Abstract
The function of the β-amyloid protein precursor (βAPP), a transmembrane molecule involved in Alzheimer pathologies, is poorly understood. We recently reported the presence of a fraction of βAPP in cholesterol and sphingoglycolipid-enriched microdomains (CSEM), a caveolae-like compartment specialized in signal transduction. To investigate whether βAPP actually interferes with cell signaling, we reexamined the interaction between βAPP and GoGTPase. In strong contrast with results obtained with reconstituted phospholipid vesicles (Okamoto et al., 1995), we find that incubating total neuronal membranes with 22C11, an antibody that recognizes an N-terminal βAPP epitope, reduces high-affinity Go GTPase activity. This inhibition is specific of Gαo and is reproduced, in the absence of 22C11, by the addition of the βAPP C-terminal domain but not by two distinct mutated βAPP C-terminal domains that do not bind Gαo. This inhibition of Gαo GTPase activity by either 22C11 or wild-type βAPP cytoplasmic domain suggests that intracellular interactions between βAPP and Gαo could be regulated by extracellular signals. To verify whether this interaction is preserved in CSEM, we first used biochemical, immunocytochemical, and ultrastructural techniques to unambiguously confirm the colocalization of Gαo and βAPP in CSEM. We show that inhibition of basal Gαo GTPase activity also occurs within CSEM and correlates with the coimmunoprecipitation of Gαo and βAPP. The regulation of Gαo GTPase activity by βAPP in a compartment specialized in signaling may have important consequences for our understanding of the physiopathological functions of βAPP.
Keywords: βAPP, Alzheimer’s disease, microdomains, signal transduction, G-proteins, nervous system
The β-amyloid protein precursor (βAPP), a transmembrane precursor with a single transmembrane domain, is normally cleaved in its extracellular domain to yield soluble APP (Selkoe, 1994). In addition to this normal processing, βAPP is a precursor for the production of the amyloid polypeptides (βA4) found in senile plaques and associated with Alzheimer’s disease. It has been proposed that βA4 peptides are primarily derived from the 695 amino acid (aa) neuronal βAPP (LeBlanc et al., 1996; Simons et al., 1996). However, the cellular compartment(s) in which this cleavage occurs, the enzymes involved and, more generally, the physiological functions of the precursor have not been clearly elucidated.
Several studies suggest that βAPP signals via the membrane (Kang et al., 1987; Schubert et al., 1989; Koo et al., 1993; Allinquant et al., 1995) and, therefore, that its cytoplasmic domain associates with molecules specialized in signal transduction. Accordingly, a few cytosolic proteins that interact with βAPP C-terminal domain have been identified (Nishimoto et al., 1993; Fiore et al., 1995; Chow et al., 1996; Guénette et al., 1996; Hardy, 1997; Yan et al., 1997;Zambrano et al., 1997).
Among the latter are heterotrimeric Go-proteins, as suggested by the following observations. First, Gαocoimmunoprecipitates with βAPP (Nishimoto et al., 1993). Second, in reconstituted phospholipid vesicles containing Go and βAPP, stimulation of βAPP with a monoclonal antibody directed against its N-terminal domain increases the turnover of GoGTPase activity (Okamoto et al., 1995). Third, a familial Alzheimer’s disease-associated mutated form of βAPP constitutively activates Go in reconstituted vesicles and, if expressed in several cell lines, induces apoptosis through a mechanism involving the G-protein βγ complex (Giambarella et al., 1997).
In this context, the presence of a fraction of βAPP in membrane microdomains with physical–chemical properties identical to those of caveolae (Bouillot et al., 1996) is highly significant. Neuronal microdomains lack the scaffolding protein caveolin, which is the signature of caveolae in many cell types (Parton, 1996), including astrocytes (Cameron et al., 1997). However, like caveolae, these cholesterol and sphingolipid-enriched membranes (CSEM) represent a site of accumulation for several cell-surface receptors, glycosyl phosphatidylinositol (GPI)-linked glycoproteins, and signaling molecules (Parton, 1996; Simons and Ikonen, 1997; Wu et al., 1997).
The presence of βAPP CSEM has been disputed (Parkin et al., 1997), but also confirmed, by three groups who reported that, within these domains, βAPP colocalizes with α-secretase (Ikezu et al., 1998) and with β1–40 and β1–42 amyloid peptides (Lee et al., 1998; Simons et al., 1998). It is very important to clarify this issue, which bears consequences for our understanding of βAPP functions, and to verify whether βAPP, within CSEM, interacts physiologically with signaling molecules. This is why we have further investigated the physiological interaction of βAPP with heterotrimeric G-proteins. Using several immunocytochemical and biochemical protocols, we demonstrate that βAPP and Gαo are colocalized within CSEM from embryonic neurons in which they interact physiologically and physically.
MATERIALS AND METHODS
Immunocytochemistry and immunoprecipitation
Polyclonal antibodies against Gαo, Gαi2, βAPP C-terminal domain, and F3/F11 were kindly provided by Drs V. Homburger [Centre National de la Recherche Scientifique (CNRS), Montpellier, France], P. Frey (Sandoz, Berne, Switzerland), and G. Rougon (CNRS, Marseille, France), respectively. The 22C11 anti-βAPP monoclonal antibody was from Boehringer Mannheim, and the anti-myc antibody was obtained from Dr. J. Bishop (University of California, San Francisco, CA) (Evan et al., 1985). The specificity of all antibodies was verified by Western blotting. CT-15 and D2–2 antibodies were obtained from Dr S. S. Sisodia (Johns Hopkins University, Baltimore, MD) (Sisodia et al., 1993; Slunt et al., 1994); they respectively recognize βAPP and amyloid precursor-like protein 2 (APLP2) and allowed us to verify by Western blotting that in the embryonic cultures or tissues [embryonic day 15–16 (E15–E16) plus 4–5 d in vitro or E19 embryos] used in this study, the 22C11 antibody and the polyclonal antibody from Dr P. Frey specifically recognize βAPP and not APLP2 (Fig. 1).
Fig. 1.
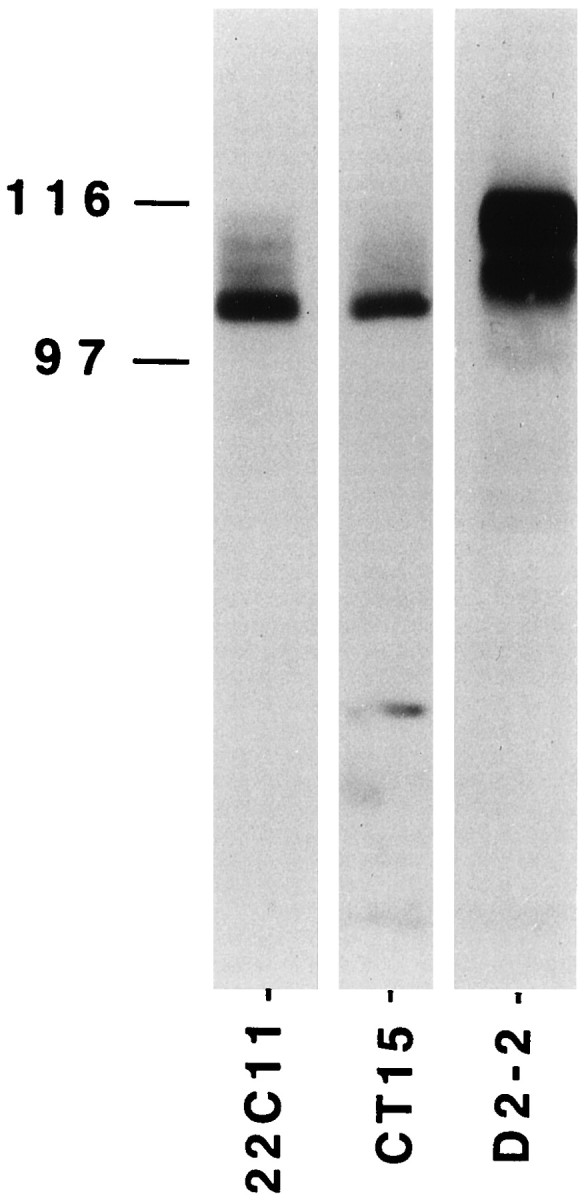
Western blotting of βAPP and APLP2. Extracts from 106 E16 rat cortical neurons cultured for 5 d were loaded on 7% SDS-PAGE and immunoblotted using either 22C11 or CT15, two antibodies recognizing βAPP or D2–2, an antibody specific to APLP2. The protein bands revealed with 22C11 and CT15 are very similar and differ from that reacting with D2–2.
