Abstract
Orbitofrontal cortex (OFC) is part of a network of structures involved in adaptive behavior and decision making. Interconnections between OFC and basolateral amygdala (ABL) may be critical for encoding the motivational significance of stimuli used to guide behavior. Indeed, much research indicates that neurons in OFC and ABL fire selectively to cues based on their associative significance. In the current study recordings were made in each region within a behavioral paradigm that allowed comparison of the development of associative encoding over the course of learning. In each recording session, rats were presented with novel odors that were informative about the outcome of making a response and had to learn to withhold a response after sampling an odor that signaled a negative outcome. In some cases, reversal training was performed in the same session as the initial learning. Ninety-six of the 328 neurons recorded in OFC and 60 of the 229 neurons recorded in ABL exhibited selective activity during evaluation of the odor cues after learning had occurred. A substantial proportion of those neurons in ABL developed selective activity very early in training, and many reversed selectivity rapidly after reversal. In contrast, those neurons in OFC rarely exhibited selective activity during odor evaluation before the rats reached the criterion for learning, and far fewer reversed selectivity after reversal. The findings support a model in which ABL encodes the motivational significance of cues and OFC uses this information in the selection and execution of an appropriate behavioral strategy.
Keywords: basolateral amygdala, orbitofrontal cortex, amygdala, prefrontal cortex, olfaction, discrimination learning, learning and memory, electrophysiology, single units, rats
Patients with damage to the orbital region of prefrontal cortex characteristically fail to use available information to appropriately guide their actions (Damasio, 1994;Bechara et al., 1997). Instead they often engage in maladaptive behavior, even when they are aware that their decisions will lead to adverse consequences. Despite knowledge regarding the outcome of their actions, it appears that such patients are inadequately motivated by that information. This deficit may be attributable, at least in part, to interruption of orbitofrontal cortex (OFC) connections with the basolateral amygdala complex (ABL) (Krettek and Price, 1977; Kolb, 1984; Price et al., 1987; McDonald, 1991), a subcortical system widely implicated in the ability to learn the motivational significance of cues (Everitt et al., 1989, 1991; Davis, 1992; Gallagher and Chiba, 1996; Hatfield et al., 1996; LeDoux, 1996; Balleine et al., 1997;Killcross et al., 1997).
The importance of ABL to adaptive behavior is apparent in many tasks that depend on associative learning. For example, in widely studied fear-conditioning paradigms, defense responses such as freezing are normally elicited by cues that predict an impending aversive event (Davis, 1992). This learning is equally impaired by damage to either ABL or the central nucleus of the amygdala. In a widely accepted model for this form of learning, projections from ABL to central nucleus provide access to brainstem systems that mediate conditioned fear responses. At the same time, recent research has shown that other learning paradigms that are sensitive to ABL damage are unaffected by central nucleus lesions. These include deficits in higher-order learning (second-order Pavlovian conditioning and instrumental learning with secondary reinforcement) (Everitt et al., 1989, 1991; Hatfield et al., 1996), an inability to adjust behavior to conditioned stimuli based on changes in reward value (Hatfield et al., 1996; Balleine et al., 1997), and a deficit in learning to direct behavior to avoid a negative outcome (Killcross et al., 1997). These adaptive behaviors, unlike the elicitation of species-typical responses, are likely to require the integrative function of the amygdala and other forebrain systems, such as ventral striatum and orbitofrontal cortex.
The current study was designed to examine the roles of OFC and ABL in adaptive instrumental learning. We recorded neural activity in OFC and ABL in rats as they learned novel olfactory discrimination problems. In some sessions, this initial training was followed by reversal training in which the response contingencies of the odors were switched. Previously we have reported that neurons in OFC and ABL, in a subset of these rats, fired selectively early in learning; this activity appeared to encode the expected outcome on a trial while the rats awaited reinforcement (Schoenbaum et al., 1998). Here we report on activity during sampling of the cues that signaled the response contingencies. Neurons in both OFC and ABL fired selectively during odor sampling to reflect task contingencies during accurate performance. Further analysis showed that the development of this activity differed in the two brain regions. In ABL, neural activity in a substantial proportion of such cells reflected the motivational significance of the odor cues early in training independent of the rat’s choice behavior. In OFC, however, activity in these neurons only emerged in conjunction with a reliable shift in the rat’s behavioral strategy (go, no-go) based on the significance of the cues.
MATERIALS AND METHODS
Subjects. Eight adult male Long–Evans rats served as subjects. The rats were housed individually, maintained on a 12 hr light/dark cycle, and given ad libitum access to food. Water access was restricted during the 24 hr preceding behavioral testing to motivate performance in the task. During testing periods, the rats received fluid during the performance of the task, amounting to ∼5–10 ml/session, and were given free access to water in a holding cage after the session was finished. During this time, food was also available.
Electrodes, surgery, and histology. Recordings of extracellular activity were obtained using a drivable bundle of 10 25-μm-diameter microwires (modified from Kubie, 1984). Rats weighed 325–375 gm at the time of surgery to implant the electrode bundle. Surgical procedures were similar to those described previously (Schoenbaum and Eichenbaum, 1995a). A single bundle was implanted in the left hemisphere in orbitofrontal cortex of four rats (3.0 mm anterior to bregma, 3.2 mm lateral, 4.0 mm ventral) and basolateral complex of amygdala of four rats (3.0 mm posterior to bregma, 5.0 mm lateral, 7.5 mm ventral). The rats were allowed two weeks to recover, during which each animal received cephalexin (40 mg · kg−1 · d−1) to guard against infection. Once recording began, the electrode bundle was advanced in 40 μm increments to acquire activity from new cells for the following day. Recording was stopped in a given rat when the estimated position of the electrode bundle was consistent with passage beyond the region of interest. The rats were then deeply anesthetized with sodium pentobarbital in preparation for perfusion. Immediately before perfusion, the final electrode position was marked by passage of a 15 μA current through each microwire for ∼10 sec to create a small iron deposit. The rats were then perfused transcardially using physiological saline followed by 10% formalin followed by 100 ml of 10% formalin-3% potassium ferrocyanide solution to visualize the iron deposit. The brains were then removed from the skulls and stored in a 10% formalin-20% sucrose-3% potassium ferrocyanide solution for several days before sectioning. Brains were cut into 30 μm sections surrounding the electrode tracks and stained with thionin, and the electrode tracks were reconstructed to determine approximate recording sites using the marks left by the iron at the tips of the electrodes.
