Abstract
Amyloid plaques that accumulate in the brains of patients with Alzheimer’s disease (AD) are primarily composed of aggregates of amyloid peptides that are derived from the amyloid precursor protein (APP). Overexpression of APP in cell cultures increases the formation of amyloidogenic peptides and causes neurodegeneration and cognitive dysfunction in transgenic mice. We now report that activation of prostaglandin E2 (PGE2) receptors increases cAMP formation and stimulates overexpression of APP mRNA and holoprotein in primary cultures of cortical astrocytes. Levels of glial fibrillary acidic protein were also increased by PGE2treatment, suggesting that these cultured astrocytes resemble reactive astrocytes found in vivo. The stimulation by PGE2 of APP synthesis was mimicked or blocked by activators or inhibitors, respectively, of protein kinase A. Actinomycin D or cycloheximide also inhibited the increase in APP holoprotein stimulated by PGE2. Treatment of astrocytes with 8-Bromo-cAMP or forskolin for 24 hr also stimulated APP overexpression in cultured astrocytes. The immunosuppressants cyclosporin A and FK-506 inhibited the increase in APP mRNA and holoprotein levels caused by PGE2 or by other treatments that elevated cellular cAMP levels; the inhibitory effect of FK-506 but not of cyclosporin A was attenuated by rapamycin. These results suggest that prostaglandins produced by brain injury or inflammation can activate APP transcription in astrocytes and that immunosuppressants may be used to prevent APP overexpression and possibly the pathophysiological processes underlying AD.
Keywords: inflammation, cAMP, astrocytes, rapamycin, cyclosporin A, FK506
Alzheimer’s disease (AD) is characterized by extracellular deposits of amyloid plaques that are associated with dystrophic neurites, reactive astrocytes, and microglia (Alzheimer, 1907). These plaques are primarily aggregates of the amyloid peptide (Aβ) that is derived from an abundant cellular protein, the amyloid precursor protein (APP). Constitutive APP processing prevents the Aβ formation by cleaving the holoprotein within the Aβ domain to release the soluble N terminus of APP (APPS) into the extracellular space (Esch et al., 1990; Sisodia et al., 1990). APPS secretion in human cells, brain slices, cultured neurons, or astrocytes is enhanced by activation of cell-surface receptors coupled to increased phosphatidylinositol hydrolysis, tyrosine phosphorylation, or protein kinase C activity (Nitsch et al., 1992; Lee et al., 1995;Slack et al., 1995; Ulus and Wurtman, 1997). The drastic alterations in neurotransmitter levels and second messenger signaling created by neurodegeneration and synapse loss observed in AD may disrupt APP processing and promote Aβ accumulation.
Overexpression of APP appears to be associated with the neuropathological findings of AD. Cell cultures overexpressing APP increase the formation of amyloid fibrils and neurotoxic peptides (Yoshikawa et al., 1992). Transgenic mice overexpressing the 751-amino acid APP isoform that contains the Kunitz-type protease inhibitor (KPI) domain also show early amyloid deposits, neurite dystrophy, and memory deficits (Cordell, 1994). Interestingly, transgenic mice overexpressing the human APP695 isoform that lacks the KPI domain do not show amyloid deposits but nevertheless do develop astrogliosis and age-related cognitive impairments (Hsiao et al., 1995).
Reactive astrocytes with increased glial fibrillary acidic protein (GFAP) levels are associated with aging. GFAP levels are elevated in brain tissue and cerebrospinal fluid of patients with AD (Wallin et al., 1996). Brain injury causes persistent and rapid elevations in APP immunoreactivity in GFAP-positive astrocytes (Siman et al., 1989;Banati et al., 1995). Synapse loss in AD is also associated with increased numbers of GFAP-positive astrocytes (Brun et al., 1995). Indeed, increased levels of the mRNA for KPI-containing APP in the frontal cortex have been attributed to the astrocytic response during neuronal damage (Golde et al., 1990). Thus, the pathological cascade in AD may include APP synthesis by reactive astrocytes.
Cytosolic phospholipase A2, which releases arachidonic acid from cellular phospholipids, is elevated in AD brain and after global ischemia (Clemens et al., 1996; Stephenson et al., 1996). The cyclooxygenation of arachidonic acid produces prostaglandins that in turn regulate neurotransmission as well as immune and inflammatory responses by activating receptors coupled to cAMP formation (Goetzl et al., 1995). We now show that activation of PGE2 receptors increases cAMP formation and stimulates the synthesis of APP mRNA and APP holoprotein.
Parts of this work have been published previously in abstract form (Lee et al., 1997a).
MATERIALS AND METHODS
Astrocyte cultures. Dissociated astrocytes were cultured from cortices of postnatal day 1–2 rat pups as described previously (McCarthy and de Vellis, 1980) with minor modifications (Lee and Wurtman, 1997a). In brief, cells from dissociated cortices were plated onto poly-l-lysine-coated 35 or 100 mm culture dishes (10–25 cells/mm2). The initial culture media, MEM (Life Technologies, Gaithersburg, MD) containing 10% horse serum (BioWhittaker, Walkersville, MD), were aspirated 2–5 hr after plating to remove unattached cells and debris and replaced with MEM containing 7.5% fetal bovine serum (FBS, BioWhittaker). Half the medium was replaced with MEM/7.5% FBS twice weekly. Astrocytes were kept at 37°C in a humidified 5%CO2/95% air incubator for 10–14 d, by which time the cultures were confluent and could be used for experiments.
Pharmacological reagents and treatments. The following drugs were stored and frozen at 10−2m stock concentrations: PGE2 (Calbiochem, La Jolla, CA); H-89 dihydrochloride and Sp-cAMP triethylamine (Research Biochemicals International, Natick, MA); actinomycin D, cycloheximide, okadaic acid, rapamycin, forskolin, and 8-Bromo-cAMP (8Br-cAMP) (from either Calbiochem or Research Biochemicals); cyclosporin A (Alexis Biochemicals); FK-506 (Fujisawa Pharmaceuticals, Osaka, Japan). The frozen aliquots were thawed and diluted in serum-free MEM (37°C) to appropriate concentrations for incubation of cultured astrocytes. When drugs were dissolved in 95% ethanol, an equivalent volume of ethanol was also added to control samples. Cells were incubated for either 1 or 24 hr with 1.5 ml of media with or without drugs at 37°C in a 5% CO2/95% air incubator. Experiments were conducted at least three times in duplicate dishes unless stated otherwise.
