Abstract
Hypocretin (orexin) has recently been shown to increase feeding when injected into the brain. Using both rat and primate brains, we tested the hypothesis that a mechanism of hypocretin action might be related to synaptic regulation of the neuropeptide Y (NPY) system. Hypocretin-immunoreactive terminals originating from the lateral hypothalamus make direct synaptic contact with neurons of the arcuate nucleus that not only express NPY but also contain leptin receptors. In addition, hypocretin-containing neurons also express leptin receptor immunoreactivity. This suggests a potential mechanism of action for hypocretin in the central regulation of metabolic and endocrine processes. The excitatory actions of hypocretin could increase NPY release, resulting in enhanced feeding behavior and altered endocrine regulation, whereas leptin, released from adipose tissue as an indicator of fat stores, would have the opposite effect on the same neurons, leading to a decrease in NPY and NPY-mediated hypothalamic functions. On the other hand, the innervation of hypocretin cells by NPY boutons raises the possibility that NPY may exert an effect on hypothalamic functions, at least in part, via mediation or feedback action on these lateral hypothalamic cells. Our data indicate that a direct interaction between leptin, hypocretin, and NPY exists in the hypothalamus that may contribute to the central regulation of metabolic and endocrine processes in both rodents and primates.
Keywords: hypocretin, orexin, neuropeptide Y, leptin receptor, synapse, hypothalamus, feeding, endocrine regulations
Destruction of distinct hypothalamic regions, particularly the ventromedial nucleus (VMH) but also the paraventricular and dorsomedial nucleus, induces hyperphagia (Brobeck, 1946; Anand and Brobeck, 1951; Powley et al., 1980; Aravich and Scalfani, 1983; Weingarten et al., 1985; Tokunaga et al., 1986;Bernadis and Berlinger, 1987). In contrast, discrete lesions placed in the lateral hypothalamus (Powley and Keesey, 1970; van den Pol, 1982) reduce food intake. During the last two decades, a substantial amount of feeding research has focused on a hypothalamic peptide, neuropeptide Y (NPY); NPY administered into the cerebral ventricles (Clark et al., 1984) or different hypothalamic sites (Stanley et al., 1985) induces food intake. Although several other hypothalamic peptides in addition to NPY were found to affect appetite and feeding behavior (for review, see Kalra, 1997; Flier and Maratos-Flier, 1998), the exact signaling modality that underlies the regulation of energy homeostasis is ill-defined. The recent revelation of the existence of a previously unknown hypothalamic peptide, hypocretin (de Lecea et al., 1998), also called orexin (Sakurai et al., 1998), which increased feeding in a manner comparable with NPY (Sakurai et al., 1998), added another layer of complexity in the interaction among hypothalamic peptidergic systems in the regulation of appetite, feeding behavior, and metabolism in general (Flier and Maratos-Flier, 1998).
Genetic mouse or rat mutants, including db/db and ob/ob mice and fa/fa rats, become strikingly obese. Molecular analysis has shown that the primary genetic defect in these animals relates to either abolished leptin production (ob/ob mice) or impaired leptin receptors (leptin-R) (db/db mice; fa/fa rats) (Campfield et al., 1995; Halaas et al., 1995;Pelleymounter et al., 1995; Leibel et al., 1997). Similar examples of obesity in humans have been found and are associated with leptin receptor mutation (Clement et al., 1998). Leptin is released by adipose tissue and has been suggested to be a key vascular signal carrying information about fat stores. Leptin receptors are found in the hypothalamus, particularly in the arcuate nucleus where leptin is thought to exert its primary feedback signaling (Mercer et al., 1996b;Schwartz et al., 1996b; Elmquist et al., 1998; Hakansson et al., 1998;Yarnell et al., 1998). During food deprivation when leptin levels rapidly decline (Saladin et al., 1995), hypothalamic NPY production is elevated (Sahu et al., 1988), suggesting that leptin levels modulate the activity of NPY neurons. Furthermore, NPY may be an effector neuron responding to changes in leptin levels; the obesity of leptin-deficient mice is reduced by elimination of NPY (Erickson et al., 1996).
The excitatory nature of hypocretin (de Lecea et al., 1998; van den Pol et al., 1998), its distinct distribution in neuronal perikarya of the lateral hypothalamus–perifornical region (de Lecea et al., 1998; Sakurai et al., 1998), its abundance of axon terminals in the arcuate nucleus (van den Pol et al., 1998), and its effect similar to that of NPY in enhancing feeding lead us to test the hypothesis that the hypocretin system may interact with the NPY system and serve as a stimulator of the NPY-producing cells in the regulation of hypothalamic mechanisms.
MATERIALS AND METHODS
Animals
Rats. Adult female and male Sprague Dawley rats (200–250 gm body weight) were used in this experiment. Animals were kept under standard laboratory conditions, with tap water and regular rat chow available ad libitum, on a 12 hr light/dark cycle. Twenty-four hours before being killed, a group of animals (n = 6) under deep Ketamine (75 mg/kg) anesthesia was fixed in a stereotaxic apparatus (David Kopf Instruments), and by the use of a Hamilton microsyringe, a single injection of colchicine (80 μg in 20 μl of saline) was placed into the lateral ventricle to enable perikaryal labeling of NPY. Rats were killed under ether anesthesia by transaortic perfusion with 50 ml of heparinized saline followed by 250 ml of fixative. The fixative consisted of 4% paraformaldehyde, 15% saturated picric acid, and 0.08% glutaraldehyde in 0.1% sodium phosphate buffer, pH 7.4 (PB). Brains were dissected out, and 3-mm-thick coronal blocks containing the hypothalamus were post-fixed for an additional 1–2 hr in glutaraldehyde-free fixative. Fifty-micrometer-thick sections were cut on a vibratome. Sections were rinsed in 1% sodium borohydride in PB for 15 min to eliminate unbound aldehydes.
Monkeys. Adult (3.5–4.0 kg) female and male African green monkeys (Cercopithecus aethiops; n = 4) and female rhesus macaques (Macacca fascicularis;n = 2) were used. To reduce the number of primates used for our general research, a number of other investigators used other regions of the brain or endocrine system for nonrelated experiments. The primate tissue was collected under animal protocols approved by the Yale University Committee on Animal Research. Procedures described below were performed while the animals were under deep ketamine anesthesia. One African green monkey received colchicine into the lateral cerebral ventricle (560 μg in 200 μl of saline). Monkeys were killed by a transcardial perfusion of 500 ml of heparinized saline (0.9%) followed by 2 l of fixative consisting of 4% paraformaldehyde, 15% saturated picric acid, and 0.08% glutaraldehyde in 0.1 m PB. The mediobasal hypothalamus was dissected out and post-fixed for an additional 1.5 hr in glutaraldehyde-free fixative. Tissue blocks were washed and stored in 0.1 m PB at 4°C. Fifty micrometer coronal sections were cut on a vibratome (Lancer, St. Louis, MO). After several rinses in PB, sections were washed for 20 min in 1% sodium borohydride in PB to eliminate unbound aldehydes. Sections processed for electron microscopy were freeze–thaw treated in 10% sucrose and PB in liquid nitrogen to increase permeability of the antibodies.
Light and electron microscopic single immunostaining
Hypothalamic sections were immunostained for hypocretin, NPY, or leptin-R. Sections were incubated in one of the primary antisera [rabbit anti-hypocretin (1:2000); rabbit anti-NPY (1:14,000); or goat anti-leptin-R (1:1000)] for 24 hr at room temperature (r.t.). After this, the material was incubated in the appropriate secondary antibody (biotinylated goat anti-rabbit IgG for hypocretin and NPY and biotinylated horse anti-goat IgG for leptin-R; 1:250 in PB; Vector Laboratories, Burlingame, CA) followed by avidin–biotin peroxidase (ABC; 1:50 in PB; ABC Elite Kit; Vector Laboratories), both for 2 hr at r.t. The tissue-bound peroxidase was visualized by a diaminobenzidine reaction (15 mg of DAB and 165 μl of 0.3% H2O2 in 30 ml of PB) for 10 min at r.t. Between each incubation step, sections were thoroughly washed (four times for 10 min each) in PB. Sections for light microscopy were mounted on gelatin-coated slides, dehydrated, cleared in xylene, and coverslipped in Permount. For electron microscopy, sections were osmicated (1% OsO4 in PB) for 30 min, dehydrated through increasing ethanol concentrations (using 1% uranyl acetate in the 70% ethanol for 30 min), and flat-embedded in araldite between liquid release-coated (Electron Microscopy Sciences, Fort Washington, PA) slides and coverslips. After capsule embedding, blocks were trimmed, and ribbons of serial ultrathin sections were collected on Formvar-coated single slot grids and examined using a Philips CM-10 electron microscope.
Light and electron microscopic double immunostaining
Light microscopic double immunostaining for hypothalamic hypocretin plus NPY, leptin-R, or β-endorphin and NPY plus hypocretin was performed according to the following protocol. Sections were immunostained for either hypocretin or NPY using the protocol described above. The first immunoreaction was visualized with a modified version of the nickel–diaminobenzidine (Ni–DAB) reaction (15 mg of DAB; 0.12 mg of glucose oxidase; 12 mg of ammonium chloride; 600 μl of 0.05m nickel ammonium sulfate; and 600 μl of 10% β-d-glucose in 30 ml of PB) for 10–30 min at r.t., resulting in a dark blue reaction product. After several rinses in PB, the sections were further incubated in either rabbit anti-NPY, rabbit anti-hypocretin, goat anti-leptin-R, or rabbit anti-β-endorphin (1:1000; Chemicon, Temecula, CA) antisera for 24 hr at 4°C, followed by secondary antibody (goat anti-rabbit IgG for NPY, hypocretin and β-endorphin or horse anti-goat IgG for leptin-R; all diluted 1:50 in PB) and rabbit or goat peroxidase–anti-peroxidase (PAP; 1:100 in PB), both steps for 2 hr at r.t. Between each incubation step, the sections were rinsed (four times for 10 min each) in PB. The tissue-bound peroxidase was visualized by a DAB reaction (see above), resulting in a light brown reaction product. The colors of the two reaction products were easily distinguishable, and in single-stained material in which one of the primaries was omitted, only one color was found. After immunostaining, the sections were thoroughly rinsed in PB and processed for correlated electron microscopy as described below.
