Abstract
Brain metastases (BMs) are usually characterised by vasogenic oedema and mass effect, but cystic appearance can rarely occur, mimicking parasitosis, such as neurocysticercosis (NCC). A woman in her mid-50s was admitted for dizziness and upper left extremity paresis. Neuroimaging showed multiple cystic lesions consistent with multiple stages of NCC evolution, and empiric albendazole was started, without any clinical improvement. A whole-body CT revealed a pulmonary lesion in the right superior lobe. Pathological analysis from brain specimen demonstrated a clear cell lung carcinoma. The patient gradually worsened and died 4 months after the diagnosis. In conclusion, multiple cystic BMs are an atypical presentation on neuroimaging; in these cases, a meticulous diagnostic workup should be performed, looking for the possible site of malignancy. Even when it is not possible to perform a biopsy from the primitive lesion, as reported in this case, a brain biopsy should be considered.
Keywords: Oncology, Neurology, Radiology, Infection (neurology)
Background
The central nervous system is a common (30%–50%) site of metastatic spread of non-small cell lung cancer (NSCLC), substantially affecting the quality of life.1 Brain metastases (BMs) present with a wide spectrum of neurological symptoms because of mass effect and oedema. Due to its atypical features and rarity,2–6 cystic BMs can appear similar to parasitosis and can be misdiagnosed. Previous studies reported multiple cystic BMs mimicking neurocysticercosis (NCC).2–7 NCC is endemic in Latin America, sub-Saharan Africa and Asia; NCC is caused by Taenia solium, acquired through faecal–oral transmission after the ingestion of the eggs.8 9 NCC is usually asymptomatic for a long time and rarely affects immunocompetent people, depending on several factors of the parasite and the host.8
MRI with contrast enhancement is the gold standard to characterise brain lesions, especially BMs.10 However, neuroimaging can generate interpretation errors, and a more accurate diagnostic workup is needed in these difficult cases.
We report the differential diagnosis process and clinical decision-making in a case of multiple cystic BMs misdiagnosed as NCC in a patient without a history of malignancy.
