Abstract
The hippocampal mossy fibers, which originate from the dentate granule cells, develop mainly in the early postnatal period and are involved in numerous pathological processes. In this study, hippocampal slices prepared from premature rats were cultivated in the presence of convulsants to evaluate the influences of epileptiform activities on mossy fiber ontogeny. Electrophysiological and histochemical analyses revealed that prolonged hyperexcitability inhibited proper growth of the mossy fibers and caused ectopic innervation to the stratum oriens and the dentate molecular layer. These phenomena were prevented by pharmacological blockade of L-type Ca2+ channels, which did not affect convulsant-evoked ictal bursts. After single-pulse stimulation of the stratum granulosum in the slices cultured under paroxysmal conditions, the dentate gyrus displayed excessive excitation, but synaptic transmission to the CA3 region was hypoactive. However, brief repetitive stimulation elicited delayed epileptiform discharges in the CA3 region that were inhibited by an NMDA receptor antagonist. Chronic treatment with an L-type Ca2+ channel blocker ameliorated such aberrant neurotransmissions. These results suggest that ictal neuron activities at the developmental stage of the mossy fibers bring about the errant maturation associated with hippocampal dysfunction, which may form a cellular basis for the sequelae of childhood epilepsy, including chronic epilepsy or cognitive deficits. Thus I propose that L-type Ca2+ channel blockers can ameliorate the aversive prognosis of childhood epilepsy.
Keywords: hippocampus, dentate gyrus, mossy fiber, childhood epilepsy, granule cell, L-type Ca2+ channel, sprouting, axon guidance, targeting
Although ontogenetic development in the CNS predominantly occurs during the embryonic period, maturation in several brain regions extends until postnatal ages. The early postnatal period is therefore a “critical stage” at which certain forms of disease or injury provoke developmental disorders in these or related brain regions.
The hippocampal formation has been implicated in learning and memory by experimental and clinical studies (Squire and Zola-Morgan, 1991;Eichenbaum et al., 1992; Zola-Morgan and Squire, 1993) and accumulating evidence indicates as well that the hippocampus plays a significant role in epileptogenesis and seizure maintenance (Schwartzkroin, 1994). Particularly, the hippocampal mossy fibers, axons of the dentate granule cells, often display high-order plasticity associated with epilepsy (Tauck and Nadler, 1985; Sutula et al., 1988; Babb et al., 1991; Van der Zee et al., 1995). Because the mossy fibers develop mainly in the early postnatal period (Stirling and Bliss, 1978; Amaral and Dent, 1981; Gaarskjaer, 1985), which corresponds to the critical stage, it is plausible that developing mossy fibers are vulnerable to epilepsy. Thus, when epilepsy, generally considered to be a disease with a satisfactory prognosis, occurs in infancy or early adolescence, it may cause severe clinical sequelae (Stores, 1971; Rodin et al., 1986; Farwell et al., 1985; Alpherts and Aldenkamp, 1990; Mizrahi, 1994). However, the influence of epileptiform activities on developing mossy fibers has not been fully understood. Jiang et al. (1998) very recently found that thorny excrescences on proximal apical dendrites of the CA3c pyramidal neuron, the major recipient site of the mossy fibers, dramatically decreased in a model of early-onset epilepsy. This result strongly suggests abnormal formation of the mossy fiber innervation. Therefore, in the present study, hippocampal slices prepared from early postnatal rats were cultivated under paroxysmal conditions to elucidate the effect of hyperexcitability on mossy fiber development and to evaluate the molecular basis underlying mossy fiber growth. Here I show for the first time that seizure-like activities caused aberrant growth of the mossy fibers via L-type Ca2+ channel activation, resulting in anomalous hippocampal neurotransmissions.
MATERIALS AND METHODS
Organotypic slice culture. The hippocampi were prepared from postnatal 6-d-old Wistar rats and were cut into 300-μm-thick slices. Sections were placed on polytetrafluoroethylene membranes that were inserted into six-well plates filled with culture medium consisting of 50% minimum essential medium (Life Technologies, Gaithersburg, MD), 25% HBSS, and 25% horse serum (Cell Culture Lab, Cleveland, OH). The cultures were kept at 37°C in a humidified and CO2-enriched atmosphere, and the culture medium was changed once every 3.5 d.