Light and electron microscopic triple immunostaining
Sections were first incubated for 24 hr at room temperature with a mixture of the hypocretin and leptin-R antisera for 48 hr at 4°C. After several washes in PB, sections were incubated in biotinylated secondary antisera (biotinylated anti-rabbit IgG for hypocretin and biotinylated anti-goat IgG for leptin-R; 1:250 in PB; Vector Laboratories) for 2 hr at r.t. This was followed by a 2 hr incubation at r.t. in avidin–biotin peroxidase (1:50 in PB; ABC Elite Kit; Vector Laboratories), and the tissue-bound peroxidase was visualized by a Ni–DAB reaction, resulting in a dark blue to black color. Subsequently, after several rinses in PB, sections were immunostained further for NPY as described above using the PAP method, and the tissue-bound peroxidase was visualized by a DAB reaction to give a light brown reaction product. After visualization of tissue antigens, sections were wet-mounted in PB and examined under the light microscope. Color photographs and images were taken of hypocretin-immunoreactive boutons making putative contact on NPY-immunoreactive cells that contained leptin-R (Figs. 7, 8). Sections were then osmicated, dehydrated, and embedded in araldite (see above). Blocks were trimmed using the color picture of previously identified cells and boutons as a guide. Ribbons of serial ultrathin sections were collected on Formvar-coated single slot grids and examined under the electron microscope.
Fig. 7.
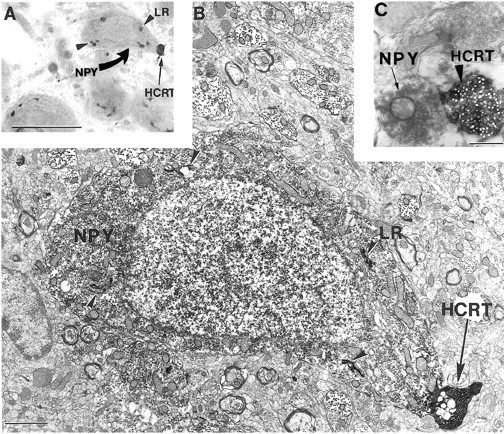
A hypocretin axon contacts a neuropeptide Y neuron containing leptin receptor in the rat arcuate nucleus. A, B, Correlated light (A; see color on Fig. 3P) and electron (B) micrographs demonstrating that in the rat arcuate nucleus, a black hypocretin (HCRT)-labeled axon terminal (black arrow on A) contacts (black arrow on B) a light brownneuropeptide Y (NPY)-immunoreactive perikaryon (curved yellow arrow on A) that containsblack Golgi-associated leptin receptor (LR) labeling (arrowheads onA, B). An unlabeled glial cell is seen to the left of the NPY-immunopositive neuron. C, Electron micrograph illustrating the distinct difference between the ultrastructural appearance of NPY(arrow) and HCRT(arrowhead) immunoreactivity. Scale bars:A, 10 μm; B, 1 μm; C, 0.5 μm.
Fig. 8.
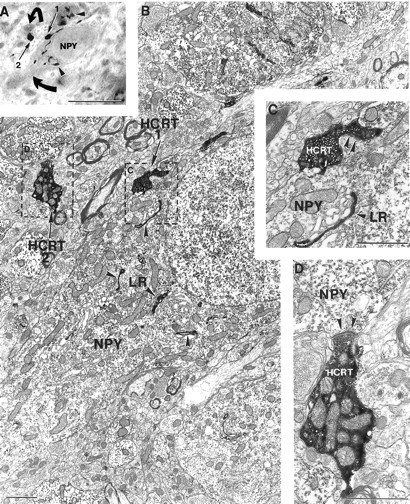
Hypocretin axons in synaptic contact with neuropeptide Y neurons containing leptin receptor in the monkey arcuate nucleus. A–D, Correlated light (A; see color on Fig. 3Q) and electron (B–D) micrographs demonstrating that in the monkey arcuate nucleus, two (arrows labeled1 and 2 on A,B) black-colored hypocretin (HCRT) axon terminals are in close apposition tolight stained neuropeptide Y (NPY)-containing neurons (curved arrows on A) HCRT axon 1 contacts a light NPY perikaryon that also expresses Golgi-associated black leptin receptor (LR) labeling (arrowheads onA–C). C and D are high-power magnifications of the boxed regions onB demonstrating that both HCRT boutons 1 and 2 establish asymmetric synaptic contacts (arrowheadson C, D) on the NPY- and leptin receptor–containing cell body (C) and on the NPY-containing dendrite (D). Note the abundance of mitochondria in theHCRT-containing axon terminal on D. Scale bars: A, 10 μm; B–D, 1 μm.
To test the validity of the triple labeling, we conducted experiments in which one or two of the primary antibodies were replaced with normal serum. Overlaps between the different immunostainings were not seen. More detailed descriptions of these control experiments and protocols describing triple labeling can be found in previous reports (Horvath et al., 1992a,b,c, 1993, 1995; Horvath, 1997, 1998).
Antisera
Rabbit antiserum against NPY was obtained from Peninsula Laboratories (Belmont, CA; lot #029078-11). This affinity-purified polyclonal antiserum was generated against porcine NPY and has been shown to have no cross-reaction with any other known hypothalamic peptides. Adsorption of the antiserum with NPY blocked immunostaining. We have used this antiserum extensively for the light and electron microscopic visualization of NPY in both rodents and monkeys (Horvath et al., 1992c, 1993, 1996).
Antiserum against leptin-R was purchased from Santa Cruz Biotechnology [Santa Cruz, CA; Ob-R (M-18); catalog #sc-1834]. This antiserum is an affinity-purified goat polyclonal antiserum raised against a peptide corresponding to amino acids 877–894 mapping at the C terminal of Ob-R of mouse origin. This antiserum has been tested extensively byHakansson et al. (1998) and was found to bind to both the short and long isoforms of leptin-R in transfected cells. In preparation for the present study, we compared the distribution pattern of leptin-R–immunoreactive cells in the rat and monkey arcuate nucleus using four different antisera generated against different portions of the mouse and human leptin-R. Each of these antisera was from Santa Cruz Biotechnology (M-18; K-20; N-20; C-20). Although the distribution pattern and the number of labeled cells were similar using all four antisera, the clearest and most distinctive labeling was achieved by the M-18 antiserum. Adsorption of the antiserum with the target peptide blocked immunostaining (Hakansson et al., 1998;Yarnell et al., 1998). The M-18 antiserum was also tested in Western blot analysis. Rats were killed by decapitation. The hypothalamus was removed and homogenized in lysis buffer containing 50 mmTris-HCl, pH 7.5, 50 mm MgCl2, 5 mm EGTA, 0.25% Triton X-100, and protease inhibitors (proteinase inhibitor cocktail tablets; Boehringer Mannheim, Indianapolis, IN) for the protein isolation. The homogenated tissues were centrifuged at 190,000 × g for 1 hr at 4°C. The resulting supernatant was normalized for total protein using the bicinchoninic acid assay (BCA Protein Assay; Pierce, Rockford, IL). Coomassie-stained SDS-polyacrylamide gels were used routinely to evaluate the concentration and quality of the extracts. Western blots were performed using 10% SDS-polyacrylamide gels run on a minigel apparatus; 30 μg of protein was loaded per lane. The gels were transferred to polyvinylidene fluoride (PDVF; Millipore, Bedford, MA) membranes by electroblotting overnight (30 V). The filters were blocked in 5% nonfat dry milk and 0.1% Tween 20 for 1 hr at room temperature. Blots were then incubated with rabbit anti-leptin-R diluted in TBS Tween 20 (TTBS; 20 mm Tris and 137 mm NaCl, pH 7.6) for 1 hr at r.t. Membranes were washed three times for 10 min each in the same buffer and were incubated for 1 hr with horseradish peroxidase–conjugated rat anti-goat IgG (Vector Laboratories) diluted 1:10,000 in TTBS. Subsequently, the blots were washed five times for 10 min each in the same buffer. Immunoreactive proteins were revealed using the enhanced chemiluminescence method (ECL; Amersham, Arlington Heights, IL). This analysis revealed multiple bands at ∼120 and 130 kDa (short isoforms) and ∼200 kDa (see Fig.1F2) and faint minor bands below 120 kDa. The 200 kDa bands correspond to the appearance of the long isoform in Western blot analysis (Bjorbaek et al., 1997; Ghilardi and Skoda, 1997).
Fig. 1.
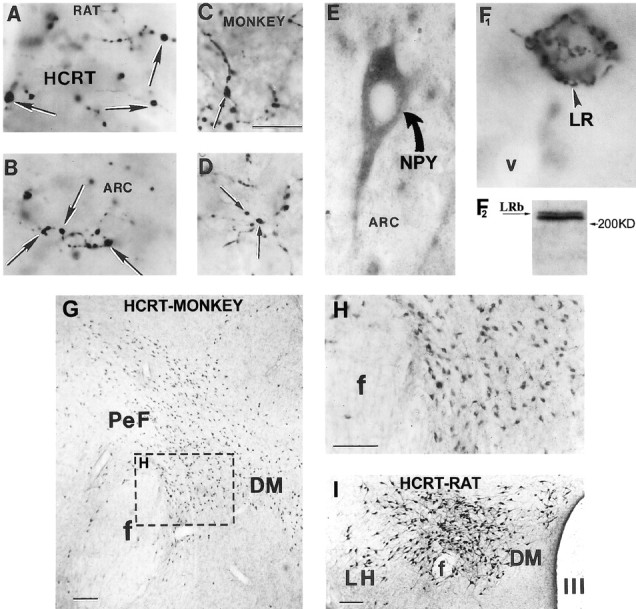
Hypocretin, neuropeptide Y, pro-opiomelanocortin, and leptin receptor immunoreactivity in the rat and monkey arcuate nucleus. A–D, Hypocretin (HCRT)-immunopositive fibers and boutons (black arrows) in the rat (A,B) and monkey (C, D) arcuate nucleus (ARC). E, Neuropeptide Y (NPY)-immunoreactive cell (curved arrow) and processes in the arcuate nucleus of a monkey.F1, Leptin receptor (LR) immunoreactivity (arrowhead) in a cell of the rat arcuate nucleus.F2, A representative Western blot of hypothalamic tissue showing distinct bands labeled with the LR antiserum at ∼200 kDa. LRb, Long leptin receptor isoform.G–I, Neurons immunoreactive for hypocretin (HCRT) in the perifornical region (PeF) of a monkey (G, H) and a rat (I). H, High magnification of the boxed area on G.f, Fornix; DM, dorsomedial hypothalamic nucleus; LH, lateral hypothalamus; III, third ventricle. Scale bars (in C andG–I): 5 μm (A–F1); 100 μm.