Immunocytochemistry on primary corticostriatal rat cultures was performed as described previously (Allinquant et al., 1994). For immunoprecipitation, 40 μg of Triton X-100-insoluble membranes in 500 μl GTPase buffer (see below) was adjusted to 100 μmMgSO4, 100 nm GTP, and 150 mm NaCl, and the Gαo antibody was added overnight at 4°C before solubilization by 2%n-octylglucoside for 1 hr at 4°C and centrifugation (14,000 × g, 4°C, 15 min). The supernatants were mixed with protein A–Sepharose saturated with 2% bovine serum albumin in 20 mm HEPES and 150 mm NaCl, pH 7.5. After 3 hr at room temperature (RT), the beads were centrifuged, washed 5 times in 20 mm HEPES and 150 mm NaCl, pH 7.5, resuspended in 5% SDS–Laemmli buffer, boiled at 100°C for 10 min, and centrifuged at RT (14,000 ×g, 15 min). Proteins in the supernatants were separated by SDS-PAGE before Western blotting. In some experiments, we used the more stringent protocol described by Rousselet et al. (1988).
For Gαo purification on βAPP C-terminal (βAPP-Cter) affinity columns, peptides fused with a myc-tag (see below) were incubated overnight at 4°C with an anti-myc monoclonal antibody protein A–Sepharose column. The beads were washed twice, incubated (4°C, 8 hr) with 30 μg of fusion peptides in the presence of protease inhibitors (1 mm Pefablock, 1 μm leupeptin, 1 μm pepstatin, and 0.3 μm aprotinin), washed twice again, and further incubated overnight at 4°C with 50 μg of membranes solubilized in 2%n-octylglucoside, 100 μmMgCl2, and protease inhibitors. After five washes in the same buffer, the proteins were eluted in 5% SDS–Laemmli buffer, and the presence of Gαo was analyzed by Western blot.
Electron microscopy
COS-7 cells were transfected with a caveolin expression plasmid as described by Joliot et al. (1997) and grown for 48 hr on golden grids coated with formvar. Alternatively, E16 rat embryonic cortex were dissociated, and the cells were cultured for 4–5 d on grids precoated with formvar and fibronectin (10 μg/ml). Cells were then treated according to Stoorvogel et al. (1996), except that peroxidase-labeled cholera toxin B subunit (CTX) was used as a cross-linking agent. To this end, the cells were incubated (5–10 min, RT) with peroxidase-labeled CTX (8 μg/ml in serum free medium), washed three times with serum free medium, and further incubated (30 min, 4°C) in freshly prepared 3–3′-diaminobenzidine (1.5 mg/ml in 20 mmHEPES, pH 7.0, 70 mm NaCl, 50 mmascorbic acid, and 0.02% H2O2). After three rinses (10 min each, 4°C) in 80 mm PIPES buffer, pH 7.0, the cells were washed (30 min, 4°C) in extraction buffer (80 mm PIPES, pH 7.0, 1 mm EGTA, 0.5 mmMgCl2, 5 mm ascorbic acid, and 0.5% Triton X-100), rinsed several times in 80 mm PIPES, pH 7.0, fixed for 1 hr with 2% paraformaldehyde plus 0.2% glutaraldehyde in phosphate buffer, and finally washed in PBS, pH 7.4 (PBS).
For immunocytochemistry, cells treated and fixed as above were incubated with 50 mm ammonium chloride in PBS for 10 min, incubated overnight in blocking buffer (PBS plus 0.5% Triton X-100, 20 mm glycine, and 0.1% gelatin), and processed for immunogold labeling as described previously (Joliot et al., 1997). Briefly, COS-7 cells transfected with the caveolin plasmid were incubated with an anti-caveolin antibody (1:500; Transduction Laboratories, Lexington, KY) subsequently detected with 10 nm of gold-labeled Protein A Gold (PAG10). For double labeling, βAPP was decorated with the anti-βAPP C-terminal domain, PAG15 protein A was then inactivated with glutaraldehyde (Stoorvogel et al., 1996), and a second incubation with anti-Gαo and PAG10 was performed. The grids were finally post-fixed with glutaraldehyde, dehydrated in ethanol, and dried using a critical point-drying apparatus.
Preparation of crude membrane and CSEM
E19 rat cortex and striatum, freed of meninges, were homogenized using 10 strokes of Dounce homogenizer and three passages through a G26 needle in cold 0.25 m sucrose buffer A (10 mmTris, pH 7.4, 100 μm EDTA, and protease inhibitors). The homogenate was loaded on a 1.7 m sucrose cushion in buffer A and centrifuged (150,000 × g, 40 min, 4°C) in a SW41 rotor (Beckman Instruments). The membrane suspension collected at the 1.7 m/0.25 m interface was loaded on a second 1.7 m sucrose step. After centrifugation (150,000 × g, 40 min, 4°C), the membranes floating over 1.7 m sucrose were collected and washed in 10 mm Tris and 100 μm EDTA, pH 7.4. The pellet collected after centrifugation (150,000 × g, 30 min, 4°C) was resuspended in buffer A with or without 1% Triton X-100 (on ice) and centrifuged (150,000 × g, 40 min, 4°C). The pellet was washed again and resuspended in GTPase buffer for GTPase activity test or in 20 mm HEPES and 150 mmNaCl, pH 7.4, for protein quantification and immunoprecipitation.
Three independent protocols were used to prepare caveolae-like microdomains
Carbonate step gradients. Carbonate step gradients were performed according to Song et al. (1996). In brief, E19 brain tissues were homogenized with a Dounce homogenizer in 500 mm sodium carbonate, pH 11.0, sonicated, made 45% in sucrose, and placed at the bottom of a 5–35% discontinuous sucrose gradient in 25 mm MES, pH 6.5, and 0.15m NaCl (MBS) containing 250 mm sodium carbonate. After centrifugation in a Beckman SW41 rotor (150,000 × g, 16 hr, 4°C), 1 ml fractions were collected, diluted in MBS, and centrifuged (150,000 × g, 30 min, 4°C), and each pellet was resuspended in GTPase buffer or in 20 mm HEPES and 150 mm NaCl, pH 7.4.
OptiPrep preparation. Membranes isolated on a Percoll (Pharmacia) step gradient (Smart et al., 1995) were sonicated and loaded at the bottom of a linear 10–20% OptiPrep (Nycomed Pharma, Oslo, Norway) gradient. After centrifugation at 52,000 ×g for 90 min, 4°C (SW41 rotor, Beckman), the top five fractions (5 ml) were made 25% in OptiPrep (9 ml total), placed under 2 ml OptiPrep 5%, and centrifuged (52,000 × g, 90 min, 4°C). The opaque band collected in the 5% OptiPrep fraction was diluted in MBS and centrifuged (150,000 × g, 16 hr, 4°C), and the final pellet was resuspended in GTPase buffer or in 20 mm HEPES and 150 mm NaCl, pH 7.4.
Sucrose gradient containing Triton X-100. According toSargiacomo et al. (1993), tissues homogenized in MBS plus 1% Triton X-100 were adjusted to 40% sucrose, placed at the bottom of a continuous 5–30% sucrose gradient in MBS, and centrifuged (150,000 × g, 16 hr, 4°C). One milliliter fractions were collected, diluted in MBS, and centrifuged. Each pellet was resuspended in GTPase buffer or in 20 mm HEPES and 150 mm NaCl, pH 7.4.
Preparation of intracytoplasmic βAPP recombinant peptides
The HoxA5 sequence present in pTmHoxA5R (Chatelin et al., 1996) was deleted (SacI and BssH2) and replaced by a synthetic oligonucleotide coding for the P spacer sequence RQIKIWFQNRRMKWKK (Prochiantz, 1996; Derossi et al., 1998). The sequence coding for βAPP cytoplasmic domain (649–695 aa) was added in 3′ of the spacer using synthetic oligonucleotides. After transformation, the bacterial (DE3Lys S) pellets of 500 ml of culture, induced by isopropyl β-d-thiogalactoside for 3 hr, were resuspended in 20 mm HEPES, pH 7.9, 1 mm EDTA, 5 mm MgCl2, and 10 μg/ml DNase, frozen and thawed three times in liquid nitrogen, and adjusted to 8m urea. After centrifugation (20,000 × g, 4°C, 1 hr), the supernatants were dialyzed against 20 mmHEPES, pH 7.9, 1 mm EDTA, 5 mmMgCl2, and 0.25 m NaCl, and loaded onto heparin–Sepharose columns. The purity of the recombinant peptides eluted in 20 mm HEPES, 1 mm EDTA, and 1m NaCl was verified by SDS-PAGE and found to be above 80%.