Extracellular recording. Cultured slices were submerged in artificial CSF (ACSF) at 32°C for >1 hr to withdraw the media’s constituents. The stratum granulosum was stimulated with a bipolar electrode, and the evoked field potential was extracellularly recorded from the CA3 stratum pyramidale with a glass capillary microelectrode filled with 0.15 m NaCl. The positive field potential (see Fig. 3B) reflected field EPSP (fEPSP) because it was blocked by 10 μm6-cyano-7-nitroquinoxaline-2,3-dione, a non-NMDA receptor antagonist. The input–output studies of field potentials indicated that the electrical rectangular pulse consisting of 100 μA intensity and 100 μsec duration gave the maximal amplitude of fEPSP. The maximal size of fEPSP was used as an index of the number of functional synaptic contacts formed as a function of time (Muller et al., 1993;Ikegaya et al., 1997). All electrophysiological experiments were conducted in the ACSF that was composed of (in mm): 127 NaCl, 1.6 KCl, 2.4 CaCl2, 2.4 MgSO4, 1.3 KH2PO4, 1.24 NaHCO3, and 10.0 glucose, and saturated with 95% O2 and 5% CO2.
Fig. 3.
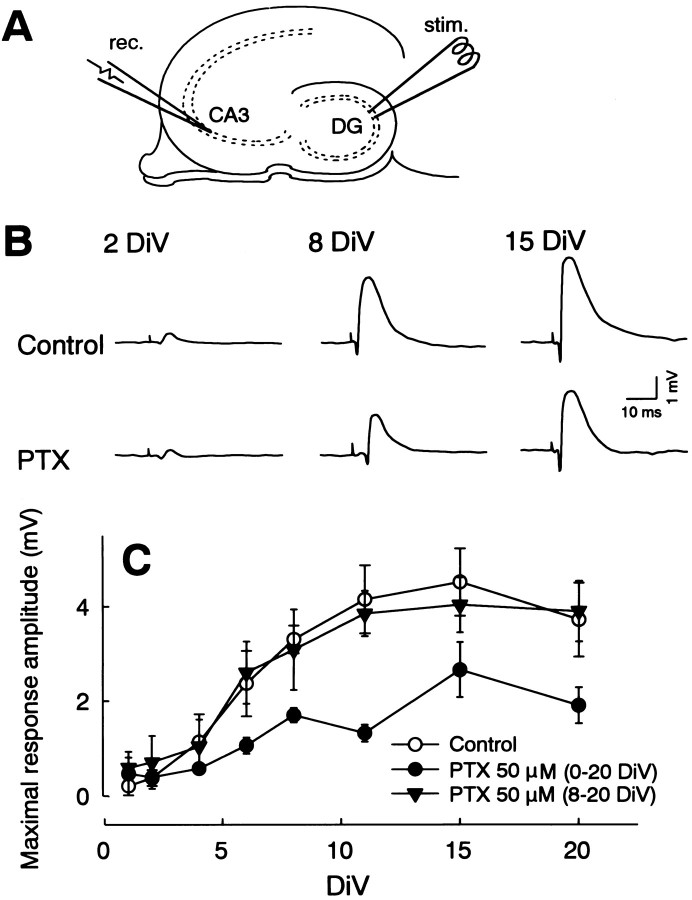
Picrotoxin inhibited mossy fiber development.A, Positions of recording (rec.) and stimulating (stim.) electrodes in slice preparation. Field potential evoked by a stimulation of the dentate stratum was recorded from the CA3 stratum pyramidale to estimate synaptic responses of the mossy fibers. DG, Dentate gyrus.B, Representative field potentials in slices that were cultured in normal medium (Control) or medium containing 50 μm picrotoxin (PTX).C, Changes in the maximal amplitude of positive field responses recorded from slices that were cultured in normal medium (○) or in the presence of picrotoxin during 0–20 DIV (•) or 8–20 DIV (▴). The development of field responses was significantly repressed in the slices that received chronic treatment (0–20 DIV) with picrotoxin (two-way ANOVA followed by Tukey’s test,p < 0.01). Data represent means ± SEM of three to seven cases.