Hypocretin antisera were made against the neuroactive peptide hypocretin 2 (orexin B; van den Pol et al., 1998). Hypocretin 2 was conjugated to keyhole limpet hemocyanin with glutaraldehyde and injected into three albino rabbits. Each of the three rabbits made antisera that stained the same cells in the lateral hypothalamus–perifornical area. Omission of the primary antisera resulted in no staining. Preadsorption of the antisera with the antigen blocked immunostaining. The pattern of cell body immunostaining was similar to that seen with two antisera made against different antigens, the C-terminal inactive fragment of preprohypocretin and the entire preprohypocretin sequence (de Lecea et al., 1998). The cells stained with the antisera used in the present study appeared to be the same cells labeled by in situ hybridization with a preprohypocretin mRNA probe (Gautvik et al., 1996; de Lecea et al., 1998).
The antiserum against β-endorphin has been characterized elsewhere (Mezey et al., 1985). We have used this antiserum extensively for the light and electron microscopic visualization of hypothalamic opiate cells (Horvath et al., 1992a,b,c, 1995).
RESULTS
Single immunolabeling for hypocretin, NPY, and leptin receptor
Hypocretin in the hypothalamus
The overall pattern of hypothalamic hypocretin immunolabeling in monkey corresponds to what we find in the rat, which has been described previously (de Lecea et al., 1998; Sakurai et al., 1998) (Fig.1). Hypocretin-immunoreactive perikarya were similar in both primate species to that found in the rat; labeled cells were present in the lateral hypothalamus–perifornical region and, to a lesser extent, in the dorsomedial hypothalamus (Fig.1G–I). The projection field of primate hypocretin neurons within the hypothalamus was also similar to that of the rat. Interestingly, although hypocretin-immunoreactive axons were found in the VMH, they did not show the abundance of terminal boutons found in the arcuate nucleus.
To compare the relative density of hypocretin-immunoreactive axons, we used a stereological analysis of intersections of immunoreactive axons in sections with a test grid composed of square grid lines 20 micrometers apart. We placed this grid in random orientations in six regions (arcuate, ventromedial, dorsomedial, and paraventricular nuclei; lateral hypothalamus; and different layers of the motor cortex) of three monkey brains to determine the relative axon density of hypocretin-immunoreactive axons in the different regions. The most abundant network of hypocretin-containing axons was present in the arcuate nucleus. When compared with other feeding-related hypothalamic sites, the number of axonal processes in the arcuate nucleus was more than twice that found in areas within the hypothalamus including the ventromedial nucleus, the lateral hypothalamus–perifornical region, the dorsomedial nucleus, and the paraventricular nucleus (Fig.2). Although a small number of hypocretin axons were found in the cortex, the density was <2% of the density in the arcuate nucleus (Fig. 2). Thus, of the areas studied, the innervation of the arcuate nucleus appeared to be the highest. We also examined the number of boutons in the same areas (Fig. 2) and found that within the test region (square with 100 μm edge), on one focal plane, the highest number of boutons could be found in the arcuate nucleus (253 ± 62) followed by the dorsomedial nucleus (68 ± 18), the paraventricular nucleus (51 ± 13), the lateral hypothalamus (47 ± 16), the ventromedial nucleus (23 ± 8), and the cortex (3 ± 3 SEM).
Fig. 2.
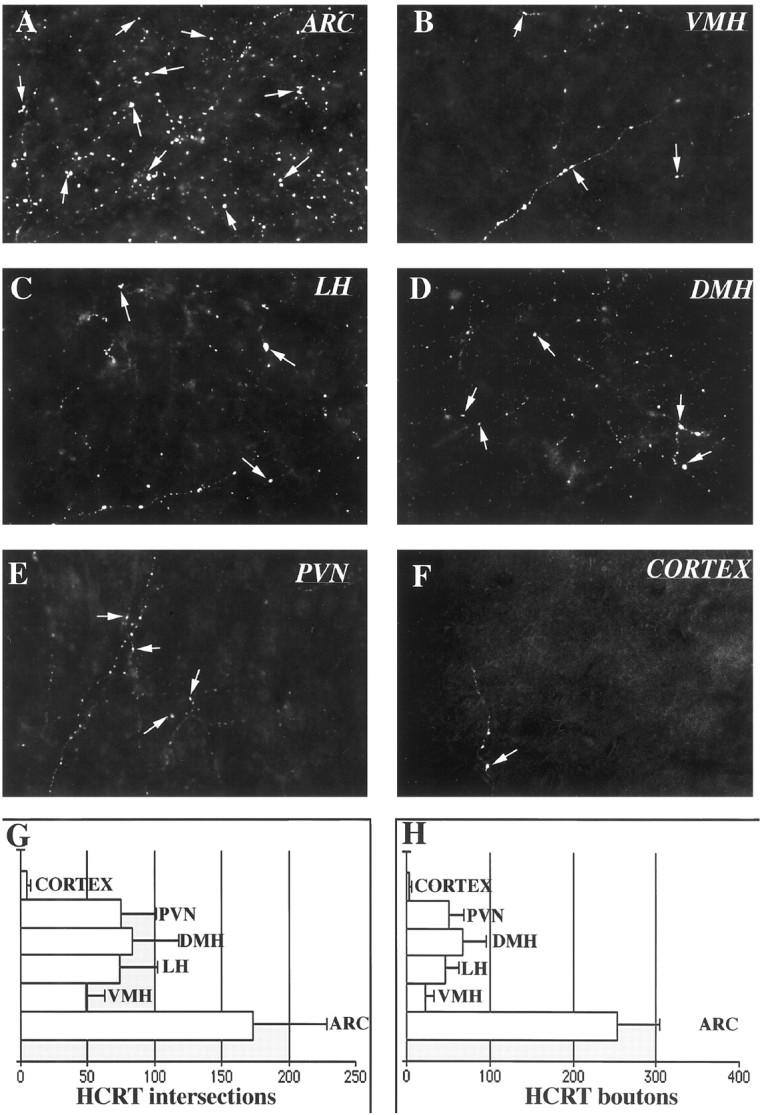
Hypocretin bouton density. These micrographs show the relative bouton density of hypocretin-immunoreactive axons (white arrows) in six different brain regions of the monkey. Additional boutons were found in other planes of focus.A, Arcuate nucleus (ARC).B, Ventromedial nucleus (VMH).C, Lateral hypothalamus (LH).D, Dorsomedial nucleus (DMH).E, Paraventricular nucleus (PVN).F, Cortex. Width of each micrograph, 200 μm.G, Axon intersections with test grid (mean of three counts in each of three monkeys. H, Hypocretin (HCRT)-immunoreactive boutons per test square that is 100 μm per side at one plane of focus. Error bars indicate SEM.
In both rats and monkeys, colchicine treatment increased the staining intensity but not the overall distribution pattern of hypocretin perikarya. In both rats and monkeys, in the electron microscope, hypocretin-immunoreactive axons were found to establish predominantly asymmetric synaptic contacts on cell bodies, proximal and distal dendrites, and dendritic spines in the arcuate nucleus (see Figs.4-8). Frequently, hypocretin axons that established synaptic contacts with dendrites or cell bodies were in direct lateral apposition to other unlabeled boutons that were also synapsing on the same postsynaptic element (see Fig.4C,D,F). Such axoaxonic contacts were observed between hypocretin-immunoreactive boutons as well (see Fig. 4E). In the lateral hypothalamus, hypocretin-immunoreactive axons made synaptic contact with perikarya that were also immunoreactive for hypocretin, suggesting that hypocretin cells send recurrent collaterals to other hypocretin cells, a possible substrate for orchestration of cellular behavior.
Fig. 4.
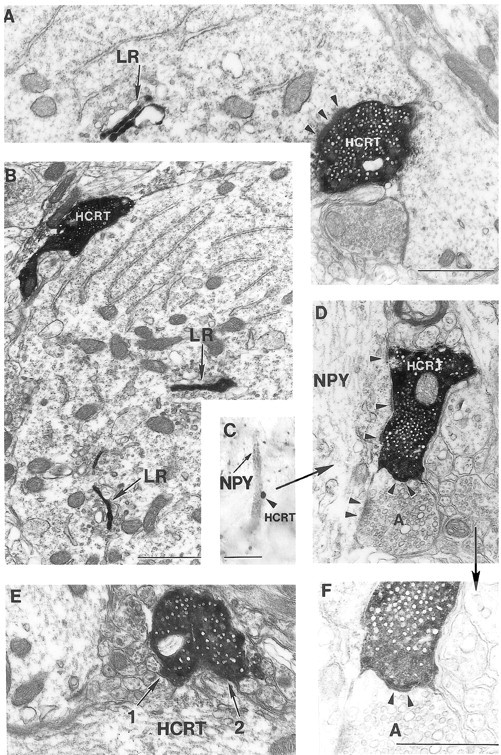
Synaptic interaction between hypocretin boutons and leptin receptor– or neuropeptide Y–producing neurons in the arcuate nucleus. A, B, Hypocretin (HCRT)-containing axon terminals establishing asymmetric (arrowheads on A) synaptic contacts with neuronal perikarya expressing leptin receptor (LR) immunolabeling associated with Golgi cisternae (black arrows) in the rat (A) and monkey (B) arcuate nucleus. C,D, F, Correlated light (C, see color on Fig. 3K) and electron (D, F) micrographs demonstrating that the black HCRT axon terminal (arrowhead on C) in contacting an NPY-containing dendrite establishes an asymmetric synapse (arrowheads on D) on thisNPY dendrite. On D, an unlabeled axon (A) next to the HCRT-labeled bouton also makes an asymmetric synapse on the same postsynaptic target. F is an underexposed, higher magnification ofD revealing the intimate relationship between theHCRT-labeled and unlabeled (A) presynaptic terminals. Note the apparent membrane specialization between these boutons (arrowheads). E, Close apposition between two HCRT-immunolabeled axon terminals in the rat arcuate nucleus. Scale bars: C, 5 μm; A, D, E, 1 μm;B, F, 1 μm.
Fig. 5.
