Abstract
Transcranial magnetic stimulation (TCMS) causes leg muscle contractions, but the neural structures in the brain that are activated by TCMS and their relationship to these leg muscle responses are not clearly understood. To elucidate this, we concomitantly recorded leg muscle responses and thoracic spinal cord-evoked potentials (SCEPs) after TCMS for the first time in 10 awake, neurologically intact human subjects. In this report we provide evidence of direct and indirect activation of corticospinal neurons after TCMS. In three subjects, SCEP threshold (T) stimulus intensities recruited both the D wave (direct activation of corticospinal neurons) and the first I wave (I1, indirect activation of corticospinal neurons). In one subject, the D, I1, and I2 waves were recruited simultaneously, and in another subject, the I1 and I2 waves were recruited simultaneously. In the remaining five subjects, only the I1 wave was recruited first. More waves were recruited as the stimulus intensity increased. The presence of D and I waves in all subjects at low stimulus intensities verified that TCMS directly and indirectly activated corticospinal neurons supplying the lower extremities. Leg muscle responses were usually contingent on the SCEP containing at least four waves (D, I1, I2, and I3).
Keywords: magnetic stimulation, motor-evoked potentials, spinal cord-evoked potentials, corticospinal tract, anesthesia, motor cortex (human)
Transcranial magnetic stimulation (TCMS) in humans and subhuman primates evokes a descending spinal cord-evoked potential (SCEP) that contains a direct (D) wave followed by several indirect (I) waves (Amassian et al., 1990; Edgley et al., 1990; Thompson et al., 1991; Burke et al., 1993; Kitigawa and Moller, 1994; Houlden et al., 1996; Kaneko et al., 1996; Nakamura et al., 1996). The D wave is thought to result from direct activation of corticospinal neurons (probably at the initial segment or at the first bend of the axon), and the I waves are thought to result from indirect activation of corticospinal neurons via interneurons excited by the stimulus (Patton and Amassian, 1954; Amassian et al., 1990, 1992;Edgley et al., 1990, 1992; Berardelli et al., 1991). The D and I waves are thought to descend in the corticospinal tracts and generate a sequence of EPSPs at the spinal motoneurons causing them to fire by temporal summation (Mills, 1991; Taylor et al., 1993). If the spinal motoneuron potential is already near threshold, then it is more likely that an earlier wave in the SCEP will cause it to fire (Day et al., 1987; Mills, 1991).
The effect of D and I waves on muscle responses evoked by TCMS has been estimated in awake humans using peristimulus time histograms (PSTHs) of motor unit firing (Day et al., 1989; Priori et al., 1993; Awiszus and Feistner, 1994). These studies have estimated that spinal motoneurons supplying hand muscles receive only I waves (Day et al., 1989), whereas spinal motoneurons supplying the tibialis anterior muscle (TA) receive D and I waves (Priori et al., 1993; Awiszus and Feistner, 1994). These conclusions are not based on actual D and I wave recordings but rather on the effect that D and I waves may have on individual motoneurons. Recently, both D and I waves were recorded directly from the cervical spinal cord after TCMS in awake humans (Kaneko et al., 1996; Nakamura et al., 1996), but it is uncertain whether those D and I waves provided input to spinal motoneurons for the upper extremities, lower extremities, or both because the recording electrode was positioned at or above the cervical enlargement. D and I waves recorded from the midthoracic spinal cord should reflect descending input primarily to spinal motoneurons supplying leg muscles.
The objective of this study was to elucidate the neural structures in the brain that are activated by TCMS and their relationship to leg muscle responses in awake human subjects. Accordingly, we characterized the SCEP waves recorded from the midthoracic spinal cord at different TCMS stimulus intensities. Then we determined the minimum number of waves necessary for activation of leg muscles during rest. Finally, we observed the effect of anesthetic on the SCEP waves.
MATERIALS AND METHODS
Patient population. Experiments were performed on 10 subjects (seven male) aged 31–62 years (mean, 49 years) who underwent surgery for dorsal column stimulator (DCS) implantation to control pain resulting from arachnoiditis after lower back surgery (three subjects), failed back syndrome (six subjects), and prostatitis (one subject). Analgesic medication ceased several hours before the experiments, and no analgesics were given during the experiments. All patients had normal motor and sensory examinations. Two other subjects were excluded from the study because of leg numbness and weakness. All experimental methods described below were approved by the Research Ethics Board at Sunnybrook Health Science Centre, and all patients gave their informed consent to participate in the experiments.
Surgical implantation of the dorsal column-stimulating electrode. Dorsal column stimulators were implanted for treatment of pain and not for the purpose of these experiments. Neuroleptic anesthesia (1–3 mg of midazolam, i.v.) was used in all patients. Each patient was positioned prone and then prepped and draped from the mid to lower thoracic spine. Local anesthesia using 1% xylocaine without adrenalin was used to infuse the subcutaneous tissue and paravertebral muscles. The paravertebral muscles were dissected from the spinous processes bilaterally through a midline incision. The inferior portion of the Th8 spinous process was removed, and the ligamentum flavum was opened laterally between Th8 and Th9 to allow for insertion of the Medtronic dorsal column stimulator (model 3586 or 3986; Medtronic Neurological, Minneapolis, MN) cephalad in the epidural space at the level of the body of Th8 in all patients (Fig.1). The Medtronic model 3986 was a later version of the model 3586 and had electrode specifications identical to that of the model 3586. The DCS electrode contained four independent electrodes arranged in a silicone rubber strip, and all four electrodes were in contact with the dura (Fig. 1). Electrode one of the DCS electrode array was most cephalad, and electrode four was most caudad. The spinal cord was stimulated through the DCS electrode, and the patient was questioned for sensations induced by stimulation. An attempt was made to place the electrode in the midline so that spinal cord stimulation induced paresthesia from the lower back into the hips and lower extremities bilaterally. When the position of the electrode was satisfactory, the incision was closed in multiple layers. The electrode cable was tunneled subcutaneously on the left side and passed through a small incision in the skin.
Fig. 1.
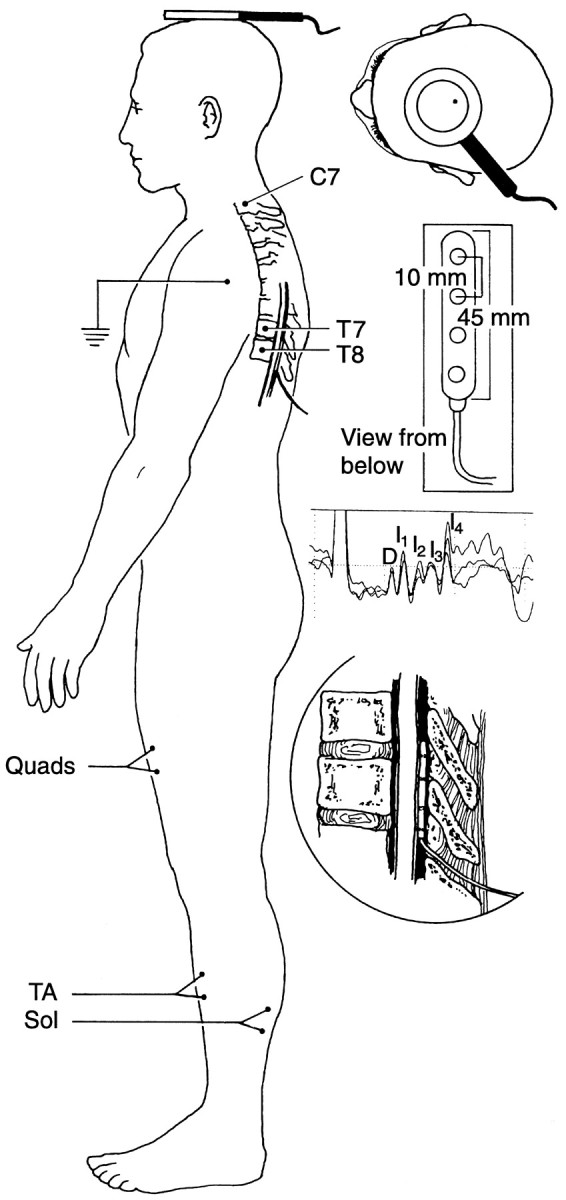
A schematic representation of the transcranial magnetic stimulation technique. The brain was stimulated by a Novametrix Magstim model 200 with a standard circular coil [internal diameter of coil, 5.4 cm; outer diameter, 11.6 cm (top right inset)]. Muscle recordings were obtained from electrode pairs over the muscle bellies of the left quadriceps (Quads), the TA, and the soleus (Sol). Spinal cord recordings were obtained from a four-lead DCS electrode positioned in the posterior epidural space at the level of the body of T8 (second inset from the top right and bottom right inset). The most rostral DCS lead (tip of the electrode) was referenced to the lead 20 mm more caudal. In this subject, TCMS produced a descending spinal cord-evoked potential that contained five peaks [D, I1,I2,I3, and I4(third inset from the top right)].