Propidium iodide labeling. The 14 d in vitro(DIV) cultures were transferred to the medium containing 10 μg/ml propidium iodide (PI). PI fluorescence imaging was performed with a confocal microscope 24 hr later.
DiI labeling. The slices were fixed with 0.1 mphosphate buffer containing 4% paraformaldehyde for 1 d before DiI crystal was placed on the dentate gyrus. After incubation in the fixative at room temperature for 7 d, DiI-labeled axons were observed using a confocal microscope.
Immunohistochemical analysis. For the immunostaining of monoclonal antibodies against anti-calbindin-D (Sigma, St. Louis, MO), 250-μm-thick slices were cultured. At 8 DIV, they were rinsed with PBS and then immersed in 4% paraformaldehyde for 30 min, followed by blocking endogenous peroxidase with 0.3% H2O2. After being washed, they were incubated overnight at 4°C with the primary antibodies (1:200) diluted with PBS containing 0.5% horse serum, and then they were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (1:50) (Amersham International, Buckinghamshire, UK) for 4 hr at 37°C. Immunofluorescent preparation was observed with a confocal microscope.
Confocal microscopy. Confocal imaging was performed with a laser scanning confocal system MRC-600 (Bio-Rad, Hercules, CA) equipped with an inverted microscope (Nikon, Tokyo, Japan), an argon ion laser, and a host computer system. All image generation and processing operations were performed with CoMOS Ver 6.01 (Bio-Rad). For the measurements of PI, DiI, or FITC fluorescence, the cultures were illuminated with the excitation wavelengths of 514 nm, and the fluorescence images were obtained through a 550 nm bandpass filter. The intensity of PI fluorescence was assessed as an averaged value of pixel intensity (0–255) in three areas (82.5 μm2) of the stratum pyramidale or the stratum granulosum (Strasser and Fischer, 1995; Nakagami et al., 1997). Three areas in each subregion were selected at even intervals across the board.
Timm staining. The cultures at 8 DIV were washed with 0.1m phosphate buffer and then immersed in 0.37% sodium sulfide solution for 10 min, immediately followed by fixation with 10% formaldehyde solution for 15 min. After being washed with 0.1m phosphate buffer, the sections were dehydrated sequentially with 70 and 96% ethanol and dried. To perform the sulfide silver staining, they were subsequently incubated with the physical developer composed of citrate-buffered 20% arabic gum solution containing 1.7% AgNO3 and 0.085% hydroquinone in a dark room at 26°C for 50 min. The slices were washed with distilled water at the end of the reaction. To quantify the intensity of Timm staining, monochrome images were obtained using a light-phase microscope, and pixel intensity values (0–255) of three areas (100 μm2) within the stratum lucidum, the CA3 stratum oriens, the CA3 stratum radiatum, the dentate hilus, or the stratum granulosum were calculated. Three areas in each subregion were selected at even intervals across the board. Timm intensity for each region was calculated by subtraction from that of the stratum radiatum.
Optical recording. The preparation cultured for 8 d was used for an optical recording. The cultures were incubated with 0.2 mg/ml RH482 (Nippon Kankoh-Shikiso Kenkyusho) for 5 min and then washed in ACSF for at least 15 min. Transmitted light with a wavelength of 700 ± 20 nm was projected, and optical data were obtained with a 128 × 128 photodiode array at a frame rate of 0.6 msec. Sixteen successive trial images (5.08 mm2, 600 msec duration) were averaged to improve the signal-to-noise ratio. The period of time during which the signal intensity lasted >50% of the maximal amplitude was used as excitation duration.