Northern blots. Total RNA was extracted from astrocytes grown on 100 mm dishes using TRI Reagent (Molecular Research Center, Cincinnati, OH) and procedures recommended by the manufacturer. In brief, the medium was aspirated and the cells were scraped in 1 ml of TRI Reagent. After incubation for 15 min at room temperature, 0.2 ml of chloroform was added, mixed vigorously, and stored for another 15 min at room temperature. After centrifugation at 13,000 rpm for 15 min, 0.5 ml of isopropanol was added to the aqueous phase of the mixture to precipitate RNA. The RNA pellet collected by centrifugation (12,000 × g, 15 min at 4°C) was washed with 70% ethanol once and solubilized in an appropriate amount of Formazol (Molecular Research Center). RNA samples (∼20 μg) were denatured by heating for 15 min at 60°C before loading onto 1.2% agarose-formaldehyde gels for electrophoresis. RNA was blotted onto Hybond polyvinyl membranes by overnight capillary transfer and fixed onto the membranes by UV light illumination. Membranes were prehybridized with Amersham Rapid-hyb (Amersham, Arlington Heights, IL) buffer for 2 hr and labeled overnight with full-length human APP695 cDNA (gift of Dr. Rachael Neve, McLean Hospital, Harvard Medical School, Belmont, MA) or with a human glyceraldehyde-3-phosphate dehydrogenase probe (G3PDH; Clontech, Cambridge, UK) labeled with [32P]dCTP using random-primed extension (Amersham Megaprime DNA labeling kit). Membranes were dried and exposed to Kodak x-ray film for 24–48 hr with an Amersham enhancer sheet. The relative amounts of mRNA obtained by hybridization were estimated using densitometric analyses of autoradiographs. The levels of APP mRNA were normalized to the amounts of G3PDH mRNA and expressed as a ratio to the levels in untreated, control cells.
Western blots. Cell-associated APP was detected from cultured astrocytes grown on 35 mm dishes. The treatment media were aspirated, and astrocytes were scraped into 50 μl of lysis buffer (60 mm Tris-HCl, 4% SDS, 20% glycerol, 1 mmdithiothreitol) and collected in Eppendorf tubes. The samples were boiled for 10 min to inhibit protease activity. The total amount of protein in each sample as estimated by the bicinchoninic acid (Sigma, St. Louis, MO) assay was not altered by pharmacological treatments. Before gel electrophoresis, 1 μl of 5% bromphenol blue solution was added to each sample.
To detect secreted APP, culture media was collected after drug treatments, and phenylmethylsulfonyl fluoride was added to a final concentration of 2 mm. The media samples were centrifuged at 13,000 rpm for 10 min to remove cell debris. The supernatant fluids were applied to Sephadex PD-10 desalting columns (Pharmacia, Piscataway, NJ) and eluted with distilled water. Column eluates were frozen and dried by vacuum centrifugation. The lyophilized proteins were reconstituted in 25 μl of water to which was added 25 μl of 2× Laemmli gel-loading buffer, and the mixture was boiled for 5 min.
The amount of medium or cell protein loaded for SDS-PAGE (10–20%) (Bio-Rad, Hercules, CA) was normalized for the amount of protein per sample. Proteins (equivalent to ∼100 μg cell protein per lane) were separated by electrophoresis, electroblotted onto polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA), and blocked in Tris-buffered saline with 0.15% Tween 20 (TBST) containing 5% powdered milk for 30 min. After two × 10 min rinses in TBST, the membranes were incubated in TBST containing an appropriate antibody. Monoclonal antibodies 22C11 and GFAP (both from Boehringer Mannheim, Indianapolis, IN) were used to detect the N terminus of APP and GFAP, respectively; antisera R37 and R98 (gifts of Dr. F. Kametani, Tokyo Institute of Psychiatry, Tokyo, Japan) were used to detected the C terminus and KPI motif of APP, respectively; and antiserum C8 (gift of Dr. D. Selkoe, Women’s Hospital, Harvard Medical School, Cambridge, MA) was used to detect the C terminus of APP.
After an overnight incubation, membranes were rinsed in TBST before being treated for 1 hr with a peroxidase-linked secondary antibody. After several rinses in TBST, protein bands were visualized on Kodak X-AR films by an enhanced chemiluminescence method (Amersham). Optical densities of the protein bands were quantitated by laser scanning densitometry (LKB, Bromma, Sweden) and normalized to the densities of those bands generated under control conditions.
cAMP assay. Levels of cyclic AMP were measured using an [8-3H]-cAMP assay kit (Amersham TRK 432) in astrocytes grown on 35 mm dishes. In brief, the media were aspirated, and the cells were rinsed twice with 1 ml of ice-cold PBS, scraped in 0.8 ml of ice-cold ethanol, and sonicated. The cell suspension was kept at room temperature for 5 min and then centrifuged, and the supernatant fluid was dried in a rotary evaporator. After resuspension in 120 μl of Tris/EDTA buffer, two duplicate samples of 50 μl each were mixed with the binding protein and a [8-3H] adenosine 3′, 5′-cyclic phosphate tracer and incubated at 2–4°C for 2 hr. A charcoal suspension (100 μl) was added to the samples before centrifugation, and 200 μl of the supernatant fluid was removed for scintillation counting. The amount of cyclic AMP (picomoles per milligram of protein) was estimated by comparisons with known standards and normalized to the amounts of whole-cell protein as determined by the bicinchoninic acid assay (Sigma).
Data analysis. Measurements of cellular and secreted proteins or of mRNA in treatment groups were normalized against those of control groups prepared in parallel and loaded onto the same blot. ANOVA and t tests were used to evaluate differences between groups (significance level, p = 0.05) using drug treatments as the independent variable. Data are presented as means ± SE; n = number of independent experiments.
