Abstract
We have shown previously that electrolytic lesions of the dorsal hippocampus (DH) produce a severe deficit in contextual fear if made 1 d, but not 28 d, after fear conditioning (Kim and Fanselow, 1992). As such, the hippocampus seems to play a time-limited role in the consolidation of contextual fear conditioning. Here, we examine retrograde amnesia of contextual fear produced by DH lesions in a within-subjects design. Unlike our previous reports, rats had both a remote and recent memory at the time of the lesion. Rats were given 10 tone–shock pairings in one context (remote memory) and 10 tone–shock pairings in a distinct context (with a different tone) 50 d later (recent memory), followed by DH or sham lesions 1 d later. Relative to controls, DH-lesioned rats exhibited no deficit in remote contextual fear, but recent contextual fear memory was severely impaired. They also did not exhibit deficits in tone freezing. This highly specific deficit in recent contextual memory demonstrated in a within-subjects design favors mnemonic over performance accounts of hippocampal involvement in fear. These findings also provide further support for a time-limited role of the hippocampus in memory storage.
Keywords: retrograde amnesia, hippocampus, context, fear, conditioning, freezing, rat, activity, consolidation, learning, memory
After damage to the hippocampal formation, humans display anterograde amnesia of declarative memory (an inability to form new memories) that is accompanied by retrograde amnesia (RA) of declarative memory (a loss of memory acquired before the damage). In amnesics, RA is typically temporally graded; it involves the loss of memories acquired just before the lesion (recent memory), but memories acquired several years before (remote memory) remain intact [Squire and Alvarez (1995); Knowlton and Fanselow (1998); but see Nadel and Moscovitch (1997)]. This effect has been observed through use of retrospective memory tests, including those examining autobiographical details, public events, famous faces, and television shows (Rempel-Clower et al., 1996; Reed and Squire, 1998).
Although many studies in animals have examined the effects of hippocampal lesions made before training (Olton et al., 1979; Morris, 1983; Phillips and LeDoux, 1992; Kim et al., 1993), relatively few studies have examined temporally graded RA after damage to the hippocampal formation (Winocur, 1990; Zola-Morgan and Squire, 1990; Cho et al., 1993; Bolhuis et al., 1994; Kim et al., 1995; Cho and Kesner, 1996; Wiig et al., 1996; Nadel and Moscovitch, 1997). Kim and Fanselow (1992) gave animals Pavlovian fear conditioning in which a tone conditional stimulus (CS) was paired with a shock unconditional stimulus (US) several times in a novel context. Rats trained in this manner develop a fear of both the tone and the training context, which can be measured as freezing, an adaptive species-specific defense reaction (Bolles, 1970; Fanselow, 1980). Electrolytic lesions of the dorsal hippocampus (DH) made 1 d, but not 28 d, after training abolished contextual freezing but spared tone freezing. That is, hippocampal lesions produced a time-limited RA of contextual fear in rats (Kim and Fanselow, 1992; Maren et al., 1997). Consistent with the proposed role of the hippocampus in other forms of memory, we have offered a mnemonic account of the deficit in context conditioning produced by hippocampal damage (Maren et al., 1998).
Despite the specificity of this deficit in recent contextual fear (and not remote contextual fear or tone fear), the mnemonic role of the hippocampus in contextual fear has recently been questioned. An alternative account suggests that the deficits observed in contextual freezing may be attributable to locomotor hyperactivity, a reliable effect of hippocampal lesions (Teitelbaum and Milner, 1963;Douglas and Isaacson, 1964; Maren and Fanselow, 1997). By this view, hippocampal animals do not exhibit normal freezing, because hyperactivity generated by the lesion disrupts inhibition and freezing directly (Blanchard et al., 1977; Good and Honey, 1997; McNish et al., 1997). This is a performance account of contextual freezing deficits, because in this view hippocampal lesions disrupt the performance of the freezing response rather than fear memory. For this account to handle the specificity of the deficit for recent contextual fear, several assumptions need to be made (McNish et al., 1997). First, it is assumed that higher levels of fear are less susceptible to disruption by hippocampal lesions than low levels of fear. Second, it is assumed that remote contextual fear is stronger than recent contextual fear because of an “incubation” of fear over time. Third, tone fear is assumed to be greater than contextual fear, because it is presumed that the tone is a better predictor of the US than the context (i.e., greater CS–US contingency) (McNish et al., 1997).
Although the specificity of the amnesic effects of hippocampal lesions observed in the Kim and Fanselow (1992) study did not appear to relate to the levels of fear observed (Maren et al., 1998), the between-groups design used did not provide an ideal test of these assumptions. Because there is considerable variability in the levels of hyperactivity produced by hippocampal lesions (Maren and Fanselow, 1997; Maren et al., 1998), an ideal design would contrast remote and recent fear within subjects.
Thus, to examine these issues, we wanted to determine whether temporally graded RA of contextual fear could be demonstrated within subjects. In Experiment 1, the same animals had both remote and recent (contextual and tone) fear memories at the time of the hippocampal lesion. This within-subjects design formally examines these performance issues. In Experiment 2, we reduced the levels of tone fear with respect to contextual fear, and in Experiment 3, we examined hippocampal lesion-induced hyperactivity. Together, these experiments should discriminate between the performance and mnemonic accounts of hippocampal lesion-induced hyperactivity and contextual fear deficits.
MATERIALS AND METHODS
Experiment 1: temporally graded retrograde amnesia of fear: within-subjects examination
Subjects. Twenty-nine female Long–Evans rats (225–250 gm, 90- to 110-d-old at initial training; 290–325 gm, 150- to 170-d-old at the time of testing) bred at University of California at Los Angeles (stock from Harlan Sprague Dawley, San Diego, CA) were used in this experiment. They were housed in individual metal cages located in a colony maintained on a 14/10 hr light/dark cycle. They had access to dry food and water ad libitum and were handled on 3 consecutive d for ∼1 min before initial training.