Behavioral methods. Behavioral testing was performed in an operant chamber using a go, no-go olfactory discrimination task in which all behavioral events and data collection were controlled and monitored by computer as described previously (Schoenbaum and Eichenbaum, 1995a). The operant chamber was constructed of aluminum and measured ∼45 cm in height, depth, and width. An odor port and a fluid well were located on the left wall of the chamber, and two panel lights were located above the odor port.
The odor port consisted of a circular opening ∼2.5 cm in diameter, behind which was a small chamber through which odorized or clean air could be passed. Air flow through the odor port was facilitated by a vacuum line drawing 1 l/min from the chamber behind the port. Odor delivery to the chamber behind the odor port was controlled by the behavioral computer via a system of flow meters and solenoid valves. The odor to be presented on a given trial was selected via activation of the appropriate solenoid valve before the start of a trial. Opening of the valve allowed a 0.5 l/min stream of clean air to pass over a 5% solution of a particular odor and then to mix with a clean air stream of 0.5 l/min. This odorized air was diverted to a vacuum dump at a final solenoid valve just outside of the odor port. This solenoid valve was then activated to deliver the odor on detection of the rat at the odor port. A photobeam across the opening to the odor port was monitored by the behavioral computer to detect entry of the rat’s snout into the port. Odor delivery was terminated by inactivation of the same solenoid when the rat left the odor port, and any remaining odor was quickly removed by the entry of clean air from the behavioral chamber due to the vacuum line in the port.
The fluid delivery well, located several centimeters below the odor port, consisted of a small depression cut in a 2.5-cm-wide Plexiglas shelf. Concealed lines in the bottom of this well allowed the delivery of sucrose and quinine for use in the task as well as water to flush the well between trials. The well was emptied by vacuum via a fourth line. Fluid delivery was also controlled by the behavioral computer via solenoid valves, and a photobeam across the top of the well was monitored by the computer to detect the presence of the rat at the fluid well.
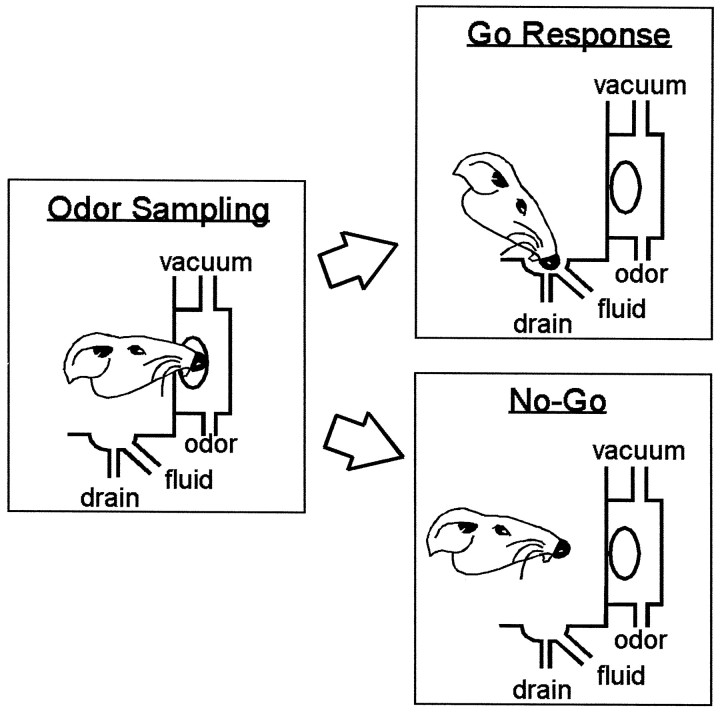
Before the sessions in which recordings were made, behavioral training was conducted using several odor discrimination problems to familiarize the rats with the procedures of the task. Odors were chosen from a pool of 64 distinct odorants (International Flavors and Fragrances, Union Beach, NJ), diluted 1:20 in propylene glycol to approximately equal intensity. In each recording session, a new odor discrimination problem was presented involving either two or four novel odors. The task is illustrated in a schematic provided in Figure1. Illumination of the panel lights signaled that a trial could be initiated, and a trial began when the rat poked its nose into the odor port to trigger odor presentation. Actual odor onset was delayed by a variable period of ∼300–800 msec after the rat’s snout interrupted a photobeam across the opening to the odor port, so that the rat was stationary in the port when the odor was delivered. The delivery of the odor cue was terminated by the rat’s decision to remove its snout from the odor port. The rat then had 3 sec after withdrawal from the port to respond by entering the nearby fluid well for reinforcement (go response). As illustrated in Figure 1, the odor port and fluid well were separated by ∼5 cm. In the two-odor task (22 sessions), one odor, designated the positive odor, signaled that a go response would produce ∼0.05 ml of a palatable 10% sucrose solution, whereas the other odor, designated the negative odor, signaled that a go response would produce ∼0.05 ml of a distasteful 0.03 m quinine solution. In the four-odor task (33 sessions), two distinct odors were associated with sucrose and two distinct odors were associated with quinine. Each rat was water-deprived overnight before a recording session and, therefore, was strongly motivated to perform for fluid reward. Because novel odors were presented in each session, the rat had to learn new associations each day.
Fig. 1.
Schematic drawings illustrate the sequence of behaviors in the go, no-go olfactory discrimination task. In this task, a water-deprived rat had to sample an odor presented at a port on each trial (odor sampling) to decide whether to respond (go response) at a nearby fluid well. Responses at both the odor port and the fluid well were registered by interruption of photo beams that detected entry of the rat’s snout into each port. A go response resulted in delivery of a rewarding sucrose solution, after presentation of a “positive” odor, or an aversive quinine solution, after presentation of a “negative” odor. A go response after a negative odor was considered an error and followed by a prolonged intertrial interval (9 vs 4 sec after a correct response). Novel odors were presented in each session; thus the animal had to learn new associations each day. The rat would begin each session by responding on every trial, irrespective of whether a positive or a negative odor was presented. Learning was evident when the rat began to withhold responses (no-go) after sampling of the negative odor to avoid quinine delivery. This shift in the rat’s behavior generally began after 15–30 trials. Stable, highly accurate performance was generally achieved after 60–100 trials, reaching a behavioral criterion defined as 90% accurate performance over a moving block of 20 trials. During postcriterion performance the rat would make very few errors.
