Abstract
The suprachiasmatic nuclei (SCN) contain the principal circadian clock governing overt daily rhythms of physiology and behavior. The endogenous circadian cycle is entrained to the light/dark via direct glutamatergic retinal afferents to the SCN. To understand the molecular basis of entrainment, it is first necessary to define how rapidly the clock is reset by a light pulse. We used a two-pulse paradigm, in combination with cellular and behavioral analyses of SCN function, to explore the speed of resetting of the circadian oscillator in Syrian hamster and mouse. Analysis of c-fos induction and cAMP response element-binding protein phosphorylation in the retinorecipient SCN demonstrated that the SCN are able to resolve and respond to light pulses presented 1 or 2 hr apart. Analysis of the phase shifts of the circadian wheel-running activity rhythm of hamsters presented with single or double pulses demonstrated that resetting of the oscillator occurred within 2 hr. This was the case for both delaying and advancing phase shifts. Examination of delaying shifts in the mouse showed resetting within 2 hr and in addition showed that resetting is not completed within 1 hr of a light pulse. These results establish the temporal window within which to define the primary molecular mechanisms of circadian resetting in the mammal.
Keywords: suprachiasmatic nucleus, circadian rhythms, entrainment, immediate-early genes, light, CREB, circadian clock
Circadian rhythms are fundamental to the behavior and physiology of all higher organisms (Pittendrigh, 1960;Aschoff, 1981), expressed at the level of organism, tissue, and cell (Welsh et al., 1995; Plautz et al., 1997; Balsabore et al., 1998). The recent identification of putative clock genes in mammals (Sun et al., 1997; Tei et al., 1997) has provided a major impetus to understanding the clock mechanism. The principal circadian oscillators of mammals are the hypothalamic suprachiasmatic nuclei (SCN) (Klein et al., 1991). Light acting via retinal afferents is the primary synchronizer (Pittendrigh and Daan, 1976; Czeisler, 1995). Although the neurochemical basis of photic entrainment is well characterized (Nelson and Takahashi, 1991; Kornhauser et al., 1996; Hastings, 1997), its molecular basis is not. To identify critical elements of the entrainment mechanism, it is necessary to define the temporal window over which resetting is completed. Responses to light that occur after this interval cannot cause resetting. Resetting of the clock is described by a phase response curve (PRC), which plots the magnitude and direction of the phase shifts of an overt rhythm as a function of the circadian times at which light is presented. Typically, delays are completed after one circadian cycle, whereas advance shifts can take several cycles to be expressed in full (Pittendrigh and Daan, 1976). However, this analysis can be misleading, because it is the position of the PRC rather than the phase of any particular output rhythm that provides a true reflection of oscillator phase. To determine the speed of resetting, it is therefore necessary to define how quickly the PRC is reset by light. This has been examined in lower organisms using a “two-pulse” protocol, the rationale being that an initial pulse causes a phase shift of the oscillator, and therefore it shifts the PRC to a new phase. This new phase can then be mapped by examining the response to “probe” pulses delivered shortly after the first. Depending on the speed, direction, and magnitude of shifts induced by the first pulse, the probe pulses will illuminate earlier (if delayed) or later (if advanced) portions of the PRC than if the first pulse had not been delivered. In Neurospora (Crosthwaite et al., 1995) and Drosophila (Pittendrigh, 1979), this has demonstrated rapid (0.75 and 3 hr, respectively) resetting of the clock. A two-pulse protocol was used in the present study to determine whether photic resetting of the mammalian clock was equally rapid. Some models of the mammalian clock suggest that exposure to light is followed by a refractory period of 4–6 hr (Kronauer et al., 1996), which would confound the two-pulse protocol. To confirm that light pulses separated by 1 or 2 hr can be resolved by the mammalian circadian system, we first examined the photic induction of c-fos (Kornhauser et al., 1990; Rusak et al., 1990) and phosphorylation of the cAMP response element-binding protein (CREB) (Ginty et al., 1993) in the retinorecipient SCN.
A preliminary account of this study was presented at the 19th International Summer School of Brain Research, Amsterdam, September 1995, and appeared in the conference proceedings (Hastings et al., 1996).
MATERIALS AND METHODS
Animals and treatments. All procedures were conducted under license, in accordance with the Animals (Scientific Procedures) Act of 1986. All reagents were obtained from Sigma (Poole, UK) unless stated otherwise. Adult male Syrian hamsters (100 gm; Wrights of Essex, Chelmsford, UK) or outbred mice [ICR(strain CD-1); Harlan Olac, Bicester, UK] were housed individually in cages equipped with running wheels, and food and water were available ad libitum. The initial photoschedule was a 16/8 hr light/dark cycle [light, 50 or 200 μW/cm2; dim red light (DR), <1 μW/cm2]. Rotation of the wheel interrupted an infrared light beam and was recorded by a personal computer (Viglen HD40V; Viglen Computers, London, UK) running Dataquest IV software (Minimitter Co.). Data were collected in 10 (hamsters) or 6 (mice) min time bins. After 3 weeks, the activity rhythms of the animals were entrained to the photoschedule, and the animals were released into constant dim red light to free-run (DR:DR) for at least 10 d. The onset of activity was defined as circadian time 12 (CT12), estimated by fitting a line by eye through at least five consecutive activity onsets on double-plotted actograms. Exposure to a light pulse (50 or 200 μW/cm2) involved moving the animal in its home cage into an adjacent room to be placed under a fluorescent strip light. In the “dark pulse” control procedure, the animal was transferred to the adjacent room, which remained in dim red light. In some cases, animals received a second light pulse 1 or 2 hr after the first pulse. Animals were killed for immunocytochemistry, in situ hybridization, or dissection of SCN tissue for Western blot, or they were allowed to free-run for 7–10 d to determine the magnitude of any phase shift.
In situ hybridization. At the indicated time points, hamsters free-running in DR:DR for 30 d were killed by cervical dislocation, and the brains were removed and frozen immediately on dry ice. Sections (16 μm) through the hypothalamus were cut with a cryostat, and slides were stored at −20°C until use. The in situ hybridization procedure was adapted from Young and Kuhar (1986) and Young et al. (1986). The slides were hybridized overnight at 37°C in buffer [deionized formamide, mix bed resin (Bio-Rad), 2× SSC, 50× Denhardt’s solution, yeast tRNA, sterile water, and dextran sulfate] containing 1 × 107 cpm/ml 35S-labeled oligoprobe in a humidified chamber (GGG AGG ATG ACG CCT CGT AGT CCG CGT TGA AAC CCG AGA ACA TCA TGG) (Vendrell et al., 1992). On the next day, the sections were washed in 1× SSC, dipped twice in distilled water at room temperature, and then left to dry. They were then exposed for 3 weeks at 4°C to Hyperfilm β max (Amersham, Arlington Heights, IL). The intensity of the autoradiographic images of c-foshybridization in the SCN was assessed by computerized densitometry using a Hamamatsu CCD (C3077) camera connected to an Apple Macintosh IIci computer running NIH Image 1.49 image analysis software (gift of Dr. Wayne Rashband, National Institutes of Health, Bethesda, MD).