Training. All of the animals received training in a remote context (with a remote tone), followed by training in a different recent context (with a different recent tone) 50 d later. Figure1 graphically depicts a sample time line of the experimental procedures. The exact contexts and tones used show little generalization between each other and were counterbalanced (see Contexts below). The training parameters were chosen from pilot work in which tone conditioning was weaker or equivalent to, but not greater than, context conditioning. For each conditioning session, the rats were placed into the conditioning chambers, and after a 4 min baseline period, the animals received 10 tone (10 sec, 2 or 8 kHz, 85 dB/A scale)–shock (2 sec, 1 mA) pairings, with each pairing separated by 64 sec. Two minutes after the last trial, the animals were returned to their home cages.
Fig. 1.
Experiment 1. Sample schematic view of within-subjects procedures. The animals were given remote conditioning in one context, and 50 d later they received recent training in a different context (with a different tone), followed by dorsal hippocampal or sham lesions 1 d later. After 10 d of recovery, they were given independent freezing tests for remote and recent, context and tone fear memory. The exact contexts and test orders used were counterbalanced. Drawings are not to scale.
Surgery. One day after recent training (50 d after remote training), all of the animals were given atropine methyl nitrate (0.04 mg/kg, i.p.), anesthetized with sodium pentobarbital (45 mg/kg, i.p.), and mounted into a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). The scalp was incised and retracted, and head position was adjusted to place bregma and lambda in the same horizontal plane. Small burr holes were drilled in the skull for placement of a stainless steel electrode (size 00 insect pin insulated with Epoxylite, except for the 0.5 mm tip). Rats were assigned to either DH (n= 14) or sham (n = 15) groups randomly but with constraints to maintain training context and tone counterbalancing. DH rats received bilateral electrolytic lesions of the dorsal hippocampus by passing anodal constant current (1.0 mA for 20 sec; direct current constant current lesion maker DCLM5A; Grass Instruments) at four sites (−2.8 mm postural to bregma; ±2.0 lateral to bregma; −4.0 ventral to the skull surface; and −4.2 posterior, ±3.0 lateral, −4.0 ventral) (Kim and Fanselow, 1992). Sham rats were treated similarly, but no current was passed.
Contexts. The context A environment consisted of aluminum (side walls) and Plexiglas (front, back, and top) chambers (28 cm wide, 21 cm high, and 22 cm deep; Lafayette Instruments, Lafayette, IN). The floor of each chamber had 18 stainless steel rods (4 mm diameter, 1.5 cm apart) connected to a shock scrambler and generator (which along with internal ventilation fans supplied background noise of 70 dB/A scale). The chambers were cleaned and scented with a 5% ammonium hydroxide solution (in collection pans below the rods). These computer-controlled (Med-Associates, Lafayette, IN) chambers were in a well lit room (the “Wilshire room”) separate from the observers, who viewed the animals on video screens and were blind to the experimental conditions. Tones were presented from a speaker in the wall of each chamber. The context B environment was in a separate room (the “Sunset room”). These chambers (same size as above) had a white rear wall inserted and two white plastic side walls (24 × 21 cm) placed at 60° to the floor, forming a triangular enclosure. The floors consisted of 17 staggered rods (two rows, 1 cm vertically apart; in each row, each rod was 2.6 cm apart). Background noise (70 dB) was supplied by a white noise generator, and the chambers were cleaned and scented with 1% acetic acid solution. This room was kept entirely dark, except for a 30 W red light bulb. The carriers used to transport the animals to this context were also different from before, and because this context was located in a different room, the room had distinctive geometric and distal features. Our previous and current experience is that rats exhibit no significant generalization between these contexts (Kim and Fanselow, 1992; Maren and Fanselow, 1997) (see Fig. 5B, BL). The two tones used were 2 and 8 kHz pure tones. Pilot studies found <15% generalization between these two tones under these training parameters. The exact contexts and tones used for remote and recent training were counterbalanced (i.e., an equal number of animals were trained in A with 2 kHz, followed by B with 8 kHz 50 d later; A8–B2, B2–A8, and B8–A2). In this manner, differences between the levels of fear attained to the different cues (which were small) were counterbalanced, and counterbalancing was maintained within each lesion group. Finally, a third context (C) was used for off-baseline tone testing. This context consisted of a stainless steel rack of hanging wire mesh cages (20 cm wide, 25 cm deep, 18 cm high; transparent in the front; opaque side and rear walls). These cages hung over deep, pine wood shavings (providing the background odor), and the entire rack was placed into the same room (Wilshire) as the context A chambers. The room was now dark, except for a 30 W red light bulb, was isolated and quiet (background noise, 55 dB), and tones (which achieved 85 dB in the individual cages) were delivered from conditioning chambers located behind the rack (although these chambers were not visible to the rats). The transport carriers used to carry the animals were those used for either the A or B context (counterbalanced). The animals did not exhibit significant generalization during baseline between this C context and the A and B contexts in which they had received training (see Fig.4A,B, BL).
Fig. 5.
Experiment 2. Further increase in the level of context fear relative to tone fear. Rats were trained only in one training context with one tone, and then given DH lesions 1 d later. A, Recent contextual fear. DH-lesioned animals exhibited a severe deficit in context freezing (% time ± SEM, for each minute of the 8 min test), although fear levels in sham animals were nearly asymptotic. B, Recent tone fear. DH-lesioned animals did not exhibit deficits in tone freezing, even as tone fear extinguished across the test period and became weaker.C, Interaction. Same data as A andB, averaged for the first 6 min of the context test and 6 min that the tone was on test (6 min was used to make the levels more comparable). DH-lesioned animals exhibited a deficit only in contextual freezing, although tone fear was substantially weaker than context fear in Sham animals.
Fig. 4.
