Abstract
Enterococci have emerged as important nosocomial pathogens due to their resistance to the most commonly used antibiotics. Alternative treatments or prevention options are aimed at polysaccharides and surface-related proteins that play important roles in pathogenesis. Previously, we have shown that 2 Enterococcus faecium proteins, the secreted antigen A and the peptidyl-prolyl cis-trans isomerase, as well as the Enterococcus faecalis polysaccharide diheteroglycan, are able to induce opsonic and cross-protective antibodies. Here, we evaluate the use of glycoconjugates consisting of these proteins and an enterococcal polysaccharide to develop a vaccine with broader strain coverage. Diheteroglycan was conjugated to these 2 enterococcal proteins. Rabbit sera raised against these glycoconjugates showed Immunoglobulin G titers against the corresponding conjugate, as well as against the respective protein and carbohydrate antigens. Effective opsonophagocytic killing for the 2 sera was observed against different E. faecalis and E. faecium strains. Enzyme-linked immunosorbent assays against whole bacterial cells showed immune recognition of 22 enterococcal strains by the sera. Moreover, the sera conferred protection against E. faecalis and E. faecium strains in a mouse infection model. Our results suggest that these glycoconjugates are promising candidates for vaccine formulations with a broader coverage against these nosocomial pathogens and that the evaluated proteins are potential carrier proteins.
Keywords: Vaccine, glycoconjugate, carrier protein, capsular polysaccharide, diheteroglycan, enterococcal proteins, Enterococcus faecalis, Enterococcus faecium, opsonophagocytic assay, mouse infection model
Enterococcus faecium immunogenic proteins, the secreted antigen A and the peptidyl-prolyl cis-trans isomerase, conjugated to the immunogenic Enterococcus faecalis polysaccharide diheteroglycan are promising vaccine antigens with cross-species opsonic and protective potential to fight infections caused by these nosocomial pathogens.
The increasing prevalence of pathogens exhibiting antimicrobial resistance has encouraged the identification of novel vaccine targets [1]. In addition to preventing infections, vaccination can help to reduce the use of broad-spectrum antibiotics by providing protection to individuals at risk [2, 3].
Enterococcus faecalis and Enterococcus faecium, respectively, are the third and fourth most commonly isolated nosocomial pathogens worldwide [4, 5]. The increased prevalence of enterococci as nosocomial pathogens has been mainly attributed to their antimicrobial resistances, their ability to acquire virulence factors, and to form biofilms on indwelling devices [6]. Several cell-surface polysaccharides and protein structures have been proposed as potential vaccine candidates to prevent and/or treat enterococcal infections [7]. Among capsular polysaccharides, diheteroglycan (DHG) has been demonstrated to elicit opsonic and protective antibodies against E. faecalis and is therefore an attractive immunogenic antigen for vaccine development [8]. DHG, present in E. faecalis CPS-C and CPS-D strains, seems to contribute to enterococcal pathogenicity by conferring resistance to opsonophagocytosis and masking antigens from detection by the host’s immune system [9]. This highlights the importance of capsular DHG in pathogenic interactions and supports its use as an antigen for vaccine development [10]. For E. faecium, several cell-surface–associated protein antigens have been proposed as vaccine candidates [11–13]. Cell wall–associated proteins have been proven to play important roles in adhesion and invasion of the host cells [14]. The secreted antigen A protein (SagA) is well conserved among E. faecium strains and has been demonstrated to be associated with biofilm formation, stress response, and adhesion to extracellular matrix proteins [11, 15]. The peptidyl-prolyl cis-trans isomerase protein (PpiC) is involved in β-lactam antibiotic resistance and has been shown to confer resistance to high salt concentrations [16]. In previous studies, we have demonstrated that both SagA and PpiC are able to induce opsonic and cross-protective antibodies that target enterococci [11, 12].
Owing to the normally T-cell–independent immune response against polysaccharides, conjugation to a carrier protein is necessary to activate T cells to induce an effective immune humoral response [17]. Currently, several licensed glycoconjugate vaccines have shown to be safe and successful in the prevention of infectious diseases [18]. However, the increasing number of conjugate vaccines relying on the same carrier proteins could cause a reduced immune response against polysaccharide antigens, resulting in vaccine interferences [19]. Therefore, in this study we evaluated the use of SagA and PpiC from E. faecium as carrier proteins conjugated to DHG from E. faecalis, to develop a cross-species vaccine with broad coverage against the 2 clinically most important enterococcal species.
METHODS
Bacterial Strains and Culture Conditions
E. faecalis type 2 was used to evaluate the polysaccharide component of the conjugates. To study the protein component in the conjugate we used the vancomycin-resistant clinical isolate E. faecium 11236/1. For cross-reactivity tests, the enterococcal strains used are listed in Figure 1. Strains were grown at 37°C in tryptic soy agar (Carl Roth). For polysaccharide purification E. faecalis type 2 was grown in Columbia broth (Becton-Dickinson) with 2% glucose at 37°C until an OD600 of 0.8 was reached. For production of recombinant proteins, Escherichia coli M15/pQE30 protein-gene strains were cultured under shaking at 37°C in Luria/Miller medium (Carl Roth) supplemented with 100 μg/mL ampicillin and 25 μg/mL kanamycin.