RESULTS
PGE2 coupled to cAMP production increases the expression of APP holoprotein and mRNA
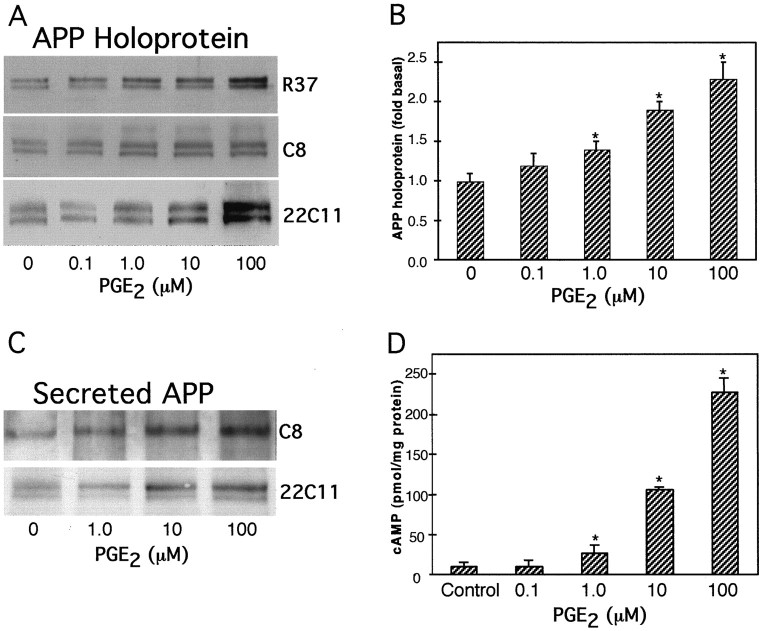
Treatment of astrocytes for 24 hr with 1, 10, or 100 μm PGE2 significantly increased the amounts of astrocytic APP holoprotein relative to those in untreated cells (allp < 0.05) (Fig.1A). Similar increases in APP holoprotein (∼110–130 kDa) were detected by monoclonal antibody (mAb) 22C11, antisera R37, or antisera R98 on Western blots (Fig. 1B). Treatment of astrocytes for 48 hr with 1, 10, or 100 μm PGE2 produced linear increases in cellular APP holoprotein that were 1.4 ± 0.1-fold (n = 3), 1.94 ± 0.06-fold (n = 5), and 2.34 ± 0.07-fold (n = 3), respectively, those in untreated, control cells (Fig. 1B). Treatment of astrocytes for 48 hr with 1, 10, or 100 μmPGE2 stimulated the expression of APP holoprotein to levels that were 1.4 ± 0.12-fold (n = 3), 1.7 ± 0.05-fold (n = 3), and 1.9 ± 0.1-fold (n = 3), respectively those in untreated cells (allp < 0.05).
Fig. 1.
Effect of PGE2 on cellular and secreted APP and on cAMP production in cultured astrocytes.A, Representative immunoblots show that 0.1, 1, 10, and 100 μm PGE2 treatment for 24 hr stimulated dose-dependent increases in cellular APP holoprotein. The levels of APP holoprotein as measured by mAb 22C11, antisera R37, or antisera R98 did not differ significantly. B, The graph represents the means and SEM of APP holoprotein levels stimulated by different concentrations of PGE2 (*p < 0.05). Densitometric analysis of APP levels using mAb 22C11 (n = 2), antiserum R37 (n = 2), or antiserum R98 (n = 1) were expressed as arbitrary values and normalized to the levels obtained from untreated, control cells. C, Representative immunoblots showing the levels of APP released into the media, as detected by mAb 22C11 or antiserum C8. Both immunoblots revealed increased amounts of secreted APP after PGE2 treatment for 24 hr compared with control cells. Similar results were obtained from a subsequent experiment.D, Dose-dependent increases in cellular cAMP levels obtained by PGE2 treatment of astrocytes. Graph represents means and SEM from a representative experiment performed on duplicate dishes (*p < 0.05).
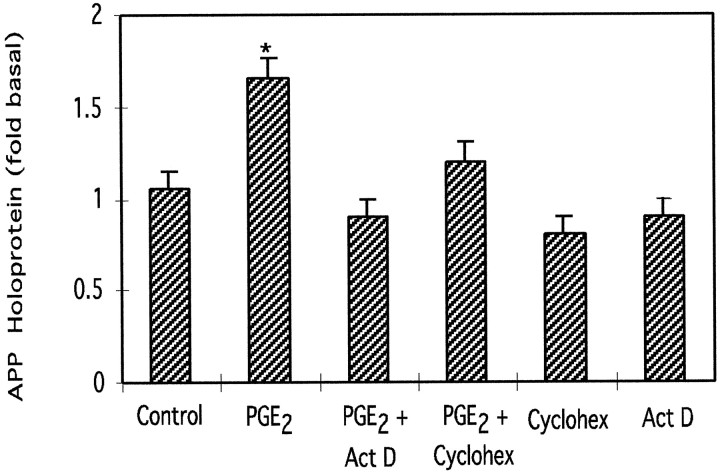
APP secreted into the media (∼110–130 kDa) was also increased by 24 hr treatment with 1, 10, or 100 μm PGE2 as assayed using mAb 22C11 or antiserum C8 (Fig. 1C). Treatment with 1, 10, or 100 μm PGE2 also stimulated dose-dependent increases in cellular cAMP levels to 27-, 106-, and 227-fold those of untreated cells (Fig. 1D); 0.1 μm PGE2 did not stimulate cAMP production and did not significantly alter APP holoprotein or mRNA levels compared with those in untreated, control astrocytes (p> 0.05). Actinomycin D and cycloheximide (both 2.5 μm) inhibited the increase in APP holoprotein stimulated by PGE2 (10 μm) (Fig.2).
Fig. 2.
Effect of actinomycin D (Act D) or cycloheximide (Cyclohex) on the increase in APP holoprotein stimulated by PGE2. Actinomycin D or cycloheximide (both 2.5 μm) effectively inhibited the increase in APP holoprotein stimulated by PGE2 (10 μm) but had no significant effect on basal APP levels. Graph represents means and SEM from four independent experiments (*p < 0.05).