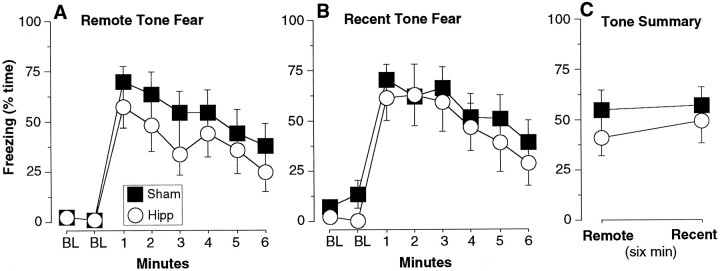
Experiment 1. Tone conditioning. For each test, the animal was placed into a novel context (C) and after a 2 min baseline (BL) period, a tone (remote or recent) played continuously for 6 min. A, Remote tone fear. DH-lesioned rats exhibited equivalent levels of freezing (% time ± SEM, for each minute of the 8 min test) as sham animals to the remotely acquired tone, for which they were trained 50 d before the lesion. B, Recent tone fear. DH-lesioned rats exhibited no deficit in freezing to the recently acquired tone, for which they were trained 1 d before the lesion. C, Tone summary. Same data as A and B, averaged for the 6 min that the tone was on. DH-lesioned rats exhibited normal levels of tone freezing.
Freezing. Freezing, an established index of conditional fear in the rat, was defined as the absence of any visible movement (including the vibrissae), except that required for respiration (only fluctuation in the volume of the thorax). It was scored according to a blind instantaneous 8 sec time sampling procedure in which each animal was observed eight times per 64 sec interval, and these were averaged to yield an estimate of the percentage time freezing. Prior study has revealed that this measure is highly amenable to parametric analysis (Fanselow and Bolles, 1979).
Tests for conditioning. After surgery (10–11 d), the animals received both a remote and a recent 8 min contextual fear test on 2 separate d (order counterbalanced). This was followed 1–2 d later by remote and recent (counterbalanced) tone tests. For each test, the rats were brought to a novel context (context C, above) for an 8 min tone fear test. The animals were placed in the wire cages, and after a 2 min baseline, either the remote or recent conditioning tone was presented for 6 min. For each test, freezing was scored continuously.
Experiment 2: further increase in the level of context fear relative to tone fear
In Experiment 1, we examined whether RA of recent contextual fear could be demonstrated even when contextual fear was higher to or equivalent to tone fear. In Experiment 2, we examined the effect of DH lesions when contextual fear levels were even higher and unambiguously greater than tone fear (compared with Experiment 1). To this end, training parameters were used (based on pilot work) that produced nearly asymptotic levels of contextual fear, with comparatively low levels of tone fear. In this experiment, only recently acquired fear was examined.
Subjects. Twenty-eight female Long–Evans rats were used (as before).
Training. The rats were given one Pavlovian fear conditioning session in the A context (above). After a 3 min baseline period, the animals received five tone (30 sec, 2 kHz, 85 dB/A scale)–footshock (2 sec, 1 mA) pairings separated by 64 sec each. Two minutes after the last pairing, the animals were returned to their home cages.
Surgery. One day after training, the animals received either DH (n = 10) or sham (n = 18) lesions as before.
Tests for conditioning. After surgery (10 d), the animals were returned to the training context for an 8 min contextual fear test. On the next day, they were brought to a novel context (B context, above), and after a 2 min baseline period, the training tone was played for 6 min. Freezing was scored continuously during the two tests.
Experiment 3: the generality of hippocampal hyperactivity
Increased locomotor activity (“hyperactivity”) after hippocampal lesions is a well established phenomenon (Douglas and Isaacson, 1964; Maren and Fanselow, 1997; Maren et al., 1998). By one view, this hyperactivity reflects a generalized loss of inhibition, of which freezing is an example (Douglas, 1967; Blanchard et al., 1977;Good and Honey, 1997; McNish et al., 1997). Consistent with this view, the performance account proposes that DH-lesioned animals fail to exhibit normal contextual freezing, because hyperactivity generated by the lesion directly interferes with the performance of the freezing response. By quite a different view, hyperactivity observed on the open field in rats with hippocampal lesions reflects aberrant exploration caused by a failure of spatial learning (Teitelbaum and Milner, 1963;Nadel, 1968). Accordingly, the mnemonic account postulates that both the contextual learning deficit and hyperactivity on an open field reflect a common spatial learning deficit (Good and Honey, 1997; Maren et al., 1998).
Thus, we examined open-field activity to test between these two accounts. Two features of open-field activity were examined for which these two views make different predictions. First, the time course of open-field activity was examined. By the performance account, DH-lesioned animals should immediately and for a sustained period of time exhibit hyperactivity. By the mnemonic account, sham and DH-lesioned rats should begin by exploring the environment to a comparable degree, but DH-lesion-induced hyperactivity should appear as a failure of normal fast habituation. Second, we examined the effect of a stimulus (anxiety-provoking bright light) that normally produces behavioral inhibition on the open field. By the performance account, DH-lesioned animals should remain hyperactive when a bright light is shined on the open field because of their generalized loss of inhibition. By the mnemonic account, an anxiety-provoking bright light should readily gain control over exploratory behavior and attenuate hyperactivity. To this end, animals were tested for activity in a very dark open field for 4 min, followed by testing in bright light for 4 min.
Subjects. Twenty-seven female Long–Evans rats were used (as before).
Surgery. Animals were given DH (n = 12) or sham (n = 15) lesions as before.
Open-field testing. After surgery (10–20 d), the animals were brought to an open field for activity testing. The open field was a translucent green polyethylene storage container (71 cm long, 36 cm wide, 30 cm high) placed in the center of a room that had been decorated with distal cues (posters in the rat’s line of sight). The container floor was separated into eight equal segments (20 × 18 cm each) using black electrical tape mounted on the underside of the open field. It was placed on a table in the center of the room and directly below an overhead camera and a 25 W red light bulb, which provided the only illumination for the dark phase of the test. Two light fixtures, each with a 100 W white bulb directly facing the outside walls of the translucent open field, were attached to the table and used to flood the open field with light during the light phase of the test. Background noise (65 dB/A scale) was supplied by a white noise generator. For each rat, the experimenter (blind to surgical condition) placed the animal in the open field, left the isolated room, then scored line crossovers (defined as the front and rear paws crossing one of the black lines, i.e., the animal entering a different segment) in the dark for 4 min, then turned on the two 100 W bulbs via remote control, and scored line crossovers for an additional 4 min (animals were observed on a video display). The animal was then returned to its home cage, and the open field was cleaned with 25% ethanol between each rat. Crossovers were tabulated for each minute of the 4 min dark and 4 min light periods.