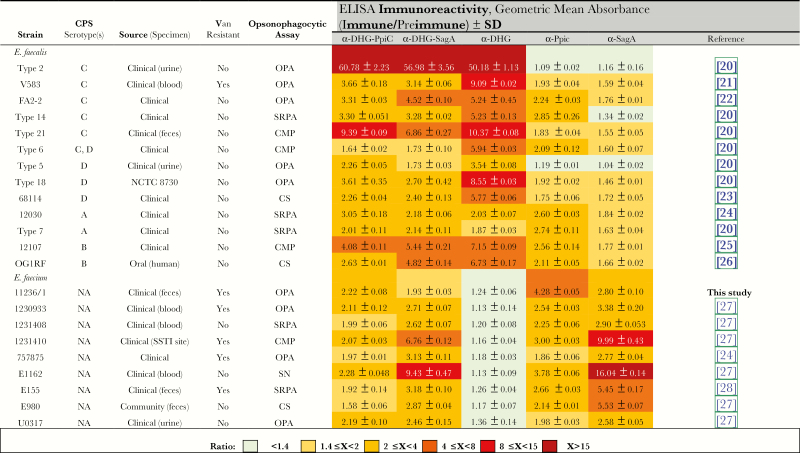
Figure 1.
Immunoreactivity detected by whole-bacterial-cell enzyme-linked immunosorbent assays (ELISAs) for α-DHG-PpiC, α-DHG-SagA, and unconjugated sera against diverse Enterococcus faecalis and Enterococcus faecium strains. CMP, complement-mediated phagocytosis in the opsonophagocytic assay; CPS, capsular polysaccharide; CS, susceptible to complement in the opsonophagocytic assay; NA, not applicable; OPA, possible to test by the opsonophagocytic assay; SRPA, susceptible to rabbit preimmune antibodies in the opsonophagocytic assay; SSTI, skin and soft-tissue infection; Van, vancomycin.
Semisynthesis of Glycoconjugates
Antigens DHG, SagA, and PpiC were produced and purified as described previously (Supplementary Materials) [8, 11, 12]. After the purification procedure, rSagA and rPpiC were used for conjugation at 5 mg/mL. DHG was covalently coupled to the proteins as described by Lees et al, using the cyanylating reagent 1-cyano-4-dimethylamino-pyridinium tetrafluoroborate (CDAP; Sigma-Aldrich) at 100 mg/mL in acetonitrile [29]. A solution of 1 mg of DHG in 100 µL of ultrapure water was slowly mixed with 10 µL of CDAP. After 30 seconds, 15 µL of 0.2 M trimethylamine was added. Final coupling was done by adding 1 mg of protein to the mixture, and the reaction was incubated overnight at room temperature. The glycoconjugates (DHG-SagA and DHG-PpiC) were cleaned up with an Amicon ultrafiltration device with a 100-kDa membrane (Merck-Millipore). The correct conjugation process was assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot (Supplementary Materials). Sugar and protein content were determined by hexose and Bradford assays to establish the polysaccharide to protein ratio in the glycoconjugates (Supplementary Materials).
Rabbit Immunzations
Rabbit immune sera raised against DHG from E. faecalis type 2 (α-DHG), SagA (α-SagA), and PpiC (α-PpiC) have been previously described [8, 11, 12]. For DHG-PpiC and DHG-SagA, New Zealand white rabbits were vaccinated with 2 subcutaneous injections of 10 µg of conjugate in incomplete Freund’s adjuvant given 2 weeks apart. An intravenous injection of 5 µg of the conjugate was given 7 days later, followed by 2 more intravenous injections of 5 µg delivered 2 days apart from each other. On day 35, a test serum was collected, and 7 and 14 days later intravenous boosts with 5 µg of antigen were administered. On day 56, a terminal immune serum was collected from each rabbit. All sera were heat inactivated at 56°C for 30 minutes. Terminal immune sera raised against the conjugates were designated α-DHG-PpiC and α-DHG-SagA. The antibodies were purified using an rProtein A GraviTrap column (GE Healthcare) in accordance with the manufacturers’ instructions. Immunoglobulin G (IgG) contents were measured by enzyme-linked immunosorbent assays (ELISAs) in purified and unpurified sera (Supplementary Materials).
IgG Titer Measurement
The antigens DHG-SagA, DHG-PpiC, DHG, SagA, and PpiC (prepared at 1 µg/mL in 0.2 M sodium carbonate/bicarbonate buffer) were used to coat Nunc-immuno Maxisorp 96-well plates (Thermo-Fisher Scientific) overnight at 4°C. Wells were washed 3 times with 200 µL of PTB (phosphate-buffered saline [PBS] with 0.05% Tween 20 at pH 7.4). Wells were blocked with 200 µL of blocking buffer (3% bovine serum albumin in PBS) at 37°C for 1 hour and washed 3 times with 200 µL of PTB. Sera were adjusted to 50 µg/mL IgG in blocking buffer, and 2-fold serial dilutions were made in the same buffer. After that, 100 µL serum dilutions were added in triplicate, and plates were incubated for 1 hour. Wells were washed 3 times with 200 µL of PTB. Afterward, 100 µL of alkaline phosphatase–conjugated anti-rabbit IgG produced in goat (Sigma-Aldrich) at 1:1000 were added to each well, and plates were incubated for 1 hour. Finally, wells were washed 4 times with 200 µL of PTB, and 100 µL of the p-nitrophenyl phosphate substrate (Sigma-Aldrich) at 1 mg/mL in glycine buffer were added to each well before incubation for 30 minutes. To stop the reaction, 50 µL/well of 3 M NaOH were added, and the absorbance was measured at 405 nm in an ELISA reader (Synergy H1 Hybrid reader, BioTek). Titers were calculated as follows: for each serum sample, the linear relationship between the OD and the log10[dilution factor] was used to extrapolate the intercept of an absorbance of 0.3 for each test, and this was taken as the ELISA end point titer.
