Abstract
The effects of membrane-permeant Ca2+ chelators on field EPSPs (fEPSPs) were measured in the hippocampal CA1 region of brain slices from young (2–4 months) and old (24–27 months) Fischer 344 rats. BAPTA-AM depressed fEPSPs in young slices by up to 70% but enhanced fEPSPs by 30% in aged slices. EGTA-AM, with slower binding kinetics, did not affect fEPSPs from young slices but enhanced fEPSPs in aged slices. BAPTA derivatives with calcium dissociation constants (Kd) of 0.2–3.5 μmreduced or enhanced fEPSPs in young and aged slices, respectively, but 5′,5′-dinitro BAPTA-AM (Kd of ∼7000 μm) had no effect. Frequency facilitation of the fEPSPs occurred in young, but not in aged, slices, except when BAPTA-AM or EGTA-AM was perfused onto aged slices. The differential effects of BAPTA-AM in young and old slices were eliminated by perfusing with a low Ca2+–high Mg2+ saline or with the calcium blocker Co2+. These data suggest that intracellular Ca2+ regulation is altered and raised in aged neurons. Cell-permeant calcium buffers may be able to “ameliorate” deficits in synaptic transmission in the aged brain.
Keywords: calcium chelator, BAPTA-AM, EGTA-AM, probenecid, hippocampus, field EPSP, frequency facilitation, synaptic transmission, aging
Calcium ions are involved in numerous neuronal signaling processes, such as the control of presynaptic neurotransmitter release (Augustine et al., 1985, 1991), the regulation of membrane excitability (Ghosh and Greenberg, 1995), long-term potentiation (Bliss and Collingridge, 1993; Nicoll and Malenka, 1995), and as a second messenger (for review, see Augustine et al., 1985; Blaustein, 1988; Simpson et al., 1995). Several lines of evidence point to alteration in Ca2+ regulation in brains of aging rodents (Landfield and Pitler, 1984; Gibson and Peterson, 1987; Verkhratsky and Toescu, 1998). In neurons from aged rat brain, altered Ca2+ extrusion, buffering, and uptake (Michaelis et al., 1984; Iacopino and Christakos, 1990;Martinez-Serrano et al., 1992) and reduced clearance of Ca2+ from aged nerve terminals (Martinez et al., 1987; Smith, 1988) have been measured. L-type Ca2+channels (Thibault and Landfield, 1996) and currents (Campbell et al., 1996) are increased in aged CA1 neurons. The above observations support the “calcium hypothesis” of aging, which implicates raised intracellular Ca2+ as the major cause of functional impairment and degeneration in aged neurons (Khachaturian, 1989, 1994;Verkhratsky and Toescu, 1998).
Recently, it was demonstrated by several groups (Scharfman and Schwartzkroin, 1989; Kudo et al., 1990; Tymianski et al., 1993, 1994a) that membrane-permeant calcium chelators may protect neurons in anin vitro model of glutamate-induced cell death (for review, see Choi, 1988, 1995) and in a rat stroke model in vivo(Tymianski et al., 1993, 1994b). These studies show that calcium buffers with fast binding kinetics and higher binding affinities (e.g., BAPTA-AM) were the most neuroprotective. The AM moiety permits cell membrane permeation, and it is then cleaved by intracellular esterases to form the active chelating calcium buffer (Tsien, 1980). We have examined the effects of concentration, Ca2+affinity, Ca2+ binding rate, and extrusion of permeant Ca2+ chelator on synaptic field potentials of hippocampal CA1 neurons in brain slices from young (20–35 d) Wistar rats (Ouanounou et al., 1996b). The application of BAPTA-AM for 15 min attenuated the synaptic field potential amplitude. Probenecid, an anion transport inhibitor, accelerated and enhanced the depression of synaptic potentials by concentrations of BAPTA-AM as low as 0.05 μm (Ouanounou et al., 1996b). We have also shown that calcium currents, which were depressed in aged dentate gyrus neurons, were enhanced by intracellularly applied EGTA (Reynolds and Carlen, 1989).
In light of these observations, we compared the effects of membrane-permeant calcium chelators on synaptic transmission in hippocampal slices taken from young-mature and aged Fischer 344 rats. We found that both BAPTA-AM and EGTA-AM enhanced the fEPSP in aged slices, suggesting that there is tonic elevation of [Ca2+]i in the aged neuron. These enhancing effects of calcium chelators could be completely reversed if Ca2+ influx was partially blocked by either reducing the extracellular Ca2+/Mg2+ ratio or incubating the slices with Co2+.
Part of this work was published previously in abstract form (Ouanounou et al., 1996a).
MATERIALS AND METHODS
Tissue preparation. Brain slices were obtained from young-adult (2–4 months) and aged (24–27 months) Fischer 344 rats. Rats were anesthetized with halothane (Halocarbon Laboratories, River Edge, NJ) and decapitated, and the brain was quickly removed, hemisected, and placed in ice-cold (4°C) artificial CSF (ACSF) for ∼3 min. Although the skulls of aged animals are somewhat thicker than those of young animals, the period required to remove the brain was not substantially longer, and we have not observed consistent differences in the viability of slices from aged and young animals. Brain slices were cut to 400 μm thickness with a Vibratome (Series 1000; Technical Products, Inc., St. Louis, MO) and incubated in ACSF at room temperature for a minimum of 1 hr before recording. ACSF contained (in mm): 120 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 25 NaHCO, and 10 d-glucose, pH 7.4, continuously bubbled with 95% O2–5% CO2.
Extracellular recordings. Slices were transferred to a submerged recording chamber and continuously perfused with bubbled ACSF at 35 ± 0.5°C. Recording pipettes were inserted into either the apical dendritic region of the Schaffer collateral–commissural termination in the stratum radiatum of the hippocampal CA1 field to record the field EPSPs (fEPSPs) or the stratum pyramidal of CA1 to record population spikes. A stimulating electrode (bipolar twisted wire) was placed on the Schaffer collateral–commissural fibers for orthodromic activation of CA1 neurons. Population spike amplitudes were measured from the onset of the spike to the negative peak. The amplitude of the fEPSP in the dendrites was measured from the baseline to the maximum negative deflection. Stable responses (±10%) for 10 min before drug application were required. Afferent fiber spike amplitude (the presynaptic volley) was measured in those dendritic records in which they were present. Signals were recorded by an Axoclamp 2A amplifier (Axon Instruments, Foster City, CA). Field potentials were evoked every 30 sec. Data were collected, digitized, and analyzed using pClamp software (version 5.1; Axon Instruments) on an IBM personal computer. Although the number of slices are noted, all statistical differences were assessed by the Student’s pairedt test comparing the number of rats (also noted for each experiment) in each group. Unless otherwise stated, mean ± SE were shown throughout the text. All drug responses were measured 40–45 min after onset of drug perfusion.
Drug preparation. BAPTA-AM was initially dissolved in DMSO and then diluted to its final concentration in the ACSF. DMSO concentration in ACSF was 0.1% for the highest concentration (50 μm) of BAPTA-AM. In addition, 2-hydroxypropyl-β-cyclodextran (0.7 mm; Research Biochemicals, Natick, MA) was used to stabilize the chelator in the aqueous ACSF, presumably protecting the AM moiety from hydrolysis. EGTA-AM, 5′,5′-dinitro BAPTA-AM, 5′5′-difluoro BAPTA-AM, and 5′5′-dibromo BAPTA-AM (Molecular Probes, Eugene, OR) were dissolved initially in DMSO. Probenecid (Sigma, St. Louis, MO) was dissolved in 1m NaOH and buffered to pH 7.4 using HCl acid. When probenecid was used, sodium concentration in the ACSF was adjusted to be the same as in the normal ACSF.
RESULTS
BAPTA-AM attenuates fEPSPs in young rats but enhances those in aged rats
Extracellular recording from the stratum radiatum of the CA1 area shows a response that is usually composed of a presynaptic volley and an fEPSP. The fEPSP that follows the presynaptic volley reflects the extracellular sum of single EPSPs at the level of the Schaffer collaterals. As shown in Table 1, the maximal fEPSP amplitudes were significantly reduced with age, consistent with previous observations (Landfield et al., 1986; Deupree et al., 1993). As shown previously in the studies mentioned above, there were no significant differences in the presynaptic volleys between young and aged rats (Table 1). These results are consistent with a reduction in the number of functional synaptic contacts made by individual Schaffer collateral axons onto old CA1 cells (Barnes et al., 1992; Barnes, 1994) or could also be caused by alterations in the postsynaptic effectiveness of released transmitter.
Table 1.
Maximal fEPSP and presynaptic volley amplitudes in young and old rats
| Maximal fEPSP amplitude (mV) | Presynaptic volley amplitude (mV) | |
|---|---|---|
| Young | 1.8 ± 0.09 (n = 12) | 0.3 ± 0.02 (n = 12) |
| Old | 1.2 ± 0.03* (n = 10) | 0.3 ± 0.01 (n = 10) |
Values are mean ± SE. As indicated, data are taken from 12 slices in young (4) rats and 10 slices in old (3) rats. *p < 0.01; Student’s paired t test.
