Abstract
OBJECTIVES
We studied the frequency and characteristics of antibiotic-induced neutropenia in otherwise healthy children receiving antibiotic therapy for hematogenous osteoarticular infections (OAIs).
METHODS
We retrospectively enrolled otherwise healthy children between 1 month and 18 years of age discharged with an OAI from our institution over an 11-year period. An absolute neutrophil count (ANC) ≤1500 cells/μL was defined as neutropenia. We recorded demographic and clinical information, as well as the value and timing of each ANC in relation to changes in antibiotic therapy. A multivariable regression model assessed the contributions of various risk factors.
RESULTS
A total of 186 children were enrolled (mean age, 7.6 years; 67.2% boys). β-Lactams represented 61.2% of all prescriptions. During treatment, 61 subjects (32.8%) developed neutropenia (median time to onset, 24 days). An ANC < 500 cells/μL occurred in 7 subjects (3.8%). Neutropenic subjects (mean age, 6.0 years) were significantly younger than those without neutropenia (mean age, 8.5 years) (OR = 0.86; 95% CI: 0.79–0.93; p < 0.001) and received significantly longer courses of total (89.3 vs. 55.8 days) and parenteral (24.6 vs. 19.9 days) antibiotic therapy (OR = 1.01; 95% CI: 1.01–1.02; p = 0.004 and OR = 1.02; 95% CI: 1.01–1.04; p = 0.041, respectively). Recurrent neutropenia occurred in 23.0% of all neutropenic subjects and was significantly more common in those with a longer mean duration of parenteral therapy (OR = 1.05; 95% CI: 1.02–1.09; p = 0.004.). No complications from neutropenia occurred.
CONCLUSIONS
Neutropenia was common in our cohort of children receiving prolonged antibiotic therapy for OAIs. Younger age and longer courses of therapy were associated with an increased risk of neutropenia.
Keywords: infectious arthritis, neutropenia, osteomyelitis
Introduction
Neutrophils are the most predominant leukocyte in human blood and are involved in the phagocytosis of a variety of organisms.1 As such, they play an important role in the prevention and control of infection. Neutropenia is a decrease in the absolute number of circulating neutrophils, typically defined as ≤1500 cells/μL.1–6 In children, this may result from a variety of insults, including medication toxicities, congenital processes, viral infections, and overwhelming bacterial sepsis.2,7 Neutropenia may predispose a patient to an increased risk of infection, particularly with some forms of medically induced immunosuppression (e.g., chemotherapy received by oncology patients).2,8
Antibiotics are one of the most commonly used medications in children, with up to one-third of all hospitalized children receiving at least 1 antibiotic during their stay.9 Although most antibiotics are perceived as relatively safe, up to one-third of children may experience adverse effects from their use, which may include neutropenia.10–13 Risk factors for antibiotic-induced neu-tropenia in adults have been hypothesized to include a prolonged duration of therapy (particularly treatment courses in excess of 3 weeks),14 use of β-lactam agents, increasing age,8 and potentially the use of higher dosages of antibiotics.14,15 Of note, the onset of antibiotic-induced neutropenia has been reported to be very low after 30 days of therapy in adults, indicating a potential high-risk period between 2 and 4 weeks of treatment in those receiving prolonged courses of therapy.