Western blotting. Hamsters free-running in DR:DR for 30 d were killed 0, 2, or 4 hr after a light pulse delivered 1 hr after activity onset (CT13), brains were rapidly removed, and coronal slices were cut through the anterior hypothalamus at the level of the SCN using a brain matrix as a guide (Agar Scientific). Tissue from the SCN was microdissected using a 14 gauge hypodermic needle and immediately frozen on dry ice. Extraction of nuclear proteins was conducted at 4°C. Tissue punches were homogenized using a Dounce homogenizer in 400 μl of homogenization buffer containing: 10 mmHEPES-KOH, pH 7.9, 1.5 mm MgCl2, 10 mm KCl, 5 mm dithiothreitol (DTT), 1 mm phenylmethylsulfonyl fluoride (PMSF), 10 μg/ml aprotinin, and 2 μg/ml pepstatin. The nuclear fraction was precipitated by centrifugation for 2 min at 14,000 rpm, and the pellet was resuspended in 36 μl of ice-cold extraction buffer (10 mm HEPES-KOH, pH 7.9, 25% glycerol, 420 mmNaCl, 1.5 mm MgCl2, 0.2 mmEDTA, 5 mm DTT, 1 mm PMSF, 10 μg/ml aprotinin, and 2 μg/ml pepstatin), and incubated on ice for 20 min. The mixture was then centrifuged at 14,000 rpm for 2 min, and the supernatant was collected and stored at −40°C. Extracts (10 μl) were separated on a 3% stacking–12% SDS- polyacrylamide gel and electrotransferred at 70 V for 2 hr to nitrocellulose. The blots were blocked for 60 min at room temperature in 4% nonfat milk (NFM) in PBS and then incubated overnight in 2.5% NFM in PBS blocking buffer containing c-fos antisera (Santa Cruz Biotechnology, Santa Cruz, CA). The blot was washed for 30 min in 2.5% NFM and 1:200 Tween 20 in PBS and then incubated for 60 min in 1:2000 biotinylated anti-rabbit antibody (Amersham). The blots were then washed in 2.5% NFM in PBS for 15 min, developed with the ECL+ system (Amersham), and exposed to film.
Immunocytochemistry. After being allowed to free-run in DR:DR for 10 d, mice were exposed to a light pulse or a control dark pulse, and at intervals after the pulse, were given an overdose of anesthetic (Euthatal; Rhone-Merieux, Harlow, UK) and perfused via the aorta with fixative (4% paraformaldehyde in PBS). Brains were removed, post-fixed for 4 hr, and then transferred to 20% sucrose solution cryoprotectant, before sectioning at 40 μm on a freezing microtome. Immunocytochemistry for Ser133phospho-CREB was conducted on free-floating sections using a specific antiserum (New England Biolabs, Beverly, MA) at 1:1000 dilution and visualized with Vectastain ABC kit (Vector Laboratories, Burlingame, CA) and diaminobenzidine as chromogen. The number of immunoreactive nuclei in the SCN was determined using the NIH Image program.
Statistical analyses. Differences between experimental groups were analyzed using ANOVA, followed by post hoc Dunnett’s t tests.
RESULTS
Transcriptional responses confirm that the SCN can resolve paired light pulses 1 or 2 hr apart
To test whether the SCN can resolve individual light pulses presented 1 or 2 hr apart in a paired-pulse paradigm, we examined the photic induction of c-fos and phosphorylation of CREB in the retinorecipient SCN. As reported previously (Kornhauser et al., 1990), the induction of c-fos mRNA in the SCN of Syrian hamster by a single light pulse (50 μW/cm2) was rapid and transient. In animals exposed to a control dark pulse during subjective night (CT13), there was no c-fos mRNA hybridization signal in the SCN (Fig. 1a). Thirty min after exposure to light at CT13 (15 min, 50 μW/cm2), there was a robust induction ofc-fos mRNA in the SCN (Fig. 1b) that was not detectable 1 hr after the light pulse (Fig. 1c). Quantification of the relative intensity of the hybridization signal revealed a significant effect of treatment (ANOVA; F = 9.7; p < 0.01), with levels being significantly elevated relative to dark control tissue 15–30 min (0.1 ± 1.9 vs 29.5 ± 4.8 gray scale units) after a single pulse but not significantly above background 1 (3.9 ± 1.9) to 3 (3.1 ± 0.1) hr after the pulse. In animals that received a double-pulse protocol, a very strong c-fos mRNA hybridization signal was evident in the SCN 30 min after the second pulse (28.1 ± 3.9 gray scale units) (Fig. 1d). This corresponded to 2.5 hr after the first pulse, a time when the signal arising from the first pulse would have dissipated. Repeated induction of c-fos was confirmed by Western blot analysis of protein extracts of SCN. In animals free-running in DR and exposed to dark pulses at CT13, the c-fos signal was very weak (Fig. 1e, lane 3). However, 2 hr after a light pulse at CT13, a strong c-fos band was evident (Fig. 1e, lane 4), and by 4 hr after the pulse at CT13, levels of c-fos had declined to those seen in dark-treated controls (lane 5). However, in animals that were killed 4 hr after the first pulse but had received a second pulse 2 hr after the first, there was a very strong c-fos signal (Fig. 1e, lane 6), comparable to that seen in animals sampled 2 hr after a single pulse. The repeated induction of c-fos demonstrates that the circadian system is able to resolve light pulses 2 hr apart.
Fig. 1.
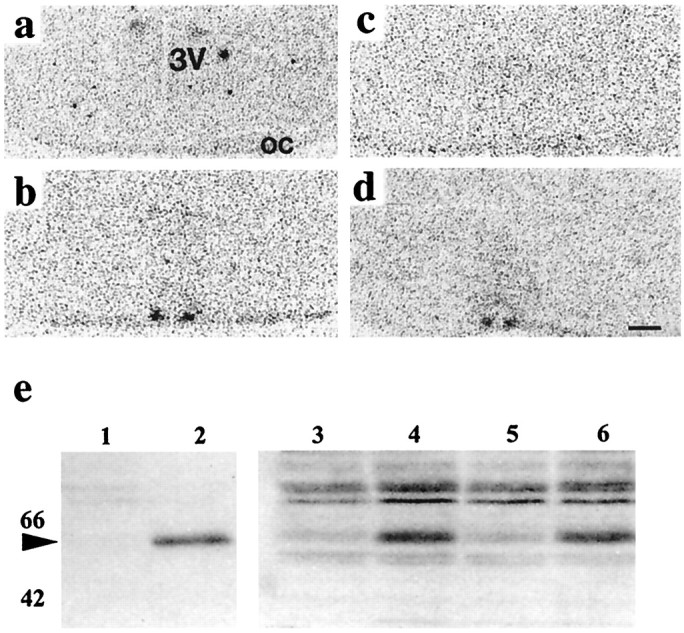
Photic induction of c-fos in hamster SCN by single and paired light pulses. Autoradiograms of coronal sections of hypothalamus of hamster SCN produced by in situ hybridization for c-fos mRNA after exposure to darkness (15 min, <1 μW/cm2) or light pulses (15 min, 50 μW/cm2) during early subjective night.a, Thirty minutes after exposure to a control dark pulse at CT13. 3V, Third ventricle; oc, optic chiasm. b, Thirty minutes after start of a light pulse at CT13. Note hybridization signal in bilateral SCN. c, One hour after start of a light pulse at CT13. Note hybridization signal is lost. d, Thirty minutes after start of second pulse in a double-pulse protocol. Note reinduction ofc-fos in SCN. Scale bar, 500 μm. e, Western blots probed for c-fos. Lanes 1,2, Negative and positive (metrazole-treated) controls from extracts of hamster cerebral cortex samples; lanes 3–6, tissue extracts from pooled SCN (5 animals per sample);lane 3, dark pulse control, weak signal; lane 4, 2 hr after light pulse at CT13, strong induction;lane 5, 4 hr after light pulse at CT13, signal lost;lane 6, 2 hr after second pulse, 4 hr after first pulse (note strong reinduction).
