Abstract
5-HT produces voltage-independent inhibition of the N-, P/Q-, and T-type Ca2+ currents in sensory neurons ofXenopus larvae by acting on 5-HT1A and 5-HT1D receptors. We have explored the underlying mechanisms further and found that the inhibition of high voltage-activated (HVA) currents by 5-HT is mediated by a pertussis toxin-sensitive G-protein that activates a diffusible second messenger. Although modulation of T-type currents is membrane-delimited, it was not affected by GDP-β-S (2 mm), GTP-γ-S (200 μm), 5′-guanylyl-imidodiphosphate tetralithium (200 μm), aluminum fluoride (AlF4−, 100 μm), or pertussis toxin, suggesting that a GTP-insensitive pathway was involved. To investigate the modulation of the T currents further, we synthesized peptides that were derived from conserved cytoplasmic regions of the rat 5-HT1A and 5-HT1D receptors. Although two peptides derived from the third cytoplasmic loop inhibited the HVA currents by activating G-proteins and occluded the modulation of HVA currents by 5-HT, two peptides from the second cytoplasmic loop and the C tail had no effect. None of the four receptor-derived peptides had any effect on the T-type currents. We conclude that 5-HT modulates T-type channels by a membrane-delimited pathway that does not involve G-proteins and is mediated by a functional domain of the receptor that is distinct from that which couples to G-proteins.
Keywords: G-proteins, 5-HT, N-type Ca2+channels, P/Q-type Ca2+ channels, T-type Ca2+ channels, receptor-derived peptide, Xenopus
Many neurotransmitters and neuropeptides mediate their biological actions by activation of receptors that are coupled to heterotrimeric G-proteins. Ligand binding to the receptor causes interaction with distinct classes of G-proteins and triggers the exchange of GTP for GDP on the α subunits, leading to the dissociation of the Gα from the βγ complex. The dissociated α-GTP or βγ subunits are then able to interact with their effector enzymes and ion channels. CNS neurons normally express many different types of receptors that transduce signals through a relatively limited repertoire of heterotrimeric G-proteins. Traditional “linear models” of signaling that require specific and highly selective coupling of receptor to G-protein to effector proteins have proven to be too simple. For example, a single neurotransmitter can activate and modulate several types of ion channels through various of mechanisms (Ciranna et al., 1996; Sun and Dale, 1998). Conversely, activation of a single receptor can modulate several types of ion channels through more than one mechanism (Diversé-Pierluissi and Dunlap, 1993; Luebke and Dunlap, 1994; Diversé-Pierluissi et al., 1995; Albillos et al., 1996; Currie and Fox, 1997; Sun and Dale, 1998). Nevertheless, the prevailing orthodoxy is that the superfamily of receptors, which includes, for example, the muscarinic, adrenergic, and serotonergic receptors, mediates their major actions on ion channels and other proteins exclusively through G-proteins [but see Hall et al. (1998)].
T-type channels are encoded by different genes and have unique kinetic characteristics compared with the high voltage-activated (HVA) channels (Perez-Reyes et al., 1998). The functions of T-type channels are also distinct from those of the HVA channels (for review, see Huguenard, 1996). Investigations into the modulation of T-type channels by neurotransmitters and G-proteins have yielded contradictory results so far. In rat spinal motoneurons and hippocampal neurons, these currents were enhanced by 5-HT and other transmitters through an unknown mechanism (Berger and Takahashi, 1990; Fraser and MacVicar, 1991), whereas in sensory neurons (Abdulla and Smith, 1997; Sun and Dale, 1997) and rat nucleus basalis neurons (Margeta-Mitrovic et al., 1997), T-type currents were inhibited by 5-HT or neuropeptides. In sensory neurons, inhibition of T-type currents can alter the neuron firing properties, and could thus be involved in analgesia (Abdulla and Smith, 1997; Sun and Dale, 1997).
In a previous report (Sun and Dale, 1997), we found that the 5-HT1A and 5-HT1D receptors inhibited both the T-type and HVA channels via voltage-independent mechanisms in sensory neurons (Sun and Dale, 1997). We report here that both 5-HT1A and 5-HT1D receptors inhibit the HVA channels through a pertussis toxin-sensitive G-protein and a diffusible second messenger. In a surprising contrast, modulation of the T-type channels, which is membrane-delimited (Sun and Dale, 1997), does not appear to occur through the actions of a G-protein. Indeed by using the peptides derived from the intracellular portions of the 5-HT receptor we have evidence that a functional domain entirely separate from that involved in coupling to the G-protein is likely to mediate the modulation of T-type channels.
MATERIALS AND METHODS
Whole-cell patch-clamp recordings. Spinal neurons were acutely isolated from Xenopus larvae [stage 40–42;Nieuwkoop and Faber (1956)] by methods based on those described byDale (1991). Conventional whole-cell recordings and cell-attached recordings were made in the primary sensory neurons [Rohon-Beard (R-B) neurons]. Because of their unique morphological characteristics, Rohon-Beard neurons were readily identifiable under phase-contrast microscopy, based on the criteria of Dale (1991): a large spherical soma, a large nucleus, and dark nucleolus. Electrodes were fabricated using a Sutter Instrument P97 puller from capillary glass obtained from World Precision Instruments (TW 150F) and Clark Electromedical Instruments (GC150F-10) and coated with Sylgard. A List L/M-PC amplifier together with a DT2831 interface (Data Translation) was used to record and digitize the voltage and current records. Data were acquired to the hard disk of an IBM-compatible PC, whereas an optical disk was used for long-term storage of experimental records. The sampling and analysis software were written by Dale (1995). The whole-cell recordings had access resistance ranging from 4 to 12 MΩ. Between 70 and 85% of this access resistance was compensated for electronically. For recording of whole-cell Ca2+currents, external solutions were composed of (in mm): 57.5 Na+, 57.5 TEA, 2.4 HCO3−, 3 K+, 10 Ca2+, 1 Mg2+, 10 HEPES, 1 4-aminopyridine(4-AP), and TTX (140 nm), pH 7.4, adjusted to 260 mOsm l−1. The pipette solution contained (in mm): 100 Cs+, 1 Ca2+, 6 Mg2+, 20 HEPES, 5 ATP, 10 EGTA, and 1 GTP, pH 7.4, adjusted to 240 mOsm l−1. Leak subtraction was performed on whole-cell recordings by either of two methods. For one method, the current of interest was blocked (Y3+ 30 μm or Cd2+ 120 μm), and the remaining leak currents were subtracted from the equivalent experimental records from the same cell. In the other method, a scaled negative version of the experimental pulse protocol was given to the same cell. This was subsequently scaled up and added to the experimental records. In both cases, the leak currents were obtained immediately before or after each set of experimental records. Drugs were applied through a multibarreled microperfusion pipette that was positioned within 1 mm of the cell. All experiments were performed at room temperature, 18–22°C.
Cell-attached patch-clamp recording. Unitary channel recordings (cell-attached mode) were made by methods described by Fox et al. (1987a,b) and Delcour and Tsien (1993). The membrane potential outside the patch was zeroed with the following external solution (in mm): 110 potassium aspartate, 10 EGTA, 10 HEPES, 1 MgCl2, and 10 glucose, pH 7.4 adjusted with KOH. The pipette solution contained 110 mm BaCl2, 10 mm TEA, 5 mm 4-AP, 10 mm HEPES, and 140 nm TTX, pH 7.4 (adjusted with TEA-OH). Leak subtractions were made by subtracting traces without channel openings or by adding a scaled negative version of the experimental pulse protocol.
Chemicals. Chemicals used were 5-hydroxytryptamine (5-HT; RBI, Natick, MA), 2-[5-[3-(4-methylsulfonylamino) benzyl-1,4-oxadiazol-5-yl [-1-H-indole-3-yl] ethylamine (L-694,247; Tocris Cookson), tetrodotoxin (TTX; Sigma, St. Louis, MO), ω-agatoxin IVA (Alomone Labs and Sigma), ω-conotoxin-GVIA (Bachem), nifedipine (Sigma), tetraethylammonium chloride (TEA; Aldrich, Milwaukee, WI), yttrium nitrate (Y3+; Sigma), 5′-guanylyl-imidodiphosphate tetralithium (GMP-PNP; Sigma), pertussis toxin (PTX; Sigma), GTP (Sigma), GDP-β-S (Sigma), and GTP-γ-S (Sigma). 5-HT1A and 5-HT1Dreceptor peptides were derived from the conserved regions of the rat 5-HT1A and 5-HT1D receptors. Peptides were synthesized by the FMOC-polyamide method of Atherton et al. (1988) and purified by reverse-phase chromatography on a C18 column equilibrated with 0.1% (v/v) trifluoracetic acid. Peptides were eluted by increasing concentrations of acetonitrile, and the sequence was confirmed using a Procise Protein Sequencer (Applied Biosystems, Foster City, CA).