The mechanisms behind antibiotic-induced neutropenia are complex, poorly studied, and potentially multifactorial.8,14,16 After initial reports of this finding emerged, early studies suggested an immunologically mediated phenomenon with the generation of antineutrophil antibodies with β-lactam agents, similar to the mechanisms underlying drug-induced hemolytic anemia.16–19 Subsequent reports highlighted a potentially direct myelosuppressive effect of antibiotics. Bone marrow aspirates of subjects with antibiotic-induced neutropenia demonstrated an increase in immature granulocyte precursors and a dearth of differentiated myeloid elements, while in vitro exposure of myeloid progenitor cell cultures to β-lactam agents resulted in a dose-dependent inhibition of granulopoiesis.15 Recent studies have described an effect of the intestinal microbiota on hematopoiesis in a murine model, with the diversity of the fecal microbiota of mice correlating with hematopoiesis and reduced stimulation of terminal granulocyte maturation.20–22 The fecal microbiota has also been shown to regulate the longevity of neutrophils in circulation (which in turn positively correlates with proinflammatory activity).22 These lines of evidence suggest that the effects of antibiotics on the microbiota of patients may also play a role in antibiotic-induced neutropenia.20,21
Osteoarticular infections (OAIs) are one of the most common indications for prolonged antibiotic therapy in children and as such, present a risk for neutropenia during treatment.23,24 Children typically receive up to 4 to 6 weeks of therapy for osteomyelitis or septic arthritis,24 and potentially longer courses should complications ensue (such as chronic osteomyelitis or suppurative complications).25 As such, this condition provides a good setting for the study of antibiotic-induced neutropenia in a relatively homogenous population. However, to our knowledge, no prior study has been dedicated to characterizing the association of antibiotic therapy and neutropenia in children in a detailed fashion. Herein we describe the frequency, severity, timing, risk factors for and outcomes of neutropenia in a cohort of otherwise healthy children receiving antibiotic treatment for hematogenous OAIs.
Materials and Methods
Subjects from 1 month to 18 years of age discharged from the University of New Mexico Health Sciences Center from January 1, 2003, to March 1, 2014, were retrospectively assessed for 1 of 40 different International Classification of Diseases, Ninth Edition codes consistent with OAI. The electronic medical record was reviewed by Pediatric Infectious Disease faculty (WD) to verify the diagnoses. Immunocompromised subjects, those with postoperative OAI and OAI associated with orthopedic implants were excluded, as were subjects with incomplete antibiotic dosing data, those who did not have a baseline absolute neutrophil count (ANC) within 24 hours of the first antibiotic dose documented, and those without subsequent ANC values after antibiotic therapy was begun. Neutropenia was defined as an ANC ≤ 1500 cells/μL, with severe neutropenia defined as an ANC < 500 cells/μL.5–8 Each episode of neutropenia recurring after normalization of the ANC was considered a separate episode. Demographic data were collected, as was every ANC value obtained during the course of antibiotic therapy from the electronic medical record, in addition to any complications felt to be related to neutropenia (assessed via review of inpatient and clinic notes, laboratory values, and home health records for each neutropenic subject by Pediatric Infectious Disease faculty [WD]). Each day of antibiotic therapy was counted as 1 day of treatment, regardless of whether the subject was receiving multiple antibiotic agents concurrently. Change in antibiotic to a new agent or a new dose was recorded as well. Any interventions used for the neutropenia were recorded (drug holiday, dose reduction, change of therapy, use of granulocyte-colony stimulating factor, observation), as well as the duration between the onset of the intervention and the resolution of the neutropenia. A failure of an intervention to resolve a neutropenic episode was defined as an ANC that did not increase above 1500 cells/μL while receiving one of the interventions above prior to cessation of therapy or introduction of a different intervention. Standard protocol at our institution during the study period was to obtain a complete blood count with differential approximately every week while on parenteral antibiotic therapy and every 2 weeks while on oral therapy, with more frequent monitoring if neutropenia was detected. Dosing for OAI for the most common antibiotic agents used was standardized at our institution and included: cephalexin and cefazolin 100 mg/kg/day, maximum 1 g/dose; clindamycin 30 to 40 mg/kg/day, maximum 600 mg/dose; vancomycin 60 mg/kg/day, maximum 1 g/dose for initial dosing, with subsequent dosing as required to maintain a serum trough concentration between 15 and 25 mg/L; ceftriaxone 100 mg/kg/day, maximum 2 g/dose; nafcillin, 200 mg/kg/day, maximum 12 g/day. Dosing of these and other antibiotics was based off of accepted references.26–47
Data were entered into an Excel spreadsheet and downloaded to Stata (v. 12.1, StataCorp, College Station, TX) for analysis. To investigate the possible association of various clinical variables with the outcome of neutropenia and recurrent neutropenia, those variables significantly associated with the binary dependent outcome of the presence of neutropenia or recurrent neutropenia on univariate analysis (Wald t test) were entered into a multivariable logistic regression model specifically created for either outcome. Independent variables included age, gender, ethnicity, duration of antibiotic therapy (total and parenteral), number of antibiotics received, use of a β-lactam or a cephalosporin, presence of concomitant bacteremia at admission, the need for surgery and the number of surgeries performed, the presence of septic arthritis, osteomyelitis or both, the length of hospital stay, and the use of concomitant prescription medications that could affect the ANC. For the latter, any prescription medication the subject was receiving that was listed as possessing the potential to produce myelosuppression as an adverse effect in the Lexi-Comp database was included.26 Odds ratios with 95% CIs were calculated for each variable included in the model, with a Wald test performed to assess whether each OR differed significantly from 1.0. Adjusted analyses were conducted with a likelihood ratio test comparing the model with and without the independent variable(s) of interest.
