Abstract
Background:
Maternal infection during pregnancy is associated with an increased risk of schizophrenia and autism in the offspring. Supporting this correlation, experimentally activating the maternal immune system during pregnancy in rodents produces offspring with abnormal brain and behavioral development. We have developed a nonhuman primate model to bridge the gap between clinical populations and rodent models of maternal immune activation (MIA).
Methods:
A modified form of the viral mimic, synthetic double-stranded RNA (polyinosinic:polycytidylic acid stabilized with poly-L-lysine) was delivered to two separate groups of pregnant rhesus monkeys to induce MIA: 1) late first trimester MIA (n = 6), and 2) late second trimester MIA (n = 7). Control animals (n = 11) received saline injections at the same first or second trimester time points or were untreated. Sickness behavior, temperature, and cytokine profiles of the pregnant monkeys confirmed a strong inflammatory response to MIA.
Results:
Behavioral development of the offspring was studied for 24 months. Following weaning at 6 months of age, MIA offspring exhibited abnormal responses to separation from their mothers. As the animals matured, MIA offspring displayed increased repetitive behaviors and decreased affiliative vocalizations. When evaluated with unfamiliar conspecifics, first trimester MIA offspring deviated from species-typical macaque social behavior by inappropriately approaching and remaining in immediate proximity of an unfamiliar animal.
Conclusions:
In this rhesus monkey model, MIA yields offspring with abnormal repetitive behaviors, communication, and social interactions. These results extended the findings in rodent MIA models to more human-like behaviors resembling those in both autism and schizophrenia.
Keywords: Animal model, autism spectrum disorder, immune activation, macaque, nonhuman primate, poly IC, schizophrenia
Autism spectrum disorder (ASD) and schizophrenia (SZ) are chronic and disabling brain disorders that each affect approximately 1% of the population (1,2) and are thought to be caused by complex interactions between genetic and environmental factors (3-5). Recent evidence suggests that the prenatal environment, and particularly the maternal immune environment, plays a critical role in some cases of ASD and SZ (6-8). Epidemiologic studies reveal that women exposed to viral, bacterial, or parasitic infections during pregnancy have an increased risk of having a child that later develops SZ (9-14). Likewise, maternal viral and bacterial infections are associated with an increased risk of ASD in the offspring (15-19). The diversity of maternal infections associated with ASD and SZ outcomes suggests that the maternal immune response is the critical link between sickness in the mother and altered neurodevelopment in her child.
Understanding the mechanism by which maternal immune activation (MIA) during pregnancy increases the risk for SZ and ASD is essential to developing novel preventative or therapeutic strategies. Rodent models have identified molecular, cellular, and behavioral abnormalities associated with prenatal immune challenge (20). Maternal influenza infection (21-24) or injection of the bacterial endotoxin lipopolysaccaride (25-27) yields offspring with behavioral abnormalities, neuropathology, and altered gene expression that are relevant to both SZ and ASD. Similar outcomes are obtained by treating pregnant rodents with the viral mimic, synthetic double stranded RNA (polyinosinic:polycytidylic acid [poly IC]), which stimulates an inflammatory response in the absence of a specific pathogen (28). Offspring born to pregnant dams treated with poly IC at mid-gestation demonstrate repetitive behaviors and deficits in social and communication behaviors that resemble features of ASD, as well as elevated anxiety, deficits in prepulse inhibition, latent inhibition, and working memory that resemble clinical features of both ASD and SZ (21,29-32). Neuropathology observed with ASD (localized loss of Purkinje cells) and SZ (enlarged ventricles) have been reported in poly IC rodent models (33-35), and there are numerous other alterations in brain structure, neurochemistry, gene expression, and immune function (36-39). The deleterious effects on brain and behavior in the mouse MIA model appear to be mediated by the maternal cytokine response, in particular interleukin-6 (40).
While rodent models have laid the foundation for understanding the effects of MIA on fetal brain development, these models have limitations. Extrapolating the timing of fetal brain development between rodents and humans is complicated by the fact that the neural events of the human third trimester occur during the early postnatal period in rodents (41). Moreover, there are challenges in relating the rodent brain to the human brain and rodent behavior to human behavior. This is particularly problematic for disorders such as ASD and SZ that are characterized by deficits in a range of complex cognitive, social, and affective functions. Indeed, portions of the human brain, such as prefrontal cortex, which mediate these functions and are heavily impacted in ASD and SZ, are poorly developed in the rodent brain (42). Understanding human disorders involving higher cognitive functions will benefit from studies in animal species more closely related to humans. Nonhuman primates, such as rhesus macaques (Macaca mulatta), demonstrate many features of human physiology, anatomy, and behavior, making them an appropriate species to study a variety of human brain disorders (43). The rhesus monkey lives in a complex, hierarchical social system and uses many forms of human-like communication such as facial expressions and social gestures (44). The rich social and cognitive repertoire of rhesus monkeys provides a framework to relate behavioral changes observed in the animal model more directly to human mental illness.
We have developed a novel, nonhuman primate model using a modified form of the viral mimic poly IC, which is adapted for use in primates (polyinosinic:polycytidylic acid stabilized with poly-L-lysine [poly ICLC]). This synthetic RNA is recognized as foreign by the primate immune system and induces a transient innate inflammatory response (45,46). Pregnant rhesus monkeys were injected with poly ICLC over a 72-hour period at the end of the first or second trimester. These gestational ages were selected based on human epidemiologic data identifying the first and second trimesters as vulnerable time points where exposure to MIA increases the risk of autism and schizophrenia (14,17). We evaluated sickness behavior, body temperature, and cytokine responses in the dams to confirm a strong immune activation and then analyzed the behavioral development of the offspring for 4 years. Here, we present our initial behavioral findings through 24 months of age, documenting the emergence of abnormal behavior in rhesus offspring exposed to MIA.
Methods and Materials
All experimental procedures were developed in consultation with the veterinary staff at the California National Primate Research Center. Protocols were approved by the University of California, Davis Institutional Animal Care and Use Committee. Detailed methods are provided in Supplement 1.
Maternal Administration of Poly ICLC
Twenty-four multiparous rhesus monkeys were assigned to one of three experimental groups: 1) first trimester MIA (MIA1), 2) second trimester MIA (MIA2), or 3) saline control animals (CONSaline) (Table S1 in Supplement 1). Pregnant animals in the MIA groups were injected with .25 mg/kg synthetic double-stranded RNA (poly ICLC) (Oncovir, Inc., Washington, DC) via intravenous injection while restrained by trained technicians on gestational days 43, 44, and 46 (MIA1) or 100, 101, and 103 (MIA2).
Rearing Conditions
Infants were raised in individual cages with their mothers, where they had visual access to other animals at all times. For 3 hours each day, one adult male and four familiar mother-infant pairs were allowed to freely interact in a large cage to provide enrichment and facilitate species-typical social development. Each group consisted of a mixture of male and female offspring of both MIA and control experimental groups. The infants were weaned from their mothers at 6 months of age but continued the same socialization routine.
Behavioral Observations
Behavioral data were collected throughout the first 2 years of life using our standardized rhesus developmental battery (Table S2 in Supplement 1) (47-50). For the sake of brevity, only behavioral assays associated with significant results are presented (Table 1). Unless noted in the material description in Supplement 1, behavioral data were collected using focal animal samples (51) in a predetermined, pseudo-random order, employing a catalog of behaviors commonly used for this species (Table S3 in Supplement 1). Behaviors initiated or received by the focal animal, as well as the behavior of other animals (i.e., mother, other adults, peers) toward the focal animal were recorded, resulting in the quantification of mother-infant and peer social interactions throughout development.