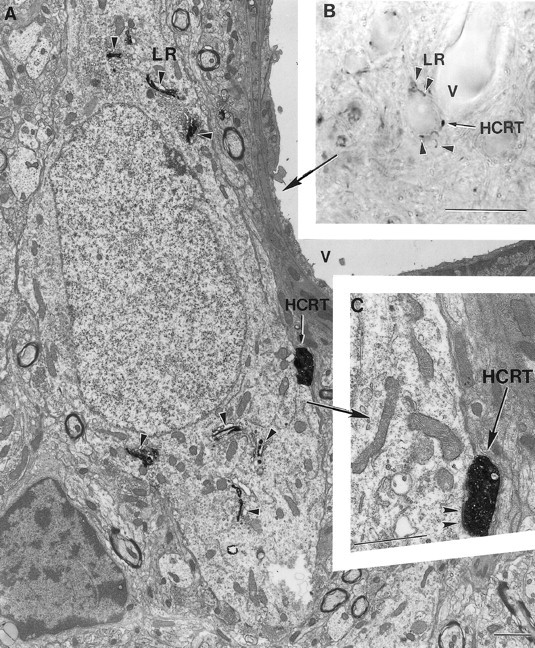
A hypocretin axon innervates a leptin receptor–containing neuron of a rat. A–C, Correlated light (B) and electron (A,C) micrographs of a leptin receptor (LR)-containing (arrowheads onA, B) neuron in close proximity to a capillary vessel (V on A,B) and a hypocretin (HCRT)-labeled bouton (black arrows on A–C).C is a high-power magnification electron micrograph showing that the HCRT bouton indicated onA and B establishes synaptic contact (arrowheads on C) on theLR-containing perikaryon. Scale bars: B, 10 μm; A, C, 1 μm.
Fig. 6.
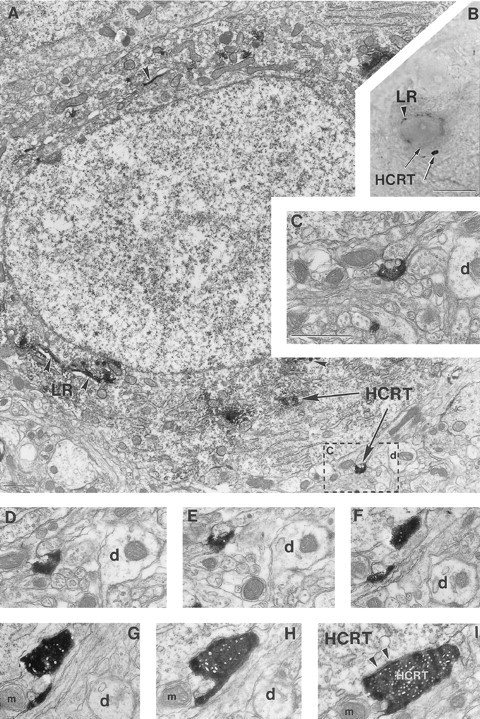
A hypocretin axon innervates a hypocretin neuron containing leptin receptor. A–I, Correlated light (B) and electron (A,C–I) micrographs demonstrating that a neuron in the perifornical region of a female rat contains homogeneously distributed cytoplasmic immunoperoxidase (HCRT immunoreactivity;black arrows on B) that is associated with the endoplasmic reticulum and ribosomes (black arrows on A), contains Golgi-associated leptin receptor (LR) labeling (arrowheads onA, B), and is in close proximity to an axon terminal that contains HCRT immunolabeling (black arrows on A, B).C is a high-power magnification of the boxed area on A. Consecutive serial ultrathin sections of this axon (D–I) reveal a synaptic membrane specialization between this HCRT bouton andHCRT perikaryon (arrowheads onI). d and/or mindicate the same dendrite (d) and/or mitochondrion (m) for orientation. Scale bars:B, 10 μm; A, 1 μm;C–I, 1 μm.
Leptin receptor in the arcuate nucleus and perifornical area of the rat and monkey
Immunostaining for leptin-R, using the antiserum corresponding to amino acids 877–894 mapping at the C terminal of mouse leptin-R, showed the same pattern of distribution in the arcuate nucleus (Fig.1F1) reported previously by Hakansson et al. (1998)using the same antiserum. As reported previously (Hakansson et al., 1998; Yarnell et al., 1998), leptin-R-like immunoreactivity was widely distributed in the monkey and rat brain including the choroid plexus, cerebral cortex, hippocampus, thalamus, and hypothalamus. In the hypothalamus, leptin-R-like immunostaining was the strongest in the arcuate nucleus and the parvicellular division of the paraventricular nucleus and dorsomedial nucleus. Immunoreactivity was also found in the periventricular area, preoptic area, and VMH. A group of cells in the lateral hypothalamus–perifornical region also showed leptin-R-like labeling (Fig.3N,O). The immunolabeling in many hypothalamic cells associated with the Golgi apparatus suggests a high level of leptin-R synthesis (Figs. 3N, 4-8) or an affinity of the antiserum to leptin-R or a precursor during its processing in the Golgi apparatus (Diano et al., 1998a). Western blot analysis of rat hypothalamic tissue confirmed the conclusions ofHakansson et al. (1998) that the leptin-R antiserum (M-18; Santa Cruz Biotechnology) recognizes both the short and long leptin-R isoforms (see Materials and Methods; Fig. 1F2).
Fig. 3.
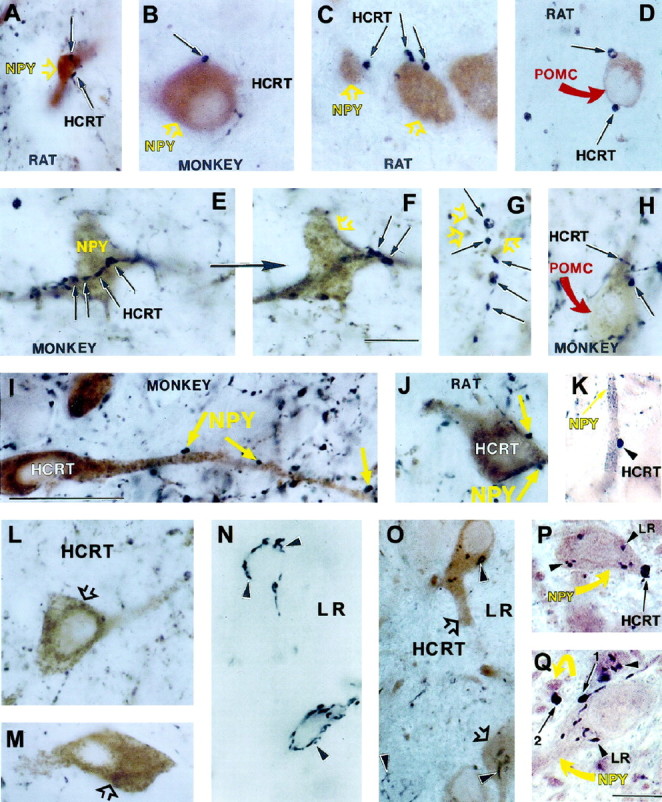
Hypocretin, NPY, and leptin receptor immunoreactivity in the rat and monkey lateral hypothalamus–perifornical region. A–C, E, F, Light brown, NPY-producing dendrites and cell bodies (open yellow arrows) in close proximity to black, HCRT-labeled putative boutons (black arrows) in the rat (A, C) and monkey (B, E, F) arcuate nucleus. The cell shown in F is the same as inE but at a different focal plane. G, Black, HCRT axon terminals (black arrows);light brown, NPY-containing axon terminal (open yellow arrows) in the monkey paraventricular nucleus. D, H, Black, HCRT axons (black arrows) in close proximity to cell bodies immunolabeled for POMC product β-endorphin (curved red arrows) in the rat (D) and monkey (H) arcuate nucleus. I, J, Light brown, HCRT-producing neural perikarya in close apposition with black, NPY-containing boutons (yellow arrows) in the perifornical region of a monkey (I) and a rat (J). L, M, Neurons in the perifornical region of a monkey (L) and rat (M) containing homogeneously distributed,light brown reaction product representing HCRT immunoreactivity (open black arrows). N, Cells in the perifornical region of a rat containingblack reaction product associated with distinct intracellular leptin-R (LR) immunoreactivity (arrowheads). O, Neurons in the perifornical region of a rat expressing immunoreactivity for both HCRT (light brown reaction product; open black arrows) and LR (black reaction product;arrowheads). K, P, Q, Black, HCRT-containing axon terminals (arrowhead onK, long arrows on P,Q) in close contact with light brown, NPY-producing dendrite (long arrow onK) and cell bodies (yellow arrows on P, Q). The NPY cells onP and Q also immunolabeled for LR (arrowheads). These exact cells and connections were processed for electron microscopy, which can be seen in Figures 4,C, D, and F (3K), 7 (3P), and 8 (3Q). Scale bars: F, Q, 5 μm; I, 10 μm.
NPY cells and axons
NPY-labeled perikarya were poorly visible in the rat hypothalamus in the absence of colchicine treatment. When colchicine was given to block axonal transport and to allow a buildup of the peptide in the cell body, NPY-immunoreactive cell bodies and dendrites were observed in the medial parts of the arcuate nucleus. No overlap was found between hypocretin-immunoreactive perikarya and NPY-immunoreactive perikarya, indicating the two peptides were not colocalized in the same cell. A dense network of NPY-immunoreactive axons and axon terminals was found throughout the hypothalamus. Immunoreactivity was stronger in the medial hypothalamus than in the lateral hypothalamus. In both monkey species, NPY-immunopositive cell bodies were detected in the ventromedial and lateral part of the arcuate nucleus without colchicine treatment. An abundant network of NPY axons was observed in the medial preoptic area, ventro- and dorsomedial hypothalamic nuclei, arcuate nucleus, paraventricular nucleus, anterior and lateral hypothalamic areas, and the periventricular area. These observations in rats and monkeys are consistent with previous descriptions of the hypothalamic NPY system (Guy and Pelletier, 1988; Pelletier, 1990; Horvath et al., 1992c, 1993, 1996).
Multiple labeling
For studies of multiple labeling, different color chromogens were used for the light microscopic examination (brown and dark blue-black). After a single bouton of one color was identified making putative contact with a cell of another color, an ultrastructural analysis was then used to study the same identifiedbouton by electron microscopy (EM). Under EM, the labeling in the bouton, which had been brown in the light microscope (Fig. 3G, yellow arrows), was diffuse and light (see Fig. 7C), whereas the bouton with dark blue-black label (Fig. 3G, black arrows) was very dense black with bright clear vesicles (see Fig. 7C).