The mean time to resolution of neutropenia was calculated for any intervention used (e.g., drug holiday, dose reduction, change of therapy, observation, etc.), and a pairwise comparison of means with a Tukey Honest Significant Difference test was conducted to assess for significant differences between means. Simple comparison of 2 independent means used a Welch's t test. This project was approved by the Human Research Review Committee at the University of New Mexico (HRRC#14-099), which determined that informed consent was not required.
Results
Of 239 subjects identified with OAI, 53 were ineligible for enrollment (9 subjects were without a baseline ANC value, 17 were missing information on antibiotic dosing, 6 were immunocompromised, 9 were neonates, 5 suffered from orthopedic implant infections, 3 suffered from postoperative infections, and 4 were without a postbaseline ANC value).
The mean age of the remaining 186 subjects was 7.6 years (median, 7.3 years; interquartile range [IQR] 0.4 years to 17.8 years). Overall, 61 subjects (32.8%) developed neutropenia, with 51 of these 61 subjects (83.6%) developing neutropenia in the first 60 days of treatment (median time to onset day 24 of therapy, IQR, 15–42 days; Figure). In addition to 14 recurrent episodes, this represented 75 separate episodes of neutropenia. Subjects with neutropenia were significantly younger than those without (mean age, 6.0 vs. 8.5 years, p < 0.001) (Table). Boys represented 67.2% of all subjects. Caucasian race was reported in 39.8% of subjects, with 21.5% Native American, 14.5% Latino, 12.9% not stated, 9.7% “other,” and 1.6% African American. There was no difference between those with neutropenia and those without regarding gender and race. Osteomyelitis was diagnosed in 109 subjects (58.6%), septic arthritis in 49 (26.3%), and septic arthritis and osteomyelitis in 28 (15.1%). Neither the presence of osteomyelitis (p = 0.452), septic arthritis (p = 0.976), concomitant septic arthritis and osteomyelitis (p = 0.437), bacteremia at admission (p = 0.375), a need for surgery (p = 0.624), the number of surgeries required (p = 0.513), the length of hospital stay (p = 0.075), nor the use of concomitant potentially myelosuppressive drugs (10 subjects, p = 0.156) were associated with the development of neutropenia. Of the antibiotics used in the cohort, 7 accounted for 83.9% of antibiotic use (vancomycin 24.6%, cefazolin 22.0%, cephalexin 12.4%, parenteral clindamycin 8.5%, ceftriaxone 6.0%, nafcillin 5.7%, and oral clindamycin 4.8%), while 18 other antibiotics accounted for the remainder. Of those patients with neutropenia, 78.7% were receiving a β-lactam antibiotic at the time of neutropenia onset, with 60.7% receiving a cephalosporin. β-Lactam agents, and specifically cephalosporins, where more commonly used by neutropenic subjects (96.7% vs. 92.0%, p = 0.221 and 93.4% vs. 86.4%, p = 0.093, respectively) at some point in their treatment regimens, though this did not reach statistical significance. The most