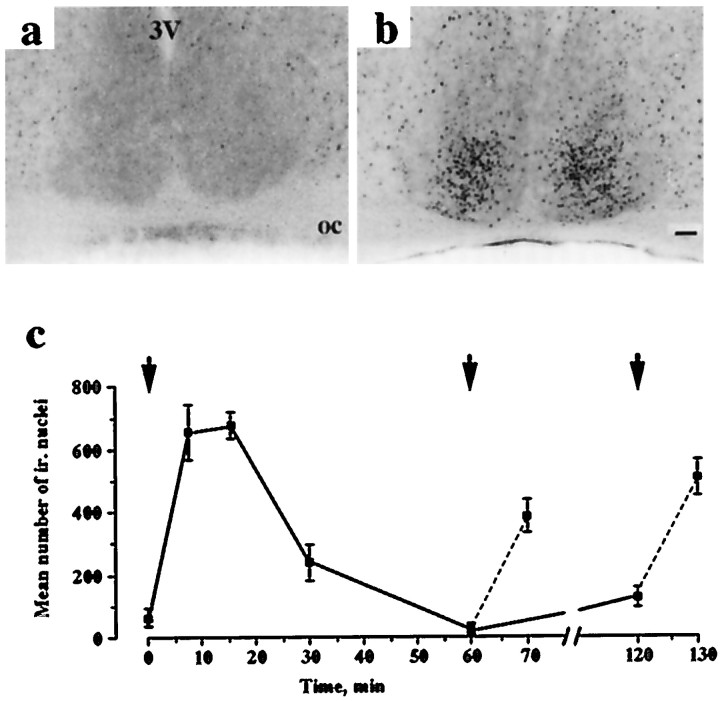
To test whether the SCN can resolve pulses separated by 1 hr, mice free-running in dim red light received a light pulse at CT13 and a second either 1 or 2 hr later. Phosphorylation of CREB was used as the index of response, because its transient nature would provide the necessary resolution. Dark-pulsed controls had low to undetectable levels of phospho-CREB (Fig.2a) nuclei in the retinorecipient SCN. Within 7.5 min of a light pulse, the number of phospho-CREB-ir nuclei was maximal (Fig. 2b,c). The peak was transient, and levels declined to baseline within 1 hr. Presentation of a second pulse 1 or 2 hr after the first was followed by a second wave of phospho-CREB induction, detectable after 10 min (Fig. 2c). These results confirm that the SCN can resolve light pulses separated by 1 or 2 hr.
Fig. 2.
Photic induction of phospho-CREB in mouse SCN by single and paired light pulses (15 min duration). Coronal sections of SCN reveal low expression in dark-pulsed control animals (a) but extensive induction in retinorecipient SCN of animals 10 min after start of a light pulse (200 μW/cm2) at CT13 (b).c, Time course for phospho-CREB induction in mouse SCN (mean ± SEM) shows rapid and transient response, with reinduction by second pulses presented 1 or 2 hr after initial pulse (arrows).
For both advance and delay shifts, the circadian clock of the Syrian hamster is reset within 2 hr of a light pulse
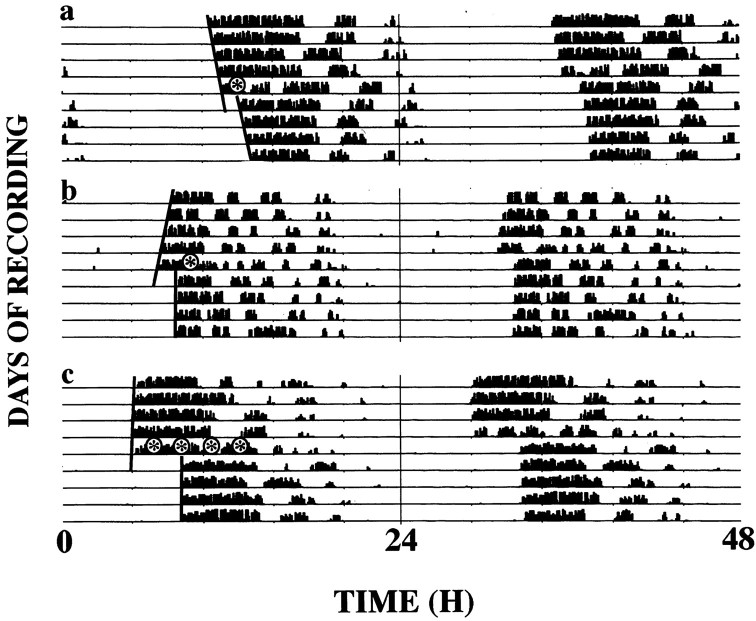
Exposure to light during subjective day or exposure to dark pulses during subjective night had no effect on the circadian rhythm of wheel-running of Syrian hamsters. Exposure to single pulses of light in early subjective night caused phase delays of the free-running activity rhythm, whereas pulses delivered later in subjective night advanced the rhythm (Figs. 3,4a). Overall, animals pulsed at CT15 showed no significant shifts; this circadian phase represents the crossover point of the PRC (Fig. 4a). Animals that received double-light pulses continued to exhibit stable free-running rhythms (Fig. 3b–d). Presentation of the first light pulse at CT13 and the second two hr later was followed by large phase delays in the free-running rhythm (Fig. 3b). When the first of the two light pulses was given at CT20 and the second was given 2 hr later, the subsequent phase advance was comparable to that observed after a single light pulse at CT20 (Fig. 3c). In contrast, when the first light pulse was administered at CT18 and the second was presented 2 hr later, it was followed by a phase advance larger than that anticipated after a single pulse at CT18 (Fig. 3d).
Fig. 3.