Transcranial magnetic stimulation. The brain was stimulated by a commercially available Novametrix Magstim model 200 (Novametrix Medical Systems) (Fig. 1). Capacitors were rapidly discharged through a circular coil (internal diameter of coil, 5.4 cm; outer diameter, 11.6 cm, consisting of 19 turns of copper wire) at a rate of less than one every 3 sec. The stimulator and coil were positioned at the head of the bed, away from the recording electrodes and amplifier head box, to reduce the recording of stimulus artifacts. The near-monophasic magnetic stimulator induced a current with a rapid rise to peak (100 μsec) that decayed to zero in <1 msec. The magnetic field strength at the center of the coil was ∼1.5 tesla. The largest induced current occurred 4.3 cm from the center of the coil (middle of coil windings) in a plane parallel to the coil (Meyer et al., 1991).
The position of the stimulating coil was measured from grid points that were marked on the scalp with a grease pencil on a line parallel to the nasion and vertex preauricular lines according to the 10–20 system (Jasper, 1958) such that the distance between points was 2 cm. Grid points were marked within a 4 cm radius of a point 4 cm anterior of Cz. When stimulating, the coil was laid flat on the scalp with the current flowing in the coil in a clockwise direction (B side of the stimulator up). The center of the coil was positioned over several scalp locations within the grid starting at the center of the grid (4 cm anterior to Cz) that is, on average, the optimal position for activation of the TA (Ingram et al., 1988; Hess et al., 1990; Meyer et al., 1991). The resting motor threshold for TA for each subject was determined by increasing the stimulus intensity in 3–5% increments. The stimulus intensity was expressed as a percentage of the maximum output of the stimulator. The coil position that produced a TA response with minimum stimulus intensity during rest was used in the experiment. The minimum stimulus intensity that produced at least three TA responses in six consecutive stimulations using a gain of 100 μV per division was termed “threshold” (T) (MacDonnell et al., 1991). The stimulus intensity was increased by 10% increments (10% of the maximum output of the stimulator) to T + 30% in three steps. The Magstim model 200 stimulator was externally triggered by a Cadwell Excel machine. The stimulus was given 9.73 msec after the onset of the sweep because the capacitors in the Magstim model 200 stimulator were discharged 9.73 msec after arrival of the trigger signal as a function of the stimulator design.
Recording techniques. The trial period for DCS was 5–7 d during which time the cable from all four electrodes was externalized. At the end of the cable were two bipolar mini phone jacks. Electrode one was connected to the tip of one phone jack, and electrode three was connected to the other tip. The centers of electrodes one and three were separated by 2.0 cm on the spinal cord (Fig. 1). The diameter of electrodes one and three was 4 mm, and the surface area was 12 mm2. The DCS electrodes were disconnected from the DCS pulse generator >1 hr before each experiment, and all patients reported no residual effects of DCS before the experiments. The DCS electrodes were changed into recording electrodes by connecting the two phone jack tips to G1 (DCS electrode one) and G2 (DCS electrode three) of a differential amplifier (Cadwell Laboratories, Kennewick, WA). The amplifier gain was 50 μV per division, and the recording bandpass was 30–5000 Hz.
Conduction velocity of the SCEP was calculated between sensorimotor cortex and Th8 for each subject. The distance between the site of activation in the cerebral cortex and the pyramidal decussation in the brainstem was estimated to be 13 cm (Rothwell et al., 1994). Accordingly, 13 cm was added to the distance measured from the inion to the body of Th8 and then divided by the latency to the initial negative deflection of the D wave.
In two subjects the conduction velocity between spinal electrodes at Th8 was determined by recording the SCEP from DCS electrode one (G1) referenced to the skin surface at Th8 (G2) and from DCS electrode three (G1) referenced to the skin surface at Th8 (G2). The surface electrode at Th8 was a Grass cup disk electrode (1 cm in diameter; Grass Instruments, Quincy, MA). For these studies, the recording bandpass was 500–5000 Hz to reduce low-frequency muscle artifacts contributed by the surface electrode. The conduction velocity of the SCEP at Th8 was calculated by dividing the distance between the two electrodes on the spinal cord (2 cm) by the difference in latency between the two SCEP waveforms recorded from each site.
Muscle responses were obtained from Grass EEG electrode pairs placed 3 cm apart over the muscle bellies of the left quadriceps (Quads), the TA, and the soleus (Sol) (Fig. 1). A ground plate electrode was placed on the shoulder.
The impedance of the DCS and muscle recording electrodes was kept <3 KΩ. The SCEP and muscle recordings were amplified and displayed using an eight channel Cadwell Excel machine with a sampling rate of 48 kHz per channel. The sweep duration was 70 msec. The sweep time could be decreased to allow for accurate measurement of the SCEP and muscle response latencies, and the display scale could be adjusted for optimum presentation of the SCEP and muscle responses.
TCMS was delivered to relaxed subjects lying supine on a bed. Subject relaxation was monitored by observing live background EMG from all three muscles and by listening to it through an audio monitor connected to the amplifiers. Audio feedback from all channels was heard simultaneously to ensure that background EMG was always absent during TCMS. For low TCMS intensities, the amplifier gain was set at 100 μV per division to detect low-amplitude muscle responses. The recording bandpass for muscle responses was 30–5000 Hz. Amplifier gain was decreased to accommodate larger muscle responses when necessary.
Measurement of SCEP and muscle responses. The SCEP was the continuous average of three to five responses. A minimum of two averages was superimposed for waveform reproducibility. A grand average containing both SCEP averages (6–10 responses) was analyzed at each stimulus intensity. A wave was defined as a negative peak followed by a positive trough. Each wave of the SCEP was measured for (1) latency to the initial negative deflection, (2) latency to negative peak, and (3) amplitude from negative peak to next positive trough (peak-to-trough). The SCEP duration and rectified area were measured from the initial negative deflection of the first wave to the positive trough of the last wave (rectified area was calculated by Digital Signal Processing software; Cadwell Laboratories). If the SCEP had more than one wave, then the interwave latency was calculated between the wave onsets (initial negative deflection) and between the negative peaks. A wave was considered present if the amplitude from the initial negative deflection-to-peak and from peak-to-trough was >0.5 μV.