Typically, a rat began each session by responding after sampling on every trial, irrespective of which odor had been presented, and then gradually learned to withhold responses (no-go) after sampling odors that signaled that quinine would be delivered. The rats became highly accurate within a single session at responding only to obtain sucrose reinforcement. The shift to the adaptive behavioral strategy of responding for the rewarding sucrose solution and withholding a response to avoid the aversive quinine solution was reflected in the acquisition of a behavioral criterion defined as 90% accurate performance in a moving block of 20 trials. The rats generally met this criterion in 60–100 trials. After reaching this criterion, rats generally made few errors. In addition, in the sessions involving two odor discrimination problems, postcriterion training was followed by reversal training when the response contingencies of the two odors were reversed so that the positive odor became associated with quinine, whereas the negative odor became associated with sucrose.
Electrophysiological methods. At the start of each recording session, each wire of the microelectrode bundle was screened for neural activity. If no activity was evident, the bundle of wires was advanced 40 or 80 μm to acquire cells for the following day. If neural activity was present on any of the wires, a recording session was conducted. Neural activity on each microwire was passed through a high-impedance JFET head stage, and then differential activity on up to eight microwires was filtered at 300–3000 Hz, amplified 5000 times using Grass P5 series preamplifiers, and recorded on analog tape along with computer-generated transistor–transistor logic pulses to mark behavioral events using a Vetter model 400 PCM data recorder (AR Vetter, Rebersburg, PA). Later, neural signals were digitized at 25 kHz, and then individual units were discriminated using a template-matching algorithm (Cambridge Electronic Design, Cambridge, England) in concert with examination of the oscilloscope tracing. Typically one to three neurons could be discriminated on an active electrode wire, and data were collected in 55 sessions in the eight rats. Data from the cells in a subset of these animals (excluding recordings in lateral nucleus) have been reported previously for a different time interval in the training trial (during a response delay after odor evaluation) (Schoenbaum et al., 1998).
Analysis of unit activity. Neural activity was examined during odor evaluation on trials after the rat reached the behavioral criterion within a time window extending from 200 msec before to 150 msec after odor offset. This sampling period was synchronized to the rat’s decision to terminate odor sampling by leaving the odor port to reflect the rat’s evaluation of the odor. The inclusion of a period 200 msec before odor offset ensured that only activity at a latency of at least 100 msec from odor onset would be included, and the sampling interval was extended slightly after odor offset to include any activity related to trace olfactory processing coincident with the rat’s decision to terminate odor evaluation. Neural activity (spikes per second) within this time interval during postcriterion training was compared on trials involving different odors using ANOVA. A statistically significant difference (p < 0.05) was further evaluated if the session involved four odors by post hoc testing to compare activity on trials with each odor. Neurons with elevated activity on trials specific to a single odor or on trials of either of a pair of odors associated with a same reinforcer were categorized similarly as either positive odor or negative odor selective (see Table 1). The populations of neurons in ABL and OFC that were selective in the postcriterion phase were further analyzed for development of that selectivity in the precriterion phase and for the effect of reversal training on selectivity.
Table 1.
Neural selectivity during odor evaluation in postcriterion training
| Trial type | Two-odor | Four-odor | ||
|---|---|---|---|---|
| Positive Odor | Negative Odor | Positive Odors | Negative Odors | |
| OFC (328)1-a | 28 | 6 | 22 | 26 |
| ABL (229)1-a | 6 | 12 | 12 | 30 |
Total number of neurons sampled in each region.
Selective activity during the precriterion trials was determined using the same statistical analysis applied to the postcriterion trials. When a similar selectivity was evident in the analysis of the precriterion trials, that phase was further subdivided to measure initial selectivity during an early segment of training. This early segment, used previously (Schoenbaum et al., 1998), included only those trials preceding the sixth negative go response (error) and included, on average, 15 trials. These trials were selected for analysis to examine neural activity before the rat began to withhold responses. Finally neural activity was also examined during reversal training, again using ANOVA (p < 0.05).
In addition to examining selective activity for each neuron, the degree of selectivity in the two populations was also analyzed. The analysis included the early and late segments of precriterion training, trials during postcriterion performance and trials during reversal training. Selectivity for each neuron in each of those phases of a session was quantified as the difference between the rates during evaluation of the preferred and nonpreferred odors divided by the sum of those rates, yielding values that ranged from −1 to 1. The contrast in activity during each phase of training was referenced to the odor preferred by each neuron during postcriterion performance. The contrasts for the four phases of training were then compared within each region using ANOVA followed by post hoc testing (p< 0.05). The population analysis (data shown in Figs. 2,3c) included 42 neurons in ABL and 43 neurons in OFC and comprised, on average, 16 (early), 73 (late), 114 (postcriterion), and 167 (reversal) trials for the data shown. It should be noted that only selective neurons from sessions (n = 44 sessions) in which the rate of learning allowed an analysis of activity during the early segment of precriterion training were included in this analysis. Neurons recorded in 11 sessions were not included, because rats did not commit at least 10 errors overall or 5 errors before the third no-go response during precriterion training. In addition, 5 neurons in OFC and 18 neurons in ABL were excluded because of a lack of activity in the early precriterion trials that were the focus of this analysis. For consistency, data from neurons excluded from the analysis of the early segment were also excluded from the other phases of training in the presentation of the results, although the values for the other phases of training were not changed significantly by the exclusion of those data. This analysis parallels that applied in an earlier report (Schoenbaum et al., 1998).
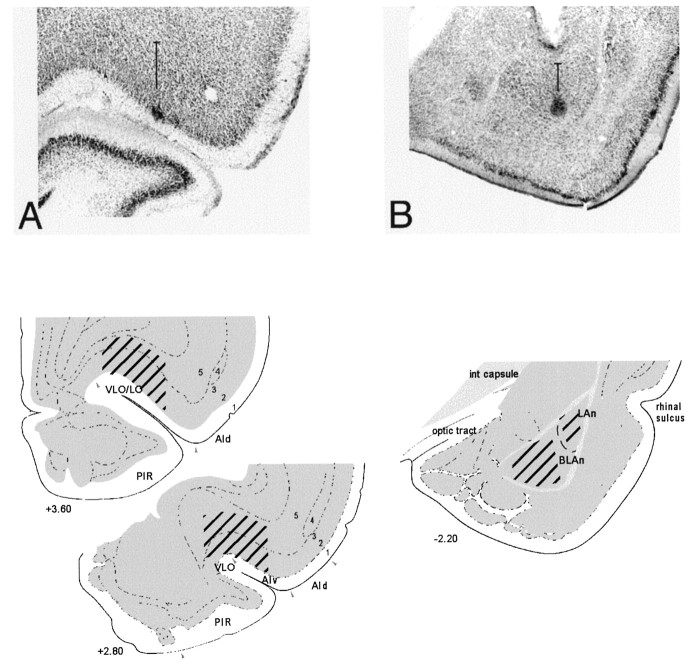
Fig. 2.