common antibiotic used at the time of neutropenia onset was cefazolin (18 subjects, 29.5%), followed by cephalexin (15 subjects, 24.6%). At the time of neutropenia onset, 59.0% of subjects were receiving a parenteral antibiotic agent. Only 1 subject was receiving more than 1 antibiotic at the time of neutropenia onset (cefotaxime and nafcillin). Linezolid was used in 4 subjects (none of whom developed neutropenia), while trimethoprim-sulfamethoxazole was used in 17 subjects, 10 of whom developed neutropenia (58.8%; 4 subjects developed neutropenia while receiving trimethoprim-sulfamethoxazole and 6 subjects developed neutropenia on a different agent after having received the drug earlier in their treatment course). Neutropenic subjects received an average of 3.1 different antibiotics during their course of therapy (range of 1–7), while non-neutropenic subjects received an average of 2.7 different antibiotics (range of 1–6, p = 0.026 in univariate analysis), though this difference did not remain significant after controlling for other factors in a regression model (p = 0.097). The overall median duration of antibiotic therapy in the cohort was 40 days (IQR, 20–69 days). Neutropenic subjects had an overall greater mean duration of antibiotic exposure (89.3 vs. 55.8 days, p = 0.004), and a greater mean duration of parenteral therapy (24.6 vs. 19.9 days, p = 0.041) than non-neutropenic subjects (Table). The relationship between the duration of parenteral antibiotic therapy and neutropenia remained significant after adjusting for the total duration of antibiotic therapy as well (p = 0.038).
Figure.
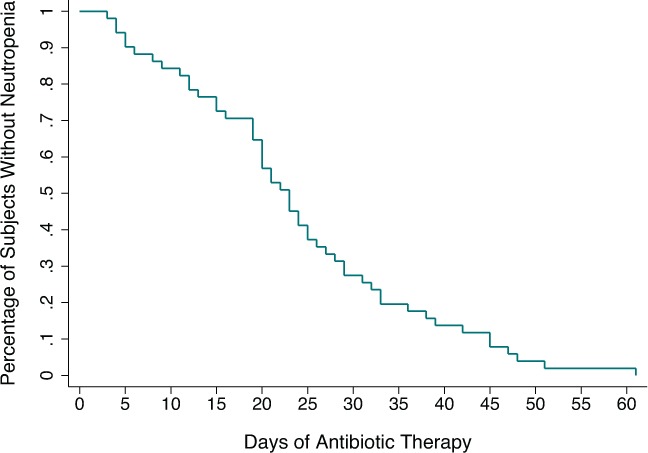
Time to neutropenia onset during the first 60 days of antibiotic treatment in 51 otherwise healthy children.
Table.
Results of Multivariable Regression Analysis of Demographic and Antibiotic-Related Factors * on the Development of Neutropenia and Recurrent Neutropenia in 186 Children
| Variable | Mean Neut. | Mean Non-Neut. | OR | 95% CI | p value |
|---|---|---|---|---|---|
| Age | 6.0 yr | 8.5 yr | 0.86 | 0.79–0.93 | <0.001 |
| Duration antibiotic therapy | 89.3 days | 55.8 days | 1.01† | 1.01–1.02 | 0.004 |
| Duration parenteral therapy‡ | 24.6 days | 19.9 days | 1.02 | 1.01–1.04 | 0.041 |
| Recurrent neutropenia | 30.2 days | 21.5 days | 1.05 | 1.02–1.09 | 0.004 |
| Number of antibiotics | 3.1 | 2.7 | 1.23 | 0.92–1.65 | 0.161 |
Neut, neutropenic
* Factors significantly associated with neutropenia or recurrent neutropenia on univariate analyses were included in the models.
† Here, an OR of 1.01 reflects a 1% increase in the odds of neutropenia per unit change in the independent variable (each day of treatment). Given the large number of potential treatment days, the change per unit increase (per day) as reflected by ORs such as these may appear smaller than their respective p values would suggest.
‡ The duration of parenteral therapy remained statistically significant (p = 0.038) after adjustment for the duration of overall antibiotic therapy.
