Human umbilical venous endothelial cells (HUVECs) have long been utilized as an in vitro model for study of endothelial cell function and their response to different stimuli. In 2017 Brodowski et al reported that preeclampsia (PE) altered endothelial function in HUVECs (1). It is well established that PE is not only associated with an increased incidence of maternal and fetal morbidity and mortality, but also greater cardiovascular (CV) disease in the offspring (2). Thus, Brodowski et al. hypothesized that reduced functional ability in HUVECs from pregnancies complicated by PE predicted greater CV risk in offspring (1). In this issue of Hypertension, Zhou and colleagues (3) extend this hypothesis to determine if fetal sex alters HUVEC function and to provide insight into potential mechanisms responsible for sex differences in increased CV risk in offspring of pregnancies complicated by PE. Using unpassaged human umbilical vein endothelial cells (HUVECs) isolated from normotensive (>39 weeks gestation) and PE pregnancies (>37 weeks gestation) coupled to RNA sequencing analysis, Zhou et al tested the hypothesis that PE results in sex-specific changes in CV disease and endothelial function associated gene pathways (2). They found that sex differences in genetic variants were particularly significant in HUVECs from PE pregnancies with genes associated with growth failure, blood pressure and chronic heart failure augmented in male HUVECs whereas genetic variants for heart disease, pulmonary hypertension, congenital heart disease and cardiac dysfunction were enhanced in female HUVECs (2). Overall, HUVECs from females were more impacted (almost 5-fold greater) than HUVECs from males in PE pregnancies and 72% of PE-dysregulated genes in female HUVECs were down-regulated whereas 90% were up-regulated in males. Pathways for eNOS signaling were enriched in male and female HUVECs alike; however, enrichment of NF-B, TGF1, and pro-inflammatory cytokines were specific to females.
Using gene arrays, a study by Lorenz et al. (4) reported greater enrichment of immune-related genes in female HUVECs. Although this study by Lorenz et al did not determine the effect of PE on sex-specific transcriptional differences in HUVECs, it does complement the sex-specific transcriptional differences observed by Zhou et al in this issue of Hypertension (3). Both studies reported that sex-specific changes in gene expression corresponded to sex differences in endothelial function. Yet, the relevance of HUVEC functionality to long-term pathophysiological outcomes in the offspring and whether sex differences in the expression of genetic factors and endothelial cell functionality in HUVECs can be interpreted to predict sex differences in long-term chronic health in the offspring is not clear.
Numerous studies indicate that blood pressure (BP) is elevated in individuals born to women with PE (5); yet, studies investigating sex differences in BP and CV risk in the offspring are very limited. Palti and Rothschild reported that systolic BP was higher at age 6 in boys and girls from mothers with PE relative to controls but the increase in systolic BP in girls was not significant (6). Seidman et al reported that SBP was higher at age 17 in girls but not boys whose mothers had PE (7). Experimental models that mimic the many facets of PE also indicate that the development of increased CV risk occurs at a younger age in male versus female offspring. The model of reduced uterine perfusion pressure in the pregnant rat mimics the many facets of PE and results in increased BP in male offspring as early as 4 weeks of age (8). However, BP is not increased in female offspring until 12 month of life (9). Prenatal hypoxia, a consequence of PE, is associated with impaired CV function in male offspring in early adulthood whereas female offspring are not compromised until one year after birth (10). The rodent model of PE induced by chronic elevations in sFlt-1 also programs a sex-specific increase in BP in the offspring with BP increased in male but not female offspring in early life (11). Collectively, these studies suggest that CV risk develops at a younger age in male offspring relative to females.
