Abstract
Hibernation in mammals such as the rodent hibernatorCitellus lateralis is a physiological state in which CNS activity is endogenously maintained at a very low, but functionally responsive, level. The neurotransmitter histamine is involved in the regulation of diurnal rhythms and body temperature in nonhibernators and, therefore, could likely play an important role in maintaining the hibernating state. In this study, we show that histamine neuronal systems undergo major changes during hibernation that are consistent with such a role. Immunohistochemical mapping of histaminergic fibers in the brains of hibernating and nonhibernating golden-mantled ground squirrels (C. lateralis) showed a clear increase in fiber density during the hibernating state. The tissue levels of histamine and its first metabolitetele-methylhistamine were also elevated throughout the brain of hibernating animals, suggesting an increase in histamine turnover during hibernation, which occurs without an increase in histidine decarboxylase mRNA expression. This hibernation-related apparent augmentation of histaminergic neurotransmission was particularly evident in the hypothalamus and hippocampus, areas of importance to the control of the hibernating state, in whichtele-methylhistamine levels were increased more than threefold. These changes in the histamine neuronal system differ from those reported for the metabolic pattern in other monoaminergic systems during hibernation, which generally indicate a decrease in turnover. Our results suggest that the influence of histamine neuronal systems may be important in controlling CNS activity during hibernation.
Keywords: hibernation, CNS, hippocampus, hypothalamus, preoptic area, septum, histamine, tele-methylhistamine, monoamine
Histamine (HA) is a multifunctional messenger that acts as a neurotransmitter or neuromodulator in the brain (Schwartz et al., 1991; Wada et al., 1991). In mammals, histaminergic perikarya are located in the tuberomammillary nucleus of the posterior hypothalamus, projecting fibers to almost all parts of the brain (Airaksinen and Panula, 1988; Inagaki et al., 1988;Airaksinen et al., 1989, 1992; Panula et al., 1989).
HA has been implicated in the regulation of the sleep–wake cycle, body temperature, energy metabolism, reproductive behavior, and neuroendocrine secretion (Schwartz et al., 1991). These functions may reflect the action of the histaminergic neuronal system on the general activity level of the brain (Wada et al., 1991).
Hibernation is a physiological state characterized by an extreme reduction of various functions, such as body temperature and metabolism. This process is under strict neuronal control involving the hypothalamus, septum, hippocampus and brainstem reticular formation (Heller, 1979; Beckman and Stanton, 1982), but the neurochemical mechanisms underlying the regulatory processes are, as yet, poorly understood. Mammalian hibernation provides a good model for studying the control of central activity by neurotransmitters/neuromodulators because the range of CNS activity displayed across the nonhibernating versus hibernating states is larger than that of any other mammalian CNS. During the nonhibernating state, CNS activity is quite representative of nonhibernators; during the hibernating state, however, it is reduced to a level that defines the lower limit of mammalian brain activity (Heller, 1979; Beckman and Stanton, 1982).
Histaminergic nerve fibers innervate many of the key areas involved in controlling hibernation (Inagaki et al., 1988; Panula et al., 1989). HA receptors are also present in these areas (Schwartz et al., 1991;Lintunen et al., 1998). The involvement of histamine in the regulation of, for example, diurnal rhythms and body temperature (Schwartz et al., 1991) renders it a good candidate transmitter for the control of hibernation, wherein brain homeostasis is substantially altered, and major changes in electrical and neurochemical activity occur. If HA is involved in hibernation, changes in histaminergic innervation, histamine levels, and turnover, as well as responsiveness of target neurons can be expected to take place in connection with this physiological state. Thus, the purpose of this study was to determine whether the histaminergic system undergoes major changes during hibernation. The golden-mantled ground squirrel, a rodent hibernator, was used in this study.
MATERIALS AND METHODS
Animals. Adult male and female golden-mantled ground squirrels (Citellus lateralis) were used in this study. The animals were trapped in the wild and kept in the laboratory for 1–4 years before killing. Nonhibernating (i.e., euthermic) animals were housed in individual cages in a colony room maintained at an ambient temperature (Ta) of 21°C with a light/dark cycle that approximated the times of sunrise and sunset. Hibernating animals were housed in individual cages in a dimly illuminated cold room maintained at a Ta of 6°C. All animals were provided with food (rat chow and sunflower seeds) and water ad libitum, and they were provided with cotton from which they constructed nests.
Hibernating animals were killed at approximately the midportion of their hibernation bout. All animals (nonhibernating and hibernating) were killed during the same period (late February to early April) of the year.
Immunocytochemical protocol. Twenty ground squirrels, ten in the nonhibernating and ten in the hibernating state (five females and five males in each group) were anesthetized with sodium pentobarbital (nonhibernating animals: 90 mg/kg, i.p.; hibernating animals: 40 mg, intracardiac injection) and perfused through the left ventricle with physiological saline followed by 4% 1-ethyl-3(3-dimethylaminopropyl)carbodiimide (Sigma, St Louis, MO) in 0.1 m phosphate buffer, pH 7.4. The brains were removed immediately and immersed in the same fixative for 24 hr, then transferred into 20% sucrose in 0.1 m phosphate buffer and kept at 4°C for 24 hr. After this, they were quick-frozen in isopentane (−30°C) and stored in sealed containers at −75°C. Cryostat sections (22-μm-thick) were collected on gelatin-coated slides and air-dried for 30–60 min. The sections were washed with PBS containing 0.25% Triton X-100 (PBS-T) and incubated with a rabbit antiserum against histamine (HA19C) diluted 1:1000 in PBS-T with 1% normal swine serum. After the incubation, which was performed overnight at 4°C, the sections were washed twice for 10 min with PBS-T and then incubated with FITC-conjugated swine anti-rabbit IgG (Dako, Glostrup, Denmark) diluted 1:40 in PBS-T, for 2 hr at room temperature. The samples were washed with PBS, coverslipped with PBS and glycerol (1:1), and examined under a Leitz Aristoplan microscope equipped for epiillumination. Other sections were processed using the Vectastain Elite kit (Vector Laboratories, Burlingame, CA). These sections were incubated for three nights with HA19C diluted 1:100,000 in PBS-T with 2% normal goat serum. The sections were washed twice for 10 min in PBS-T and incubated with a biotinylated antibody to rabbit IgG diluted 1:600 for 2 hr at room temperature. After washing twice for 10 min in PBS-T, the sections were incubated with an avidin–biotin solution, and both reagents were diluted 1:500 for 2 hr at room temperature. The sections were washed with 0.05 m Tris-HCl, pH 7.6, twice for 10 min, and the staining was developed with 0.025% diaminobenzidine tetrahydrochloride, 0.3% NiSO4, and 0.01% H2O2 in Tris-HCl. The reaction was terminated with several changes in distilled water, the sections were air-dried, dehydrated, and coverslipped with Permount. These sections were used for evaluation of fiber density.
The specificity of the antiserum has been tested extensively with dot-blot assays and preabsorption tests with histamine,l-histidine, and l-histidine-containing peptides (Panula et al., 1990). All immunoreactivity was abolished by preincubation of the antiserum with a histamine–protein conjugate (1 μg/ml), whereas preincubation with l-histidine protein conjugates (up to 100 μg/ml) did not affect the staining. Preincubation of the antiserum with thyrotropin-releasing hormone, luteinizing hormone, vasoactive intestinal polypeptide, or substance K (1–10 μg/ml) did not affect the staining. When the specific histamine antiserum was replaced by normal rabbit serum, no immunofluorescence was detected.
Brain atlas. Because published brain maps of Citellus lateralis with sufficient anatomical details are not available, one was prepared by cutting the brain of one nonhibernating male ground squirrel into 22-μm-thick cryosections and staining every third section with toluidine blue. The sections were photographed, and the pictures were scanned into a computer. Using these pictures, a brain atlas was prepared using Adobe Photoshop and Corel Draw computer programs. The nuclei of the ground squirrel brain were identified according to a rat brain atlas (Paxinos and Watson, 1982) and a brain atlas of the ground squirrel Citellus tridecemlineatus(Joseph et al., 1966). The nomenclature was adopted from the atlas ofPaxinos and Watson (1982).