Case presentation
A Caucasian woman in her mid-50s, a smoker, was admitted to our department for dizziness and upper left extremity paresis lasting from 2 weeks. Neurological examination revealed distal upper left arm weakness, diffuse hyper-reflexia and normal sensitivity and cranial nerves. The patient denied any recent travel to endemic regions for NCC and tuberculosis, and she referred a past diagnosis of human papillomavirus infection, uterine myomas and irritable bowel syndrome.
Investigations
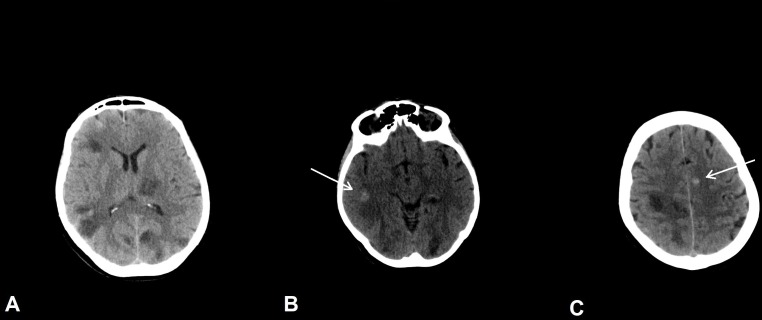
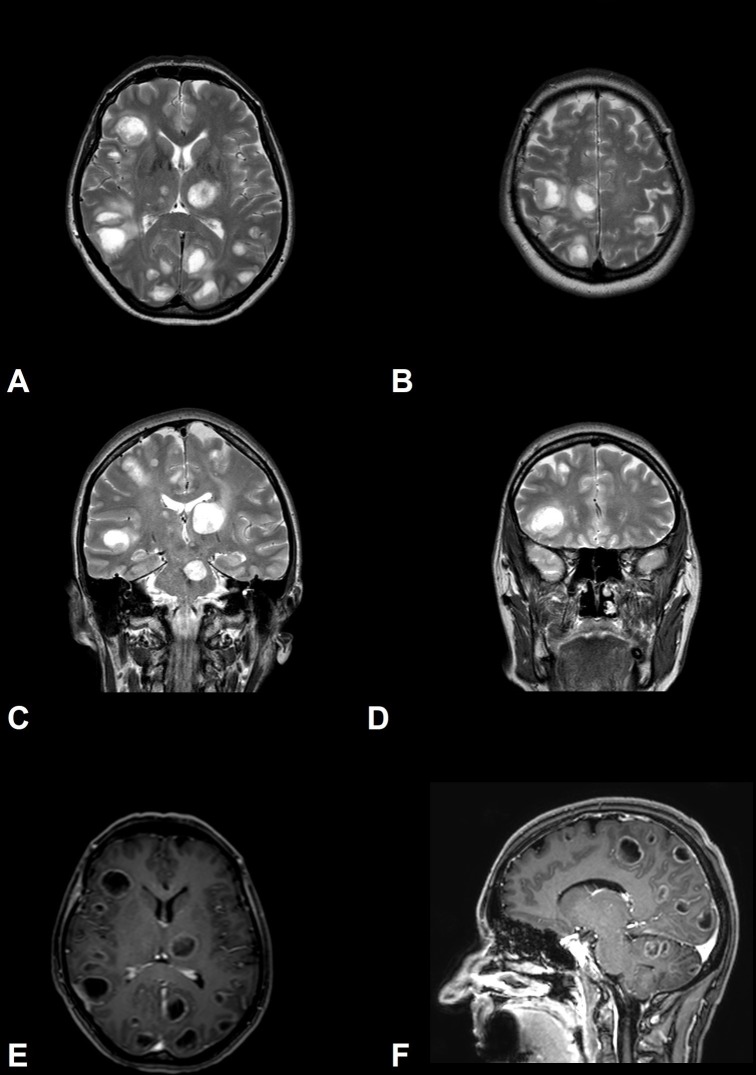
A brain CT showed multiple cystic lesions with calcifications (figure 1). The thorough investigation, conducted by a brain MRI, showed multiple supratentorial and infratentorial cystic lesions localised at the grey and white matter junction, thalamus, basal ganglia and brainstem, characterised by mild perifocal oedema and ring enhancement (figure 2). Furthermore, there was hypointense signal within many cystic lesions as often reported in NCC (‘hole-in-dot’ sign).11 Diagnostic workup for HIV, borreliosis, echinococcosis, toxoplasmosis and tuberculosis was negative. Increased serum levels of carcinoembryonic antigen (CEA=913.4 mg/dL) and microcytic hypochromic anaemia were observed. A whole-body CT revealed a pulmonary lesion of 3 cm in diameter in the right superior lobe. Diagnostic workup consisted of CT-guided needle lung biopsy with a non-diagnostic result and, in the second phase, of right parietal lobe brain lesion biopsy.
Figure 1.
Non-enhanced CT of the brain. (A) Multiple cystic lesions with low signal in CT. (B,C) Calcifications (hyperdense signal) are shown (arrows).
Figure 2.
MRI of the brain. T2 sequences (A–D) and T1 postcontrastographic sequences (E,F). (A,B) Axial images demonstrate high signal on T2 sequences of multiple disseminated cystic lesions. (C,D) Coronal T2 sequences showing a hypointense signal within cystic lesions can mimic the ‘hole-in-dot’ sign in neurocysticercosis. (E,F) T1 postcontrastographic sequences show mild ring enhancement of cystic lesions.
The pathological analysis demonstrated the presence of clear cell lung carcinoma without actionable oncogenic driver: epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1).