RESULTS
Synchronous epileptiform activities
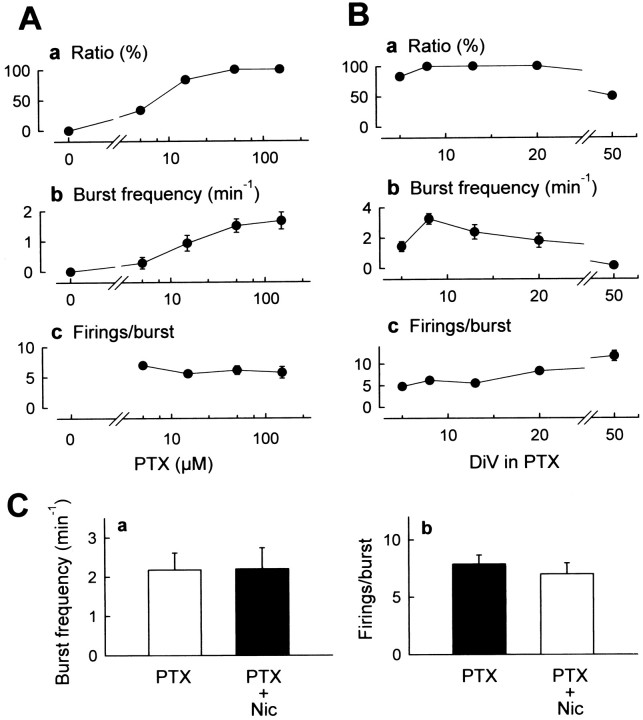
Although apparent spontaneous activities were not seen in normal ACSF (Fig. 1A), all 32 cultures superfused with 50 μm picrotoxin, a GABAA receptor channel blocker, showed continuous synchronous epileptiform bursts in the CA3 stratum pyramidale, which individually consisted of five to nine repetitive firings (Fig.1B). The bursts were completely blocked by 0.5 μm tetrodotoxin, a voltage-sensitive Na+ channel blocker (Fig. 1C). Figure2A represents the effects of varying concentrations of picrotoxin on the number of the slices that displayed epileptiform bursts per the number of all tested slices (percentage), the number of bursts per min, and the number of firings per burst. The effect of picrotoxin was dose dependent and was saturated at a concentration of 50 μm, but the number of firings per burst was not concentration dependent. Epileptiform activities similar to those seen in the CA3 region were elicited both in the CA1 stratum pyramidale and in the stratum granulosum (data not shown).
Fig. 1.
Iterative epileptiform discharges induced by picrotoxin in cultured hippocampal slices. Field potentials were recorded from the CA3 stratum pyramidale in normal ACSF (A) or in the presence of 50 μmpicrotoxin (PTX) (B), a combination of 50 μm picrotoxin and 0.5 μmtetrodotoxin (TTX) (C), or a combination of 50 μm picrotoxin and 10 μmnicardipine (Nic) (D). The burst indicated by • in a is expanded in b. Picrotoxin showed continual synchronous paroxysmal bursts, which consisted of several repetitive firings. The bursts were completely blocked by tetrodotoxin but were not influenced by nicardipine.
Fig. 2.
Characterization of picrotoxin-induced epileptiform activities. A, Rates of the slices that displayed ictal bursts (a), the number of bursts per minute (b), and the number of firings per burst (c) were plotted versus picrotoxin (PTX) concentration. Picrotoxin induced discharges in a dose-dependent manner. B, Ictal bursts induced by 50 μm picrotoxin were recorded from the cultures that received chronic exposure to 50 μmpicrotoxin. The abscissa indicates the duration of exposure to picrotoxin. Picrotoxin was added at 0 DIV. Epileptiform activities did not decline in slices cultured in the presence of picrotoxin for at least 20 d. C, Burst frequency (a) and the number of firings per burst (b) were monitored immediately before and 30 min after superfusion of 10 μm nicardipine (Nic). Nicardipine did not alter either parameters of 50 μm picrotoxin-induced discharges (paired ttest; p = 0.915 and p = 0.260, respectively). Data represent means ± SEM of five to seven cases.
Next, I examined the time course of the appearance of epileptiform activities exhibited by hippocampal slices cultured in the chronic presence of 50 μm picrotoxin (Fig. 2B). The decline of epileptiform activities was not observed until at least 20 DIV. Rather, burst frequency was dramatically increased in slices treated with picrotoxin for 8 d, suggesting that the hippocampus grown under paroxysmal conditions became epileptogenic.