Overall, 1304 ANC values were recorded (an average of 7 per subject). The overall mean ANC was 5067 cells/μL (median, 3588 cells/μL). The mean ANC at baseline was 6625 cells/μL (median, 6179 cells/μL; IQR, 3188–9180 cells/μL), with a mean corresponding baseline white blood cell count of 11,400 cells/μL (median, 10,100 cells/μL; IQR, 7600–14,500 cells/μL). The lowest ANC recorded was 0 cells/μL in 2 different subjects.
The mean of all neutropenic values was 1050.6 cells/μL (median, 1152 cells/μL; IQR, 880–1360 cells/μL; range, 0–1500 cells/μL). The mean nadir of neutropenic values was 1048 cells/μL, with a range of 0 to 1496 cells/μL. An ANC nadir < 1000 cells/μL was experienced by 21 subjects during therapy (11.3% of cohort as a whole, 34.4% of those with neutropenia), while 7 subjects (3.8% of the cohort as a whole, 11.5% of those with neutropenia) developed severe neutropenia, with an ANC nadir < 500 cells/μL.
The mean time required overall for ANC recovery was 16.0 days (median, 15.0 days; IQR, 6.5–21.5 days). Among the 75 episodes of neutropenia, 26 episodes (34.7%) experienced a spontaneous resolution of the neutropenia with observation alone (with 3 failures), with a mean time to resolution of 17 days (median, 18.5 days; IQR, 8–22 days). The lowest ANC value associated with spontaneous recovery in the cohort was 702 cells/μL. A change in antibiotic therapy successfully treated 19 episodes of neutropenia (with 1 failure), with a mean time to resolution of 15.2 days (median, 12 days; IQR, 6–24 days). A drug holiday was used successfully in 5 episodes of neutropenia (with no failures), with a mean time to resolution of 6.8 days (median, 6 days; IQR, 4–7 days). A dose reduction of the antibiotic was used in 2 episodes, which resulted in resolution of the neutropenia in 14 and 10 days. Neutropenia occurred at the end of therapy in 13 episodes, with no interventions used and no further ANC values obtained. Granulocyte-colony stimulating factor was used in 1 case (with an ANC of 0 cells/μL), with normalization of the ANC after 2 days. The 5 remaining cases were lost to follow-up after documentation of neutropenia. Use of a drug holiday resulted in faster resolution of neutropenia than other treatment approaches, despite the fact that the mean ANC at the time of the drug holiday (530.2 cells/μL) was significantly lower than the ANC nadir in the observation group (1282.3 cells/μL; p = 0.0018) and the antibiotic change group (971.9 cells/μL; p = 0.016). However, this failed to reach statistical significance for any comparisons.
Recurrent neutropenia occurred in 14 neutropenic patients (23.0% of neutropenic patients, 7.5% of the cohort overall) while on therapy after resolution of a prior episode of neutropenia. Resolution of this recurrent neutropenia occurred after a mean of 14.6 days (median, 13 days; IQR, 7–20 days), not significantly different than the time to resolution of the original episodes of neutropenia (p = 0.352). Those with recurrent neutropenia presented with an initial mean neutropenic value of 858.5 cells/μL during their first episode, which was not significantly lower than the overall mean neutropenic ANC value (p = 0.100). The first episode of recurrent neutropenia consisted of a mean ANC of 1130.9 cells/μL. The median time between neutropenic episodes was 20.5 days (IQR, 13–56 days). When compared with neutropenic subjects without recurrence, those with recurrence were not significantly younger (mean age, 5.0 vs. 6.2 years; p = 0.422) but did receive a longer duration of parenteral therapy (30.2 vs. 21.5 days; p = 0.004) (Table). There were no gender differences (p = 0.675), differences in the mean duration of antibiotic therapy (p = 0.862), nor differences in the mean number of antibiotic agents received (3.1 vs. 3.1; p = 0.820) among those with and without recurrent neutropenia. Of note, half of those subjects with recurrent neutropenia had their initial neutropenia addressed with a change in antibiotic coverage, which represented a higher percentage than that in the overall cohort (50.0% vs. 25.7%; p = 0.059). Only 1 subject with recurrent neutropenia had their initial episode treated by a drug holiday. Overall, no adverse outcomes suspected to be related to the neutropenia occurred in any subject, including infections and readmissions.