Recently, we showed that BAPTA-AM attenuated synaptic field potentials recorded from the stratum radiatum in a concentration-dependent manner in young (20–35 d) Wistar rats (Ouanounou et al., 1996b). BAPTA-AM was more efficient when applied together with probenecid (1 mm), an anion transport blocker, which presumably blocks the extrusion of BAPTA from the presynaptic terminal. Following the same strategy, BAPTA-AM (1 μm) was applied in the presence of 1 mm probenecid after a stable baseline was achieved. To control for the possible effects of DMSO, cyclodextran, and probenecid (see Materials and Methods), the slices were perfused with ACSF containing the same concentrations of the above agents until a stable baseline was achieved before the application of BAPTA-AM. BAPTA-AM application for 20–25 min attenuated the fEPSPs in young slices by 58 ± 4% (n = 5 slices from 5 rats); however, it enhanced the fEPSP in aged slices by 31 ± 6% (n = 9 slices from 6 rats) (Fig.1). The maximal effect was achieved within 10–12 min from the application time. fEPSP attenuation (or enhancement) by BAPTA was reversible on washout once probenecid had been removed and was reproduced by a second BAPTA application (young,n = 4 slices from 4 rats; aged, n = 6 slices from 4 rats; data not shown).
Fig. 1.
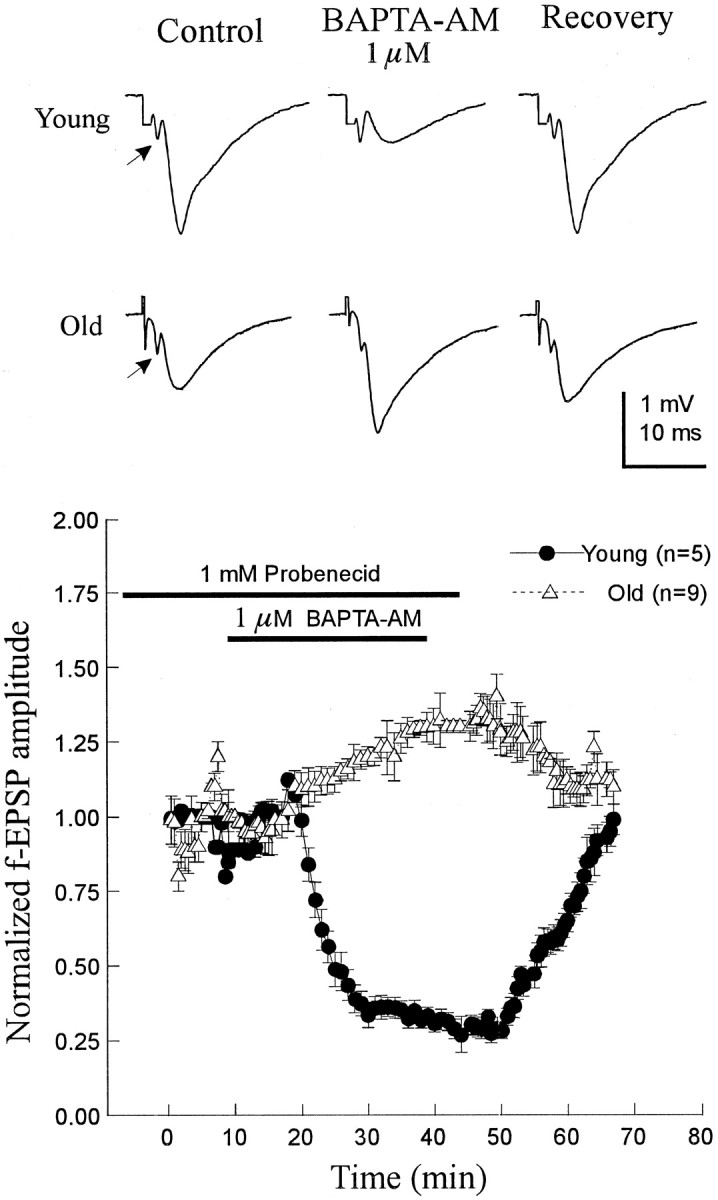
BAPTA-AM attenuates the fEPSPs in young slices but enhances fEPSPs in aged slices. Top, Sample tracing recording of fEPSPs during control condition, during BAPTA-AM and probenecid application, and after removal of the drugs.Bottom, Normalized fEPSP (±SE) plotted against time. Slices (5 young from 5 rats; 9 old from 6 rats) were incubated in ACSF with probenecid (see Materials and Methods), and, after a stable baseline was achieved, BAPTA-AM was applied. fEPSPs were reduced in the young and enhanced in the aged slices without a clear effect on the presynaptic volley (arrows).
When the Schaffer collateral pathway was stimulated, there was a population spike amplitude of ∼2 mV in the stratum pyramidal (somatic region), representing postsynaptic action potentials in response to the excitatory stimulus. Superfusion of the young brain slices with ACSF containing 1 μm BAPTA-AM and 1 mm probenecid for 20 min attenuated the population spike amplitude by 68 ± 8% (p < 0.01; Student’s t test) when measurements were taken 20 min after the onset of the BAPTA application. However, when aged slices were perfused with the same concentration of BAPTA-AM, the population spike amplitude was enhanced by 28 ± 3% (n = 4 slices from 3 rats).
To determine whether the action of BAPTA might be attributed to altered inhibitory synaptic transmission, the experiments in both the young-adult and aged slices were repeated using ACSF containing the GABAA blocker bicuculline (10 μm). Results similar to those shown in Figure 1 were obtained (young, 52 ± 4% depression; n = 5 slices from 4 rats; aged, 27 ± 5% enhancement; n = 6 slices from 5 rats), supporting the notion that BAPTA-AM directly attenuates excitatory responses in young animals and enhances those in aged. In the present study, the attenuation (or enhancement) of the fEPSPs occurred without clearly affecting the presynaptic volley amplitude (Fig. 1, arrows; see also Fig. 4, arrows), suggesting that BAPTA is unlikely to act on axonal spike invasion into the presynaptic terminal.
Fig. 4.
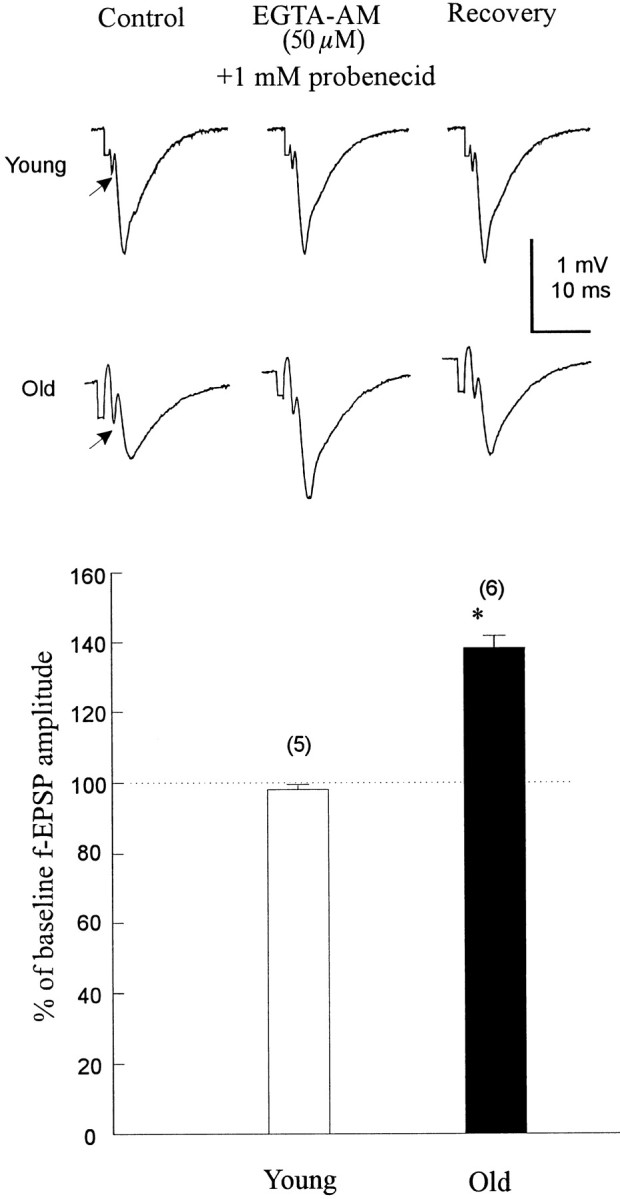
EGTA-AM (50 μm), a Ca2+ chelator with slow Ca2+-binding kinetics, enhanced the fEPSP amplitude in slices from aged animals but did not attenuate fEPSPs when applied to young-mature brain slices for 45 min. Top, Single recordings from young and aged slices during control, EGTA-AM application, and washout. Bottom, Barsindicate mean ± SE. Numbers inparentheses represent the number of slices in each group. *p < 0.01, significant difference from baseline; paired Student’s t test. Note the significant effect that EGTA-AM had on the fEPSPs, but not on the presynaptic volleys, in aged slices (arrows).