Inhibition of mossy fiber development in epileptiform hippocampus
After single-pulse stimulation of the stratum granulosum in cultured hippocampal slices, a positive field potential was evoked in the CA3 stratum radiatum (Fig. 3). The maximal amplitude of the synaptic response gradually increased according to DIV and almost stabilized by 11 DIV. This time course is quite adequate because the mossy fibers are generated mainly in the postnatal second week in rats (Stirling and Bliss, 1978; Amaral and Dent, 1981; Gaarskjaer, 1985) and strongly suggests that the positive field potentials reflect EPSP elicited by the developed mossy fibers. When the slices were cultured in the chronic presence of 50 μm picrotoxin, the development of the response amplitude was partially but significantly inhibited. However, picrotoxin had no effects when applied from 8 to 20 DIV. The maximal response size at 8 DIV was used for diagnostics of the formed mossy fibers in subsequent experiments.
The inhibitory effects of picrotoxin on the response increment was dose dependent, which was saturated at a concentration of 50 μm, and the development of synaptic responses could not be completely inhibited even by 150 μm picrotoxin (Table1). The effect of picrotoxin was blocked in slices that received co-treatment with 1 μmtetrodotoxin, whereas tetrodotoxin alone did not affect the response development (Table 1). The effects of other convulsants that had pharmacological properties different from picrotoxin were also examined. Bicuculline (10 μm), pentylenetetrazol (1 mm), 4-aminopyridine (2 mm), or pilocarpine (10 μm) evoked paroxysmal discharges in cultured hippocampus (data not shown) and inhibited the development of synaptic responses (Table 1). These actions were quite similar to the effects of picrotoxin.
Table 1.
Epileptiform activities evoked by convulsants inhibit mossy fiber development
| Drugs | n | Maximal response amplitude | |
|---|---|---|---|
| (mV) | (%) | ||
| Control | 8 | 3.50 ± 0.30 | 100.0 ± 8.6 |
| PTX | |||
| 5 μm | 6 | 3.02 ± 0.15 | 86.3 ± 4.3 |
| 15 μm | 5 | 2.10 ± 0.46* | 60.0 ± 13.1 |
| 50 μm | 6 | 1.61 ± 0.12* | 46.0 ± 3.4 |
| 150 μm | 5 | 1.92 ± 0.28* | 54.9 ± 8.0 |
| TTX 0.5 μm | 5 | 3.21 ± 0.35 | 91.7 ± 10.0 |
| PTX 50 μm + TTX 0.5 μm | 6 | 3.49 ± 0.33** | 99.7 ± 9.4 |
| Bicuculline 10 μm | 6 | 1.68 ± 0.11* | 48.0 ± 3.1 |
| Pentylenetetrazol 1 mm | 6 | 1.74 ± 0.12* | 50.3 ± 3.4 |
| 4-aminopyridine 2 mm | 6 | 1.67 ± 0.09* | 47.7 ± 2.6 |
| Pilocarpine 10 μm | 6 | 1.66 ± 0.32* | 47.4 ± 9.1 |
The maximal amplitude of mossy fiber synaptic responses was measured in hippocampal slice cultures that were fostered in the continuous presence of drugs for 8 d. Data represent means ± SEM of n cases. Values in the right column are expressed as percentage of the amplitude in control slices. PTX, Picrotoxin; TTX, tetrodotoxin. *p < 0.01 vs control, **p < 0.01 vs PTX 50 μm; ANOVA followed by Tukey’s test.
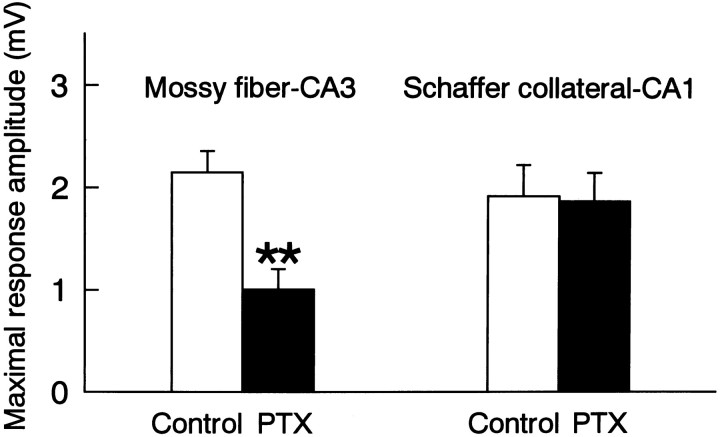
To determine whether the effects of picrotoxin were restricted to the mossy fibers, synaptic responses evoked by stimulating the mossy fibers or the Schaffer collaterals were recorded from the CA3 or CA1 stratum pyramidale, respectively. Chronic treatment with 50 μmpicrotoxin reduced the amplitude of synaptic responses of the mossy fibers but not that of the Schaffer collaterals (Fig.4).