Determination of histamine and tele-methylhistamine.Nonhibernating (n = 6; three female and three male) and hibernating (n = 7; three female and four male) animals were killed by decapitation. The brains were rapidly removed, dissected on ice into 10 regions, and each of the ten regions was split in half and quick frozen in isopentane at −25 to −30°C. The samples were weighed in the frozen state and stored at −75°C until assay. One half of each region was used for HPLC determination of HA content; the other half was used for measurement of tele-methylhistamine (tmHA) by mass spectrometry (see below). The regions dissected for analysis were: cortex, striatum, preoptic area/septum, hippocampus, hypothalamus, thalamus, midbrain, medulla, pons, and cerebellum.
The HA content in the tissue was determined by an automated HPLC-fluorimetric method (Yamatodani et al., 1985). The brain tissue was thawed and homogenized in 10 volumes of 2% perchloric acid. The homogenates were centrifuged at 10,000 × g for 30 min, and 20 ml of the supernatant was injected onto the column (4 mm inner diameter × 60 mm) packed with cation exchanger (TSKgel Histaminepak; Tosoh). The fluorescence intensity was measured at 450 nm with excitation at 360 nm in a spectrofluorometer (F-1050; Hitachi). The detection limit for the assay was 0.05 pmol per injection.
tele-Methylhistamine in the ten regions of hibernating and nonhibernating ground squirrel brain was measured with gas chromatography–mass spectrometry by the method of Hough et al. (1981), with some modifications (Tuomisto et al., 1996).
Statistical analysis of the total difference in HA and tmHA content between nonhibernating and hibernating ground squirrel brains was performed using two-way ANOVA. The difference between the two states in each individual brain region was analyzed by separate two-tailedt tests.
In situ hybridization. An oligonucleotide probe (CCG TGT CTG ACA TGT GCT TGA AGA TTC TTC ACC CCG AAG GAC CGA ATC AC) complementary to a highly conserved region of the histidine decarboxylase (HDC) gene was generated according to the published sequences of rat (Joseph et al., 1990), mouse (Yamamoto et al., 1990), and human (Yamauchi et al., 1990) HDC. The probe has a 98% identity with the human sequence and a 100% with the rat and mouse sequences. A 50-mer oligonucleotide probe complementary to theStaphylococcus aureus chloramphenicol acetyltransferase gene was used as a control. The probes were labeled with deoxyadenosine 5′-α(-thio)triphosphate (35S, NEG-034H; DuPont NEN, Boston, MA) at their 3′-ends using terminal deoxynucleotide transferase (Promega, Madison, WI). The purifications were done in Sephadex G-50-columns. The labeled probes with specific activities of 1–2 × 109 cpm/μg were stored at −20°C in 10 mm dithiotreitol until used.
Unfixed ground squirrel brains were cryosectioned (10 μm) and thaw-mounted onto poly-l-lysine-coated slides. The slides were illuminated with UV light for 5 min at a distance of 25 cm. Sections were hybridized for 20 hr at 50°C in a humidified chamber with 200 μl of hybridization buffer containing 50% deionized formamide, 4× SSC (0.6 m sodium chloride, 0.06m sodium citrate), 1× Denhardt’s solution (0.02% polyvinylpyrrolidone, 0.02% Ficoll, and 0.02% bovine serum albumin), 1% sarcosyl (N-lauroylsarcosine), 0.02 m sodium phosphate, pH 7.0, 10% dextran sulfate, 500 μg/ml denatured salmon sperm DNA, 250 μg/ml tRNA, 200 mm dithiotreitol, and 107 cpm/ml of labeled probe. After hybridization, the slides were dipped in 1× SSC at room temperature, shortly washed with 1× SSC at 56°C and then three times 20 min at 56°C in 1× SSC. They were left to cool to room temperature in fresh 56°C 1× SSC before dehydration with ethanol. Tissue sections were then apposed to Kodak BioMax MR-film (Eastman Kodak, Rochester, NY) for 4 d.
RESULTS
Histamine immunoreactivity in the ground squirrel brain
The HA-immunoreactive fiber system was very widespread in the ground squirrel brain both in the nonhibernating and in the hibernating state, and a dense network of fibers and nerve terminals was found in almost all parts of the brain.
Histamine-immunoreactive cell bodies
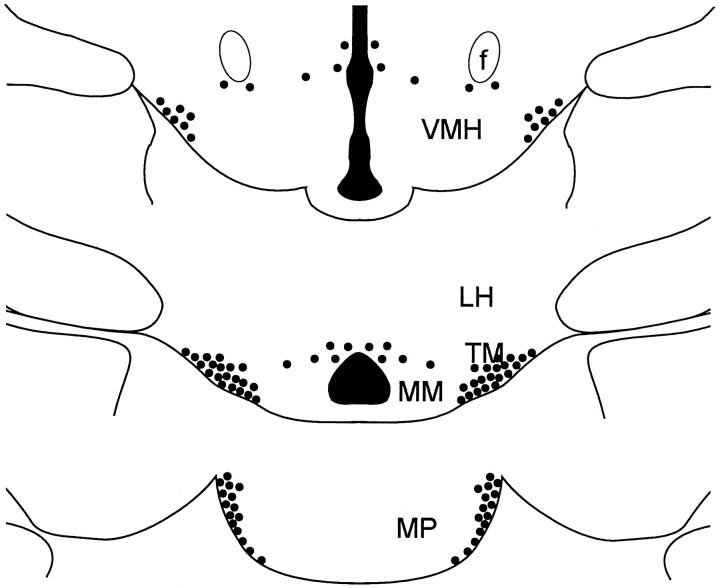
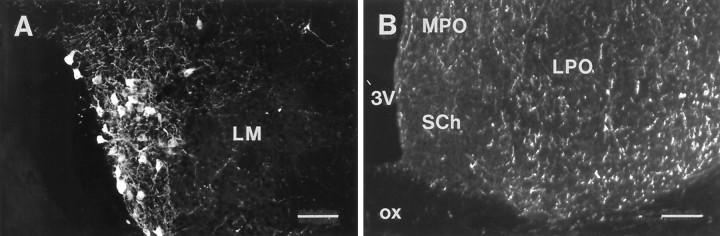
HA-immunoreactive cell bodies were found exclusively in the posterior hypothalamus, in a region corresponding to the tuberomammillary nucleus in the rat brain. Figure1 indicates the location of the cells schematically at consecutive frontal levels from rostral to caudal. A few cell bodies were found close to the third ventricle and scattered around the mammillary nuclei, but the majority of the histaminergic cell bodies comprised a cluster in the ventrolateral part of the posterior hypothalamus (Fig.2A). The compartments of the tuberomammillary neurons thus resembled those found in the rat brain (Panula et al., 1984).
Fig. 1.
Schematic drawing of the distribution of histaminergic neurons in the ground squirrel brain. The frontal sections are consecutively arranged from rostral to caudal. Onedot represents approximately two cell bodies. Themiddle panel corresponds to level d in Figure 3.
Fig. 2.
A, Histamine-immunoreactive tuberomammillary neurons of the posterior hypothalamus.LM, Lateral mammillary nucleus. Scale bar, 100 μm.B, Histaminergic fibers in the most anterior part of the hypothalamus at the level of the suprachiasmatic nucleus.3V, Third ventricle; LPO, lateral preoptic nucleus; MPO, medial preoptic nucleus;SCh, suprachiasmatic nucleus; ox, optic chiasm. Scale bar, 200 μm.
Telencephalon
Most neocortical and allocortical areas contained moderately dense fiber networks, except for the deep areas of the most anterior parts of the neocortex, where the fiber density was low (Fig.3a–f). The striatum (caudate putamen) had low to moderate fiber density (Fig.3a–c). The most anterior and posterior parts displayed low density of fibers, whereas moderate fiber density was observed in the medial part. Adjacent to the lateral ventricle, the density was even higher. Low to moderate density of fibers was seen in the nucleus accumbens (Fig. 3a,b). The bed nucleus of the stria terminalis displayed moderate fiber density (Fig.3b). A very high density of fibers was observed in the medial septal nucleus and the nucleus of the diagonal band, whereas the lateral septal nuclei had high fiber density (Fig. 3a). A high density of fibers was seen in the fornix and the medial preoptic nucleus (Fig. 3b). High fiber density was observed in the fimbria (Fig. 3c), whereas the anterior parts of the hippocampus displayed relatively low fiber density. In the more posterior parts, there were larger differences between the hippocampal areas (Fig. 3e,f). The density of fibers was high in the subiculum, moderate in the dentate gyrus and CA4, moderate to high in CA3, low to moderate in CA1, and low in CA2. The amygdaloid nuclei had high fiber density (Fig. 3c).