Two compound muscle action potentials (CMAPs) from each muscle at each stimulus intensity were superimposed for waveform reproducibility. Onset latency and peak-to-trough amplitude were calculated from the average of the two CMAPs. If the CMAP had more than one peak or trough, then the maximum peak-to-trough amplitude was measured. Typically, the muscle recordings were obtained at the beginning of each SCEP average. A response was considered present if it had a maximum peak-to-trough amplitude of >20 μV.
Intraoperative studies. After the trial period for DCS, surgical internalization of the DCS apparatus was performed in the patients for whom DCS alleviated their pain. TCMS studies were repeated during surgery in three of these patients. After induction of anesthesia, inhalation agents (0.5–1.0% isoflurane, 55–66% N2O, and O2) were used to maintain a constant level of anesthesia in all three patients. Experiments were performed after they were on inhalation agents. For each patient, SCEP recordings were obtained from the same DCS electrode used in the awake experiments. Furthermore, for each patient, the recording variables and the position of the stimulating coil were the same as those used in the awake experiment so the effects of anesthesia on the SCEPs could be determined. The TCMS intensity was T + 40% in all three subjects.
RESULTS
Awake studies
The mean optimal stimulator coil position for activation of the left TA was 4 ± 0.7 cm anterior to Cz (International 10–20 system) and 1.6 ± 0.7 cm to the left of Cz. The stimulus intensity necessary for activation of the TA during rest varied from 50 to 100% of the maximum output of the stimulator (mean, 69 ± 18%).
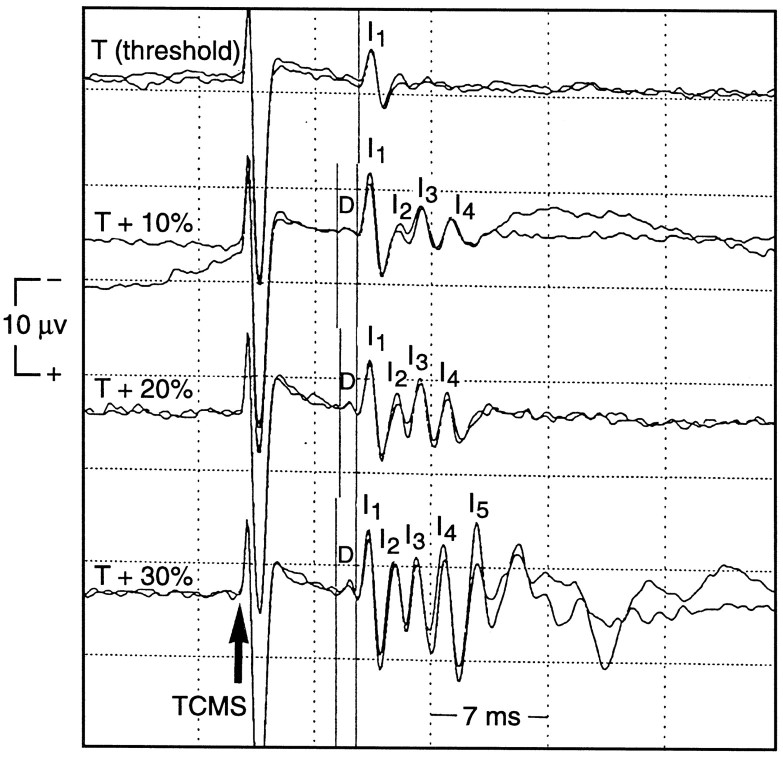
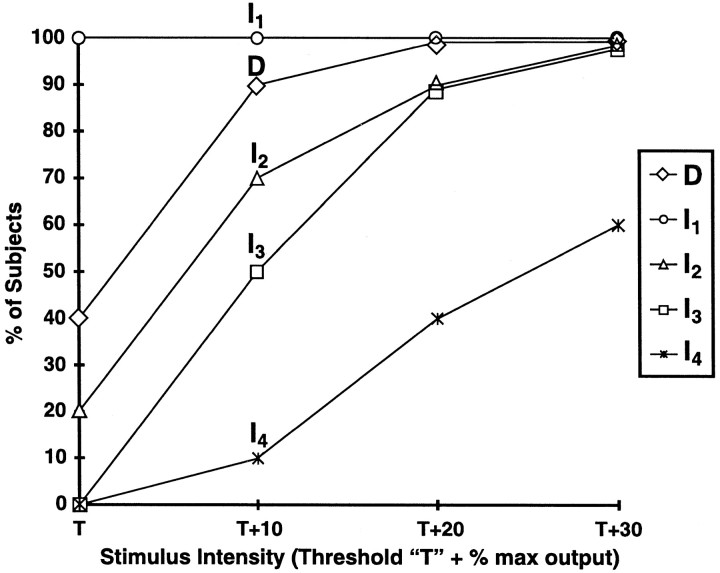
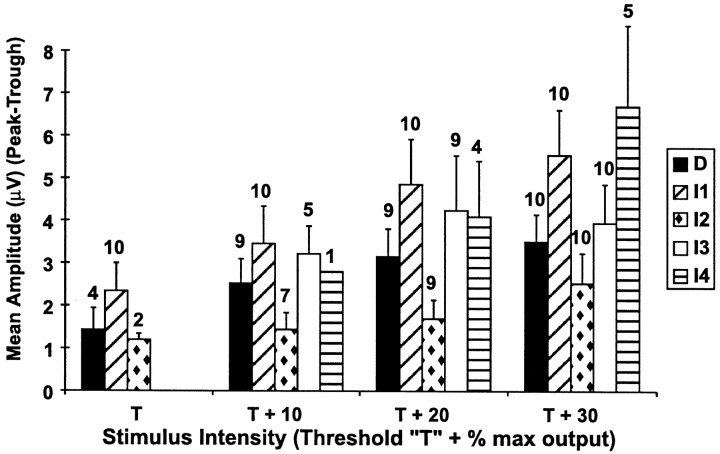
The T for activation of the SCEP varied between 40 and 60% of the maximum output of the stimulator (mean, 49 ± 7.5%). In three subjects, the D wave and the first I wave of the SCEP were recruited simultaneously. In one subject, the D, I1, and I2 waves were recruited simultaneously, and in another subject, the I1 and I2 waves were recruited simultaneously. In the remaining five subjects, only the I1 wave was recruited first. Increasing the stimulus intensity to T + 10% recruited the D wave in all subjects but one. At T + 10%, the I1 wave amplitude (peak-to-trough) was greater than the D wave amplitude in seven subjects (one subject did not have a D wave), equal to the D wave amplitude in two subjects, and less than the D wave amplitude in one subject. The pattern of D and I wave recruitment for one subject is shown in Figure 2. The order of activation of D and I waves for all subjects is shown in Figure3. The effect of TCMS intensity on the mean amplitude of each wave at each stimulus intensity is shown in Figure 4.
Fig. 2.
The pattern of activation of D andI waves recorded from an epidural electrode at Th8 afterTCMS in one subject. The stimulating coil was positioned for optimal activation of the left tibialis anterior. The stimulus intensity was adjusted until only a liminal but reproducible SCEP could be recorded. This stimulus intensity was termedT. Only the I1 wave was recruited at T (top trace). The stimulus intensity was increased by 10% increments (10% of the maximum output of the stimulator) to T + 30% in three steps. The amplitude of each wave contained in the SCEP (D,I1,I2,I3, andI4) greatly increased, and more waves were recruited as the stimulus intensity increased, but the latency of each wave did not greatly change. Each trace is an average of five responses. Two traces were superimposed for waveform reproducibility.
Fig. 3.
Order of activation of D andI waves evoked by transcranial magnetic stimulation in 10 control subjects. T was the minimum intensity necessary to elicit a reproducible spinal cord-evoked response recorded from an epidural electrode at Th8. I1 was recruited at T in all subjects, but the Dwave was recruited at T in only 40% of the subjects. More waves were recruited as TCMS intensity increased.