High-affinity GTPase activity assay
GTPase assay was performed according to Charpentier et al. (1993). Briefly, 2–5 μg of membranes or microdomains were incubated for 30 min at 30°C in a total volume of 100 μl containing 20 mm HEPES, pH 7.5, 0.1 mm EGTA, 0.5 mm adenyl-5′-yl β,γ-imidodiphosphate, 0.1 mm ATP, 3 mm creatine phosphate, 0.2 mg/ml creatine kinase, and protease inhibitors. The 22C11 βAPP antibody was preincubated with the membranes 2 hr at 4°C or 1 hr at 37°C, and the reaction was started by adding GTP (100 nm or 20 μm final concentration), 200,000 cpm of [γ32P]GTP (Dupont NEN, Boston, MA), and MgSO4 (10 μm final concentration). Controls were incubated in the same conditions without 22C11. When indicated, 22C11 was preincubated (1 hr at RT) with its epitope (2 mm). The reaction was stopped with 400 μl of cold charcoal (5% washed several times in 20 mmNaH2PO4, pH 2.0). The mixture was kept on ice for 2–3 min and centrifuged at 13,000 × g for 10 min. Radioactivity in the supernatant was measured by scintillation counting. High-affinity GTPase activity was calculated by substracting the radioactivity released in the presence of 100 nm and 20 μm GTP, and the results were expressed in femtomoles of inorganic phosphate released per milligram of protein per minute. When indicated, the membranes were first incubated for 1 hr at 37°C with 7 μm recombinant peptides in 10 mm Tris, 100 μm EDTA, 200 mmNaCl, and protease inhibitors, centrifuged, and resuspended in GTPase buffer. All results presented in this study correspond to high-affinity GTPase activity. All reagents used in the GTPase experiments are from Sigma (St. Louis, MO) and Boehringer Mannheim.
ADP ribosylation
ADP ribosylation with pertussis toxin (PTX) or C3 (gift from Dr. P. Boquet, Institute National de la Santé et de la Recherche Médicale, Nice, France) was as described in Brabet et al. (1990). Membranes (25–50 μg) were incubated (1 hr, 37°C) in 100 μl containing 70 mm Tris-HCl, pH 7.5, 25 mmdithiothreitol, 20% glycerol, 1 mm EDTA, 0.1 mm MgCl2, 1 mm ATP, 10 mm thymidine, 10 mm nicotinamide, 1 μCi [32P]NAD, PTX (2.5 μg/100 μl) or C3 (the appropriate dilution of C3 was established for each batch of enzyme), and 0.5 μm NAD. For [32P]ADP ribosylation with CTX, membranes (25 μg of protein) were incubated for 1 hr at 37°C with 2–3 μCi of [32P]NAD (Dupont NEN) in 100 μl containing 50 μg of toxin, 100 mm phosphate buffer, pH 7.5, 1 mm ATP, 10 mm thymidine, 10 mm arginine, 100 μm GTP, and 100 μm MgCl2. The reaction was stopped with 400 μl of cold stop buffer (10 mm Tris-HCl, pH 7.5, 100 μm EDTA, and 200 μm NAD), and the membranes were washed twice in the same buffer. ADP ribosylated samples were alkylated withN-ethylmaleimide before separation by SDS-PAGE and quantification by phosphoimaging (Fuji). All reagents were from Sigma and Boehringer Mannheim.
RESULTS
Gαo interacts physiologically with the C-terminal part of βAPP
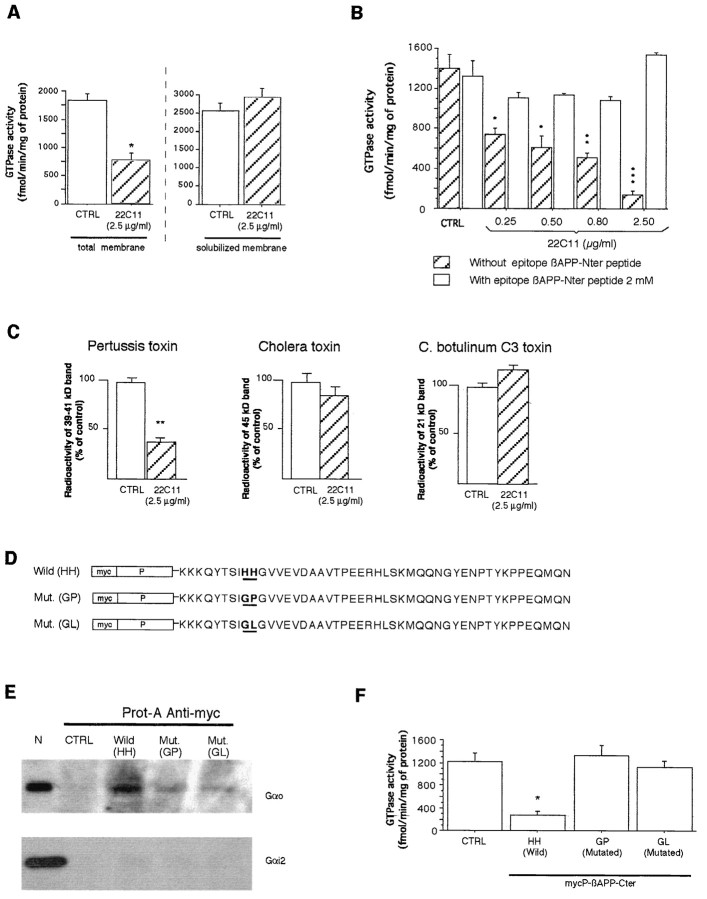
In a reconstituted system associating Go and βAPP within phospholipid vesicles, the anti-βAPP 22C11 antibody, which binds to a specific epitope in the extracellular domain of the protein, upregulates Go GTPase activity (Okamoto et al., 1995). To verify whether we could use the regulation of high-affinity GTPase activities to follow the interaction of βAPP with G-proteins, we applied a similar protocol to membranes prepared from the cortex/striatum of E19 rat embryos (the source of biological material used thereafter). In strong contrast with the findings of Okamoto et al. (1995), the addition of 22C11 on brain membranes decreased the high-affinity GTPase activity (Fig.2A). This effect was lost after membrane solubilization (Fig. 2A), was dose-dependent (Fig. 2B), and was specific because it was blocked at all 22C11 concentrations in the presence of the peptidic epitope recognized by the antibody (Fig.2B). One possible explanation for this difference between our results and those reported earlier is the presence in whole membranes of βAPP and/or Gαo molecular partners not present in the reconstituted system of Okamoto et al. (1995).
Fig. 2.
βAPP and Gαo interaction in total neuronal membranes. A, 22C11-induced decrease in GTPase activity (left) is lost after membrane solubilization (right). B, GTPase inhibition by 22C11 is dose-dependent and is abolished after incubation with the 22C11 epitope. Control (CTRL) is without 22C11.C, 22C11 decreases ADP ribosylation by PTX but not by CTX and C3. The radiolabeled substrates of PTX and CTX run with electrophoretic mobilities of 39–45 kDa and that of C3 with an electrophoretic mobility of 21 kDa (data not shown). D, Primary structures of the three recombinant peptides. In two constructions, the histidine doublet (HH) present in the wild-type βAPP cytoplasmic domain has been replaced by a GP or GL doublet. E, Gαo and Gαi2are present in neuronal extracts (N), but only Gαo binds to the wild-type βAPP cytoplasmic domain [Wild (HH)]. Gαo does not bind to protein A–agarose (CTRL) and binds only poorly to mutated C-terminal domains [Mut. (GP), Mut. (GL)]. F, Only the wild-type βAPP cytoplasmic domain gives a significant decrease in GTPase activity. Values are expressed as mean ± SEM; unpaired Student’s ttest or one-way ANOVA with post hoc Scheffe F test was used; *p < 0.01; **p < 0.001; ***p < 0.0001.
To identify the G-protein(s) involved in the downregulation of GTPase activity by 22C11, we analyzed the effect of the antibody on the ADP ribosylation of Gαo/Gαi, Gαs, and Rho by PTX, CTX, and C3, respectively (Gill and Merens, 1978; Li, 1992; Hauser et al., 1993). 22C11 only interfered with the ADP ribosylating activity of PTX, suggesting a preferential interaction with Gαo/Gαi (Fig. 2C). To investigate whether the C-terminal domain of βAPP interacts with Gαo/Gαi and whether this interaction downregulates the high-affinity GTPase, we cloned the intracytoplasmic domain (βAPP 649–695 aa) downstream of a myc-tag. We also cloned and produced two versions of the C-terminal domain in which the histidines doublet involved in Gαo/βAPP interaction (Nishimoto et al., 1993) were replaced with a glycine–proline (GP) or a glycine–leucine (GL) doublet (Fig. 2D). The three recombinant polypeptides were attached to an anti-myc–protein A matrix, and neuronal extracts solubilized in 2%n-octylglucoside were loaded onto the column. Figure2E illustrates that Gαo strongly binds to the wild-type C-terminal domain of βAPP and that mutating the histidine doublet dramatically reduces this interaction. In contrast, Gαi2 did not bind the C-terminal domain significantly. When wild-type and mutated domains were incubated with neuronal membranes, only the wild-type domain was able to reduce the high-affinity GTPase activity (Fig. 2F). The latter experiments strongly suggest that GTPase inhibition requires a specific recognition between Gαo and the βAPP C-terminal domain and raise the possibility that 22C11 modifies this interaction, resulting in a downregulation of Gαo GTPase activity.