Fig. 3.
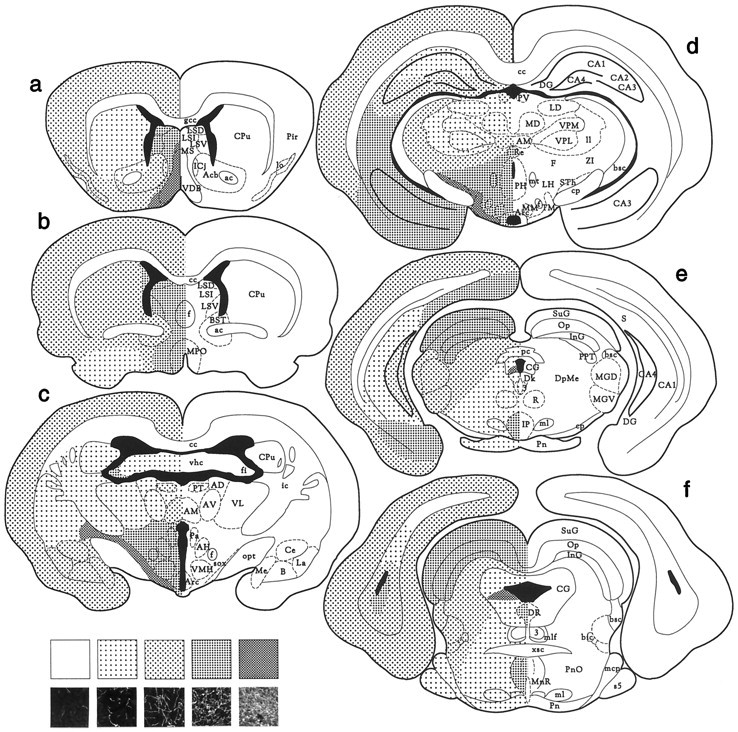
Distribution of HA-immunoreactive fibers in the ground squirrel brain. The frontal sections are arranged from rostral to caudal. The small photomicrographs at the bottom of the panel show the fiber densities representative of the different patterns used in the map. The area shown in the micrographs is 250 × 250 μm. 3, Principal oculomotor nucleus;Me, medial amygdaloid nucleus; ac, anterior commissure; MGD, medial geniculate nucleus, dorsal part; Acb, nucleus accumbens; MGV, medial geniculate nucleus, ventral part; AD, anterodorsal thalamic nucleus; ml, medial lemniscus;AH, anterior hypothalamic nucleus; AM, anteromedial thalamic nucleus; mlf, medial longitudinal fasciculus; Arc, arcuate nucleus; MM, medial mammillary nucleus, medial part; AV, nucleus anterior ventralis thalami; MnR, median raphe nucleus;B, basal amygdaloid nucleus; MPO, medial preoptic nucleus; bic, brachium of the inferior colliculus; MS, medial septal nucleus;bsc, brachium of the superior colliculus;mt, mammillothalamic tract; BST, bed nucleus of the stria terminalis; Op, optic nerve layer of the superior colliculus; CA1-4, fields CA1–4 of ammons horn; opt, optic tract;cc, corpus callosum; Pa, paraventricular hypothalamic nucleus; Ce, central amygdaloid nucleus;pc, posterior commissure; CG, central gray; PH, posterior hypothalamic nucleus;cp, cerebral peduncle; Pir, piriform cortex; CPu, caudate putamen (striatum);Pn, pontine nuclei; DG, dentate gyrus;PnO, pontine reticular nucleus, oral part;Dk, nucleus of Darkschewitsch; PT, paratenial thalamic nucleus; DpMe, deep mesencephalic nucleus; PV, paraventricular thalamic nucleus;DR, dorsal raphe nucleus; R, red nucleus;F, fields of Forel; Re, reuniens thalamic nucleus; f, fornix; S, subiculum;fi, fimbria; s5, sensory root of the trigeminal nerve; gcc, genu corpus callosi;sox, supraoptic decussation; ic, internal capsule; STh, subthalamic nucleus; ICj, islands of Calleja; SuG, superficial gray layer of the superior colliculus; InG, intermediate gray layer of the superior colliculus; TM, tuberomammillary nucleus;IP, interpenduncular nucleus; VDB, nucleus of the ventral limb of the diagonal band of Broca;La, lateral amygdaloid nucleus; LD, laterodorsal thalamic nucleus; vhc, ventral hippocampal commissure; ll, lateral lemniscus; VL, ventrolateral thalamic nucleus; lo, lateral olfactory tract; VMH, ventromedial hypothalamic nucleus;LSD, lateral septal nucleus, dorsal part;VPL, ventral posterolateral thalamic nucleus;LSI, lateral septal nucleus, intermediate part;VPM, ventral posteromedial thalamic nucleus;LSV, lateral septal nucleus, ventral part;xsc, decussation of the superior cerebellar peduncle;mcp, middle cerebral peduncle; MD, mediodorsal thalamic nucleus; ZI, zona incerta.
Diencephalon
In the thalamus, low density of fibers was observed in the anteroventral, ventrolateral (Fig. 3c), dorsal, ventroposterior (Fig. 3e), posterior, pretectal, parafascicular, and lateral geniculate nuclei. Moderate fiber density was found in the paratenial (Fig. 3c), anteromedial (Fig.3c,d), periventricular (Fig. 3d), mediodorsal, habenular, and medial geniculate nuclei (Fig.3e,f). The reuniens thalamic nucleus displayed high fiber density (Fig. 3d). The densest fiber network in the whole brain was found in the hypothalamus. The fiber density was even and high throughout most areas of the hypothalamus and very high in the supraoptic commissure and the posterior basal parts (Fig. 3c,d). In the suprachiasmatic nucleus the fiber density was low to moderate (Fig. 2b).
Mesencephalon
The density of HA-immunoreactive fibers was high in the substantia nigra and very high in the ventral tegmental area and the supramammillary nucleus and commissure. The fiber density was low in the red nucleus and high in the interpenduncular nucleus (Fig.3e). In the superior (Fig.3e,f) and inferior colliculus, a high density of fibers was observed.
Rhombencephalon
A moderate density of fibers was observed in the central gray, except for the caudal part, wherein fibers were highly concentrated in a zone beginning from the ventricle and spreading laterally (Fig.3e,f). A low density of fibers was observed in the pons, medial lemniscus (Fig.3e,f), reticular nuclei (Fig.3e), inferior olive, and the cuneate nucleus. The density of fibers in the raphe nuclei varied. In the dorsal and median raphe nuclei a high fiber density was found (Fig. 3f), whereas the raphe obscurus nucleus contained a low density of fibers. A moderate density of fibers was observed in the nucleus of the solitary tract and the spinal trigeminal nucleus. A low density of fibers was observed in the cerebellum.
Comparison of brains from hibernating and nonhibernating ground squirrels
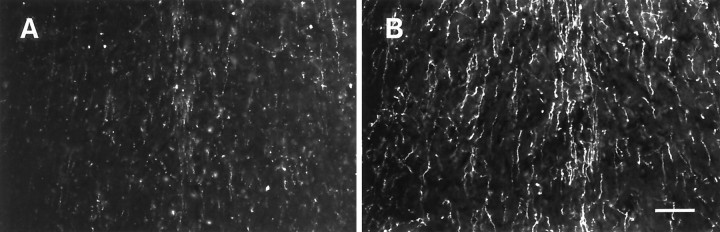
HA-immunoreactive nerve fibers displayed a higher density and were more strongly stained in hibernating ground squirrels than in nonhibernating ones. This was evident, for example in the cortex (Fig.4), septum (Fig.5), hippocampus (Fig.6), anterior hypothalamus (Fig.7), central gray (Fig.8), and the dorsal raphe nucleus (Fig.9). In addition to increased fiber density, the fibers in the hibernating brains appeared to be thicker with fewer varicosities. No obvious differences could be seen in the number of histaminergic perikarya.