Common to all experiments
Histology. Histological verification of lesion location was performed after behavioral testing was completed. Rats were perfused across the heart with 0.9% saline, followed by 10% formalin. After extraction from the skull, the brains were post-fixed in 10% formalin for several days and in 10% formalin–30% sucrose until sectioning. Coronal sections (50 μm thick, taken every 200 μm) were cut on a cryostat (−16°C) and mounted on glass microscope slides with 70% ethanol. After drying, the sections were stained with 0.25% thionin. Lesions were verified by visual inspection of the stained sections reconstructed on rat brain atlas templates (Swanson, 1992).
Data analysis
Percentage freezing averaged over several minutes was entered into a general multivariate ANOVA (MANOVA), followed by multiplepost hoc comparisons. For unpaired comparisons, the MANOVA generates a post hoc equivalent to an unpaired two-tailed t test. For paired comparisons, the post hoc is equivalent to a paired two-tailed t test (Woodward et al., 1990). Time courses are presented for visualization (see Figs. 3A,B,4A,B,5A,B), but unless indicated otherwise, group × minutes interactions were not significant; thus, to simplify data analysis, statistics are presented only for summary data (see Figs. 3C, 4C,5C). In all cases, time course analyses yielded equivalent findings. Where there was a meaningful group × time interaction (see Fig. 6), a full time course analysis is given. Any additional analyses are as indicated in the text.
Fig. 3.
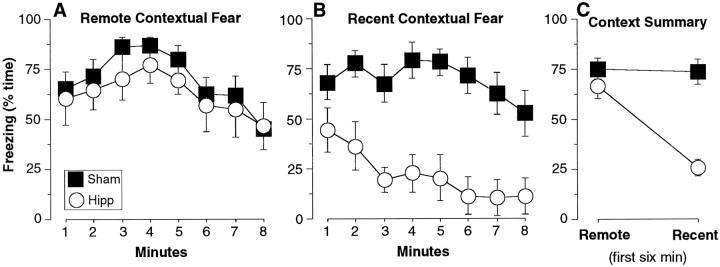
Experiment 1. Temporally graded retrograde amnesia of contextual fear: within-subjects examination. A, Remote contextual fear. Rats that received DH lesions exhibited equivalent levels of freezing (% time ± SEM, for each minute of the 8 min test) as sham animals to the remotely acquired context, for which they were trained 50 d before the lesion.B, Recent contextual fear. The very same DH-lesioned rats exhibited a severe amnesia of contextual memory that was 1 d old at the time of the lesion. C, Context summary. This is the same data as in A and B, averaged for the first 6 min of each test (6 min was used to make the levels more comparable to the tone tests; see Fig. 4). DH-lesioned rats exhibited a severe but time-limited retrograde amnesia of contextual fear.
Fig. 6.
Experiment 3. The generality of hippocampal hyperactivity. Rats were placed on a dark open field (lit only by a 25 W red bulb), and crossovers were scored for 4 min. Two bright lights (two 100 W white bulbs) were then shined onto the open field, and crossovers were scored for another 4 min. Open-field activity was assessed by scoring segment crossovers (mean ± SEM), which are depicted for each minute of the dark and light periods. DH-lesioned animals exhibited a robust hyperactivity during the dark phase of the test, but this hyperactivity disappeared when the lights were turned on. Moreover, hippocampal lesion-induced hyperactivity observed during the dark phase appeared to result from a lack of habituation that was seen in sham animals.
RESULTS
Histology: all experiments
Histological reconstruction from a representative Hippocampal rat is shown in Figure 2. Rats in this group exhibited damage throughout the rostral-caudal extent of the DH. The lesions were variable in size, but included damage to CA1, CA3, and dentate gyrus. Of the included rats, extrahippocampal damage, when present, primarily included minimal damage to neocortex overlying hippocampus [a previous study has indicated that damage to these cortical regions does not impact fear conditioning (Kim and Fanselow, 1992)]. In Experiment 1, three rats were excluded from the final analysis: one rat had a unilateral lesion and two rats had damage that extended ventrally into the thalamus (final DH, n = 11). In Experiment 2, two rats were excluded, because only very small or unilateral lesions were apparent (final DH, n = 8). In Experiment 3, one animal was excluded because it died from unknown causes before it could be perfused (final DH, n = 11). However, exclusion of these rats did not affect any statistical conclusions or the qualitative appearance of any of the figures.
Fig. 2.

Histological reconstruction of representative electrolytic lesion of the dorsal hippocampus.
Experiment 1: temporally graded retrograde amnesia of fear: within-subjects examination
Context fear
After surgery (10–11 d), the rats were returned to the remote and recent training contexts for two separate 8 min contextual freezing tests (order counterbalanced) (Fig. 3). Figure 3A depicts the time course for the freezing response for remote contextual fear that had been acquired 50 d before the lesion. Figure 3B depicts the time course of recent contextual fear, learned 1 d before the lesion (note that despite the appearance of Figure 3B, the lesion × min interaction for this test was not significant; MANOVA,F(7,168) = 1.2, p > 0.3; moreover, DH rats were significantly impaired for every minute of this test; F(1,24) values > 7.2,p < 0.02). Figure 3C depicts the average freezing observed during the first 6 min of the two tests (6 min was used to make the levels more comparable to the tone tests; see below). Lesions of the hippocampus produced an obvious and severe but time-limited RA of contextual fear. There was a significant lesion × time interaction (F(1,24) = 16,p < 0.001). Post hoc comparisons revealed that time-limited RA of contextual fear was robust whether tested within-subjects or between-groups. Remote contextual fear differed from recent contextual fear for hippocampal rats (F(1,10) = 28, p < 0.001) but not for sham rats (F(1,14) = 0.06,p > 0.8), which, importantly, exhibited an equivalent average level of fear to the two contexts. Hippocampal rats differed from sham rats for recent (F(1,24) = 34,p < 0.0001) but not remote (F(1,24) = 1.3, p > 0.25) contextual fear. Thus, unambiguous time-limited RA of contextual fear can be demonstrated within-subjects.