Table 1.
Behavioral Phenotyping Assays
| Behavioral Assay | Brief Description | Relevance to Autism Spectrum Disorders and Schizophrenia |
|---|---|---|
| 6–12 Months of Age | ||
| Mother preference a | Following weaning, each infant was tested for 4 days to evaluate one aspect of mother-infant attachment, the infant’s preference for its own mother versus another familiar adult female (12 2-minute trials/subject). | Measures of attachment serve as control parameters for species-typical development and response to separation (48). |
| Postweaning solo Observations b | At approximately 10 months of age, the animals were observed alone in a large, unfamiliar cage for two 5-minute focal samples on 2 separate days to screen for abnormal behaviors such as motor stereotypies or self-directed behaviors. | Solo observations are conducted to screen for a wide array of stereotyped behaviors produced by rhesus monkeys (49,53,58). |
| 12–18 Months of Age | ||
| Juvenile Y-maze | At approximately 18 months of age, animals were given visual access to a novel conspecific in one arm of a Y-maze test apparatus. Each animal was tested for six 2-minute trials on 2 separate days, meeting an opposite-sex conspecific on the first day and a same-sex conspecific on the second day. | Initial social assays with novel conspecifics were carried out using the Y-maze testing apparatus and later followed with the three-chambered social approach assay described below. |
| Juvenile solo Observationsb | At approximately 22 months of age, the animals were observed alone in a large, unfamiliar cage for two 5-minute focal samples on 2 separate days to screen for abnormal behaviors such as motor stereotypies or self-directed behaviors. | Solo observations are conducted to screen for a wide array of stereotyped behaviors produced by rhesus monkeys (49,53,58). |
| Juvenile social approachc | At approximately 24 months of age, social interactions with a novel conspecific were evaluated using a modified version of the mouse three-chambered social approach assay (20 minutes/subject). | The high-throughput social approach assay used in mouse models (54) paired with the fine-grained focal observations utilized in our nonhuman primate studies (47,48) provide a screen for sociability as indexed by the amount of time spent in a chamber with a constrained, novel conspecific. |
ASD, autism spectrum disorders; SZ, schizophrenia.
Assays used to control for changes in physical development, reflexes, fear response development, maternal attachment, and activity levels that are not directly related to the core features of ASD and SZ.
Behavioral assays targeting repetitive behaviors and restricted interests.
Behavioral assays targeting social and communication domains.
Statistical Analysis
Preliminary analyses revealed that the behavioral profiles of the saline-treated monkeys and the untreated control monkeys were very similar. They were therefore pooled to form a single control group. Mixed-effects linear models (52) were used to analyze the frequency and duration of the behaviors, since all the experiments involved repeated observations. Suitable transformations were performed for the variables that violated the assumption of normality. All core models included fixed effects for group (MIA1, MIA2, and control) and gender (to adjust for gender imbalance across groups and account for its potential effect on frequency and duration of the behaviors) and a random effect for animal (to account for the correlated nature of the data). For experiments involving stimulus monkeys or where time effects were detected, additional fixed terms (for stimulus monkey gender, time, interaction of time with group, etc.) were also added to the core model and tested. These terms were retained in the models only if they were significant. All tests were two-sided, with α = .05.
Results
Sickness behavior, temperature and cytokine profiles of the pregnant monkeys confirmed a strong inflammatory response to poly ICLC (Figures S1 and S2 and Tables S4-S7 in Supplement 1). For the sake of brevity, only significant behavioral results from the offspring are presented in detail. There were no consistent differences across offspring in physical growth, motor or reflex development, adrenal activity, interactions with mothers, or development of threat detection in the first 6 months of postnatal life (Table S8 in Supplement 1).
Mother Preference
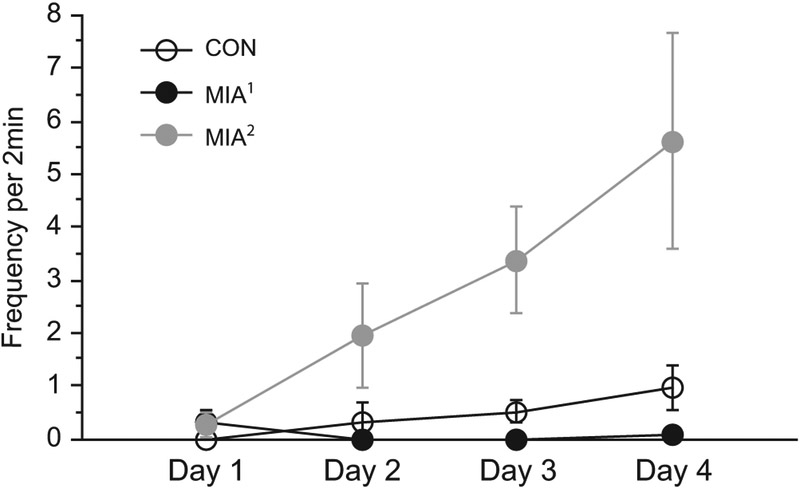
Following weaning at 6 months of age, MIA offspring differed from control animals during a test designed to evaluate infant attachment to the mother. While all animals, irrespective of treatment condition, demonstrated a species-typical attachment to their own mother, we detected differences in the patterns of the animals’ responses to the test. Offspring in the MIA2 treatment group produced significantly more distress/self-soothing behaviors that are commonly observed during the attachment assay (i.e., tantrums, convulsive jerk, self-clasp, infant crook tail) than MIA1 or control offspring. Group differences were not apparent on the first day but emerged over the 4 consecutive days of testing (Figure 1; Table 2; Figure S9 in Supplement 1). On the final day, MIA2 treatment offspring were highly reactive and control offspring were moderately reactive, while MIA1 treatment offspring displayed almost no evidence of reactivity.
Figure 1.
Maternal immune activation (MIA) offspring exhibit abnormal responses to weaning. Although all animals demonstrate a species-typical attachment to their own mother, MIA offspring exhibit an unusual response in the attachment test. Second trimester MIA (MIA2) offspring produce significantly more distress or self-soothing behaviors (i.e., tantrums, convulsive jerk, self-clasp, infant crook tail) than control (CON) offspring. This group difference emerges over the 4 days of testing, with both MIA groups showing a different pattern over time than control animals (p < .001 and p < .003 for the differences in slopes, respectively). Thus, on the final day, MIA2 offspring are highly reactive, control animals are moderately reactive, and first trimester MIA (MIA1) offspring display little evidence of reactivity (p < .01 for difference from control animals for both MIA1 and MIA2 groups on day 4).
Table 2.
Mother Preference
| Estimate (SE) | p Value | |
|---|---|---|
| Estimated Trajectory for the Control Group | ||
| Baseline (day 1) | −.1 (.1) | .60 |
| Linear change with time (per day) | .2 (.0) | <.001 |
| Estimated Difference between MIA1 and Control Animals | ||
| Baseline (day 1) | .2 (.2) | .31 |
| Linear change with time (per day) | −.2 (.1) | <.001 |
| Estimated Difference between MIA2 and Control Animals | ||
| Baseline (day 1) | .3 (.2) | .09 |
| Linear change with time (per day) | .1 (.1) | .003 |
Summary (parameter estimates and standard errors) of the mixed-effects models assessing the relationship of group and time with frequency of reactive behaviors.a Differences from control animals are estimated from mixed-effects regression models fitted to the frequency of behaviors and adjusted for gender, day, and the interaction between group and day.
MIA1, first trimester maternal immune activation; MIA2, second trimester maternal immune activation.