RESULTS
Both the HVA channels and the T-type channels can be distinguished in cell-attached patch recordings
In whole-cell recordings, T-type currents represent only a very small amount of total Ca2+ current elicited from a holding potential of −90 mV (Sun and Dale, 1997). This current density can be produced by some 1000 T-type channels. In cell-attached patch recordings, T-type channels are nonuniformly distributed and were not observed at a holding potential of −90 mV in the majority of patches (∼70%, n = 120). In the remaining 30% of patches, recorded near the neuronal process but not the nucleus, we observed some large T-type currents. These ranged in amplitude from 3 to 20 pA at a test potential of −10 mV. Assuming a single channel conductance of 8 pS (Fox et al., 1987a,b) and an effective reversal potential of +50 mV, the currents were therefore produced by approximately 6–40 channels (Fig. 1A). This suggests that the T-type channels are clustered with a nonuniform spatial distribution.
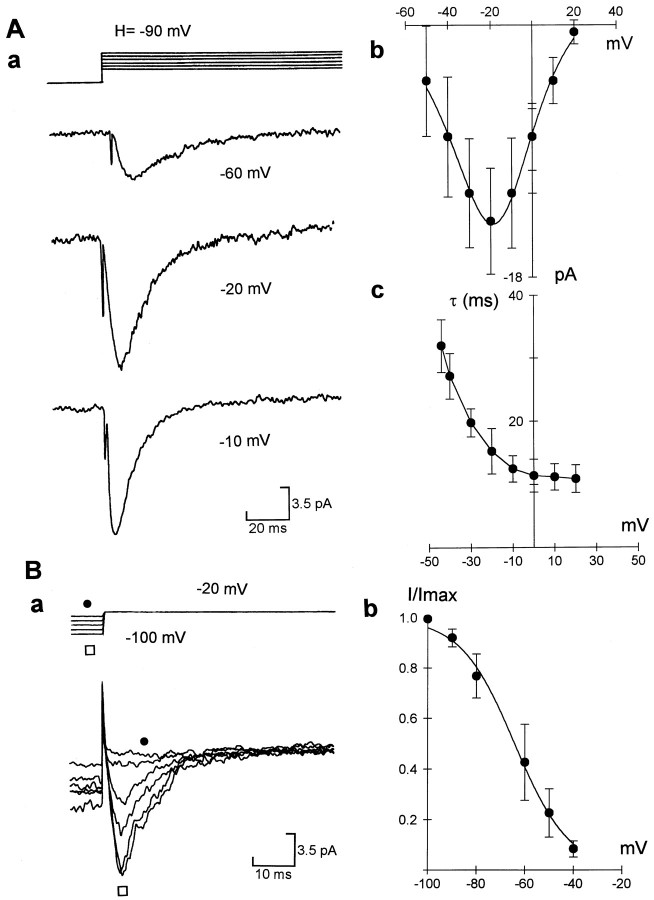
Fig. 1.
Kinetic characteristics of T-type channel currents in cell-attached patches. Aa, T-type currents were elicited by steps of +10 mV from a holding potential of −90 mV; representative traces were elicited by steps to −60, −20, and −10 mV, respectively. Ab, Current–voltage relation of T-type currents (n = 5). Solid lineis the best fit of the product of the Goldman-Hodgkin-Katz equation and Boltzmann equation (cf. Dale, 1995). Ac, Graph showing time constant of inactivation of T-type currents versus test voltage in four cells. Ba, T-type currents were elicited by steps to −20 mV from holding potential varied from −100 to −40 mV.Bb, Steady-state inactivation of T-type currents.Solid line is the best fit of Boltzmann relation,I = {1 + exp[(V +V1/2)/k]−1}−1, where V1/2 = −62 ± 6.3 mV, andk = 11.6 ± 3.2 (n = 4). Error bars represent SEM in this and all figures unless stated otherwise.
By fitting the current–voltage relation with the Boltzmann equation, we obtained a half-activation voltage for the T-type currents (110 mm BaCl2) of −35 ± 4.5 mV (n = 6) (Fig. 1Aa,b) and an activation slope (k) of 9.1 ± 2.4 mV (n = 6) (Fig. 1Aa,b). The inactivation of T-type currents was very fast, with a time constant that ranged from 33.6 ± 3.8 msec (at test potential to −40 mV) to 14 ± 2.6 msec (at + 10 mV) (Fig. 1Aa,c), which is similar to the time constant of inactivation of cloned mammalian T channels [α-1G channels; Perez-Reyes et al. (1998)]. In some patches that contained more than 50 T-type channels (Fig. 1Ba), we tested the steady-state inactivation properties of the T-type and obtained a half-inactivation voltage of −62 ± 4.3 mV (n = 4) (Fig. 1Bb). These kinetic parameters of T-type channels are also similar to those unitary kinetics of T-type channels recorded under cell-attached patches in chick sensory neurons (Fox et al., 1987a,b). However, their voltage-dependence differs from those seen in whole-cell recordings (Fox et al., 1987a,b; Sun and Dale, 1997), and this is probably because of the high levels of Ba2+ that will screen surface charge and alter channel gating. Based on our previous whole-cell recordings (Sun and Dale, 1997), only 5% of the whole-cell Ca2+ conductance could be mediated by R-type channels. This, in addition to the voltage-sensitivity of these channels, makes it very unlikely that the transient semi-macroscopic currents in our cell-attached patches were produced via R channels.
By contrast to the T currents, HVA currents had very different characteristics. HVA currents were elicited at more depolarized membrane potentials (>+10 mV) in the cell-attached patches (Figs.2A,3). In Xenopus R-B neurons, N- and P/Q-type channels comprised the majority of HVA channels. N currents represent ∼70% of whole-cell currents, and P/Q currents represent ∼25% (Sun and Dale, 1997). In most patches recorded, the HVA currents resembled the characteristic kinetic pattern of N-type HVA channels (Figs. 2A, 3A). Like N-type channels in chick sensory neurons (Fox et al., 1987a,b),Xenopus N channels also appear to be nonuniformly distributed and spatially clustered. HVA currents were recorded in only 35% of the patches, with currents produced by some 10–200 channels. In cell-attached patches (110 mm BaCl2), N-channel currents showed strong inactivation at a holding potential of −50 mV, but this had a time constant that was twice as long as the T-type currents recorded at the same test voltage (τ = 35.8 ± 6.2 msec at +20 mV; n = 5).
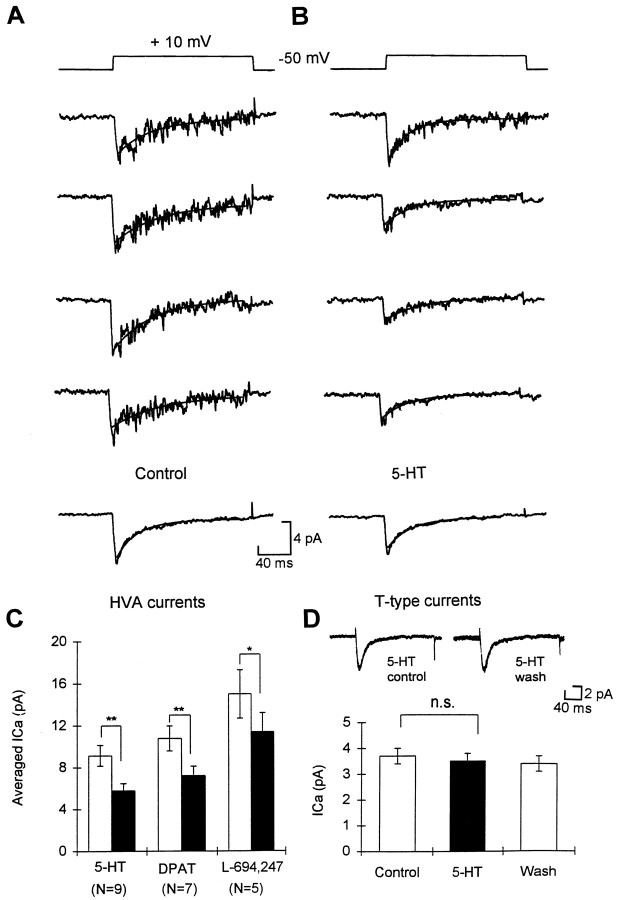
Fig. 2.
Modulation of the HVA channel currents by 5-HT and 5-HT agonists in cell-attached patches. A, Four consecutive recordings of HVA channel currents in a cell-attached patch. Bottom, Averaged trace from 30 consecutive recordings. The solid line is the best fit of a single exponential equation. B, Equivalent traces from the same patch after 5-HT was applied to the cell. C, Summary of the effects of 5-HT, 5-HT1A agonist 8-OH-DPAT, and 5-HT1D agonist L-694,247 on the HVA currents recorded in cell-attached patches. D, Summary of the effects of 5-HT on T-type currents recorded in the same batch of neurons.Inset, Representative traces of T-type currents (averaged from 20 consecutive recordings) recorded in the cell-attached patches in control, 5-HT, and after wash. n.s., Not significant; *p < 0.05, **p < 0.01 in this and all figures.