Hyperactivity cannot account for the present data, because hyperactivity would be expected to similarly impact both measures of context fear, taken within-subjects. Moreover, there is no evidence that remote contextual fear is stronger than recent contextual fear because of “incubation,” or some other temporal process (cf.McNish et al., 1997). Indeed, the average remote and recent contextual fear in sham animals differed by only ∼1%.
Tone fear
After contextual fear testing (1–2 d), the animals were brought to a novel context for remote and recent tone fear testing (order counterbalanced). For each test, after a 2 min baseline period, either the recent or remote tone was played for 6 min (Fig.4). Figure 4A depicts the time course for the freezing response of remote tone fear, whereas Figure 4B depicts the time course for recent tone fear. None of the animals exhibited appreciable baseline freezing. Figure 4C depicts the average responses during the 6 min periods when the tones were on. Hippocampal lesions failed to produce any significant effect in tone fear (MANOVA, main effect of lesion,F(1,24) = 0.6, p > 0.4; lesion × time interaction, F(1,24) = 0.6,p > 0.4). For summary data, there were no significantpost hoc comparisons (p > 0.2).
Tone compared with context fear
Although such a comparison is theoretically problematic, it has been assumed that tone fear is stronger than contextual fear (McNish et al., 1997), whereas the present study was specifically designed to provide lower or equivalent levels of tone fear.
Average analysis
Compare Figures 3C and 4C. For sham rats, context fear was indeed higher than tone fear at both the remote (F(1,14) = 11.7, p < 0.01) and recent (F(1,14) = 5.0, p < 0.05) time points (first 6 min of context test vs 6 min of tone test when the tone was on).
Peak analysis
Because the time course for context and tone fear are different (compare Figs. 3A,B and4A,B), it is possible that the average analysis underestimated tone fear. Thus, we computed the peak continuous freezing for each rat during any 1 min interval for each of the remote and recent context and tone tests (Maren et al., 1997). These data and post hoc comparisons are reported in Table1 (Experiment 1). Nonparametricpost hoc comparisons were used throughout the peak analysis because of heterogeneity of variance (substantial compression against the ceiling for context freezing). For sham rats, peak context freezing was indeed significantly higher than peak tone freezing at both the remote and recent time points. Moreover, consistent with the results of the average analysis, DH rats were significantly impaired relative to sham rats in peak recent context freezing, but not peak remote context, peak remote tone, or peak recent tone fear (Table 1, Experiment 1). Thus, average and peak analyses yielded equivalent findings. Although it is difficult to directly compare levels of tone and context fear, in this study, tone fear was weaker than or equivalent to but not greater than context fear (cf. McNish et al., 1997).
Table 1.
Peak fear
| Context | Tone | ||
|---|---|---|---|
| Experiment 1 | |||
| Remote | |||
| Sham | 95.8 ± 2.0 | 75.8 ± 8.2 | p = 0.02* |
| DH | 96.6 ± 2.4 | 75.0 ± 11.7 | p = 0.2 |
| p > 0.6 | p > 0.9 | ||
| Recent | |||
| Sham | 95.8 ± 1.6 | 80.0 ± 6.3 | p = 0.01* |
| DH | 71.6 ± 8.4 | 71.6 ± 12.5 | p > 0.99 |
| p = 0.02* | p > 0.9 | ||
| Experiment 2 | |||
| Sham | 99.3 ± 0.7 | 84.0 ± 7.6 | p = 0.03* |
| DH | 79.7 ± 9.1 | 92.2 ± 4.7 | p = 0.2 |
| p < 0.01* | p > 0.8 |
Peak freezing (mean ± SEM) was computed by taking each rat’s maximum freezing for any continuous minute during each of the 8-min context and 6-min (post-baseline) tone fear tests. Paired comparisons contrast context and tone freezing within-subjects using the Wilcoxon signed-rank post hoc; unpaired comparisons contrast Sham and DH groups for each test using the Mann–Whitney U post hoc. For both Experiments 1 and 2, DH lesions produced significant impairments in average (Figs. 3-5) and peak (above) recent context fear but not remote context or tone fear. For Sham animals, peak (above) and average (Figs. 3-5) context fear was greater than, or equivalent to, but not weaker than tone fear.
As such, in the present study, the deficit produced by hippocampal lesions was highly specific to recent contextual fear. It was not observed in remote contextual fear, nor was it observed in weaker tone-elicited freezing.
Experiment 2: further increase in the level of context fear relative to tone fear
In Experiment 2, we sought to further increase (through manipulation of training parameters) the amount of contextual fear relative to tone fear. Figure5A depicts the time course for the 8 min contextual fear test, Figure 5B depicts the time course of the tone fear test, and Figure 5C depicts the interaction (first 6 min of the context test, and 6 min from the tone test when the tone was on). There was an obvious lesion × test type interaction (MANOVA, F(1,24) = 9,p < 0.01) because DH lesions produced a substantial deficit for context freezing (ANOVA, F(1,24) = 25, p < 0.0001) but not tone freezing (F(1,24) = 0.2, p > 0.6). This was despite the fact that average contextual fear levels were nearly asymptotic in the present study and considerably greater than tone fear [Figure 5C (sham rats, tone versus context, average of first 6 min; F(1,17) = 16.5, p< 0.001)]. As in Experiment 1, we also computed peak continuous freezing for each rat during any 1 min interval of the context and tone tests (Table 1, Experiment 2). For sham rats, peak context freezing was again significantly greater than peak tone freezing; moreover, DH rats were significantly impaired relative to sham rats for peak context but not peak tone fear (Table 1, Experiment 2).
Finally, examination of Figure 5B reveals that even as tone fear extinguishes and becomes weaker, hippocampal deficits still fail to appear. For example, in min 6, sham fear has diminished to 31.2 ± 10.5%, but DH rats still fail to show any significant deficit (34.4 ± 17.0%, F(1,24) = 0.03,p > 0.8). Taken with the data from Experiment 1, it is apparent that hippocampal lesion-induced deficits in freezing cannot be predicted based simply on levels of fear (McNish et al., 1997).