The outcome was first transformed using the fourth root to improve its normality.
Solo Observations
At 10 months of age, we conducted postweaning solo observations of the animals alone in a large cage to screen for abnormal motor stereotypic and/or self-directed behaviors that are common to captive rhesus monkeys (see Table S1 in Supplement 1 for definitions) (53). Compared with control animals, the MIA2 animals produced motor stereotypic and/or self-directed behaviors more frequently than control animals (p = .002) (Figure 2A; Table 3). First trimester MIA animals displayed a trend level increase in these behaviors compared with control animals (p = .06). We also detected trend level differences in the frequency of affiliative contact “coo” calls produced by the MIA1 offspring when observed alone in the large cage (p = .08). Juvenile solo observations were repeated at 22 months of age. Both MIA groups produced significantly more motor stereotypic and/or self-directed behaviors than control animals (p = .03, .01, respectively) (Figure 2A; Table 3). As observed in the postweaning period, MIA1 offspring produced fewer affiliative contact coo calls than control animals, although the difference remained at trend level. At this later time point, however, MIA2 offspring produced significantly fewer coo calls than control animals (Figure 2B, Table 3).
Figure 2.
(A) Maternal immune activation (MIA) offspring exhibit increased frequency of motor stereotypies and self-directed behaviors. Left panel: When observed alone in a large cage at 10 months of age, second trimester MIA (MIA2) animals produce significantly more repetitive behaviors than control animals (CON) (**p ≤ .01). The first trimester MIA (MIA1) offspring also produce more repetitive behaviors than control animals, but this difference does not reach statistical significance at 10 months (p = .06). Middle panel: When observed alone at 22 months of age, MIA1 offspring produce significantly more repetitive behaviors (*p ≤ .05). Second trimester MIA animals also produce significantly more repetitive behaviors than control animals at 22 months (**p ≤ .01). Right panel: When tested at 17 months of age in the Y-maze social preference assay, MIA2 treatment animals produce significantly more repetitive behaviors than control animals (**p ≤ .01). (B) Maternal immune activation offspring display decreased affiliative vocalizations. Left panel: At 22 months, MIA2 offspring produce significantly fewer coo calls than control animals (**p < .01). Right panel: When observed with a novel conspecific at 24 months of age, MIA1 offspring produce significantly fewer coo calls than control animals (*p ≤ .05). (C) Maternal immune activation offspring exhibit inappropriate interactions with unfamiliar conspecifics. Left panel: First trimester MIA offspring demonstrate inappropriate social interactions with an unfamiliar animal, as indexed by high frequency of approaching (*p <0.05) and more frequently moving within arm’s reach of the unfamiliar animal (**p < .01). Right panel: First trimester MIA offspring remained near the unfamiliar animal, as indexed by the duration of time spent in physical contact or within arm’s reach of the unfamiliar animal (*p < .05)
Table 3.
Behaviors During Postweaning and Juvenile Solo Observations
| Average Group Frequency |
Difference from Control Group |
||||||
|---|---|---|---|---|---|---|---|
| MIA1 Mean (SD) |
MIA2 Mean (SD) |
Control Group Mean (SD) |
MIA1 vs. Control Group |
MIA2 vs. Control Group |
|||
| Behavior | Estimate (SE) | p Value | Estimate (SE) | p Value | |||
| Postweaning | |||||||
| Coo | 27.5 (8.0) | 34.6 (9.5) | 38.5 (10.9) | −10.2 (5.5) | .08 | −3.5 (5.0) | .48 |
| Stereotypy a | 5.8 (8.3) | 9.1 (7.6) | .5 (.7) | 1.4 (.7) | .06 | 2.2 (.6) | .002 |
| Juvenile | |||||||
| Coo | 24.7 (12.1) | 21.5 (6.9) | 36.1 (9.6) | −8.5 (4.8) | .09 | −14.8 (4.4) | .003 |
| Stereotypy a | 10.5 (11.4) | 9.5 (8.9) | 1.8 (2.3) | 1.7 (.7) | .03 | 1.8 (.7) | .01 |
Descriptive statistics and summary (parameter estimates and standard errors) of the mixed-effects models assessing the relationship between group and frequency of behavior variables. Average group behaviors are based on observed frequency of behaviors. Differences from control groups are estimated from mixed-effects regression models fitted to the frequency of behaviors and adjusted for gender.
MIA1, first trimester maternal immune activation; MIA2, second trimester maternal immune activation.
Variable square-root transformed to improve its normality.
Interaction with Novel Conspecifics (Y-Maze)
At 17 months of age, we conducted an exploratory assay designed to evaluate social interactions with an unfamiliar conspecific, using a Y-shaped testing chamber in which the experimental animal had access to two chutes. A novel stimulus animal was housed in a holding cage at the end of one chute; the other arm led to an empty cage. While there were no differences in the amount of time spent in the social versus nonsocial arms of the cage (Table 4) and there were few interactions with the novel animal, we did detect differences in coo vocalization and repetitive behaviors (Table 4). While there were no differences in the total number of coo vocalizations, the MIA1 offspring exhibited a trend level difference in the frequency of affiliative contact coo calls produced when alone in the nonsocial arm of the Y-maze (p = .06). Paralleling the results from postweaning and juvenile experiments, the MIA2 offspring produced significantly more motor stereotypic and/or self-directed behaviors than control animals (p = .002; Figure 2B).
Table 4.
Duration and Frequency of Behaviors in Juvenile Y-Maze Paradigm
| Average Group Duration |
Difference from Control Group |
||||||
|---|---|---|---|---|---|---|---|
| MIA1 Mean (SD) |
MIA2 Mean (SD) |
Control Group Mean (SD) |
MIA1 vs. Control Group |
MIA2 vs. Control Group |
|||
| Behavior | Estimate (SE) | p Value | Estimate (SE) | p Value | |||
| Startbox a | 19.7 (11.7) | 21.7 (12.2) | 17.1 (9.7) | 5.4 (5.9) | .37 | 5.8 (5.3) | .29 |
| Social Arm a | 51.1 (15.4) | 50.7 (10.4) | 48.5 (21.0) | 2.7 (9.7) | .78 | 2.3 (8.7) | .80 |
| Nonsocial Arm | 49.2 (19.7) | 47.6 (8.7) | 54.4 (24.5) | −8.1 (11.1) | .48 | −8.1 (10.0) | .43 |
| Average Group Frequency | Difference from Control Group | ||||||
| Coo Alone b,c | 2.3 (3.0) | 3.7 (2.9) | 4.9 (2.0) | −.8 (.4) | .06 | .6 (.4) | .14 |
| Coo to Novel c Animal2 | 2.1 (1.7) | 2.3 (1.4) | 3.0 (2.2) | −.2 (.4) | .53 | −.3 (.3) | .45 |
| Total Coob | 4.4 (4.5) | 6.0 (4.2) | 7.9 (3.3) | −2.3 (1.9) | .24 | −2.0 (1.8) | .28 |
| Stereotypies c | 2.5 (4.2) | 4.2 (2.9) | .7 (.6) | .6 (.4) | .14 | 1.3 (.4) | .002 |
Average group behaviors are based on observed duration or frequency of behaviors over 2-minute trials. Differences from control groups are estimated from mixed-effects regression models fitted to the duration or frequency of behaviors and adjusted for gender. Descriptive statistics and summary (parameter estimates and standard errors) of the mixed-effects models assessing the relationship between group and duration and frequency of behavioral variables.
MIA1, first trimester maternal immune activation; MIA2, second trimester maternal immune activation.
Analyses for these duration variables further adjusted for the day of the trial and gender of the stimulus monkey.