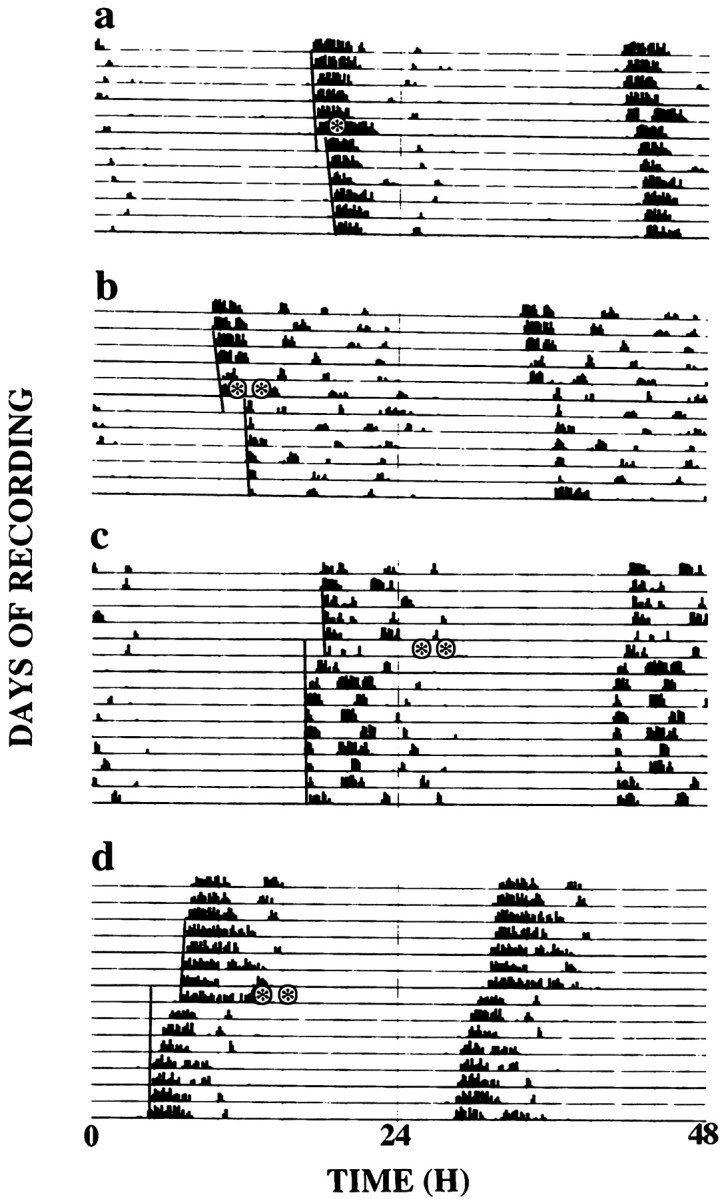
Representative double-plotted actograms of wheel-running activity of Syrian hamsters held in continuous dim red light and exposed to brief pulses of light (15 min,asterisks) at one pulse only at CT13 (a), paired pulses first at CT13 and a second 2 hr later (b), at CT20 and a second 2 hr later (c), and at CT18 and a second 2 hr later (d). Lines on leftside indicate phase shift of activity onset. Pulses ina, b, 15 min, 50 μW/cm2; pulses in c,d, 15 min, 200 μW/cm2.
Fig. 4.
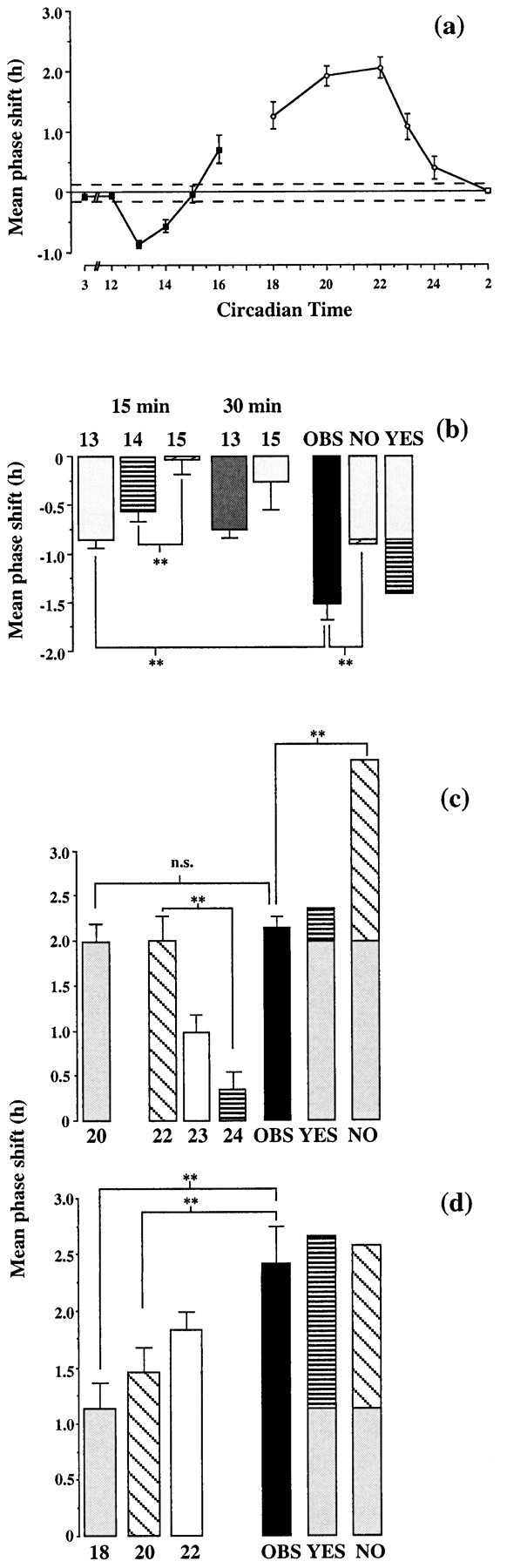
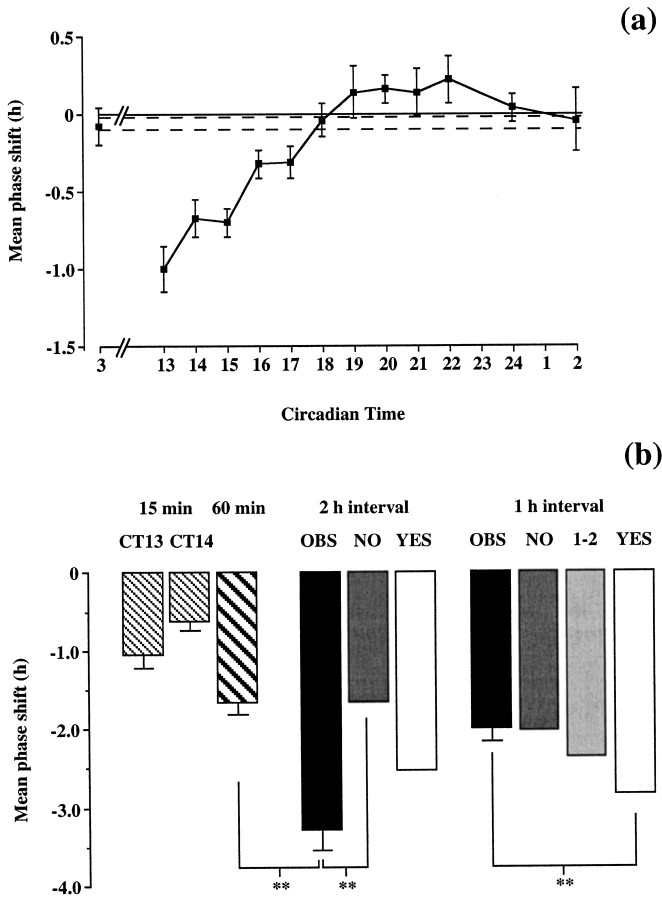
a, PRCs (mean ± SEM) of the circadian activity rhythm of Syrian hamsters held in continuous dim red light and exposed to a brief pulse of light (15 min) of 50 (filled symbols; n = 84) or 200 (open symbols; n = 45) μW/cm2. The dotted line about the abscissa is the SEM for control dark pulses (mean shift, −0.01 hr;n = 15). b, Predicted and observed resetting to paired pulses in delaying phase of PRC. Shifts to individual pulses (15 or 30 min, 50 μW/cm2) delivered at CT13, CT14, or CT15. NO, Predicted shift if no resetting within 2 hr of first pulse; YES, predicted composite shift if delay arising from pulse at CT13 is completed before second pulse is given; OBS, observed data.n = 11–25; n = 99. **p < 0.01, pairwise comparison. c,d, Predicted and observed resetting to paired pulses in advancing phase of PRC. Shifts to individual pulses (15 min, 200 μW/cm2) delivered at CT18, CT20, CT22, CT23, or CT24. NO, Predicted shift if no resetting within 2 hr of first pulse; YES, predicted composite shift if advance arising from pulse at CT20 or CT18 is completed before second pulse is given; OBS, observed data. n = 6–11; n = 74. **p < 0.01, pairwise comparison.