Fig. 4.
The effect of TCMS intensity on the amplitude of the D and I waves recorded from an epidural electrode at Th8. The amplitude of each wave was measured from its negative peak to the following positive trough (peak-to-trough). Each bar represents the mean amplitude of each wave, and the error bars represent the SEM. The number above each bar is the number of subjects in the sample. The I1 wave was recruited at spinal cord-evoked potential T in all 10 subjects, and theD wave was concomitantly recruited withI1 in 4 of these. At T + 10% the D wave was recruited in 9 of 10 subjects.
The I2 and I3 waves were present at T + 10% (5 of 10 subjects), T + 20% (9 of 10 subjects), or T + 30% (10 of 10 subjects). The TA and Sol muscle responses were contingent on the presence of at least four SCEP waves (D, I1, I2, and I3) in all subjects except three. One had a TA and Sol response with only an I1wave. The other two subjects did not have a TA response at T + 30% despite having an SCEP that contained four waves. One of these had very low-amplitude I2 and I3 waves. The TCMS intensity was increased to T + 40% in this subject, the amplitude of I2 and I3 increased, and a TA response was evoked. Increasing the TCMS intensity to T + 40% in the other subject activated another I wave (I5) and evoked a TA response.
SCEP and muscle response data were complete for all subjects at all stimulus intensities up to T + 30%. T + 30% produced an SCEP with five waves (D, I1, I2, I3, and I4) in five subjects and an SCEP with four waves in the other five subjects (absent I4).
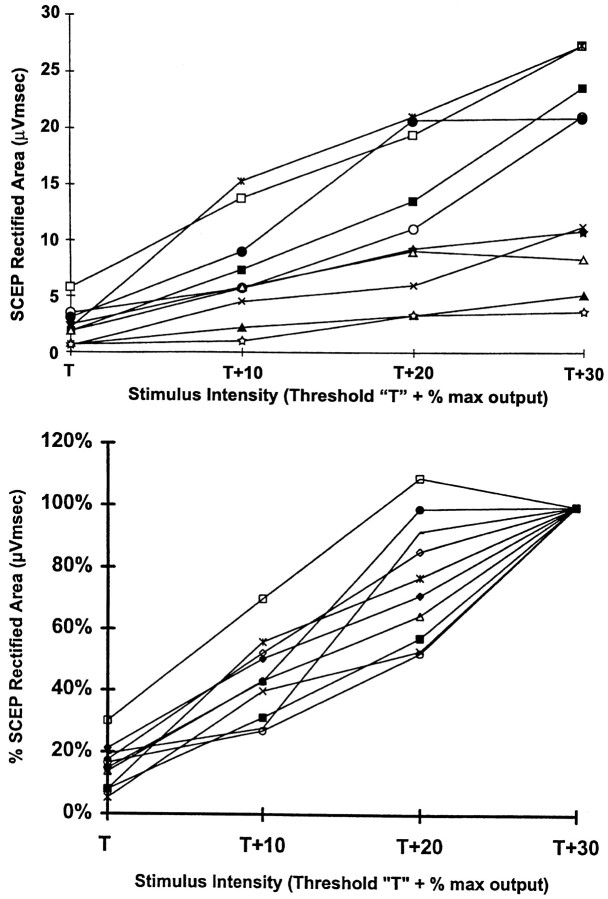
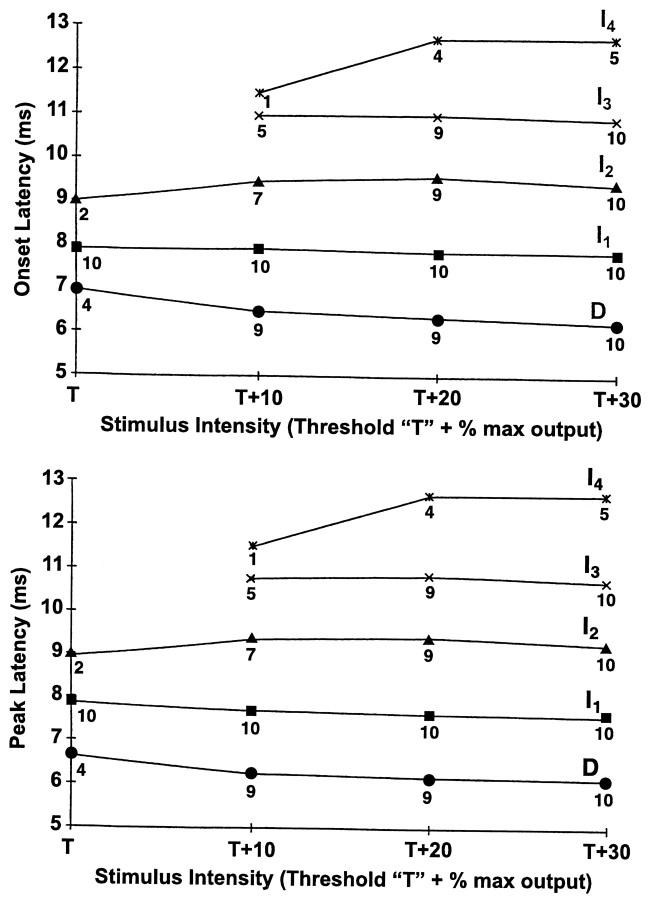
The absolute SCEP rectified area at a given stimulus intensity differed greatly among subjects (Fig. 5), but when the data were normalized by converting absolute SCEP rectified area values (obtained at each stimulus intensity) into a percentage of the SCEP rectified area obtained at T + 30%, the SCEP rectified area increased proportional to stimulus intensity in all subjects. In contrast, the latency of the D and individual I waves did not greatly change as TCMS intensity increased from T to T + 30% (Fig.6).
Fig. 5.
The effect of TCMS intensity on the rectified area of the SCEP recorded from an epidural electrode at Th8. Each line represents data from a single subject. TheSCEP rectified area at each stimulus intensity was calculated from the average of eight responses for each subject.Top, The absolute SCEP rectified area at a given stimulus intensity was greatly different among subjects. Bottom, The SCEP rectified area was normalized by dividing the SCEP rectified area atT + 30% by that obtained at lower stimulus intensities for each subject. The SCEP rectified area increased with increasing stimulus intensity in a similar way in all subjects.
Fig. 6.
The effect of TCMS intensity on the latency of theD and I waves recorded from an epidural electrode at Th8. The number under each datapoint represents the number of subjects in the sample. The mean onset latency (initial negative deflection) (top) and the mean peak latency (negative peak) (bottom) of the D andI waves did not greatly change as TCMS intensity increased from T to T + 30%.
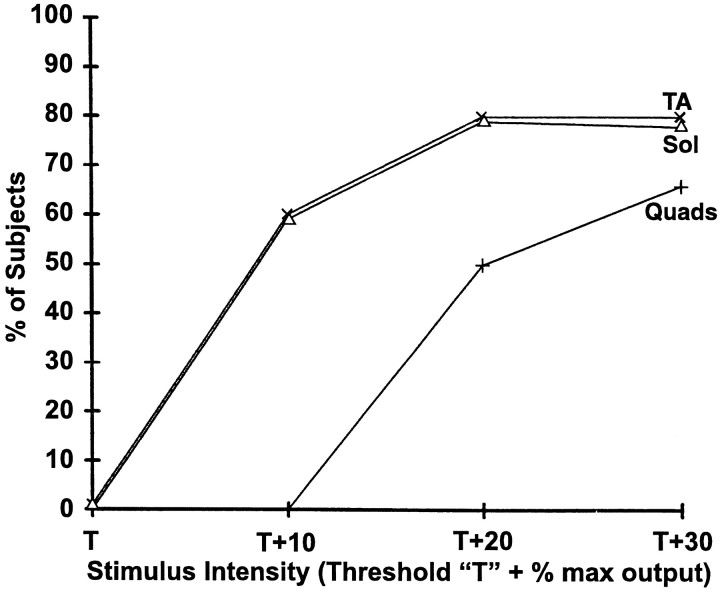
No subjects had Quads, TA, or Sol recruited at T. The effect of stimulus intensity on muscle activation is shown in Figure7.