Analyses for these frequency variables further adjusted for the gender of the stimulus monkey.
Frequency variables square-root transformed to improve their normality.
Interaction with Novel Conspecifics (Two-Chamber Social Approach)
This test was modeled after the sensitive assay of sociability used for mouse models of ASD (54-57). All subjects, irrespective of experimental condition, spent significantly more time in the social chamber than in the nonsocial chamber (Table 5). The MIA1 offspring, however, differed from control animals in several behavioral measures. They produced fewer total affiliative contact coo calls (Figure 2B, Table 5), and they approached the stimulus cage more frequently than control animals and initiated proximity with the unfamiliar animal more than twice as frequently as the control animals (Figure 2C, Table 5). Differences were also detected in the amount of time spent in contact or proximity (i.e., within arm’s reach) of the stimulus cages within the chambers. Compared with control subjects, both MIA groups spent more time near the small empty cage in the nonsocial chamber. However, only MIA1 offspring spent more time near the small cage containing an unfamiliar conspecific in the social chamber (Figure 2C). There were no differences in the frequency of entering or exiting the social and nonsocial chambers or in the frequency of approaching the empty stimulus cage, suggesting that the differences in approach frequency were specific to the social stimulus and not reflective of global changes in activity.
Table 5.
Duration and Frequency of Behaviors in Juvenile Social Approach Paradigm
| Average Group Duration |
Difference from Control Group |
||||||
|---|---|---|---|---|---|---|---|
| MIA1 Mean (SD) |
MIA2 Mean (SD) |
Control Group Mean (SD) |
MIA1 vs. Control Group |
MIA2 vs. Control Group |
|||
| Behavior | Estimate (SE) | p Value | Estimate (SE) | p Value | |||
| Proximity/Contact to Empty Cagea | 67.7 (29.1) | 71.1 (42.8) | 39.1 (24.3) | 2.9 (1.2) | .02 | 2.6 (1.0) | .02 |
| Proximity/Contact to Subject Cage | 207.7 (23.9) | 101.0 (47.1) | 109.9 (85.9) | 96.3 (36.8) | .02 | −9.6 (33.0) | .77 |
| Social Chamber | 427.2 (48.9) | 379.8 (75.3) | 425.2 (75.0) | .9 (39.2) | .98 | −45.9 (35.2) | .21 |
| Nonsocial Chamber | 172.8 (48.9) | 220.2 (75.3) | 174.8 (75.0) | −.9 (39.2) | .98 | 45.9 (35.2) | .21 |
| Average Group Frequency | Difference from Control Group | ||||||
| Coo | 9.3 (11.4) | 22.4 (12.9) | 27.4 (12.0) | −15.9 (6.7) | .03 | −4.0 (6.0) | .51 |
| Approach | 15.5 (3.4) | 10.1 (5.3) | 9.3 (3.6) | 4.9 (2.2) | .04 | .2 (2.0) | .91 |
| Contact | 14.7 (3.4) | 9.5 (6.1) | 8.8 (4.5) | 4.6 (2.6) | .09 | .1 (2.3) | .95 |
| Proximity | 7.4 (2.1) | 2.3 (2.0) | 2.0 (1.3) | 5.1 (.9) | <.001 | .2 (.8) | .84 |
Average group behaviors are based on observed duration or frequency of behaviors over 10-minute trials. Differences from control groups are estimated from mixed-effects regression models fitted to the duration of behaviors and adjusted for gender. Descriptive statistics and summary (parameter estimates and standard errors) of the mixed-effects models assessing the relationship between group and duration or frequency of behavior variables.
MIA1, first trimester maternal immune activation; MIA2, second trimester maternal immune activation.
Variable square-root transformed to improve its normality.
Discussion
Rhesus monkey offspring exposed to MIA in utero differ from control offspring in measures of repetitive behaviors, vocal communication, and social interactions. These alterations in behavior overlap with the core diagnostic domains of ASD, and the latter behaviors may also be relevant for SZ. The development of some abnormal behaviors (increased reactivity in MIA2 offspring and abnormal social behavior in MIA1 offspring) depends on the specific period of MIA exposure during pregnancy, while other abnormal behaviors (decreased affiliative vocalizations and increased repetitive behaviors) are present in both MIA groups (Figure S3 in Supplement 1).
While the majority of rodent MIA models have reported behavioral abnormalities in adult offspring, here we describe the emergence of behavior over the first 2 years of life in a nonhuman primate model. This period for rhesus monkeys is roughly equivalent to early childhood in humans. Although group differences were not consistently detected at the early time points, by 2 years of age the MIA monkey offspring began to demonstrate consistent patterns of behavioral changes. The first indication of differences between the experimental groups occurred immediately after weaning, at 6 months of age, during an assessment of emotional attachment to the mother. While all animals, irrespective of treatment condition, demonstrated a species-typical attachment to their own mother, the MIA animals’ responses to the test were different from control animals. The MIA2 offspring displayed a dramatic increase in distress/self-soothing behaviors over the 4-day testing period that was not observed in the control animals. In contrast, the MIA1 offspring produced almost none of these behaviors. Differences in the animals’ responses to the test were most pronounced on the fourth consecutive day of testing, suggesting that this particular repeated assay can reveal changes in distress/self-soothing behaviors that are not detected in other paradigms. While we do not know why the MIA2 offspring responded with increased distress/self-soothing behaviors, mouse MIA models also exhibit behaviors indicative of heightened anxiety (i.e., less time in the center of the open field paradigm and reluctance to explore novel objects) that may provide insight into this atypical response in the monkey (21).
Additional behavioral changes in monkey MIA offspring began to emerge during the postweaning (6–12 months) and juvenile (12–24 months) periods. It is important to note that these early changes in behavior were subtle, as there were no group differences detected in daily home cage observations or in weekly observations of the animals interacting with familiar peers. However, when the MIA animals were removed from these familiar environments and observed alone, they consistently produced more motor stereotypic and/or self-directed behaviors than control animals. These behavioral pathologies were most pronounced in the MIA2 group, as indexed by a high frequency in three different testing paradigms. Animals in the MIA1 group also appeared to produce more repetitive behaviors than control animals, although these differences did not attain statistical significance until the animals reached 2 years of age. It is well established that restricted rearing environments, small cage size, and stress-inducing events can trigger stereotypies in laboratory animals (53,58,59) and we designed our protocols to minimize these factors. The fact that the control animals exhibited a low frequency of motor stereotypic and/or self-directed behaviors indicates that we can reasonably attribute these behaviors to MIA, rather than general socioenvironmental restrictions. The results from this nonhuman primate model parallel findings of increased repetitive and compulsive behaviors of mouse MIA offspring that exhibit high levels of repetitive behaviors in marble burying and self-grooming tests (29).
When the animals were removed from their home cages where they had constant visual access to familiar animals, we also collected data on any social signals, including vocalizations, that were produced. During these temporary separations, young monkeys often produced affiliative coo calls that are thought to serve the function of reestablishing contact with conspecifics (60-63). Compared with control offspring, both groups of MIA offspring produced fewer coo calls, although only the MIA2 group differed significantly from control animals under these conditions. Interestingly, the MIA1 offspring continued to exhibit reduced coo calling when removed from their home cage and introduced to an unfamiliar peer, suggesting that the presence of an unfamiliar animal may differentially impact social buffering for the MIA groups (64,65). The reduced affiliative vocalizations observed in macaque MIA offspring are consistent with data from male MIA mice, which display a reduced number of vocalizations as pups when they are isolated from their littermates and mother and as adults in the presence of a female (29).