Electrode recording sites. Photomicrographs of histological sections showing the reconstruction of recording sites in representative subjects in OFC (A) and ABL (B). In each photomicrograph, a vertical line represents the dorsoventral range along the electrode track from which neurons were recorded in the case shown. Below each photomicrograph is a drawing that shows the approximate area in which recordings were obtained in each group. The OFC encompasses the orbital regions and agranular insular cortex. Recordings were localized to ventrolateral and lateral orbital regions (VLO/LO) and ventral agranular insular cortex (AIv) in the four rats in the OFC group. Recordings were localized to the basolateral nucleus in three of the rats in the ABL group (pictured in photomicrograph and as BLAn in drawing) and lateral nucleus in the fourth rat (LAn). (Drawings adapted from Swanson, 1992; photomicrographs adapted from Schoenbaum et al., 1998.)
Fig. 3.
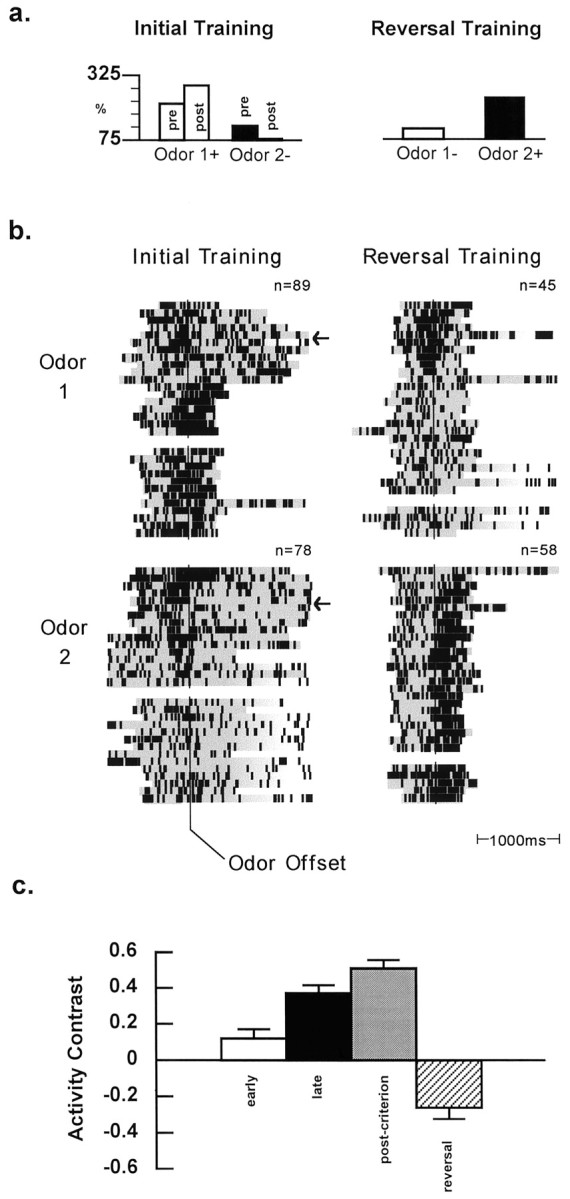
Selective activity in ABL during odor evaluation.a, Selective activity for an ABL neuron during evaluation of odor 1 (open bars) and odor 2 (closed bars) represented as a percentage of the pretrial baseline firing rate (24.1 spikes/sec). On initial training, this neuron fired more strongly during evaluation of the positive odor (odor 1) during postcriterion (post) performance [F(1,86) = 83.05; p < 0.001], and similar selectivity was present during precriterion (pre) training [F(1,77) = 4.00; p < 0.05]. During reversal training the neuron changed selectivity based on the new contingencies, developing a higher relative firing rate to the odor signaling sucrose availability [F(1,101) = 22.52; p < 0.001]. Please note that although this example shows a neuron with greater activity to the positive odor, the data shown in Table 1indicate that other cells in ABL fired more strongly during evaluation of the negative odor(s). b, Raster displays showing neural activity on 30 representative trials (n = total trials) during evaluation of each odor before and after reversal, presented in the left and right panels, respectively. Neural activity, with spikes shown as black tick marks within the shaded regions, begins with odor onset and is synchronized to odor offset corresponding to withdrawal from the odor port (thin vertical line). Activity is truncated at the go response when a response was made or after 1500 msec in the event of a no-go. Trials in which a no-go occurred are evident in fading of the shaded region at the end of each raster. Precriterion and postcriterion trials are separated by a small empty space in the displays of both the initial training and reversal training. Note that during initial training, the rat begins the session responding on every trial but gradually starts to withhold responses on the negative trials; very few responses were made to negative odors after criterion is achieved. During postcriterion performance, this neuron is strongly selective for the positive odor. This selectivity is also present during precriterion trials; however, the cell does not fire selectively during the initial block of precriterion trials corresponding to the early segment of training (trials preceding the arrows). During reversal training, the selective activity of the neuron rapidly shifts after only a few trials to reflect the new response contingencies. At that point in reversal training, however, the rat continues to respond after sampling of the formerly positive odor that now signals quinine. Thus the reversal of selective activity in the neuron develops before a reliable change in the rat’s behavioral strategy. It is also clear that this selectivity reflects the significance of an odor cue rather than its sensory features. c, Contrast in activity during evaluation of positive and negative odors during the early (open bars) and late (closed bars) segments of precriterion training, during postcriterion performance (gray bars), and during reversal training (striped bars) for the neurons with postcriterion selectivity in ABL (see Materials and Methods). The activity contrast was calculated as the difference in firing rate during the evaluation of positive and negative odors divided by the sum of those rates, yielding values that ranged from -1 to 1. The calculation was referenced to the selectivity established during postcriterion training. Firing activity between the trials was used to calculate a baseline activity contrast of −0.01 (data not shown). The degree of selectivity changed significantly in ABL [F(4,184) = 39.44; p < 0.000001] during training. Post hoc tests revealed that the degree of selectivity differed from baseline in each phase of training except the early segment. Relative to the early segment, the activity contrast increased significantly in the late segment of precriterion training but was not significantly different between the late segment and the postcriterion performance phase. During reversal the selectivity in the ABL population reversed; the negative values in the contrast indicate that the odor that elicited greater firing activity after reversal differed from the odor that was preferred during initial training. This contrast for reversal trials differed significantly from the contrasts for each other phase, including baseline and the early precriterion segment that showed low selectivity.