Fig. 4.
Distinct effects of picrotoxin on the mossy fiber–CA3 synapses and Schaffer collateral–CA1 synapses. After chronic treatment with picrotoxin (PTX) for 8 d, the synaptic response evoked by a stimulation of the stratum granulosum or the CA1 stratum radiatum was recorded from the CA3 or CA1 stratum pyramidale, respectively. Picrotoxin reduced the response amplitude of the mossy fiber synapses but not that of Schaffer collateral synapses. **p < 0.01 versusControl; ANOVA followed by Tukey’s test. Data represent means ± SEM of five to six cases.
Because excessive excitatory activities often lead to neuron death, termed excitotoxicity (Ikonomidou and Turski, 1995; Greene and Greenamyre, 1996), it was possible that the decrease in the mossy fiber response in picrotoxin-treated slices was a result of massive loss of the CA3 pyramidal cells. To verify this possibility, the slices were incubated with PI fluorescent dye, an indicator for damaged cells. The confocal imaging indicated that chronic treatment with 50 μm picrotoxin did not induce neuronal degeneration (Fig.5). In the same figure, the slice that was transiently exposed to 400 μm kainate is shown as a positive control. Kainate induced severe damages in all hippocampal areas, particularly in the CA3 region.
Fig. 5.
Picrotoxin did not produce neuronal cell death in hippocampal slice cultures. To assess the possibility that picrotoxin induced cell loss, confocal images of PI fluorescence were obtained from hippocampal slices cultured in normal medium (A) or in medium containing 50 μmpicrotoxin (PTX) (B).C, The confocal image of 400 μmkainate-treated slices, which revealed massively damaged cells in the overall hippocampus, was shown as a positive control. D, Intensity of PI fluorescence is measured in the dentate gyrus (DG) (open column), the CA3 region (closed column), or the CA1 region (hatched column). Picrotoxin-treated group displays no apparent cell damage. **p < 0.01 versus Control; two-way ANOVA followed by Tukey’s test. Data represent means ± SEM of four cases.
The mossy fiber development is roughly divided into two processes, axon outgrowth and synaptogenesis. To examine which process was affected under the epileptiform conditions, the mossy fibers were orthodromically labeled with DiI, a neuronal tracer. Although few mossy fibers were observed at 2 DIV, they elongated toward the CA3 region at 8 DIV, which was independent of the presence of picrotoxin (Fig.6A). Therefore, outgrowth process was not likely to be inhibited by hyperexcitability. However, some of the DiI-labeled mossy fibers in the picrotoxin-treated slice showed ectopic innervation out of the stratum lucidum into the stratum pyramidale, even into the stratum oriens (SO) (Fig.6Ac). Because this result suggests that picrotoxin abolished the normal guidance of the mossy fibers, the 8 DIV slices were immunostained with anti-calbindin-D antibody to label the mossy fibers (Fig. 6B). The stratum lucidum was selectively stained in the intact slice (Fig. 6Ba), but the mossy fiber tract was sparsely distributed in the stratum lucidum, the stratum pyramidale, and the stratum oriens in the picrotoxin-treated slice (Fig. 6Bb).
Fig. 6.