Protein kinase A and cAMP regulate APP expression
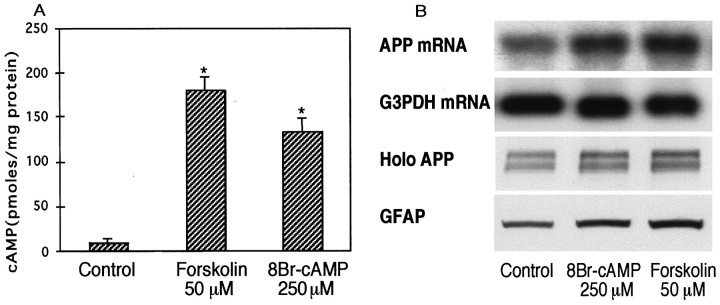
Treatment of astrocytes for 1 hr with membrane-permeant 8Br-cAMP (250 μm) or with forskolin (10, 50, or 100 μm) significantly increased cellular cAMP levels (Fig.3A) and stimulated increases in APP mRNA (∼3.5 kb) and holoprotein after 24 hr treatments (Fig.3B). Astrocytes treated with 8Br-cAMP (250 μm) expressed APP mRNA, APP holoprotein, and GFAP levels that were 1.6 ± 0.1-fold (n = 4), 2.1 ± 0.2-fold (n = 5), and 1.8 ± 0.1-fold (n = 5), respectively, those of untreated, control cells (allp < 0.05). Similarly, astrocytes treated with forskolin (50 μm) expressed APP mRNA, APP holoprotein, and GFAP levels that were 1.8 ± 0.07-fold (n = 3), 1.9 ± 0.06-fold, (n = 4) and 1.8 ± 0.1-fold (n = 4), respectively, those of untreated, control cells (all p < 0.05).
Fig. 3.
Effect of 8Br-cAMP or forskolin on cAMP production and APP synthesis in cultured astrocytes. A, Cellular cAMP levels in astrocytes stimulated by 8Br-cAMP (250 μm) or forskolin (50 μm). Graph represents means and SEM from a representative experiment performed on duplicate dishes (*p < 0.05). B, Representative Northern and Western blots show that APP mRNA, APP holoprotein (Holo APP), and GFAP levels are increased by 8Br-cAMP (250 μm) or forskolin (50 μm). The amount of RNA loaded for each lane on Northern blots was not different as measured by G3PDH mRNA levels.
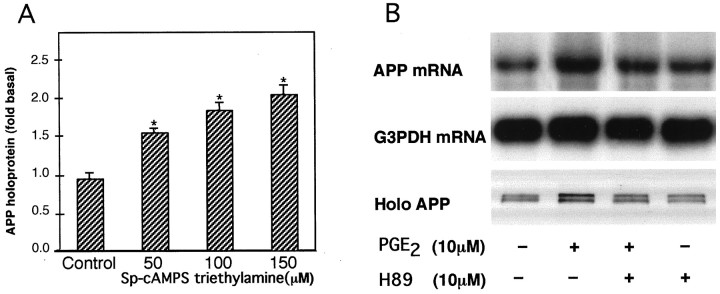
Activation of protein kinase A by 24 hr treatment with 50, 100, or 150 μm Sp-cAMP triethylamine increased cellular levels of APP holoprotein to 1.6-, 1.9-, and 2.2-fold compared with those in untreated cells (Fig.4A). Treatment with PGE2 (10 μm) alone resulted in astrocytic APP mRNA and holoprotein levels that were 1.7 ± 0.1-fold (n = 3) and 2.0 ± 0.1-fold (n = 3), respectively, those of untreated, control cells (all p<0.05). Co-treatment with both PGE2 and the protein kinase A inhibitor H-89 (100 μm) resulted in APP mRNA and holoprotein levels that were not significantly different from those of control cells (p > 0.05) (Fig.4B).
Fig. 4.
Effect of PKA activation and inhibition on APP synthesis in cultured astrocytes. A, APP holoprotein (Holo APP) levels are increased by activation of PKA with Sp-cAMPS triethylamine. Graph represents means and SEM from three independent experiments (*p < 0.05).B, APP mRNA and APP holoprotein increases stimulated by PGE2 (10 μm) are blocked by the PKA inhibitor H-89 (10 μm). APP was detected with antiserum R98 directed at the KPI motif of APP. These results were replicated in subsequent experiments using mAb 22C11 or R37 directed at the N and C termini of APP, respectively.
Immunosuppressants cyclosporin A and FK-506 inhibit APP synthesis stimulated by PGE2 or cAMP elevations
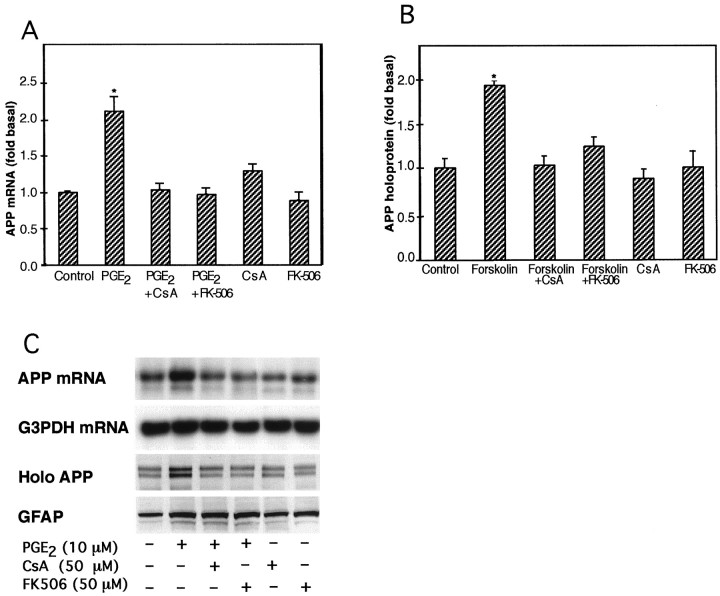
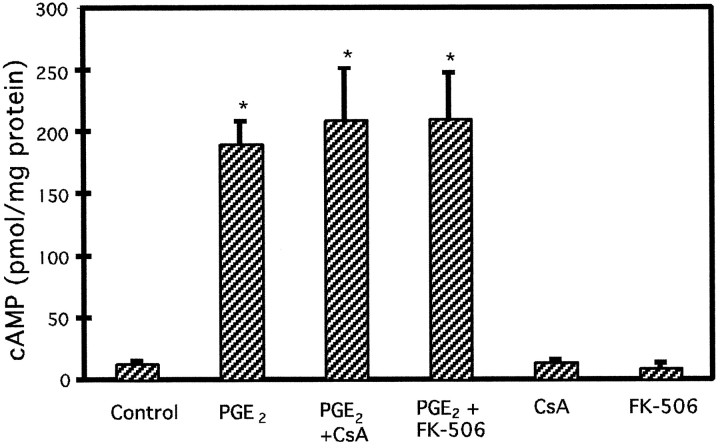
The increases in astrocytic APP holoprotein and mRNA stimulated by 24 hr treatments with 50 μm forskolin or 10 μm PGE2, respectively, were significantly inhibited by co-treatment with either 50 μmcyclosporin A or 50 μm FK-506 (Fig.5). Increases in GFAP levels stimulated by 10 μm PGE2 were not altered by co-treatments with cyclosporin A or FK-506 (both 50 μm). Also, neither cyclosporin A nor FK-506 modified the increase in cellular cAMP levels produced by 10 μm PGE2(Fig. 6). Treatment of astrocytes with either cyclosporin A or FK-506 (both 50 μm) alone had no significant effect on basal APP holoprotein or on cAMP levels (p > 0.05).