Analysis of the grouped data for single and double pulses presented between CT13 and CT15 revealed a significant overall effect of treatment (F = 14.3; p < 0.01). The prediction was that if the circadian clock had not delayed before the second light pulse was presented, the PRC would retain its phase and the second pulse would fall at CT15, and so the response to the double-pulse treatment would be a composite of the delay associated with light at CT13 and the nonsignificant shift associated with light at CT15. This is indicated in Figure 4b, NO(i.e., no shift within 2 hr). This composite value would not be significantly different from the delay to light at CT13 alone. Alternatively, if the PRC had been shifted within 2 hr of the first pulse at CT13, the second pulse would fall not at CT15 but at CT14. This would produce a composite shift to the double-pulse protocol, representing the delay associated with light at CT13 and at approximately CT14. This composite shift would be significantly greater than that seen after light at CT13 alone (Fig. 4b,YES). Post hoc comparison revealed that the observed shifts (Fig. 4b, OBS) after the double-pulse procedure were significantly greater than those after light at CT13 alone. The increased shifts could not be attributed to the longer total exposure to light associated with two pulses, because exposure to light for 30 min did not cause a significant increase in the size of the delay.
For phase advances, there was also a significant effect of treatment (F = 13.2; p < 0.01). Single pulses at CT20 and CT22 caused large phase advances of ∼2 hr, whereas light at CT24 caused only a small advance not significantly different from that seen after a dark pulse. The prediction was that if the circadian clock had not advanced before the second light pulse was presented, the response to the double-pulse treatment would be a composite of the advance associated with light at CT20 and the shift associated with light at CT22. This is indicated in Figure 4c, NO(i.e., no shift within 2 hr). Alternatively, resetting within 2 hr of the first pulse at CT20 would cause a composite shift, representing the advance associated with light at CT20 and the minimal shift associated with light at CT24 (i.e., CT20 + 2 hr advance + 2 hr interval). This composite shift would not be significantly greater than that seen after light at CT20 alone (Fig. 4c, YES). The observed shifts (Fig. 4c, OBS) were significantly different from the NO, but not the YES, prediction (Figs. 3c, 4c).
To demonstrate that large composite advances can be induced by the double-light pulse protocol, in a further experiment, animals received a light pulse at CT18, followed by a second pulse 2 hr later. Regardless of how rapidly the clock reset to the first pulse, this ensured that the second pulse would fall in the advance portion of the PRC, and so it should induce a further advance, with the response to the double-pulse protocol being significantly larger than the advance to single pulses at CT18. The observed shifts (Fig. 3d) confirmed this to be the case, with a significant difference between the advances after two pulses and those receiving one at either CT18 or CT20 (Fig. 4d).
In the current double-pulse protocol, the consequences of rapid resetting were different for advance and delay shifts, insofar as advances were not associated with large cumulative shifts, whereas the delay shifts were. Nevertheless, the data from both types of shift are consistent with resetting of the circadian clock within 2 hr of exposure to light.
Delays of the circadian clock of the mouse are completed between 1 and 2 hr after a light pulse
Exposure to light during subjective night caused significant phase delays, but not advances, of the circadian activity rhythm of mice (Figs. 5a,6a). The PRC could therefore only be used to examine the speed of delay resetting. Moreover, the slope of the delay portion of the PRC and the maximum size of phase delays were not adequate to support a simple two-pulse paradigm; unlike the hamster, it was not possible to distinguish between resetting at CT14 and CT15, which would be necessary to examine the speed of a 1 hr delay after a single pulse at CT13. Consequently, a modification of the protocol was that four light pulses were presented at intervals of 1 or 2 hr, the first pulse falling at CT13 or CT14, respectively. The PRC to single pulses was then used to calculate the composite shifts expected whether resetting did or did not occur in the interval between serial pulses. Between CT13 and CT19, the PRC was linear (r2 = 0.96; p < 0.01), and so a regression function was fitted to the data so that predicted phase shifts could be calculated by direct interpolation. As for the previous study with hamsters, if delays were occurring rapidly, serial pulses would continue to fall in the delay portion of the PRC. Conversely, if resetting was slow, the later pulses would fall around or beyond the crossover point. Consequently, rapid resetting predicted larger cumulative delays than slow resetting. For example, for the 2 hr series, if no resetting occurred at all, the cumulative delay would represent the sum of shifts associated with light at CT13, CT15, CT17, and CT19, which was predicted to be 1.65 hr (Fig. 6b,NO). However, if the oscillator was reset within 2 hr of every pulse, the cumulative delay would be at least 2.53 hr (Fig.6b, YES). As anticipated from the hamster study, the four serial 15 min pulses separated by 2 hr caused large delays (Fig. 5c, 6b, OBS), significantly greater than single pulses at either CT13 or CT14 and significantly greater than predicted if resetting was not rapid (Fig. 6b). The large shifts could not be attributed to a greater total exposure to light, because they were considerably larger than those seen after a single light pulse of 60 min at either CT13 or CT14 (Fig.6b). These data are therefore consistent with rapid (<2 hr) resetting of the clock.
Fig. 5.
Representative double-plotted actograms of wheel-running activity of ICR(CD-1) mice held in continuous dim red light and exposed to one or a series of four light pulses (200 μW/cm2; asterisks).Lines on left side indicate phase shift of activity onset. a, Single pulse (15 min) at CT13;b, single pulse (1 hr) at CT13; c, series of four pulses of 15 min presented at 2 hr intervals, first at CT13.
Fig. 6.
a, PRC (mean ± SEM) of the circadian activity rhythm of ICR(CD-1) mice held in continuous dim red light and exposed to a brief pulse of light (15 min, 200 μW/cm2) (n = 153). Thedotted line about the abscissa is the SEM for control dark pulses (mean shift, −0.05 hr; n = 15).b, Predicted and observed resetting to single and serial pulses (4 pulses, delivered at intervals of once every 1 hr or once every 2 hr) in delaying phase of PRC. Shifts to individual pulses (15 or 60 min, 200 μW/cm2) delivered at CT13 or CT14 as indicated. NO, Predicted shift after four pulse series if resetting does not occur until final pulse is delivered; YES, predicted composite shift if resetting does occur in the interval of either 1 or 2 hr between pulses;1–2, predicted shift if resetting occurs within 2 hr but not within 1 hr; OBS, observed data.n = 11–17; n = 73. **p < 0.01, pairwise comparison.