Fig. 4.
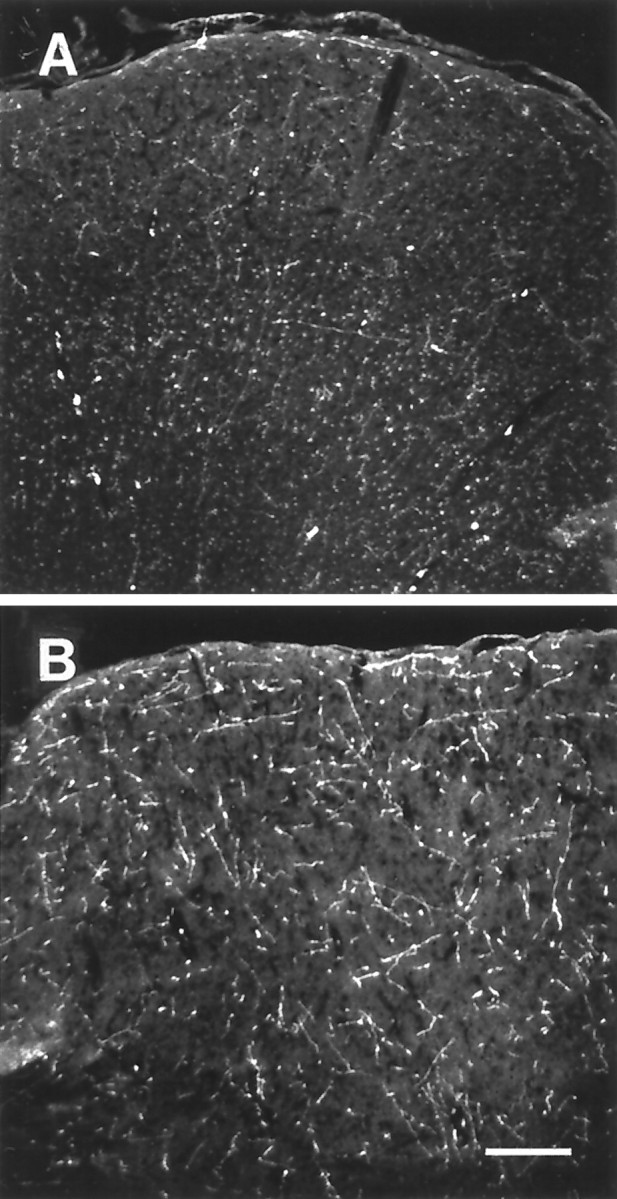
Histamine immunohistochemical staining of the frontal cortex of nonhibernating (A) and hibernating (B) ground squirrels using an antibody against histamine. Scale bar, 200 μm.
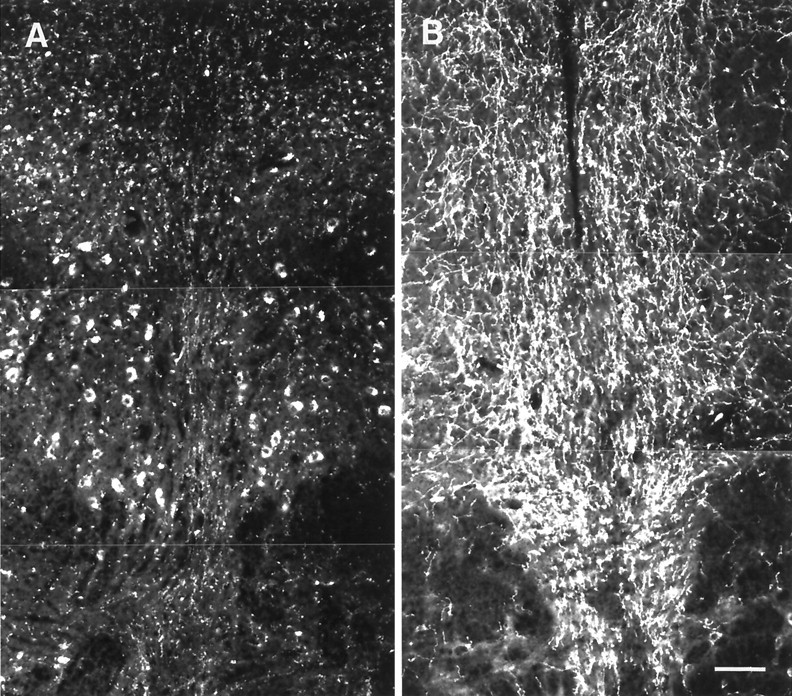
Fig. 5.
Histamine immunohistochemical staining of the medial septum of nonhibernating (A) and hibernating (B) ground squirrels. Scale bar, 100 μm.
Fig. 6.
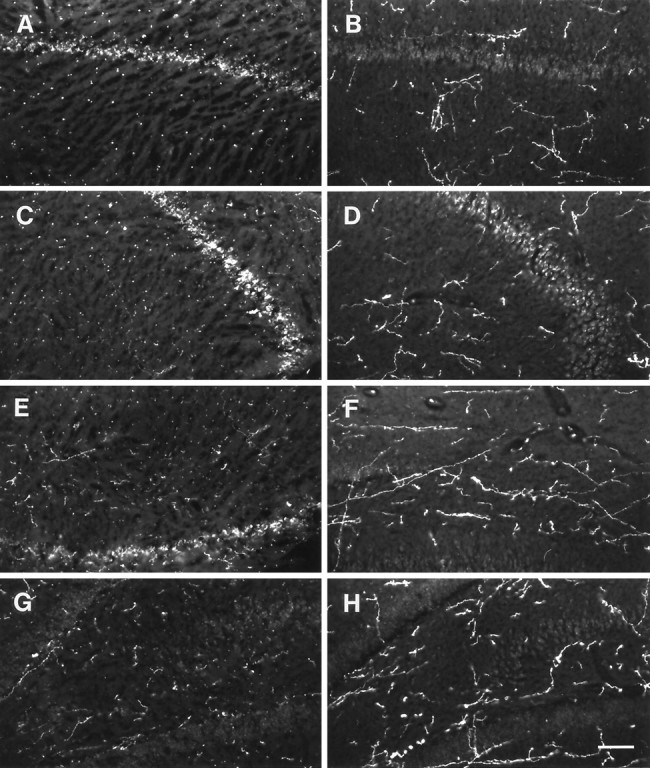
Areas from the rostral part of the ground squirrel hippocampus stained with an antibody against histamine.A, C, E, andG show areas from nonhibernating animals, whereasB, D, F, andH show areas from hibernating animals. Aand B show hippocampal area CA1, C andD area CA2, E and F area CA3, G and H area CA4. Scale bar, 100 μm.
Fig. 7.
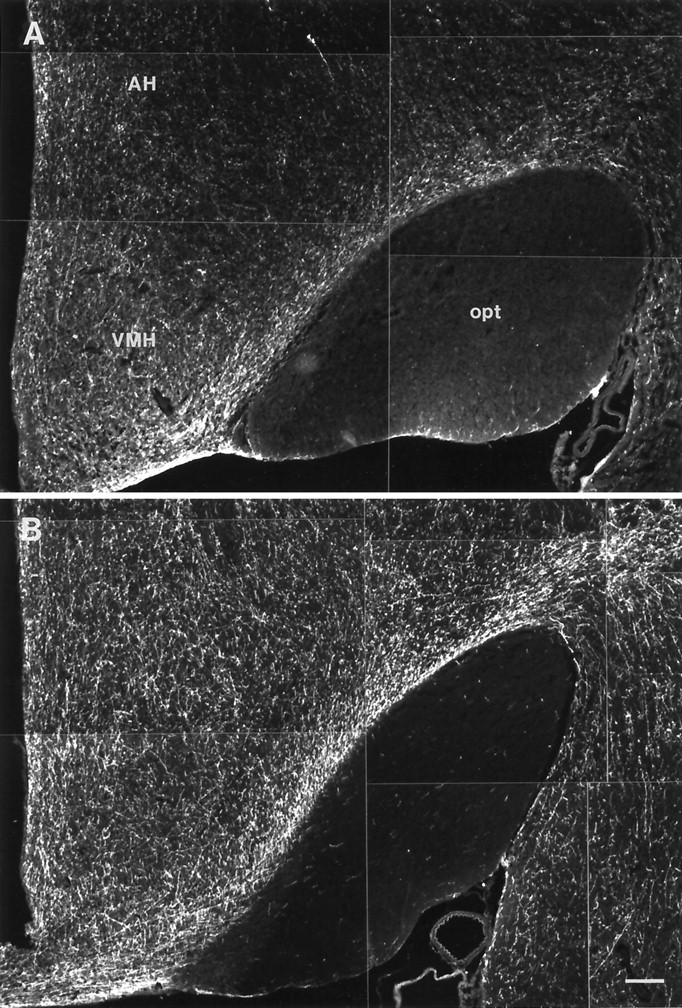
Histamine-immunoreactive fibers in the anterior hypothalamus nonhibernating (A) and hibernating (B) ground squirrels. Scale bar, 200 μm.