Opsonophagocytic Assay (OPA)
OPAs were performed as previously described [11]. The bacterial strain, complement, white blood cells and rabbit sera were prepared individually prior the assay. The bacterial suspension was diluted in RPMIF (Roswell Park Memorial Institute 1640 medium with 15% fetal bovine serum) to yield a final concentration of 2 × 106 cells/mL. Lyophilized baby rabbit complement (Cedarlane) was dissolved in RPMIF at 6.7%, absorbed for 60 minutes at 4°C with the target bacterial strain, and sterilized by filtration before use. Rabbit sera or purified antibodies were diluted in RPMIF at the indicated concentration. White blood cells were freshly prepared from human blood specimens and adjusted to a final concentration of 2 × 106 cells/mL. For opsonophagocytic inhibition assays, purified antibodies were inhibited with the corresponding DHG-protein conjugate, protein alone, or DHG alone. Concentrations ranging from 0.08 to 200µg/mL of inhibitor were incubated overnight at 4°C with an equal volume of a purified antibody at the indicated concentration. After incubation, the mixture of inhibitor/serum was used as a source of antibodies in the OPA as described above.
Whole-Bacterial-Cell ELISA
Immunoreactivity against the enterococcal strains listed in Figure 1 was measured by whole-cell ELISAs with α-DHG, α-protein, and α-DHG-protein sera. Bacteria were grown on tryptic soy agar plates and incubated overnight at 37°C. Colonies were collected from the plate and inoculated in 50 mL of tryptic soy broth at an OD650 of 0.1. Cultures were grown to an OD650 of 0.4 and harvested by centrifugation (at 7450 xg and 4°C for 10 minutes). Cell pellets were washed twice with 50 mL of PBS, resuspended in 25 mL of 8% paraformaldehyde (Sigma-Aldrich) in PBS, and incubated at 4°C for 30 minutes under gentle shaking. Then, cells were washed twice with 25 mL of PBS and finally resuspended in 10 mL of 0.2 M sodium carbonate/bicarbonate buffer. Nunc-immuno Maxisorp 96-well plates were coated with 100 µL/well of cell suspension and incubated overnight at 4°C. ELISAs were performed as described above to measure the IgG titer. The immunoreactivity with a specific serum sample was calculated as the ratio of absorbance of the terminal immune serum to the absorbance of the preimmune serum.
Intravenous Mouse Infection Model
The mouse sepsis infection model was performed as described previously with some modifications [13, 30, 31]. Briefly, male BALB/c mice (weight range, 20–25 g; Harlan, Italy) were randomly separated into 4 groups of 6 and intraperitoneally injected 3 times with 200 µL of either normal rabbit serum, α-DHG-PpiC, or α-DHG-SagA 48 hours before, 24 hours before, and 4 hours after the bacterial challenge. Overnight cultures of E. faecalis type 2 and vancomycin-resistant E. faecium 11236/1 grown in brain heart infusion broth (Sigma-Aldrich) supplemented with 40% heat-inactivated horse serum (Sigma-Aldrich) were centrifuged, and the resulting pellets were resuspended in sterile PBS to achieve final concentrations of 109 bacteria/mL. Mice were first anaesthetized with 100 mg/g ketamine (Merial) and 12 mg/g xylazine (Bayer) via intraperitoneal injection. Aliquots of 100 µL from each strain suspension were injected intravenously into the corresponding group of mice. The animals were monitored twice per day before being euthanized by cervical dislocation 48 hours after the bacterial challenge. Kidneys and livers were aseptically removed, weighed, and homogenized in PBS for 120 seconds at high speed in a Stomacher (Pbi International). Serial dilutions were plated onto Enterococcus Selective Agar (Fluka Analytical) to determinate the number of colony-forming units.
Statistical Analysis
For statistical analysis, Prism, version 7.00 (GraphPad), was used. The percentage of opsonophagocytic killing and absorbance detected by whole-cell ELISA was expressed as the geometrical mean and standard error of the mean. For OPAs and opsonophagocytic inhibition assays, statistical significance was determined by the nonparametric Kruskal-Wallis test, followed by the Dunn post hoc test for multiple comparisons. Results of in vivo experiments were subjected to statistical analysis by using 1-way analysis of variance with a Dunnett multiple comparison test. P values of <.05 were considered statistically significant.
Ethics Statement
Rabbits were housed, immunized, and had serum samples collected by Biogenes (Berlin, Germany), in accordance with national and international animal welfare regulations. Rabbit immunizations were performed under approval and with assurance from the National Institutes of Health Office of Laboratory Animal Welfare (identifier A5755-01). Mouse experiments were conducted under a protocol approved by the Institutional Animal Use and Care Committee at Università Cattolica del Sacro Cuore, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, and authorized by the Italian Ministry of Health (protocol 1F295.37, 11/05/2017; authorization 903/2017-PR, 11/05/2017) according to Legislative Decree 116/92, which implemented the European Directive 86/609/EEC on laboratory animal protection in Italy.
RESULTS
DHG-Protein Conjugate Synthesis
After purifying the conjugates, protein and sugar amounts in the conjugates were determined. The ratios of protein to polysaccharide in the DHG-PpiC and DHG-SagA conjugates were 1:8.7 and 1:7.5, respectively. To verify the conjugation, gels used in SDS-PAGE were either stained with InstantBlue or Stains-All or were blotted into a membrane for Western blot analysis (Supplementary Materials). All glycoconjugates showed broad bands, with staining showing greater molecular weights than those of the unconjugated polysaccharide and protein. Western blot analysis using sera raised against the unconjugated DHG and proteins showed that the conjugates were recognized by these antibodies. This demonstrates that, in the polysaccharide and protein parts of the conjugate, the important immunogenic epitopes remained intact after the conjugation process.