Fig. 5.
Effect of the immunosuppressant cyclosporin A or FK-506 on APP synthesis caused by PGE2 or forskolin treatment of cultured astrocytes. A, Increases in APP mRNA caused by PGE2 (10 μm) are inhibited by the immunosuppressant cyclosporin A (CsA) or FK-506 (both 50 μm). Graph and SEM represent data collected from three independent experiments (*p < 0.05).B, Increases in APP holoprotein caused by forskolin (50 μm) are inhibited by the immunosuppressant cyclosporin A (CsA) or FK-506 (both 50 μm). Graph and SEM represent data collected from three independent experiments (*p < 0.05). C, Representative Northern and Western blots show that the increases in APP mRNA and APP holoprotein stimulated by PGE2 (10 μm), but not the increases in GFAP levels, were inhibited by cyclosporin A (CsA) or FK-506 (both 50 μm).
Fig. 6.
Effect of the immunosuppressant cyclosporin A or FK-506 on cAMP production caused by PGE2 treatment of cultured astrocytes. Cellular cAMP levels stimulated by PGE2 are not inhibited by the immunosuppressant cyclosporin A (CsA) or by FK-506 (both 50 μm). Graph represents means and SEM from a typical experiment conducted on duplicate dishes (*p < 0.05).
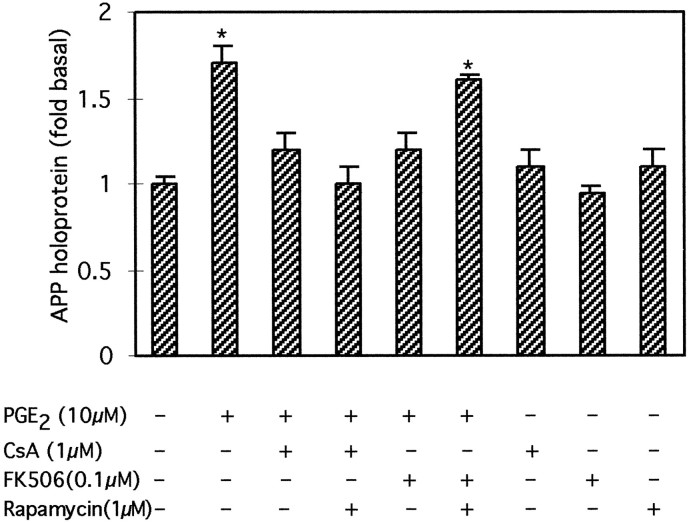
The increase in astrocytic APP holoprotein stimulated by 24 hr treatment with 10 μm PGE2 were also significantly inhibited by co-treatment with either 1 μmcyclosporin A or 0.1 μm FK-506; the inhibitory effect of FK-506 but not of cyclosporin A was blocked by rapamycin (1 μm) (Fig. 7).
Fig. 7.
Effect of rapamycin on the FK506- or cyclosporin A-mediated inhibition of APP increases in astrocytes treated with PGE2. Rapamycin (1 μm) antagonized the inhibitory effect of FK-506 (0.1 μm) but not of cyclosporin A (1 μm) on PGE2-mediated increases in APP holoprotein. Graph represents means and SEM from three independent experiments (*p < 0.05).
DISCUSSION
Our results show that PGE2 stimulates APP synthesis in cultured astrocytes. Increases in APP mRNA and holoprotein were increased by astrocytes treated with 1, 10, or 100 μmPGE2 for 24 hr. The increase in APP holoprotein stimulated by PGE2 was inhibited by actinomycin D or cycloheximide, indicating that this increase in APP is mediated by transcription or translation of the APP gene. APP holoprotein was also increased by prolonged treatment (i.e., 48 hr) of astrocytes with 1, 10, or 100 μm PGE2; however, shorter duration treatment (6 or 12 hr) with 10 μm PGE2 did not reliably increase APP synthesis. APP synthesis in astrocytes is probably mediated by the increases in cAMP production stimulated by PGE2 treatment because dose-dependent increases in APP holoprotein were paralleled by concentration-dependent elevations in cAMP levels in astrocytes treated with 1, 10, or 100 μmPGE2. Neither cAMP nor APP holoprotein levels were increased by 0.1 μm PGE2. Furthermore, the stimulatory effect of PGE2 on APP synthesis was mimicked by membrane-permeant 8Br-cAMP (250 μm) or by activating adenylate cyclase with forskolin (10, 50, or 100 μm). Activation of cAMP-dependent protein kinase (PKA) by Sp-cAMP triethylamine in the absence of PGE2 was sufficient to stimulate increases in astrocytic APP holoprotein. Furthermore, inhibition of PKA by H-89 dihydrochloride blocked the stimulatory effect of PGE2 on APP mRNA production. These data provide strong support for PKA in mediating the stimulatory effect of cAMP on APP synthesis.
Astrocytes and microglia express low levels of APP751/770 isoforms in the resting state but upregulate these KPI-containing APP isoforms after brain injury or neurodegeneration (Siman et al., 1989; Soláet al., 1993; Banati et al., 1995). Furthermore, KPI-containing APP mRNA is not usually expressed in the brain but is upregulated in frontal cortex of AD patients (Tanaka et al., 1989; Golde et al., 1990). Antiserum R98 (Kametani et al., 1993) revealed increases in KPI-containing APP isoforms after PGE2 treatments. Increases in cellular APP holoprotein were also detected by antisera R37 directed at the C terminus of APP (Kametani et al., 1993), indicating that the KPI-containing APP are full-length holoproteins harboring intact and potentially amyloidogenic Aβ peptides. Because mAb 22C11 recognizes the N termini of both APP and APP-like proteins (Weidemann et al., 1989; Slunt et al., 1994), it is possible that PGE2 treatment may also stimulate transcriptional regulation of other members of the APP gene family. To the extent that astrocytes proliferate and upregulate APP synthesis during aging and neuronal injury, these cells may contribute to the neuronal dysfunction and pathology of AD.