Fig. 8.
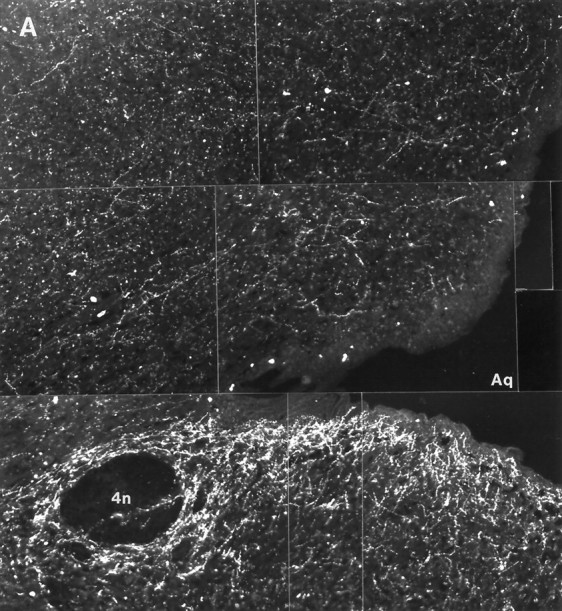
Histaminergic fibers in the central gray of nonhibernating (A) and hibernating (B) ground squirrels. Scale bar, 100 μm.
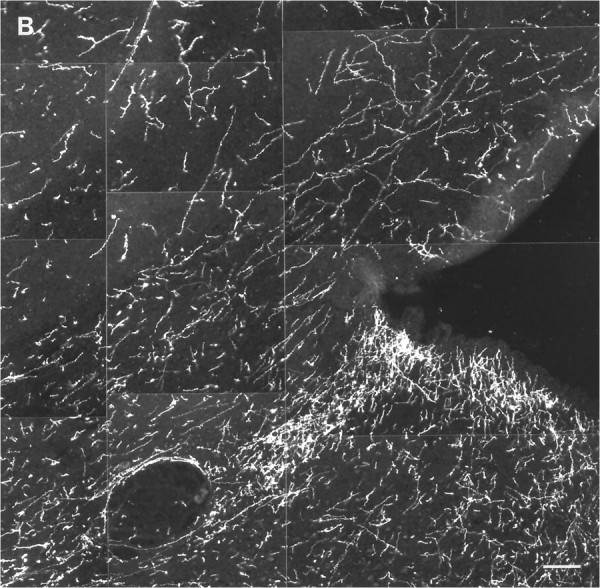
Fig. 9.
Histaminergic fibers in the dorsal Raphe nucleus of nonhibernating (A) and hibernating (B) ground squirrels. The cell bodies visible inA are nonspecifically autofluorescent. Scale bar, 100 μm.
Histamine and tele-methylhistamine concentrations in the ground squirrel brain
HPLC analysis showed that HA levels were higher in all brain areas of hibernating ground squirrels compared with those of nonhibernating ones (Fig. 10A). The two-way ANOVA showed that there was a significant difference in HA content between the hibernating and the nonhibernating brains in total (p < 0.0001; F = 57.433; df = 1) and a significant interaction between the two states and the brain areas (p < 0.0001; F= 6.122; df = 9). The t tests proved the differences in HA concentrations very highly significant in the pons (p < 0.0001; t = 6.321; df = 11), highly significant in the cortex (p = 0.0035; t = 4.648; df = 6), thalamus (p = 0.0064; t = 4.089; df = 6), midbrain (p = 0.0050; t = 4.023; df = 7), medulla (p = 0.0023;t = 4.395; df = 8), and cerebellum (p = 0.0012; t = 4.348; df = 11), and significant in the striatum (p = 0.0199; t = 3.147; df = 6), preoptic area/septum (p = 0.0152; t = 3.362; df = 6), hippocampus (p = 0.0125; t= 2.980; df = 11), and hypothalamus (p = 0.0295; t = 3.016; df = 5). However, if the α level was adjusted by the Bonferroni correction, yielding a significance level of p < 0.005, only the cortex, thalamus, midbrain, medulla, pons, and cerebellum were significant. During the hibernating state the mean HA levels were 93% higher in the striatum, 95% in the pons, 109% in the medulla, 113% in the cerebellum, 125% in the cortex, 126% in the preoptic area/septum, 133% in the hippocampus, 138% in the midbrain, 150% in the hypothalamus, and 186% in the thalamus.
Fig. 10.
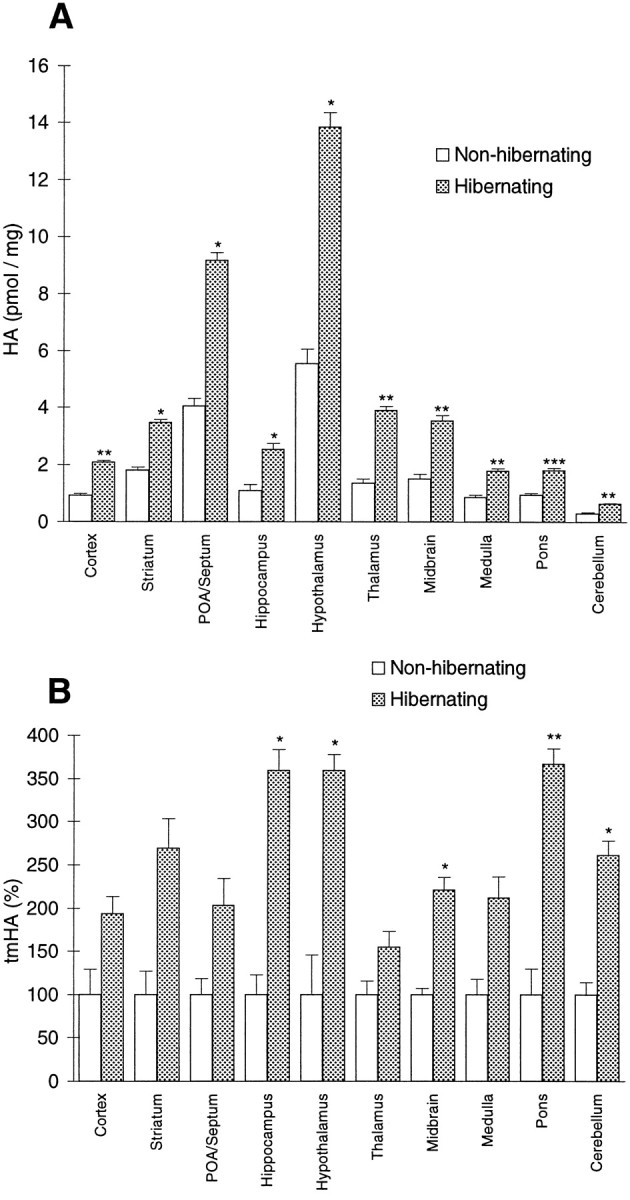
A, HA concentrations.B, tmHA concentrations in different brain areas of hibernating and nonhibernating ground squirrels. Values show mean ± SEM. *p < 0.05; **p < 0.001; ***p < 0.0001. POA, Preoptic area.