Colocalization of βAPP and Gαo in axonal microdomains
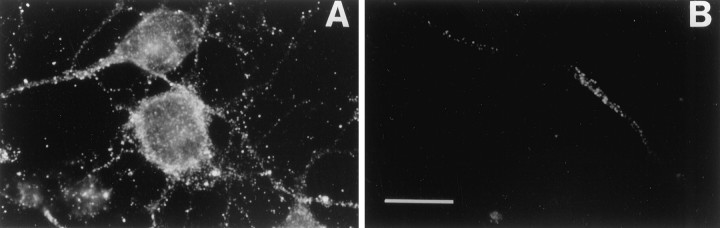
We have reported previously that βAPP is distributed into two distinct pools. As illustrated in Figure3A, a large pool can be visualized after permeabilization with Triton X-100, whereas, in the absence of detergent (Fig. 3B), only a small amount of βAPP can be decorated with the 22C11 antibody. The latter restricted pool is primarily axonal, with some staining of the cell body, and within membrane domains specialized in signal transduction and enriched in cholesterol and glycosphingolipids (Allinquant et al., 1994;Bouillot et al., 1996). The restricted pool present at the cell surface, although it only corresponds to ∼5% of total βAPP, is physiologically important because it localizes near or at the cell surface (Allinquant et al., 1994). Furthermore, all neosynthesized βAPP transits through this pool before being redistributed in other compartments via transcytosis (Simons et al., 1995; Yamazaki et al., 1995; Tienari et al., 1996).
Fig. 3.
βAPP is distributed in two pools. E15 neurons were fixed with 4% paraformaldehyde and processed for βAPP immunolocalization using the 22C11 antibody. A, Permeabilization with Triton X-100 demonstrates the presence of βAPP in all cell compartments. B, In the absence of Triton X-100, only short neurite segments are labeled (Allinquant et al., 1994). Scale bar, 5 μm.
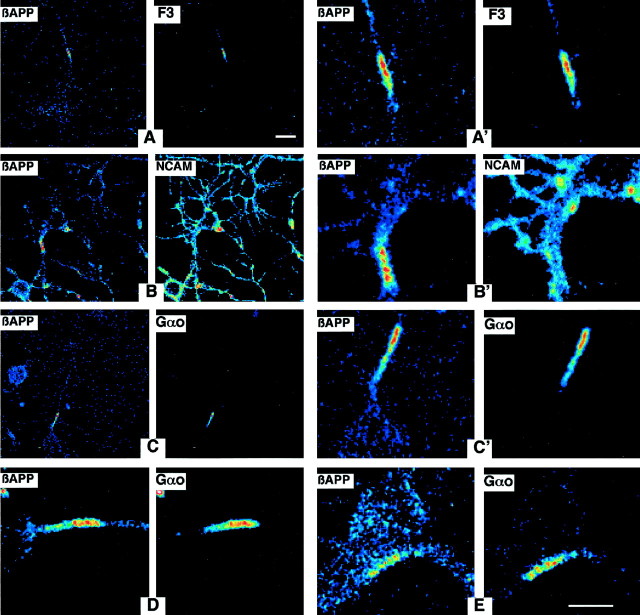
Figure 4, A and A′, illustrates, in corticostriatal neurons from E15 or E16 rat embryos cultured for 4–5 d and fixed with paraformaldehyde without detergent, the colocalization of βAPP and the GPI-linked glycoprotein F3/F11, taken as a CSEM marker (Bouillot et al., 1996). Using the same procedure, we observed that βAPP is often colocalized with Gαo within short axonal segments (Fig. 4C–E). These results strongly suggest that Gαo and βAPP are colocalized in axonal microdomains. This pattern of staining differs strikingly from that obtained with an anti-neural cell adhesion molecule (NCAM) antibody, which at this stage exclusively recognizes the non-GPI-linked NCAM isoforms. Indeed, as shown in Figure4, B and B′, NCAM is ubiquitously distributed on the membrane and, as opposed to F3/F11 or Gαo, does not colocalize with βAPP.
Fig. 4.
Colocalization of βAPP with F3 and Gαo in neurons in vitro. Cells cultured for 5 d in vitro were fixed with paraformaldehyde and double-stained for βAPP (22C11) and F3 (A,A′) or NCAM (B,B′), or Gαo (C–E). The confocal section with the highest pseudocolor intensity is presented for each double-labeling. Double detections (CY3 for βAPP and FITC for F3, NCAM, or Gαo) are shown at low (A,B, C) and high (A′,B′, C′, D,E) magnification. The low magnification illustrates that only limited areas of the axons are labeled (except for NCAM). The high magnification demonstrates a colocalization of βAPP with either F3 (A′) or Gαo (C′,D, E). In E, the strong staining corresponds to an axon in close apposition with a faintly βAPP-positive cell body. Scale bars:A, B, C, 20 μm; A′, B′, C′,D, E, 10 μm.
To verify the association of Gαo and βAPP in CSEM at the ultrastructural level, we adapted the technology recently developed by Stoorvogel et al. (1996), which permits the visualization and immunolabeling of specific compartment on nonsectioned cells. The original technology, aimed at the identification of proteins enriched in endosomes, is based on the use of horseradish peroxydase (HRP)–transferrin. Here, because we wanted to verify the presence of βAPP and Gαo in CSEM, we have used HRP-CTX, which binds to GM1, a glycosphingolipid highly enriched in CSEM. HRP-CTX was internalized by live cells grown on electron microscopy grids, and DAB was added. DAB polymerization at the surface of membrane domains enriched in GM1 (CSEM) makes them electron dense and cross links their proteins, thus leading to their specific fixation. Cytosolic proteins can then be removed by detergent treatment (Triton X-100), and the grids can be processed for whole-mount immunogold labeling.
We validated the technology by demonstrating that it permits the selective fixation and visualization of caveolae in COS-7 cells. Indeed, Figure 5A illustrates that a large number of structures preserved in this procedure can be labeled with an antibody directed against caveolin, a marker of caveolae in fibroblasts. Nonlabeled electron-dense material corresponds primarily to cytoskeleton (Stoorvogel et al., 1996). The same technique applied to neurons (Fig. 5B) demonstrates the presence and, in several cases, the colocalization, of βAPP and Gαoin electron-dense structures. Based on 13 pictures similar to that in Figure 5B, we counted that, of 747 Gαo beads, 15.7 ± 2.3% (mean ± SEM) colocalized with βAPP. Interestingly, when the cells were incubated with both Triton X-100 and saponin, a detergent that complexes cholesterol and therefore disrupts CSEM, this percentage (11 pictures and 446 beads) was reduced to 6.9 ± 0.8%, although for technical reasons, the detergents had to be added after DAB polymerization and thus presented a reduced efficiency. The percentage of βAPP associated with Gowithin caveolae is also ∼15%. Therefore, βAPP and Gocan be covisualized in caveolae-like vesicles at the ultrastuctural level. The percentage of βAPP and Go not colocalized suggests the existence of GM1-enriched membranes primarily containing either βAPP or Go and the possible association of Go and βAPP to the cytoskeleton.
Fig. 5.