Fig. 7.
Order of muscle activation after TCMS in 10 control subjects. T was the minimum stimulus intensity necessary to elicit a reproducible spinal cord-evoked response recorded from an epidural electrode at Th8. No subjects hadQuads, TA, or Solrecruited at T.
Spinal cord conduction velocity
The latencies of the D and I waves recorded from the rostral DCS electrode (electrode one referenced to the skin surface at Th8) were shorter than were those recorded from the more caudal DCS electrode (electrode three referenced to the Th8 surface), verifying that the SCEP was conducted down the long tracts of the spinal cord (Fig.8). The change in latency of the D and I waves was similar. The conduction velocity of the D, I1, and I2 peaks was 78 m/sec in one subject and 84 m/sec in the other. This was similar to the conduction velocity from the sensorimotor cortex to the Th8 spinal cord in both subjects (79 and 87 m/sec, respectively). For all 10 subjects, the mean conduction velocity from the sensorimotor cortex to the Th8 spinal cord was 78 ± 8 m/sec (range, 69–89 m/sec).
Fig. 8.
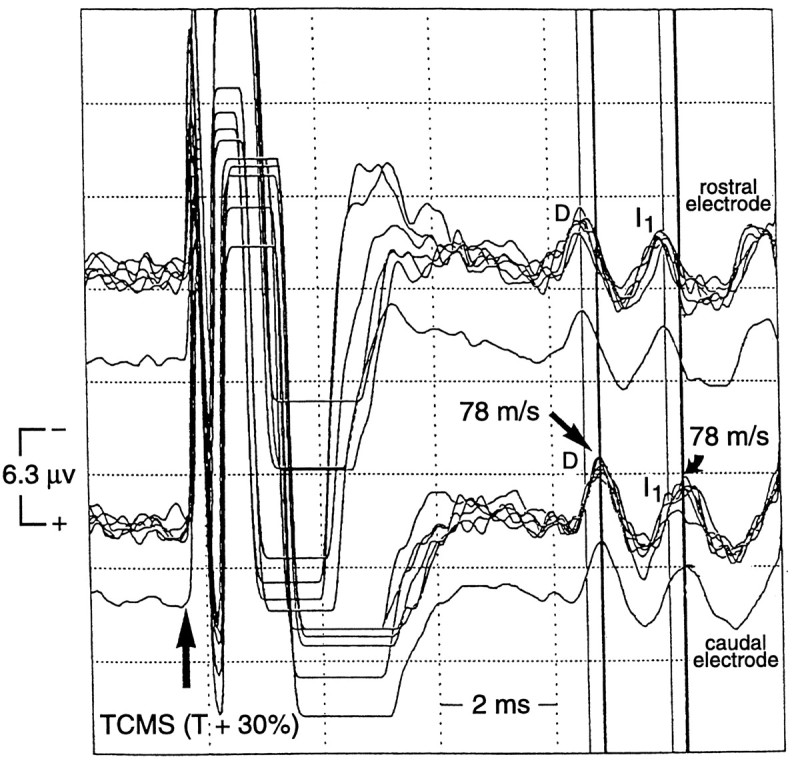
Descending SCEPs recorded from epidural electrodes at two different spinal cord levels (near Th8) afterTCMS at SCEP T + 30% in one alert subject. The epidural recording electrodes were rostrocaudally separated by 2.0 cm, and each was referenced to a surface electrode (G2) over the Th8 vertebrae. The high bandpass filter was 500 Hz (bandpass, 500 Hz to 5 kHz) on both channels to reduce muscle artifacts contributed by the surface electrode. The SCEP waves recorded from the rostral epidural electrode had shorter latencies than did those recorded from the caudal electrode. The spinal cord conduction velocity of the D and I1 wave was 78 m/sec.
Intraoperative studies
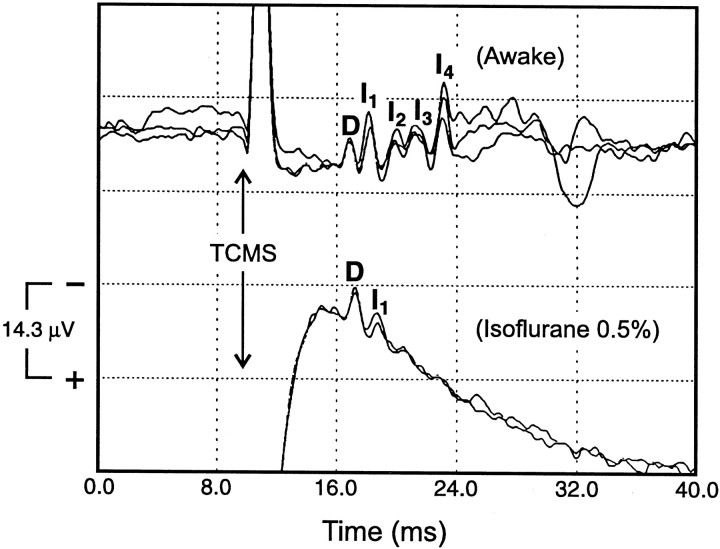
In the three subjects who had studies performed during inhalation anesthesia, the D wave peak latency was slightly increased (0.29, 0.23, and 0.4 msec, respectively) (Fig. 9). The D wave amplitude (peak-to-trough) was unchanged in two subjects and only slightly reduced in the third subject (12%) when compared with that of the preoperative recordings obtained at the same stimulus intensity (T + 40%). The I1 wave was absent in one subject but present in the other two. In these two subjects, the I1peak latency was increased (0.74 and 0.53 msec, respectively), and the I1 wave amplitude (peak-to-trough) was greatly diminished (68 and 63%, respectively) compared with that of the preoperative recordings obtained at the same stimulus intensity (T + 40%). All subsequent I waves were either absent or ill-defined in all three subjects. Patient temperature during intraoperative recordings was 36.1, 36.2, and 36.2°C, respectively.
Fig. 9.
The effect of general anesthetic on SCEPs recorded from an epidural electrode at Th8 after TCMS. When the subject was awake and at rest, the SCEP had a D wave followed by four I waves (I1,I2,I3, andI4) (top traces). After the subject was anesthetized (0.5% isoflurane, 66% N2O, and 33% O2), theI1 wave was delayed and diminished, and theI2,I3, and I4waves were absent (bottom traces) when compared with that obtained during the awake state. In contrast, the Dwave was relatively unaffected by anesthesia. TCMSintensity (SCEP T + 40% of the maximum output of the stimulator) and stimulus coil position were the same in the awake and anesthetized conditions.