Specific Antibodies Were Generated During Immunization
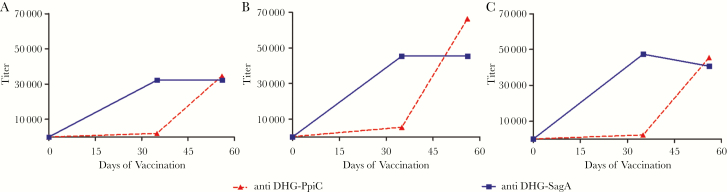
To determine antibody titers raised against DHG, carrier proteins, or DHG-protein conjugates during immunization, sera collected at different time points of the immunization regimen were analyzed against the unconjugated molecules and glycoconjugates. Figure 2 shows that increasing amounts of IgG antibodies against all evaluated molecules were generated during the immunization procedure. The IgG titer against DHG-PpiC with the terminal immune serum α-DHG-PpiC was 11% higher than the titer against DHG-SagA exhibited by α-DHG-SagA terminal immune serum (Figure 2C). Thereafter, in all subsequent experiments, terminal immune serum α-DHG-SagA was used 1.1 times more concentrated than that from α-DHG-PpiC terminal immune serum, and preimmune serum were used at the same IgG concentration as the terminal immune serum.
Figure 2.
Immunoglobulin G (IgG) titer curves of sera raised against different DHG-protein glycoconjugates. Rabbit sera α-DHG-PpiC (solid line) or α-DHG-SagA (dotted line) were examined during the immunization schedule for specificity toward the native DHG (A), the respective carrier proteins SagA and PpiC (B), and the respective different DHG-protein glycoconjugates (C). IgG titers were measured by enzyme-linked immunosorbent assays, using 1 µg per well of antigen. Rabbit sera were plated in 2-fold serial dilutions, starting at an IgG concentration of 50 µg/mL.
Raised Antibodies Mediate Opsonophagocytosis
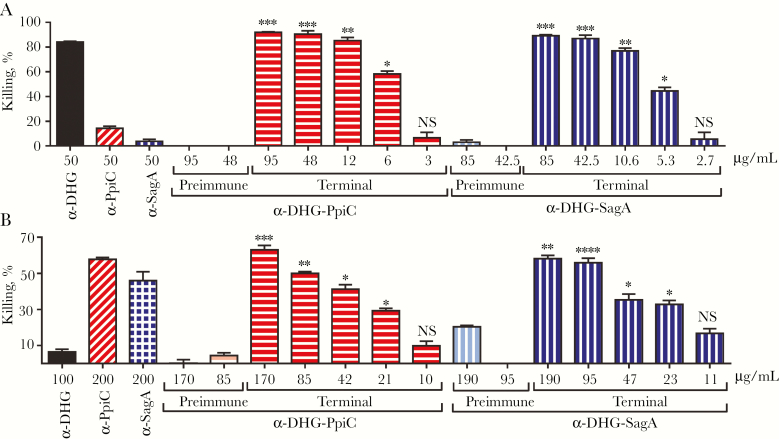
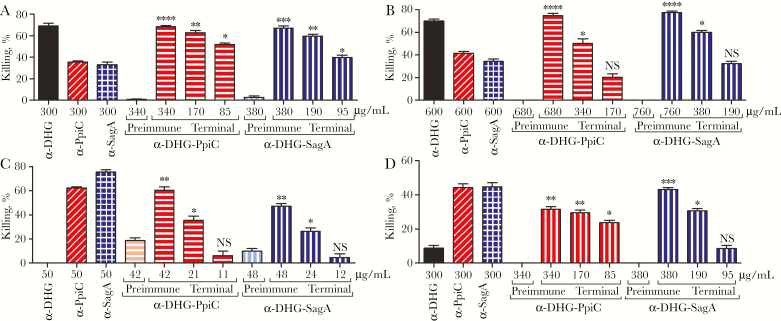
Antibodies targeting DHG were tested using E. faecalis type 2 because this strain was previously demonstrated to possess this antigen and to be targeted by α-DHG opsonic antibodies [8]. Vancomycin-resistant clinical isolate E. faecium 11236/1 was used as target strain for anti-protein antibodies, since most E. faecium strains encode and expresses the 2 immunogenic proteins SagA and PpiC [11, 12]. Percentages of opsonophagocytic killing against E. faecalis Type 2 and E. faecium 11236/1 elicited by α-DHG-PpiC and α-DHG-SagA were comparable and concentration dependent (Figure 3). Control preimmune sera from α-DHG-protein did not significantly mediate opsonophagocytic killing.
Figure 3.
Opsonophagocytic killing activity of anti glycoconjugate rabbit sera against prototype enterococcal strains. Enterococcus faecalis type 2 (A) and vancomycin-resistant Enterococcus faecium 11236/1 (B) were tested with antibodies raised against the conjugates DHG-PpiC (horizontal stripes) and DHG-SagA (vertical stripes), used at the same titer concentration ratio. The absolute immunoglobulin G (IgG) concentration of the sera is shown in the x-axis. Sera raised against the native DHG (black bar) and the recombinant proteins PpiC (diagonal stripes) and SagA (square grids) were used as controls. The effectiveness of opsonophagocytic killing by the anti-conjugate rabbit sera obtained from terminal immune sera (terminal) was compared to that by the preimmune rabbit sera (preimmune; lighter color). Comparisons of preimmune and terminal immune sera at the same concentration were made by the nonparametric Kruskal-Wallis test, followed by the Dunn multiple comparisons post hoc test. Bars and whiskers denote mean values ± standard errors of the mean. NS, not significant (P ≥ .05). *P < .05, **P < .01, ***P < .001, and ****P < .0001.