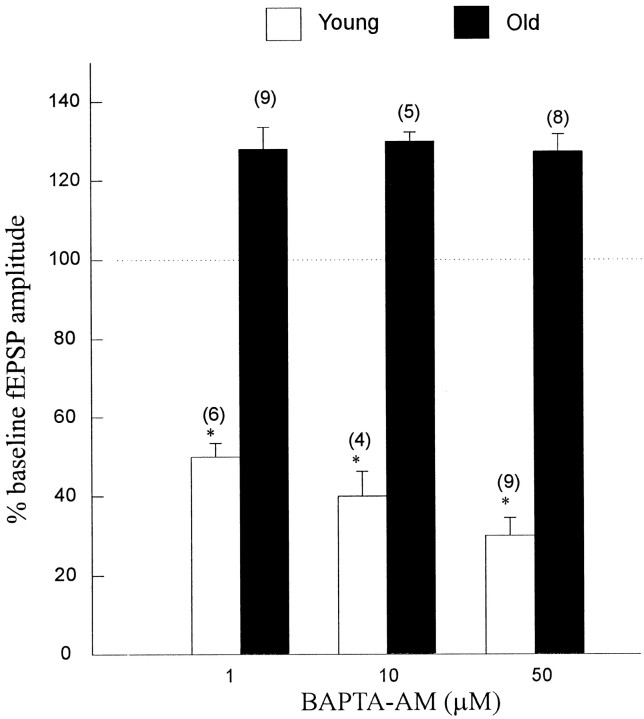
We next asked whether the enhancing effects that we observed in the aged neurons were attributable to failure to accumulate sufficient BAPTA intracellularly. For instance, Robitaille and Charlton (1992) andRobitaille et al. (1993a) found that for a short time, when its intracellular concentrations would be small, BAPTA-AM actually enhanced transmitter release at the frog neuromuscular junction, but later, when intracellular BAPTA concentration should have increased, transmitter release was inhibited. They showed that this enhancement was caused by block of Ca2+-gated K+ channels in the presynaptic terminal. If enhancement in aged slices is attributable to the inability to accumulate BAPTA sufficiently, then increasing the BAPTA-AM concentration would cause a more rapid increase in intracellular BAPTA and attenuate the fEPSP, even in the aged slices. However, BAPTA-AM application to the young slices at a concentration of 1, 10, or 50 μm caused fEPSP depressions of 52 ± 7 (1 μm; n = 6 slices from 4 rats), 66 ± 5 (10 μm; n = 4 slices from 4 rats), and 71 ± 11% (50 μm;n = 9 slices from 6 rats) (Fig.2) when measured 40 min after the onset of the BAPTA-AM application. These results show that increasing concentrations of BAPTA-AM can have larger effects in young, but not old, slices, confirming our previous observations in brain slices from young Wistar rats (Ouanounou et al., 1996b). Application of 1 (n = 9 slices from 6 rats), 10 (n = 5 slices from 3 rats), or 50 μm (n = 8 slices from 5 rats) BAPTA-AM with 1 mm probenecid for 40 min enhanced the fEPSP by ∼30% (Fig. 2). At these concentrations, it is unlikely that BAPTA-AM entry was inhibited or was not active, particularly because 1 mm probenecid was added to the ACSF (Ouanounou et al., 1996b). Furthermore, aged slices perfused with 50 μm BAPTA-AM for 1 hr showed enhancement of the fEPSP amplitude (n = 6 slices from 5 rats; 25 ± 3% enhancement).
Fig. 2.
Effects of different concentrations of BAPTA-AM on the fEPSP amplitude in young and aged Fischer 344 rats. Slices were perfused for 15–20 min with ACSF until stable recordings (changes in the baseline fEPSPs of <±10% for 10 min before drug application) were achieved. In the young animals (open bars), the application of 1, 10, or 50 μm and 1 mmprobenecid for 40 min caused a reduction in the fEPSP amplitude, but in the aged (filled bars), BAPTA-AM caused enhancement of the fEPSP. Numbers inparentheses are the number of brain slices used to generate the average plotted values (mean ± SE). *p < 0.001; paired Student’s ttest.
BAPTA-AM effects in young and aged slices occurred at all stimulus intensities
We questioned whether the excitatory synapses would be affected by BAPTA-AM at all stimulation intensities, i.e., lower intensities may show different effects than higher intensities during application of BAPTA-AM to the slices. To test this hypothesis, the effect of 50 μm BAPTA-AM and 1 mm probenecid at different stimulation intensities was examined in young and aged slices. The stimulus strength was altered to determine the range of synaptic responses when different numbers of presynaptic axons were stimulated. Responses to different stimuli between threshold and maximum were obtained to construct an input–output (I–O) curve from averaged potentials (n = 4 at each stimulus). After generation of the baseline I–O curve, BAPTA-AM and probenecid were applied. The BAPTA effect, i.e., reduction or enhancement of the field amplitude in young and aged slices, respectively, was observed at all stimulation intensities (Fig. 3), suggesting that this phenomenon is not dependent on a particular stimulation intensity and is therefore not peculiar to a small number of synapses.
Fig. 3.
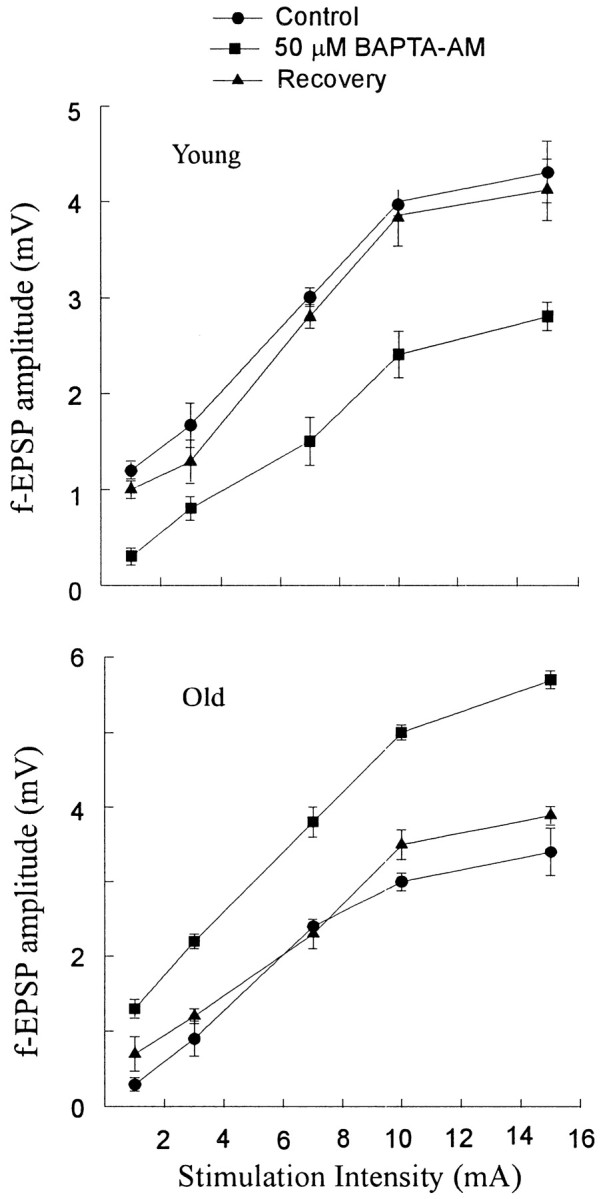
BAPTA-AM reduced or enhanced the fEPSP amplitude in young and aged slices, respectively, at all stimulation intensities. I–O curves for fEPSP amplitude versus the stimulation intensity. Data were generated by averaging the fEPSP amplitudes at the different stimulation intensities in six slices from four young rats and in 10 slices from five aged rats. BAPTA effects were significantly different (p < 0.05) from control and recovery (30 min after drug washout) conditions at each stimulus intensity.
EGTA-AM enhances the fEPSP in aged slices
The importance of the Ca2+ binding rate on the ability of the chelator to block synaptic transmission in young animals and to enhance that in aged was examined next. BAPTA binds Ca2+ ions ∼100 times faster than EGTA, although both have similar Ca2+ affinities (Smith et al., 1984; Kao and Tsien, 1988; Pethig et al., 1989; Augustine et al., 1991). In contrast to BAPTA-AM, the application of 50 μmEGTA-AM with 1 mm probenecid (n = 5 slices from 4 rats) (Fig. 4) caused no significant change in the fEPSP amplitude as measured 45 min after EGTA-AM bath application to the young slices. The lack of effect of EGTA-AM, the slow Ca2+ chelator, is unlikely to be caused by poor loading of this compound given the concentration used. The probable explanation remains that the kinetics of calcium binding by EGTA are too slow relative to [Ca2+]i stimulation of evoked transmitter release (Adler et al., 1991; Augustine et al., 1991).
If aging is associated with tonically elevated intracellular calcium concentration, application of EGTA-AM, as well as BAPTA-AM, might also enhance the fEPSP in the aged slices, regardless of the binding kinetics. When aged slices were incubated with 50 μmEGTA-AM and 1 mm probenecid, the fEPSP was enhanced by 36 ± 5% (n = 6 slices from 4 rats) (Fig. 4), suggesting that fast association and dissociation binding kinetics are not required for this effect in aged animals.