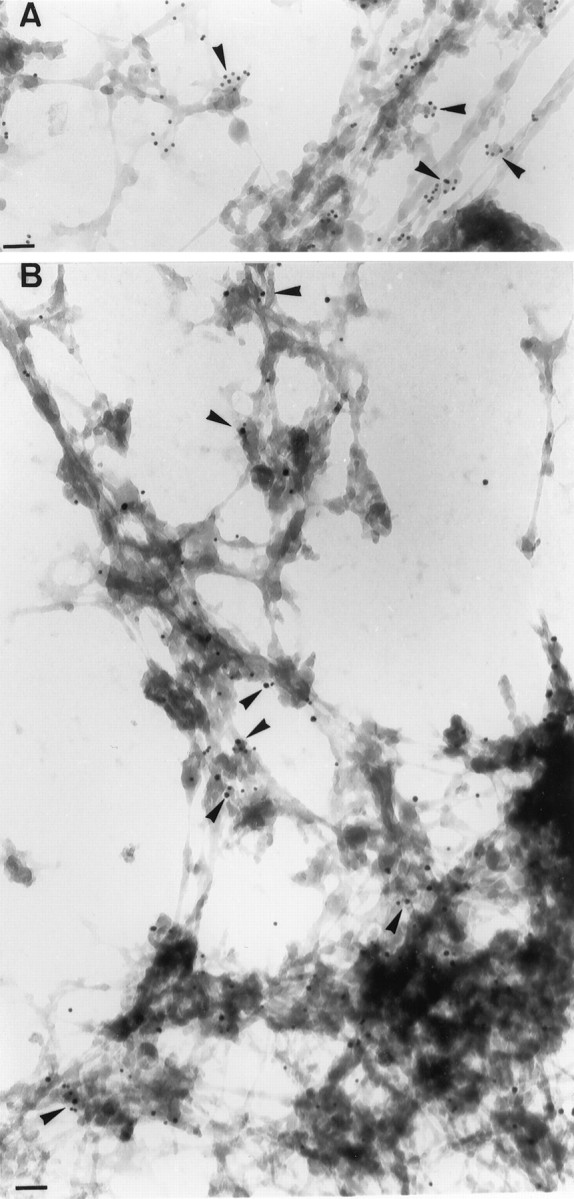
Colocalization of βAPP and Gαo in CTX-HRP stabilized structures. Cells were incubated with CTX-HRP and processed for transmission electron microscopy as indicated in Material and Methods. A, Caveolin expressed in COS-7 cells by transfection is associated with CTX-HRP crossed-linked structures (arrowheads, 10 nm beads). B, Examples of the intracellular neuronal colocalization of βAPP (15 nm beads) and Gαo (10 nm beads) in HRP crossed-linked structures are indicated by arrowheads. Scale bars, 100 nm.
Gαo and βAPP interactions are preserved in CSEM
The presence of βAPP and Gαo proteins in microdomains illustrated in Figures 4 and 5 was further verified by cell fractionation using three different protocols for caveolae or microdomain purification: carbonate step-gradient (Fig.6A) (Song et al., 1996), OptiPrep gradients (Fig. 6B) (Smart et al., 1995), and Triton-resistance plus sucrose gradient (Fig. 6C) (Bouillot et al., 1996). Figure 6 demonstrates that (1) glycoprotein F3/F11, βAPP, and Gαo colocalize in microdomains purified according to the three protocols, (2) a basal GTPase activity is present in the microdomains, (3) the intensity of the GTPase activity correlates with the amount of Gαo (Fig.6A,C), and (4) 22C11 antibody antagonizes this GTPase activity in the three preparations (Fig.6A–C).
Fig. 6.
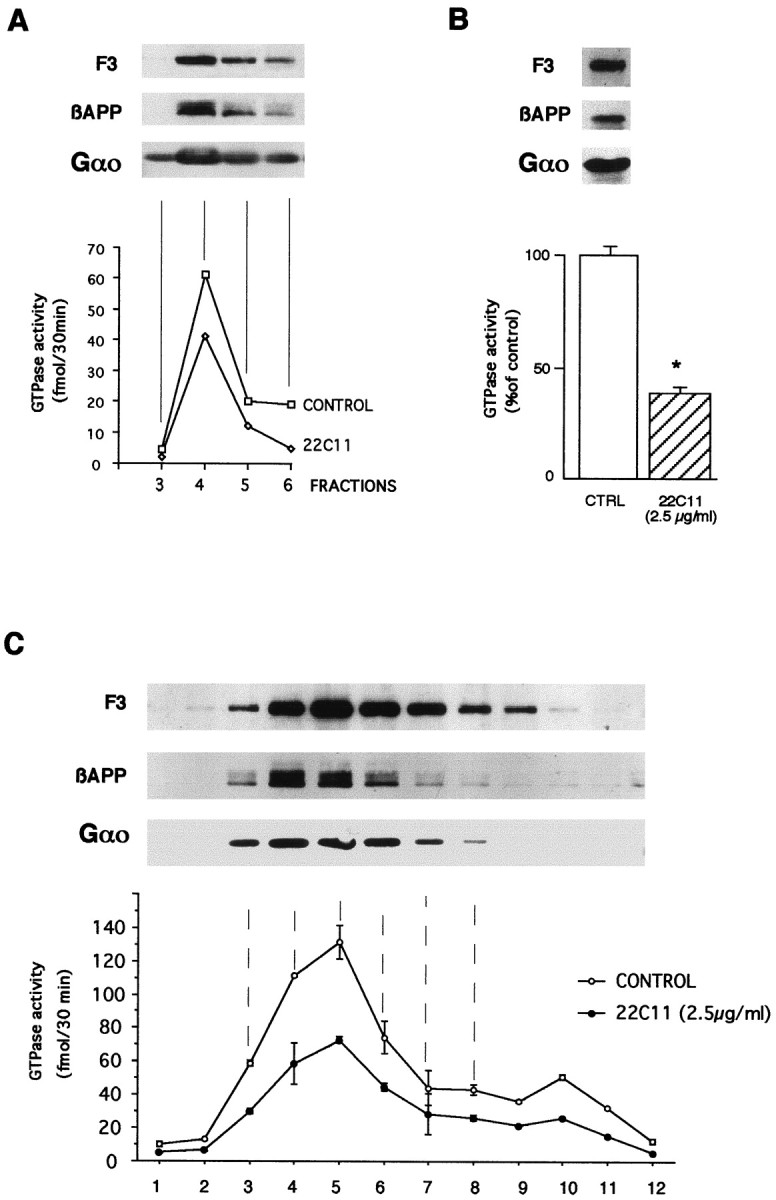
GTPase activity present in caveolar microdomains, isolated according to three different protocols, is decreased by 22C11. The presence of F3, βAPP, and Gαo was observed in the high-speed pellets of microdomains isolated by carbonate step (A), OptiPrep (B), and sucrose gradient in the presence of Triton X-100 (C,fractions 3–8). In the three types of preparation, the high-affinity GTPase activity present in microdomains was decreased by 22C11 (although the percentage of inhibition can vary between preparations). Note that the second step in the OptiPrep protocol (B) corresponds to the concentration within one fraction of cholesterol-rich membranes; *p < 0.01. In C, the GTPase activity in fractions 9–12 corresponds to the presence of Go in the latter fractions (data not shown), although this does not appear with the revelation time selected here.
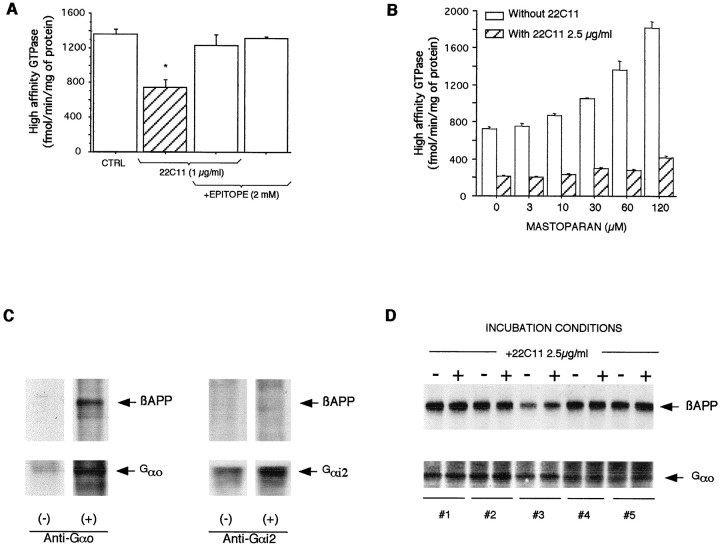
Microdomains isolated in the presence of the nonionic detergent Triton X-100 (Fig. 6C, fractions 3–8) contained >80% of total GTPase activity present in total insoluble Triton X-100 material. This confirms previous results (Allinquant et al., 1994) and justifies the use of the Triton-insoluble pellet from E19 cortex/striatum as an easy source of CSEM. Figure7A demonstrates that the basal GTPase activity present in this Triton-insoluble material is significantly inhibited by the addition of 22C11 and that this inhibition is antagonized by the 22C11 epitope and is thus specific.
Fig. 7.
The GTPase activity present in Triton X-100-resistant membranes decreased by 22C11 primarily corresponds to Gαo. A, The decrease in GTPase activity (*p < 0.005) induced by 22C11 is antagonized by the 22C11 epitope. B, Basal GTPase activity is stimulated by mastoparan, and the stimulation is dose-dependent. Inhibition by 22C11 is similar (∼70%) in the absence of mastoparan and at all mastoparan concentrations used in this experiment.C, βAPP is coimmunoprecipitated with Gαo. No βAPP immunoprecipitation is observed in the absence of antibody (−) or with an anti-Gαi2 antibody (+). D, Membranes from five independent experiments incubated with (+) or without (−) 22C11 in the conditions of the GTPase assay were immunoprecipitated with the anti-Gαo antibody. The immunoprecipitates were run on a gel, and both Gαo and coimmunoprecipitated βAPP were revealed with the appropriate antibodies.