Discussion
Neutropenia occurred frequently in our cohort, affecting nearly one-third of subjects. Approximately 11% of subjects experienced an ANC < 1000 cells/μL during therapy as well. However, severe neutropenia was rare, with only 3.8% of subjects developing an ANC < 500 cells/μL. Olson et al10 reported similar findings in a study of 298 children receiving antibiotics, where 3.0% of subjects developed an ANC < 500 cells/μL and 12.1% developed ANC values < 1000 cells/μL. The population in this cohort was similar to ours, with a median duration of therapy of 45 days, a median duration of parenteral therapy of 24 days, and a mean age of 7.4 years.10 However, as the focus of this study was not on neutropenia, additional analyses and details were not presented. Subjects with neutropenia in our cohort were significantly younger than those without, which, to our knowledge, is the first time this has been reported. Longer courses of total and parenteral antibiotic therapy were significantly associated with neutropenia. Recurrent neutropenia was common, occurring in approximately 23% of neutropenic subjects, and was significantly associated with longer courses of parenteral therapy. To our knowledge, no other study has reported on the frequency or characteristics of recurrent neutropenia during prolonged antibiotic treatment.
Importantly, no subjects experienced any adverse events from neutropenia, which is consistent with other studies of transient neutropenia in otherwise healthy children (though these studies did not assess the association with antibiotic-induced neutropenia specifically).2,48,49 The onset of neutropenia occurred late in the treatment course in our cohort, after a median of 24 days of therapy, consistent with prior reports.13,14 Gomez et al12 reported a mean time to neutropenia onset in 30 children receiving outpatient parenteral antibiotic therapy of 20 days, while Olaison et al14 noted a mean time to neutropenia onset of 21 days among 18 adults who developed neutropenia while on prolonged antibiotic therapy. Notably, 34.7% of all episodes of neutropenia in our cohort spontaneously resolved, though none in subjects with ANC values < 700 cells/μL. Use of a drug holiday provided the most rapid resolution of neutropenia (6.8 days) compared with a change in antibiotic therapy or observation alone, despite the fact that subjects receiving a drug holiday demonstrated significantly lower ANC values at the time of the intervention than other intervention groups (though the findings were not significant, potentially due to the small numbers of subjects analyzed). Interestingly, Olaison et al14 and Neftel et al15 reported similar findings in courses of prolonged antibiotic therapy in adults, with resolution of neutropenia within 4 to 7 days of drug withdrawal. This suggests that, provided the patient is stable and clinically able to tolerate such an intervention, a drug holiday may be an option for treatment of antibiotic-induced neutropenia. In addition, the brief period of time required for resolution of neutropenia with this approach is unlikely to worsen an underlying OAI, particularly as most patients develop neutropenia several weeks into a prolonged treatment course, at a time when the infection is likely stable and the patient would be able to tolerate a brief period off therapy. To our knowledge, these findings have not been well described in children.
Our study had several limitations. The number of subjects analyzed was small, and as a retrospective study, we were unable to control for variables that may affect the natural evolution of neutropenia. Some variables, such as a family history of blood dyscrasias and compliance with antibiotic therapy, were not assessed. In addition, as with most studies addressing this topic, the timing of ANC determination was not predetermined, but at the whim of providers, precluding a more specific characterization of the exact time course of the neutropenia. The treatment of osteomyelitis in children typically involves at least 1 antibiotic change as patients are transitioned from a parenteral agent to an oral agent. However, frequently several such changes are made in an effort to respond to culture results, antibiotic intolerance, and/or selecting agents with appropriate outpatient dosing schedules. Subjects in our cohort received an average of 3 different antibiotics during the course of their therapy. As such, assigning a causative role for a specific antibiotic after the development of neutropenia was difficult when a patient may have taken several such agents in the preceding weeks. This precluded us from accurately determining the risks of neutropenia with specific agents. However, our data still provide the first overview and epidemiologic description of this phenomenon in children.