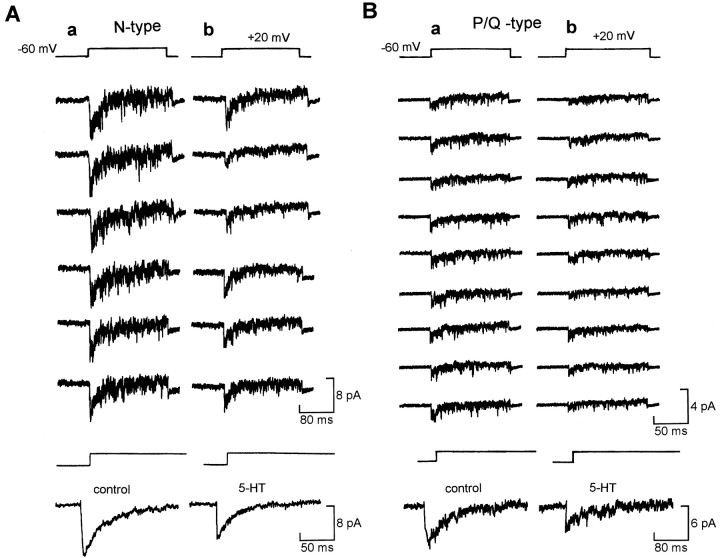
Fig. 3.
Modulation of N- and P/Q-type channels via diffusible second messengers. A, Representative consecutive records of N-type Ca2+ channel currents (200 nm ω-agatoxin-IVA in pipette) in control (a) and 5-HT (b).Bottom, Averaged trace from 50 consecutive sweeps.B, Representative records of P/Q-type Ca2+ channel currents (1 μmω-conotoxin-GVIA in pipette) in control (a) and 5-HT (b). Bottom, Averaged trace from 30 consecutive sweeps.
Modulation of HVA channels but not T-type channels involves a freely diffusible second messenger
To confirm our earlier findings (Sun and Dale, 1997), we studied whether 5-HT could modulate the T-type currents recorded in the cell-attached patch mode. Once again we found that 5-HT had no effect on the T channels (n = 6) (Fig. 2D), showing that modulation of whole-cell T-type currents by 5-HT is indeed membrane-delimited. In contrast to the T-type currents, the HVA currents recorded in cell-attached patches were inhibited by 5-HT applied outside the pipette (p < 0.01,n = 9) (Fig. 2A–C). The selective 5-HT1A agonist 8-OH-DPAT (p < 0.01,n = 7) (Fig. 2C) and 5-HT1Dagonist L-694,247 (p < 0.05, n= 5) (Fig. 2C) also caused inhibition. The inhibition of HVA currents by both 5-HT1A and 5-HT1D receptors must therefore be mediated through a freely diffusible second messenger. The inhibition of HVA currents by 5-HT was 35.6 ± 4.2% (n = 9), which was much higher than the inhibition in whole-cell recordings [16%; Sun and Dale (1997)]. This is probably because of greater preservation of the cell structure and content in the cell-attached form of recordings.
We next tested whether both types of HVA channels were individually modulated via diffusible second messengers. Patches that contained L-type channel activity (readily distinguished by unitary conductance and voltage dependence of activation and inactivation) were excluded from analysis. Pure N- or P/Q-channel recordings were obtained by including in the patch pipette either ω-conotoxin-GVIA (1 μm) to block N channels or ω-agatoxin-IVA (200 nm) to block P/Q channels. To check whether the blocking effects of ω-conotoxin-GVIA might be lessened by high concentrations of divalent ions (McDonough et al., 1995), we performed whole-cell recordings with 110 mm BaCl2 in the external saline. We found, as expected, that the whole-cell currents greatly increased in amplitude after changing the external saline to one containing 110 mm Ba2+. However, ω-conotoxin-GVIA at 1 μm was still very effective at blocking the whole-cell Ca2+ currents [65 ± 5.2%, n = 3; compare Sun and Dale (1997)]. Thus even under conditions of high divalents, ω-conotoxin-GVIA could be used to block the N channels and effectively isolate the P/Q channels.
In cell-attached patches, both N-type channels (Fig. 3A, three neurons) and P/Q channels (Fig. 3B, two neurons) were inhibited by 5-HT. Our results therefore suggest that N and P/Q channels are inhibited via a diffusible second messenger that can be activated by both 5-HT1A and 5-HT1Dreceptors.
Modulation of HVA but not T-type currents involves a pertussis toxin-sensitive G-protein
We examined the effects of preincubation of PTX on the modulation of T-type and HVA currents by 5-HT. PTX (500 ng–1 μg/ml) was added to dishes of neurons and left for ∼12 hr before patch-clamp recordings were commenced. Control dishes of neurones dissociated from the same batch of spinal cords were left for the same period but without addition of PTX. PTX greatly reduced the inhibition of the HVA currents by 5-HT (Fig. 4) but had no effect on the inhibition of the T-type currents by 5-HT (Fig. 4). This suggests that the inhibition of HVA currents is mediated by a PTX-sensitive G-protein, whereas modulation of T-type currents in the same neuron is insensitive to PTX.
Fig. 4.
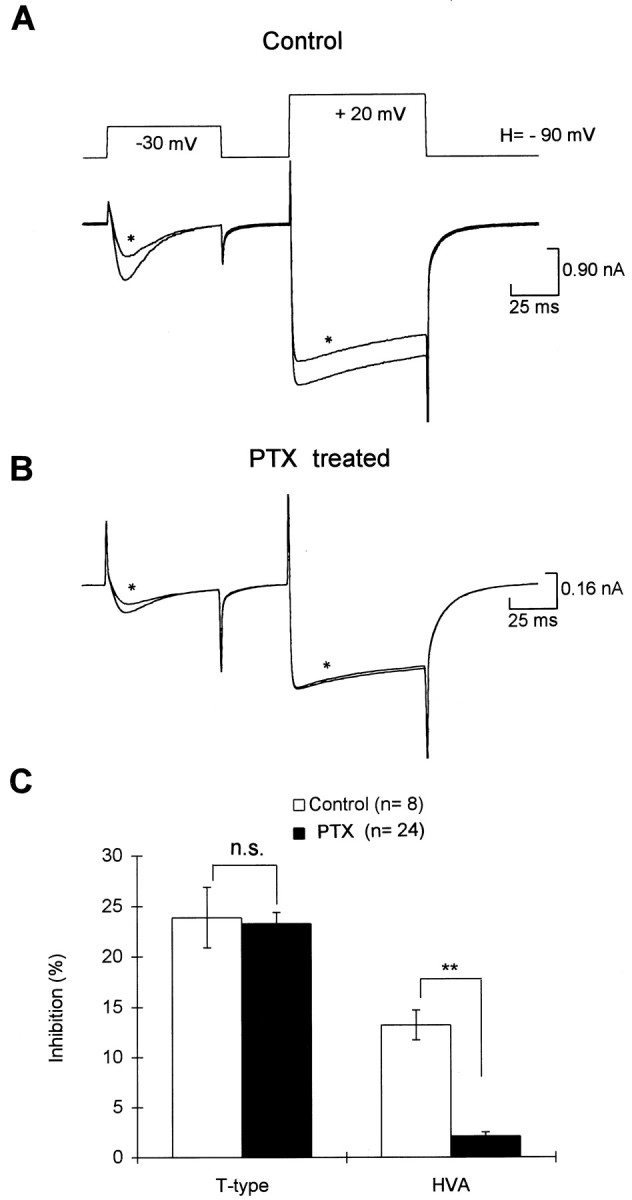
Effects of overnight preincubation of pertussis toxin (PTX) on the inhibition of Ca2+ currents by 5-HT. A, 5-HT produced reversible inhibition on both T-type and HVA currents in a neuron that had been incubated overnight but without the addition of PTX. B, In a neuron that was incubated with PTX (1 ng/ml) for 12 hr, 5-HT had very little effect on the HVA currents but still inhibited the T-type currents. C, Summary of the effects of 5-HT on both T-type and HVA currents in the control and PTX-treated neurons.
Nonhydrolyzable analogs of GTP and GDP modify modulation of HVA but not T-type currents
To test further the possible involvement of G-proteins on the modulation of Ca2+ currents by 5-HT, 500 μm-2 mm GDP-β-S was added to the pipette to replace the 1 mm GTP included in the control. A twin pulse protocol from a holding potential of −90 mV was used to elicit both the T-type and the HVA currents (Fig.5Aa). Unlike in mammalian dorsal root ganglion neurons, where intracellular addition of GDP-β-S caused an enhancement of the transient Ca2+ currents (Dolphin and Scott, 1987), we found that GDP-β-S had very little effect on either the HVA currents or the T-type currents (n = 20), suggesting that there was little or no tonic modulation of these currents by G-proteins in R-B neurons.