Antibodies Are Specific Against DHG or Protein Antigens
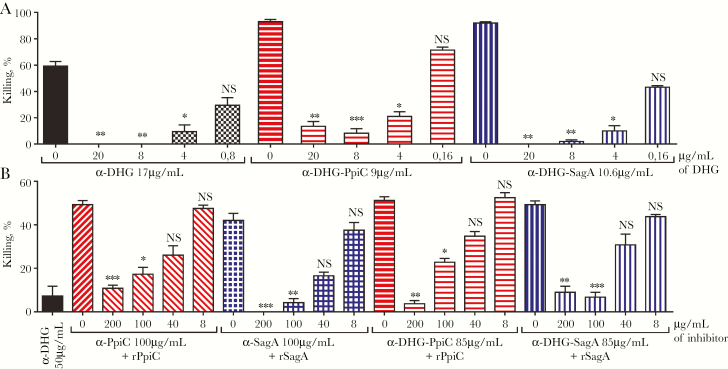
Specificity was evaluated by incubating the antibodies with different amounts of unconjugated molecules. As observed in Figure 4A, opsonophagocytic killing of E. faecalis type 2 by α-DHG, α-DHG-PpiC, and α-DHG-SagA sera was inhibited by high amounts of DHG and restored at lower amounts of antigen. For vancomycin-resistant E. faecium 11236/1, activities of α-PpiC, α-SagA, α-DHG-PpiC, and α-DHG-SagA sera were inhibited in a dose-dependent manner with increasing amounts of rPpiC or rSagA proteins (Figure 4B).
Figure 4.
Inhibition of opsonophagocytic killing activity of α-DHG-protein rabbit sera by polysaccharide and protein components. The purified antibodies against the conjugates DHG-PpiC (horizontal stripes) and DHG-SagA (vertical stripes) were preincubated with different inhibitors. A, The different α-DHG-protein antibodies at a concentration yielding opsonic killing activities between 60% and 90% against Enterococcus faecalis type 2 were incubated with different amounts of native DHG. B, The antibodies at a concentration yielding opsonic killing activities ranging from 40% to 60% against vancomycin-resistant Enterococcus faecium 11236/1 were incubated with different amounts of the corresponding protein. As controls, α-DHG serum (black bars) was incubated with native DHG, whereas α-PpiC serum (diagonal stripes) and α-SagA serum (square grids) were incubated with recombinant PpiC and SagA proteins, respectively. The final inhibitor concentration is shown below the x-axis, as well as the sera concentration used for each set of samples. Statistical significance was performed by the nonparametric Kruskal-Wallis test, followed by the Dunn multiple comparisons post hoc test. Bars and whiskers denote mean values ± standard errors of the mean. NS, not significant (P ≥ .05). *P < .05, **P < .01, and ***P < .001 for comparison of the mean killing by control serum without inhibitor to that of the serum sample incubated with the corresponding inhibitor.
Antibodies Against Conjugates Are Cross-reactive Against Other E. faecium and E. faecalis Strains
Cross-reactivity of antibodies targeting DHG was evaluated against E. faecalis from serotypes CPS-C and CPS-D, ie E. faecalis V583 and E. faecalis type 5, respectively, strains that are susceptible to α-DHG sera in OPA (Figure 5A and 5B) [8]. Antibodies targeting proteins in the α-DHG-protein sera were evaluated against E. faecium E155 and E. faecium 757875 (Figure 5C and 5D), previously reported to be effectively phagocytosed by α-SagA and α-PpiC sera [11, 12]. α-SagA, α-PpiC, and α-DHG-protein mediated opsonophagocytic killing of the 4 enterococcal strains tested, while α-DHG only mediated the opsonophagocytosis of E. faecalis strains. Nevertheless, higher opsonophagocytic killing activities against the E. faecalis strains were observed for α-DHG-protein sera when compared to α-protein sera. To further examine the cross-reactivity and to determine surface availability of our antigenic determinants in a broader bacterial collection, we performed whole cell ELISAs (Figure 1). α-DHG-protein sera recognized all enterococcal strains tested, whereas α-DHG sera only recognized E. faecalis strains. Even though some immunoreactivity with the E. faecalis strains was observed for α-protein sera, it was much lower than the one observed with α-DHG-protein sera. As expected, E. faecium strains immunoreacted only with α-protein and α-DHG-protein antibodies. For E. faecalis strains, some opsonophagocytic killing activity was observed by α-protein, as previously described [11, 12].
Figure 5.
Opsonophagocytic assay of anti-glycoconjugate rabbit sera against different enterococcal strains. The opsonophagocytic activity was tested against Enterococcus faecalis V583 (A), E. faecalis type 5 (B), Enterococcus faecium E155 (C), and E. faecium 757875 (D). The antibodies raised against DHG-PpiC (horizontal stripes) and DHG-SagA (vertical stripes) were used at the same titer concentration ratio. Serum raised against the native DHG (black bars) and the recombinant proteins PpiC (diagonal stripes) and SagA (square grids) were used as controls. Effective opsonophagocytic killing in the α-DHG-protein rabbit sera from terminal immune sera (terminal) was compared to preimmune rabbit sera (preimmune; lighter color) by the nonparametric Kruskal-Wallis test, followed by the Dunn multiple comparisons post hoc test. Bars and whiskers denote mean values ± standard errors of the mean. NS, not significant (P ≥ .05). *P < .05, **P < .01, ***P < .001, and ****P < .0001.