DISCUSSION
TCMS produced D and I waves that were recorded from a DCS electrode at Th8 in awake humans. The first descending wave was verified as a D wave because it had a short latency and was relatively resistant to the effects of anesthesia when compared with the later I waves (Patton and Amassian, 1954; Katayama et al., 1988; Edgley et al., 1990, 1992; Burke et al., 1993). The D wave amplitude was less affected because it likely reflected direct activation of the corticospinal axon (at the first bend) and/or the corticospinal cell itself that was not dependent on synaptic transmission, unlike the corticocortical connections necessary for I wave generation (Amassian et al., 1990,1992; Edgley et al., 1990). Although I waves were recruited at lower intensities than were D waves in 6 of 10 awake subjects, simultaneous recruitment of D and I waves occurred first in 4 subjects. D waves were recruited at marginally higher stimulus intensities (T + 10%) in all subjects. These findings from the thoracic spinal cord in awake humans are in accordance with those obtained from the cervical spinal cord (Kaneko et al., 1996; Nakamura et al., 1996) and from nonhuman primates anesthetized by pentobarbital (Amassian et al., 1990).
The D and I waves we recorded at Th8 represented cortical outflow from corticospinal neurons supplying the trunk and lower extremities. The pyramidal cells for the lower extremities are located in the anterior bank of the central sulcus (paracentral lobule), so some may lie parallel to the induced current when the coil is flat on the top of the head (Amassian et al., 1989) because pyramidal cells and their apical dendrites are orientated perpendicular to the cortical surface. The amount of cellular polarization is increased when the stimulus current flow is parallel to the cell’s neural axis (Rushton, 1927;Toleikis et al., 1974; Roth and Basser, 1990), so a coil oriented flat on the top of head may directly excite corticospinal neurons for the lower extremity or their axons at the first bend (Amassian et al., 1992). The coil position we used (mean, 4 cm anterior and 1.6 cm to the left of vertex) was suitable for optimal activation of corticospinal neurons in the leg area of the motor cortex (near vertex) because, for our coil, the largest induced current occurs 4.3 cm from the axis of the coil in a plane parallel to the coil (Meyer et al., 1991). I waves also occurred at low stimulus intensities, suggesting that the corticocortical axons activated by TCMS were orientated parallel to the current flow, probably running posteriorly from the premotor cortex or anteriorly from the postcentral to precentral gyrus (Day et al., 1986,1989; Amassian et al., 1989, 1990). Alternately, it is possible that the descending pathways from the postcentral gyrus contributed to the SCEP because the primary sensory cortex is located beside the primary motor cortex and contains corticospinal neurons that project to the dorsal horn (Brodal, 1981; Willis and Grossman, 1981). This was unlikely because motor cortex ablation in monkeys obliterated all D and I waves evoked by stimulation of the primary sensory cortex (Patton and Amassian, 1960). Furthermore, dorsal column transection did not affect the SCEP originating from the sensorimotor cortex after TCMS in cats (Kawai and Nagao, 1992).
The D and I1 waves evoked by TCMS had similar conduction velocities suggesting both were conducted in the same pathway. The conduction velocity of the D and I1 waves at the Th8 spinal cord was fast at 78 m/sec in one subject and 84 m/sec in another, with the mean conduction velocity (cortex to spinal cord) for all subjects being 78 m/sec. There is considerable overlap in the range of conduction velocity for fast-conducting pathways like the corticospinal, rubrospinal, vestibulospinal, reticulospinal, and dorsal spinocerebellar pathways (Woolsey and Chang, 1948; Patton and Amassian, 1954; Lund and Pompeiano, 1965; Eccles et al., 1974; Bantli and Bloedel, 1975; Bloedel and Bantli, 1978; Brodal, 1981; Levy, 1983; Levy et al., 1986). It is possible that other descending motor pathways, in addition to the corticospinal ones, may have been indirectly or directly activated by TCMS and contributed to the SCEP and muscle responses. For example, the lateral vestibulospinal pathway is fast conducting with mono- and polysynaptic connections in the spinal cord that are predominantly ipsilateral (Nyberg-Hansen and Mascitti, 1964), but the muscle responses evoked by TCMS are primarily contralateral to the side of stimulation (Toleikis et al., 1991; Terao et al., 1994). The reticulospinal pathway is also fast conducting with mono- and polysynaptic connections in the spinal cord that activate primarily proximal muscles bilaterally (Peterson et al., 1979), but this is in contrast to the pattern of activation after TCMS (Brouwer and Ashby, 1990; Rothwell et al., 1987). Our knowledge of other descending motor pathways (i.e., rubrospinal, interstitiospinal, and tectospinal) in man is limited (Rothwell et al., 1987). The conduction velocity of D and I waves in the spinal cord was similar to that from sensorimotor cortex to spinal cord in our subjects, suggesting there was insufficient time for activation of subcortical structures via cortical-brainstem connections. Accordingly, the pathways mediating the earliest effects of cortical stimulation have been termed “corticomotoneuronal” (Bernhard and Bohm, 1954; Rothwell et al., 1987).
The interwave latencies and conduction velocities of D and I waves we recorded from the surface of the thoracic spinal cord in awake humans closely resemble those recorded from single fibers in the lateral corticospinal tract and from the surface of the spinal cord after direct electrical stimulation of the motor cortex in baboons (Kernell and Wu, 1967). The I waves Kernell and Wu recorded originated from synchronous repetitive discharges in the same group of pyramidal cells that created the D wave. Consequently, the D and I waves recorded from single fibers had similar amplitudes. In surface recordings, the compound D wave Kernell and Wu recorded was larger than were the compound I waves probably because of variability of I wave response latencies resulting in phase cancellation when recorded from the surface of the spinal cord (Patton and Amassian, 1954; Amassian et al., 1987). In contrast, we and others have found the amplitude of the I waves evoked by TCMS (coil oriented flat on the scalp at vertex) was often greater than that of the D wave when recorded from the surface of the spinal cord in humans (Burke et al., 1993; Kaneko et al., 1996;Nakamura et al., 1996). This suggests that many of the corticospinal neurons contributing to the I waves may not have contributed to the D wave evoked by TCMS (Edgley et al., 1992; Burke et al., 1993). This discrepancy is not attributable to the difference between single fiber and surface recordings because surface recordings underestimate I responses (Patton and Amassian, 1954; Amassian et al., 1987). Instead, this finding may be related to the difference in stimulating techniques because direct electrical stimulation of the motor cortex may excite corticospinal neurons differently (i.e., more discretely, more directly) than does TCMS, thereby creating larger D waves. Conversely, TCMS (coil oriented flat on the scalp at vertex) may indirectly activate corticospinal neurons better than does direct electrical stimulation by exciting more corticocortical inputs to corticospinal neurons and/or by creating more synchronous indirect activation of corticospinal neurons. Nevertheless, we found the interwave latencies between D and I waves did not change greatly as TCMS intensity increased, suggesting that the synchronous repetitive discharges of corticospinal neurons had a rigorous temporal code. Furthermore, the onset latencies to the D and I waves did not greatly change as TCMS intensity increased, suggesting that descending pathways located more caudally in the brain were not excited at higher stimulus intensities. The D and I wave conduction velocity is higher than that recorded previously from the epidural spinal cord during surgery (Inghilleri et al., 1989). Lower spinal cord temperatures during surgery (resulting in conduction slowing) may account for this difference.
More than three SCEP waves were necessary for activation of the TA in 9 of 10 subjects at rest. This supports previous work that has shown that depolarization of α motoneurons results from the summation effects of later I waves (Day et al., 1987; Mills, 1991). Previous investigators have estimated the effect of D and I waves on the TA motoneuron pool in man after low-intensity TCMS using PSTHs (Awiszus and Feistner, 1994). They recorded two PSTH subpeaks after TCMS that were thought to represent the effect of the D wave (first positive peak) followed 3–4 msec later by the effect of the I3 wave (second positive peak). These conclusions are based on the assumption that the form of the PSTH reflects the differential of the time course of the compound EPSP at the spinal motoneuron created by the arrival of individual D and I waves (Day et al., 1989). Our results confirm the importance of I waves (especially I3) for activation of TA, but our D–I3 interwave latency after low-intensity TCMS (mean, 4.6 ± 0.3 msec) was longer than the 3–4 msec estimated previously by the PSTHs (Awiszus and Feistner, 1994). This may have been attributable to latency shifts caused by the overlap of one wave’s positive trough and the next wave’s negative peak resulting in a delay in the next wave’s negative peak. It is possible that the residual effects of DCS may have affected interwave latency, but this has not been reported previously, and the patients did not feel any residual effects of DCS before the experiments. It is also possible that the PSTH-positive peak attributed previously to the D wave was related to I1 because our I1–I3interwave latency was between 3 and 4 msec (mean, 3.2 ± 0.2) and I1 was recruited first at low TCMS intensities in 6 of our 10 subjects.