The APP promoter contains several sequences for regulatory elements that are responsive to cAMP signaling (Salbaum et al., 1988). A consensus sequence for cAMP response element-binding protein exists within the 3.7 kb region upstream from the APP transcription start site (Salbaum et al., 1988). Although elevations in cAMP can activate AP-1 or AP-2 sites on the APP promoter, APP synthesis in NG108–15 and HepG2 cells stimulated by dibutyryl cAMP appears not to depend on these sites (Bourbonniére et al., 1997; Shekarabi et al., 1997). At least two other cAMP-responsive regions have been identified within the APP promoter of NG-108 cells (Bourbonniére et al., 1997). It is not yet known whether these cis-acting regulators are functional for regulating APP synthesis in astrocytes or whether the induction of APP synthesis is mediated by trans-acting elements acting through the expression of other cAMP-responsive genes.
We found previously that the immunosuppressant cyclosporin A, at 1, 5, or 10 μm, effectively inhibited APP synthesis in astrocytes treated with 8Br-cAMP. Cyclosporin A at these concentrations had no significant effect on basal APP or GFAP levels, suggesting that cell death or cell viability was not altered by cyclosporin treatments (Lee et al., 1997b). We did not determine in our previous study whether cyclosporin A would also inhibit APP synthesis mediated by receptor activation or whether other immunosuppressants such as FK-506 would be effective in regulating APP synthesis. We now show that cyclosporin A (1 or 50 μm) or FK-506 (0.1 or 50 μm) completely suppresses APP overexpression stimulated by PGE2or by forskolin. At 0.1 μm concentrations, FK-506 but not cyclosporin A suppressed PGE2-stimulated APP overexpression, indicating that FK-506 is a more potent inhibitor than cyclosporin A. Lower concentrations of FK-506 (< 0.1 μm) did not reliably suppress the increase in APP holoprotein stimulated by PGE2 or 8Br-cAMP. Protein contents of astrocytes treated in the presence or absence of cyclosporin A (1 or 50 μm) or FK-506 (0.1 or 50 μm) assayed using the bichinchoninic assay did not differ, indicating that these immunosuppressants stimulated neither cell death nor cell proliferation. Toxicity produced by cyclosporin A or FK-506 might be expected to alter the production of second messengers (e.g., cAMP) in astrocytes. However, neither cyclosporin A nor FK-506 (both 50 μm) affected basal cAMP levels in cultured astrocytes, and neither drug inhibited the increase in cAMP caused by PGE2. These results indicate that neither immunosuppressant was toxic, and that the inhibitory effect of cyclosporin A or FK-506 appears to lie downstream from cAMP production and possibly is mediated by direct interference with gene transcription (Schwaninger et al., 1995). Unlike cyclosporin A, FK-506 does not bind to cyclophilin receptors but instead selectively binds to the FK-506 binding protein (Marks, 1996). Rapamycin, an antagonist of FK-506 (Sabatini et al., 1994), blocked the inhibitory effect of FK-506 but not of cyclosporin A on APP overexpression stimulated by PGE2, without producing significant changes in basal APP levels. These data suggest that inhibition of calcineurin by cyclosporin A or FK-506 (Steiner et al., 1997) may underlie the inhibitory effect of these immunosuppressants on APP synthesis stimulated by PGE2 or elevations in cAMP production. Okadaic acid (1 μm), another phosphatase inhibitor, did not suppress APP overexpression stimulated by PGE2 but instead caused astrocytes to detach from the culture dishes, suggesting that okadaic acid may induce toxicity (our unpublished data).
Treatment with PGE2 also increased the levels of GFAP in our cultured astrocytes (this study) and decreased the levels of β-actin mRNA (Lee et al., 1997b), suggesting that these cultured astrocytes resemble reactive astrocytes. GFAP-positive reactive astrocytes exhibited rapid and persistent increases in APP immunoreactivity after brain lesions or ischemia (Siman et al., 1989;Banati et al., 1996). Although the phagocytic activity of astrocytes or microglia can increase APP immunoreactivity (Paresce et al., 1996), our study suggests that GFAP-positive astrocytes can actively upregulate APP synthesis after brain injury. The loss of synapses appears to be an early event in the pathology of AD and is related to the extent of reactive astrogliosis (Brun et al., 1995; Heinonen et al., 1995). The invasion and proliferation of reactive astrocytes within degenerating regions may explain the increased levels of GFAP in the brains and cerebrospinal fluid of AD patients (Wallin et al., 1996). Indeed, the proliferation of astrocytes associated with neurodegeneration in the frontal cortex and hippocampus of AD brains can cause an upregulation of β-adrenergic receptors (Kalaria et al., 1989). Increased circulating levels of norepinephrine after brain injury (Hodges-Savola et al., 1996) may result in the aberrant activation of β-adrenergic receptors coupled to cAMP signaling to stimulate APP overexpression in astrocytes (Lee et al., 1997b). These studies, together with our present finding that activation of PGE2 receptors can stimulate APP synthesis, underscore the contribution of receptor activation in the overproduction of APP.
APP overexpression in cultured astrocytes treated with PGE2was associated with increased secretion of APP holoprotein. Although secreted APP is usually truncated at the C terminus, antisera C8 that is directed at the C terminus of APP (Selkoe et al., 1988) detected increased amounts of APP holoprotein (∼130 kDa) in the media of astrocytes treated with PGE2 for 24 hr. Similarly, Chinese hamster ovary cells overexpressing APP751 also exhibited increased secretion of APP holoprotein (Efthimiopoulos et al., 1996). APP holoprotein is found in human cerebrospinal fluid and can be actively released from secretory vesicles in response to receptor stimulation or neuronal depolarization (Efthimiopoulos et al., 1996). It is not known whether secreted APP holoprotein is reinternalized for subsequent processing or whether it can be metabolized in the extracellular space.
Neuronal damage or amyloid deposits in AD may trigger inflammatory or immune processes and accelerate neuropathology. Epidemiological data suggest that such anti-inflammatory therapies such as nonsteroidal anti-inflammatory drugs or dapsone may be effective in slowing the progression of neuropathology in AD (McGeer and McGeer, 1995). Increased lipid peroxidation and formation of prostaglandins have been detected in AD (Iwamoto et al., 1989; Subbarao et al., 1990).