Zhou et al. addressed the caveat that sex differences in genetic consequences and fetal cell functionality in HUVECs from women with PE may not reflect long-term sex specific increased CV risk in the offspring (3). Yet, interpretation of fetal cell gene expression and functionality on sex differences in CV risk in the offspring was not extrapolated. Genes related to increased CV risk were more enriched in female HUVECs from PE pregnancies than male HUVECs (3) suggesting that CV risk could be greater in females born to women with PE than their male counterparts. In contrast, female HUVECs from women with PE were transcriptionally more responsive to PE stress than were male HUVECs (3). This could indicate that females born to women with PE may be better able to up-regulate stress-related genes in response to a biological stress whereas male offspring may lack the ability to up-regulate stress response genes resulting in the development of early CV risk. It is also possible that a “second hit” may be necessary for females exposed to PE during fetal life to develop CV diseases. Certainly pregnancy may be one of those “second hits” since women whose mothers had PE are at increased risk for development of PE during their pregnancies (12)
The association between sex-specific fetal cell dysfunction and adverse maternal outcome was not a hypothesized component of the study by Zhou and colleagues (3). However, numerous clinical studies indicate that sex of the fetus is linked to the severity of disease in PE. In a study by Taylor and colleagues, women with early preterm PE (<34 weeks) had a greater probability of a female fetus whereas male fetal sex was associated with a normotensive preterm birth (13). Shiozaki et al found that women pregnant with a female fetus had a significantly higher incidence of pregnancy-induced hypertension (PIH) and PE compared to those pregnant with a male fetus (14). The Global Pregnancy Collaboration reported that there were no difference in the female to male fetal distribution if the pregnancy was delivered closer to term (> 37 weeks), but in pregnancies delivered <34 weeks, the prevalence was higher for a female fetus than a male fetus (15). Similarly, Elsmen et al reported that male fetal sex was associated with a greater occurrence of PE in later gestation (<37 weeks) whereas female sex was associated with higher risk of PE in very preterm births (<32 weeks) (16). In the study by Zhou and colleagues, HUVECs were collected at 37 weeks or greater in the PE cohort; thus, not preterm (3). Male HUVECs were less sensitive to endothelial dysfunction; pro-inflammatory pathways were dysregulated in female HUVECs (3). Zhou et al did not clarify if the PE cohort developed early or late onset manifestations of disease. Whether use of HUVECs from preterm pregnancies complicated by PE would enhance sex-specific gene expression and fetal cell dysfunction is not clear. However, this study emphasizes the need to further explore the relationship between fetal sex, the severity of PE and the contribution of these factors in the development of increased CV risk in the offspring.
There are additional limitations of the study by Zhou and colleagues that are important to contemplate. Although this study used freshly isolated primary HUVECs and not immortalized cells, venous endothelial cells are phenotypically different from arterial endothelial cells. Dysfunctional arteries, rather than veins, are associated with cardiovascular disease suggesting that findings form this study may not recapitulate long-term pathophysiological relevance in the offspring or the mother. Also, genetic predisposition may not accurately reflect susceptibility to chronic disease. Epigenetic mechanisms can contribute to increased risk that has its origins in early life. An additional limitation involves the use of HUVECs from a Caucasian cohort. Whether the same genetic findings would be present in HUVECs from African American pregnancies is not clear. The incidence of PE in African American women is significantly higher (approximately 12%) than in Caucasians. Low birth weight, a predictor of increased CV risk, is also significantly greater (approximately 2-fold) in African Americans compared to Caucasians. Lastly, the cohort was either normal weight or slightly overweight, but none were obese. With the epidemic of obesity in the US, there is a significant correlation with incidence of PE and body mass index with the higher the body mass index, the higher the incidence of PE. Despite these caveats, this publication serves as a baseline for future studies and highlights a critical consideration in the field of PE as it relates to the developmental origins of chronic health and disease. It emphasizes the need for more-in-depth investigation in the clinical setting to clarify the long-term consequences of fetal exposure to PE and whether the sex of the fetus alters the degree or sex-specific timing of CV risk in the offspring.
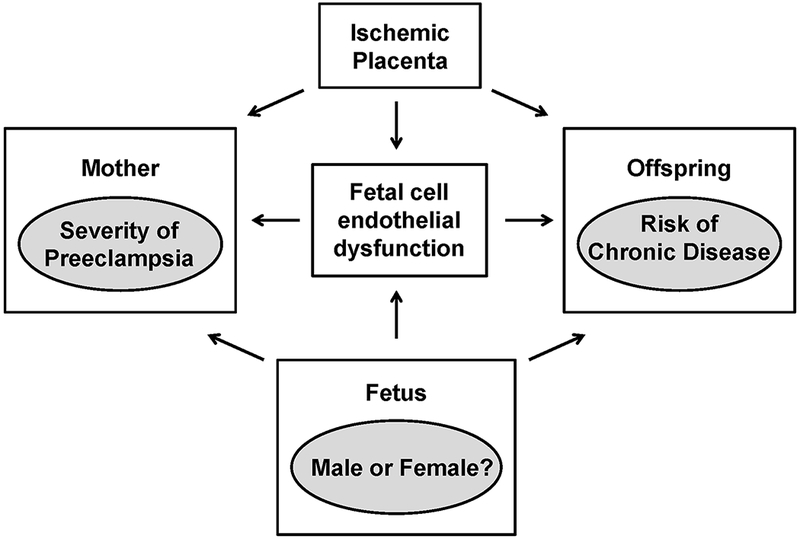
The study by Zhou and colleagues leads to the following question: Can HUVECs be used as indicators of fetal cell dysfunction and early predictors of cardiovascular risk in offspring? How does sex alter the severity of preeclampsia in the mother and the long-term risk of chronic disease in the offspring? What interactions between the maternal and fetal milieu during preeclampsia affect disease progression in the mother during pregnancy and postpartum as well as later risk for chronic disease in the offspring?