To further identify the GTPase activity, we used mastoparan, a peptide that directly activates Gαo (at low and high concentrations) and Gαi (only at high concentrations) (Higashijima et al., 1988). Figure 7B illustrates that basal high-affinity GTPase activity is stimulated by mastoparan and that inhibition by 22C11 is high even at low mastoparan concentrations. This experiment, added to the fact that Go GTPase turnover is 10 times that of Gi (Neer et al., 1984), is in agreement with an inhibition of Gαo GTPase activity by 22C11 in CSEM. The latter proposed physiological interaction between the Gαo and βAPP correlates with a specific physical interaction in Triton X-100-insoluble membranes as shown by the coimmunoprecipitation of βAPP with Gαo, but not with Gαi2 (Fig. 7C), or with Gαi1 and Gαi3 using an antibody that recognizes the three proteins (data not shown). This interaction is very strong because coimmunoprecipitation occurs not only in the conditions of the GTPase assay but also in highly stringent conditions developed for the immunoprecipitation of transmembrane proteins (Rousselet et al., 1988).
Finally, we needed to verify that the decrease in GTPase activity could not be attributed to a specific degradation of Gαo after a conformation change induced by 22C11. To this end, CSEM was incubated with or without 22C11 in the conditions used for the GTPase assay, and Gαo was immunoprecipitated. Figure 7Dillustrates for five different experiments that incubation with 22C11 does not modify the amount of Gαo in the preparation nor that of βAPP coimmunoprecipitated with Gαo.
DISCUSSION
In this report, we demonstrate that β-APP and Gαocolocalize in neuronal CSEM and interact physically and physiologically in both total membranes and CSEM. Physical interactions are demonstrated by the coimmunoprecipitation of βAPP with Gαo and by the direct binding of Gαo on a βAPP C-terminal domain affinity column. The latter binding is specific because it is abolished by mutating a histidine doublet critical for βAPP/Gαo interaction (Nishimoto et al., 1993). The physiological interaction is demonstrated by the downregulation of Gαo GTPase activity after either addition of 22C11, a monoclonal antibody directed against a N-terminal epitope of βAPP, or by that of the wild-type βAPP cytoplasmic domain. We propose that (1) βAPP interacts directly with Gαo, (2) in total membranes and CSEM, the binding of βAPP to Gαo inhibits the basal Gαo GTPase activity, and (3) extracellular signals, here mimicked by 22C11, can regulate this interaction. The fact that this interaction also occurs within a compartment specialized in signal transduction raises the possibility that one of the physiological functions of βAPP is to regulate signal transduction.
The addition of 22C11 downregulates GTPase basal activity. This downregulation is dose-dependent and antagonized by the 22C11 epitope. Although we do not exclude that several GTPases could interact with βAPP, the evidence for a βAPP/Gαo interaction is strong. First, the incubation with 22C11 partially inhibits the ADP ribosylation by PTX, demonstrating interaction of βAPP with Gαo and/or Gαi. The absence of effect on the level of ADP ribosylation by CTX or C3 eliminates an implication of Gs and Rho. Second, the amphiphilic peptide mastoparan is a well known activator of Gαo and Gαi GTPase activity. However, the levels of mastoparan required for the activation of the two GTPases are very different, and below 10 μm, mastoparan preferentially activates Gαo. The fact that 22C11 inhibits the GTPase activity induced at low mastoparan concentrations demonstrates that Gαo is the 22C11 target. Third, Gαo, but not Gαi2, binds to the βAPP cytoplasmic domain, and this binding is lost if two histidines necessary for the latter binding are mutated. Accordingly, the inhibition of basal GTPase activity by the βAPP cytoplasmic domain requires that this histidine doublet be present. Finally, βAPP coimmunoprecipitates with Gαobut not with Gαi2. The identification of Gαo as the 22C11 target allowed us to verify that the addition of 22C11 does not provoke its specific degradation.
An interaction between βAPP and Gαo has already been reported by Okamoto and colleagues (1995), who demonstrated that in a reconstituted system associating phospholipids and purified Go and βAPP, the addition of 22C11 stimulates Go GTPase activity. In embryonic neuronal membranes and CSEM, our own observations confirm that 22C11 modulates an interaction between Gαo and βAPP. However, in strong contrast with the results of Okamoto and colleagues (1995), we find that the addition of 22C11 downregulates Go GTPase activity. The striking difference between the two set of data are probably attributable to the presence, in the brain membranes, of molecules that interact with βAPP and/or Go and are absent in the reconstituted system developed by Okamoto and colleagues (1995). In fact, although Go GTPase stimulation is the general rule, downregulations have also been reported, which can imply an interaction of a cytosolic protein with the βγ subunits (Schröder and Lohse, 1996) or a classical stimulation of G-protein-coupled receptors (Giguère et al., 1996; Ueda et al., 1996). The fact that the same downregulation is observed in the absence of 22C11 when the βAPP cytoplasmic domain is added to the preparation raises the possibility that, in our conditions, 22C11 acts by inducing a conformational change of the C-terminal domain or by freeing it from previous interactions.
In this study, we have followed the approach of Okamoto et al. (1995), and we have used 22C11 as a mean to stimulate βAPP. This does not give any clue on the natural βAPP ligands, if any. Several nonmutually exclusive possibilities exist. First, βAPP might interact with matrix components (Koo et al., 1993; Mattson et al., 1993; Lee et al., 1995; Williamson et al., 1996) or with a real ligand as yet unidentified and may directly transduce a signal by recruiting trimeric G-proteins and/or other signaling partners. Second, βAPP could interact in cis with other receptors or adhesion molecules and could regulate their signaling activity by interacting with cytoplasmic proteins, in particular Gαo. The possibility for a molecule with a single transmembrane domain to interact with a receptor coupled to a G-protein has been demonstrated in the case of the epidermal growth factor receptor, which signaling through a heterotrimeric G-protein can involve G-coupled receptors such as endothelin-1, lisophosphatidic acid, and thrombin receptors (Daub et al., 1996) or the muscarinic m1 receptor (Tsai et al., 1997). Similar interactions have been reported between PDGF and angiotensin II receptors (Linseman et al., 1995).
Many data presented here are centered on a subcellular compartment with specific biochemical and biophysical properties and specialized in cell signaling as illustrated by its content in several kinases and G-proteins (Olive et al., 1995; Li et al., 1996; Solomon et al., 1996;Song et al., 1996; Wu et al., 1997). They establish without ambiguity that βAPP and Gαo are present in this compartment. Indeed, this presence was observed using three different fractionation protocols, by immunocytochemistry in the absence of detergent, and by a whole immunoelectron microscopy technology allowing the specific preservation of GM1-enriched microdomains. In earlier reports (Allinquant et al., 1994; Bouillot et al., 1996), we have quantified the percentage of βAPP in the Triton-insoluble fraction and found that it corresponded only to 5% of total βAPP expressed in the cells. Thus, according to the gradient in Figure 5, βAPP in CSEM only represents 4–5% of total βAPP.
This percentage, although small, is functionally highly significant for the following reasons. First, what is most important in a signaling molecule is the percentage of protein accessible to the extracellular space. Although the amount of βAPP visualized in the absence of permeabilization and colocalized at the cell surface with Gαo is small, it is the only one in position to signal with molecules present in the extracellular space. Second, recent reports (Simons et al., 1995; Yamazaki et al., 1995; Tienari et al., 1996) strongly suggest that most neosynthesized βAPP is first sent to the axon and then endocytosed and transported retrogradely into the dendrites. The mechanism for axonal addressing involves the binding of the intramembranous domain of βAPP to the sphingolipid GM1 (Tienari et al., 1996). Because in the anterograde pathway GM1 is primarily present in caveolae and CSEM (Parton, 1996), it is likely that, even if at a given time microdomains only contain 5% of total βAPP, a much higher percentage of βAPP transits through CSEM before redistribution into the entire neuron.
Dysregulation of Gαo activity by βAPP may have important consequences through its downstream effects on the signaling activity of several receptors. In this context, it is interesting that Alzheimer’s disease might be associated with signaling defaults. Particularly striking examples of the latter association are (1) the impaired learning and long-term potentiation observed in transgenic mice overexpressing the C-terminal domain of βAPP (Naibantoglu et al., 1997), and (2) the abnormal amounts of free βγ subunits, which initiate apoptosis and DNA fragmentation in the case of βAPP mutations found in familial cases (Yamatsuji et al., 1996;Giambarella et al., 1997). Reciprocally, mediators that modulate the intracellular GTPase activity can interfere with the maturation or degradation of βAPP, as observed for some muscarinic or glutamatergic receptors (Nitsch et al., 1992; Lee et al., 1995; Slack et al., 1995). Finally and most importantly, interactions with Go or other molecular targets within microdomains could regulate βAPP processing and the production of secreted βAPP and of the amyloidogenic fragments (Ikezu et al., 1998; Lee et al., 1998).