Conclusions
Neutropenia is a frequent complication associated with prolonged courses of antibiotic therapy for OAIs in otherwise healthy children, though complications are rare. Such neutropenia is more common in younger children and is associated with longer courses of both total and parenteral antibiotic therapy.
Acknowledgment
This research was presented at the 2018 Western Society for Pediatric Research in Carmel, CA (Abstract #320).
ABBREVIATIONS
- ANC
absolute neutrophil count
- IQR
interquartile range
- OAI
osteoarticular infection
Footnotes
Disclosure The authors declare no conflicts or financial interest in a product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data and take responsibility for the integrity and accuracy of the data analysis.
REFERENCES
- 1.Bhatt V, Saleem A. Review: drug-induced neutropenia—pathophysiology, clinical features, and management. Ann Clin Lab Sci. 2004;34(2):131–138. [PubMed] [Google Scholar]
- 2.Barg AA, Kozer E, Mordish Y et al. The risk of serious bacterial infection in neutropenic immunocompetent febrile children. J Pediatr Hematol Oncol. 2015;37:e347–e351. doi: 10.1097/MPH.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 3.Segel BG, Halterman S. Neutropenia in pediatric practice. Pediatr Rev. 2008;29(1):12–27. doi: 10.1542/pir.29-1-12. [DOI] [PubMed] [Google Scholar]
- 4.Newburger PE, Dale DC. Evaluation and management of patients with isolated neutropenia. Semin Hematol. 2013;50(3):198–206. doi: 10.1053/j.seminhematol.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsieh MM, Everhart JE, Byrd-Hold DD et al. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Int Med. 2007;146(7):486–498. doi: 10.7326/0003-4819-146-7-200704030-00004. [DOI] [PubMed] [Google Scholar]
- 6.Dale DC. How I manage children with neutropenia. Br J Hematol. 2017;178(3):351–363. doi: 10.1111/bjh.14677. [DOI] [PubMed] [Google Scholar]
- 7.Knight K. Unexpected neutropenia in a febrile, but immunocompetent child. Arch Dis Child. 2015;100(11):1093–1095. doi: 10.1136/archdischild-2015-309497. [DOI] [PubMed] [Google Scholar]
- 8.Andres E, Maloisel F. Idiosyncratic drug-induced agranulocytosis or acute neutropenia. Curr Opin Hematol. 2008;15(1):15–21. doi: 10.1097/MOH.0b013e3282f15fb9. [DOI] [PubMed] [Google Scholar]
- 9.Tribble A, Lee B, Handy L et al. Appropriateness of antibiotic prescribing in U.S. children's hospitals: a national prevalence survey. Open Forum Infect Dis. 2017;4(S1):S497. [Google Scholar]
- 10.Olson SC, Smith S, Weissman SJ, Kronman MP. Adverse events in pediatric patients receiving long-term outpatient antimicrobials. J Pediatr Infect Dis Soc. 2015;4(2):119–125. doi: 10.1093/jpids/piu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maraqa NF, Gomez MM, Rathore M. Outpatient parenteral antimicrobial therapy in osteoarticular infections in children. J Pediatr Orthop. 2002;22(4):506–510. [PubMed] [Google Scholar]
- 12.Gomez M, Maraqa N, Alvarez A, Rathore M. Complications of outpatient parenteral antibiotic therapy in childhood. Pediatr Infect Dis J. 2001;20(5):541–543. doi: 10.1097/00006454-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007;146(9):657–665. doi: 10.7326/0003-4819-146-9-200705010-00009. [DOI] [PubMed] [Google Scholar]
- 14.Olaison L, Belin L, Hogevik H, Alestig K. Incidence of β-lactam-induced delayed hypersensitivity and neutropenia during treatment of infective endocarditis. Arch Intern Med. 1999;159(6):607–615. doi: 10.1001/archinte.159.6.607. [DOI] [PubMed] [Google Scholar]
- 15.Neftel KA, Hauser SP, Müller MR. Inhibition of granulopoiesis in vivo and in vitro by β-lactam antibiotics. J Infect Dis. 1985;152(1):90–98. doi: 10.1093/infdis/152.1.90. [DOI] [PubMed] [Google Scholar]
- 16.Murphy MF, Metcalfe P, Grint PC et al. Cephalosporin-induced immune neutropenia. Br J Hematol. 1985;59(1):9–14. doi: 10.1111/j.1365-2141.1985.tb02957.x. [DOI] [PubMed] [Google Scholar]