Acknowledgments
SOURCES OF FUNDING
The authors would like to acknowledge NIH grants R01HL135089 (JFR), R56HL143459 (BTA), P01HL051971 (JFR, BTA), P20GM121334 (JFR, BTA, BL), P20GM104357 (BTA, JFR), R01HDO67541 (BL) and R01HL 136348 (VDG).
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Brodowski L, Burlakov J, Hass S, von Kaisenberg C, von Versen-Höynck F. Impaired functional capacity of fetal endothelial cells in preeclampsia. PLoS One. 2017;12(5):e0178340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stojanovska V, Scherjon SA, Plösch T. Preeclampsia As Modulator of Offspring Health. Biol Reprod. 2016;94(3):53. [DOI] [PubMed] [Google Scholar]
- 3.Zhou C, Uan Q, Zou Q-Y, Zhong X-q, Tyler CT, Magness RR, Bird IM, Zheng J Sexual dimrophism of preeclampsia-dysregulated transcrptomic profiles and cell function in fetal endothelial cells. Hypertension. 2019; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenz M, Koschate J, Kaufmann K, Kreye C, Mertens M, Kuebler WM, Baumann G, Gossing G, Marki A, Zakrzewicz A, Mieville C, Benn A, Horbelt D, Wratil PR, Stangl K, Stangl V. Does cellular sex matter? Dimorphic transcriptional differences between female and male endothelial cells. Atherosclerosis. 2015;240:61–72. [DOI] [PubMed] [Google Scholar]
- 5.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C, Leeson P. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129(6):e1552–61. [DOI] [PubMed] [Google Scholar]
- 6.Palti H, Rothschild E. Blood pressure and growth at 6 years of age among offsprings of mothers with hypertension of pregnancy. Early Hum Dev. 1989;19(4):263–9. [DOI] [PubMed] [Google Scholar]
- 7.Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. Pre-eclampsia and offspring’s blood pressure, cognitive ability and physical development at 17-years-of-age. Br J Obstet Gynaecol. 1991;98(10):1009–14. [DOI] [PubMed] [Google Scholar]
- 8.Alexander BT. Placental insufficiency leads to development of hypertension in growth restricted offspring. Hypertension, 2003;41(3):457–462. [DOI] [PubMed] [Google Scholar]
- 9.Intapad S, Tull FL, Brown AD, Dasinger JH, Ojeda NB, Fahling JM, Alexander BT. Renal Denervation Abolishes the Age-Dependent Increase in Blood Pressure in Female Intrauterine Growth-Restricted Rats at 12 Months of Age. Hypertension. 2013;61(4):828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rueda-Clausen CF, Morton JS, Davidge ST. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc Res. 2009;81(4):713–22. [DOI] [PubMed] [Google Scholar]
- 11.Lu F, Bytautiene E, Tamayo E, Gamble P, Anderson GD, Hankins GD, Longo M, Saade GR. Gender-specific effect of overexpression of sFlt-1 in pregnant mice on fetal programming of blood pressure in the offspring later in life. Am J Obstet Gynecol. 2007;197(4):418.e1–5. [DOI] [PubMed] [Google Scholar]
- 12.Sherf Y, Sheiner E, Shoham Vardi I, Sergienko R, Klein J, Bilenko N. Like mother like daughter: low birth weight and preeclampsia tend to reoccur at the next generation. J Matern Fetal Neonatal Med. 2019;32(9):1478–1484. [DOI] [PubMed] [Google Scholar]
- 13.Taylor BD, Ness RB, Klebanoff MA, Tang G, Roberts JM, Hougaard DM, Skogstrand K, Haggerty CL. The impact of female fetal sex on preeclampsia and the maternal immune milieu. Pregnancy Hypertens. 2018;12:53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiozaki A, Matsuda Y, Satoh S, Saito S. Impact of fetal sex in pregnancy-induced hypertension and preeclampsia in Japan. J Reprod Immunol. 2011;89(2):133–9. [DOI] [PubMed] [Google Scholar]
- 15.Global Pregnancy Collaboration:, Schalekamp-Timmermans S, Arends LR, Alsaker E, Chappell L, Hansson S, Harsem NK, Jälmby M, Jeyabalan A, Laivuori H, Lawlor DA, Macdonald-Wallis C, Magnus P, Myers J, Olsen J, Poston L, Redman CW, Staff AC, Villa P, Roberts JM, Steegers EA. Fetal sex-specific differences in gestational age at delivery in pre-eclampsia: a meta-analysis. Int J Epidemiol. 2017;46(2):632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elsmén E, Källén K, Marsál K, Hellström-Westas L. Fetal gender and gestational-age-related incidence of pre-eclampsia. Acta Obstet Gynecol Scand. 2006;85(11):1285–91. [DOI] [PubMed] [Google Scholar]