Hypocretin axons synapse on NPY cells
In the arcuate nucleus of both rats and monkeys, close apposition was observed between dark black hypocretin-immunoreactive axon terminals and light brown NPY-immunopositive cell bodies and proximal dendrites (Figs. 3A–C,E–G,K,4C,D,F). The cell body and proximal dendrites were contacted by as many as 10–12 hypocretin boutons (Fig. 3E,F). If the same high level of interaction exists on distal dendrites that are 15–20 times longer than the short length of proximal dendrites labeled with NPY antisera (van den Pol and Cassidy, 1982), some NPY neurons could receive synaptic contact from many more hypocretin-containing boutons. We examined the frequency of hypocretin contacts on NPY cells in the primate material only, because colchicine was not needed for the visualization of NPY-immunoreactive perikarya in the primate brain. In the rat, however, colchicine is necessary for perikaryal labeling of NPY that, in turn, compromises the fiber staining of hypocretin and thus renders the quantitation of hypocretin axons useless. An axosomatic or axodendritic contact was noted only if a bouton-like structure was in close proximity to a cell body or dendrite and was found as a continuation of its axon by changing the focus plane. Of 500 NPY-immunoreactive cells, 354 (70%) were contacted by hypocretin-immunoreactive axon terminals. This is probably a significant underestimate of the true percentage of contacts (see below), because our investigation was limited to the cell bodies and proximal dendrites of NPY-producing cells and could not detect hypocretin terminals contacting distal NPY dendrites and their spines that do not show NPY immunoreactivity.
When EM was used to determine whether an actual synaptic relationship was present between hypocretin axons and NPY cells, we found abundant presynaptic hypocretin-immunoreactive boutons (labeled dense black) making asymmetric Gray type I synaptic contacts, typical of excitatory synapses, on NPY-immunoreactive cell bodies and proximal dendrites that showed a diffuse light immunolabeling (Figs. 3K,4C, D). The hypocretin-immunoreactive presynaptic axon contained many small, clear, round synaptic vesicles, also typical of excitatory synapses, and an occasional mitochondrion. These data provide direct evidence of a projection from hypocretin cells to NPY-containing cells in the arcuate nucleus.
Detection of boutons by light microscopy is suggestive of synaptic interaction, but in the final analysis, only EM can detect the synapse. The number of boutons could be an overestimate of the number of synapses if some boutons are not involved in synaptic interaction but could represent an underestimate if more than one synapse is made by a single bouton. To correlate the ratio of the number of hypocretin boutons detected at the light microscopic level with the actual number of synapses they established in the arcuate nucleus where NPY cells are located, we randomly selected 90 hypocretin-labeled boutons in trimmed blocks of embedded sections from three monkeys (3 monkeys × 30 boutons each). EM analysis of serial sections of these boutons revealed that these 90 boutons established 145 synapses. Multiple synapses established by the same axon were confirmed if the postsynaptic membrane specializations were clearly separate or if two synaptic membrane specializations were clearly apart from each other and did not merge. Of the 145 synapses, 71 were made between HCRT boutons and NPY-immunoreactive postsynaptic elements. The ratio of axodendritic to axosomatic hypocretin synapses on NPY postsynaptic neurons was ∼4:1 (56 axodendritic vs 15 axosomatic).
In our analysis of axon terminals, we often found adjacent axon terminals, with one axon containing immunoreactivity for NPY and the other containing that for hypocretin (Figs. 3G,7C). Some of these pairs contacted a common NPY-immunoreactive perikaryon. This close juxtaposition may be relevant if presynaptic axons contain HCRT receptors, suggesting that HCRT could have a direct effect on an adjacent axon. It is also relevant to our previous finding that many hypothalamic axon terminals do contain functional NPY receptors that modulate transmitter release (Chen and van den Pol, 1996; van den Pol et al., 1996); if HCRT terminals do express NPY receptors, then release could be modulated by presynaptic NPY receptors.
NPY axons contact hypocretin cells in the lateral hypothalamus
NPY-immunoreactive axons were abundant in the lateral hypothalamus–perifornical region in both rat and primate brains. In these sites, numerous NPY-immunoreactive boutons were in close proximity to hypocretin-immunopositive perikarya (Fig.3I,J). Under the electron microscope, synapses could be detected between these NPY boutons and hypocretin cells (data not shown). These may represent a feedback from the NPY neurons of the arcuate nucleus, the primary source of NPY-containing neurons in the hypothalamus, but could also represent NPY fibers from other brain areas, including different brain stem nuclei.
Hypocretin axons terminate on pro-opiomelanocortin–producing cells
NPY has been shown to enhance feeding, and above, we described the direct synaptic innervation of NPY cells by hypocretin axons. Another group of cells in the arcuate nucleus that also plays a role in feeding is the pro-opiomelanocortin (POMC) cells (Morley et al., 1983; Fan et al., 1997). These cells make melanocortin, β-endorphin, and ACTH from the same precursor, and all three peptides are coexpressed in the same cells (Mezey et al., 1985) and can be detected with antisera against one or another of these peptides. Double labeling for hypocretin and pro-opiomelanocortin cells (using β-endorphin antiserum) revealed boutons of hypocretin axons making contact with pro-opiomelanocortin cells (Fig. 3D,H). Although these contacts are suggestive of synaptic contact, we have not verified this by EM.
Hypocretin axons in synaptic contact with neurons containing leptin-like immunoreactivity
In all regions of the rat and monkey hypothalamus where leptin-R-like immunoreactivity was detected (see above), hypocretin-immunoreactive boutons were in close apposition to leptin-R–containing cells. Ultrastructural analysis of boutons identified at the light microscope level showed hypocretin-immunoreactive boutons making asymmetric synaptic contact with arcuate neurons immunoreactive for leptin-R (Figs.4A,B,5). Because leptin is released by adipose tissue into the vascular system, it is of interest that arcuate neurons with leptin-R-like immunoreactivity that received hypocretin innervation were frequently observed in the immediate vicinity of capillaries (Fig. 5).
In the lateral hypothalamus–perifornical region, hypocretin-immunoreactive boutons established asymmetric synapses on leptin-R–containing cells that expressed cytoplasmic labeling for hypocretin (Fig. 6), suggesting that local axon collaterals connect hypocretin neurons.
NPY cells with leptin receptor immunoreactivity receive synapses from hypocretin neurons
The differential subcellular distribution of leptin-R and hypocretin or NPY and the availability of antisera generated in different species against hypocretin or NPY and leptin-R made it possible to achieve the visualization of these three antigens in the same tissue section by both light and electron microscopy (Figs.3P,Q, 7,8). Because leptin-R immunoreactivity is not present in axon terminals and hypocretin immunoreactivity is absent from cell bodies of the arcuate nucleus, the same chromogen (dark blue-black Ni–DAB reaction) was used for visualization of hypocretin (axons) and leptin-R (cell bodies) (Figs. 3P,Q, 4-8). NPY-producing cells were visualized using a different color, a light brown chromogen (Figs. 3P,Q, 7, 8). In accordance with the observations of Hakansson et al. (1998), who used the same leptin-R antiserum that we did, all NPY-producing perikarya that we examined in the dorsomedial arcuate nucleus expressed immunoreactivity for leptin-R. Frequent close appositions were observed between hypocretin axons and the perikaryal membrane of NPY-immunoreactive cells that contained leptin-R in both rats and monkeys (Fig. 3P,Q). Many contacts were found between hypocretin-immunoreactive axon terminals and the perikaryal membrane of leptin-R–containing NPY cells (Fig. 7). Serial ultrathin sections of these contacts revealed the typical asymmetric type of synaptic contacts between hypocretin-immunoreactive axons and postsynaptic NPY neurons that expressed leptin-R immunoreactivity (Fig. 8).
DISCUSSION
By the use of correlated double and triple label light and electron microscopic analysis, the present study provides direct evidence of synaptic contact between the hypocretin and NPY-producing neural systems of both the rodent and primate hypothalamus. Furthermore, both the hypocretin-containing neurons and the NPY-producing postsynaptic targets of hypocretin axon terminals in the arcuate nucleus express leptin receptor immunoreactivity.
Leptin receptor immunostaining
The leptin-R antiserum we used binds to both long and short isoforms of the leptin receptor as revealed by Hakansson et al. (1998) and by our Western blot analysis. Thus, the possibility that some of the cells we visualized contained leptin-R with a reduced signaling capacity cannot be excluded. However, according to in situ hybridization studies, the active form of the leptin-R is dominant in the arcuate nucleus and the lateral hypothalamus (Mercer et al., 1996a; Lollmann et al., 1997; Elmquist et al., 1998). Furthermore, recent evidence from different laboratories revealed that short leptin receptor isoforms retain signaling abilities to induce transcriptional events (Bjorbaek et al., 1997; Murakami et al., 1997; Yamashita et al., 1998). Therefore, our demonstration of leptin-R immunoreactivity in different hypothalamic cells may represent cells that are influenced by circulating leptin. However, because multiple bands were recognized by this antiserum and others showed that leptin receptors travel with multiple bands in Western blots (Bjorbaek et al., 1997; Ghilardi and Skoda, 1997), it is also possible that some of the immunostaining we detected may be related to processed or premature nonfunctional leptin-R or some other protein with a sequence similarity. The appearance of leptin-R immunoreactivity in the Golgi apparatus could also reflect a high turnover of leptin-R in the hypothalamus (Diano et al., 1998a). Supporting the notion that leptin could reach cells in the lateral hypothalamus where hypocretin cell bodies are located but the blood–brain barrier exists, recent studies have revealed leptin receptors in capillaries (Bjorbaek et al., 1998; Sierra-Honigmann et al., 1998). In particular, mRNA of the short leptin-R isoform was detected in vessels of the lateral hypothalamus, and the suggestion was made that these leptin-binding elements could provide a transport mechanism for leptin to pass the blood–brain barrier (Bjorbaek et al., 1998).
Evolutionary conservation of the hypocretin system in rodents and primates
Within the hypothalamus, the general innervation patterns and the location of the hypocretin-immunoreactive cell bodies were relatively similar in both the rodents and primates. Importantly, the strong innervation by hypocretin-immunoreactive axons of NPY cells that contained leptin-R was found in both rats and primates, suggesting that this synaptic interaction has been conserved over long periods of mammalian evolution, and this finding underlines the potential importance of this hypothalamic circuit. In both species, blocking axonal transport of the peptide with colchicine enhanced the intensity of cellular and dendriticlabeling but did not reveal hypocretin-immunoreactive neuronal perikarya in other regions of the brain outside the lateral hypothalamic area, thus providing further support for the interpretation that the hypocretin axons in the arcuate nucleus that innervate the leptin-R–containing NPY neurons originate solely from cell bodies in the lateral hypothalamus and perifornical area, as originally described (de Lecea et al., 1998; Sakurai et al., 1998).