Differential diagnosis
The presence of multiple cystic brain lesions carries a wide range of differential diagnosis that includes BMs, primary brain tumours, cerebral abscess and parasitic infections. The diagnostic workup depends on anamnesis, clinical correlation, imaging findings (whole-body CT and brain MRI), diagnostic tests for infectious diseases, cerebrospinal fluid (CSF) studies and, in selected cases, brain biopsy. Treatment with antiparasitics could be the first step for patients with a probable diagnosis of NCC. Furthermore, lack of improvement with albendazole is a useful tool for differential diagnosis. In this case, pyogenic brain abscess was not considered, as the patient did not have a fever.
Treatment
Neuroimaging findings were consistent with multiple stages of NCC evolution, and an empiric albendazole 400 mg twice daily was started, without any clinical improvement. During hospitalisation, the patient developed tonic–clonic generalised seizures, treated with levetiracetam 500 mg twice daily in association with dexamethasone 4 mg twice daily (for vasogenic oedema). Unexpectedly, serology for T. solium resulted negative and albendazole was interrupted.
After the histological diagnosis of BMs from lung cancer, the patient underwent whole-brain radiation therapy without clinical improvement.
Outcome and follow-up
She progressively worsened with confusion, dizziness, weakness, disorientation and visual hallucinations and developed a partial status epilepticus. The spread of the malignancy continued, leading to death 4 months after diagnosis.
Discussion
We report an unusual case of metastatic cystic brain lesions with MRI finding that mimic NCC.
The typical neuroimaging of BMs is characterised by single or multiple well-circumscribed lesions with perilesional vasogenic oedema, contrast enhancement and mass effect. BMs are usually localised in the grey-white junction, being one of the most important features to make a diagnosis.10 In our case, lesions were diffusely localised in the grey-white junction but were also diffused to the pons, basal ganglia, thalamus and cerebral white matter. Furthermore, the cystic appearance of lesions, slight vasogenic oedema and absence of mass effect were atypical for BMs and arose other differential diagnoses.
On the other hand, the imaging findings of NCC are quite variable and heterogeneous, depending on the stage of the disease. There are four recognised stages: vesicular, colloidal vesicular, granular nodular and nodular calcified.12 The vesicular pattern is characterised by a cyst with a thin wall, no perilesional oedema or contrast enhancement and an intralesional eccentric bright dot representing the scolex, appearing hypointense within hyperintense cystic lesions in T2 sequences (hole-in-dot sign).11 13 The ring enhancement with perilesional oedema is typical of the colloidal vesicular stage NCC, whereas calcifications are a hallmark of the final evolutionary stage of the cysticercus, but sometimes they raise suspicion of tuberculosis.11 13 Cysts can occur in the brain parenchyma (parenchymal NCC) or CSF compartments (extraparenchymal NCC), and occasionally, patients have both localisations.11 13 Furthermore, many lesions in different stages and locations can be frequently detected and are considered a typical feature of NCC. A particular challenge is posed for intraventricular cysts, because of the similar signal intensity of the cyst in the CSF spaces. As a result, NCC is often missed by conventional MRI sequences, but the diagnosis can be achieved thanks to the more recent neuroimaging techniques.13
Some authors described eccentric diffusion-weighted imaging (DWI) hyperintense signal in the scolex of many cysts during both the vesicular and colloidal vesicular stages.14 The mechanism underlying DWI-hyperintense scolices is still unknown, probably reflecting the earliest sign of cyst degeneration. This feature is necessary for the diagnosis, as the detection of the scolex is an absolute diagnostic criterion of the disease.9
High-resolution and heavily T2-weighted MRI (fast imaging employing steady-state acquisition (FIESTA) or three-dimensional constructive interference in steady state) sequences are very helpful in delineating the walls and scolex of NCC, which are indistinguishable on T2-weighted images.13 15 Fluid in live cysts has an MRI signal very similar to CSF on T2-weighted sequences; however, on FIESTA sequence, cyst content showed slightly lower signals, making it stand out in the background of CSF. These neuroimaging advances have significant clinical value because intraventricular cysticercal cysts constitute up to 33% of NCC infections, and they are potentially fatal and missed on routine MRI studies in the majority of cases.16 Another group described increased visibility of scolex when susceptibility weighted imaging (SWI) was added to conventional sequences.17 In our patient, there were no available FIESTA and SWI sequences, but DWI sequences did not demonstrate hyperintensity in the correspondent suspected scolex, as expected in the case of metastases.