Effect of BAPTA derivatives with different calcium affinities
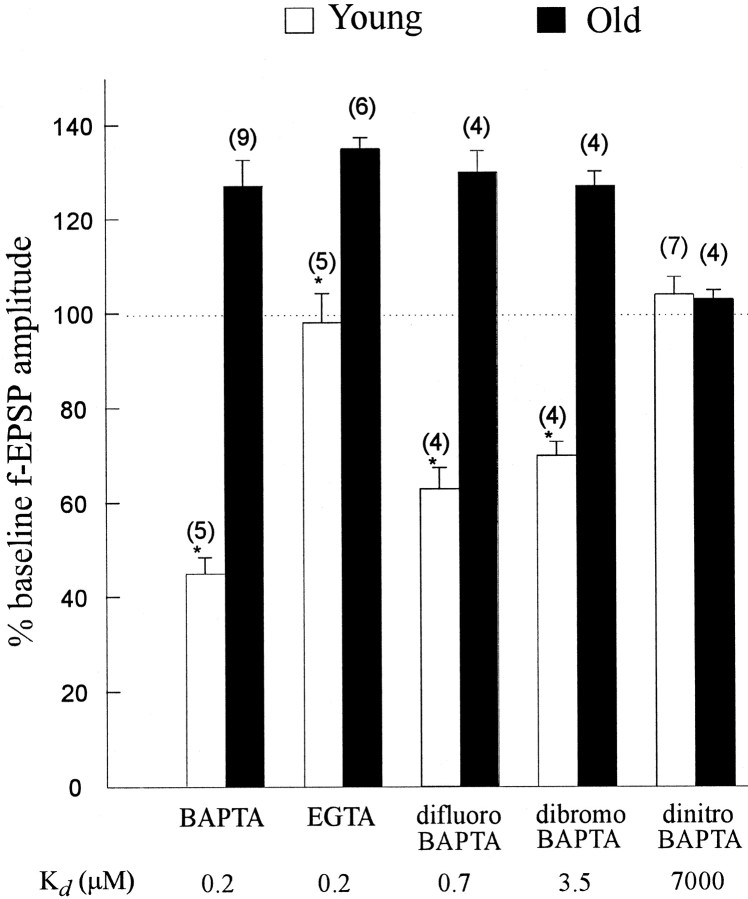
We investigated the effects of several BAPTA derivatives with different calcium affinities, including 5′,5′-difluoro BAPTA-AM (0.7 μm estimated Kd), 5′,5′-dibromo BAPTA-AM (3.5 μm estimatedKd), and 5′,5′-dinitro BAPTA-AM, a low affinity BAPTA analog (7000 μm estimatedKd) (Pethig et al., 1989). All compounds were applied at 1 μm with 1 mm probenecid (Ouanounou et al., 1996b), and, except for 5′,5′-dinitro BAPTA-AM, were capable of reducing the fEPSP amplitude in young slices and enhancing those in aged slices (Fig. 5). The mean reduction in transmission produced by 5′,5′-difluoro BAPTA-AM in the young slices was 43 ± 3%, and the mean enhancement in the aged slices was 26 ± 2%. As for 5′5′-dibromo BAPTA-AM, there was a 38 ± 2% depression in fEPSP amplitude in young slices and a 23 ± 3% enhancement in the aged slices.
Fig. 5.
Relative ability of several BAPTA derivatives to reduce or enhance synaptic transmission in young and aged slices, respectively. Values are mean ± SE. *p < 0.01; paired Student’s t test. Numbersin parentheses indicate the number of slices used.Kd, Estimated dissociation constant. The numbers of animals for each condition are as follows (young and old, respectively): BAPTA, four and five; EGTA, four and five; difluoro BAPTA, four and three; dibromo BAPTA, four and three; and dinitro BAPTA, six and four.
The effects seen were attributable to the BAPTA analogs rather than to the hydrolyzed AM ester moiety, because treating the slices with 50 μm 5′,5′-dinitro BAPTA-AM, a permeant BAPTA analog with a low affinity (Kd values in the micromolar range) (Pethig et al., 1989) had no effect in young and aged slices (young,n = 7 slices from 6 rats; aged, n = 4 slices from 4 rats) (Fig. 5).
BAPTA-AM and EGTA-AM permit frequency facilitation in aged neurons
In some central systems, EPSP amplitude increases substantially during repetitive stimulation (“frequency facilitation”), as does the probability of spike generation. We examined the effects of a 1 Hz repetitive stimulation for 16 sec in young and aged slices. In younger slices, the amplitude of the fEPSP tended to increase with increasing number of stimuli; after 16 stimuli, the fEPSP was increased by 44 ± 4% (n = 7 slices from 4 rats; p < 0.01). In aged slices, only the first one to two stimuli caused an increase in the fEPSP, and after 16 stimuli, no significant frequency facilitation was noted (n = 8 slices from 5 rats).
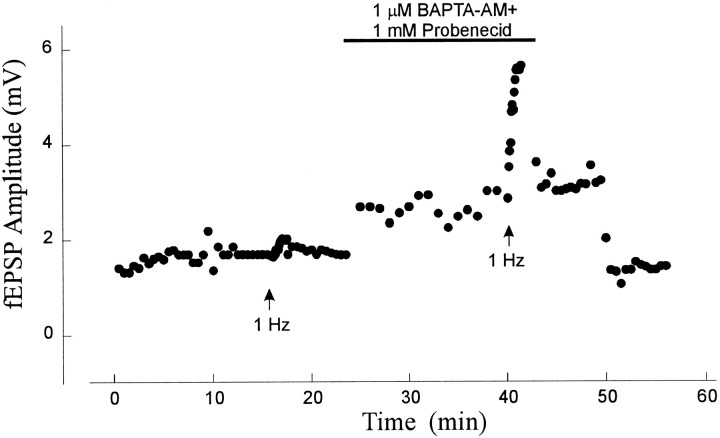
We next tested the effect of repetitive stimulation (1 Hz for 16 sec) in the presence of BAPTA-AM. In seven slices examined, we observed an 83 ± 3% increase in the fEPSP amplitude in the aged slice in the presence of BAPTA-AM (Fig. 6). Similar effects occurred in aged slices in the presence of EGTA-AM (n = 6 slices from 6 rats; a 49 ± 5% increase in the fEPSP amplitude).
Fig. 6.
Repeated stimulation (1 Hz for 16 sec) causes an increase in the fEPSP amplitude in the presence of BAPTA-AM in the aged slice. Repetitive stimulation before the application of 1 μm BAPTA and 1 mm probenecid had no effect, but in the presence of BAPTA-AM, it caused a significant increase in the fEPSP amplitude. Arrows indicate the times at which 1 Hz stimulation for 16 sec was applied. Similar results were obtained from eight slices.
Restricted Ca2+ entry causes calcium chelators to depress fEPSP in aged slices
Although there may be a number of potential sites of altered Ca2+ homeostasis in aged neurons, several electrophysiological studies have pointed to a specific potential source of disregulated neuronal calcium, namely excessive Ca2+ influx (Landfield and Pitler, 1984; Campbell et al., 1996; Thibault and Landfield, 1996). To examine this hypothesis, we lowered the extracellular calcium concentration to 0.5 mm (from 2 mm), and increased Mg2+ to 4 mm (from 2 mm). This maneuver has been shown to reduce neurotransmitter release (Mg2+ by blocking the Ca2+channels and other nonspecific channels) (Martin, 1977; Hagiwara and Byerly, 1981; Lansman et al., 1986; Katz et al., 1997). Moreover, the probability of neurotransmitter release will be decreased, presumably because both resting Ca2+ levels and Ca2+ entry are reduced. Application of the above medium caused a significant reduction in the fEPSPs in the young animals (Fig. 7A,left, open bar, B). In seven young slices, the low Ca2+–high Mg2+saline caused a 62 ± 5% reduction of the fEPSP, compared with a reduction of only 31 ± 7% in the aged slices (p < 0.01; paired Student’s t test) (Fig. 7A, left, filled bar). The time required to cause the depression was ∼15–20 min (Fig.7B). Similar time was required to obtain complete reversal in the normal perfusate.
Fig. 7.