RESULTS
Recordings were obtained from 328 neurons in OFC and 229 neurons in ABL. Figure 2 illustrates the electrode placement in a photomicrograph from a representative animal in each group and drawings depicting the area within which recordings were obtained in each region. Cells recorded in the OFC group were located in the ventrolateral and lateral orbital regions and also ventral agranular insular cortex. Cells recorded in the ABL group were located in the basolateral nucleus in the case of three of the rats and in the lateral nucleus in the case of a fourth rat. Neuronal characteristics were similar between groups, and we observed no significant differences in either the characteristics or activity during odor sampling of the neurons recorded in ABL based on their localization in basolateral or lateral nucleus. Overall the cells included in this report tended to have low baseline firing rates and relatively wide spike widths consistent with classification as regular spiking cells thought to be pyramidal-type neurons in these areas (McCormick et al., 1985; Connors and Gutnick, 1990; Taira and Georgopoulos, 1993). Average baseline firing rates were 3.73 in OFC and 2.08 in ABL [F(2,552) = 10.38;p < 0.0001]. No complex spike or intrinsic bursting cells were included in the analyses.
Neural activity in OFC and ABL during accurate performance
Neurons in both OFC and ABL fired differentially during odor evaluation after rats had achieved the behavioral criterion for learning; such differences were observed in analyses based on ∼100 postcriterion trials on average. During this postcriterion phase, comparison of neural activity in trials involving different odors revealed that 96 (or 29%) of the 328 neurons recorded in OFC and 60 (or 26%) of the 229 neurons recorded in ABL fired selectively during evaluation of the odor cues. As indicated in Table1, some neurons in each region had higher rates of firing during evaluation of cues predicting sucrose delivery, whereas other neurons fired more strongly during evaluation of cues predicting quinine delivery. Of the neurons recorded in the four-odor task with selectivity during odor evaluation (Table 1), 20 (41%) in OFC and 25 (60%) in ABL responded equally to either of the two odors associated with a particular outcome; thus the outcome associated with the odor appeared to influence firing activity in these cases during accurate performance. In subsequent analyses, the responses of the neurons that had selective activity during accurate performance were examined over the course of learning before rats reached criterion and, where possible, during reversal training.
Characteristics of the development of selective encoding in ABL
Consistent with the proposal that ABL is involved in associative learning, a considerable number of the ABL neurons that fired selectively in the postcriterion phase developed that selective activity during precriterion training. In fact, 22 (or 37%) of the 60 ABL neurons represented in Table 1 had selectivity during the precriterion trials similar to that observed in the postcriterion phase. These neurons developed selectivity before accurate postcriterion performance was achieved, as illustrated by the ABL neuron shown in Figure 3. During initial training this neuron had significantly higher activity when the rat sampled the positive odor relative to the negative odor during both precriterion and postcriterion phases of the session (Fig. 3a, left panel). The development of this neuron’s selectivity is illustrated in the trials shown in Figure 3b, left panel. Note that this neuron was not selective during an early segment of the precriterion phase (Fig. 3b, left panel, arrows; see Materials and Methods for definition of early segment) but developed selectivity well before the behavioral criterion was achieved (indicated by the break in the display of trials for each odor). Furthermore, the selective activity of this neuron reversed to reflect the new reinforcement contingencies during reversal training (Fig.3a,b, right panels). It is apparent in the trials shown for reversal training that the neuron initially had selectivity for the formerly positive odor but then rapidly developed selectivity for the formerly negative odor that signaled a positive contingency after reversal. As observed during initial training, selectivity developed after reversal well before the behavioral criterion for reversal learning was achieved. Indeed, reversal of the neuron’s selectivity occurs well before the rat makes the initial no-go response to the new contingencies.
The example shown in Figure 3 illustrates the importance of associative significance in the selective activity observed in ABL. Clearly the neuron’s selectivity was not tied to the sensory features of a particular odor cue but rather depended on the associated outcome. In fact, none of the ABL cells that developed selectivity during initial learning maintained the same selectivity during reversal training. Of the 18 selective ABL neurons (two-odor sessions only) that were recorded during reversal training, 10 cells (or 55%) reversed firing selectivity to reflect the new contingencies, as illustrated by the neuron in Figure 3; the remainder lost the selectivity that had developed during initial learning.
The main features of the ABL neuron illustrated in Figure 3 were representative of the population of ABL neurons that displayed selective activity during postcriterion performance. The relative selectivity for that population of cells was examined within the early and late segments of precriterion training (see Materials and Methods for description), during postcriterion training, and during reversal training. The selectivity measure for each of those phases of a training session was calculated as the difference in firing rate during evaluation of the preferred and nonpreferred odors divided by the sum of these rates, yielding a measure of the contrast in activity that ranged from −1 to 1. The calculation was referenced to the postcriterion selectivity of each neuron for each of the other phases. The results of this analysis for the cells in ABL are presented in Figure 3c. As in the example of the ABL neuron shown, the relative selectivity of the population of neurons in ABL increased significantly between early and late precriterion segments of training but did not change significantly between the late precriterion trials and the postcriterion phase. Although some correct no-go responses were observed during the late precriterion phase, the same results depicted in Figure 3c were obtained irrespective of whether the criterion block of 20 trials was included in the late phase (contrast = 0.37) or not (contrast = 0.38); thus the increase in relative selectivity appears to be independent of the emerging change in behavioral strategy. In addition, the relative selectivity of the population in ABL changed polarity during reversal training relative to the postcriterion phase, indicating a change from selectivity for one odor during initial training to selectivity for the other odor after reversal. Indeed, the relative selectivity during reversal training was significantly different from that observed in each of the earlier phases of training, including both baseline (data not shown) and the early segment of precriterion training.
Characteristics of the development of selective encoding in OFC
The pattern of encoding in OFC differed from that observed in ABL. Among the neurons that fired selectively in the postcriterion phase, few exhibited similar selectivity in the precriterion phase; only 9 (or 9%) of the 96 OFC neurons represented in Table 1 had a similar selectivity during precriterion training. Instead, the vast majority (91%) of these neurons developed selectivity only at the phase of training when the rat made a reliable shift to the go, no-go response strategy. This pattern is apparent in Figure4, a and b, left panels, which illustrates a neuron that fired selectively during evaluation of the positive odor but only during postcriterion training when the rat was reliably discriminating between the odors. Also, in contrast to the results for ABL, a smaller proportion of these neurons reversed selectivity when the contingencies were changed. Of the 34 selective OFC neurons recorded during reversal training (two-odor sessions), only 8 (or 23%) reversed firing selectivity, a proportion significantly smaller than that in ABL [χ2 = 12.43;p = 0.0004]. Rather than reversing selectivity, the majority of these cells (22 cells or 65%) lost the selectivity that was manifest after initial learning, as was the case for the example shown in Figure 4, a and b, right panels. Note that this neuron fails to reverse even when the rat begins to perform well at the reversed discrimination.