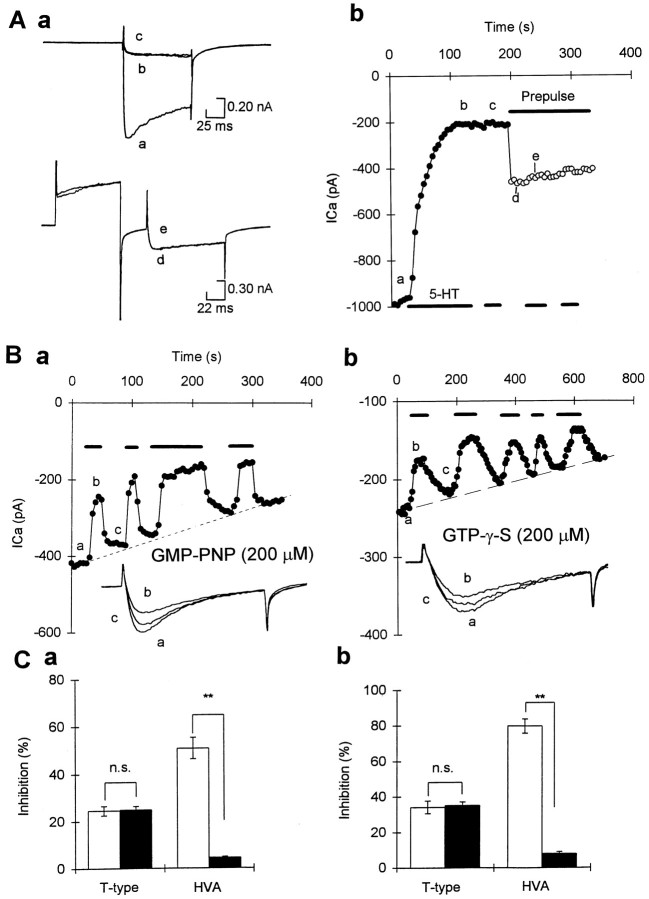
Fig. 5.
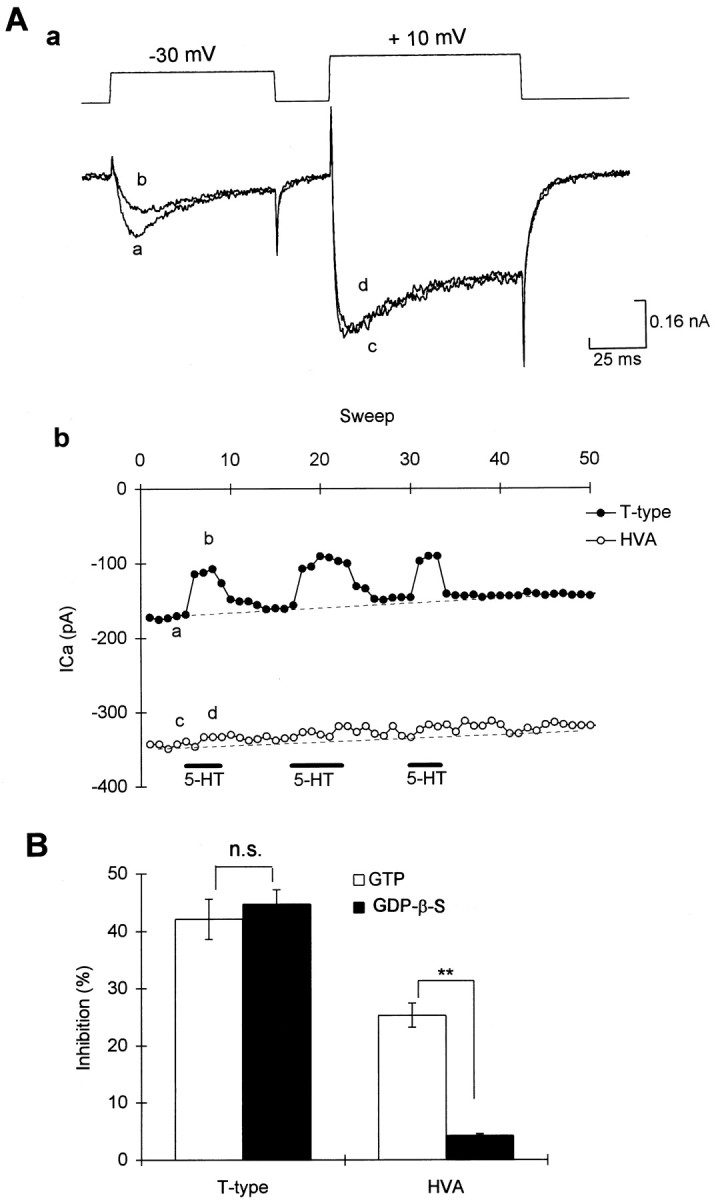
Intracellular dialysis of GDP-β-S diminished modulation of HVA but not T-type Ca2+currents. A, In a cell loaded with GDP-β-S (2 mm), 5-HT produced reversible inhibition on T-type currents but had very little effect on the HVA currents; Aa, representative traces of the Ca2+ currents;Ab, graph showing amplitude of current versus time in the same cell. Symbols in graph correspond to those on the current traces. B, Summary of the effects of 5-HT on T-type currents and HVA currents in cells loaded with either GTP (control) or GDP-β-S.
In all recordings in which GDP-β-S was added into the pipette (n = 14) (Fig. 5A,B), 5-HT (1–10 μm) still reversibly inhibited the T-type currents. The amount of inhibition evoked by 5-HT in these neurons was similar to those recordings in the same dish in which GTP (1 mm) instead of GDP-β-S (1 mm) loaded into patch pipette (Fig.5C). However, GDP-β-S did prevent the inhibition of HVA currents (n = 14) (Fig. 5A–C). The amount of inhibition produced by 5-HT in those neurons loaded with GDP-β-S was much smaller than those neurons loaded with 1 mm GTP (p < 0.01, GDP-β-S vs control,n = 14) (Fig. 5C).
GMP-PNP is a nonhydrolyzable analog of GTP that was first used to demonstrate that GTP regulates adenylyl cyclase (Londos et al., 1974). In pipettes loaded with GMP-PNP (200 μm), there was very little change in the T-type current (n = 8) (Fig.6Ba), and 5-HT still produced reversible inhibition even 10 min after the start of recordings (Fig. 6Ba). The amount of inhibition of T currents produced by 5-HT (1 μm) remained similar to that in neurons with control pipette solution recorded in the same dish (1 mm GTP; n = 8, p > 0.1) (Fig. 6Ba,Ca).
Fig. 6.
Effects of intracellular dialysis of GMP-PNP or GTP-γ-S on the modulation of whole-cell Ca2+currents by 5-HT. A, In a cell loaded with GMP-PNP (200 μm), 5-HT produced irreversible inhibition of HVA currents that was accompanied by slowing of activation. Further application of 5-HT did not have any additional effect, even in the presence of prepulse to remove the voltage-dependent inhibition.Aa, HVA currents elicited by test potential to +10 mV from a holding potential of −90 mV, without (top traces) or with prepulse to +120 mV (bottom traces). Ab, Time series recordings in the same cell; symbols correspond to current traces inAa. Filled circles represent recordings made before applying prepulse, and open circlesrepresent recordings made during prepulse application;symbols correspond to current traces.B, Time series measurements and T-type currents in the same cell showing that in a cell loaded with GMP-PNP (a, 200 μm) or GTP-γ-S (b, 200 μm), 5-HT reversibly inhibited the T-type currents. Insets, T-type currents elicited by a test potential of −30 mV from a holding potential of −90 mV in control (trace a), 5-HT (trace b), and after wash (trace c). C, The first application of 5-HT caused increased inhibition of the HVA but not the T-type currents (shown by white bar) in neurons loaded with GMP-PNP (Ca) or 200 μm GTP-γ-S (Cb). The filled bars show the effects of subsequent addition of 5-HT (5 min after the first addition) on the T-type and the HVA currents.