Hypocretin synapses in the arcuate nucleus
Hypocretin axons made asymmetric synapses on NPY cells that contained leptin-R immunoreactivity. Asymmetric synapses are considered to be indicative of an excitatory signal transmission (Eccles, 1964). This is consistent with electrophysiological reports that hypocretin evokes an increase in synaptic activity in hypothalamic neurons (de Lecea et al., 1998). A stimulatory action of hypocretin on arcuate nucleus NPY cells is also consistent with the robust induction of feeding induced by intraventricular administration of hypocretin (Sakurai et al., 1998). This stimulatory effect of hypocretin or orexin on rat feeding behavior has been confirmed recently by other researchers (Dube et al., 1998; Jain et al., 1998). Substantial increases in food intake are also induced by hypothalamic NPY in the rat (Clark et al., 1984; Stanley et al., 1985). Furthermore, periods of starvation cause an increase in hypocretin mRNA (Sakurai et al., 1998) and NPY levels (Sahu et al., 1988). Together, these data suggest that in the rat, hypocretin may act to increase food intake, at least in part, by enhancing the activity of the arcuate nucleus NPY system. Furthermore, fasting-induced c-fos expression in the monkey hypothalamus is present in lateral hypothalamic hypocretin-producing perikarya as well as in postsynaptic targets of hypocretin axons (Diano et al., 1998d), providing evidence that in the primate hypothalamus, similar to rodents, hypocretin may participate in the regulation of energy homeostasis.
Presynaptic interactions of hypocretin axons in the arcuate nucleus
Hypocretin boutons making synaptic contact with arcuate nucleus NPY cells were frequently in a close relationship to other boutons establishing asymmetric contacts on the same postsynaptic target. Similar axonal appositions were found making synaptic contact with neurons of uncharacterized transmitter phenotype. That the proximity of these axons was not simply a circumstance of probability was suggested by the novel finding of membrane specializations between pairs of presynaptic boutons. This pairing of hypocretin axon terminals with other stimulatory axons raises the possibility that hypocretin might act presynaptically to enhance the release of other excitatory orexigenic peptides and neurotransmitters, including melanin concentrating hormone (MCH) and glutamate. In fact, all fast excitatory synaptic input to the arcuate nucleus appears to arise from glutamatergic neurons (van den Pol et al., 1990; van den Pol and Trombley, 1993), with some arising within the arcuate nucleus (Belousov and van den Pol, 1997). The idea of axoaxonic interaction is supported by our whole-cell recording experiments showing that hypocretin increased the frequency of miniature EPSCs and IPSCs in the presence of tetrodotoxin and supporting the view that hypocretin can increase the release of glutamate or GABA by hypocretin receptors on presynaptic terminals of hypothalamic neurons (van den Pol et al., 1998). These possibilities for the amplification by hypocretin of the input to arcuate NPY neurons could explain further the effect of hypocretin administration on feeding (Sakurai et al., 1998).
Leptin receptors, hypocretin cells, and synapses in the lateral hypothalamus
The fact that arcuate neurons express functional leptin-R is supported by several independent lines of evidence (Mercer et al., 1996a,b; Guan et al., 1997; Lollmann et al., 1997; Elmquist et al., 1998). Our data indicate that hypocretin cells in the lateral hypothalamus also express leptin receptors, consistent with localization of leptin-R mRNA in this region (Mercer et al., 1996b;Elmquist et al., 1998). The functional nature of leptin receptors in the hypocretin neurons remains to be clarified. However, if functionally active, hypocretin neurons may be able to detect this signal from adipose tissue. Another indicator of metabolic state is glucose. Previous studies have shown that some neurons in the lateral hypothalamus have a unique sensitivity to glucose that reduces electrical activity (Oomura, 1983). Because these glucose-sensing cells were found in the same part of the lateral hypothalamus in which hypocretin cells are located, this raises the possibility that hypocretin neurons themselves may express both glucose and leptin receptors; this remains to be tested physiologically. Recurrent collaterals from hypocretin axons make asymmetric synaptic contact with hypocretin-immunoreactive cells; recurrent collaterals may act to synchronize the activity of these neurons. Increased hypocretin synthesis during food deprivation (Sakurai et al., 1998) may serve to enhance the output intensity of hypocretin neurons both at their postsynaptic NPY/leptin-R target neurons in the arcuate nucleus and by feedback excitation on the population of hypocretin neurons in the lateral hypothalamus. The fact that we find a large number of hypocretin terminals in synaptic contact with other neurons of the lateral hypothalamus suggests that even if hypocretin does not sense these metabolic indicators directly, hypocretin neurons are in an excellent position to modulate excitability of other neurons that may have these sensing capabilities. Other such lateral hypothalamic systems may include neurons synthesizing MCH (Bittencourt et al., 1992), a peptide that also stimulates food intake and is upregulated in ob/ob mice (with no leptin production) and after fasting (Qu et al., 1996). An interaction of hypocretin with MCH in the lateral hypothalamus and in the arcuate nucleus (see above) may also be an important component of metabolic regulations. Further studies are needed to clarify this hypothesis.
Hypocretin–NPY signaling in metabolic regulations
When mice or rats lack leptin or its receptors, they become extremely obese (Campfield et al., 1995; Halaas et al., 1995;Pelleymounter et al., 1995), suggesting that leptin acts to inhibit food intake regulated by the brain. Increased circulating leptin levels inhibit the arcuate nucleus NPY neurons (Stephens et al., 1995;Schwartz et al., 1996a). Of importance is the observation that when leptin-deficient mice are crossed with NPY knock-out mice, the absence of NPY ameliorates the obesity, underlying the potential importance and interaction of NPY and leptin in the regulation of feeding. However, although NPY knock-out reduces the obesity, it does not eliminate it totally, indicating other transmitters are involved in the signal from leptin-receptive neurons. One reason for this could be that hypocretin cells compensate for the loss of NPY by increasing their own activity. This may be enhanced further by leptin signaling directly at receptor expressed by hypocretin cells, as described in Results. In fact, because NPY can inhibit the release of the excitatory transmitter glutamate from hypothalamic axons (van den Pol et al., 1996), the loss of NPY in fibers that innervate hypocretin cells may lead to an increased activity in those cells, which then may increase feeding. However, it should be noted that the NPY innervation of the lateral hypothalamic HCRT cells may derive from different regions, including the arcuate nucleus, brainstem catecholaminergic cells, and the intergeniculate leaflet of the lateral geniculate body (Everitt and Hokfelt, 1989; Horvath, 1998). Nevertheless, although NPY is absent from hypothalamic cells in these knock-out animals, there are probably other transmitters in the same cell where NPY has been deleted. Because hypocretin activity may remain tightly coupled to the metabolic state, the same neuronal circuits that are stimulated in normal animals may be regulated adequately by hypocretin in NPY knock-out mice, as well. For example, a population of neurons that produce NPY in the arcuate nucleus also contains GABA (Horvath et al., 1997) that itself can stimulate feeding if administered into the paraventricular nucleus or the cerebral ventricles (Grandison and Guidotti, 1977; Kelly et al., 1977; Morley et al., 1981). An increased release of GABA from these neurons by enhanced stimulation of hypocretin neurons may be sufficient to adjust feeding behavior to subtle changes in metabolic state but requires NPY as well to respond to more drastic changes. In addition, compensatory adjustments in other hypothalamic orexigenic and anorexigenic signals may also be expected in the NPY knock-out animals. For example, although not the primary focus of the present study, we also found many boutons from hypocretin axons in contact with opiate cells, another type of arcuate nucleus neuron involved in the regulation of daily energy homeostasis and other hypothalamic regulations. Thus, hypocretin appears to innervate at least two arcuate nucleus cell types, the NPY and pro-opiomelanocortin cells, both of which have been strongly implicated in the central regulation of energy homeostasis and endocrine mechanisms (Morley et al., 1983; Clark et al., 1984; Stanley et al., 1985; Fan et al., 1997).
Hypocretin–NPY signaling in endocrine regulations
It is interesting to note that although hypocretin has gained attention for its putative role in regulating feeding (Sakurai et al., 1998), the finding that synaptic activity to physiologically identified neuroendocrine neurons in the arcuate nucleus is enhanced by hypocretin suggests that this peptide may play a role in endocrine regulations (van den Pol et al., 1998). This is consistent with another role of NPY in the arcuate nucleus, that is, the central regulation of anterior pituitary hormones (McDonald et al., 1987; Leibowitz, 1991; Kalra and Crowley, 1992; McDonald and Koenig, 1993). In fact, a recent report revealed that in parallel with its effect on feeding, intraventricular administration of hypocretin elevates circulating luteinizing hormone levels (Jain et al., 1998). Furthermore, during fasting, the activation of arcuate nucleus NPY cells has been suggested to underlie suppressed thyrotropin-releasing hormones and thyroid-stimulating hormone production (Harfstrand et al., 1987; Legradi et al., 1997; Diano et al., 1998b,c). Therefore, our present observation of the hypocretin innervation of arcuate nucleus NPY cells may provide a signaling modality via which hypocretin could regulate endocrine systems.
Conclusions
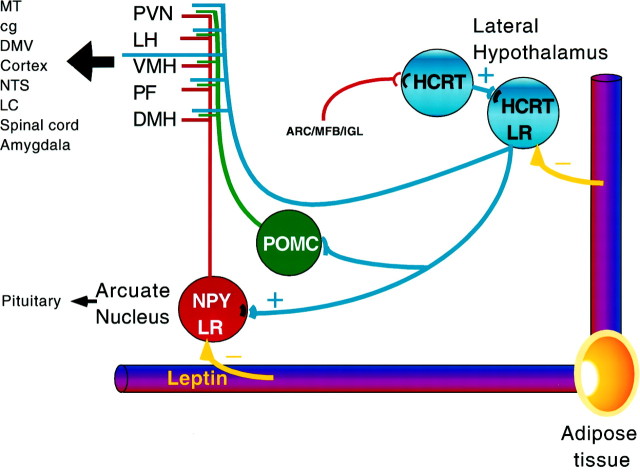
Our work has suggested a circuit within the hypothalamus that ties together three systems that individually have been shown to participate in energy homeostasis and endocrine regulations. Ultimately, the question remains as to how the hypothalamus regulates energy homeostasis and food intake. Although there is no simple answer, the role of the hypothalamus as a hub that can integrate information from many different neuronal and blood-derived sources and can then send complex signals to both regions of the brain controlling motivation and behavior, as well as areas of the endocrine hypothalamus and autonomic nervous system that send and receive signals from all the organ systems involved in metabolic regulation, is probably central. Some of the signaling pathways that might be involved in the efferent control of energy homeostasis and endocrine functions related to hypocretin, NPY, and leptin are depicted in a simplified diagram shown in Figure9.