Antibodies Against Conjugates Promotes Clearance of Bacteria in Mice
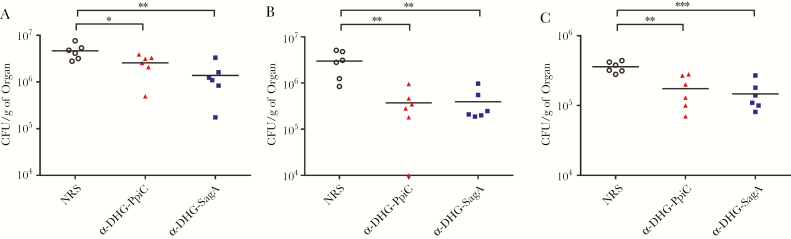
To evaluate the protective efficacy of antibodies raised against the DHG-protein conjugates, we used a mouse infection model, in which we challenged mice with E. faecalis type 2 and vancomycin-resistant E. faecium 11236/1. For E. faecalis type 2, the protection conferred by the sera raised against the different DHG-protein conjugates was comparable in the livers and kidneys (Figure 6A and 6B) and significantly better than that conferred by control sera (normal rabbit sera). Mice challenged with E. faecium 11236/1 were protected in the livers (Figure 6C) when passively vaccinated with the α-DHG-protein sera. However, no protection in mouse kidneys against the E. faecium 11236/1 was observed for any of the sera (data not shown).
Figure 6.
Intravenous mouse infection model. Mice were passively immunized with the sera raised against DHG-PpiC (triangles) and DHG-SagA (squares) conjugates and challenged with Enterococcus faecalis type 2 and Enterococcus faecium 11236/1. After 48 hours of challenge, mice were euthanized, and their kidneys and livers were removed to assess viable counts. A and B, Viable counts in mice livers and kidneys challenged with E. faecalis type 2, respectively. C, Viable counts in mice livers challenged with E. faecium 11236/1. Each point represents the bacterial counts from a single mouse. Bars indicate the median number of colony-forming units (CFU) per gram of organ for the group. Statistical analysis was done by 1-way analysis of variance with the Dunnett post hoc test for comparison of the animals immunized with the antibodies raised against the DHG-protein conjugates to the control animals immunized with normal rabbit serum (NRS; circles). Horizontal bars represent geometric means. *P < .05, **P < .01, ***P < .001, and ****P < .0001.
DISCUSSION
Enterococci are the second most common cause of nosocomial infections [32]. The pathogenicity of enterococci is greatly enhanced by their genetic versatility and ability to acquire antimicrobial resistance and virulence determinants [33]. Immunoprophylactic approaches are of great importance and could provide protection against infections caused by antibiotic-resistant bacteria [3]. Therefore, the development of a vaccine against enterococci would be a valuable approach to fight this opportunistic pathogen [7].
Key points in the development of an antibacterial vaccine have been already pursued in enterococci, such as serotyping and elucidation of carbohydrates responsible of serodiversity. For E. faecalis, 4 different serotypes (CPS A–D) have been previously described, and 2 polysaccharides responsible for most of this serodiversity have been proposed as antigens for the development of an enterococcal vaccine (ie, lipoteichoic acid for CPS-A/B and DHG for CPS-C/D) [8, 24]. A study evaluated the genetic diversity of E. faecalis strains and showed that half or more CPS-C strains appear to be more virulent than CPS-A/B strains [10]. On the other hand, for E. faecium several cell-surface associated proteins have been studied as antigens for vaccine development [11–13]. However, none of them have been evaluated for their dual role as a carrier protein for conjugation with a polysaccharide and as vaccine antigen.
In this work, we semisynthetized 2 enterococcal conjugates consisting of the immunogenic E. faecalis polysaccharide DHG and either PpiC or SagA, 2 immunogenic proteins of E. faecium. We demonstrated that DHG-PpiC and DHG-SagA not only induced polysaccharide specific antibodies toward DHG but also elicited antibodies against the protein immunogens. The resulting sera, α-DHG-PpiC and α-DHG-SagA, showed good and specific opsonophagocytic killing against both clinically relevant enterococcal species. We observed that the α-DHG-protein sera had cross-reactive opsonophagocytic activities against E. faecalis strains expressing the DHG polysaccharide and E. faecium strains expressing PpiC and SagA proteins.
Some bacterial strains cannot be evaluated by OPA because they can be susceptible to rabbit preimmune antibodies or to complement alone, while others undergo complement-mediated phagocytosis. To further analyze the sera coverage, we performed whole-cell ELISA with a collection of 13 E. faecalis and 9 E. faecium strains. Similar immunoreactivities toward E. faecalis strains were observed with α-DHG-protein sera when compared to α-DHG sera. For the E. faecium strains, sera raised against the conjugates showed reduced immunoreactivities in comparison to α-protein sera, which may be caused by the fact that the concentration was adjusted to the conjugate titers and not to protein titers. Lower immune responses have been also observed for protein carriers, since the conjugation process may affect immunogenic protein epitopes involved in the linkage of protein-polysaccharide molecules [34]. Nevertheless, α-DHG-protein sera had a broader immunoreactivity toward the enterococcal collection than the sera raised against the unconjugated antigens. Although immunoreactivity does not completely correlate with phagocytic killing, whole-cell ELISA can be used to determine whether the antigen/pathogen is recognized by antibodies [35].