Fig. 4.
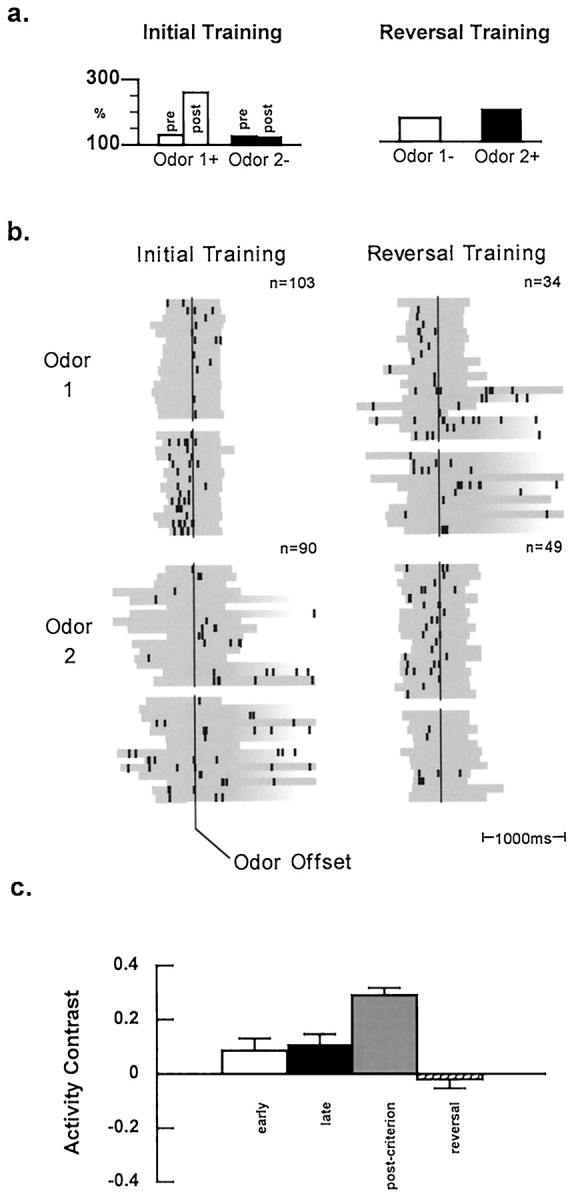
Selective activity in OFC during odor evaluation.a, Neural activity during evaluation of odor 1 (open bars) and odor 2 (closed bars) represented as a percentage of the pretrial baseline firing rate (1.35 spikes/sec). This neuron fired more strongly during evaluation of the positive odor (odor 1) during postcriterion (post) training [F(1,83) = 5.31 (5.08);p < 0.05]. That selectivity was not evident during precriterion (pre) training when the rat had not yet adopted a reliable response strategy to reflect the learned significance of the odors. Furthermore, the selectivity disappeared during reversal training. Please note that although this example shows a neuron with greater activity to the positive odor, the data shown in Table 1 indicate that other cells in OFC fired more strongly during evaluation of the negative odor(s). b, Raster displays showing neural activity on 30 representative trials (n = total trials) during evaluation of each odor before and after reversal, presented in the left andright panels, respectively (for details, see Fig. 3). Again note that the rat begins the session responding on every trial but gradually begins to withhold (striped bars) for the neurons with postcriterion selectivity in OFC (forresponses on the negative trials. During the precriterion phase, the rat makes several intermittent no-go responses, but the neuron fires very little during evaluation of either odor. The selective activity of this neuron develops only after a reliable change in the rat’s behavioral strategy; during postcriterion performance, the neuron fires strongly during evaluation of the positive odor (odor 1). After reversal, the activity during odor evaluation is no longer selective either before or after the rat achieves criterion on the reversed discrimination problem.c, Contrast in activity during evaluation of positive and negative odors during the early (open bars) and late (closed bars) segments of precriterion training, during postcriterion performance (gray bars), and during reversal training details, see Materials and Methods and Fig. 3legend). The degree of selectivity changed significantly in OFC [F(4,192) = 10.16; p < 0.000001] during training. Post hoc tests revealed that the degree of selectivity only differed from the baseline of 0.04 (data not shown) during the postcriterion phase of training. In other words, the activity contrast in OFC was not significantly different between the early and late segments of precriterion training, increasing significantly only during the postcriterion performance phase. During reversal training, the activity contrast decreased significantly relative to postcriterion performance, returning to a value not significantly different from baseline or either the early or the late segment of precriterion training.
The relative selectivity for the population of OFC cells, shown in Figure 4c, exhibits a pattern consistent with the example shown for that region. The selectivity observed during postcriterion training was not evident in either the early or late segments of the precriterion phase. Again selectivity during the late segment of precriterion training did not differ significantly whether the criterion block of trials was included (contrast = 0.09) or excluded from the analysis (contrast = 0.13). In addition, the relative selectivity in this population of OFC neurons decreased significantly from the postcriterion phase during reversal training but did not reverse as it had in ABL (Fig. 3c). Instead, selectivity during reversal training did not differ significantly from the values obtained for baseline (data not shown) or during either the early or late precriterion training phases. Notably, the loss of selectivity in this population of OFC neurons was accompanied by the emergence of selectivity in a separate population of neurons in OFC. Of the 92 remaining neurons, recorded during reversal training and not selective during initial training, 22 (or 24%) developed selective firing after criterion was attained on the reversed discrimination.
DISCUSSION
The current findings are consistent with the interpretation that ABL encodes the motivational significance of cues and OFC serves an integrative function for guiding adaptive goal-directed behavior based on information accessed through its connections with ABL and other structures. This view agrees with other evidence that ABL provides a critical function in associative learning and that OFC integrates information needed for decision making.
This report differs from previous studies that have separately examined neural activity in either OFC (Thorpe et al., 1983; Schoenbaum and Eichenbaum, 1995a; Critchley and Rolls, 1996; Rolls et al., 1996) or ABL (Sanghera et al., 1979; Nishijo et al., 1988; Muramoto et al., 1993; Quirk et al., 1995). Although such studies have reported that neurons in each region encode associative information, they provide little information about the relationship between the development of encoding and behavior, because recordings were made in well trained animals or in very rapidly acquired behavioral tasks.
