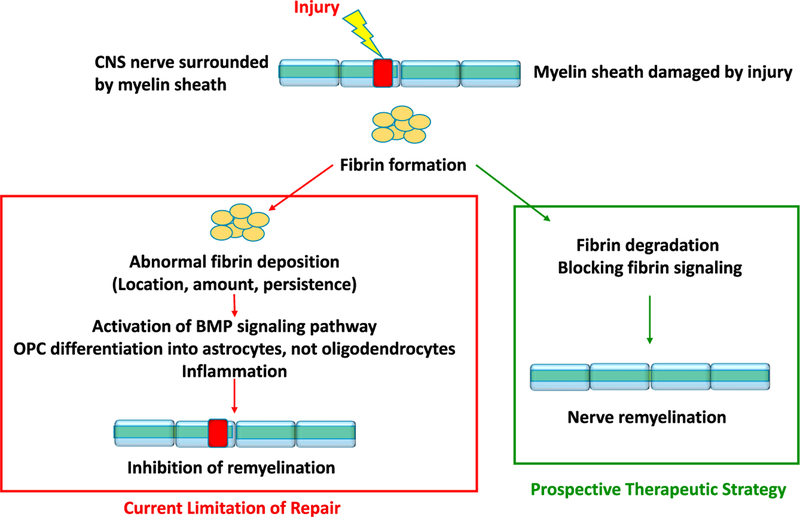
A critical test of the hypothesis was determining whether fibrin could have effects on OPCs in vivo. Petersen et al. (2017) injected lysolecithin (LPC) into the spinal cords of mice to induce focal demyelination and found abundant fibrin deposition. To determine if this fibrin plays a role in repair, they lesioned mice with LPC and treated them 3 days later with ancrod, a protein that can deplete fibrinogen. Ancrod treatment reduced fibrin deposition as expected and also reduced phosphorylated Smad in the lesioned area, indicating less BMP pathway activation. Ancrod treatment also increased the number of mature oligodendrocytes in the nerve, and electron micrographs of the axons in the lesioned area showed enhanced re-myelination in the fibrin-depleted mice as opposed to the controls. This in vivo study supports the conclusions derived from cell culture and suggests that fibrinogen depletion might be a useful therapeutic strategy after nervous system injury (Figure 1).
Figure 1. Fibrin Inhibits Re-myelination of Injured CNS Nerves.
Fibrin activates the BMP signaling pathway and prevents the differentiation of oligodendrocyte progenitor cells into myelinating oligodendrocytes, thereby inhibiting repair after injury. Blocking fibrin-mediated signaling may promote re-myelination and repair.
Future Prospects
Several interesting questions are prompted by this elegant and provocative work.
First, what is the role of inflammation in fibrin-mediated neuronal disease? Fibrin promotes inflammation through binding to leukocytes. The αMβ2 binding site on fibrin is located at position 377–395 on the γ-chain of fibrinogen. To study the relative contribution of occlusion and inflammation in fibrin pathology, a mouse was created that carries a mutated γ-chain with alanines replacing amino acids 390–396. Mice homozygous for this mutation have generally normal clotting but have an impaired host inflammatory response (Flick et al., 2004). These mice have been used to show that the αMβ2 binding site regulates collagen-induced arthritis, experimental autoimmune encephalomyelitis, bile duct injury, colitis, and liver injury. Thus, the pro-inflammatory properties of fibrin drive many of its pathological effects.
Second, what are the circumstances that can lead to abnormal fibrin deposition in the CNS? One possibility is that disease processes such as inflammation damage the BBB, allowing blood proteins—including fibrinogen—to leak into normally protected areas like the brain parenchyma or the perivascular space (Zhao et al., 2015). Once blood is in contact with these surfaces, clotting might be initiated and fibrin deposited in amounts that are difficult for the fibrinolytic system to clear. A second possibility is the clots are structurally altered and resistant to degradation. Aβ, a peptide that can drive Alzheimer’s disease (AD) pathology, binds to fibrin and inhibits its lysis (Cortes-Canteli et al., 2010). A third possibility is inappropriate downregulation of components of the fibrinolytic system leading to the inability to degrade deposited fibrin. Knowing the cause of persistent fibrin in individual diseases might allow targeted intervention to help clear the protein.
Third, why does fibrin have an inhibitory function on tissues? A rationale for fibrin’s inhibitory action might be that it acts to signal the state of the tissue damage so that repair does not commence too soon. If an area was still bleeding and/or the inflammatory system had not yet cleared cell debris, it would not be useful to initiate differentiation of cells to restore normal function. The continued presence of fibrin would put the brakes on the repair process, and its eventual removal would allow regenerative mechanisms to begin.
Defining extracellular signals that can inhibit nervous system repair has obvious therapeutic implications. Drugs that block the interaction of fibrin with its cellular receptors could have utility in reducing the inflammation that is a driving force in disease pathology in several neurological diseases. Since the clotting properties of fibrin are separable from its pro-inflammatory properties, targeting the inflammatory pathway would leave hemostasis intact. As details emerge from studies such as these about the mechanism of fibrin’s effects, it may be possible to target specific pathways to promote repair and regeneration in chronic neurological diseases such as MS and AD.
REFERENCES
- Adams RA, Schachtrup C, Davalos D, Tsigelny I, and Akassoglou K (2007). Curr. Med. Chem 14, 2925–2936. [DOI] [PubMed] [Google Scholar]
- Akassoglou K, Kombrinck KW, Degen JL, and Strickland S (2000). J. Cell Biol 149, 1157–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akassoglou K, Yu WM, Akpinar P, and Strickland S (2002). Neuron 33, 861–875. [DOI] [PubMed] [Google Scholar]
- Akassoglou K, Adams RA, Bauer J, Mercado P, Tseveleki V, Lassmann H, Probert L, and Strickland S (2004). Proc. Natl. Acad. Sci. USA 101, 6698–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugge TH, Kombrinck KW, Flick MJ, Daugherty CC, Danton MJ, and Degen JL (1996). Cell 87, 709–719. [DOI] [PubMed] [Google Scholar]
- Cortes-Canteli M, Paul J, Norris EH, Bronstein R, Ahn HJ, Zamolodchikov D, Bhuvanen dran S, Fenz KM, and Strickland S (2010). Neuron 66, 695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick MJ, Du X, Witte DP, Jirousková M, Soloviev DA, Busuttil SJ, Plow EF, and Degen JL (2004). J. Clin. Invest 113, 1596–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MA, Ryu JK, Chang KJ, Etxeberria A, Bardehle S, Mendiola AS, Kamau-Devers W, Fancy SPJ, Thor A, Bushong EA, et al. (2017). Neuron 96, this issue, 1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JK, Davalos D, and Akassoglou K (2009). J. Thromb. Haemost 7 (Suppl 1 ), 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Nelson AR, Betsholtz C, and Zlokovic BV (2015). Cell 163, 1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]