Given that impaired social functioning is a hallmark feature of both ASD and SZ, we would expect a valid animal model to also produce impairments in social processing. While MIA offspring did not differ from control animals during daily interactions with familiar peers, group differences were detected during interactions with an unfamiliar social partner, which is considered to be a more challenging social encounter. It is important to point out that the nature of behavioral perturbations in an animal model may be complex and species-specific, especially in challenging social interactions. In mice, for example, the default response to an unfamiliar conspecific is to approach and investigate. Thus, decreased time spent investigating a novel animal is taken as evidence of diminished sociability (66) and is a common behavioral outcome of MIA mouse models (21,29,40). For rhesus monkeys, the decision to approach and interact with another animal depends on a number of internal (i.e., individual temperament differences) and external (i.e., characteristics of the unfamiliar animal, presence or absence of kin) factors (67-71). For many species of nonhuman primates, immediately approaching an unfamiliar conspecific or behaving impulsively with familiar animals is met with negative outcomes and physical aggression (72-79). The default for rhesus monkeys is to approach an unfamiliar conspecific with caution and after considerable evaluation at a distance. However, when evaluated with an unfamiliar conspecific at 2 years of age, MIA1 offspring exhibited a clear deviation from the species-typical social protocol for rhesus monkeys by frequently approaching, contacting, and staying within arm’s reach of the unfamiliar animal. Thus, both mouse and monkey MIA models result in deviation from species-typical social norms.
Behavioral changes in mouse MIA models have been interpreted as bearing resemblance to features of both ASD and SZ (20,80-82), although the timing of the prenatal challenge likely determines the ultimate consequences of MIA exposure (83-90). The 165-day macaque monkey pregnancy provides an opportunity to further delineate vulnerable periods of gestation during which MIA alters specific neural networks and ultimately leads to distinct behavioral trajectories over a relatively protracted period of postnatal development. Our results indicate that experimentally inducing MIA at either late first trimester or late second trimester produces offspring with overlapping alterations in repetitive behaviors and affiliative vocalizations, as well as distinct changes in reactivity and social interactions. While it is premature to determine if MIA in the primate model is related specifically to ASD or SZ or to more general neurodevelopmental issues (91), we can begin to evaluate the nature and timing of the behavioral outcomes of the monkey MIA model.
Stereotypic behaviors, for example, are one of the diagnostic features of ASD and were consistently observed throughout postnatal development in the MIA2 offspring and to a lesser extent in the MIA1 offspring. While these behaviors support the face validity of the model, it is important to recognize that stereotypies are observed in a variety of developmental, psychiatric, and neurological disorders and are not specific to ASD. However, both ASD and SZ are characterized by changes in social cognition and emotion (92), which were also altered in the macaque MIA offspring compared with control animals. While both MIA groups exhibited decreased frequency of the affiliative contact coo calls when observed alone, only the MIA1 offspring produced fewer coos in a social context. Likewise, only the MIA1 offspring exhibited inappropriate social interactions with a novel conspecific. We suggest that the inappropriate social approach behaviors observed in the animal model may be reminiscent of the active but odd subtype of social interaction style described in ASD (93) and the complex social functioning impairments in SZ (94). We have initiated an eye-tracking study to evaluate social processing in the monkey model and will utilize these data to further clarify the nature of the social impairments and the relevance to ASD and SZ.
The timing of behavioral alterations is another important consideration. Autism spectrum disorder, for example, is diagnosed in early childhood (95), while the onset of psychotic symptoms of SZ typically occurs during the transition from adolescence to adulthood (96). In the present study, we first detected group differences in response to weaning at 6 months of age, which is roughly equivalent to a 2-year-old child. While this time frame is more consistent with the early symptom onset of ASD, prospective studies of patients who develop SZ also have social and neurocognitive impairments that emerge long before psychiatric SZ symptoms (97-100). Observations of macaque offspring will continue as they mature, which is needed to interpret the emergence of symptoms over time, as well as the long-term effects of MIA in primates and the relevance to human neurodevelopmental disorders.
While the rhesus monkey provides an animal model that closely parallels human brain organization and cognitive and social functioning, there are ethical and pragmatic limitations in the development of a nonhuman primate model. The primary limitation of the current study is the sample size. A second limitation is that we must wait until the conclusion of the behavioral studies (approximately 4 years) before initiating brain pathology studies that are often simultaneously carried out in rodent models. Thus, the data presented here describe behavioral outcomes but do not provide a mechanistic neural basis for the specific abnormalities. Mouse MIA models, however, have identified several plausible mechanisms by which poly IC-induced immune responses can disrupt fetal brain development (101-104). The maternal cytokine response to poly IC, in particular interleukin-6 (40), plays a critical role in triggering immune activation and endocrine changes in the placenta (105) and altered cytokine expression in the fetal brain, as well as long-lasting changes in cytokine expression in the brains of MIA mouse offspring as they mature (36). In the present study, we utilized a modified form of poly IC (poly ICLC), which stimulates comparable inflammatory responses in humans and nonhuman primates (45,46,106). While other nonhuman primate models of MIA have explored maternal immune challenges in the third trimester (107,108), we focused our efforts on the first and second trimesters, as human studies have identified these as the gestational windows of vulnerability for ASD and SZ associated with maternal immune challenge (109). This time frame of early fetal brain development captures the peak period of macaque neurogenesis (110-117). Short et al. (107) report that rhesus offspring born to mothers exposed to influenza in the early third trimester demonstrate reduced gray matter volume throughout the cortex and increased white matter in the parietal cortex at 1 year of age. We predict that MIA exposure in the late first and second trimesters also produce changes in brain development of the offspring. We are currently exploring brain pathology in these animals to determine if MIA offspring demonstrate structural or functional brain pathologies characteristic of ASD or SZ and will initiate a comprehensive histological evaluation of the brain at the conclusion of the behavioral studies.
While experimentally inducing MIA in the primate model alters behavioral development, it is important to emphasize that sickness during human pregnancy is not uncommon (118,119), and clearly not all women who experience infection during pregnancy have children later diagnosed with a neurodevelopmental disorder (120). A number of factors, including genetic susceptibility, the intensity of the infection, and the maternal and/or fetal response, as well as the precise timing of the immune challenge, likely influence the degree to which MIA alters fetal brain development and may ultimately determine which disease phenotype (ASD or SZ) is expressed. With mounting evidence of the increased risk of psychiatric disorders in offspring exposed to MIA, increased efforts to understand MIA-induced alterations in brain development are clearly needed.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Simons Foundation (SFARI [9900060] to PHP). Additional support was provided by the base Grant (RR00169) of the California National Primate Research Center.
Biobehavioral assessments at 3 months of age were carried out by Dr. John Capitanio and funded in part by the National Center for Research Resources (R24RR019970 to JPC and P51RR000169 to California National Primate Research Center) and are currently supported by the Office of Research Infrastructure Programs/Office of the Director (R24OD010962 and P51OD011157, respectively). SEPS is currently affiliated with the Department of Immunology, Mayo Clinic, Rochester, Minnesota. CB is currently affiliated with the Department of Neurosurgery, University Hospital Basel, Basel, Switzerland.
We acknowledge the Research Services and Primate Medicine staff at the California National Primate Research Center for care of the animals and to J. Buser, G. Moadab, and A. Lopez for behavioral data collection and assistance with manuscript preparation.
Footnotes
Polyinosinic:polycytidylic acid stabilized with poly-L-lysine was kindly provided by Dr. Andres Salazar, M.D., Oncovir, Washington. DC.
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2013.06.025.