- 17.Murphy MF, Riordan T, Minchinton RM et al. Demonstration of an immune-mediated mechanism of penicillin-induced neutropenia and thrombocytopenia. Br J Hematol. 1983;55(1):155–160. doi: 10.1111/j.1365-2141.1983.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 18.Salama A, Schütz B, Kiefel V et al. Immune-mediated agranulocytosis related to drugs and their metabolites: mode of sensitization and heterogeneity of antibodies. Br J Hematol. 1989;71(2):127–132. doi: 10.1111/j.1365-2141.1989.tb07672.x. [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood CF, Smith LL, Rustagi PK, Schentag JJ. Neutropenia associated with beta-lactam antibiotics. Clin Pharm. 1983;2(6):569–578. [PubMed] [Google Scholar]
- 20.Josefsdottir KS, Baldridge MT, Kadmon CS, King KY. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood. 2017;129(6):729–741. doi: 10.1182/blood-2016-03-708594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balmer ML, Schürch CM, Saito Y et al. Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. J Immunol. 2014;193(100):5273–5283. doi: 10.4049/jimmunol.1400762. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D, Chen G, Manwani D et al. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525(7570):528–532. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peltola H, Pååkkønen M. Acute osteomyelitis in children. N Engl J Med. 2014;370(14):352–360. doi: 10.1056/NEJMra1213956. [DOI] [PubMed] [Google Scholar]
- 24.Keren R, Shah SS, Srivastava R et al. Comparative effectiveness of intravenous vs. oral antibiotics for post-discharge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120–128. doi: 10.1001/jamapediatrics.2014.2822. [DOI] [PubMed] [Google Scholar]
- 25.Johnson JJ, Murray-Krezan C, Dehority W. Suppurative complications of acute hematogenous osteomyelitis in children. J Pediatr Orthop B. 2017;26(6):491–496. doi: 10.1097/BPB.0000000000000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 27.Vancomycin. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 28.Cefazolin. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 29.Clindamycin. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 30.Ampicillin-sulbactam. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 31.Cefotaxime. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 32.Cephalexin. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 33.Trimethoprim-sulfamethoxazole. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 34.Linezolid. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 35.Amoxicillin-clavulonate. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 36.Nafcillin. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 37.Amoxicillin. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 38.Ampicillin. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 39.Ceftriaxone. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 40.Doxycycline. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 41.Piperacillin-tazobactam. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 42.Cefepime. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 43.Gentamicin. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 44.Rifampin. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 45.Penicillin G (Parenteral/Aqueous). Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 46.Meropenem. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 47.Ciprofloxacin. Lexicomp. Hudson, OH: Wolters Kluwer Clinical Drug Information; Updated May 21, 2019. https://online.lexi.com/lco/action/home Accessed January 11, 2019. [Google Scholar]
- 48.Hussain EH, Mullah-Ali A, Al-Sharidah S et al. Infectious etiologies of transient neutropenia in previously healthy children. Pediatr Infect Dis J. 2012;31(6):575–577. doi: 10.1097/INF.0b013e318250084a. [DOI] [PubMed] [Google Scholar]
- 49.Pérez-Méndez C, Molinos-Norniella C, Moran-Poladura M et al. Low-risk of bacteremia in otherwise healthy children presenting with fever and severe neutropenia. Pediatr Infect Dis J. 2010;29(7):671–672. doi: 10.1097/inf.0b013e3181d7a486. [DOI] [PubMed] [Google Scholar]