In contrast, GMP-PNP (200 μm) greatly enhanced the inhibition of HVA currents by 5-HT. However, the inhibition in most cells was not reversible during wash (n = 8) (Fig.6A), whereas in a few other cells (n= 4) there was a partial reversal. Subsequent applications of 5-HT gave virtually no further inhibition (Fig. 6A,Ca). Unlike cells that had been loaded with control pipette solution (1 mm GTP) and where inhibition is not associated with slowing and shifting of voltage-dependence (Sun and Dale, 1997), the inhibition of the HVA currents in the presence of GMP-PNP was associated with slowing of activation that could be relieved partially by a strong depolarizing prepulse to +120 mV (Fig. 6A). After loading with GMP-PNP, the onset of the inhibition of HVA currents by 5-HT was rather slow, ranging from 100 to 200 sec (Fig.6Ab). Because the inhibition of HVA currents by 5-HT was not only enhanced but was also accompanied by a kinetic change and voltage dependence, this suggests that new modulatory components were activated in the presence of GMP-PNP (cf. Sun and Dale, 1998). To observe the effects of GMP-PNP on the voltage-independent suppression of HVA currents in R-B neurons, we eliminated the voltage-dependent interaction by applying a prepulse to +120 mV, and we found that 5-HT still had no further effect (n = 3) (Fig.6A).
GTP-γ-S is another nonhydrolyzable analog of GTP that has effects similar to those of GMP-PNP on G-proteins but with a higher affinity for G-proteins (Olate and Allende, 1991). Like GMP-PNP, GTP-γ-S had very little effect on T-type currents by itself (Fig.6Bb). The mean peak current in neurons loaded with GTP-γ-S (100–200 μm at −30 mV test potential) was 228 ± 18.8 pA (n = 29), which was not significantly different from the mean T-type currents in control recordings (196 ± 16.8 pA, n = 30,p > 0.1). This is in contrast to the effects of photo-release of GTP-γ-S on T-type currents in cultured rat dorsal root ganglion neurons (Dolphin et al., 1989) and suggests that activation of G-proteins by intracellular dialysis of GTP-γ-S or GMP-PNP had no effects on the T-type currents in R-B neurons.
After the GTP-γ-S (200 μm) was allowed to diffuse into the cytoplasm (>5 min), 5-HT still produced reversible inhibition of ∼24.5 ± 2% (n = 7) of the T-type currents. This is similar to that recorded under control pipette solution (24.9 ± 1%, 1 mm GTP, n = 8) (Fig.6Bb,Cb). In contrast to its effects on the T-currents, GTP-γ-S (200 μm) caused 5-HT to produce much stronger inhibition on the HVA currents (Fig. 6Cb). Like the effects of 5-HT in cells loaded with GMP-PNP, the effects of 5-HT in cell loaded with GTP-γ-S were not reversible, and subsequent addition of 5-HT evoked no further inhibition (Fig.6Cb).
The effects of 5-HT on the T-type and HVA currents were also examined in neurons loaded with aluminum fluoride (AlF4−), which can permanently stimulate the GTP hydrolysis. Although AlF4− greatly enhanced the effects of 5-HT on the HVA currents in a partially reversible manner (38 ± 4.2% in AlF4−, n = 6, vs 18.2 ± 4.2% in control, n = 20,p < 0.01). The modulation of T-type currents by 5-HT remained totally reversible and was unaffected by loading AlF4− (35 ± 6.4% in ALF4−, n = 6, vs 32 ± 4.8% in control, n = 6).
The very different responses of HVA currents and T-type currents to 5-HT in cells loaded with GDP-β-S, GMP-PNP, GTP-γ-S, or AlF4− suggest that although G-proteins are involved in the modulation of HVA currents they may not be involved in the modulation of T-type currents.
Receptor-derived peptides activate G-proteins and modulate HVA but not T-type currents
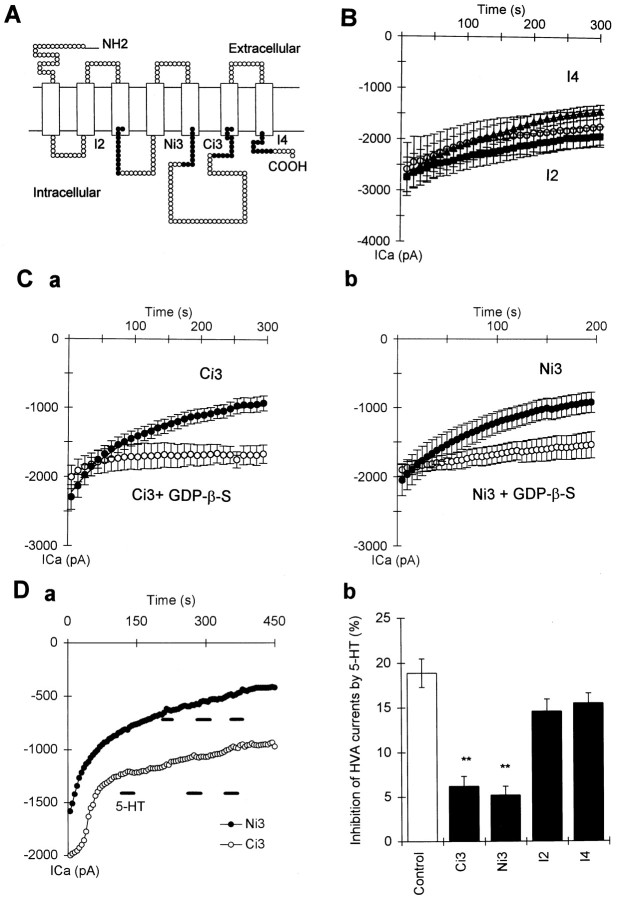
Because GDP-β-S, GMP-PNP, and GTP-γ-S all act competitively at the GTP binding site of G-proteins, the lack of effects of these substances on the modulation of T-type currents could still be explained by involvement of a novel G-protein with a very much higher affinity for GTP and GDP than for the nonhydrolyzable GTP and GDP analogs. G-protein activation involves a conformational change of receptor that enables the G-protein to interact with previously inaccessible regions on the receptor protein (cf. Wess, 1997). Biochemical studies from several laboratories suggest that peptides corresponding to the second intracellular loop (hereafter referred to as I2), and N- and C-terminal regions of the third intracellular loop (hereafter referred to as Ni3 and Ci3) can mimic or inhibit the receptor/G-protein interaction (cf. Savarese and Fraser, 1992; Strader et al., 1994; Wess, 1997; Zhu et al., 1997). We therefore synthesized four highly conserved segments of 5-HT1A and 5-HT1D receptor from regions that are generally thought to be involved in receptor G-protein interaction with other types of receptors (Fig. 7A). These peptides were applied via patch pipette to see whether they could induce inhibition of the T-type and HVA channels. We used four peptides: the first peptide was derived from the carboxy end of the third cytoplasmic loop and had an amino acid sequence ofRKRISAARERKATK (Ci3 peptide), the second was from the amino end of the third cytoplasmic loop with an amino acid sequence ofLYGRIYVAARSRI (Ni3 peptide), the third was from the second cytoplasmic loop (IALDRYWAITD: I2 peptide), and the fourth was from the cytoplasmic carboxy tail (DFRQAFQRVV: I4 peptide).
Fig. 7.
Effects of 5-HT receptor-derived peptides on the HVA currents and their modulation by 5-HT. A, A topographical model of the 5-HT1 receptor to show the location of the four synthesized cytoplasmic peptides (•).B, Time series measurements of HVA currents showing the rundown of the HVA currents in cells loaded with control recording solution (○, n = 16), I4 (▴,n = 9), and I2 peptide (▪, n= 6). The solid line is the best fit of single exponential curve. Ca, Time series measurements of HVA currents in cells loaded with Ci3 peptide alone (•,n = 8) and in cells loaded with Ci3 peptide plus 1 mm GDP-β-S (○, n = 6).Cb, Time series measurements of HVA currents in cells loaded with Ni3 peptide alone (•, n = 10) and in cells loaded with Ni3 peptide plus 1 mm GDP-β-S (○,n = 6). Da, Example of time series measurements in cells loaded with Ci3 (○) and Ni3 peptides (•) showing bath administration of 5-HT (1 μm) caused virtually no further inhibition. Db, Summary of the inhibition of HVA currents produced by 5-HT in cells loaded with the four synthetic peptides (**p < 0.01 vs control).