Contributor Information
Melissa D. Bauman, Department of Psychiatry and Behavioral Sciences, California National Primate Research Center, University of California, Davis, Davis; The M.I.N.D. Institute, University of California, Davis, Sacramento, Center for Neuroscience, University of California, Davis, Davis, California
Ana-Maria Iosif, Department of Public Health Sciences, Division of Biostatistics, University of California, Davis, Davis.
Stephen E.P. Smith, Division of Biology, California Institute of Technology, Pasadena
Catherine Bregere, Division of Biology, California Institute of Technology, Pasadena.
David G. Amaral, Department of Psychiatry and Behavioral Sciences, California National Primate Research Center, University of California, Davis, Davis; The M.I.N.D. Institute, University of California, Davis, Sacramento; Center for Neuroscience, University of California, Davis, Davis, California
Paul H. Patterson, Division of Biology, California Institute of Technology, Pasadena
References
- 1.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators; Centers for Disease Control and Prevention (2012): Prevalence of autism spectrum disorders–Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ 61:1–19. [PubMed] [Google Scholar]
- 2.Saha S, Chant D, Welham J, McGrath J (2005): A systematic review of the prevalence of schizophrenia. PLoS Med 2:e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Os J, Kenis G, Rutten BP (2010): The environment and schizophrenia. Nature 468:203–212. [DOI] [PubMed] [Google Scholar]
- 4.Newschaffer CJ, Fallin D, Lee NL (2002): Heritable and nonheritable risk factors for autism spectrum disorders. Epidemiol Rev 24:137–153. [DOI] [PubMed] [Google Scholar]
- 5.Piper M, Beneyto M, Burne TH, Eyles DW, Lewis DA, McGrath JJ (2012): The neurodevelopmental hypothesis of schizophrenia: Convergent clues from epidemiology and neuropathology. Psychiatr Clin North Am 35:571–584. [DOI] [PubMed] [Google Scholar]
- 6.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. (2011): Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 68:1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagberg H, Gressens P, Mallard C (2012): Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol 71:444–457. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg RE, Law JK, Yenokyan G, McGready J, Kaufmann WE, Law PA (2009): Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch Pediatr Adolesc Med 163:907–914. [DOI] [PubMed] [Google Scholar]
- 9.Brown AS, Schaefer CA, Quesenberry CP Jr, Liu L, Babulas VP, Susser ES (2005): Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry 162:767–773. [DOI] [PubMed] [Google Scholar]
- 10.Brown AS, Schaefer CA, Wyatt RJ, Goetz R, Begg MD, Gorman JM, Susser ES (2000): Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: A prospective birth cohort study. Schizophr Bull 26:287–295. [DOI] [PubMed] [Google Scholar]
- 11.Sorensen HJ, Mortensen EL, Reinisch JM, Mednick SA (2009): Association between prenatal exposure to bacterial infection and risk of schizophrenia. Schizophr Bull 35:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babulas V, Factor-Litvak P, Goetz R, Schaefer CA, Brown AS (2006): Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Am J Psychiatry 163:927–929. [DOI] [PubMed] [Google Scholar]
- 13.Buka SL, Cannon TD, Torrey EF, Yolken RH (2008): Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol Psychiatry 63:809–815. [DOI] [PubMed] [Google Scholar]
- 14.Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, et al. (2004): Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry 61:774–780. [DOI] [PubMed] [Google Scholar]
- 15.Brown AS, Sourander A, Hinkka-Yli-Salomaki S, McKeague IW, Sundvall J, Surcel HM (2013): Elevated maternal C-reactive protein and autism in a national birth cohort [published online ahead of print January 22]. Mol Psychiatry. doi: 10.1038/mp.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atladottir HO, Henriksen TB, Schendel DE, Parner ET (2012): Autism after infection, febrile episodes, and antibiotic use during pregnancy: An exploratory study. Pediatrics 130:e1447–e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET (2010): Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 40:1423–1430. [DOI] [PubMed] [Google Scholar]
- 18.Abdallah MW, Larsen N, Mortensen EL, Atladottir HO, Norgaard-Pedersen B, Bonefeld-Jorgensen EC, et al. (2012): Neonatal levels of cytokines and risk of autism spectrum disorders: An exploratory register-based historic birth cohort study utilizing the Danish Newborn Screening Biobank. J Neuroimmunol 252:75–82. [DOI] [PubMed] [Google Scholar]
- 19.Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, et al. (2011): Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Mol Autism 2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson PH (2009): Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav Brain Res 204:313–321. [DOI] [PubMed] [Google Scholar]
- 21.Shi L, Fatemi SH, Sidwell RW, Patterson PH (2003): Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci 23:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fatemi SH, Emamian ES, Sidwell RW, Kist DA, Stary JM, Earle JA, Thuras P (2002): Human influenza viral infection in utero alters glial fibrillary acidic protein immunoreactivity in the developing brains of neonatal mice. Mol Psychiatry 7:633–640. [DOI] [PubMed] [Google Scholar]
- 23.Fatemi SH, Sidwell R, Kist D, Akhter P, Meltzer HY, Bailey K, et al. (1998): Differential expression of synaptosome-associated protein 25 kDa [SNAP-25] in hippocampi of neonatal mice following exposure to human influenza virus in utero. Brain Res 800:1–9. [DOI] [PubMed] [Google Scholar]
- 24.Fatemi SH, Reutiman TJ, Folsom TD, Huang H, Oishi K, Mori S, et al. (2008): Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: Implications for genesis of neurodevelopmental disorders. Schizophr Res 99:56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coyle P, Tran N, Fung JN, Summers BL, Rofe AM (2009): Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav Brain Res 197:210–218. [DOI] [PubMed] [Google Scholar]
- 26.Fortier ME, Joober R, Luheshi GN, Boksa P (2004): Maternal exposure to bacterial endotoxin during pregnancy enhances amphetamine-induced locomotion and startle responses in adult rat offspring. J Psychiatr Res 38:335–345. [DOI] [PubMed] [Google Scholar]
- 27.Fortier ME, Luheshi GN, Boksa P (2007): Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res 181:270–277. [DOI] [PubMed] [Google Scholar]
- 28.Traynor TR, Majde JA, Bohnet SG, Krueger JM (2004): Intratracheal double-stranded RNA plus interferon-gamma: A model for analysis of the acute phase response to respiratory viral infections. Life Sci 74:2563–2576. [DOI] [PubMed] [Google Scholar]
- 29.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH (2012): Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun 26:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuckerman L, Weiner I (2003): Post-pubertal emergence of disrupted latent inhibition following prenatal immune activation. Psychopharmacology (Berl) 169:308–313. [DOI] [PubMed] [Google Scholar]
- 31.Zuckerman L, Weiner I (2005): Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res 39:311–323. [DOI] [PubMed] [Google Scholar]
- 32.Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, et al. (2013): Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 339:1095–1099. [DOI] [PubMed] [Google Scholar]