Fig. 9.
Schematic representation of the projection from hypocretin-containing neurons to neuropeptide Y neurons that may regulate a number of efferent pathways. Leptin is released from adipose tissue into the circulatory system. Both hypocretin (HCRT; blue) and NPY (red) neurons receiving hypocretin innervation contain leptin receptor (LR) immunoreactivity. The arcuate NPY neurons that receive hypocretin innervation project to a number of regions of the brain, particularly those listed that also have been implicated in feeding mechanisms, including the paraventricular nucleus (PVN), lateral hypothalamus (LH), ventromedial nucleus (VMH), perifornical region (PF), and dorsomedial nucleus (DMH). The same regions may also receive direct hypocretin projections. These regions in turn project (large black arrow) widely throughout the brain to loci that include the medial thalamic nuclei (MT), central gray (cg), dorsal motor nucleus of the vagus (DMV), cortex, nucleus of the solitary tract (NTS), locus coeruleus (LC), spinal cord, and amygdala. Some of these areas also receive hypocretin innervation. Hypocretin-targeted arcuate nucleus NPY cells may also affect neuroendocrine cells that are responsible for the regulation of pituitary hormone secretions. The NPY innervation of hypocretin cells in the lateral hypothalamus may originate in the arcuate nucleus (ARC), in the median forebrain bundle (MFB) carrying brainstem catecholaminergic fibers, and/or in the intergeniculate leaflet (IGL). Arcuate nucleus POMC cells (green) are also contacted by hypocretin axons, and the efferents of these opiate cells may target the same regions as the projections of the NPY cells.
Footnotes
This study was supported by the National Science Foundation Grant IBN-9728581 and by the National Institutes of Health Grant NS-34887. We thank M. Shanabrough, K. Szigeti, A. Evans, and Y. Yang for their excellent technical support.
Parts of this paper have been presented previously at the 28th Annual Meeting of the Society for Neuroscience, Los Angeles, CA, 1998, abstract 11.7.
Correspondence should be addressed to Dr. Tamas L. Horvath, Department of Obstetrics and Gynecology, Yale Medical School, 333 Cedar Street, New Haven, CT 06520.
REFERENCES
- 1.Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951;24:123–146. [PMC free article] [PubMed] [Google Scholar]
- 2.Aravich PF, Scalfani A. Paraventricular hypothalamic lesions and medial hypothalamic cuts produce similar hyperphagia syndromes. Behav Neurosci. 1983;97:970–983. doi: 10.1037//0735-7044.97.6.970. [DOI] [PubMed] [Google Scholar]
- 3.Belousov AB, van den Pol AN. Local synaptic release of glutamate from neurons in the rat hypothalamic arcuate nucleus. J Physiol (Lond) 1997;499:747–761. doi: 10.1113/jphysiol.1997.sp021966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernadis LL, Berlinger LL. The dorsomedial hypothalamic nucleus revisited. Brain Res. 1987;434:321–381. doi: 10.1016/0165-0173(87)90004-x. [DOI] [PubMed] [Google Scholar]
- 5.Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon J-L, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 6.Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;51:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 7.Bjorbaek C, Elmquist JK, Michl P, Ahima RS, Vanbueren A, Mccall AL, Flier JS. Expression of leptin receptor isoform in rat brain microvessels. Endocrinology. 1998;139:3485–3491. doi: 10.1210/endo.139.8.6154. [DOI] [PubMed] [Google Scholar]
- 8.Brobeck JR. Mechanism of the development of obesity in animals with hypothalamic lesions. Physiol Rev. 1946;26:541–559. doi: 10.1152/physrev.1946.26.4.541. [DOI] [PubMed] [Google Scholar]
- 9.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, van den Pol AN. NPY Y1- and Y2-like receptors coexist in pre- and postsynaptic sites: inhibition of GABA release in isolated self-innervating SCN neurons. J Neurosci. 1996;16:7711–7724. doi: 10.1523/JNEUROSCI.16-23-07711.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 12.Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacrote JM, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Gygrand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 13.de Lecea L, Kilduff TS, Peyron C, Gao XB, Foye PE, Danielson PE, Fukuhara C, Battenberg ELF, Gautvik VT, Bartlett FS, II, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: two hypothalamic peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diano S, Kalra SP, Horvath TL. Leptin receptor immunoreactivity is associated with the Golgi apparatus of hypothalamic cells. J Neuroendocrinol. 1998a;9:647–650. doi: 10.1046/j.1365-2826.1998.00261.x. [DOI] [PubMed] [Google Scholar]
- 15.Diano S, Naftolin F, Goglia F, Horvath TL. Fasting-induced increase in type II iodothyronine deiodinase activity and messenger ribonucleic acid levels is not reversed by thyroxine in the rat hypothalamus. Endocrinology. 1998b;139:2879–2884. doi: 10.1210/endo.139.6.6062. [DOI] [PubMed] [Google Scholar]
- 16.Diano S, Naftolin F, Goglia F, Horvath TL. Segregation of intra- and extrahypothalamic neuropeptide Y and catecholaminergic inputs on paraventricular neurons, including those producing thyrotropin-releasing hormone. Regul Pept. 1998c;75–76:117–125. doi: 10.1016/s0167-0115(98)00060-3. [DOI] [PubMed] [Google Scholar]
- 17.Diano S, van den Pol AN, Horvath TL. Hypocretin (orexin)-containing neuronal network in the primate hypothalamus and its relationship to fasting-induced c-Fos expressing cells. Soc Neurosci Abstr. 1998d;24:12. [Google Scholar]
- 18.Dube MG, Kalra SP, Kalra PS. Food intake elicited by central administration of orexins: identification of hypothalamic sites of action. Soc Neurosci Abstr. 1998;24:448. doi: 10.1016/s0006-8993(99)01824-7. [DOI] [PubMed] [Google Scholar]
- 19.Eccles J. The physiology of synapses. Springer; Berlin: 1964. [Google Scholar]
- 20.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- 21.Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- 22.Everitt BJ, Hokfelt T. The coexistence of neuropeptide Y with other peptides and amines in the central nervous system. In: Mutt V, Hokfelt T, Fuxe K, Lundberg JM, editors. Neuropeptide Y. Raven; New York: 1989. pp. 61–72. [Google Scholar]
- 23.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 24.Flier JS, Maratos-Flier E. Obesity and the hypothalamus–novel peptides for new pathways. Cell. 1998;92:437–440. doi: 10.1016/s0092-8674(00)80937-x. [DOI] [PubMed] [Google Scholar]
- 25.Gautvik KM, de Lecea L, Gautvik VT, Danielson PE, Tranque P, Dopazo A, Bloom FE, Sutcliffe JG. Overview of the most prevalent hypothalamus-specific mRNAs, as identified by directional tag PCR subtraction. Proc Natl Acad Sci USA. 1996;93:8733–8738. doi: 10.1073/pnas.93.16.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghilardi N, Skoda RC. The leptin receptor activates janus kinase 2 and signals for proliferation in a factor-dependent cell line. Mol Endocrinol. 1997;11:393–399. doi: 10.1210/mend.11.4.9907. [DOI] [PubMed] [Google Scholar]
- 27.Grandison L, Guidotti A. Stimulation of food intake by muscimol and beta endorphin. Neuropharmacology. 1977;16:533–536. doi: 10.1016/0028-3908(77)90019-3. [DOI] [PubMed] [Google Scholar]
- 28.Guan XM, Hess JF, Yu H, Hey PJ, van der Ploeg LH. Differential expression of mRNA for leptin receptor isoforms in the rat brain. Mol Cell Endocrinol. 1997;133:1–7. doi: 10.1016/s0303-7207(97)00138-x. [DOI] [PubMed] [Google Scholar]
- 29.Guy J, Pelletier G. Neuronal interactions between neuropeptide Y (NPY) and catecholaminergic system in the rat arcuate nucleus as shown by dual immunocytochemistry. Peptides. 1988;9:567–570. doi: 10.1016/0196-9781(88)90165-9. [DOI] [PubMed] [Google Scholar]
- 30.Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci. 1998;18:559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halaas JL, Gajiwala KS, Maffei SL, Cohen BT, Chaib D, Rabanowitz RL, Lallone SK, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 32.Harfstrand A, Eneroth P, Agnatyi L, Fuxe K. Further studies on the effects of central administration of neuropeptide Y on neuroendocrine function in the male rat: relationship to hypothalamic catecholamines. Regul Pept. 1987;17:167–179. doi: 10.1016/0167-0115(87)90026-7. [DOI] [PubMed] [Google Scholar]
- 33.Horvath TL. Suprachiasmatic efferents avoid phenestrated capillaries but innervate neuroendocrine cells including those producing dopamine. Endocrinology. 1997;138:1312–1320. doi: 10.1210/endo.138.3.4976. [DOI] [PubMed] [Google Scholar]
- 34.Horvath TL. An alternate pathway for visual signal integration into the hypothalamo-pituitary axis: retinorecipient intergeniculate neurons project to various regions of the hypothalamus and innervate neuroendocrine cells including those producing dopamine. J Neurosci. 1998;18:1546–1558. doi: 10.1523/JNEUROSCI.18-04-01546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horvath TL, Naftolin F, Leranth C. β-Endorphin innervation of dopamine neurons in the rat hypothalamus; a light and electron microscopic double immunostaining study. Endocrinology. 1992a;131:1547–1555. doi: 10.1210/endo.131.3.1354605. [DOI] [PubMed] [Google Scholar]
- 36.Horvath TL, Naftolin F, Leranth C. GABAergic and catecholaminergic innervation of mediobasal hypothalamic β-endorphin cells projecting to the medial preoptic area. Neuroscience. 1992b;51:391–399. doi: 10.1016/0306-4522(92)90323-t. [DOI] [PubMed] [Google Scholar]
- 37.Horvath TL, Naftolin F, Kalra SP, Leranth C. Neuropeptide Y innervation of β-endorphin-containing cells in the rat mediobasal hypothalamus. A light and electron microscopic double-immunostaining study. Endocrinology. 1992c;131:2461–2467. doi: 10.1210/endo.131.5.1425443. [DOI] [PubMed] [Google Scholar]