Footnotes
This work was supported by Centre National de la Recherche Scientifique, Ecole Normale Supérieure, European Community Grant BIOMED 950524, and Direction des Recherches et Etudes Techniques Grant DRET 95166. D.G. is a Fondation pour la Recherche Médicale fellow. We thank Drs. Vincent Homburger, Graça Raposo, and Philippe Vernier for their advise during the course of this study.
Correspondence should be addressed to Alain Prochiantz, Centre National de la Recherche Scientifique, Unité de Recherche Associée 1414, Ecole Normale Supérieure, 46 rue d’Ulm, 75230 Paris Cedex 05, France.
REFERENCES
- 1.Allinquant B, Moya KL, Bouillot C, Prochiantz A. Amyloid precursor protein in cortical neurons: coexistence of two pools differentially distributed in axons and dendrites and association with cytoskeleton. J Neurosci. 1994;14:6842–6854. doi: 10.1523/JNEUROSCI.14-11-06842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allinquant B, Hantraye P, Mailleux P, Moya K, Bouillot C, Prochiantz A. Downregulation of amyloid precursor protein inhibits neurite outgrowth in vitro. J Cell Biol. 1995;128:919–927. doi: 10.1083/jcb.128.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouillot C, Prochiantz A, Rougon G, Allinquant B. Axonal amyloid precursor protein expressed by neurons in vitro is present in a membrane fraction with caveolae-like properties. J Biol Chem. 1996;271:7640–7644. doi: 10.1074/jbc.271.13.7640. [DOI] [PubMed] [Google Scholar]
- 4.Brabet P, Pantaloni C, Rodriguez M, Martinez J, Bockaert J, Homburger V. Neuroblastoma differentiation involves the expression of two isoforms of the α-subunit of Go. J Neurochem. 1990;54:1310–1320. doi: 10.1111/j.1471-4159.1990.tb01964.x. [DOI] [PubMed] [Google Scholar]
- 5.Cameron PL, Ruffin JW, Bollag R, Rasmussen H, Cameron RS. Identification of caveolin and caveolin-related proteins in the brain. J Neurosci. 1997;17:9520–9535. doi: 10.1523/JNEUROSCI.17-24-09520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charpentier N, Prézeau L, Carette J, Bertorelli R, Le Cam G, Manzoni O, Bockaert J, Homburger V. Transfected Go1α inhibits the calcium dependence of β-adrenergic stimulated cAMP accumulation in C6 glioma cells. J Biol Chem. 1993;268:8980–8989. [PubMed] [Google Scholar]
- 7.Chatelin L, Volovitch M, Joliot AH, Perez F, Prochiantz A. Transcription factor Hoxa-5 is taken up by cells in culture and conveyed to their nuclei. Mech Dev. 1996;55:111–117. doi: 10.1016/0925-4773(95)00478-5. [DOI] [PubMed] [Google Scholar]
- 8.Chow N, Korenberg JR, Chen X-N, Neve RL. APP-BP1, a novel protein that binds to the carboxy-terminal region of the amyloid precursor protein. J Biol Chem. 1996;271:11339–11346. doi: 10.1074/jbc.271.19.11339. [DOI] [PubMed] [Google Scholar]
- 9.Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of EGF receptor in signalling by G-protein-coupled receptor. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 10.Derossi D, Chassaing G, Prochiantz A. Trojan peptides: the penetratin system for intracellular delivery. Trends Cell Biol. 1998;8:84–87. [PubMed] [Google Scholar]
- 11.Evan GI, Lewis GK, Ramsay G. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiore F, Zambrano N, Minopoli G, Donini V, Duilio A, Russo T. The regions of the Fe65 protein homologous to the phosphotyrosine binding domain of the Shc bind the intracellular domain of the Alzheimer’s amyloid precursor protein. J Biol Chem. 1995;270:30853–30856. doi: 10.1074/jbc.270.52.30853. [DOI] [PubMed] [Google Scholar]
- 13.Giambarella U, Yamatsuji T, Okamoto T, Matsui T, Ikezu T, Murayama Y, Levine MA, Katz A, Gautam N, Nishimoto I. G-protein βγ complex-mediated apoptosis by familial Alzheimer’s disease mutant of APP. EMBO J. 1997;16:4897–4907. doi: 10.1093/emboj/16.16.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giguère A, Fortier S, Beaudry C, Gallo-Payet N, Bellabarba D. Effect of thyroid hormones on G-proteins in synaptosomes of chick embryo. Endocrinology. 1996;137:2558–2564. doi: 10.1210/endo.137.6.8641209. [DOI] [PubMed] [Google Scholar]
- 15.Gill DM, Merens R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci USA. 1978;75:3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guénette SY, Chen J, Jondro PD, Tanzi RE. Association of a novel human FE65-like protein with the cytoplasmic domain of the β-amyloid precursor protein. Proc Natl Acad Sci USA. 1996;93:10832–10837. doi: 10.1073/pnas.93.20.10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy J. Amyloid, the presenilins and Alzheimer’s disease. Trends Neurosci. 1997;20:154–159. doi: 10.1016/s0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- 18.Hauser D, Gibert M, Eklund MW, Boquet P, Popoff MR. Comparative analysis of C3 and botulinal neurotoxin genes and their environment in Clostridium botulinum types C and D. J Bacteriol. 1993;175:7260–7268. doi: 10.1128/jb.175.22.7260-7268.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higashijima T, Uzu S, Nakajima T, Ross EM. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G-proteins). J Biol Chem. 1988;263:6491–6494. [PubMed] [Google Scholar]
- 20.Ikezu T, Trapp BD, Song KS, Schlegel A, Lisanti MP, Okamoto T. Caveolae, plasma membrane microdomains for a-secretase-mediated processing of the amyloid precursor protein. J Biol Chem. 1998;273:10485–10495. doi: 10.1074/jbc.273.17.10485. [DOI] [PubMed] [Google Scholar]
- 21.Joliot A, Trembleau A, Raposo G, Calvet S, Volovitch M, Prochiantz A. Association of engrailed homeoproteins with vesicles presenting caveolae-like properties. Development. 1997;124:1865–1875. doi: 10.1242/dev.124.10.1865. [DOI] [PubMed] [Google Scholar]
- 22.Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Müller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 23.Koo EH, Park L, Selkoe DJ. Amyloid β-protein as a substrate interacts with extracellular matrix to promote neurite outgrowth. Proc Natl Acad Sci USA. 1993;90:4748–4752. doi: 10.1073/pnas.90.10.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeBlanc AC, Xue R, Gambetti P. Amyloid precursor protein metabolism in primary cell cultures of neurons, astrocytes and microglia. J Neurochem. 1996;66:2300–2310. doi: 10.1046/j.1471-4159.1996.66062300.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee RKK, Wurtman RJ, Cox AJ, Nitsch RM. Amyloid precursor protein processing is stimulated by metabotropic glutamate receptors. Proc Natl Acad Sci USA. 1995;92:8083–8087. doi: 10.1073/pnas.92.17.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S-J, Liyanage U, Bickel PE, Xia W, Lansbury PT, Kosik KS. A detergent-insoluble membrane compartment contains Aβ in vivo. Nat Med. 1998;4:730–734. doi: 10.1038/nm0698-730. [DOI] [PubMed] [Google Scholar]
- 27.Li J. Bacterial toxins. Curr Opin Struct Biol. 1992;2:545–556. [Google Scholar]
- 28.Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by Src tyrosine kinases. J Biol Chem. 1996;271:3863–3868. [PubMed] [Google Scholar]
- 29.Linseman DA, Benjamin CW, Jones DA. Convergence of angiotensin II and platelet-derived growth factor receptor signaling cascades in vascular smooth muscle cells. J Biol Chem. 1995;270:12563–12568. doi: 10.1074/jbc.270.21.12563. [DOI] [PubMed] [Google Scholar]
- 30.Mattson MP, Barger SW, Cheng B, Lieberburg I, Smith-Swintosky VL, Rydel RE. β-Amyloid precursor protein metabolites and loss of neuronal Ca2+ homeostasis in Alzheimer’s disease. Trends Neurosci. 1993;16:409–414. doi: 10.1016/0166-2236(93)90009-b. [DOI] [PubMed] [Google Scholar]
- 31.Naibantoglu J, Tirado-Santiago G, Lahsaïni A, Poirier J, Goncalves O, Verge G, Momoli F, Weiner SA, Massicotte G, Julien J-P, Shapiro ML. Impaired leaning and LTP in mice expressing the carboxy terminus of the Alzheimer amyloid precursor protein. Nature. 1997;387:500–505. doi: 10.1038/387500a0. [DOI] [PubMed] [Google Scholar]