For the series of pulses separated by 1 hr and starting at CT14, the predicted cumulative delay if no resetting occurred over the 3 hr between first and final pulse was 2.00 hr (Fig. 6b,NO). If resetting did occur within 2 hr but not after 1 hr, a cumulative delay of 2.39 hr was predicted (Fig. 6b,1–2). Finally, if resetting did occur within 1 hr of a light pulse, the predicted delay was 2.81 hr (Fig. 6b,YES). The phase delays produced by serial pulses separated by 1 hr (Fig. 6b, OBS) were larger than those after single 15 min pulses at CT13 or CT14 but were not significantly greater than delays produced by a single 60 min pulse at CT13 or CT14. Moreover, they were significantly smaller than those predicted for resetting within 1 hr. In contrast, they were not significantly different from the prediction of no resetting at all or resetting that occurred between 1 and 2 hr after a pulse (Fig. 6b). Finally, the composite shifts after four pulses once every 1 hr were significantly less than those seen with four pulses every 2 hr, which is also consistent with rapid resetting within 2 hr but not 1 hr. Together, the behavioral data from the mice indicate that delays of the circadian clock take more than 1 hr but less than 2 hr to complete after a light pulse.
DISCUSSION
Our behavioral and biochemical results demonstrate that the circadian clock of the SCN of mammals can resolve light pulses separated by 1 and 2 hr. They also show that for both phase advances and phase delays, the oscillator is reset to a new phase within 2 hr of exposure to light. Moreover, despite the SCN being able to resolve light pulses presented 1 hr apart, the oscillator is not reset to a new phase within 1 hr of a light pulse. The current study therefore identifies the interval of 1–2 hr after presentation of a brief pulse of light as the critical period during which the molecular events that constitute resetting are completed. A mechanistic explanation of photic entrainment should therefore focus on those light-induced responses of the SCN that occur during this interval.
The PRCs generated in the current study are consistent with earlier work. Whereas hamsters show both large delays and large advances, mice typically exhibit small advance shifts (DeCoursey, 1964; Daan and Pittendrigh, 1976; Elliott, 1981; Schwartz and Zimmerman, 1990; Nelson and Takahashi, 1991; Grosse et al., 1995). Hamsters were therefore more suitable to demonstrate equally rapid resetting in both directions. Mice were also used in the current study to define the rate of delay resetting, because the circadian and light-regulated expression of putative clock genes are described more extensively in this species. The assumptions behind the protocol, including additive phase shifts and nondistortion of the PRC during resetting, are best fulfilled by the type 1 resetting, characterized by relatively small (<3 hr) shifts and a defined crossover point, as seen in the Syrian hamster and mouse (Lakin-Thomas, 1995). Nevertheless, the current findings complement studies in Drosophila (Pittendrigh, 1967, 1979) and Neurospora (S. Dharmananda thesis cited inCrosthwaite et al., 1995), showing that the oscillator was reset within 3 or 0.75 hr, respectively, although they exhibit type 0 resetting. In double-pulse experiments with sparrows (Binkley and Mosher, 1987), photic resetting was observed within 8 hr, but because 4 hr pulses were used, the time course could not be defined more precisely. In a different context examining the behavior of transient phase shifts during advances of the hamster circadian system, Elliott and Pittendrigh (1996) concluded that although full delays were completed within one cycle and advances of behavioral rhythm were not completed until ∼6 d, detectable shifts of the oscillator could be observed between 2 and 4 hr after a pulse, as reported here. Finally, a recent study in wild-caught field mice (Mus booduga) has also identified resetting of the oscillator to delaying pulses within 2 hr (Sharma and Chandrashekaran, 1997), although advance resetting that exhibits the most pronounced transients was not explored.
The molecular basis of the mammalian clock is unknown, and so the recent cloning of mammalian homologs of the insect periodgene is an important development (Sun et al., 1997; Tei et al., 1997). Whatever their nature, it is clear that resetting mechanisms are engaged very quickly, between 1 and 2 hr after a pulse. Thus, the rapid photic induction of mPer1 and mPer2 expression, which peaks after 1 hr and 2 hr, respectively (Albrecht et al., 1997;Shigeyoshi et al., 1997; Zylka et al., 1998), provides a molecular correlate of the overt resetting reported here. Increases in their expression and subsequent abundance of their putative protein products may be the cause of resetting to a new phase. Such a model is based on the Neurospora clock in which rapid resetting is associated with equally rapid induction of the gene frq, which has been shown to encode a state variable of the oscillation, i.e., the relative abundance of this transcript, and its protein products actually define circadian phase (Crosthwaite et al., 1995). The report that light-induced advances remain sensitive to blockade by inhibitors of protein synthesis for the 2–4 hr after a pulse (Zhang et al., 1996) supports the possibility that the mammalian clock is based on such an autoregulatory transcriptional cycle.
The rapid response of the SCN to light is dependent on glutamatergic signaling by retinal ganglion cell afferents (Ebling et al., 1991;Vindlacheruvu et al., 1992; Rea et al., 1993; Abe and Rusak, 1994). Light also causes rapid phosphorylation of CREB in retinorecipient SCN neurons (Ginty et al., 1993), probably via NMDA-mediated calcium influx (Kornhauser et al., 1996; Schurov et al., 1998). Phosphorylated CREB is a positive regulator of the c-fos gene via the calcium response element (Ginty et al., 1993; Hardingham et al., 1997;Johnson et al., 1997), although its potential role in the induction ofmPer1 and mPer2 awaits clarification. The changes in phospho-CREB expression are extremely rapid, occurring significantly before increases in c-fos or mPer mRNA, although the magnitude of the response is not related to the size or direction of a phase shift. Moreover, in both hamsters and mice, it occurs after presentation of a light pulse at all phases of circadian night but is not induced by light during the subjective day (Ginty et al., 1993; von Gall et al., 1998), closely matching the temporal induction ofmPer1 regardless of whether light advances or delays the clock. If mPer1 does encode a state variable of the oscillator, it should be induced repeatedly by serial resetting light pulses. Moreover, the observation that photic induction ofmPer2 is a marker for delaying pulses (early subjective night) but not advancing pulses (late subjective night) (Albrecht et al., 1997; Zylka et al., 1998) suggests a novel means for mapping the phase of the oscillator during serial resetting by using a molecular index. If the inducibility of mPer2 is a marker for early subjective night (delay phase of PRC), it should be possible to show that serial pulses delivered in the delay zone hold the oscillator at early subjective night, i.e., mPer2 induction is sustained. This approach is comparable to using the onset and offset of photic induction of c-fos to define the total limits of subjective night (Mead et al., 1992; Grosse et al., 1995).