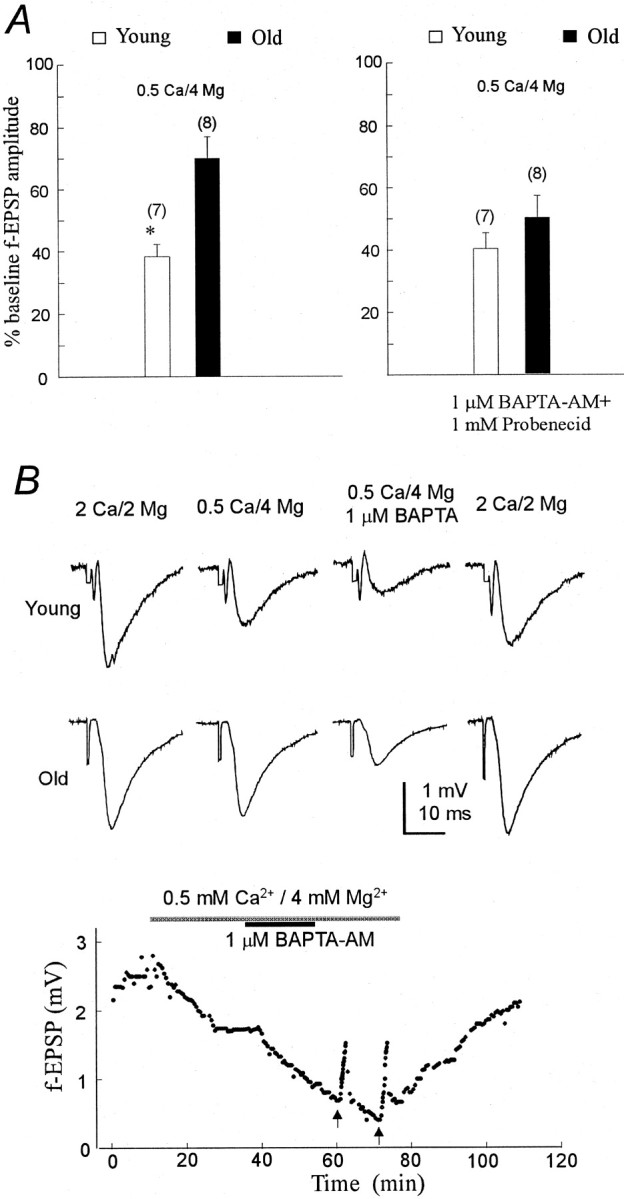
Effects of low Ca2+–high Mg2+ saline on the fEPSP amplitude in young and aged slices. A, Slices were perfused initially with normal ACSF and, after stable control responses were achieved, with 0.5 mm Ca2+ and 4 mmMg2+ ACSF. Left, The percent of baseline fEPSP in the low Ca2+–high Mg2+ saline. Open bars represent the percent (mean ± SE) of the baseline response of the fEPSP amplitude when switching from the normal ACSF to the 0.5 mmCa2+–4 mm Mg2+ ACSF in young slices, and filled bars represent those in old slices. Note that aged slices were less affected by the reduction of Ca2+–Mg2+ than young slices. *p < 0.01; paired Student’s ttest. Numbers in parentheses represent the number of slices used in each group. Right, Application of 1 μm BAPTA-AM and 1 mmprobenecid in the presence of low Ca2+–high Mg2+ causes further attenuation of the fEPSP in both the young and old slices. Slices were perfused as described above. After stability was achieved in the low Ca2+–high Mg2+, 1 μm BAPTA-AM and 1 mm probenecid were applied, causing a reduction of ∼40–50% in the fEPSP amplitude in the slices taken from both young and aged Fischer 344 rats (open bars, young;filled bars, old). B, Top, Sample fEPSP recordings from stratum radiatum. Bottom, Effect of low Ca2+–high Mg2+saline and BAPTA-AM in one old slice. One micromolar probenecid was applied at the beginning of the experiment and was removed at the same time as the low Ca2+–high Mg2+ saline. Note that 1 Hz repetitive stimulation for 16 sec (arrows) facilitated the fEPSP after BAPTA-AM application. In addition, after perfusion with normal ACSF, the amplitude of the fEPSP returned toward the control level. Similar results were obtained from eight slices.
We next asked whether application of BAPTA-AM can cause a depression of the fEPSPs in the aged slices when calcium influx is reduced. Application of 1 μm BAPTA-AM and 1 mmprobenecid (in the above saline containing 0.5 mmCa2+ and 4 mm Mg2+) depressed the fEPSP in the aged slices by 51 ± 8% (n = 8 slices from 5 rats) (Fig. 7A,right, filled bar). Similar results were obtained when BAPTA-AM and the anion blocker probenecid were applied to the younger slices (n = 7 slices from 4 rats; depression by 61 ± 5.8%) (Fig. 7A, right, open bar). Also shown in Figure 7B, bottom, is the time course for this effect in one aged slice. As can be seen from this figure, recovery from the BAPTA-induced synaptic inhibition in the presence of the above mentioned saline occurred after application of a 1 Hz repetitive stimulation for 16 sec (Fig. 7B,bottom, arrows). After perfusion with normal ACSF, the amplitude of the fEPSP returned toward the control level.
If after perfusing with low Ca2+–high Mg2+ saline the aged slice takes on “younger” characteristics, the observed depressive effects of BAPTA-AM will not be mimicked by EGTA-AM, which has no effect on younger animals (Ouanounou et al., 1996b). If this hypothesis is correct, then it is very likely that elevated Ca2+ in aged animals might be caused by excessive Ca2+ influx. In a set of five experiments on aged slices, application of EGTA-AM in low Ca2+–high Mg2+ saline caused no significant change of the fEPSP amplitude (n = 5 slices from 4 rats).
The voltage-gated calcium channel blocker cobalt has different effects on slices from aged and young animals
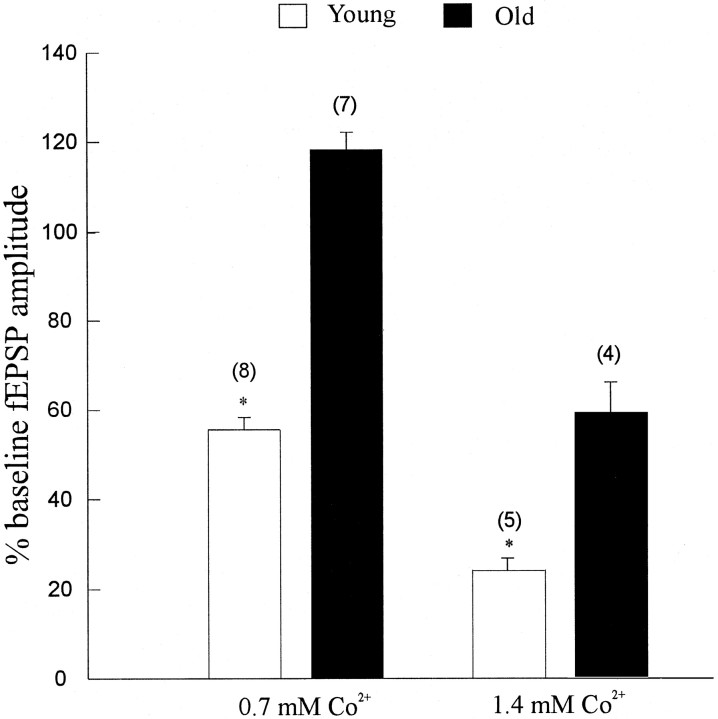
From the above results, it seemed that in this preparation aging could be associated with greater influx of Ca2+. Although there appears to be a number of potential sites for altered Ca2+ influx, some studies suggest enhanced voltage-gated Ca2+ influx (Campbell et al., 1996;Thibault and Landfield, 1996). Divalent metals, such as cobalt, have been used in neuropharmacological studies to block synaptic transmission presynaptically (Kretz, 1984; Kaneko and Tachibana, 1986;Dickie and Davies, 1992). In the present study, 0.7 mmcobalt application to young and aged slices revealed strikingly different results. In eight slices from young-mature rats, 0.7 mm cobalt depressed the fEPSPs by 43 ± 2%, but in seven slices from the aged rats, 0.7 mm cobalt caused a 19 ± 4% enhancement of the fEPSP (Fig.8). Prolonging the incubation time of 0.7 mm Co2+ in the aged slices to 1 hr produced the same result. We therefore decided to increase the concentration of Co2+ to 1.4 mm, which depressed synaptic transmission in the aged slices by ∼45% (Fig. 8). The same concentration of cobalt (1.4 mm) depressed synaptic transmission in young slices by 78 ± 2.3% (n = 5 slices from 5 rats) (Fig. 8). These results suggest significant modification of the number and function of the voltage-gated calcium channels during the process of normal aging.
Fig. 8.
The voltage-gated calcium channel blocker cobalt causes different effects on the fEPSP in young and aged slices. Slices were perfused with normal ACSF and later with ACSF containing 0.7 mm Co2+. Bars represent mean ± SE change from baseline. As can be viewed, 0.7 mm Co2+ caused ∼45% reduction in the fEPSP amplitude in the young (open bars), whereas the same concentration of Co2+ caused 20% enhancement in the fEPSP amplitude (filled bars). Increasing the concentration of Co2+ to 1.4 mmcaused remarkable attenuation of the fEPSP in young slices (open bars), and the reduction in aged was similar to the effect of 0.7 mm in young. Numbers inbrackets indicate the number of slices in each group. *p < 0.01; paired Student’s ttest.
Application of 1 μm BAPTA-AM and 1 mmprobenecid with 1.4 mm cobalt in the aged slices caused a depression of 50 ± 4% (n = 7 slices from 4 rats; data not shown), demonstrating, as before, that by blocking Ca2+ influx and thereby presumably lowering intracellular calcium, BAPTA “resumes” its role of depressing synaptic transmission as in the young slices.
DISCUSSION
The BAPTA-AM effects of enhancing fEPSPs in aged slices were striking, because BAPTA-AM attenuates synaptic transmission in various preparations (Charlton and Iwanchshyn, 1986; Adler et al., 1991; Niesen et al., 1991; Robitaille and Charlton, 1992; Robitaille et al., 1993a,b; Hunt et al., 1994; Tymianski et al., 1994b; Winslow et al., 1994; Blundon et al., 1995; Ouanounou et al., 1996b). Hippocampal synaptic physiology appears to be different in slices from aged compared with young animals in their response to Ca2+ chelators or to diminishing inward Ca2+ flux, supporting the hypothesis that aging results in a persistent increase in the free cytoplasmic calcium concentration (Verkhratsky and Toescu, 1998). These differential effects of calcium chelators cannot be caused by poor loading, because increasing the concentration and exposure time of BAPTA-AM produced similar results, and BAPTA-AM was able to reduce fEPSPs in aged slices once treatments to markedly reduce presynaptic Ca2+ influx were used.