Analysis of tmHA concentrations showed higher levels during hibernation than during the nonhibernating state (Fig. 10B). The two-way ANOVA showed a significant difference between hibernating and nonhibernating brains in total (p < 0.0001;F = 38.418; df = 1) but no significant interaction between state and brain region (p = 0.733;F = 0.671; df = 9). The t test showed that the tmHA levels were significantly higher in the hippocampus (p = 0.0281; t = 2.880; df = 6), hypothalamus (p = 0.0126;t = 3.034; df = 10), midbrain (p = 0.0108; t = 3.646; df = 6), pons (p = 0.0044; t = 3.774; df = 9), and cerebellum (p = 0.0112;t = 3.414; df = 7), but not in the cortex (p = 0.0957; t = 1.822; df = 11), striatum (p = 0.1563; t = 1.743; df = 4), preoptic area/septum (p = 0.1933; t = 1.562; df = 4), thalamus (p = 0.1336; t = 1.620; df = 11), and medulla (p = 0.0825;t = 2.025; df = 7). If the α level was adjusted by the Bonferroni correction, none of the areas were significant. In brains from hibernating animals the mean tmHA levels were 55% higher in the thalamus, 93% in the cortex, 103% in the preoptic area/septum, 112% in the medulla, 121% in the midbrain, 162% in the cerebellum, 169% in the striatum, 260% in the hypothalamus and hippocampus, and 267% in the pons.
Expression of HDC mRNA
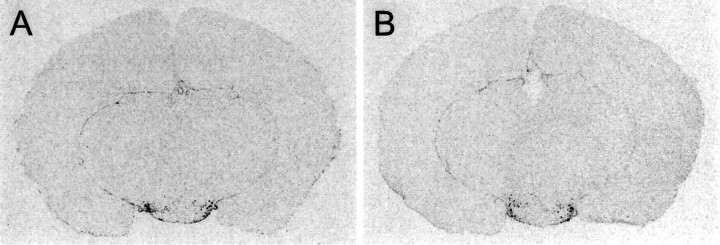
The oligonucleotide probe from a highly conserved region of the HDC gene gave a positive signal in the cells corresponding to the tuberomammillary neurons but in no other location of the brain. Hybridizations with the control oligonucleotide complementary toStaphylococcus aureus chloramphenicol acetyltransferase gave no signal. Although the sequence of the HDC gene is unknown in this species, we consider the HDC signal to be specific, because of its location and the fact that the probe is complementary to a sequence highly conserved among diverse mammalian species. No apparent difference was found in the level of expression between the hibernating and nonhibernating states (Fig.11).
Fig. 11.
Expression of the histamine-synthesizing enzyme HDC in the hypothalamus of nonhibernating (A) and hibernating (B) ground squirrels, shown byin situ hybridization with a radioactively labeled oligonucleotide probe, complementary to a highly conserved region of the HDC gene. Expression is seen exclusively in the tuberomammillary neurons. The anteroposterior level is that between the twobottom panels in Figure 1. The level of expression does not differ between A and B.
DISCUSSION
We present here the first comprehensive immunohistochemical study of the CNS of a hibernator for a brain monoamine. An important outcome of this study is the demonstration of major changes in the central histaminergic system during hibernation. We examined the distribution of histaminergic nerve fibers in the brain of the golden-mantled ground squirrel and evaluated the differences in fiber density between the nonhibernating and hibernating states. In addition, we examined the nonhibernating versus hibernating state differences in HA and tmHA concentrations and HDC mRNA expression. This study demonstrated that the histaminergic neuronal system in the ground squirrel brain is more extensive than in some nonhibernators such as the rat (Panula et al., 1989). The fiber density was clearly higher, for example, in the septum, hippocampus, and hypothalamus of ground squirrels than in corresponding areas of the rat brain. This could be interpreted as an indication of an enhanced importance of HA in the CNS of this animal, compared with other species. Moreover, our demonstration of an increase in HA-immunoreactive fibers, HA content, and HA metabolite (tmHA) content during the hibernating versus the nonhibernating state suggests that HA neuronal systems may play an important role in the control of the hibernating state. Our results raise some interesting issues regarding the possible mechanisms underlying the changes we observed.
For example, do the hibernation-related changes in the histaminergic system occur as a result of an increase in the activity of HA-releasing neurons? The increased fiber density we observed may result from an increase in fiber HA concentration consequent to a decrease in HA release, with the intraneuronally accumulated HA thereby bringing more fibers to a detectable level. Alternatively, we note that tmHA levels are considered to be strongly correlated with HA turnover in mammalian brain (Hough et al., 1984) and, in accordance with this, our findings of elevated regional tmHA levels during hibernation suggest increased histaminergic activity (i.e., histamine release) in this state. In this view, the elevated regional HA concentrations we observed would be expected to result from an enhanced level of HA synthesizing activity by the enzyme HDC that outpaces that of the HA-metabolizing enzyme histamine N-methyltransferase.
With regard to this latter point, we do not know the status of HDC activity during hibernation. Our HDC in situ hybridization data do, however, show that the amount of HDC mRNA remained unchanged during hibernation, making any increased synthesis of HA most likely a consequence of increased enzyme activity rather than to increased production of HDC itself. Although a general decline in enzyme activity might be expected to be associated with hibernation, there is evidence in the literature for increased as well as decreased enzyme activity in the brain during the hibernating versus the nonhibernating state (Robinson and Bradley, 1963; Stanton and Johnson, 1987).
Thus, it remains that if the elevation of tmHA levels found throughout the brain of hibernating C. lateralis is indeed a consequence of increased HA turnover, it follows that histaminergic transmission is increased in this state. We hold this interpretation with caution, however, because the possibility does exist that tmHA levels increased during hibernation as a consequence of changes in enzyme activity downstream in the degradation pathway of HA. For example, levels of homovanillic acid, a monoamine oxidase (MAO)-dependent dopamine metabolite, have been shown to decrease during hibernation (Salzman et al., 1985; Haak et al., 1991), indicating a decline in the activity of MAO (which also metabolizes tmHA). The extracellular level of 3-methoxy-4-hydroxyphenethanol has been shown to increase, suggesting a shift in metabolizing enzymes from aldehyde dehydrogenase to alcohol dehydrogenase (Salzman et al., 1985).tele-Methylimidazol acetic acid, the end product of the metabolic pathway of HA, is also a product of aldehyde dehydrogenase. Hence, the increase in tmHA levels we report here could be a result of a shift in enzyme activity that alters the clearance of tmHA from the brain. If this is the case, the activity of the histaminergic system may in fact be decreased rather than increased during hibernation. We note, however, that there are two types of MAO enzymes, MAO-A and MAO-B, in which dopamine is metabolized by both types and tmHA is metabolized by MAO-B. It is possible that the decrease in dopamine metabolites observed during hibernation results from a decline in MAO-A activity only.
Given these considerations, our results could either indicate an increase or a decrease in histaminergic activity. It may seem most natural to assume that the HA neuronal system would be inactive during hibernation in view of its well known role in promoting wakefulness. The brain levels of HA are known to show diurnal (Orr and Quay, 1975;Tuomisto and Tuomisto, 1982) and circadian (Garbarg et al., 1974) variation, and several studies have shown that HA increases wakefulness and decreases slow-wave sleep through the action of H1receptors (Schwartz et al., 1991). HA depolarizes human cortical neurons via blockade of a voltage-independent potassium conductance (Reiner and Kamondi, 1994) and switches thalamic neuronal activity from rhythmic burst firing to single-spike activity (McCormick and Williamson, 1991). HA activates cholinergic nucleus basalis neurons (Khateb et al., 1995) and increases EEG activity in the mesopontine tegmentum, presumably through interaction with cholinergic neurons (Lin et al., 1996). These findings support the interpretation that HA-releasing neural systems are inactive in the hibernating state. Given this, the high levels of intracellular HA accumulating in the fibers and terminals during the maintenance phase of the hibernation bout may be rapidly released during the arousal phase as part of the neuronal drive that activates and sustains this portion of the hibernation cycle. In concert with this, it seems plausible to speculate that a constant, but very low, level of histamine release during the maintenance phase of hibernation could be ongoing to keep CNS activity at a minimal functional level.