Effects of the peptides on HVA currents and T-type currents
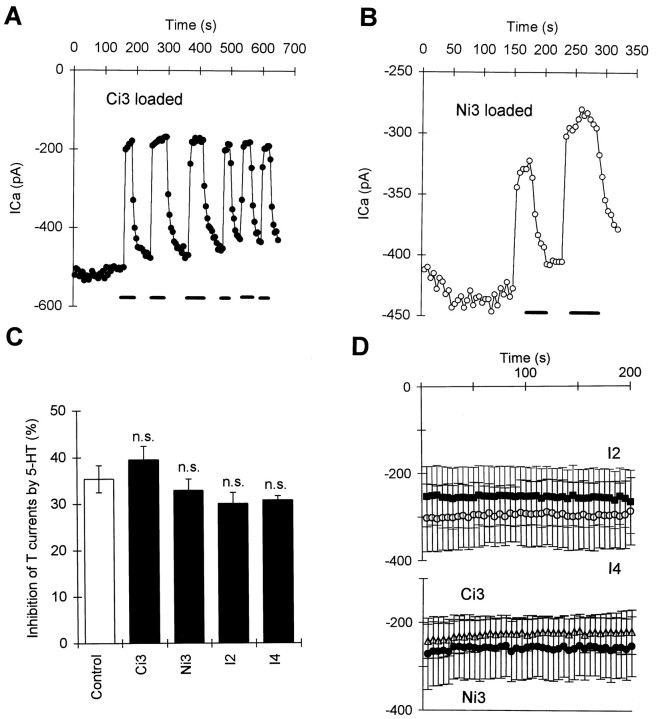
We first observed whether addition of the four 5-HT receptor-derived peptides via the patch pipette could alter the HVA and T-type currents directly. We found that addition of peptides from the third cytoplasmic loop (Ci3 and Ni3 peptides), but not the other regions (I2 and I4), caused inhibition of the HVA currents manifested as abnormally fast rundown of the HVA currents (Fig. 7B,C). Rundown of HVA currents can also occur in control pipette solutions and is exacerbated if resealing and blocking of the pipette tip occurs. We therefore excluded recordings that were accompanied by changes in electrode access resistance. The amount and rate of rundown were compared between neurons loaded with control pipette solutions (with 1 mm GTP) and neurons loaded with the synthetic peptides. Both the Ci3 and Ni3 peptides, but not the I2 and I4 peptides (Fig.7B,C), caused a much bigger amount of rundown of the HVA currents as measured at 5 min after the start of recording (Fig.7Ca,b vs B). The time course of the rundown could be fitted by a single exponential equation (Fig. 7B,C). The mean time constant of rundown for cells injected with Ci3 and Ni3 peptides was significantly shorter (77.5 ± 14.5 sec in Ci3 and Ni3, n = 16) than the control (184.5 ± 47 sec,n = 11, p < 0.05). However, the time constant for run down of HVA currents in neurons injected with the other two 5-HT receptor-derived peptides (I2 and I4 peptides) remained similar to control (197 ± 27 sec for I4, n = 9, and 224 ± 21 sec for I2, n = 6). To test whether the abnormal rundown of the HVA currents was mediated via G-proteins, we measured the rundown in cells loaded with the Ni3 (or the Ci3) peptides with GDP-β-S (1 mm) instead of GTP. GDP-β-S (1 mm) significantly reduced the rundown of HVA currents caused by both peptides (Fig. 7C) and also slowed the time constant of rundown (180 ± 41.5 sec, n = 12,p < 0.05 vs peptides only). These results suggests that both peptides activated G-proteins and mimicked the effects of 5-HT. However, unlike the effects of 5-HT, whose effects on the HVA currents in R-B neurons were mediated via PTX-sensitive G-proteins, the effects of the Ni3 and Ci3 peptides on the HVA currents were not changed by preincubation with PTX overnight. The rundown of HVA currents at 5 min in neurons treated with PTX (>12 hr, 1 μg/ml) remained similar (64.9 ± 5.2%, n = 6) to that without PTX incubation (60.3 ± 7.3%, n = 8,p > 0.5). This treatment, however, did significantly reduce the effects of 5-HT on HVA currents in neurons loaded with control pipette solution (5.8 ± 2.6% inhibition in PTX,n = 6, and 16.7 ± 3.5% inhibition in control,n = 8, p < 0.05). These receptor-derived peptides must therefore strongly activate several types of G-protein.
In contrast to the effects of Ci3 and Ni3 peptides on the HVA currents, none of the four synthetic peptides had any direct effect on the T-type currents (Fig. 8D), which also did not undergo rundown during control recordings (data not shown in figures). These observations together with the observations of the effects of nonhydrolyzable G-protein activators suggest that T-type channels in R-B neurons seem to be unaffected by G-protein activation.
Fig. 8.
Effects 5-HT receptor-derived peptides on the T-type currents and their modulation by 5-HT. A, Time series measurements of T-type currents in a neuron loaded with Ci3 peptide. There was no rundown during the time series recordings, and 5-HT (1 μm) still reversibly inhibited the T-type currents. B, Time series measurements of T-type currents in a neuron loaded with Ni3 peptide. There is very little rundown during the time series recordings; 5-HT (1 μm) also caused reversible inhibition on T-type currents. C, Summary of the inhibition of T-type currents by 5-HT in cells loaded with different synthetic peptides (n.s. vsControl). D, The mean time series measurements in cells loaded with different synthetic peptides (○, I4; •, Ni3; ▵, Ci3; ▴, I2). No rundown of the T current is apparent with any peptide.
Effects of synthetic peptides on modulation of HVA currents and T-type currents
We next examined whether the synthetic 5-HT receptor peptides could alter the modulation of T-type and HVA currents by 5-HT. The effects of 5-HT on the T-type currents were not altered by any of these peptides (Fig. 8). However, the effect of 5-HT on the HVA currents was almost totally abolished by the Ci3 (n = 8) and Ni3 (n = 10) peptides derived from the third cytoplasmic loop of 5-HT receptors (Fig. 7Da,b), but not by peptides from the other cytoplasmic regions of 5-HT receptor (I2,n = 6; I4, n = 9) (Fig.7Db). Because fragments of the third cytoplasmic loop mimicked and occluded the effects of 5-HT on the HVA channels but had no effect on T-type channels or their modulation by 5-HT, we suggest that receptor domains distinct from those involved in activation of G-proteins are required for modulation of T channels by 5-HT.
DISCUSSION
GTP-insensitive T-type channel modulation
We previously found that 5-HT inhibited the T-type currents inXenopus R-B neurons (Sun and Dale, 1997). This is not a direct effect of 5-HT on the T-type channels themselves for several reasons. The inhibition of T-type currents is dose dependent with an IC50 of <1 nm and can be mimicked and blocked by selective 5-HT1A and 5-HT1D agonists and antagonists, respectively. The inhibition is never complete (∼25%), and the time course for wash occurs within several seconds (Sun and Dale, 1997). Our previous evidence showed that the inhibition was membrane-delimited and did not involve a diffusible messenger. Membrane-delimited inhibition of HVA channels occurs through a direct interaction between Gβγ subunits and the channels themselves (Herlitze et al., 1996; Ikeda et al., 1996; De Waard et al., 1997). Surprisingly, however, activation of G-proteins did not appear to be required for the modulation of T-type currents by 5-HT: in neurons loaded with GDP-β-S, GTP-γ-S, GMP-PNP, or AlF4−, the inhibition of T-type currents by 5-HT was not diminished or enhanced. This is unlikely to be caused by lack of access of these GTP analogs, because these agents were effective in altering inhibition of HVA currents in the same cell. However, these GTP analogs act as competitive ligands at the GTP binding site on the Gα subunit, and the inability of these agents to block modulation does not rule out involvement of a novel G-protein with a very high affinity for GTP that could be activated at very low concentrations of GTP (cf. Sprang, 1997). Nevertheless, our results with the receptor-derived peptides make this interpretation unlikely (see below).
The opioid-like peptide nociceptin (orphanin FQ), like 5-HT, inhibits the HVA and the T-type currents (Abdulla and Smith, 1997). The inhibition of the HVA currents involves a G-protein, but the inhibition of T currents was not altered by nonhydrolyzable analogs of GTP and GDP. The voltage sensitivity of the inhibition of the T-type currents by nociceptin has not been studied, and it remains unknown whether the inhibition is membrane-delimited. The possibility still remains, however, that an unidentified G-protein with a higher affinity to GTP than nonhydrolyzable GTP analogs could mediate the actions of nociceptin.
Receptor-derived peptides and receptor–G-protein coupling
Biochemical experiments using synthetic peptides have been used to study the receptor activation and selectivity of G-protein recognition, and the results generally agree well with studies that use chimeric or mutated receptors (Wess, 1997). The majority of such studies indicate that the selectivity of G-protein recognition is primarily determined by amino acids located in the I2 loop and the amino and carboxy ends of the third cytoplasmic loop (Ci3 and Ni3) (cf. Hedin et al., 1993; Wess, 1997). Several laboratories have shown that peptides corresponding to the I2, Ni3, and Ci3 regions (in some cases also the membrane-proximal portion of the C-terminal I4 region) can mimic or inhibit receptor–G-protein interaction in various receptors (cf. Savarese and Fraser, 1992; Strader et al., 1994; Wess, 1997; Zhu et al., 1997). Attempts have also been made to determine the critical amino acid sequences that determine specificity of 5-HT receptor G-protein coupling. Current evidence suggests important roles for the second loop (Varrault, 1994; Lembo et al., 1997), the carboxyl end of the third intracellular loop (Varrault, 1994; Oksenberg et al., 1995). However, the role of individual amino acid residues in determining the receptor–G-protein activation is not clear.