- 38.Horvath TL, Shanabrough M, Naftolin F, Leranth C. Neuropeptide Y innervation of estrogen-induced progesterone receptor-containing dopamine cells in the monkey hypothalamus: a triple labeling light and electron microscopic study. Endocrinology. 1993;133:405–414. doi: 10.1210/endo.133.1.8100520. [DOI] [PubMed] [Google Scholar]
- 39.Horvath TL, Kalra SP, Naftolin F, Leranth C. Morphological evidence for a galanin-opiate interaction in the rat mediobasal hypothalamus. J Neuroendocrinol. 1995;7:579–588. doi: 10.1111/j.1365-2826.1995.tb00795.x. [DOI] [PubMed] [Google Scholar]
- 40.Horvath TL, Naftolin F, Leranth C, Sahu A, Kalra SP. Morphological and pharmacological evidence for neuropeptide Y-galanin interaction in the rat hypothalamus. Endocrinology. 1996;137:3069–3077. doi: 10.1210/endo.137.7.8770933. [DOI] [PubMed] [Google Scholar]
- 41.Horvath TL, Bechmann I, Kalra SP, Naftolin F, Leranth C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res. 1997;756:283–286. doi: 10.1016/s0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- 42.Jain MR, Pu S, Kalra SP. Orexin-B: a novel neuropeptide activates hypothalamic axes regulating appetite and reproduction. Soc Neurosci Abstr. 1998;24:269. [Google Scholar]
- 43.Kalra SP. Appetite and body weight regulation: is it all in the brain? Neuron. 1997;19:227–230. doi: 10.1016/s0896-6273(00)80934-4. [DOI] [PubMed] [Google Scholar]
- 44.Kalra SP, Crowley WR. Neuropeptide Y: a novel neuroendocrine peptide in the control of pituitary hormone secretion, and its relation to luteinizing hormone. Front Neuroendocrinol. 1992;13:1–46. [PubMed] [Google Scholar]
- 45.Kelly J, Alheid GF, Newberg A, Grossman SP. GABA stimulation and blockade in the hypothalamus and midbrain: effects on feeding and locomotor activity. Pharmacol Biochem Behav. 1977;7:537–541. doi: 10.1016/0091-3057(77)90250-7. [DOI] [PubMed] [Google Scholar]
- 46.Legradi G, Emerson CH, Ahima RS, Flier JS, Lechan RM. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997;138:2569–2576. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- 47.Leibel RL, Chung WK, Chua SC., Jr The molecular genetics of rodent single gene obesities. J Biol Chem. 1997;272:31937–31940. doi: 10.1074/jbc.272.51.31937. [DOI] [PubMed] [Google Scholar]
- 48.Leibowitz SF. Brain neuropeptide Y: an integrator of endocrine, metabolic and behavioral processes. Brain Res Bull. 1991;27:333–337. doi: 10.1016/0361-9230(91)90121-y. [DOI] [PubMed] [Google Scholar]
- 49.Lollmann B, Gruninger S, Stricker-Krongrad A, Chiesi M. Detection and quantification of the leptin receptor splice variants Ob-Ra, b, and e in different mouse tissues. Biochem Biophys Res Commun. 1997;238:648–652. doi: 10.1006/bbrc.1997.7205. [DOI] [PubMed] [Google Scholar]
- 50.McDonald JK, Koenig JI. Neuropeptide Y actions on reproductive and endocrine functions. In: Colmers WF, Wahlestedt C, editors. The biology of neuropeptide Y and related peptides. Humana; Totowa, NJ: 1993. pp. 419–456. [Google Scholar]
- 51.McDonald JK, Koenig JI, Gibbs DM, Collins P, Noe BD. High concentrations of neuropeptide Y in pituitary portal blood of rats. Neuroendocrinology. 1987;46:538–541. doi: 10.1159/000124877. [DOI] [PubMed] [Google Scholar]
- 52.Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Morgan PJ, Trayhurn P. Coexpression of leptin receptor and preproneuropeptide Y mRNA in arcuate nucleus of mouse hypothalamus. J Neuroendocrinol. 1996a;8:733–735. doi: 10.1046/j.1365-2826.1996.05161.x. [DOI] [PubMed] [Google Scholar]
- 53.Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Morgan PJ, Trayhurn P. Localization of leptin receptor mRNA and the long splice variant (O-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996b;387:113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- 54.Mezey E, Kiss JZ, Mueller GP, Eskay R, Odonohue TL, Palkovits M. Distribution of pro-opiomelanocortin derived peptides, adrenocorticotrope hormone, α-melanocyte-stimulating hormone and β-endorphin (ACTH, α-MSH, β-END) in the rat hypothalamus. Brain Res. 1985;328:341–347. doi: 10.1016/0006-8993(85)91046-7. [DOI] [PubMed] [Google Scholar]
- 55.Morley JE, Levine AS, Kneip J. Muscimol induced feeding: a model to study the hypothalamic regulation of appetite. Life Sci. 1981;29:1213–1218. doi: 10.1016/0024-3205(81)90225-3. [DOI] [PubMed] [Google Scholar]
- 56.Morley JE, Levine AS, Yim GK, Lowy MT. Opioid modulation of appetite. Neurosci Biobehav Rev. 1983;7:281–305. doi: 10.1016/0149-7634(83)90020-9. [DOI] [PubMed] [Google Scholar]
- 57.Murakami T, Yamashita T, Iida M, Kuwajima M, Shima K. A short form of leptin receptor performs signal transduction. Biochem Biophys Res Commun. 1997;231:26–29. doi: 10.1006/bbrc.1996.6030. [DOI] [PubMed] [Google Scholar]
- 58.Oomura Y. Glucose as a regulator of neuronal activity. Adv Metab Disord. 1983;10:31–65. doi: 10.1016/b978-0-12-027310-2.50008-6. [DOI] [PubMed] [Google Scholar]
- 59.Pelletier G. Ultrastructural localization of neuropeptide Y in the hypothalamus. Ann NY Acad Sci. 1990;611:232–246. doi: 10.1111/j.1749-6632.1990.tb48935.x. [DOI] [PubMed] [Google Scholar]
- 60.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 61.Powley TL, Keesey RE. Relationship of body weight to the lateral hypothalamic feeding syndrome. J Comp Physiol Psychol. 1970;70:25–36. doi: 10.1037/h0028390. [DOI] [PubMed] [Google Scholar]
- 62.Powley TL, Opsahl CH, Cox JE, Weingarten HP. The role of the hypothalamus in energy homeostasis. In: Morgane PJ, Panskepp J, editors. Handbook of the hypothalamus. Part A: behavioral studies of the hypothalamus. Dekker; New York: 1980. pp. 211–298. [Google Scholar]
- 63.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Pryzpek J, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behavior. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 64.Sahu A, Kalra PS, Kalra SP. Food deprivation and ingestion induced reciprocal changes in neuropeptide Y concentrations in the paraventricular nucleus. Peptides. 1988;9:83–86. doi: 10.1016/0196-9781(88)90013-7. [DOI] [PubMed] [Google Scholar]
- 65.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu W-S, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 66.Saladin R, De Vos P, Guerre-Millo M, Leturque A, Girard J, Staels B, Auwerx J. Transient increase in obese gene expression after food intake or insulin administration. Nature. 1995;377:527–529. doi: 10.1038/377527a0. [DOI] [PubMed] [Google Scholar]
- 67.Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte D, Jr, Woods SC, Seeley RJ, Weigle DS. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide gene expression in ob/ob mice. Diabetes. 1996a;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 68.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of leptin action in rat hypothalamus. J Clin Invest. 1996b;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 70.Stanley BG, Chin AS, Leibovitz SF. Feeding and drinking elicited by central injection of neuropeptide Y: evidence for a hypothalamic site(s) of action. Brain Res Bull. 1985;14:521–524. doi: 10.1016/0361-9230(85)90100-5. [DOI] [PubMed] [Google Scholar]
- 71.Stephens TW, Basinki M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffman J, Hsiung HM, Kriauciunas A, MacKellar W, Rosteck PR, Jr, Schoner B, Smith D, Tinsley FC, Zhang X-Y, Heiman M. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377:530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- 72.Tokunaga K, Fukushima M, Kemnitz JW, Bray GA. Comparison of ventromedial and paraventricular lesions in rats that become obese. Am J Physiol. 1986;251:R1221–R1227. doi: 10.1152/ajpregu.1986.251.6.R1221. [DOI] [PubMed] [Google Scholar]
- 73.van den Pol AN. Lateral hypothalamic damage and body weight regulation: role of gender,diet, and lesion placement. Am J Physiol. 1982;243:R265–R274. doi: 10.1152/ajpregu.1982.242.3.R265. [DOI] [PubMed] [Google Scholar]
- 74.van den Pol AN, Cassidy JR. The hypothalamic arcuate nucleus of rat–a quantitative Golgi analysis. J Comp Neurol. 1982;203:65–98. doi: 10.1002/cne.902040108. [DOI] [PubMed] [Google Scholar]
- 75.van den Pol AN, Trombley PQ. Glutamate neurons in hypothalamus regulate excitatory transmission. J Neurosci. 1993;13:2829–2836. doi: 10.1523/JNEUROSCI.13-07-02829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van den Pol A, Waurin J, Dudek F. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990;250:1276–1278. doi: 10.1126/science.1978759. [DOI] [PubMed] [Google Scholar]
- 77.van den Pol AN, Obrietan K, Chen G, Belousov A. NPY mediated long term depression of excitatory activity in suprachiasmatic nucleus neurons. J Neurosci. 1996;16:5883–5895. doi: 10.1523/JNEUROSCI.16-18-05883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van den Pol AN, Gao XB, Obrietan K, Kilduff T, Belousov A. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weingarten HP, Chang P, McDonald TJ. Comparisons of the metabolic and behavioral disturbances following paraventricular and ventromedial hypothalamic lesions. Brain Res Bull. 1985;14:1551–1559. doi: 10.1016/0361-9230(85)90104-2. [DOI] [PubMed] [Google Scholar]
- 80.Yamashita T, Murakami T, Otani S, Kuwajima M, Shima K. Leptin receptor signal transduction: ONRa and OBRb of fa type. Biochem Biophys Res Commun. 1998;246:752–759. doi: 10.1006/bbrc.1998.8689. [DOI] [PubMed] [Google Scholar]
- 81.Yarnell DO, Knight DS, Hamilton K, Tulp O, Tso P. Localization of leptin receptor immunoreactivity in the lean and obese zucker rat brain. Brain Res. 1998;785:80–90. doi: 10.1016/s0006-8993(97)01388-7. [DOI] [PubMed] [Google Scholar]