The immediate early gene c-fos is also a potential component of the photic resetting pathway. Phase shifts are impaired inc-fos knock-out mice (Honrado et al., 1996), and central infusion of antisense oligonucleotides to c-fos andjun-B are reported to block light-induced resetting in the rat (Wollnik et al., 1995). Induction of c-fos mRNA by a light pulse peaks after 30 min and is undetectable after 60 min (Kornhauser et al., 1990; Rusak et al., 1990; current study). Moreover, the induction of c-fos protein, which was detectable after 7.5 min and peaks after 1 to 2 hr (J. D. Best and M. H. Hastings unpublished data), is simultaneous with the induction ofmPer1 mRNA, raising the possibility that c-fos contributes to resetting by modulating expression of the mPer1 gene via changes in AP-1 activity, which is known to occur after a light pulse in subjective night (Kornhauser et al., 1992; Takeuchi et al., 1993; Best and Hastings, unpublished data). In situ hybridization and Western blots revealed that the second resetting pulse causedde novo induction of c-fos, although the second pulse was presented when c-fos protein levels were high. In other contexts, it has been shown that c-fos protein can downregulate expression of the c-fos gene via upstream promoters (Sassone-Corsi et al., 1988; Konig et al., 1989), although it is clear that autoregulation of c-fos did not occur in the SCN.
Rapid resetting has also been reported in the hamster and rat in response to nonphotic cues, such as behavioral arousal (Mead et al., 1992) and injection of melatonin (Sumova and Illnerová, 1996). Nonphotic cues act via a mechanism distinct from phosphorylation of CREB and induction of c-fos (Mead et al., 1992; Sumova et al., 1994) and are most potent during subjective day when expression ofmPer1, mPer2, and mPer3 is maximal, suggesting a possible resetting mechanism based on suppression of these genes and/or the activity of their products. The current demonstration of rapid advance and delay resetting by light in the mammalian clock provides the context for comparative analyses of the molecular basis of resetting by different cues.
Footnotes
This work was supported by Wellcome Trust Project Grant 037667/Z/93 and Biotechnology and Biological Sciences Research Council Studentships to J.D.B. and K.L.S. We are grateful to J. Bashford, A. Newman, I. Bolton (Audio-visual Media Group, Department of Anatomy, University of Cambridge), C. Cardinal, and M. Carlson (Biomedical Services, University of Cambridge) for excellent technical assistance, and to I. Schurov for advice on Western blot analyses.
Correspondence should be addressed to Dr. Michael H. Hastings, Reader in Neuroscience, Department of Anatomy, University of Cambridge, Downing Street, Cambridge CB2 3DY, United Kingdom.
REFERENCES
- 1.Abe H, Rusak B. Physiological mechanisms regulating photic induction of Fos-like protein in hamster suprachiasmatic nucleus. Neurosci Biobehav Rev. 1994;18:531–536. doi: 10.1016/0149-7634(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. doi: 10.1016/s0092-8674(00)80495-x. [DOI] [PubMed] [Google Scholar]
- 3.Aschoff J. Biological rhythms. Handbook of behavioral neurobiology, Vol 4 (Aschoff J, ed). Plenum; New York: 1981. [Google Scholar]
- 4.Balsabore A, Damiola F, Schibler U. A serum-shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 5.Binkley S, Mosher K. Circadian rhythm resetting in sparrows: early response to doublet light pulses. J Biol Rhythms. 1987;2:1–11. doi: 10.1177/074873048700200101. [DOI] [PubMed] [Google Scholar]
- 6.Crosthwaite SK, Loros JJ, Dunlap JC. Light-induced resetting of a circadian clock is mediated by a rapid increase in frequency. Cell. 1995;81:1003–1012. doi: 10.1016/s0092-8674(05)80005-4. [DOI] [PubMed] [Google Scholar]
- 7.Czeisler CA. The effect of light on the human circadian pacemaker. In: Chadwick DJ, Ackrill K, editors. Circadian clocks and their adjustment, Ciba Foundation Symposium 183. Wiley; New York: 1995. pp. 254–290. [DOI] [PubMed] [Google Scholar]
- 8.Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J Comp Physiol [A] 1976;106:255–266. [Google Scholar]
- 9.DeCoursey PJ. Function of a light response rhythm in hamsters. J Cell Comp Physiol. 1964;63:189–196. doi: 10.1002/jcp.1030630208. [DOI] [PubMed] [Google Scholar]
- 10.Ebling FJP, Staley K, Maywood ES, Humby T, Hancock DC, Waters CM, Evan GI, Hastings MH. The role of NMDA-type glutamatergic neurotransmission in the photic induction of immediate-early gene expression in the suprachiasmatic nuclei of the Syrian hamster. J Neuroendocrinol. 1991;3:641–652. doi: 10.1111/j.1365-2826.1991.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 11.Elliott JA. Circadian rhythms, entrainment and photoperiodism in the Syrian hamster. In: Follett BK, Follett DE, editors. Biological clocks in reproductive seasonal cycles. Wright; Bristol, UK: 1981. pp. 203–217. [Google Scholar]
- 12. Elliott JA, Pittendrigh CS. 1996. Time course of hamster clock resetting following single light pulses. Fifth meeting of the Society for Research on Biological Rhythms [Abstr] 157:105
- 13.Ginty DD, Kornhauser JM, Thompson MA, Bading H, Mayo KE, Takahashi JS, Greenberg ME. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- 14.Grosse J, Loudon ASI, Hastings MH. Behavioural and cellular responses to light of the circadian system of Tau mutant and wild-type Syrian hamsters. Neuroscience. 1995;65:587–597. doi: 10.1016/0306-4522(94)00403-r. [DOI] [PubMed] [Google Scholar]
- 15.Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385:260–265. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- 16.Hastings MH. Central clocking. Trends Neurosci. 1997;20:459–464. doi: 10.1016/s0166-2236(97)01087-4. [DOI] [PubMed] [Google Scholar]
- 17.Hastings MH, Best JD, Ebling FJP, Maywood ES, McNulty S, Schurov I, Selvage D, Sloper P, Smith KL. Entrainment of the circadian clock. Prog Brain Res. 1996;111:147–174. doi: 10.1016/s0079-6123(08)60406-9. [DOI] [PubMed] [Google Scholar]
- 18.Honrado GI, Johnson RS, Golombek DA, Spiegelman BM, Papaioannou VE, Ralph MR. The circadian system of c-fos deficient mice. J Comp Physiol [A] 1996;178:563–570. doi: 10.1007/BF00190186. [DOI] [PubMed] [Google Scholar]
- 19.Johnson CM, Hill CS, Chawla S, Treisman R, Bading H. Calcium controls gene expression via three distinct pathways that can function independently of the Ras/mitogen-activated protein kinases (ERKs) signalling cascade. J Neurosci. 1997;17:6189–6202. doi: 10.1523/JNEUROSCI.17-16-06189.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein DC, Moore RY, Reppert SM. Suprachiasmatic nucleus: the mind’s clock. Oxford UP; New York: 1991. [Google Scholar]
- 21.Konig H, Ponta H, Rahmsdorf U, Buscher M, Schonthal A, Rahmsdorf HJ, Herrlich P. Autoregulation of fos: the dyad symmetry element as the major target of repression. EMBO J. 1989;8:2559–2566. doi: 10.1002/j.1460-2075.1989.tb08394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornhauser JM, Nelson DE, Mayo KE, Takahashi JS. Photic and circadian regulation of c-fos gene expression in the hamster suprachiasmatic nucleus. Neuron. 1990;5:121–134. doi: 10.1016/0896-6273(90)90303-w. [DOI] [PubMed] [Google Scholar]