Another important outcome of the present study, which bears on whether HA activity is enhanced or reduced during hibernation, is the demonstration that hibernation-related changes in the metabolism of HA follow a unique pattern compared with that of other monoamines reported in the literature. That is, whereas HA metabolite levels increase during hibernation, the turnover of norepinephrine (Cai et al., 1989), dopamine (Salzman et al., 1985), and 5-hydroxytryptamine (Popova and Voitenko, 1981) decrease during hibernation. Given these results of decreased turnover, it seems reasonable to assume that the maintenance of brain activity in the hibernation mode requires activity in these respective monoamine systems be held at a low level and that increases in the activity of these systems would trigger arousal from hibernation. Indeed, intracerebroventricular microinjections of norepinephrine (Glass and Wang, 1978, 1979) and intrahypothalamic microinjections of acetylcholine (Stanton and Beckman, 1977), norepinephrine, and 5-hydroxytryptamine (Beckman and Satinoff, 1972) have been shown to induce arousal in hibernating ground squirrels. From these microinjection findings, it may again be concluded that each of these neurotransmitter systems seems to be subjected to inhibitory influence during hibernation and that arousal occurs when this inhibition ceases and the transmitter systems are reactivated.
Histamine is known to both enhance and inhibit the release of other neurotransmitters through different histamine receptor mechanisms. Histamine H3 heteroreceptors have been shown to inhibit the release of 5-hydroxytryptamine (Schlicker et al., 1988; Fink et al., 1990), norepinephrine (Schlicker et al., 1989), dopamine (Schlicker et al., 1993), and acetylcholine (Arrang et al., 1995). On the other hand, HA evokes the release of 5-hydroxytryptamine and norepinephrine through H1 and H2 receptors (Young et al., 1988). The net effect of HA in a given brain area is therefore likely to be dependent on the relative constitution of receptor subtypes in that area. If the results of the present study are taken to indicate an increase in histaminergic activity during hibernation, it would follow that the role of HA activity would be to inhibit other neurotransmitter systems, such as those noted above, through a H3receptor-related mechanism. A shift in HA receptor constitution during hibernation or alternatively a downregulation of HA turnover could then contribute to reactivating the brain and inducing arousal. Receptor-binding studies in progress should provide further insight into this issue.
Our results showing that tmHA levels are increased more than threefold in the hypothalamus and hippocampus of hibernating animals may be of particular importance, if one considers the role of these areas in controlling hibernation. First, the preoptic/anterior hypothalamus is known to be of central importance for the regulation of body temperature. During hibernation, activity within this region shifts, producing a decline in the regulated level of body temperature (Heller, 1979; Beckman and Stanton, 1982) that provides the hallmark of mammalian hibernation (i.e., decreased body temperature and metabolic rate). Given that brain histaminergic neuronal systems have been implicated in the regulation of body temperature (Sakata et al., 1997), it is likely that the histaminergic system in the ground squirrel brain is actively involved in controlling this aspect of hypothalamic function during hibernation. Second, the hippocampus has been hypothesized to be involved in controlling entrance into, maintenance of, and arousal from hibernation (Heller, 1979; Beckman and Stanton, 1982). Studies of the effect of HA on synaptic transmission in hippocampal slices from Turkish hamsters have shown that augmentation of population spikes by HA is significantly stronger in slices from hibernating hamsters than in slices from warm-acclimated ones (Nikmanesh et al., 1996), indicating that HA neuronal activity in the hippocampus during hibernation should exert a strong influence on the manner in which the hippocampus contributes to the control of the hibernation state.
In conclusion, our results demonstrate that dramatic changes occur in the histaminergic system during hibernation. However, the mechanisms underlying these changes, and therefore our understanding of their significance for the role of HA neuronal activity in controlling the hibernation state, requires further clarification.
Footnotes
This work was supported by the Academy of Finland, the Erna and Viktor Hasselblad foundation, the Signal Transduction Program of Åbo Akademi University, and the California State University, Long Beach, CA. We thank Ms. Hannele Jaatinen and Mr. Esa Nummelin for skillful technical help.
Correspondence should be addressed to Pertti Panula, Department of Biology, Åbo Akademi University, Artillerigatan 6, BioCity, 20520 Åbo, Finland.
REFERENCES
- 1.Airaksinen MS, Panula P. The histaminergic system in the guinea pig central nervous system: an immunocytochemical mapping study using an antiserum against histamine. J Comp Neurol. 1988;273:163–186. doi: 10.1002/cne.902730204. [DOI] [PubMed] [Google Scholar]
- 2.Airaksinen MS, Flügge G, Fuchs E, Panula P. Histaminergic system in the tree shrew brain. J Comp Neurol. 1989;286:289–310. doi: 10.1002/cne.902860302. [DOI] [PubMed] [Google Scholar]
- 3.Airaksinen MS, Alanen S, Szabat E, Visser TJ, Panula P. Multiple neurotransmitters in the tuberomammillary nucleus: comparison of rat, mouse and guinea pig. J Comp Neurol. 1992;323:103–116. doi: 10.1002/cne.903230109. [DOI] [PubMed] [Google Scholar]
- 4.Arrang J-M, Drutel G, Schwartz J-C. Characterization of histamine H3 receptors regulating acetylcholine release in rat entorhinal cortex. Br J Pharmacol. 1995;114:1518–1522. doi: 10.1111/j.1476-5381.1995.tb13379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckman AL, Satinoff E. Arousal from hibernation by intrahypothalamic injections of biogenic amines in ground squirrels. Am J Physiol. 1972;222:875–879. doi: 10.1152/ajplegacy.1972.222.4.875. [DOI] [PubMed] [Google Scholar]
- 6.Beckman AL, Stanton TL. Properties of the CNS during the state of hibernation. In: Beckman AL, editor. The Neural Basis of Behavior. Spectrum; New York: 1982. pp. 19–45. [Google Scholar]
- 7.Cai YP, Zhao HQ, Huang QH. Reevaluation of involvement of norepinephrine and serotonin in initiation of hibernation. In: Malan A, Canguilhem B, editors. Living in the Cold II. Libbey; London: 1989. pp. 477–484. [Google Scholar]
- 8.Fink K, Schlicker E, Neise A, Göthert M. Involvement of presynaptic H3 receptors in the inhibitory effect of histamine on serotonin release in the rat brain cortex. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:513–519. doi: 10.1007/BF00169038. [DOI] [PubMed] [Google Scholar]
- 9.Garbarg M, Julien C, Schwartz J-C. Circadian rhythm of histamine in the pineal gland. Life Sci. 1974;14:539–543. doi: 10.1016/0024-3205(74)90368-3. [DOI] [PubMed] [Google Scholar]
- 10.Glass JD, Wang LC. Thermoregulatory effects of central injection of noradrenaline in the Richardson’s ground squirrel. Comp Biochem Physiol. 1978;61C:347–351. doi: 10.1016/0306-4492(78)90067-9. [DOI] [PubMed] [Google Scholar]
- 11.Glass JD, Wang LC. Effects of central injections of biogenic amines during arousal from hibernation. Am J Physiol. 1979;236:R162–R167. doi: 10.1152/ajpregu.1979.236.3.R162. [DOI] [PubMed] [Google Scholar]
- 12.Haak LL, Mignot E, Kilduff TS, Dement WC, Heller HC. Regional changes in central monoamine and metabolite levels during the hibernation cycle in the golden-mantled ground squirrel. Brain Res. 1991;563:215–220. doi: 10.1016/0006-8993(91)91536-a. [DOI] [PubMed] [Google Scholar]
- 13.Heller HC. Hibernation: neural aspects. Annu Rev Physiol. 1979;41:305–321. doi: 10.1146/annurev.ph.41.030179.001513. [DOI] [PubMed] [Google Scholar]