Our findings that the Ni3 and Ci3 peptides inhibit HVA currents are therefore consistent with these general ideas. However, our peptides, derived from the Ni3 and Ci3 regions of 5-HT1 receptors, activated various G-proteins in a nonspecific manner. Thus, if there is specificity in the interaction between receptor and G-proteins, it must reside in some other part of the receptor. Although some evidence suggests that the I2 loop is important to determine the signaling specificity of 5-HT1A receptor (Lembo et al., 1997), we did not find that the I2 peptide alone had any direct effect on HVA currents or on the inhibition of HVA currents by 5-HT. However, peptides derived from different regions of the receptor may need to act in a cooperative manner to allow specificity of signaling.
By applying these peptides via the patch pipette, we have demonstrated that the activation of G-protein by these peptides caused substantial voltage-independent inhibition of the HVA currents (in some cells >80% inhibition of total HVA currents). Nevertheless these peptides still had no effects on the T-type current recorded in the same neurons. Because this is a way of manipulating G-protein activation that is mechanistically completely distinct from the use of GTP analogs, this strongly suggests that G-proteins are not involved in modulating the T-type channels in R-B neurons. Furthermore our results imply that a functional domain of the receptor that is distinct from that involved in activating G-proteins may cause inhibition of T channel either directly or through an unknown intermediate.
The effects of 5-HT receptors (except for 5-HT3 receptor) and the activation of other G-protein-coupled receptors are thought to be mediated exclusively via activation of G-proteins. This was challenged by recent findings of an agonist-promoted association of the β2-adrenergic receptor with the Na+/H+ exchanger regulatory factor (Hall et al., 1998). This regulatory protein binds to the β2-adrenergic receptor and interacts specifically with the last few residues of the C-terminal cytoplasmic domain of the receptor (Hall et al., 1998).
Two general alternatives are possible for the G-protein-independent inhibition of T channels given that it is membrane-delimited and nonvoltage dependent. (1) The inhibition may mediated by a protein that interacts with both the receptor and the T channels [cf. β2 adrenergic modulation of the Na+/H+ exchanger in Hall et al. (1998)]. (2) The receptor may interact directly with the T channels. The lack of voltage dependence to the modulation suggests that if such a direct interaction were to occur, it would probably involve the intracellular domains of the receptor rather than the transmembrane regions because these might be sensitive to the transmembrane voltage.
Voltage-independent inhibition of N and P/Q currents
Voltage-independent inhibition is characterized by an incomplete ability of a depolarizing prepulse to reverse the inhibition (Diversé-Pierlussi and Dunlap, 1993; Page et al., 1997) and the continuing presence of inhibition measured from the tail current amplitude at large depolarizations (Diversé-Pierlussi and Dunlap, 1993). As agreed by many researchers (Dolphin, 1998), the voltage-independent inhibition of HVA channels is a unique form of modulation that occurs either independently or in conjunction with the well known voltage-dependent inhibition. However, much less is known about the mechanisms underlying the voltage-independent inhibition. Although the whole-cell recordings have shown a reduction in the occurrence of maximum HVA channel conductance (Diversé-Pierlussi and Dunlap, 1993; Sun and Dale, 1998), this could be produced either via a modification of the single channel conductance or through reduction of the number of functional Ca2+channels.
The second messengers underlying the voltage-independent inhibition are not clear either. In some circumstances, this inhibition may be mediated via phosphorylation of N-type channels by protein kinase C (PKC) (Diversé-Pierlussi and Dunlap, 1993). However, PKC appears to inhibit Ca2+ currents only in sensory neurons (Rane et al., 1989; Boland et al., 1991), whereas in sympathetic neurons channel activity is enhanced by PKC activation (Swartz, 1993;Zhu and Ikeda, 1994). Attempts to identify the second messengers in these sympathetic neurons have so far been unsuccessful. Some evidence supports the involvement of diffusible second messengers: muscarinic receptors are thought to suppress Ca2+ channels in rat sympathetic neurons via a slow, diffusible second messenger (Bernheim et al., 1991), and angiotensin receptors act through a PTX-insensitive G-protein and a diffusible second messenger to suppress HVA currents (Shapiro et al., 1994). Our experiments provide a further example of involvement of a diffusible second messenger but in voltage-independent inhibition of N and P/Q currents.
Nonhydrolyzable GTP analogs enhance the inhibition of HVA currents
Intracellular loading with GTP-γ-S, GMP-PNP, or AlF4− did not produce much change in the amplitude of HVA currents by itself but did produce a great enhancement of the effects of 5-HT on the HVA currents. Furthermore, the inhibition produced by 5-HT in cells loaded with nonhydrolyzable GTP analogs was also accompanied by slowing of activation kinetics and could be partially relieved by strong depolarizing prepulses. This is very different from the effects of 5-HT on R-B neurons loaded with GTP, where the inhibition of N and P/Q currents is purely voltage independent (Sun and Dale, 1997). Although GTP-γ-S-mediated direct and voltage-dependent inhibition of the HVA currents have been reported (Elmslie et al., 1990; Page et al., 1997), our results differ from these reports in that the effects of GTP-γ-S were only produced in the presence of extracellular 5-HT. The additional modulation seen when G-proteins were irreversibly activated may result from higher local concentrations of free Gα and Gβγ subunits, which could drift in the plane of membrane further and act directly on more distant HVA channels, that would normally be out of reach under control conditions (cf. Herlitze et al., 1996; Ikeda, 1996; De Waard et al., 1997).
Footnotes
We are grateful to the Committee of Vice Chancellors and Principals for an Overseas Research Studentship award to Q.Q.S. We thank Dr. Graham Kemp of the School of Biomedical Sciences for synthesizing the 5-HT receptor-derived peptides.
Correspondence should be addressed to Dr. Nicholas Dale, School of Biological and Medical Science, University of St. Andrews, Scotland KY16 9TS, UK.
REFERENCES
- 1.Abdulla FA, Smith PA. Nociceptin inhibits T-type Ca2+ channel current in rat sensory neurons by a G-protein-independent mechanism. J Neurosci. 1997;17:8721–8728. doi: 10.1523/JNEUROSCI.17-22-08721.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albillos A, Carbone E, Gandia L, Garcia AG, Pollo A. Opioid inhibition of Ca2+ channel subtypes in bovine chromaffin cells: selectivity of action and voltage-dependence. Eur J Neurosci. 1996;8:1561–1570. doi: 10.1111/j.1460-9568.1996.tb01301.x. [DOI] [PubMed] [Google Scholar]
- 3.Atherton E, Cameron LR, Sheppard RC. Peptide synthesis, Part 10. Use of pentafluorophenyl esters of fluorenylmethoxycarbonylamino acids in solid phase synthesis. Tetrahedron. 1988;44:843–857. [Google Scholar]
- 4.Berger AJ, Takahashi T. Serotonin enhances a low-voltage-activated Ca2+ current in rat spinal motoneurons. J Neurosci. 1990;10:1922–1928. doi: 10.1523/JNEUROSCI.10-06-01922.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernheim L, Beech DJ, Hille B. A diffusible second messenger mediates one of the pathways coupling receptors to calcium channels in rat sympathetic neurons. Neuron. 1991;6:859–867. doi: 10.1016/0896-6273(91)90226-p. [DOI] [PubMed] [Google Scholar]
- 6.Boland LM, Allen AC, Dingledine R. Inhibition by bradykinin of voltage-activated barium current in a rat dorsal root ganglion cell line: role of protein kinase C. J Neurosci. 1991;11:1140–1149. doi: 10.1523/JNEUROSCI.11-04-01140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciranna L, Feltz P, Schlichter R. Selective inhibition of high voltage-activated L-type and Q-type Ca2+ currents by serotonin in rat melanotrophs. J Physiol (Lond) 1996;490:595–609. doi: 10.1113/jphysiol.1996.sp021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Currie KMP, Fox AP. Comparison of N- and P/Q- type voltage-gated Ca2+ channel current inhibition. J Neurosci. 1997;17:4570–4579. doi: 10.1523/JNEUROSCI.17-12-04570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dale N. The isolation and identification of spinal neurones that control movement in the Xenopus embryo. Eur J Neurosci. 1991;3:1025–1035. doi: 10.1111/j.1460-9568.1991.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 10.Dale N. Kinetic characterization of the voltage-gated currents possessed by Xenopus embryo spinal neurons. J Physiol (Lond) 1995;489:472–488. doi: 10.1113/jphysiol.1995.sp021066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delcour AH, Tsien TW. Altered prevalence of gating modes in neurotransmitter inhibition of N-type calcium channels. Science. 1993;259:980–984. doi: 10.1126/science.8094902. [DOI] [PubMed] [Google Scholar]