- 33.Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH (2009): Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun 23:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piontkewitz Y, Assaf Y, Weiner I (2009): Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biol Psychiatry 66:1038–1046. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Cheung C, Wei R, Hui ES, Feldon J, Meyer U, et al. (2009): Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: Evidence from MRI in a mouse model. PloS One 4:e6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garay PA, Hsiao EY, Patterson PH, McAllister AK (2013): Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun 31:54–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garbett KA, Hsiao EY, Kalman S, Patterson PH, Mirnics K (2012): Effects of maternal immune activation on gene expression patterns in the fetal brain. Transl Psychiatry 2:e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richetto J, Calabrese F, Riva MA, Meyer U (2013): Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome [published online ahead of print January 17]. Schizophr Bull. doi: 10.1093/schbul/sbs195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH (2012): Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci U S A 109:12776–12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH (2007): Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 27:10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clancy B, Finlay BL, Darlington RB, Anand KJ (2007): Extrapolating brain development from experimental species to humans. Neuro-toxicology 28:931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preuss T (1995): Do rats have a prefrontal cortex? The Rose-Woolsey-Akert reconsidered. J Cogn Neurosci 7:1–24. [DOI] [PubMed] [Google Scholar]
- 43.Capitanio JP, Emborg ME (2008): Contributions of non-human primates to neuroscience research. Lancet 371:1126–1135. [DOI] [PubMed] [Google Scholar]
- 44.Altmann S (1967): The structure of primate social communication In: Altmann S, editor. Social Communication Among Primates. Chicago: University of Chicago Press. [Google Scholar]
- 45.Levy HB, Baer G, Baron S, Buckler CE, Gibbs CJ, Iadarola MJ, et al. (1975): A modified polyriboinosinic-polyribocytidylic acid complex that induces interferon in primates. J Infect Dis 132:434–439. [DOI] [PubMed] [Google Scholar]
- 46.Caskey M, Lefebvre F, Filali-Mouhim A, Cameron MJ, Goulet JP, Haddad EK, et al. (2011): Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J Exp Med 208:2357–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG (2004): The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. J Neurosci 24:711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG (2004): The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci 16:1388–1411. [DOI] [PubMed] [Google Scholar]
- 49.Bauman MD, Toscano JE, Babineau BA, Mason WA, Amaral DG (2008): Emergence of stereotypies in juvenile monkeys (Macaca mulatta) with neonatal amygdala or hippocampus lesions. Behav Neurosci 122:1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bauman MD, Toscano JE, Mason WA, Lavenex P, Amaral DG (2006): The expression of social dominance following neonatal lesions of the amygdala or hippocampus in rhesus monkeys (Macaca mulatta). Behav Neurosci 120:749–760. [DOI] [PubMed] [Google Scholar]
- 51.Altmann J (1974): Observational study of behavior: Sampling methods. Behaviour 49:227–267. [DOI] [PubMed] [Google Scholar]
- 52.Laird NM, Ware JH (1982): Random-effects models for longitudinal data. Biometrics 38:963–974. [PubMed] [Google Scholar]
- 53.Lutz C, Well A, Novak M (2003): Stereotypic and self-injurious behavior in rhesus macaques: A survey and retrospective analysis of environment and early experience. Am J Primatol 60:1–15. [DOI] [PubMed] [Google Scholar]
- 54.Silverman JL, Yang M, Lord C, Crawley JN (2010): Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci 11:490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crawley JN (2008): Behavioral phenotyping strategies for mutant mice. Neuron 57:809–818. [DOI] [PubMed] [Google Scholar]
- 56.Crawley JN (2007): Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol 17:448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crawley JN (2004): Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev 10:248–258. [DOI] [PubMed] [Google Scholar]
- 58.Vandeleest JJ, McCowan B, Capitanio JP (2011): Early rearing interacts with temperament and housing to influence the risk for motor stereotypy in rhesus monkeys (Macaca mulatta). Appl Anim Behav Sci 132:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Capitanio J (1986): Behavioral pathology In: Mitchell G, Erwin J, editors. Comparative Primate Biology: Behavior, Conservation, and Ecology. New York: Alan R. Liss, 411–454. [Google Scholar]
- 60.Kalin NH, Shelton SE (1998): Ontogeny and stability of separation and threat-induced defensive behaviors in rhesus monkeys during the first year of life. Am J Primatol 44:125–135. [DOI] [PubMed] [Google Scholar]
- 61.Owren MJ, Dieter JA, Seyfarth RM, Cheney DL (1993): Vocalizations of rhesus (Macaca mulatta) and Japanese (M. fuscata) macaques cross-fostered between species show evidence of only limited modification. Dev Psychobiol 26:389–406. [DOI] [PubMed] [Google Scholar]
- 62.Erwin J, Mitchell G (1973): Analysis of Rhesus monkey vocalizations: Maturation-related sex differences in clear-cell frequency. Am J Phys Anthropol 38:463–467. [DOI] [PubMed] [Google Scholar]
- 63.Kalin NH, Shelton SE, Snowdon CT (1992): Affiliative vocalizations in infant rhesus macaques (Macaca mulatta). J Comp Psychol 106:254–261. [DOI] [PubMed] [Google Scholar]
- 64.Gilbert MH, Baker KC (2011): Social buffering in adult male rhesus macaques (Macaca mulatta): Effects of stressful events in single vs. pair housing. J Med Primatol 40:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR (2003): Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology 28:910–918. [DOI] [PubMed] [Google Scholar]
- 66.Yang M, Silverman JL, Crawley JN (2011): Automated three-chambered social approach task for mice. Curr Protoc Neurosci Chapter 8:Unit 8.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCowan B, Beisner BA, Capitanio JP, Jackson ME, Cameron AN, Seil S, et al. (2011): Network stability is a balancing act of personality, power, and conflict dynamics in rhesus macaque societies. PloS One 6:e22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Capitanio JP (2011): Individual differences in emotionality: Social temperament and health. Am J Primatol 73:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suomi SJ (1997): Early determinants of behaviour: Evidence from primate studies. Br Med Bull 53:170–184. [DOI] [PubMed] [Google Scholar]
- 70.Novak MA, Suomi SJ (1991): Social interaction in nonhuman primates: An underlying theme for primate research. Lab Anim Sci 41:308–314. [PubMed] [Google Scholar]
- 71.Capitanio JP (1999): Personality dimensions in adult male rhesus macaques: Prediction of behaviors across time and situation. Am J Primatol 47:299–320. [DOI] [PubMed] [Google Scholar]
- 72.Fairbanks LA (2001): Individual differences in response to a stranger: Social impulsivity as a dimension of temperament in vervet monkeys (Cercopithecus aethiops sabaeus). J Comp Psychol 115:22–28. [DOI] [PubMed] [Google Scholar]
- 73.Fairbanks LA, Jorgensen MJ, Huff A, Blau K, Hung YY, Mann JJ (2004): Adolescent impulsivity predicts adult dominance attainment in male vervet monkeys. Am J Primatol 64:1–17. [DOI] [PubMed] [Google Scholar]
- 74.Fairbanks LA, Melega WP, Jorgensen MJ, Kaplan JR, McGuire MT (2001): Social impulsivity inversely associated with CSF 5-HIAA and fluoxetine exposure in vervet monkeys. Neuropsychopharmacology 24:370–378. [DOI] [PubMed] [Google Scholar]
- 75.Manuck SB, Kaplan JR, Rymeski BA, Fairbanks LA, Wilson ME (2003): Approach to a social stranger is associated with low central nervous system serotonergic responsivity in female cynomolgus monkeys (Macaca fascicularis). Am J Primatol 61:187–194. [DOI] [PubMed] [Google Scholar]
- 76.Fairbanks LA, Newman TK, Bailey JN, Jorgensen MJ, Breidenthal SE, Ophoff RA, et al. (2004): Genetic contributions to social impulsivity and aggressiveness in vervet monkeys. Biol Psychiatry 55:642–647. [DOI] [PubMed] [Google Scholar]
- 77.Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, et al. (1995): Correlation of CSF 5-HIAA concentration with sociality and the timing of emigration in free-ranging primates. Am J Psychiatry 152:907–913. [DOI] [PubMed] [Google Scholar]