Encoding in ABL reflects the motivational significance of olfactory cues
In the course of training, ABL neurons developed selective responses during odor sampling. This selective activity was not present during the initial training trials but developed rapidly, well before accurate choice performance was achieved. Moreover, a large proportion of these cells also rapidly reversed selectivity when the reinforcement contingencies were switched.
These findings support the proposal that neural activity in ABL reflects the acquired significance of the olfactory cues based on associations between the originally neutral odors and the motivational properties of reinforcement. Rapid conditioning of neural responses in rat amygdala has also been reported in auditory discrimination training (Muramoto et al., 1993) and fear conditioning (Quirk et al., 1995) and in visual discrimination in primates (Sanghera et al., 1979; Nishijo et al., 1988). In each of these cases neural activity was recorded in conjunction with conditioned responses that were also rapidly acquired. The present study extends on those reports by showing that a change in neural activity in ABL can occur independently of a reliable change in choice behavior. Most of the neurons reported here developed selective firing before the rat had begun to consistently use that information in performing the go, no-go discrimination. This was evident both during initial training and after reversal.
The reversal of many ABL neurons in our study also extends on earlier work in clearly demonstrating that neural encoding of cue significance in ABL can change rapidly to reflect changes in task contingencies. Somewhat equivocal findings have been previously reported regarding the ability of neural correlates in amygdala to change after initial training. Quirk et al. (1995) reported that conditioned neural responses in the lateral nucleus of amygdala disappeared during extinction procedures in a fear-conditioning task. Similarly, Nishijo et al. (1988) reported that neural responses in primate amygdala to the visual presentation of a food item were eliminated when the food item was made unpalatable. During reversal training in a visual discrimination task, however, Sanghera et al. (1979) reported that neurons in primate amygdala maintained their responses to items that were paired with either rewarding or aversive fluid delivery after reversal. In their study, none of the nine visually selective neurons reversed firing selectivity when the contingencies were reversed. In the present study, 10 of the 18 selective neurons in the two-odor task reversed, a phenomenon that often occurred rapidly after only a few trials. In comparing our results with those of Sanghera et al. (1979), it may be important to note that the reversal of neural activity reported here occurred in the same session in which a new odor discrimination was originally learned, whereas Sanghera et al. (1979)conducted a separate reversal session after considerable experience with the visual items used in the task. Perhaps reversal of neural activity is more readily achieved with relatively new learning, and extensive training makes reversal of the conditioned neural responses more difficult. Nevertheless, our data clearly demonstrate that encoding in ABL remains plastic for some time after modification by new learning.
Encoding in OFC reflects integration of motivational significance into behavioral strategy
In contrast to the neural activity observed in ABL, selective responses during odor sampling in OFC developed in a manner that was more clearly related to the change in choice behavior. These findings extend on previous neurophysiological studies performed in well trained animals that have reported selective neural activity in rat and primate OFC during stimulus sampling within discrimination tasks (Schoenbaum and Eichenbaum, 1995a; Thorpe et al., 1983; Critchley and Rolls, 1996;Rolls et al., 1996). As noted previously, these studies were performed in well trained animals. In the current report, neurons in OFC that were selective during accurate performance rarely developed that selectivity during the earlier phases of training. Moreover, during reversal training, the selective activity of these neurons was more likely to be eliminated rather than reversed, and a largely separate set of neurons emerged to perform the function that this population had performed during initial training. Therefore, selective activity in OFC did not consistently represent the identity of particular odors, the motivational characteristics of the associated reinforcer, or preparation for the motoric response. Instead it would appear that the selective activity in OFC during accurate performance represents the integration of information regarding the significance of a particular cue (or cues) with subsequent behavior. These findings are consistent with the view that neural activity in OFC reflects the information used to guide a behavioral strategy.
Interactions between OFC and ABL
Our findings indicate that neurons encoding the associative significance of cues predominate in ABL during odor sampling, whereas the vast majority of OFC neurons active during odor sampling are tied to the use of that information in the rats’ behavioral strategy. Interconnections between these regions may allow cells in ABL to signal networks in OFC regarding the particular motivational significance of cues as this information becomes relevant to selection of behavioral options. Dependence of OFC on ABL for associative information is consistent with other neural correlates we have found in this task. As reported elsewhere, a largely different (nonoverlapping) population of neurons in OFC fired selectively in anticipation of a particular outcome when these same rats responded at the fluid well after odor sampling (Schoenbaum et al., 1998). Those neurons had significantly different firing rates depending on whether sucrose or quinine delivery was imminent during a delay instituted after the response. The selective activity developed early in training when rats had not yet modified their behavior; it was present before cells in OFC had begun to fire selectively during actual sampling of the odor cues, as reported here. Selective firing in OFC during responding may depend, in part, on the encoding observed in ABL during odor sampling.
By the same token, reciprocal connections may allow processing in OFC to regulate networks in ABL. In our previous report, neurons in ABL also fired selectively during the delay after responding (Schoenbaum et al., 1998). This population of ABL neurons might reflect a working memory function that is supported by OFC. Moreover, neural activity in anticipation of a response-dependent outcome, as described in our previous report, would require registration that a response had been executed. An indicator of responding in the task might be provided directly or indirectly via networks in OFC. In this manner, these two regions would function cooperatively, along with other interconnected structures, in the production of goal-directed behavior that reflects the motivational significance of cues.
OFC as a model for prefrontal cortex
Within prefrontal cortex, different subdivisions can be distinguished based on anatomical and cytoarchitectonic criteria. One view is that these subdivisions perform comparable processing functions on domain-specific information determined by anatomical connectivity with other neural systems (Goldman-Rakic, 1987). For example, adjacent areas within primate dorsolateral prefrontal cortex process specialized attributes of visual input, but a similar working memory function is evident their operation (for review, see Goldman-Rakic, 1996).
Within this framework, the connectivity of OFC is consistent with its role in processing information concerning the emotional and motivational significance of cues. OFC is heavily involved in circuits related to olfactory processing as well as limbic structures such as the amygdala (Krettek and Price, 1977; Kolb, 1984; Price et al., 1987,1991; McDonald, 1991). Accordingly, the response properties of cells in OFC reflect this specialized information domain but also share certain features with other regions of prefrontal cortex. For example, during discrimination task performance, cells in both OFC (Thorpe et al., 1983; Schoenbaum and Eichenbaum, 1995a) and dorsolateral prefrontal cortex (Watanabe, 1990) encode the identity and significance of the cues presented to the animal. Moreover, we have recently demonstrated selective activity in OFC during a delay (Schoenbaum et al., 1998), indicating that the cells in this region are able to maintain a representation similar to the working memory function proposed for other subdivisions of prefrontal cortex in primates (Goldman-Rakic, 1996). Finally, the current report and previous data (Schoenbaum and Eichenbaum, 1995b) demonstrate that neurons in OFC, like those in primate prefrontal cortex (Rainer et al., 1998), best represent information of direct relevance to ongoing behavior. Viewed from this perspective, OFC provides a potentially useful model for the study of prefrontal systems in the rat.