- 14.Hough LB, Khandelwal JK, Green JP. Histamine turnover in regions of rat brain. Brain Res. 1984;291:103–109. doi: 10.1016/0006-8993(84)90655-3. [DOI] [PubMed] [Google Scholar]
- 15.Hough LB, Khandelwal JK, Morrishow AM, Green JP. An improved GCMS method to measure tele-methylhistamine. J Pharmacol Methods. 1981;5:143–148. doi: 10.1016/0160-5402(81)90006-1. [DOI] [PubMed] [Google Scholar]
- 16.Inagaki N, Yamatodani A, Ando-Yamamoto M, Tohyama M, Watanabe T, Wada H. Organization of histaminergic fibers in the rat brain. J Comp Neurol. 1988;273:283–300. doi: 10.1002/cne.902730302. [DOI] [PubMed] [Google Scholar]
- 17.Joseph SA, Knigge KA, Kalejs LM, Hoffman RA, Reid P. A stereotaxic atlas of the brain of the 13-line ground squirrel (Citellus tridecemlineatus). Edgewood Arsenal Special Publications; Edgewood, MD: 1966. [Google Scholar]
- 18.Joseph DR, Sullivan PM, Wang Y-M, Kozak C, Fenstermacher DA, Behrendsen ME, Zahnow CA. Characterization and expression of the complementary DNA encoding rat histidine decarboxylase. Proc Natl Acad Sci USA. 1990;87:733–737. doi: 10.1073/pnas.87.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khateb A, Fort P, Pegna A, Jones BE, Mühlethaler M. Cholinergic nucleus basalis neurons are excited by histamine in vitro. Neuroscience. 1995;69:495–506. doi: 10.1016/0306-4522(95)00264-j. [DOI] [PubMed] [Google Scholar]
- 20.Lin J-S, Hou Y, Sakai K, Jouvet M. Histaminergic descending outputs to the mesopontine tegmentum and their role in the control of cortical activation and wakefulness in the cat. J Neurosci. 1996;16:1523–1537. doi: 10.1523/JNEUROSCI.16-04-01523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lintunen M, Sallmen T, Karlstedt K, Fukui H, Eriksson KS, Panula P. Postnatal expression of H1-receptor mRNA in the rat brain: correlation to l-histidine decarboxylase expression and local upregulation in limbic seizures. Eur J Neurosci. 1998;10:2287–2301. doi: 10.1046/j.1460-9568.1998.00240.x. [DOI] [PubMed] [Google Scholar]
- 22.McCormick DA, Williamson A. Modulation of neuronal firing mode in cat and guinea pig LGNd by histamine: possible cellular mechanism of histaminergic control of arousal. J Neurosci. 1991;11:3188–3199. doi: 10.1523/JNEUROSCI.11-10-03188.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikmanesh FG, Spangenberger H, Igelmund P. Histamine enhances synaptic transmission in hippocampal slices from hibernating and warm-acclimated Turkish hamsters. Neurosci Lett. 1996;210:119–120. doi: 10.1016/0304-3940(96)12672-0. [DOI] [PubMed] [Google Scholar]
- 24.Orr E, Quay WB. Hypothalamic 24-hour rhythms in histamine, histidine decarboxylase and histamine-N-methyltransferase. Endocrinology. 1975;96:941–945. doi: 10.1210/endo-96-4-941. [DOI] [PubMed] [Google Scholar]
- 25.Panula P, Yang H- YT, Costa E. Histamine containing neurons in the rat hypothalamus. Proc Natl Acad Sci USA. 1984;81:2572–2576. doi: 10.1073/pnas.81.8.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panula P, Pirvola U, Auvinen S, Airaksinen MS. Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience. 1989;28:585–610. doi: 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- 27.Panula P, Airaksinen MS, Pirvola U, Kotilainen E. A histamine-containing neuronal system in human brain. Neuroscience. 1990;34:127–132. doi: 10.1016/0306-4522(90)90307-p. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1982. [DOI] [PubMed] [Google Scholar]
- 29.Popova NK, Voitenko NN. Brain serotonin metabolism in hibernation. Pharmacol Biochem Behav. 1981;14:773–777. doi: 10.1016/0091-3057(81)90360-9. [DOI] [PubMed] [Google Scholar]
- 30.Reiner PB, Kamondi A. Mechanisms of antihistamine-induced sedation in the human brain: H1 receptor activation reduces a background leakage potassium current. Neuroscience. 1994;59:579–588. doi: 10.1016/0306-4522(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 31.Robinson JD, Bradley RM. Cholinesterase and glutamic acid decarboxylase levels in the brain of the hibernating hamster. Nature. 1963;197:389–390. [Google Scholar]
- 32.Sakata T, Yoshimatsu H, Kurokawa M. Thermoregulation modulated by histamine in rats. Inflamm Res [Suppl 1] 1997;46:S35–S36. [PubMed] [Google Scholar]
- 33.Salzman SK, Llados-Eckman C, Beckman AL. In vivo analysis of dopamine and its metabolites in the caudate nucleus during euthermia and hibernation. Brain Res. 1985;343:95–103. doi: 10.1016/0006-8993(85)91162-x. [DOI] [PubMed] [Google Scholar]
- 34.Schlicker E, Betz R, Göthert M. Histamine H3 receptor-mediated inhibition of noradrenaline release in the rat brain cortex. Naunyn Schmiedebergs Arch Pharmacol. 1988;337:588–590. doi: 10.1007/BF00182737. [DOI] [PubMed] [Google Scholar]
- 35.Schlicker E, Fink K, Hinterthaner M, Göthert M. Inhibition of noradrenaline release in the rat brain cortex via presynaptic H3 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:633–638. doi: 10.1007/BF00717738. [DOI] [PubMed] [Google Scholar]
- 36.Schlicker E, Fink K, Detzner M, Göthert M. Histamine inhibits dopamine release in the mouse striatum via presynaptic H3 receptors. J Neural Transm Gen Sect. 1993;93:1–10. doi: 10.1007/BF01244933. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz J-C, Arrang J-M, Garbarg M, Pollard H, Ruat M. Histaminergic transmission in the mammalian brain. Physiol Rev. 1991;71:1–51. doi: 10.1152/physrev.1991.71.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Stanton TL, Beckman AL. Thermal changes produced by intrahypothalamic injections of acetylcholine during hibernation and euthermia in Citellus lateralis. Comp Biochem Physiol. 1977;59A:143–150. [Google Scholar]
- 39.Stanton TL, Johnson GVW. In vitro measurements of cholinergic activity in brain regions of hibernating ground squirrels. Brain Res Bull. 1987;18:663–668. doi: 10.1016/0361-9230(87)90136-5. [DOI] [PubMed] [Google Scholar]
- 40.Tuomisto L, Tuomisto J. Diurnal variations in brain and pituitary histamine and histamine-N-methyltransferase in the rat and guinea pig. Med Biol. 1982;60:204–209. [PubMed] [Google Scholar]
- 41.Tuomisto L, Ylinen M, Onodera K, Tacke U, Airaksinen M. Comparison of regional brain histamine and tele-methylhistamine levels in genetically epilepsy-prone rats and in rats resistant to epilepsy. Meth Find Exp Clin Pharmacol [Suppl A] 1996;18:155–159. [Google Scholar]
- 42.Wada H, Inagaki N, Yamatodani A, Watanabe T. Is the histaminergic system a regulatory center for whole-brain activity? Trends Neurosci. 1991;14:415–418. doi: 10.1016/0166-2236(91)90034-r. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto J, Yatsunami K, Ohmori E, Sugimoto Y, Fukui T, Katayama T, Ichikawa A. cDNA-derived amino acid sequence of l-histidine decarboxylase from mouse mastocytoma P-815 cells. FEBS Lett. 1990;276:214–218. doi: 10.1016/0014-5793(90)80545-t. [DOI] [PubMed] [Google Scholar]
- 44.Yamatodani A, Fukuda H, Wada H, Iwaeda T, Watanabe T. High-performance liquid chromatographic determination of plasma and brain histamine without previous purification of biological samples: cation-exchange chromatography coupled with post-column derivatization fluorometry. J Chromatogr. 1985;344:115–123. doi: 10.1016/s0378-4347(00)82012-5. [DOI] [PubMed] [Google Scholar]
- 45.Yamauchi K, Ruriko S, Ohkawara Y, Tanno Y, Maeyama K, Watanabe T, Satoh K, Yoshizawa M, Shibahara S, Takishima T. Nucleotide sequence of the histidine decarboxylase cDNA derived from human basophilic leukemia cell line, KU-812-F. Nucleic Acids Res. 1990;18:5891–5891. doi: 10.1093/nar/18.19.5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young CS, Mason R, Hill SJ. Studies on the mechanisms of histamine-induced release of noradrenaline and 5-hydroxytryptamine from slices of rat cerebral cortex. Biochem Pharmacol. 1988;37:2799–2805. doi: 10.1016/0006-2952(88)90043-3. [DOI] [PubMed] [Google Scholar]