In summary, the neurophysiological outflow evoked by TCMS was recorded directly from the thoracic spinal cord in awake human subjects for the first time. The descending volleys evoked by TCMS had fast conduction velocities. TCMS evoked a descending volley consisting of a D wave followed by three or four I waves that were reliably recorded by the DCS electrode in all subjects. The presence of the D and I waves in all subjects at low stimulus intensities verified that TCMS directly and indirectly activated corticospinal neurons in the sensorimotor cortex supplying the trunk and lower extremities. Leg muscle responses evoked by TCMS in awake subjects were usually contingent on a descending SCEP containing at least four waves.
Footnotes
We would like to thank Dr. G. Vanderlinden and Dr. M. Fazl for patient referral and Shar Leilabadi, Ming Wei Li, and Rene Henriquez for data management. We would especially like to acknowledge the helpful comments and suggestions from Dr. V. Amassian. This work was presented as the Herbert Jasper Prize Lecture at the Canadian Congress of Neurological Sciences, Saskatoon, Saskatchewan, Canada, June 26, 1997.
Correspondence should be addressed to Dr. David Houlden, Sunnybrook Health Science Centre, Suite D416, 2075 Bayview Avenue, Toronto, Ontario, Canada M4N 3M5.
REFERENCES
- 1.Amassian VE, Stewart M, Quirk GJ, Rosenthal JL. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93. [PubMed] [Google Scholar]
- 2.Amassian VE, Cracco RQ, Maccabee PJ. Focal stimulation of human cerebral cortex with the magnetic coil: a comparison with electrical stimulation. Electroencephalogr Clin Neurophysiol. 1989;74:401–416. doi: 10.1016/0168-5597(89)90029-4. [DOI] [PubMed] [Google Scholar]
- 3.Amassian VE, Quirk GJ, Stewart M. A comparison of corticospinal activation by magnetic coil and electrical stimulation of monkey motor cortex. Electroencephalogr Clin Neurophysiol. 1990;77:390–401. doi: 10.1016/0168-5597(90)90061-h. [DOI] [PubMed] [Google Scholar]
- 4.Amassian VE, Eberle L, Maccabee PJ, Cracco RQ. Modelling magnetic coil excitation of human cerebral cortex with a peripheral nerve immersed in a brain-shaped volume conductor: the significance of fiber bending in excitation. Electroencephalogr Clin Neurophysiol. 1992;85:291–301. doi: 10.1016/0168-5597(92)90105-k. [DOI] [PubMed] [Google Scholar]
- 5.Awiszus F, Feistner H. Quantification of D- and I-wave effects evoked by transcranial magnetic brain stimulation on the tibialis anterior motoneuron pool in man. Exp Brain Res. 1994;101:153–158. doi: 10.1007/BF00243225. [DOI] [PubMed] [Google Scholar]
- 6.Bantli H, Bloedel JR. Monosynaptic activation of direct reticulospinal pathway by the dentate nucleus. Pflügers Arch. 1975;357:237–242. doi: 10.1007/BF00585978. [DOI] [PubMed] [Google Scholar]
- 7.Berardelli A, Inghilleri M, Rothwell JC, Cruccu G, Manfredi M. Multiple firing of motoneurones is produced by cortical stimulation but not by direct activation of descending motor tracts. Electroencephalogr Clin Neurophysiol. 1991;81:240–242. doi: 10.1016/0168-5597(91)90078-c. [DOI] [PubMed] [Google Scholar]
- 8.Bernhard CG, Bohm E. Cortical representation and functional significance of the corticomotoneuronal system. AMA Arch Neurol Psychiatr. 1954;72:473–502. doi: 10.1001/archneurpsyc.1954.02330040075006. [DOI] [PubMed] [Google Scholar]
- 9.Bloedel JR, Bantli H. The spinal action of the dentate nucleus mediated by descending systems originating in the brain stem. Brain Res. 1978;153:602–607. doi: 10.1016/0006-8993(78)90345-1. [DOI] [PubMed] [Google Scholar]
- 10.Brodal A. Neurological anatomy in relation to clinical medicine. Oxford UP; New York: 1981. [Google Scholar]
- 11.Brouwer B, Ashby P. Corticospinal projections to upper and lower limb spinal motoneurons in man. Electroencephalogr Clin Neurophysiol. 1990;76:509–519. doi: 10.1016/0013-4694(90)90002-2. [DOI] [PubMed] [Google Scholar]
- 12.Burke D, Hicks RG, Gandevia SC, Stephen J, Woodworth I, Crawford M. Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. J Physiol (Lond) 1993;470:383–393. doi: 10.1113/jphysiol.1993.sp019864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day BL, Dick JP, Marsden CD, Thompson PD. Differences between electrical and magnetic stimulation of the human brain. J Physiol (Lond) 1986;378:36P. [Google Scholar]
- 14.Day BL, Rothwell JC, Thompson PD, Dick JPR, Cowan JMA, Berardelli A, Marsden CD. Motor cortex stimulation in intact man. 2. Multiple descending volleys. Brain. 1987;110:1191–1209. doi: 10.1093/brain/110.5.1191. [DOI] [PubMed] [Google Scholar]
- 15.Day BL, Dressler D, Maertens de Noordhout A, Marsden CS, Mills K, Murray NMF, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol (Lond) 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eccles JC, Nicoll RA, Schwarz DWF, Taborikova H. Cerebello-spinal pathway via the fastigial nucleus and the medial reticular nucleus. Brain Res. 1974;66:525–530. [Google Scholar]
- 17.Edgley SA, Eyre JA, Lemon RN, Miller S. Excitation of the corticospinal tract by electromagnetic and electrical stimulation of the scalp in the macaque monkey. J Physiol (Lond) 1990;425:301–320. doi: 10.1113/jphysiol.1990.sp018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edgley SA, Eyre JA, Lemon RN, Miller S. Direct and indirect activation of corticospinal neurones by electrical and magnetic stimulation in the anaesthetized macaque monkey. J Physiol (Lond) 1992;446:224P. [Google Scholar]
- 19.Hess CW, Rosler K, Heckmann R, Ludin HP. Magnetic stimulation of the human brain: influence of the size and shape of the stimulating coil. In: Berardelli A, Benecke R, Manfredi M, Marsden CD, editors. Motor disturbances II. Academic; London: 1990. pp. 31–42. [Google Scholar]
- 20.Houlden DA, Schwartz ML, Tator CH, Ashby P, MacKay WA, Fazl M. Motor evoked potentials recorded directly from the spinal cord following transcranial magnetic stimulation in awake human subjects: direct evidence that human motor cortical excitability does not change during voluntary contraction. Can J Neurol Sci. 1996;23:S11. [Google Scholar]
- 21.Inghilleri M, Berardelli A, Cruccu G, Priori A, Manfredi M. Corticospinal potentials after transcranial stimulation in humans. J Neurol Neurosurg Psychiatry. 1989;52:970–974. doi: 10.1136/jnnp.52.8.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingram DA, Thompson AJ, Swash M. Central motor conduction in multiple sclerosis: evaluation of abnormalities revealed by transcutaneous magnetic stimulation of the brain. J Neurol Neurosurg Psychiatry. 1988;51:487–494. doi: 10.1136/jnnp.51.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jasper H. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- 24.Kaneko K, Kawai S, Fuchigami Y, Morita H, Ofuji A. The effect of current direction induced by transcranial magnetic stimulation on the corticospinal excitability in human brain. Electroencephalogr Clin Neurophysiol. 1996;101:478–482. doi: 10.1016/s0013-4694(96)96021-x. [DOI] [PubMed] [Google Scholar]