Calcium hypothesis of aging
The calcium hypothesis of aging (for review, see Khachaturian, 1989, 1994; Disterhoft et al., 1993; Landfield, 1994; Verkhratsky and Toescu, 1998) suggests that basal intracellular calcium is increased in aged animals, altering Ca2+-dependent processes, such as neurotransmitter release, synaptic plasticity, and protease activity. In aged CA1 neurons, a direct mechanism for increasing intracellular calcium has been found; L-type Ca2+channels (Thibault and Landfield, 1996) and currents (Campbell et al., 1996) are increased. In hippocampal dentate granule cells of aged rats, L-type Ca2+ currents were reduced, possibly because of an age-related increase in Ca2+-mediated inactivation of Ca2+ channels (Reynolds and Carlen, 1989). Furthermore, calcium currents were enhanced in the dentate neurons of aged, but not young, Fischer 344 rats by intracellular application of EGTA. By analogy to the effects on postsynaptic L-type currents, we hypothesize that buffering of presynaptic intracellular calcium is impaired in aged animals, leading to tonically raised calcium, which might impair rather than enhance synaptic function
Calcium buffering and extrusion is impaired in aging
Cytosolic calcium-binding (buffering) proteins are decreased in the hippocampus, but not the cerebellum or cortex, of aged rats (Villa, 1994; Papazafiri et al., 1995). The number of calbindin-immunoreactive neurons is decreased in the hippocampus of aged rats (Krzywkowski et al., 1995). Aged Fischer rats have lower Ca2+extrusion through both the Na/Ca2+ exchanger and the Ca2+ ATPase (Martinez-Serrano et al., 1992).Michaelis et al. (1984) found a decrease in theVmax of the Ca2+ ATPase and an increase in the Km of the Na/Ca2+ exchanger in synaptic membranes from 23- to 25-month-old rats compared with younger rats. The ameliorative effects of the addition of a membrane-permeable calcium buffer may be attributable to enhanced calcium buffering.
Ca2+ binding kinetics of BAPTA compared with EGTA
BAPTA has rapid Ca2+ binding kinetics compared with EGTA (Tsien, 1980; Neher, 1986). BAPTA can markedly attenuate neurotransmitter release in squid synaptic terminals (Adler et al., 1991), frog synaptic terminals (Robitaille and Charlton, 1992;Robitaille et al., 1993a), crayfish nerve terminals (Winslow et al., 1994), and in the Calyx of Held (Helmchen et al., 1997), presumably by shuttling calcium ions away from synaptic active zones where transmitter release is triggered. BAPTA-AM, but not EGTA-AM, attenuates excitatory neurotransmission in hippocampal slices, possibly by acting presynaptically (Niesen et al., 1991; Tymianski et al., 1994b;Ouanounou et al., 1996b). The kinetics of EGTA–calcium binding are slow relative to the initiation of transmitter release so that EGTA may be unable to reduce calcium concentration rapidly enough to reduce transmitter release in this preparation, although EGTA does affect release at crayfish neuromuscular junction (Winslow et al., 1994) and the Calyx of Held, as demonstrated by Helmchen et al.(1997). However, in our study, fEPSP enhancement was also achieved when the aged slices were perfused with EGTA-AM, an effect that we did not observe in the young slices (Fig. 4). This supports the notion that cytoplasmic calcium is elevated in aged neurons, because fast association and dissociation calcium binding kinetics were not required here.
Presynaptic or postsynaptic actions
We hypothesize that the major effects of membrane-permeant calcium chelators on the fEPSPs could be on the presynaptic terminals, in part because of the overwhelming amount of evidence showing that alterations in presynaptic calcium effects neurotransmitter release. Loading the postsynaptic neuron with high concentrations of BAPTA did not alter the effects of BAPTA-AM on fEPSPs in the CA1 region in slices from young-mature rats (Velumian et al., 1998). In CA1 presynaptic terminals of aged animals, there is increased calcium that was decreased by preadministration of BAPTA-AM (Morris et al., 1998). However, we cannot exclude a postsynaptic action, because there are several studies showing that alterations in postsynaptic calcium-dependent events can effect neurotransmission. An L-type calcium channel blocker enhanced hippocampal long-term potentiation in aged animals, an effect correlated with depression of the calcium-activated afterhyperpolarization measured postsynaptically (Norris et al., 1998). In CA1 neurons, increased postsynaptic calcium entry via voltage-sensitive calcium channels transiently potentiates EPSPs (Kullmann et al., 1992). Inhibition of postsynaptic calcineurin activity induced postsynaptic calcium-dependent synaptic potentiation in adult CA1 neurons (Wang and Kelly, 1997).
Frequency facilitation
The increase in EPSP that occurs during or after repetitive activation has long been viewed as a possible substrate of behavioral or functional plasticity (Massicotte and Baudry, 1991; Muller et al., 1991). However, the mechanisms of central frequency facilitation remain unclear. The amount of frequency facilitation in the aged hippocampus responses is markedly reduced (Landfield and Lynch, 1977). The deficit appears to involve both presynaptic and postsynaptic components; moreover, alterations in postsynaptic membrane hyperpolarization may well contribute to impaired transmission, particularly during periods of high frequency activity (Pitler and Landfield, 1987; Landfield, 1994). In our experiments, aged slices exhibited significantly less frequency facilitation than young-mature ones, which markedly improved after application of BAPTA-AM (or EGTA-AM) to the aged slices. Because frequency facilitation itself is Ca2+-dependent, it appears paradoxical that excess Ca2+ can reduce frequency facilitation. However, elevated calcium might impair frequency facilitation of the fEPSP by Ca2+-dependent inactivation of subsequent Ca2+ influx (Landfield et al., 1986) or by increased Ca2+-dependent hyperpolarization of the axon terminals or dendrites, resulting in presynaptic action potential failure or a “shunt” of the dendritic EPSP (Landfield, 1994). Frequency facilitation is usually greater at lower quantal content. Therefore, lowering extracellular Ca2+ or applying BAPTA, both of which lower quantal content, might increase facilitation. Although the ratio of the first to second fEPSP (paired-pulse facilitation) and the quantal content may not change with aging (Landfield and Lynch, 1977; Barnes et al., 1992; Deupree et al., 1993), the facilitation that we measured was over a period of 16 stimuli at 1 Hz. With BAPTA-AM, the absolute size of the fEPSPs grew markedly during the 16 stimuli, whereas the ratio of the first two fEPSPs could remain constant. Further experiments are required to properly investigate these phenomena. A Ca2+receptor for facilitation could be saturated in old neurons (Stanley, 1986; Yamanda and Zucker, 1992). Reducing [Ca2+]i may unsaturate the facilitation Ca2+ receptor so that it can function again. In aged neurons, a chronic Ca2+ leak may elevate [Ca2+]i. Thus, application of BAPTA is less effective, because it becomes saturated with Ca2+. BAPTA may reduce presynaptic [Ca2+]i, thus reducing spontaneous release and making more vesicles available for evoked release. BAPTA may also reduce activation of KCachannels, making the presynaptic action potential longer, thereby admitting more Ca2+, causing increased neurotransmitter release (Robitaille and Charlton, 1992; Robitaille et al., 1993a,b).
Reducing calcium influx reverses “aging” effects
High Ca2+ might impair frequency potentiation by saturation of binding sites for release or by rapid transmitter depletion (Landfield, 1994). Because Mg2+competitively inhibits these calcium actions, the beneficial effects of Mg2+ on hippocampal synaptic potentiation in aged rats suggests that calcium may be elevated in these cells. Magnesium facilitates maze reversal learning and improves hippocampal frequency facilitation in aged rats (Landfield and Morgan, 1984). Blocking calcium influx by decreasing the Ca2+/Mg2+ ratio improved intracellular and extracellular measures of frequency facilitation in aged hippocampal slices (Landfield et al., 1986). We showed that low Ca2+–high Mg2+ caused a reversal of the effect of the BAPTA-AM (but not that of EGTA-AM) in aged slices, here showing synaptic transmission resembling that of young slices. These data suggest that reducing calcium influx can reverse some of the functional effects of aging. Also, in younger slices, 0.7 mm Co2+ attenuated fEPSPs by ∼50%, whereas in aged slices, it enhanced fEPSPs by 20%. Only after doubling the Co2+ concentration to 1.4 mm were similar results obtained in the aged slices, i.e., ∼50% depression of the fEPSP (Fig. 8). Recently, we showed that the volatile anesthetic isoflurane depressed fEPSPs significantly more in aged than in young slices (Ouanounou et al., 1998a). However, when the slices were exposed to low Ca2+–high Mg 2+ or to cobalt (Ouanounou et al., 1998b), the isoflurane depression was similar in young and old slices. Overall, these results in old neurons suggest Ca2+-dependent modifications in the number of presynaptic Ca2+ channels, the number of postsynaptic receptors, or altered function of these channels, possibly from increased cytoplasmic calcium.
Calcium-mediated cellular dysfunction
Thus, neuronal dysfunction in aging could be attributable to the buildup of intracellular Ca2+ via NMDA receptors, voltage-gated Ca2+ channels, impaired membrane pumps or exchangers, leakage from intracellular stores, and/or by impaired intracellular Ca2+ buffering. Increased free cytosolic Ca2+ could then activate several Ca2+-dependent processes, leading to neuronal damage.