Footnotes
This work was supported by funding from the National Institutes of Health. We thank T. Lam for assistance in figure preparation and Dr. M. Burchinal and E. Neebe for statistical consultation.
Correspondence should be addressed to Dr. Geoffrey Schoenbaum, Johns Hopkins University, Department of Psychology, 3400 North Charles Street, 25 Ames Hall, Baltimore, MD 21218.
REFERENCES
- 1.Balleine BW, Leibeskind JC, Dickinson A. Effect of cell body lesions of the basolateral amygdala on instrumental conditioning. Soc Neurosci Abstr. 1997;23:786. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1294. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- 3.Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- 4.Critchley HD, Rolls ET. Olfactory neuronal responses in the primate orbitofrontal cortex: analysis in an olfactory discrimination task. J Neurophysiol. 1996;75:1659–1672. doi: 10.1152/jn.1996.75.4.1659. [DOI] [PubMed] [Google Scholar]
- 5.Damasio AR. Decartes error. Putnam; New York: 1994. [Google Scholar]
- 6.Davis M. The role of the amygdala in conditioned fear. In: Aggleton J, editor. The amygdala: neurological aspects of emotion, memory, and mental dysfunction. Wiley; Chichester, UK: 1992. pp. 255–306. [Google Scholar]
- 7.Everitt BJ, Cador M, Robbins TW. Interactions between the amygdala and ventral striatum in stimulus-reward associations: studies using a second-order schedule of sexual reinforcement. Neuroscience. 1989;30:63–75. doi: 10.1016/0306-4522(89)90353-9. [DOI] [PubMed] [Google Scholar]
- 8.Everitt BJ, Morris KA, O’Brien A, Burns L, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher M, Chiba AA. The amygdala and emotion. Curr Opin Neurobiol. 1996;6:221–227. doi: 10.1016/s0959-4388(96)80076-6. [DOI] [PubMed] [Google Scholar]
- 10.Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Mountcastle VB, Plum F, Geiger SR, editors. Handbook of physiology: the nervous system V. Waverly; Bethesda, MD: 1987. pp. 373–417. [Google Scholar]
- 11.Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- 12.Hatfield T, Han J-S, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Killcross S, Robbins TW, Everitt BJ. Different types of fear conditioned behavior mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- 14.Kolb B. Functions of the frontal cortex of the rat: a comparative review. Brain Res Rev. 1984;8:65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- 15.Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol. 1977;172:687–722. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- 16.Kubie JL. A driveable bundle of microwires for collecting single-unit data from freely-moving rats. Physiol Behav. 1984;32:115–118. doi: 10.1016/0031-9384(84)90080-5. [DOI] [PubMed] [Google Scholar]
- 17.LeDoux JE. The emotional brain. Simon and Schuster; New York: 1996. [Google Scholar]
- 18.McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- 19.McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- 20.Muramoto K, Ono T, Nishijo H, Fukuda M. Rat amygdaloid neuron responses during auditory discrimination. Neuroscience. 1993;52:621–636. doi: 10.1016/0306-4522(93)90411-8. [DOI] [PubMed] [Google Scholar]
- 21.Nishijo H, Ono T, Nishino H. Single neuron responses in alert monkey during complex sensory stimulation with affective significance. J Neurosci. 1988;8:3570–3583. doi: 10.1523/JNEUROSCI.08-10-03570.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price JL, Russchen FT, Amaral DG. The limbic region. II. The amygdaloid complex. In: Bjorklund A, Hokfelt T, Swanson LW, editors. Integrated systems of the CNS, Pt I. Handbook of chemical neuroanatomy, Vol 5. Elsevier; Amsterdam: 1987. pp. 279–388. [Google Scholar]
- 23.Price JL, Carmichael ST, Carnes KM, Clugnet M, Kuroda M, Ray JP. Olfactory input to the prefrontal cortex. In: Davis J, Eichenbaum H, editors. Olfaction: a model system for computational neuroscience. MIT; Cambridge, MA: 1991. pp. 101–120. [Google Scholar]
- 24.Quirk GJ, Repa JC, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 25.Rainer G, Asaad WF, Miller EK. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 1998;393:577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- 26.Rolls ET, Critchley HD, Mason R, Wakeman EA. Orbitofrontal cortex neurons: role in olfactory and visual association learning. J Neurophysiol. 1996;75:1970–1981. doi: 10.1152/jn.1996.75.5.1970. [DOI] [PubMed] [Google Scholar]
- 27.Sanghera MK, Rolls ET, Roper-Hall A. Visual responses of neurons in the dorsolateral amygdala of the alert monkey. Exp Neurol. 1979;63:610–626. doi: 10.1016/0014-4886(79)90175-4. [DOI] [PubMed] [Google Scholar]
- 28.Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I. Single neuron activity in orbitofrontal cortex compared with that in piriform cortex. J Neurophysiol. 1995a;74:733–750. doi: 10.1152/jn.1995.74.2.733. [DOI] [PubMed] [Google Scholar]
- 29.Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. II. Ensemble activity in orbitofrontal cortex. J Neurophysiol. 1995b;74:750–762. doi: 10.1152/jn.1995.74.2.751. [DOI] [PubMed] [Google Scholar]
- 30.Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 31.Swanson LW. Brain maps: structure of the rat brain. Elsevier; New York: 1992. [Google Scholar]
- 32.Taira M, Georgopoulos AP. Cortical cell types from spike trains. Neurosci Res. 1993;17:39–45. doi: 10.1016/0168-0102(93)90027-n. [DOI] [PubMed] [Google Scholar]
- 33.Thorpe SJ, Rolls ET, Maddison S. The orbitofrontal cortex: neuronal activity in the behaving monkey. Exp Brain Res. 1983;49:93–115. doi: 10.1007/BF00235545. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe M. Prefrontal unit activity during associative learning in the monkey. Exp Brain Res. 1990;80:296–309. doi: 10.1007/BF00228157. [DOI] [PubMed] [Google Scholar]