There is renewed interest in studying the dual role (carrier/protective antigen) of protein moieties in glycoconjugate vaccines to reduce vaccine formulation complexity, increase vaccine coverage, and simplify vaccination schedules [34, 36]. Dual-role carrier proteins have been studied in several bacterial pathogens, such as Streptococcus pneumoniae, Staphylococcus aureus, Clostridium difficile, Neisseria meningitidis, group B Streptococcus, Salmonella enterica, Klebsiella pneumoniae, and Pseudomonas aeruginosa [37–45]. In most of these studies, protein and polysaccharide from the same pathogen have been conjugated to increase either the protection and/or coverage against same bacterial species [37–45]. However, most of these studies have not assessed the potential cross-reactive coverage that this kind of glycoconjugates may have, since they evaluate 1–2 strains for each part of the glycoconjugate but no larger strain collections. Cross-immunoreactivity was assessed only for N. meningitidis, K. pneumoniae, and P. aeruginosa, for 12, 11, and 3 different bacterial strains, respectively [41, 45]. Only 1 study has conjugated 2 antigens from different bacterial pathogens (ie, O-antigen from K. pneumoniae and flagellin proteins from P. aeruginosa) to increase the coverage of the potential vaccine against gram-negative bacterial infections [45]. In our work, we conjugated antigens from the 2 most clinically relevant enterococcal species and immunized rabbits with the resulting glycoconjugates. The sera were evaluated by in vitro assays against 6 strains, whereas their cross-immunorecognition was tested against 23 enterococcal strains. Also, an in vivo infection model showed that the α-DHG-protein sera promoted clearance of bacteria in mice livers (E. faecalis type 2 and E. faecium 11236/1) and kidneys (E. faecalis type 2), comparable to previous experiments with unconjugated antigens [8, 12, 13]. Nevertheless, further studies should assess whether these conjugates are more potent than the single antigens in the same animal models or if they offer protection in others (ie, rat endocarditis or urinary tract infection). Additionally, different conjugation strategies between DHG, SagA, and PpiC (ie, selective conjugation and noncovalent association), as well as the physicochemical and immunological characterization of our carrier proteins, should be used to improve the immunogenicity of the glycoconjugates and to ensure their controlled production [34].
In summary, the glycoconjugates evaluated here are potential vaccine candidates that offer a broad coverage against infections caused by the 2 most clinically relevant enterococcal species. Furthermore, the SagA and PpiC proteins are promising immunogenic carrier proteins that could be used either conjugated to DHG or to other polysaccharides to develop a multivalent vaccine against enterococci.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. F. R.-S., D. L., C. M., R. T., E. K., and D. M.-M. performed the experiments. F. R.-S., D. L., C. M., R. T., M. S., and J. H. analyzed the results. F. R.-S., D. L., and J. H. wrote the manuscript. All authors read and approved the final manuscript.
Financial support. This work was supported by GLYCOVAX (grant 675671), an European Union Horizon 2020 Research and Innovation Programme funded under the Marie Skłodowska-Curie program.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Astronomo RD, Burton DR. Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat Rev Drug Discov 2010; 9:308–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berghman LR, Abi-Ghanem D, Waghela SD, Ricke SC. Antibodies: an alternative for antibiotics? Poult Sci 2005; 84:660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jansen KU, Knirsch C, Anderson AS. The role of vaccines in preventing bacterial antimicrobial resistance. Nat Med 2018; 24:10–9. [DOI] [PubMed] [Google Scholar]
- 4. Zarb P, Coignard B, Griskeviciene J, et al. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Eurosurveillance 2012; 17:pii=20316. [DOI] [PubMed] [Google Scholar]
- 5. Werner G, Coque TM, Hammerum AM, et al. Emergence and spread of vancomycin resistance among enterococci in Europe. Eurosurveillance 2008; 13:19046. [PubMed] [Google Scholar]
- 6. Hollenbeck BL, Rice LB. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 2012; 3:421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koch S, Hufnagel M, Huebner J. Treatment and prevention of enterococcal infections–alternative and experimental approaches. Expert Opin Biol Ther 2004; 4:1519–31. [DOI] [PubMed] [Google Scholar]
- 8. Theilacker C, Kaczyński Z, Kropec A, et al. Serodiversity of opsonic antibodies against Enterococcus faecalis—glycans of the cell wall revisited. Lu S, ed. PLoS One 2011; 6:e17839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thurlow LR, Thomas VC, Fleming SD, Hancock LE. Enterococcus faecalis capsular polysaccharide serotypes C and D and their contributions to host innate immune evasion. Infect Immun 2009; 77:5551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McBride SM, Fischetti VA, Leblanc DJ, Moellering RC Jr, Gilmore MS. Genetic diversity among Enterococcus faecalis. PLoS One 2007; 2:e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kropec A, Sava IG, Vonend C, Sakinc T, Grohmann E, Huebner J. Identification of SagA as a novel vaccine target for the prevention of Enterococcus faecium infections. Microbiology 2011; 157:3429–34. [DOI] [PubMed] [Google Scholar]
- 12. Romero-Saavedra F, Laverde D, Wobser D, et al. Identification of peptidoglycan-associated proteins as vaccine candidates for enterococcal infections. PLoS One 2014; 9:e111880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Romero-Saavedra F, Laverde D, Budin-Verneuil A, et al. Characterization of two metal binding lipoproteins as vaccine candidates for enterococcal infections. PLoS One 2015; 10:e0136625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grandi G. Bacterial surface proteins and vaccines. F1000 Biol Rep 2010; 2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paganelli FL, de Been M, Braat JC, et al. Distinct SagA from hospital-associated Clade A1 Enterococcus faecium strains contributes to biofilm formation. Schaffner DW, ed. Appl Environ Microbiol 2015; 81:6873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reffuveille F, Connil N, Sanguinetti M, et al. Involvement of peptidylprolyl cis/trans isomerases in Enterococcus faecalis virulence. Camilli A, ed. Infect Immun 2012; 80:1728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ada G. Vaccines and vaccination. Mackay IR, Rosen FS, eds. N Engl J Med 2001; 345:1042–53. [DOI] [PubMed] [Google Scholar]