Footnotes
This work was supported by grants from the Medical Research Council (to P.L.C.), the Network on Neuronal Recovery, and Regeneration of the Networks of Centres of Excellence of Canada (to P.L.C. and M.P.C.). L.Z. is a Research Scholar of the Heart and Stroke Foundation of Canada and Ontario. We thank Frank Vidic for his assistance with electronics and computerized data processing and Drs. Michael Tymianski, Hossam El-Beheiry, Giovanni Facciponte, and Patrick McDonald for helpful discussions throughout this study.
Correspondence should be addressed to Dr. Peter L. Carlen, Room 12-413, Playfair Neuroscience Unit, Toronto Hospital–Western Division, 399 Bathurst Street, Toronto, Ontario M5T 2S8, Canada.
REFERENCES
- 1.Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelator attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991;11:1469–1477. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augustine GJ, Charlton MP, Smith SJ. Calcium entry and transmitter release at voltage-clamped terminals of squid. J Physiol (Lond) 1985;367:163–181. doi: 10.1113/jphysiol.1985.sp015819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustine GJ, Adler EM, Charlton MP. The calcium signal for transmitter secretion from presynaptic nerve terminals. Ann NY Acad Sci. 1991;636:365–381. doi: 10.1111/j.1749-6632.1991.tb36505.x. [DOI] [PubMed] [Google Scholar]
- 4.Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994;17:13–18. doi: 10.1016/0166-2236(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 5.Barnes CA, Rao G, Foster TC, McNaughton BL. Region-specific age effects on AMPA sensitivity: electrophysiological evidence for loss of synaptic contacts in hippocampal CA1 field. Hippocampus. 1992;2:457–468. doi: 10.1002/hipo.450020413. [DOI] [PubMed] [Google Scholar]
- 6.Blaustein MP. Calcium transport and buffering in neurons. Trends Neurosci. 1988;11:438–443. doi: 10.1016/0166-2236(88)90195-6. [DOI] [PubMed] [Google Scholar]
- 7.Bliss TVP, Collingridge GL. A synaptic model of memory: long term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 8.Blundon JA, Wright SN, Brodwick MS, Bittner GD. Presynaptic calcium activated potassium channels and calcium channels at a crayfish neuromuscular junction. J Neurophysiol. 1995;73:178–189. doi: 10.1152/jn.1995.73.1.178. [DOI] [PubMed] [Google Scholar]
- 9.Campbell LW, Su-Yang H, Thibault O, Blalock EM, Landfield PW. Aging changes in voltage-gated calcium currents in hippocampal CA1 neurons. J Neurosci. 1996;16:6286–6295. doi: 10.1523/JNEUROSCI.16-19-06286.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlton MP, Iwanchshyn G. Exogenous calcium buffer reduces synaptic transmitter release and facilitation. Soc Neurosci Abstr. 1986;12:817. [Google Scholar]
- 11.Choi DW. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988;11:465–467. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- 12.Choi DW. Calcium: still centre in hypoxic–ischemic neuronal death. Trends Neurosci. 1995;18:58–60. [PubMed] [Google Scholar]
- 13.Deupree DL, Bradley J, Turner DA. Age-related alterations in potentiation in CA1 region in F344 rats. Neurobiol Aging. 1993;14:249–258. doi: 10.1016/0197-4580(93)90009-z. [DOI] [PubMed] [Google Scholar]
- 14.Dickie BGM, Davies JA. Calcium channel blocking agents and potassium-stimulated release of glutamate from cerebellar slices. Eur J Pharmacol. 1992;229:97–99. doi: 10.1016/0014-2999(92)90291-b. [DOI] [PubMed] [Google Scholar]
- 15.Disterhoft JF, Moyer JR, Thompson LT, Kowaslka M. Functional aspects of calcium channel modulation. Clin Neuropharmacol. 1993;16:S12–S24. doi: 10.1097/00002826-199316001-00003. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh A, Greenberg ME. Calcium signalling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 17.Gibson GE, Peterson C. Calcium and the aging nervous system. Neurobiol Aging. 1987;8:329–344. doi: 10.1016/0197-4580(87)90072-8. [DOI] [PubMed] [Google Scholar]
- 18.Hagiwara S, Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- 19.Helmchen F, Borst JG, Sakman B. Calcium dynamics associated with a single action potential in a CNS presynaptic terminal. Biophys J. 1997;72:1458–1471. doi: 10.1016/S0006-3495(97)78792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt JM, Redman RS, Silinsky EM. Reduction by intracellular calcium chelation of acetylcholine secretion without occluding the effects of adenosine at frog motor nerve endings. Br J Pharmacol. 1994;111:753–758. doi: 10.1111/j.1476-5381.1994.tb14802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iacopino AM, Christakos S. Specific reduction of calcium binding protein (28-kilodalton calbindin-D) gene expression in aging and neurodegenerative disease. Proc Natl Acad Sci USA. 1990;87:4078–4082. doi: 10.1073/pnas.87.11.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneko A, Tachibana M. Blocking effects of cobalt and related ions on the γ-aminobutyric acid-induced current in turtle retinal cones. J Physiol (Lond) 1986;373:463–479. doi: 10.1113/jphysiol.1986.sp016058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kao JP, Tsien RY. Ca2+ binding kinetics of Fura-2 and Azo-1 from temperature-jump relaxation measurements. Biophys J. 1988;53:635–639. doi: 10.1016/S0006-3495(88)83142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz E, Protte DA, Ferro PA, Rosato MD, Uchitel OD. Effects of Ca2+ channel blocker neurotoxins on transmitter release and presynaptic currents at the mouse neuromuscular junction. Br J Pharmacol. 1997;121:1531–1540. doi: 10.1038/sj.bjp.0701290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khachaturian ZC. The role of calcium regulation in brain aging: reexamination of a hypothesis. Aging. 1989;1:17–34. doi: 10.1007/BF03323872. [DOI] [PubMed] [Google Scholar]
- 26.Khachaturian ZC. Calcium hypothesis of Alzheimer’s disease and brain aging. Ann NY Acad Sci. 1994;747:1–11. doi: 10.1111/j.1749-6632.1994.tb44398.x. [DOI] [PubMed] [Google Scholar]
- 27.Kretz R. Local cobalt injection: a method to discriminate presynaptic axonal from postsynaptic neuronal activity. J Neurosci Methods. 1984;11:129–135. doi: 10.1016/0165-0270(84)90030-x. [DOI] [PubMed] [Google Scholar]
- 28.Krzywkowski P, Debilbao F, Senut MC, Lamour Y. Age-related changes in Parvalbumin-immunoreactive and GABA-immunoreactive cells in the rat septum. Neurobiol Aging. 1995;16:29–40. doi: 10.1016/0197-4580(95)80005-c. [DOI] [PubMed] [Google Scholar]
- 29.Kudo Y, Takeda K, Yamazaki K. Quin2 protects against neuronal cell death due to Ca2+ overload. Brain Res. 1990;528:48–54. doi: 10.1016/0006-8993(90)90193-f. [DOI] [PubMed] [Google Scholar]
- 30.Kullmann DM, Perkel DJ, Manabe T, Nicoll RA. Ca2+ entry via postsynaptic voltage-sensitive Ca2+ channels can transiently potentiate excitatory synaptic transmission in the hippocampus. J Neurosci. 1992;9:1175–1183. doi: 10.1016/0896-6273(92)90075-o. [DOI] [PubMed] [Google Scholar]
- 31.Landfield PW. Increased hippocampal Ca2+ channel activity in brain aging and dementia. Ann NY Acad Sci. 1994;747:351–364. doi: 10.1111/j.1749-6632.1994.tb44422.x. [DOI] [PubMed] [Google Scholar]
- 32.Landfield PW, Lynch GS. Impaired monosynaptic potentiation in vitro hippocampal slices from aged, memory-deficient rats. J Gerontol. 1977;32:523–533. doi: 10.1093/geronj/32.5.523. [DOI] [PubMed] [Google Scholar]
- 33.Landfield PW, Morgan G. Chronically elevating plasma Mg2+ improves hippocampal frequency potentiation and reversal learning in aged and young rats. Brain Res. 1984;322:167–171. doi: 10.1016/0006-8993(84)91199-5. [DOI] [PubMed] [Google Scholar]
- 34.Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarization in hippocampal neurons of aged rats. Science. 1984;226:1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- 35.Landfield PW, Pitler TA, Applegate MD. The effects of high Mg 2+ to Ca2+ ratios on frequency potentiation in hippocampal slices of young and aged rats. J Neurophysiol. 1986;56:797–811. doi: 10.1152/jn.1986.56.3.797. [DOI] [PubMed] [Google Scholar]
- 36.Lansman JB, Hess P, Tsien RW. Blockade of current through single calcium channels by Cd2+, Mg2+ and Ca2+ voltage and concentration dependence of calcium entry into the pore. J Gen Physiol. 1986;88:321–347. doi: 10.1085/jgp.88.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin AR. Handbook of physiology. The nervous system, Chap 10, pp 329–355. American Physiology Society; Bethesda, MD: 1977. Junctional transmission. II. Presynaptic mechanisms. [Google Scholar]