- 32.Neer EJ, Lok JM, Wolf LG. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984;259:14222–14229. [PubMed] [Google Scholar]
- 33.Nishimoto I, Okamoto T, Matsuura Y, Takahashi S, Okamoto T, Murayama Y, Ogata E. Alzheimer amyloid protein precursor complexes with brain GTP-binding protein Go. Nature. 1993;362:75–79. doi: 10.1038/362075a0. [DOI] [PubMed] [Google Scholar]
- 34.Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992;258:304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto T, Takeda S, Murayama Y, Ogata E, Nishimoto I. Ligand-dependent G-protein coupling function of amyloid transmembrane precursor. J Biol Chem. 1995;270:4205–4208. doi: 10.1074/jbc.270.9.4205. [DOI] [PubMed] [Google Scholar]
- 36.Olive S, Dubois C, Schachner M, Rougon G. The F3 neuronal glycosylphosphatidylinositol-linked molecule is localized to glycolipid-enriched membrane subdomains and interacts with L1 and Fyn kinase in cerebellum. J Neurochem. 1995;65:2307–2317. doi: 10.1046/j.1471-4159.1995.65052307.x. [DOI] [PubMed] [Google Scholar]
- 37.Parkin ET, Hussain I, Turner AJ, Hooper NM. The amyloid precursor protein is not enriched in caveolae-like, detergent-insoluble membrane microdomains. J Neurochem. 1997;69:2179–2188. doi: 10.1046/j.1471-4159.1997.69052179.x. [DOI] [PubMed] [Google Scholar]
- 38.Parton RG. Caveolae and caveolins. Current Opin Cell Biol. 1996;8:542–548. doi: 10.1016/s0955-0674(96)80033-0. [DOI] [PubMed] [Google Scholar]
- 39.Prochiantz A. Getting hydrophilic compounds into cells: lessons from homeopeptides. Curr Opin Neurobiol. 1996;6:629–634. doi: 10.1016/s0959-4388(96)80095-x. [DOI] [PubMed] [Google Scholar]
- 40.Rousselet A, Fetler L, Chamak B, Prochiantz A. Rat mesencephalic neurons in culture exhibit different morphological traits in the presence of media conditioned on mesencephalic or striatal astroglia. Dev Biol. 1988;129:495–504. doi: 10.1016/0012-1606(88)90395-8. [DOI] [PubMed] [Google Scholar]
- 41.Sargiacomo M, Sudol M, Tang Z, Lisanti MP. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993;122:789–808. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schröder S, Lohse ML. Inhibition of G-protein βγ-subunit functions by phosducin-like protein. Proc Natl Acad Sci USA. 1996;93:2100–2104. doi: 10.1073/pnas.93.5.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schubert D, Jin LW, Saitoh T, Cole G. The regulation of amyloid β protein precursor secretion and its modulatory role in cell adhesion. Neuron. 1989;3:689–694. doi: 10.1016/0896-6273(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 44.Selkoe D. Normal and abnormal biology of the β-amyloid precursor protein. Annu Rev Neurosci. 1994;17:489–517. doi: 10.1146/annurev.ne.17.030194.002421. [DOI] [PubMed] [Google Scholar]
- 45.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 46.Simons M, Ikonen E, Tienari PJ, Cid-Arregui A, Mönning U, Beyreuther K, Dotti CG. Intracellular routing of human amyloid protein precursor: axonal delivery followed by transport to the dendrites. J Neurosci Res. 1995;41:121–128. doi: 10.1002/jnr.490410114. [DOI] [PubMed] [Google Scholar]
- 47.Simons M, De Strooper B, Multhaup G, Tienari PJ, Dotti CG, Beyreuther K. Amyloidogenic processing of the human amyloid precursor protein in primary cultures of rat hippocampal neurons. J Neurosci. 1996;16:899–908. doi: 10.1523/JNEUROSCI.16-03-00899.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc Natl Acad Sci USA. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sisodia SS, Koo EK, Hoffman PN, Perry G, Price DL. Identification and transport of full-length amyloid precursor proteins in rat peripheral nervous system. J Neurosci. 1993;13:3136–3142. doi: 10.1523/JNEUROSCI.13-07-03136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slack BE, Breu J, Petryniak MA, Srivastava K, Wurtman RJ. Tyrosine phosphorylation-dependent stimulation of amyloid precursor protein secretion by the m3 muscarinic acetylcholine receptor. J Biol Chem. 1995;270:8337–8344. doi: 10.1074/jbc.270.14.8337. [DOI] [PubMed] [Google Scholar]
- 51.Slunt HH, Thinakaran G, Von Koch C, Lo ACY, Tanzi RE, Sisodia SS. Expression of a ubiquitous, cross-reactive homologue of the mouse β-amyloid precursor protein (APP). J Biol Chem. 1994;269:2637–2644. [PubMed] [Google Scholar]
- 52.Smart EJ, Ying Y-S, Mineo C, Anderson RGW. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci USA. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solomon KR, Rudd CE, Finberg RW. The association between glycosylphosphatidylinositol-anchored proteins and heterotrimeric G-protein α subunits in lymphocytes. Proc Natl Acad Sci USA. 1996;93:6053–6058. doi: 10.1073/pnas.93.12.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. J Biol Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- 55.Stoorvogel W, Oorschot V, Geuze HJ. A novel class of clathrin-coated vesicles budding from endosomes. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tienari PJ, De Strooper B, Ikonen E, Simons M, Weidemann A, Czech C, Hartmann T, Ida N, Multhaup G, Masters CL, Van Leuven F, Beyreuther K, Dotti C. The β-amyloid domain is essential for axonal sorting of amyloid precursor protein. EMBO J. 1996;15:5218–5229. [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai W, Morielli AD, Peralta EG. The m1 muscarinic acetylcholine receptor transactivates the EGF receptor to modulate ion channel activity. EMBO J. 1997;16:4597–4605. doi: 10.1093/emboj/16.15.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ueda H, Misawa H, Fukushima N, Katada T, Ui M, Satoh M. A subtype of κ-opioid receptor mediates inhibition of high-affinity GTPase inherent in Gi1 in guinea pig cerebellar membranes. J Neurochem. 1996;66:845–851. doi: 10.1046/j.1471-4159.1996.66020845.x. [DOI] [PubMed] [Google Scholar]
- 59.Williamson TG, San Mok S, Henry A, Cappai R, Lander AD, Nurcombe V, Beyreuther K, Masters CL, Small DH. Secreted glypican binds to the amyloid precursor protein of Alzheimer’s disease (APP) and inhibits APP-induced neurite outgrowth. J Biol Chem. 1996;271:31215–31221. doi: 10.1074/jbc.271.49.31215. [DOI] [PubMed] [Google Scholar]
- 60.Wu C, Butz S, Ying Y-S, Anderson RGW. Tyrosine kinase receptors concentrated in caveolae-like domains from neuronal plasma membranes. J Biol Chem. 1997;272:3554–3559. doi: 10.1074/jbc.272.6.3554. [DOI] [PubMed] [Google Scholar]
- 61.Yamatsuji T, Matsui T, Okamoto T, Komatsuzaki K, Takeda S, Fukumoto H, Iwatsubo T, Suzuki N, Asami-Odaka A, Ireland S, Kinane B, Giambarella U, Nishimoto I. G-protein-mediated neuronal DNA fragmentation induced by familial Alzheimer disease-associated mutants of APP. Science. 1996;272:1349–1352. doi: 10.1126/science.272.5266.1349. [DOI] [PubMed] [Google Scholar]
- 62.Yamazaki T, Selkoe DJ, Koo EH. Trafficking of cell surface β-amyloid precursor protein: retrograde and transcytosis transport in cultured neurons. J Cell Biol. 1995;129:431–442. doi: 10.1083/jcb.129.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan SD, Soto C, Chen X, Zhu H, Al-Mohanna F, Collison K, Zhu A, Stern E, Saido T, Tohyama M, Ogawa S, Roher A, Stern D. An intracellular protein that binds amyloid-β peptide and mediates neurotoxicity in Alzheimer’s disease. Nature. 1997;389:689–695. doi: 10.1038/39522. [DOI] [PubMed] [Google Scholar]
- 64.Zambrano N, Buxbaum JD, Minopoli G, Fiore F, De Candia P, De Renzis S, Faraonio R, Sabo S, Cheetham J, Sudol M, Russo T. Interaction of the phosphotyrosine interaction/phosphotyrosine binding-related domains of Fe65 with wild-type and mutant Alzheimer’s β-amyloid precursor proteins. J Biol Chem. 1997;272:6399–6405. doi: 10.1074/jbc.272.10.6399. [DOI] [PubMed] [Google Scholar]