- 23.Kornhauser JM, Nelson DE, Mayo KE, Takahashi JS. Regulation of jun-B messenger RNA and AP-1 activity by light and a circadian clock. Science. 1992;255:1581–1584. doi: 10.1126/science.1549784. [DOI] [PubMed] [Google Scholar]
- 24.Kornhauser JM, Mayo KE, Takahashi JS. Light, immediate early genes, and circadian rhythms. Behav Genet. 1996;26:221–240. doi: 10.1007/BF02359382. [DOI] [PubMed] [Google Scholar]
- 25. Kronauer RE, Rimmer D, Czeisler CA. 1996. Circadian phase is shifted most efficiently by brief light pulses; critical pulse durations and intervals are comparable in mosquito, hamster and man. Fifth meeting of the Society for Research on Biological Rhythms [Abstr] 202:128
- 26.Lakin-Thomas PL. A beginner’s guide to limit cycles, their uses and abuses. Biol Rhythm Res. 1995;26:216–232. [Google Scholar]
- 27.Mead S, Ebling FJP, Maywood ES, Humby T, Herbert J, Hastings MH. A nonphotic stimulus causes instantaneous phase advances of the light-entrainable circadian oscillator of the Syrian hamster but does not induce the expression of c-fos in the suprachiasmatic nuclei. J Neurosci. 1992;12:2516–2522. doi: 10.1523/JNEUROSCI.12-07-02516.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus). J Physiol (Lond) 1991;439:115–145. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Pittendrigh CS. Circadian Systems. I. The driving oscillation and its assay in Drosophila pseudoobscura. Proc Natl Acad Sci USA. 1967;58:1762–1767. doi: 10.1073/pnas.58.4.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pittendrigh CS. Some functional aspects of circadian pacemakers. In: Suda M, Hayaishi O, Nakagawa H, editors. Biological rhythms and their central mechanism. Elsevier; Amsterdam: 1979. pp. 3–12. [Google Scholar]
- 32.Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. V. Pacemaker structure: a clock for all seasons. J Comp Physiol [A] 1976;106:333–335. [Google Scholar]
- 33.Plautz JD, Kaneko M, Hall JC, Kay SA. Independent photoreceptive circadian clocks throughout Drosophila. Science. 1997;278:1632–1635. doi: 10.1126/science.278.5343.1632. [DOI] [PubMed] [Google Scholar]
- 34.Rea MA, Buckley B, Lutton LM. Local administration of EAA antagonists blocks light-induced phase shifts and c-fos expression in hamster SCN. Am J Physiol. 1993;265:R1191–R1198. doi: 10.1152/ajpregu.1993.265.5.R1191. [DOI] [PubMed] [Google Scholar]
- 35.Rusak B, Robertson HA, Wisden W, Hunt SP. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990;243:1237–1240. doi: 10.1126/science.2112267. [DOI] [PubMed] [Google Scholar]
- 36.Sassone-Corsi P, Sisson JC, Verma IV. Transcriptional autoregulation of proto-oncogene fos. Nature. 1988;334:314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- 37.Schurov IL, McNulty S, Best JD, Sloper PJ, Hastings MH. Glutamatergic induction of CREB phosphorylation and Fos expression in the suprachiasmatic hypothalamus in vitro is mediated by co-ordinate activity of NMDA and non-NMDA receptors. J Neuroendocrinol. 1999;11:43–52. doi: 10.1046/j.1365-2826.1999.00289.x. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz WJ, Zimmerman P. Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J Neurosci. 1990;10:3685–3694. doi: 10.1523/JNEUROSCI.10-11-03685.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma VK, Chandrashekaran MK. Rapid phase resetting of a mammalian circadian rhythm by brief light pulses. Chronobiol Int. 1997;14:537–548. doi: 10.3109/07420529709001445. [DOI] [PubMed] [Google Scholar]
- 40.Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC, Okamura H. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997;91:1043–1053. doi: 10.1016/s0092-8674(00)80494-8. [DOI] [PubMed] [Google Scholar]
- 41.Sumova A, Illnerová H. Melatonin instantaneously resets intrinsic circadian rhythmicity in the rat suprachiasmatic nucleus. Neurosci Lett. 1996;218:181–184. doi: 10.1016/s0304-3940(96)13159-1. [DOI] [PubMed] [Google Scholar]
- 42.Sumova A, Ebling FJP, Maywood ES, Herbert J, Hastings MH. Non-photic circadian entrainment in the Syrian hamster is not associated with phosphorylation of the transcriptional regulator CREB within the suprachiasmatic nucleus, but is associated with adrenocortical activation. Neuroendocrinology. 1994;59:579–589. doi: 10.1159/000126708. [DOI] [PubMed] [Google Scholar]
- 43.Sun ZS, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee CC. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell. 1997;90:1003–1011. doi: 10.1016/s0092-8674(00)80366-9. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi J, Shannon W, Aronin N, Schwartz WJ. Compositional changes of AP-1 DNA-binding proteins are regulated by light in a mammalian circadian clock. Neuron. 1993;11:825–836. doi: 10.1016/0896-6273(93)90112-5. [DOI] [PubMed] [Google Scholar]
- 45.Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature. 1997;389:512–516. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- 46.Vendrell M, Pujol MJ, Tusell JM, Serratosa J. Effect of different convulsants on calmodulin levels and protooncogene c-fos expression in the central nervous system. Mol Brain Res. 1992;14:285–292. doi: 10.1016/0169-328x(92)90095-s. [DOI] [PubMed] [Google Scholar]
- 47.Vindlacheruvu RR, Ebling FJP, Maywood ES, Hastings MH. Blockade of glutamatergic neurotransmission in the suprachiasmatic nucleus prevents cellular and behavioural responses of the circadian system to light. Eur J Neurosci. 1992;4:673–679. doi: 10.1111/j.1460-9568.1992.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 48.von Gall C, Duffield GE, Hastings MH, Kopp MDA, Dehghani F, Korf H-W, Stehle JH. CREB in the mouse SCN: a molecular interface coding the phase-adjusting stimuli light, glutamate, PACAP, and melatonin for clockwork access. J Neurosci. 1999;18:10389–10397. doi: 10.1523/JNEUROSCI.18-24-10389.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 50.Wollnik F, Brysch W, Uhlmann E, Gillardon F, Bravo R, Zimmermann M, Schlingensiepen KH, Herdegen T. Block of c-fos and junB expression by antisense-oligonucleotides inhibits light-induced phase shifts of the mammalian circadian clock. Eur J Neurosci. 1995;7:388–393. doi: 10.1111/j.1460-9568.1995.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 51.Young WS, III, Kuhar MJ. Quantitative in situ hybridization and determination of mRNA content. In: Uhl GR, editor. In situ hybridization in brain. Plenum; New York: 1986. pp. 243–248. [Google Scholar]
- 52.Young WS, III, Mezey E, Siegel RE. Quantitative in situ hybridization histochemistry reveals increased levels of corticotrophin-releasing factor messenger-RNA after adrenalectomy in rats. Neurosci Lett. 1986;70:198–203. doi: 10.1016/0304-3940(86)90463-5. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Takahashi JS, Turek FW. Critical period for cycloheximide blockade of light-induced phase advances of circadian locomotor activity rhythms in golden hamster. Brain Res. 1996;740:285–290. doi: 10.1016/s0006-8993(96)00900-6. [DOI] [PubMed] [Google Scholar]
- 54.Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]