Our findings show that PGE2 can stimulate GFAP expression, APP synthesis, and the secretion of amyloidogenic APP holoprotein from cultured astrocytes. APP overexpression in cell cultures and in transgenic mice is associated with disorders of the CNS and the production of neurotoxic or amyloidogenic APP fragments (Yoshikawa et al., 1992; Cordell, 1994; Hsiao et al., 1995). We suggest that anti-inflammatory agents (e.g., aspirin or indomethacin), inhibitors of PLA2 (e.g., dexamethasone), or inhibitors of prostaglandin synthase (cyclooxygenase) may prevent the neuropathologies associated with APP overexpression.
Footnotes
This work was supported by National Institutes of Health Grant MH-28783, the Center for Brain Sciences and Metabolism Charitable Trust, and a Deutsche Forschungsgemeinschaft grant (S.K.). Antisera R37 and R98 were gifts from Dr. F. Kametani (Tokyo Institute of Psychiatry). Antisera C8 and APPcDNA were kindly provided by Drs. D. Selkoe and R. Neve, respectively (both from Harvard Medical School). We are grateful to J. P. Shi, J. Breu, and J. Zarach for technical assistance.
Correspondence should be addressed to Dr. Robert K. Lee, Division of Health Sciences and Technology, E25-604, Massachusetts Institute of Technology, Cambridge, MA 02139.
REFERENCES
- 1.Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychiatr Psychol-Gerichtl Med. 1907;64:146–148. [Google Scholar]
- 2.Banati RB, Gehrmann J, Wieβner C, Hossman K-A, Kreutzberg GW. Glial expression of the β-amyloid precursor protein (APP) in global ischemia. J Cereb Blood Flow Metab. 1995;12:257–269. doi: 10.1038/jcbfm.1995.80. [DOI] [PubMed] [Google Scholar]
- 3.Bourbonniére M, Shekarabi M, Nalbantoglu J. Enhanced expression of amyloid precursor protein in response to dibutyryl cyclic AMP is not mediated by the transcription factor AP-2. J Neurochem. 1997;68:909–916. doi: 10.1046/j.1471-4159.1997.68030909.x. [DOI] [PubMed] [Google Scholar]
- 4.Brun A, Liu X, Erikson C. Synapse loss and gliosis in the molecular layer of cerebral cortex in Alzheimer’s disease and in frontal lobe degeneration. Neurodegeneration. 1995;4:171–177. doi: 10.1006/neur.1995.0021. [DOI] [PubMed] [Google Scholar]
- 5.Clemens JA, Stephenson DT, Smalstig EB, Roberts EF, Johnstone EM, Sharp JD, Little SP, Kramer R. Reactive glia express cytosolic phospholipase A2 after transient global forebrain ischemia in the rat. Stroke. 1996;27:527–535. doi: 10.1161/01.str.27.3.527. [DOI] [PubMed] [Google Scholar]
- 6.Cordell B. β-amyloid formation as a potential therapeutic target for Alzheimer’s disease. Annu Rev Pharmacol Toxicol. 1994;34:69–89. doi: 10.1146/annurev.pa.34.040194.000441. [DOI] [PubMed] [Google Scholar]
- 7.Efthimiopoulos S, Vassilacopoulou D, Ripellino JA, Tezapsidis N, Robakis N. Cholinergic agonists stimulate secretion of soluble full-length amyloid precursor protein in neuroendocrine cells. Proc Natl Acad Sci USA. 1996;93:8046–8050. doi: 10.1073/pnas.93.15.8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esch FS, Keim PS, Beattie EC, Blacher RW, Culwell AR, Oltersdorf T, McClure D, Ward PJ. Cleavage of amyloid β peptide during constitutive processing of its precursor. Science. 1990;248:1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- 9.Goetzl EJ, An S, Smith WL. Specificity of expression and effects of eicosanoid mediators in normal physiology and human diseases. FASEB J. 1995;9:1051–1058. doi: 10.1096/fasebj.9.11.7649404. [DOI] [PubMed] [Google Scholar]
- 10.Golde TE, Estus S, Usiak M, Younkin LH, Younkin SG. Expression of β amyloid protein precursor mRNAs: recognition of a novel alternatively spliced form and quantitation in Alzheimer’s disease using PCR. Neuron. 1990;4:253–267. doi: 10.1016/0896-6273(90)90100-t. [DOI] [PubMed] [Google Scholar]
- 11.Heinonen O, Soininen H, Sorvari H, Kosunen O, Paljärvi L, Koivisto E, Riekkinen PJ. Loss of synaptophysin-like immunoreactivity in the hippocampal formation is an early phenomenon in Alzheimer’s disease. Neuroscience. 1995;64:375–384. doi: 10.1016/0306-4522(94)00422-2. [DOI] [PubMed] [Google Scholar]
- 12.Hodges-Savola C, Rogers SD, Ghilardi JR, Timm DR, Mantyh PW. β-adrenergic receptors regulate astrogliosis and cell proliferation in the central nervous system in vivo. Glia. 1996;17:52–62. doi: 10.1002/(SICI)1098-1136(199605)17:1<52::AID-GLIA5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Hsiao KK, Borchelt DR, Olson K, Johannsdottir R, Kitt C, Yunis W, Xu S, Eckman C, Younkin S, Price D, Iadecola C, Clark HB, Carlson G. Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins. Neuron. 1995;15:1203–1218. doi: 10.1016/0896-6273(95)90107-8. [DOI] [PubMed] [Google Scholar]
- 14.Iwamoto N, Kobayashi K, Kosaka K. The formation of prostaglandins in the postmortem cerebral cortex of Alzheimer-type dementia patients. J Neurol. 1989;236:80–84. doi: 10.1007/BF00314401. [DOI] [PubMed] [Google Scholar]
- 15.Kalaria RN, Andorn AC, Tabaton M, Whitehouse PJ, Harik SI, Unnerstall JR. Adrenergic receptors in aging and Alzheimer disease: increased β2-receptors in prefrontal cortex and hippocampus. J Neurochem. 1989;53:1772–1781. doi: 10.1111/j.1471-4159.1989.tb09242.x. [DOI] [PubMed] [Google Scholar]
- 16.mcKametani F, Tanaka K, Ishii T, Ikeda S, Kennedy HE, Allsop D. Secretory form of Alzheimer amyloid precursor protein 695 in human brain lacks β/A4 amyloid immunoreactivity. Biochem Biophys Res Commun. 1993;191:392–398. doi: 10.1006/bbrc.1993.1230. [DOI] [PubMed] [Google Scholar]