Experiment 3: the generality of hippocampal hyperactivity
In Experiment 3 we examined the generality of DH lesion-induced hyperactivity. It was predicted that although DH animals would be hyperactive in a dark open field, bright anxiety-provoking lights might be able to substantially attenuate open-field activity. Figure6 depicts the average cage crossovers during each minute of the dark and light phases of the open-field activity test. There was a lesion × time interaction (MANOVA,F(7,168) = 3.2, p < 0.01), so each minute was considered separately. DH-lesioned animals were not significantly more active than controls during the first minute of the test (F(1,24) = 0.9, p > 0.3; however, they were numerically elevated: sham = 12.8 ± 1.7 crossovers; DH = 15.6 ± 2.5), but were markedly hyperactive during the next 3 min (F(1,24) values > 6.7,p < 0.02). DH-lesioned animals also failed to show significant habituation during this phase (first vs fourth minute,F(1,10) = 0.4, p > 0.5), whereas sham animals showed a marked decrease in activity from the first to the fourth minute (F(1,14) = 11.7,p < 0.01). Surprisingly, when the bright lights were shined onto the maze during the last 4 min of the test, DH-lesioned animals were not hyperactive relative to shams (F(1,24) values < 3, p ≥ 0.1). Finally, both groups of animals exhibited a marked inhibition of crossovers when the lights were shined onto the open field relative to the dark phase (total of 4 min dark phase vs total of 4 min light phase; sham, F(1,14) = 10.4, p< 0.01; DH, F(1,10) = 31, p < 0.001).
These data provide support for the view that rats with DH lesions are not universally hyperactive; rather, external stimuli seem to control when hyperactivity is observed. The determinants of this hyperactivity remain unknown, but it does not appear to represent a generalized and uncontrollable loss of inhibition. Indeed, the present results suggest that DH lesion-induced hyperactivity can be readily abolished by external activity-suppressing stimuli. Moreover, hippocampal hyperactivity at least partially reflects a failure of habituation to a novel environment (and perhaps a spatial learning deficit), because their hyperactivity seemed to partially reflect a lack of the rapid habituation seen in sham animals.
DISCUSSION
In the present study, we determined whether time-limited RA of contextual fear could be demonstrated within-subjects. It was found that despite having a severe deficit in recently acquired contextual freezing, the same DH-lesioned rats exhibited no deficit in remotely acquired contextual fear or tone fear. These findings are consistent with the view that has emerged from work in animals and humans that the hippocampus plays a time-limited role in the consolidation of some forms of memory [Zola-Morgan and Squire (1990); Squire and Alvarez (1995); Knowlton and Fanselow (1998); but see Nadel and Moscovitch (1997)].
The within-subjects design used in the present study also affords considerable control because the deficit in recent contextual fear cannot be attributed to a compromised freezing response, as has been proposed recently (Good and Honey, 1997; McNish et al., 1997). By the performance view, hippocampal lesion-induced hyperactivity directly interferes with the freezing response. This account deals with the specificity of the deficit for recent contextual fear by assuming that high levels of freezing are resistant to disruption by hyperactivity, and further assuming that remote context fear and tone fear exceed recent context fear. The present data do not support these assumptions. Although the RA of recent contextual fear was severe, reducing average freezing by more than half, it was also quite specific. The very same animals did not exhibit any significant deficit in remote contextual fear or in tone-elicited fear. Moreover, in sham controls, remote and recent contextual fear differed by ∼1%, and tone fear was actually weaker than context fear. Thus, the amnesic effects of hippocampal lesions on fear conditioning cannot be predicted simply on the basis of the levels of fear observed. The present results are actually quite similar to the observations of Kim and Fanselow (1992), in which the temporal gradient was manipulated between-groups. Indeed, because of the substantial training given in the present experiments (5–10 trials with high-intensity shocks) and in Kim and Fanselow [(1992) 15 trials], the levels of contextual fear under which RA after hippocampal lesions have been observed are actually quite high.
Moreover, we have shown recently that even when lesions are made before training, where deficits in contextual fear are less substantial, hyperactivity cannot predict freezing deficits (Maren and Fanselow, 1997; Maren et al., 1998). Because it is proposed that hyperactivity directly disrupts freezing, a necessary condition for this view is that there be a strong, negative, within-group activity-freezing correlation, and there is not (Maren et al., 1998). That is, a direct relationship between each individual animal’s activity and freezing deficit should be observed. Because not all animals with hippocampal lesions exhibit hyperactivity (Maren and Fanselow, 1997; Maren et al., 1998), those without hyperactivity should exhibit no freezing deficit, and those with the most severe hyperactivity should have the most severe deficit. In a sample of 48 DH-lesioned rats, there was no significant correlation between hyperactivity and freezing deficit (Maren et al., 1998). Because hyperactivity and contextual freezing deficits produced by hippocampal lesions do not correlate, hyperactivity and freezing deficits are not causally related, and a basic condition of response competition is not met (Maren et al., 1998). The evidence that the deficit in the present study was specific to recent contextual fear and was not observed in equivalent levels of remote contextual fear or in weaker levels of tone fear further bolsters this conclusion. Indeed, it is apparent from examination of Figures 3A, 4, A and B, and5B that DH-lesioned rats can in fact stand still and freeze at the same levels as sham controls. The evidence that this hyperactivity can be abolished by external stimuli (Fig. 6) further strengthens the view that it need not compete with freezing behavior.
The discussion so far has focused on lack of support for the hyperactivity account of contextual freezing deficits; however, McNish et al. (1997) presented data showing that context-potentiated startle was not affected by electrolytic DH lesions made immediately after training. This suggests that the deficits in recent contextual freezing that we have found may be specific to freezing, because no deficit was observed with another index of fear: potentiated startle. However, there are problems in the design used by McNish et al. (1997) that require qualification of this conclusion. Because the measure of context-potentiated startle was a comparison of the presurgery training baseline and postsurgery testing, it is possible that hippocampal lesions themselves (through hyperactivity) elevated startle as an unconditional effect of the lesion (J. J. Kim, personal communication). This elevation of baseline may have occluded any amnesic effect on context-potentiated startle. Indeed, there is evidence that startle is elevated unconditionally in hippocampal rats (Coover and Levine, 1972; Tilson et al., 1987). In fact, although the direct comparison was not given, Lee and Davis (1997) reported data supporting this view. Electrolytic DH and knife-cut fornix lesions substantially elevated numerical baseline startle scores relative to electrolytic shams (Lee and Davis, 1997, their Table 1), although animals had been matched for startle performance scores before the lesion.