- 78.Westergaard GC, Suomi SJ, Chavanne TJ, Houser L, Hurley A, Cleveland A, et al. (2003): Physiological correlates of aggression and impulsivity in free-ranging female primates. Neuropsychopharmacology 28:1045–1055. [DOI] [PubMed] [Google Scholar]
- 79.Kinnally EL, Whiteman HJ, Mason WA, Mendoza SP, Capitanio JP (2008): Dimensions of response to novelty are associated with social engagement and aggression in adult male rhesus macaques (Macaca mulatta). J Comp Psychol 122:195–203. [DOI] [PubMed] [Google Scholar]
- 80.Meyer U, Feldon J, Dammann O (2011): Schizophrenia and autism: Both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res 69:26R–33R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meyer U, Yee BK, Feldon J (2007): The neurodevelopmental impact of prenatal infections at different times of pregnancy: The earlier the worse? Neuroscientist 13:241–256. [DOI] [PubMed] [Google Scholar]
- 82.Patterson PH (2006): Pregnancy, immunity, schizophrenia, and autism. Eng Sci LXIX:10–21. [Google Scholar]
- 83.Meyer U, Feldon J, Schedlowski M, Yee BK (2005): Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev 29:913–947. [DOI] [PubMed] [Google Scholar]
- 84.Meyer U, Feldon J, Schedlowski M, Yee BK (2006): Immunological stress at the maternal-foetal interface: A link between neurodevelopment and adult psychopathology. Brain Behav Immun 20:378–388. [DOI] [PubMed] [Google Scholar]
- 85.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. (2006): The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci 26:4752–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J (2008): Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun 22:469–486. [DOI] [PubMed] [Google Scholar]
- 87.Vuillermot S, Weber L, Feldon J, Meyer U (2010): A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J Neurosci 30:1270–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meyer U, Schwendener S, Feldon J, Yee BK (2006): Prenatal and postnatal maternal contributions in the infection model of schizophrenia. Exp Brain Res 173:243–257. [DOI] [PubMed] [Google Scholar]
- 89.Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U (2010): Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology 35:2462–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bitanihirwe BK, Weber L, Feldon J, Meyer U (2010): Cognitive impairment following prenatal immune challenge in mice correlates with prefrontal cortical AKT1 deficiency. Int J Neuropsychopharmacol 13:981–996. [DOI] [PubMed] [Google Scholar]
- 91.Harvey L, Boksa P (2012): Prenatal and postnatal animal models of immune activation: Relevance to a range of neurodevelopmental disorders. Dev Neurobiol 72:1335–1348. [DOI] [PubMed] [Google Scholar]
- 92.King BH, Lord C (2011): Is schizophrenia on the autism spectrum? Brain Res 1380:34–41. [DOI] [PubMed] [Google Scholar]
- 93.Wing L, Gould J (1979): Severe impairments of social interaction and associated abnormalities in children: Epidemiology and classification. J Autism Dev Disord 9:11–29. [DOI] [PubMed] [Google Scholar]
- 94.Horan WP, Green MF, DeGroot M, Fiske A, Hellemann G, Kee K, et al. (2012): Social cognition in schizophrenia, Part 2: 12-month stability and prediction of functional outcome in first-episode patients. Schizophr Bull 38:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mandell DS, Novak MM, Zubritsky CD (2005): Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics 116:1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ziermans TB, Schothorst PF, Sprong M, van Engeland H (2011): Transition and remission in adolescents at ultra-high risk for psychosis. Schizophr Res 126:58–64. [DOI] [PubMed] [Google Scholar]
- 97.Bachman P, Niendam TA, Jalbrzikowski M, Park CY, Daley M, Cannon TD, Bearden CE (2012): Processing speed and neurodevelopment in adolescent-onset psychosis: Cognitive slowing predicts social function. J Abnorm Child Psychol 40:645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Niendam TA, Jalbrzikowski M, Bearden CE (2009): Exploring predictors of outcome in the psychosis prodrome: Implications for early identification and intervention. Neuropsychol Rev 19:280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, Cannon TD (2007): Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull 33:688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Niendam TA, Bearden CE, Rosso IM, Sanchez LE, Hadley T, Nuechterlein KH, Cannon TD (2003): A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. Am J Psychiatry 160:2060–2062. [DOI] [PubMed] [Google Scholar]
- 101.Meyer U, Feldon J (2009): Prenatal exposure to infection: A primary mechanism for abnormal dopaminergic development in schizophrenia. Psychopharmacology (Berl) 206:587–602. [DOI] [PubMed] [Google Scholar]
- 102.Meyer U, Feldon J (2010): Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol 90:285–326. [DOI] [PubMed] [Google Scholar]
- 103.Patterson PH (2011): Maternal infection and immune involvement in autism. Trends Mol Med 17:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patterson PH (2012): Maternal infection and autism. Brain Behav Immun 26:393. [DOI] [PubMed] [Google Scholar]
- 105.Hsiao EY, Patterson PH (2011): Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun 25:604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Packard AE, Hedges JC, Bahjat FR, Stevens SL, Conlin MJ, Salazar AM, Stenzel-Poore MP (2012): Poly-IC preconditioning protects against cerebral and renal ischemia-reperfusion injury. J Cereb Blood Flow Metab 32:242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Short SJ, Lubach GR, Karasin AI, Olsen CW, Styner M, Knickmeyer RC, et al. (2010): Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiatry 67:965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Willette AA, Lubach GR, Knickmeyer RC, Short SJ, Styner M, Gilmore JH, Coe CL (2011): Brain enlargement and increased behavioral and cytokine reactivity in infant monkeys following acute prenatal endotoxemia. Behav Brain Res 219:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brown AS (2012): Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol 72:1272–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rakic P (1974): Neurons in rhesus monkey visual cortex: Systematic relation between time of origin and eventual disposition. Science 183:425–427. [DOI] [PubMed] [Google Scholar]
- 111.Rakic P (1975): Timing of major ontogenetic events in the visual cortex of the rhesus monkey. UCLA Forum Med Sci 18:3–40. [DOI] [PubMed] [Google Scholar]
- 112.Rakic P, Nowakowski RS (1981): The time of origin of neurons in the hippocampal region of the rhesus monkey. J Comp Neurol 196:99–128. [DOI] [PubMed] [Google Scholar]
- 113.van Eerdenburg FJ, Rakic P (1994): Early neurogenesis in the anterior hypothalamus of the rhesus monkey. Brain Res Dev Brain Res 79:290–296. [DOI] [PubMed] [Google Scholar]
- 114.Zecevic N, Rakic P (2001): Development of layer I neurons in the primate cerebral cortex. J Neurosci 21:5607–5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kordower JH, Piecinski P, Rakic P (1992): Neurogenesis of the amygdaloid nuclear complex in the rhesus monkey. Brain Res Dev Brain Res 68:9–15. [DOI] [PubMed] [Google Scholar]
- 116.Zecevic N, Rakic P (1991): Synaptogenesis in monkey somatosensory cortex. Cereb Cortex 1:510–523. [DOI] [PubMed] [Google Scholar]
- 117.Smart IH, Dehay C, Giroud P, Berland M, Kennedy H (2002): Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex 12:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Laibl VR, Sheffield JS (2005): Influenza and pneumonia in pregnancy. Clin Perinatol 32:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Longman RE, Johnson TR (2007): Viral respiratory disease in pregnancy. Curr Opin Obstet Gynecol 19:120–125. [DOI] [PubMed] [Google Scholar]
- 120.Selten JP, Frissen A, Lensvelt-Mulders G, Morgan VA (2010): Schizophrenia and 1957 pandemic of influenza: Meta-analysis. Schizophr Bull 36:219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.