On CT imaging, several BMs characteristically show calcifications, especially in the frontal lobe,18 and multiple intracranial calcified cysts are often seen in the chronic phase of NCC.19 In our case, the presence of calcifications in the first CT study could be misinterpreted as mature cysts (figure 1). However, the calcifications were not within a cyst, but in the near parenchyma. Interestingly, this finding has already been reported in a case of BMs misdiagnosed with NCC.20
Moreover, DWI has high sensitivity and specificity in differentiating brain abscesses from other intracranial cystic lesions; DWI sequences show diffusion facilitation in tumours and restriction in abscess.21
According to current guidelines, treatment for multiple metastases (>3) from NSCLC consists of whole-brain radiotherapy.1 For patients with symptomatic BMs and/or significant oedema, dexamethasone is recommended.22
To our knowledge, this is the first case of clear cell lung carcinoma presenting with multiple cystic BMs. This case is also unusual because of its clinical presentation. Indeed, there is a strong discrepancy between paucisymptomatic manifestations and the severity of the radiological picture at diagnosis: the patient had no respiratory complaints with mild neurological dysfunction, in spite of disseminated brain lesions.
In conclusion, multiple cystic brain lesions may be misdiagnosed as NCC, especially if there is no known history of primary malignancy. More awareness of atypical patterns of BMs and neuroimaging features is needed for a non-invasive differential diagnosis. DWI and volumetric heavily T2-weighted MRI enable a differential diagnosis with NCC in most cases, especially in the assessment of intraventricular cysts. However, in certain cases, invasive procedures are needed for timely diagnosis and appropriate management.
Learning points.
Cystic brain lesions are an uncommon presentation of brain metastases (BMs) and raise the possibility of other diseases affecting the brain, such as primary brain tumours, cerebral abscess and parasitic infections.
BMs could cause a mild neurological dysfunction, in spite of severe and disseminated radiological involvement.
Histology should be obtained when atypical brain lesions are present on MRI, or when there is clinical suspicion of BMs with unknown primary tumour.
Diffusion-weighted imaging and volumetric heavily T2-weighted MRI enable a differential diagnosis with neurocysticercosis, especially in the assessment of intraventricular cysts.
Footnotes
Contributors: FB, VDS and MV provided clinical care to the patient, conception and design, acquisition of the data, analysis and interpretation of the data; MP revised the article critically for intellectual content; all authors contributed to and approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. NCCN Guidelines version 3 Non-Small cell lung cancer 2019.