- 38.Martinez A, Vitorica J, Bogonez E, Satrustegui J. Differential effects of age on the pathways of calcium influx into nerve terminals. Brain Res. 1987;435:249–257. doi: 10.1016/0006-8993(87)91608-8. [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Serrano A, Blanco P, Satrustegui J. Calcium binding to the cytosol and calcium extrusion mechanisms in intact synaptosomes and their alterations with aging. J Biol Chem. 1992;267:4673–4679. [PubMed] [Google Scholar]
- 40.Massicotte G, Baudry M. Triggers and substrates of hippocampal synaptic plasticity. Neurosci Biobehav Rev. 1991;15:415–423. doi: 10.1016/s0149-7634(05)80034-x. [DOI] [PubMed] [Google Scholar]
- 41.Michaelis ML, Johe K, Kitos TE. Age-dependent alterations in synaptic membrane systems for calcium regulation. Mech Ageing Dev. 1984;25:215–225. doi: 10.1016/0047-6374(84)90142-8. [DOI] [PubMed] [Google Scholar]
- 42.Morris ME, Carlen P, Jahromi SS, Pivneva TA. Ultrastructural Ca2+ stores during aging: modulation by BAPTA. Soc Neurosci Abstr. 1998;24:1973. [Google Scholar]
- 43.Muller D, Buchs PA, Stoppini L, Boddeke H. Long term potentiation, protein kinase C, and glutamate receptors. Mol Neurobiol. 1991;5:277–288. doi: 10.1007/BF02935551. [DOI] [PubMed] [Google Scholar]
- 44.Neher E. Concentration profiles of intracellular calcium in the presence of a diffusible chelator. In: Klee M, Neher E, editors. Calcium electrogenesis and neuronal functioning. Springer-Verlag; Berlin: 1986. pp. 80–96. [Google Scholar]
- 45.Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 46.Niesen C, Charlton MP, Carlen PL. Postsynaptic and presynaptic effects of the calcium chelator BAPTA on synaptic transmission in rat hippocampal dentate granule neurons. Brain Res. 1991;555:319–325. doi: 10.1016/0006-8993(91)90358-3. [DOI] [PubMed] [Google Scholar]
- 47.Norris CM, Halpain S, Foster TC. Reversal of aged-related alterations in synaptic plasticity and blockade of L-type Ca2+channels. J Neurosci. 1998;18:3171–3179. doi: 10.1523/JNEUROSCI.18-09-03171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ouanounou A, Zhang L, Charlton MP, Carlen PL. Excitatory synaptic transmission is enhanced in aged hippocampal neurons by calcium chelator. FASEB J [Abstr] 1996a;10:3914. [Google Scholar]
- 49.Ouanounou A, Zhang L, Tymianski M, Charlton MP, Wallace CM, Carlen PL. Accumulation and extrusion of permeant Ca 2+ chelator in attenuation of synaptic transmission at hippocampal CA1 neurons. Neuroscience. 1996b;75:99–109. doi: 10.1016/0306-4522(96)00319-3. [DOI] [PubMed] [Google Scholar]
- 50.Ouanounou A, El-Beheiry H, Carlen PL. Enhanced isoflurane suppression of excitatory synaptic transmission in the aged rat hippocampus. Br J Pharmacol [Abstr] 1998a;124:1075–1083. doi: 10.1038/sj.bjp.0701911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouanounou A, El-Beheiry H, Carlen PL. Suppression of calcium influx reverses anaesthetic actions in old neurons. J Dent Res. 1998b;77:143. [Google Scholar]
- 52.Papazafiri P, Podini P, Meldolesi J, Yamaguchi T. Aging affects cytosolic Ca2+ binding-proteins and synaptic markers in the retina but not in cerebral cortex neurons of the rat. Neurosci Lett. 1995;186:65–68. doi: 10.1016/0304-3940(95)11285-5. [DOI] [PubMed] [Google Scholar]
- 53.Pethig RR, Kuhn M, Payne R, Adler EM, Chen TH, Jaffe JH. On the dissociation constants of BAPTA-AM type calcium buffers. Cell Calcium. 1989;10:491–498. doi: 10.1016/0143-4160(89)90026-2. [DOI] [PubMed] [Google Scholar]
- 54.Pitler TA, Landfield PW. Probable calcium-mediated inactivation in Ca 2+ currents in mammalian brain neurons. Brain Res. 1987;410:147–153. doi: 10.1016/s0006-8993(87)80037-9. [DOI] [PubMed] [Google Scholar]
- 55.Reynolds JN, Carlen PL. Diminished calcium currents in aged hippocampal dentate gyrus granule neurones. Brain Res. 1989;479:384–390. doi: 10.1016/0006-8993(89)91646-6. [DOI] [PubMed] [Google Scholar]
- 56.Robitaille R, Charlton MP. Presynaptic calcium signals and transmitter release are modulated by calcium activated potassium channels. J Neurosci. 1992;12:297–305. doi: 10.1523/JNEUROSCI.12-01-00297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robitaille R, Adler EM, Charlton MP. Calcium and calcium gated potassium channels at the frog neuromuscular junction. J Physiol (Paris) 1993a;87:15–24. doi: 10.1016/0928-4257(93)90020-t. [DOI] [PubMed] [Google Scholar]
- 58.Robitaille R, Garcia ML, Kaczorowski GJ, Charlton MP. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron. 1993b;11:645–655. doi: 10.1016/0896-6273(93)90076-4. [DOI] [PubMed] [Google Scholar]
- 59.Scharfman HE, Schwartzkroin PA. Protection of dentate hilar cells from prolonged stimulation by intracellular calcium chelation. Science. 1989;246:257–260. doi: 10.1126/science.2508225. [DOI] [PubMed] [Google Scholar]
- 60.Simpson PB, Challiss RAJ, Nahorski SR. Neuronal Ca2+ stores: activation and function. Trends Neurosci. 1995;18:299–306. doi: 10.1016/0166-2236(95)93919-o. [DOI] [PubMed] [Google Scholar]
- 61.Smith DO. Muscle-specific decrease in presynaptic calcium dependence and clearance during and neuromuscular transmission in aged rats. J Neurophysiol. 1988;59:1069–1082. doi: 10.1152/jn.1988.59.4.1069. [DOI] [PubMed] [Google Scholar]
- 62.Smith PD, Liesegang GW, Berger RL, Czerlinski G, Podolsky RJ. A stopped-flow investigation of calcium ion binding by ethylene glycol bis(β-aminoethyl ether)-N,N′-tetraacetic acid. Anal Biochem. 1984;143:188–195. doi: 10.1016/0003-2697(84)90575-x. [DOI] [PubMed] [Google Scholar]
- 63.Stanley EF. Decline in calcium cooperativity as the basis of facilitation at the squid giant synapse. J Neurosci. 1986;6:782–789. doi: 10.1523/JNEUROSCI.06-03-00782.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;272:1017–1020. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- 65.Tsien RY. New calcium indicators and buffers with high selectivity against Mg and protons: design, synthesis, and prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- 66.Tymianski M, Wallace MC, Spigelman I, Uno M, Carlen PL, Tator CH, Charlton MP. Cell permeant Ca2+ chelators reduce early exitotoxic and ischemic neuronal damage in vitro and in vivo. Neuron. 1993;11:221–235. doi: 10.1016/0896-6273(93)90180-y. [DOI] [PubMed] [Google Scholar]
- 67.Tymianski M, Charlton MP, Carlen PL, Tator CH. Properties of neuroprotective cell permeant Ca2+ chelator: effects on [Ca2+]i and glutamate neurotoxicity in vitro. J Neurophysiol. 1994a;72:1973–1991. doi: 10.1152/jn.1994.72.4.1973. [DOI] [PubMed] [Google Scholar]
- 68.Tymianski M, Spigelman I, Zhang L, Carlen PL, Tator CH, Charlton MP, Wallace WC. Mechanism of action and persistence of neuroprotection by cell permeant Ca2+ chelator. J Cereb Blood Flow Metab. 1994b;35:1–13. doi: 10.1038/jcbfm.1994.122. [DOI] [PubMed] [Google Scholar]
- 69.Velumian AA, Ouanounou A, Carlen PL. The site of action of BAPTA-AM on synaptic transmission in rat hippocampus is presynaptic. Soc Neurosci Abstr. 1998;24:568. [Google Scholar]
- 70.Verkhratsky A, Toescu EC. Calcium and neuronal ageing. Trends Neurosci. 1998;21:2–7. doi: 10.1016/s0166-2236(97)01156-9. [DOI] [PubMed] [Google Scholar]
- 71.Villa A, Podini P, Panzeri MC, Raccheti G, Meldolesi J. Cytosolic Ca2+ binding proteins during rat brain aging loss of calbindin and calretinin in the hippocampus, with no change in the cerebellum. Eur J Neurosci. 1994;6:1491–1499. doi: 10.1111/j.1460-9568.1994.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 72.Wang JH, Kelly PT. Postsynaptic calcineurin activity downregulates synaptic transmission by weakening intracellular Ca2+ signaling mechanisms in hippocampal CA1 neurons. J Neurosci. 1997;17:4600–4611. doi: 10.1523/JNEUROSCI.17-12-04600.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winslow JL, Duffy SN, Charlton MP. Homosynaptic facilitation of transmitter release in crayfish is not affected by mobile calcium chelator implications for the residual ionized calcium hypothesis from electrophysiological and computational analyses. J Neurophysiol. 1994;72:1769–1793. doi: 10.1152/jn.1994.72.4.1769. [DOI] [PubMed] [Google Scholar]
- 74.Yamanda WM, Zucker RS. Time course of transmitter release calculated from simulations of a calcium diffusion model. Biophys J. 1992;61:671–682. doi: 10.1016/S0006-3495(92)81872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]