Thus, to establish that elevated startle in hippocampal rats was actually potentiated by contextual fear, a comparison of postsurgical startle between a trained and untrained context, or with an untrained hippocampal-lesioned control, is necessary. Alternatively, other behavioral indices of contextual fear may be helpful in resolving this discrepancy.
Nonetheless, some caution should be exercised when examining freezing in animals that exhibit hyperactivity. Although hippocampal hyperactivity was not sufficient to disrupt freezing under the conditions in the present study, it is possible that this hyperactivity, under other conditions, may interfere with freezing or contribute to the magnitude of the observed deficit. For example, we have reported that fornix lesions made before training produce greater contextual freezing deficits and greater hyperactivity than DH lesions (Maren and Fanselow, 1997). Moreover, it is obvious that a manipulation that produced more profound movement disturbance than DH lesions may disrupt freezing directly.
There is at least some evidence, however, that hippocampal lesion-induced hyperactivity observed on an open field may not be a disorder of motor inhibition, but may at least partially reflect a learning deficit similar to the contextual learning deficit. In Experiment 3, the robust hyperactivity observed on an open field appeared to be partially attributable to impaired habituation to the test environment (Fig. 6), although hippocampal rats may eventually habituate after repeated presentation (Nadel, 1968). Interestingly, one can speculate that the 1–2 min time course required to habituate for sham animals is similar to what we have argued is the time for the animal to form a representation of the context. Rats given electric shocks with brief context placement-to-shock intervals (<1.5 min) exhibit a deficit in context conditioning known as the immediate shock deficit. In fact, if the placement-to-shock interval is short enough, rats may not exhibit any context conditioning at all (Fanselow, 1986,1990). We have argued that it is during this time that the intact hippocampus may form the representation of the contextual CS (Young et al., 1994; Maren et al., 1998).
In the present study, electrolytic lesions of the dorsal hippocampus produced a severe RA of recently acquired contextual fear. In contrast, these lesions did not produce deficits in remotely acquired contextual fear or tone fear. We have argued previously that the hippocampus plays a role in assembling a unified spatial or configural representation of the contextual CS, which is then consolidated and stored permanently elsewhere (Kim and Fanselow, 1992; Young et al., 1994). As such, hippocampal rats may have a deficit specifically in recognizing the recently acquired context. By one view, this is a reflection of a generalized spatial learning deficit produced by hippocampal lesions (Nadel and Willner, 1980; Nadel et al., 1985). Indeed, in our preparation, context includes not only aspects of the conditioning chamber but also distal features and geometry of the room, which partially control contextual freezing (our unpublished observations). Conditioning context may also be viewed as configural in nature, because the assembly of a unified contextual CS may involve the configuration of multiple elemental cues (Sutherland and Rudy, 1989;Frankland et al., 1998).
Nadel and Moscovitch (1997), however, have argued recently that time-limited RA is observed only after partial damage to the hippocampus, although this conclusion remains in dispute (Knowlton and Fanselow, 1998). Thus, because only the effects of DH lesions were examined in the present study, the role of ventral hippocampus in fear conditioning remains unexplored.
Nonetheless, the present study found time-limited RA after damage to the hippocampal formation. Although many studies in rodents have involved the manipulation of temporal intervals between-groups, the present study examined the temporal gradient within-subjects (Zola-Morgan and Squire, 1990). The present data provide further support for the view that the hippocampus plays a time-limited role in the storage of some forms of memory (Squire and Alvarez, 1995; Knowlton and Fanselow, 1998). The statistical power and control afforded by this Pavlovian contextual fear-conditioning preparation may be a useful method to advance our knowledge of this process.
Footnotes
This research was supported by National Science Foundation Grant IBN 9723295 (M.S.F). S.A. was supported by a University of California at Los Angeles (UCLA) C. M. Kernan Dissertation Year Fellowship. S.M. was supported by individual National Institute of Mental Health National Research Service Award MH 11061. We thank Bernard Balleine, Paul Frankland, Jeansok Kim, Barbara Knowlton, Frank Krasne, Tom O’Dell, Jennifer Sage, Alcino Silva, and two anonymous reviewers for their thoughtful comments on an earlier version of this manuscript. We also thank Jennifer Sage and Jennifer Spooner for excellent technical assistance. An earlier version of this manuscript was part of S.A.’s UCLA doctoral dissertation.
Correspondence should be addressed to Stephan Anagnostaras, University of California at Los Angeles, Department of Psychology, 1285 Franz Hall, Los Angeles, CA 90095-1563.