- 2. Surov A, Hainz M, Kornhuber M. Multiple cystic metastases in the brain from adenocarcinoma of the lung. Am J Med 2009;122:e3–4. 10.1016/j.amjmed.2009.02.030 [DOI] [PubMed] [Google Scholar]
- 3. Mota PC, Reis C, Pires NF, et al. Lung cancer: atypical brain metastases mimicking neurocysticercosis. Int J Clin Oncol 2011;16:746–50. 10.1007/s10147-011-0221-7 [DOI] [PubMed] [Google Scholar]
- 4. Choi HJ, Choi SK. Multiple cystic brain metastases from adenocarcinoma mimicking cysticercosis. Clin Neuroradiol 2012;22:105–7. 10.1007/s00062-011-0074-5 [DOI] [PubMed] [Google Scholar]
- 5. Costa R, Costa RB, Bacchi C, et al. Adenocarcinoma of the lung presenting with atypical cystic brain lesions. Case Reports 2014;2014 10.1136/bcr-2013-203506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Essenmacher AC, Watal P, Bathla G, et al. Brain metastases from adenocarcinoma of the lung with truly cystic magnetic resonance imaging appearance. Clin Imaging 2018;52:203–7. 10.1016/j.clinimag.2018.07.023 [DOI] [PubMed] [Google Scholar]
- 7. Troiani C, Lopes CCB, Scardovelli CA, et al. Cystic brain metastases radiologically simulating neurocysticercosis. Sao Paulo Med J 2011;129:352–6. 10.1590/S1516-31802011000500011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. The Lancet Neurology 2014;13:1202–15. 10.1016/S1474-4422(14)70094-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Del Brutto OH. Neurocysticercosis. Continuum 2012;18:1392–416. [DOI] [PubMed] [Google Scholar]
- 10. Davis PC, Hudgins PA, Peterman SB, et al. Diagnosis of cerebral metastases: double-dose delayed CT vs contrast-enhanced MR imaging. AJNR Am J Neuroradiol 1991;12:293–300. [PMC free article] [PubMed] [Google Scholar]
- 11. Delgado-García G, Méndez-Zurita VA, Bayliss L, et al. Neurocysticercosis: mimics and chameleons. Pract Neurol 2018. [DOI] [PubMed] [Google Scholar]
- 12. Garcıa HH. Del Brutto OH: imaging findings in neurocysticercosis. Acta Trop 2003;87:71–8. [DOI] [PubMed] [Google Scholar]
- 13. Neyaz Z, Patwari S, Paliwal V. Role of FIESTA and Swan sequences in diagnosis of intraventricular neurocysticercosis. Neurol India 2012;60:646–7. 10.4103/0028-3886.105205 [DOI] [PubMed] [Google Scholar]
- 14. Santos GT, Leite CC, Machado LR, et al. Reduced diffusion in neurocysticercosis: circumstances of appearance and possible natural history implications. AJNR Am J Neuroradiol 2013;34:310–6. 10.3174/ajnr.A3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mont'Alverne Filho FEF, Machado LdosR, Lucato LT, et al. The role of 3D volumetric Mr sequences in diagnosing intraventricular neurocysticercosis: preliminar results. Arq Neuropsiquiatr 2011;69:74–8. 10.1590/S0004-282X2011000100015 [DOI] [PubMed] [Google Scholar]
- 16. Govindappa SS, Narayanan JP, Krishnamoorthy VM, et al. Improved detection of intraventricular cysticercal cysts with the use of three-dimensional constructive interference in steady state Mr sequences. AJNR Am J Neuroradiol 2000;21:679–84. [PMC free article] [PubMed] [Google Scholar]
- 17. Verma A, Awasthi R, Prasad KN, et al. Improved detection of parenchymal cysticercal lesions in neurocysticercosis with T2*-weighted angiography magnetic resonance imaging. Acad Radiol 2012;19:958–64. 10.1016/j.acra.2012.03.019 [DOI] [PubMed] [Google Scholar]
- 18. Smits M. Imaging of oligodendroglioma. Br J Radiol 2016;89:20150857 10.1259/bjr.20150857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gasparetto EL, Alves-Leon S, Domingues FS, et al. Neurocysticercosis, familial cerebral cavernomas and intracranial calcifications: differential diagnosis for adequate management. Arq Neuropsiquiatr 2016;74:495–500. 10.1590/0004-282x20160054 [DOI] [PubMed] [Google Scholar]
- 20. Fantini J, Sartori A, Manganotti P. Avoiding misdiagnosis: cystic calcified brain metastases of uterine cervical cancer mimicking neurocysticercosis. BMJ Case Rep 2017;2017:pii: bcr2016217952 10.1136/bcr-2016-217952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reddy JS, Mishra AM, Behari S, et al. The role of diffusion-weighted imaging in the differential diagnosis of intracranial cystic mass lesions: a report of 147 lesions. Surg Neurol 2006;66:246–50. discussion 250-1 10.1016/j.surneu.2006.03.032 [DOI] [PubMed] [Google Scholar]
- 22. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019. [DOI] [PubMed] [Google Scholar]