- 17.Lee RKK, Wurtman RJ, Cox AJ, Nitsch RM. Amyloid precursor protein processing is stimulated by metabotropic glutamate receptors. Proc Natl Acad Sci USA. 1995;92:8083–8087. doi: 10.1073/pnas.92.17.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee RKK, Knapp S, Zarach J, Wurtman RJ. Prostaglandin E2 stimulates overexpression of amyloid precursor protein in astrocyte cultures: inhibition by immunosuppressants. J Neurochem [Suppl] 1997a;69:S103B. [Google Scholar]
- 19.Lee RKK, Araki W, Wurtman RJ. Stimulation of amyloid precursor protein synthesis by adrenergic receptors coupled to cAMP formation. Proc Natl Acad Sci USA. 1997b;94:5422–5426. doi: 10.1073/pnas.94.10.5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marks AR. Cellular functions of immunophilins. Physiol Rev. 1996;76:631–649. doi: 10.1152/physrev.1996.76.3.631. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGeer P, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 23.Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992;258:304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- 24.Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer’s disease amyloid β-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 25.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 26.Salbaum JM, Weidemann A, Lemaire H-G, Masters CL, Beyreuther K. The promoter of Alzheimer’s disease amyloid A4 precursor gene. EMBO J. 1988;7:2807–2813. doi: 10.1002/j.1460-2075.1988.tb03136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwaninger M, Blume R, Krüger M, Lux G, Oetjen E, Knepel W. Involvement of the Ca2+-dependent phosphatase calcineurin in gene transcription that is stimulated by cAMP through cAMP response elements. J Biol Chem. 1995;270:8860–8866. doi: 10.1074/jbc.270.15.8860. [DOI] [PubMed] [Google Scholar]
- 28.Selkoe DJ, Podlisny MB, Joachim CL, Vickers EA, Lee G, Fritz LC, Oltersdorf T. β-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane associated proteins in neural and nonneuronal tissues. Proc Natl Acad Sci USA. 1988;85:7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shekarabi M, Bourbonniere M, Dagenais A, Nalbantoglu J. Transcriptional regulation of amyloid precursor protein during dibutyryl cyclic AMP-induced differentiation of NG108-15 cells. J Neurochem. 1997;68:970–978. doi: 10.1046/j.1471-4159.1997.68030970.x. [DOI] [PubMed] [Google Scholar]
- 30.Siman R, Card JP, Nelson RB, Davis LG. Expression of β-amyloid precursor protein in reactive astrocytes following neuronal damage. J Neurosci. 1989;3:275–285. doi: 10.1016/0896-6273(89)90252-3. [DOI] [PubMed] [Google Scholar]
- 31.Sisodia SS, Koo EH, Beyreuther K, Unterbeck A, Price D. Evidence that β-amyloid protein in Alzheimer’s disease is not derived by normal processing. Science. 1990;248:492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- 32.Slack BE, Breu J, Petryniak MA, Srivastava K, Wurtman RJ. Tyrosine phosphorylation-dependent stimulation of amyloid precursor protein secretion by the m3 muscarinic acetylcholine receptor. J Biol Chem. 1995;270:8337–8344. doi: 10.1074/jbc.270.14.8337. [DOI] [PubMed] [Google Scholar]
- 33.Slunt HH, Thinakaran G, Koch C, Lo ACY, Tanzi RE, Sisodia SS. Expression of a ubiquitous, cross-reactive homologue of the mouse β-amyloid precursor protein (APP). J Biol Chem. 1994;269:2637–2644. [PubMed] [Google Scholar]
- 34.Solá C, Garcia-Ladona FJ, Mengod G, Probst A, Frey P, Palacios JM. Increased levels of the Kunitz protease inhibitor-containing βAPP mRNAs in rat brain following neurotoxic damage. Mol Brain Res. 1993;17:41–52. doi: 10.1016/0169-328x(93)90071-v. [DOI] [PubMed] [Google Scholar]
- 35.Steiner JP, Connolly MA, Valentine HL, Hamilton GS, Dawson TM, Hester L, Snyder SH. Neurotrophic actions of nonimmunosuppressive analogues of immunosuppressive drugs FK506, rapamycin and cyclosporin A. Nat Med. 1997;3:421–428. doi: 10.1038/nm0497-421. [DOI] [PubMed] [Google Scholar]
- 36.Stephenson DT, Lemere CA, Selkoe DJ, Clemens JA. Cytosolic phospholipase A2 (cPLA2) immunoreactivity is elevated in Alzheimer’s disease brain. Neurobiol Dis. 1996;3:51–63. doi: 10.1006/nbdi.1996.0005. [DOI] [PubMed] [Google Scholar]
- 37.Subbarao KV, Richardson JS, Ang LC. Autopsy samples of Alzheimer’s cortex show increased peroxidation in vitro. J Neurochem. 1990;55:342–345. doi: 10.1111/j.1471-4159.1990.tb08858.x. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka S, Shiojiri S, Takahashi Y, Kitaguchi N, Ito H, Kameyama M, Kimura J, Nakamura S, Ueda K. Tissue-specific expression of three types of β-protein precursor mRNA: enhancement of protease inhibitor-harboring types in Alzheimer’s disease brains. Biochem Biophys Res Commun. 1989;165:1406–1414. doi: 10.1016/0006-291x(89)92760-5. [DOI] [PubMed] [Google Scholar]
- 39.Ulus IH, Wurtman RJ. Metabotropic glutamate receptor agonists increase release of soluble amyloid precursor protein derivatives from rat brain cortical and hippocampal slices. J Pharmacol Exp Ther. 1997;281:149–154. [PubMed] [Google Scholar]
- 40.Wallin A, Blennow K, Rosengren LE. Glial fibrillary acidic protein in the cerebrospinal fluid of patients with dementia. Dementia. 1996;7:267–272. doi: 10.1159/000106891. [DOI] [PubMed] [Google Scholar]
- 41.Weidemann A, König G, Bunke D, Fischer P, Salbaum JM, Masters CL, Beyreuther K. Identification, biogenesis and localization of precursors of Alzheimer’s disease A4 amyloid protein. Cell. 1989;57:115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- 42.Yoshikawa K, Aizawa T, Hayashi Y. Degeneration in vitro of post-mitotic neurons overexpressing the Alzheimer amyloid protein precursor. Nature. 1992;359:64–67. doi: 10.1038/359064a0. [DOI] [PubMed] [Google Scholar]