- 12.De Waard MD, Liu H, Walker D, Scott VES, Gurnett CA, Campbell KP. Direct binding of G-protein βγ complex to voltage-dependent calcium channels. Nature. 1997;385:446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- 13.Diversé-Pierlussi M, Dunlap K. Distinct convergent second messenger pathways modulate neuronal calcium currents. Neuron. 1993;10:753–760. doi: 10.1016/0896-6273(93)90175-q. [DOI] [PubMed] [Google Scholar]
- 14.Diversé-Pierlussi M, Goldsmith PK, Dunlap K. Transmitter-mediated inhibition of N-type calcium channels in sensory neurons involves multiple GTP-binding proteins and subunits. Neuron. 1995;14:191–200. doi: 10.1016/0896-6273(95)90254-6. [DOI] [PubMed] [Google Scholar]
- 15.Dolphin AC. Mechanisms of modulation of voltage-dependent calcium channels by G-proteins. J Physiol (Lond) 1998;506:3–11. doi: 10.1111/j.1469-7793.1998.003bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolphin AC, Scott RH. Calcium-channel currents and their inhibition by (−)-baclofen in rat sensory neurons. J Physiol (Lond) 1987;386:1–17. doi: 10.1113/jphysiol.1987.sp016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolphin AC, Scott RH, Wootton JF. Photo-release of GTP-γ-S inhibits the low threshold calcium-channel current in cultured rat dorsal-root ganglion (DRG) neurons. J Physiol (Lond) 1989;423:89P. [Google Scholar]
- 18.Elmslie KS, Zhou W, Jones SW. LHRH and GTP-γ-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990;5:75–80. doi: 10.1016/0896-6273(90)90035-e. [DOI] [PubMed] [Google Scholar]
- 19.Fox AP, Nowycky MC, Tsien RW. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurons. J Physiol (Lond) 1987a;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox AP, Nowycky MC, Tsien RW. Single-channel recordings of three types of calcium channels in chick sensory neurons. J Physiol (Lond) 1987b;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser DD, MacVicar BA. Low-threshold transient calcium current in rat hippocampal lacunosum-moleculare interneurons: kinetics and modulation by neurotransmitters. J Neurosci. 1991;11:2812–2820. doi: 10.1523/JNEUROSCI.11-09-02812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall RA, Premont RT, Chow CW, Blitzer JT, Pitcher JA, Claing A, Stoffel RH, Barak LS, Shenolikar S, Weinman EJ, Grinstein S, Lefkowitz RJ. The beta2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- 23.Hedin KE, Duerson K, Clapham DE. Specificity of receptor-G protein interactions: searching for structure behind the signal. Cell Signal. 1993;5:505–518. doi: 10.1016/0898-6568(93)90046-o. [DOI] [PubMed] [Google Scholar]
- 24.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein βγ subunits. Nature. 1996;380:255–258. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 25.Huguenard JR. Low-threshold calcium currents in central-nervous-system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature. 1996;380:258–262. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 27.Lembo PM, Ghahremani MH, Morris SJ, Albert PR. A conserved threonine residue in the second intracellular loop of the 5-hydroxytryptamine 1A receptor directs signalling specificity. Mol Pharmacol. 1997;52:164–171. doi: 10.1124/mol.52.1.164. [DOI] [PubMed] [Google Scholar]
- 28.Londos C, Salomon Y, Lin MC, Harwood JP, Schramm M, Wolff J, Rodbell M. 5′-Guanylylimidodiphosphate, a potent activator of adenylate cyclase systems in eukaryotic cells. Proc Natl Acad Sci USA. 1974;71:3087–3090. doi: 10.1073/pnas.71.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luebke JI, Dunlap K. Sensory neuron N-type calcium currents are inhibited by both voltage-dependent and independent mechanisms. Pflügers Arch. 1994;428:499–507. doi: 10.1007/BF00374571. [DOI] [PubMed] [Google Scholar]
- 30.Margeta-Mitrovic M, Grigg JJ, Koyano K, Nakajima Y, Nakajima S. Neurotensin and substance P inhibit low- and high-voltage-activated Ca2+ channels in cultured newborn rat nucleus basalis neurons. J Neurophysiol. 1997;78:1341–1352. doi: 10.1152/jn.1997.78.3.1341. [DOI] [PubMed] [Google Scholar]
- 31.McDonough SI, Swartz KJ, Mintz IM, Boland LM, Bean BP. Inhibition of calcium channels in rat central and peripheral neurons by ω-conotoxin MVIIC. J Neurosci. 1995;16:2612–2623. doi: 10.1523/JNEUROSCI.16-08-02612.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nieuwkoop PD, Faber J. Normal tables of Xenopus laevis (Daudin). North Holland; Amsterdam: 1956. [Google Scholar]
- 33.Oksenberg D, Havlik S, Peroutka SJ, Ashkenazi A. The third intracellular loop of the 5-hydroxytryptamine2A receptor determines effector coupling specificity. J Neurochem. 1995;64:1440–1447. doi: 10.1046/j.1471-4159.1995.64041440.x. [DOI] [PubMed] [Google Scholar]
- 34.Olate J, Allende JE. Structure and function of G-proteins. Pharmacol Ther. 1991;51:403–419. doi: 10.1016/0163-7258(91)90068-w. [DOI] [PubMed] [Google Scholar]
- 35.Page KM, Stephens GJ, Berrow NS, Dolphin AC. The intracellular loop between domains I and II of the B-type calcium channel confers aspects of G-protein sensitivity to the E-type calcium channel. J Neurosci. 1997;17:1330–1338. doi: 10.1523/JNEUROSCI.17-04-01330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee JH. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–899. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- 37.Rane SG, Walsh MP, McDonald JR, Dunlap K. Inhibitors of protein kinase C block transmitter-induced modulation of sensor neuron calcium current. Neuron. 1989;3:239–245. doi: 10.1016/0896-6273(89)90037-8. [DOI] [PubMed] [Google Scholar]
- 38.Savarese TM, Fraser CM. In vitro mutagenesis and the search for structure-function relationships among G-protein-coupled receptors. Biochem J. 1992;283:1–19. doi: 10.1042/bj2830001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shapiro MS, Wollmuth LP, Hille B. Modulation of Ca2+ channels by PTX-sensitive G-proteins is blocked by N-ethylmaleimide in rat sympathetic neurons. Neuron. 1994;12:1319–1329. doi: 10.1523/JNEUROSCI.14-11-07109.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sprang SP. G-protein mechanisms: insights from structural analysis. Annu Rev Biochem. 1997;66:639–678. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- 41.Strader CD, Fong TM, Tota MR, Underwood D, Dixon RAF. Structure and function of G-protein-coupled receptors. Annu Rev Biochem. 1994;63:101–132. doi: 10.1146/annurev.bi.63.070194.000533. [DOI] [PubMed] [Google Scholar]
- 42.Sun QQ, Dale N. Serotonergic inhibition of the T-type and HVA Ca2+ currents in the primary sensory neurons of Xenopus larvae. J Neurosci. 1997;17:6839–6849. doi: 10.1523/JNEUROSCI.17-18-06839.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun QQ, Dale N. Differential inhibition of N and P/Q Ca2+ currents by 5-HT1A and 5-HT1D receptors in spinal neurons of Xenopus larvae. J Physiol (Lond) 1998;510:103–120. doi: 10.1111/j.1469-7793.1998.103bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swartz KJ. Modulation of Ca2+ channels by protein kinase C in rat central and peripheral neurons: disruption of G-protein mediated inhibition. Neuron. 1993;11:305–320. doi: 10.1016/0896-6273(93)90186-u. [DOI] [PubMed] [Google Scholar]
- 45.Turner TJ, Adams ME, Dunlap K. Multiple Ca2+ channel types coexist to regulate synaptosomal neurotransmitter release. Proc Natl Acad Sci USA. 1993;90:9518–9522. doi: 10.1073/pnas.90.20.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varrault A, Le Nguyen D, McClue S, Harris B, Jouin P, Bockaert J. 5-Hydroxytryptamine1A receptor synthetic peptides. Mechanisms of adenylyl cyclase inhibition. J Biol Chem. 1994;269:16720–16725. [PubMed] [Google Scholar]
- 47.Wess J. G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J. 1997;11:346–354. [PubMed] [Google Scholar]
- 48.Zhu M, Neubig RR, Wade SM, Posner P, Gelband CH, Sumners C. Modulation of K+ and Ca2+ currents in cultured neurons by an angitensin II type 1a receptor peptide. Am J Physiol. 1997;273:C1040–C1048. doi: 10.1152/ajpcell.1997.273.3.C1040. [DOI] [PubMed] [Google Scholar]
- 49.Zhu Y, Ikeda SR. Modulation of Ca2+-channel currents by protein kinase C in adult rat sympathetic neurons. J Neurophysiol. 1994;72:1549–1560. doi: 10.1152/jn.1994.72.4.1549. [DOI] [PubMed] [Google Scholar]