REFERENCES
- 1.Blanchard DC, Blanchard RJ, Lee MC, Fukunaga KK. Movement arrest and the hippocampus. Physiol Psychol. 1977;5:331–335. [Google Scholar]
- 2.Bolhuis JJ, Stewart CA, Forrest EM. Retrograde amnesia and memory reactivation in rats with ibotenate lesions to the hippocampus or subiculum. Q J Exp Psychol B Comp Physiol Psychol. 1994;47:129–150. [PubMed] [Google Scholar]
- 3.Bolles RC. Species–specific defense reactions and avoidance learning. Psychol Rev. 1970;77:32–48. [Google Scholar]
- 4.Cho YH, Kesner RP. Involvement of entorhinal cortex or parietal cortex in long-term spatial discrimination memory in rats: retrograde amnesia. Behav Neurosci. 1996;110:436–442. doi: 10.1037//0735-7044.110.3.436. [DOI] [PubMed] [Google Scholar]
- 5.Cho YH, Beracochea D, Jaffard R. Extended temporal gradient for the retrograde and anterograde amnesia produced by ibotenate entorhinal cortex lesions in mice. J Neurosci. 1993;13:1759–1766. doi: 10.1523/JNEUROSCI.13-04-01759.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coover GD, Levine S. Auditory startle response of hippocampectomized rats. Physiol Behav. 1972;9:75–77. doi: 10.1016/0031-9384(72)90268-5. [DOI] [PubMed] [Google Scholar]
- 7.Douglas RJ. The hippocampus and behavior. Psychol Bull. 1967;67:416–442. doi: 10.1037/h0024599. [DOI] [PubMed] [Google Scholar]
- 8.Douglas RJ, Isaacson RL. Hippocampal lesions and activity. Psychon Sci Sect Anim Physiol Psychol. 1964;1:187–188. [Google Scholar]
- 9.Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 10.Fanselow MS. Associative vs topographical accounts of the immediate shock-freezing deficit in rats: implications for the response selection rules governing species-specific defense reactions. Learn Motiv. 1986;17:16–39. [Google Scholar]
- 11.Fanselow MS. Factors governing one-trial contextual conditioning. Anim Learn Behav. 1990;18:264–270. [Google Scholar]
- 12.Fanselow MS, Bolles RC. Naloxone and shock-elicited freezing in the rat. J Comp Physiol Psychol. 1979;93:736–744. doi: 10.1037/h0077609. [DOI] [PubMed] [Google Scholar]
- 13.Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998;112:1–12. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- 14.Good M, Honey RC. Dissociable effects of selective lesions to hippocampal subsystems on exploratory behavior, contextual learning, and spatial learning. Behav Neurosci. 1997;111:487–493. doi: 10.1037//0735-7044.111.3.487. [DOI] [PubMed] [Google Scholar]
- 15.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 16.Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- 17.Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- 18.Knowlton BJ, Fanselow MS. The hippocampus, consolidation, and on-line memory. Curr Opin Neurobiol. 1998;8:293–296. doi: 10.1016/s0959-4388(98)80154-2. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y, Davis M. Role of the septum in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6424–6433. doi: 10.1523/JNEUROSCI.17-16-06424.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maren S, Fanselow MS. Electrolytic lesions of the dorsal hippocampus, fimbria-fornix, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- 21.Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 22.Maren S, Anagnostaras SG, Fanselow MS. The startled seahorse: is the hippocampus necessary for contextual fear conditioning? Trends Cogn Sci. 1998;2:39–42. doi: 10.1016/s1364-6613(98)01123-1. [DOI] [PubMed] [Google Scholar]
- 23.McNish KA, Gewirtz JC, Davis M. Evidence of contextual fear after lesions of the hippocampus: a disruption of freezing but not fear-potentiated startle. J Neurosci. 1997;17:9353–9360. doi: 10.1523/JNEUROSCI.17-23-09353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris RGM. An attempt to dissociate “spatial mapping” and “working memory” theories of hippocampal function. In: Seifert W, editor. The neurobiology of the hippocampus. Academic; London: 1983. pp. 405–432. [Google Scholar]
- 25.Nadel L. Dorsal and ventral hippocampus lesions and behavior. Physiol Behav. 1968;3:891–900. [Google Scholar]
- 26.Nadel L, Moscovitch M. Memory consolidation, retrograde am- nesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 27.Nadel L, Willner J. Context and conditioning: a place for space. Physiol Psychol. 1980;8:218–228. [Google Scholar]
- 28.Nadel L, Willner J, Kurz EM. Cognitive maps and environmental context. In: Balsam PD, Tomie A, editors. Context and learning. Erlbaum; London: 1985. pp. 285–406. [Google Scholar]
- 29.Olton DS, Becker JT, Handelmann GE. The hippocampus, space, and memory. Behav Brain Sci. 1979;2:313–365. [Google Scholar]
- 30.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 31.Reed JM, Squire LR. Retrograde amnesia of facts and events: findings from four new cases. J Neurosci. 1998;18:3943–3954. doi: 10.1523/JNEUROSCI.18-10-03943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;15:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol. 1995;5:178–183. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 34.Sutherland RJ, Rudy RJ. Configural association theory: the role of the hippocampal formation in learning, memory, and amnesia. Psychobiology. 1989;17:129–144. [Google Scholar]
- 35.Swanson LW. Brain maps: structure of the rat brain. Elsevier; New York: 1992. [Google Scholar]
- 36.Teitelbaum H, Milner P. Activity changes following partial hippocampal lesions in rats. J Comp Physiol Psychol. 1963;56:281–289. doi: 10.1037/h0047052. [DOI] [PubMed] [Google Scholar]
- 37.Tilson HA, Rogers BC, Grimes L, Harry GJ, Peterson NJ, Hong JS, Dyer RS. Time-dependent neurobiological effects of colchicine administered directly into the hippocampus of rats. Brain Res. 1987;408:163–172. doi: 10.1016/0006-8993(87)90368-4. [DOI] [PubMed] [Google Scholar]
- 38.Wiig KA, Cooper LN, Bear MF. Temporally graded retrograde amnesia following separate and combined lesions of the perirhinal cortex and fornix in the rat. Learn Mem. 1996;3:313–325. doi: 10.1101/lm.3.4.313. [DOI] [PubMed] [Google Scholar]
- 39.Winocur G. Anterograde and retrograde amnesia in rats with dorsal hippocampal or dorsomedial thalamic lesions. Behav Brain Res. 1990;38:145–154. doi: 10.1016/0166-4328(90)90012-4. [DOI] [PubMed] [Google Scholar]
- 40.Woodward JA, Bonett DG, Brecht ML. Introduction to linear models and experimental design. Academic; San Diego: 1990. [Google Scholar]
- 41.Young SL, Bohenek DL, Fanselow MS. NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: immunization against amnesia by context preexposure. Behav Neurosci. 1994;108:19–29. doi: 10.1037//0735-7044.108.1.19. [DOI] [PubMed] [Google Scholar]
- 42.Zola-Morgan SM, Squire LR. The primate hippocampal formation: evidence for a time-limited role in memory storage. Science. 1990;250:288–290. doi: 10.1126/science.2218534. [DOI] [PubMed] [Google Scholar]