- 18. Rappuoli R. Glycoconjugate vaccines: principles and mechanisms. Sci Transl Med 2018; 10:1–7. [DOI] [PubMed] [Google Scholar]
- 19. Siegrist C-A. Vaccine immunology. In: Orenstein WA, Offit PA, Edwards KM, Plotkin SA, eds. Plotkin’s vaccines. Philadelphia, PA: Elsevier, 2018:16–34.e7. [Google Scholar]
- 20. Maekawa S, Yoshioka M, Kumamoto Y. Proposal of a new scheme for the serological typing of Enterococcus faecalis strains. Microbiol Immunol 1992; 36:671–81. [DOI] [PubMed] [Google Scholar]
- 21. Sahm DF, Kissinger J, Gilmore MS, et al. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob Agents Chemother 1989; 33:1588–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jacob AE, Hobbs SJ. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol 1974; 117:360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hufnagel M, Hancock LE, Koch S, Theilacker C, Gilmore MS, Huebner J. Serological and genetic diversity of capsular polysaccharides in Enterococcus faecalis. J Clin Microbiol 2004; 42:2548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huebner J, Wang Y, Krueger WA, et al. Isolation and chemical characterization of a capsular polysaccharide antigen shared by clinical isolates of Enterococcus faecalis and vancomycin-resistant Enterococcus faecium. Infect Immun 1999; 67:1213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huebner J, Quaas A, Krueger WA, Goldmann DA, Pier GB. Prophylactic and therapeutic efficacy of antibodies to a capsular polysaccharide shared among vancomycin-sensitive and -resistant enterococci. Infect Immun 2000; 68:4631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murray BE, An FY, Clewell DB. Plasmids and pheromone response of the beta-lactamase producer Streptococcus (Enterococcus) faecalis HH22. Antimicrob Agents Chemother 1988; 32:547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palmer KL, Carniol K, Manson JM, et al. High-quality draft genome sequences of 28 Enterococcus sp. isolates. J Bacteriol 2010; 192:2469–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leendertse M, Willems RJ, Giebelen IA, Roelofs JJ, Bonten MJ, van der Poll T. Neutrophils are essential for rapid clearance of Enterococcus faecium in mice. Infect Immun 2009; 77:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lees A, Nelson BL, Mond JJ. Activation of soluble polysaccharides with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate for use in protein-polysaccharide conjugate vaccines and immunological reagents. Vaccine 1996; 14:190–8. [DOI] [PubMed] [Google Scholar]
- 30. Zhao C, Hartke A, La Sorda M, et al. Role of methionine sulfoxide reductases A and B of Enterococcus faecalis in oxidative stress and virulence. Infect Immun 2010; 78:3889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gentry-Weeks C, Estay M, Loui C, Baker D. Intravenous mouse infection model for studying the pathology of Enterococcus faecalis infections. Infect Immun 2003; 71:1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012; 10:266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller WR, Munita JM, Arias CA. Mechanisms of antibiotic resistance in enterococci. Expert Rev Anti Infect Ther 2014; 12:1221–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Micoli F, Adamo R, Costantino P. Protein carriers for glycoconjugate vaccines: history, selection criteria, characterization and new trends. Molecules 2018; 23:1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Romero-Steiner S, Libutti D, Pais LB, et al. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol 1997; 4:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Micoli F, Costantino P, Adamo R. Potential targets for next generation antimicrobial glycoconjugate vaccines. FEMS Microbiol Rev 2018; 42:388–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Michon F, Fusco PC, Minetti CA, et al. Multivalent pneumococcal capsular polysaccharide conjugate vaccines employing genetically detoxified pneumolysin as a carrier protein. Vaccine 1998; 16:1732–41. [DOI] [PubMed] [Google Scholar]
- 38. Pozzi C, Wilk K, Lee JC, Gening M, Nifantiev N, Pier GB. Opsonic and protective properties of antibodies raised to conjugate vaccines targeting six Staphylococcus aureus antigens. PLoS One 2012; 7:e46648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wacker M, Wang L, Kowarik M, et al. Prevention of Staphylococcus aureus infections by glycoprotein vaccines synthesized in Escherichia coli. J Infect Dis 2014; 209:1551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Romano MR, Leuzzi R, Cappelletti E, et al. Recombinant Clostridium difficile toxin fragments as carrier protein for PSII surface polysaccharide preserve their neutralizing activity. Toxins (Basel) 2014; 6:1385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pinto VB, Burden R, Wagner A, Moran EE, Lee CH. The development of an experimental multiple serogroups vaccine for Neisseria meningitidis. PLoS One 2013; 8:e79304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nilo A, Passalacqua I, Fabbrini M, et al. Exploring the effect of conjugation site and chemistry on the immunogenicity of an anti-group B Streptococcus Glycoconjugate vaccine based on GBS67 pilus protein and type V polysaccharide. Bioconjug Chem 2015; 26:1839–49. [DOI] [PubMed] [Google Scholar]
- 43. Nilo A, Morelli L, Passalacqua I, et al. Anti-group B Streptococcus glycan-conjugate vaccines using pilus protein GBS80 as carrier and antigen: comparing lysine and tyrosine-directed conjugation. ACS Chem Biol 2015; 10:1737–46. [DOI] [PubMed] [Google Scholar]
- 44. Simon R, Tennant SM, Wang JY, et al. Salmonella enterica serovar enteritidis core O polysaccharide conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with S. enteritidis. Infect Immun 2011; 79:4240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hegerle N, Choi M, Sinclair J, et al. Development of a broad spectrum glycoconjugate vaccine to prevent wound and disseminated infections with Klebsiella pneumoniae and Pseudomonas aeruginosa. PLoS One 2018; 13:e0203143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.