- 25.Katayama Y, Tsubokawa T, Maejima S, Hirayama T, Yamamoto T. Corticospinal direct response in humans: identification of the motor cortex during intracranial surgery under general anaesthesia. J Neurol Neurosurg Psychiatry. 1988;51:50–59. doi: 10.1136/jnnp.51.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawai N, Nagao S. Origins and conducting pathways of motor evoked potentials elicited by transcranial magnetic stimulation in cats. Neurosurgery. 1992;31:520–527. doi: 10.1227/00006123-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Kernell D, Wu CP. Responses of the pyramidal tract to stimulation of the baboon’s motor cortex. J Physiol (Lond) 1967;191:653–672. doi: 10.1113/jphysiol.1967.sp008273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitigawa H, Moller AR. Conduction pathways and generators of magnetic evoked spinal cord potentials: a study in monkeys. Electroencephalogr Clin Neurophysiol. 1994;93:57–67. doi: 10.1016/0168-5597(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 29.Levy WJ. Spinal evoked potential from the motor tracts. J Neurosurg. 1983;58:38–44. doi: 10.3171/jns.1983.58.1.0038. [DOI] [PubMed] [Google Scholar]
- 30.Levy WJ, McCaffrey M, Goldman D, York DH. Nonpyramidal motor activation produced by stimulation of the cerebellum, direct or transcranial: a cerebellar evoked potential. Neurosurgery. 1986;19:163–176. doi: 10.1227/00006123-198608000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Lund S, Pompeiano O. Descending pathways with monosynaptic action on motoneurones. Experientia. 1965;21:602–603. doi: 10.1007/BF02151558. [DOI] [PubMed] [Google Scholar]
- 32.MacDonnell RAL, Shapiro BE, Chiappa KH, Helmers SL, Cros D, Day BJ, Shahani BT. Hemispheric threshold differences for motor evoked potentials produced by magnetic coil stimulation. Neurology. 1991;41:1441–1444. doi: 10.1212/wnl.41.9.1441. [DOI] [PubMed] [Google Scholar]
- 33.Meyer BU, Britton TC, Kloten H, Steinmetz H, Benecke R. Coil placement in magnetic brain stimulation related to skull and brain anatomy. Electroencephalogr Clin Neurophysiol. 1991;81:38–46. doi: 10.1016/0168-5597(91)90102-4. [DOI] [PubMed] [Google Scholar]
- 34.Mills KR. Magnetic brain stimulation: a tool to explore the action of the motor cortex on single human spinal motoneurones. Trends Neurosci. 1991;14:401–405. doi: 10.1016/0166-2236(91)90029-t. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Direct and indirect activation of human corticospinal neurons by transcranial magnetic and electrical stimulation. Neurosci Lett. 1996;210:45–48. doi: 10.1016/0304-3940(96)12659-8. [DOI] [PubMed] [Google Scholar]
- 36.Nyberg-Hansen R, Mascitti TA. Sites and mode of termination of fiber on the vestibulospinal tract in the cat: an experimental study with silver impregnation methods. J Comp Neurol. 1964;122:369–383. doi: 10.1002/cne.901220307. [DOI] [PubMed] [Google Scholar]
- 37.Patton HD, Amassian VE. Single- and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954;17:345–363. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- 38.Patton HD, Amassian VE. Handbook of physiology, neurophysiology, Vol 11, pp 837–861. American Physiology Society; Washington, DC: 1960. The pyramidal tract: its excitation and functions. [Google Scholar]
- 39.Peterson BW, Pitts NG, Fukushima K. Reticulospinal connections with limb and axial motoneurons. Exp Brain Res. 1979;36:1–20. doi: 10.1007/BF00238464. [DOI] [PubMed] [Google Scholar]
- 40.Priori A, Bertolasi L, Dressler D, Rothwell JC, Day BL, Thompson PD, Marsden CD. Transcranial electric and magnetic stimulation of the leg area of the human motor cortex: single motor unit and surface EMG responses in the tibialis anterior muscle. Electroencephalogr Clin Neurophysiol. 1993;89:131–137. doi: 10.1016/0168-5597(93)90095-7. [DOI] [PubMed] [Google Scholar]
- 41.Roth BJ, Basser PJ. A model of the stimulation of a nerve fiber by electromagnetic induction. IEEE Trans Biomed Eng. 1990;37:588–597. doi: 10.1109/10.55662. [DOI] [PubMed] [Google Scholar]
- 42.Rothwell JC, Day BL, Thompson PD, Dick JPR, Marsden CD. Some experiences of techniques for stimulation of the human cerebral cortex through the scalp. Neurosurg. 1987;20:156–163. doi: 10.1097/00006123-198701000-00032. [DOI] [PubMed] [Google Scholar]
- 43.Rothwell JC, Burke D, Hicks R, Stephen J, Woodforth I, Crawford M. Transcranial electrical stimulation of the motor cortex in man: further evidence for the site of activation. J Physiol (Lond) 1994;481:243–250. doi: 10.1113/jphysiol.1994.sp020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rushton WAH. Effect upon the threshold for nervous excitation of the length of nerve exposed and the angle between current and nerve. J Physiol (Lond) 1927;63:272–286. doi: 10.1113/jphysiol.1927.sp002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor BA, Fennelly ME, Taylor A, Farrell J. Temporal summation—the key to motor evoked potential spinal cord monitoring in humans. J Neurol Neurosurg Psychiatry. 1993;56:104–106. doi: 10.1136/jnnp.56.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terao Y, Ugawa Y, Sakai K, Uesaka Y, Kohara N, Kanazawa I. Transcranial stimulation of the leg area of the motor cortex in humans. Acta Neurol Scand. 1994;89:378–383. doi: 10.1111/j.1600-0404.1994.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 47.Thompson PD, Day BL, Crockard HA, Calder I, Murray NMF, Rothwell JC, Marsden CD. Intra-operative recording of motor tract potentials at the cervico-medullary junction following scalp electrical and magnetic stimulation of the motor cortex. J Neurol Neurosurg Psychiatry. 1991;54:618–623. doi: 10.1136/jnnp.54.7.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toleikis JR, Sances A, Larson SJ. Effects of diffuse transcerebral electrical currents on cortical unit potential activity. Anesth Analg. 1974;53:48–55. [PubMed] [Google Scholar]
- 49.Toleikis JR, Sloan TB, Ronai AK. Optimal transcranial magnetic stimulation sites for the assessment of motor function. Electroencephalogr Clin Neurophysiol. 1991;81:443–449. doi: 10.1016/0013-4694(91)90006-p. [DOI] [PubMed] [Google Scholar]
- 50.Willis WD, Grossman RG. Medical neurobiology, Third Edition, pp 273–346. Mosby; St. Louis: 1981. Sensory systems. [Google Scholar]
- 51.Woolsey CN, Chang HT. Activation of cerebral cortex by antidromic volleys in pyramidal tract. Res Publ Assoc Res Nerv Ment Dis. 1948;27:146–161. [PubMed] [Google Scholar]