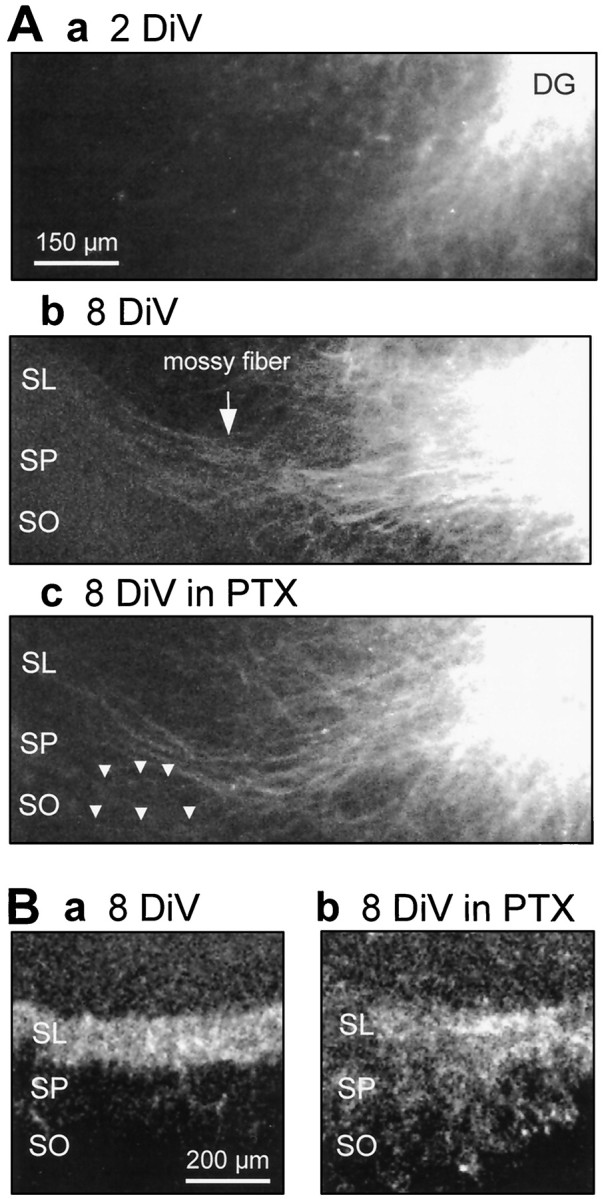
Picrotoxin produced aberrant guidance of the mossy fibers. A, The mossy fibers that were labeled with DiI placed on the dentate gyrus (DG) were observed using a confocal microscope at 2 DIV (Aa) or 8 DIV (Ab, Ac). The mossy fibers showed fundamentally regular elongation even in the presence of 50 μm picrotoxin (Ac), but several fibers showed ectopic innervation out of the stratum lucidum (SL) into the stratum pyramidale (SP), even into the stratum oriens (SO) (indicated byarrowheads). B, The mossy fibers were labeled with anti-calbindin-D antibody at 8 DIV. The stratum lucidum was selectively stained in the intact slice (Ba), but the mossy fiber tract was sparsely distributed in the stratum lucidum, the stratum pyramidale, and the stratum oriens in the picrotoxin-treated slice (Bb).
Blockade of the effect of picrotoxin by L-type Ca2+ channel blockers
Epileptiform bursts elicit a prolonged depolarization shift of neuronal membrane potential, which may allow Ca2+influx through voltage-sensitive Ca2+-permeable channels. Therefore, I investigated the effects of nicardipine or nifedipine, L-type Ca2+ channel blockers, NiCl2, a T-type Ca2+ channel blocker, and 2-amino-5-phosphonopentanoic acid (AP5), an NMDA receptor antagonist, on picrotoxin-induced inhibition of mossy fiber growth (Table 2). Nicardipine reduced picrotoxin-induced inhibition of mossy fiber development in a dose-dependent manner. Nifedipine (10 μm) also showed the ameliorative effect. Because nicardipine changed neither burst frequency nor the number of firings in picrotoxin-evoked discharges (Figs. 1D, 2C), its ameliorative effect was not caused by blockade of epileptiform activities. It is somewhat surprising that nicardipine exerted similar potency even when applied only for the latter 5 d of the treatment with picrotoxin. By contract, 50 μm NiCl2 and 50 μmAP5 did not affect the effects of picrotoxin. These channel blockers alone had no effect on the development of mossy fiber responses. Taken together, these results suggest that Ca2+ influx through L-type Ca2+ channels during ictal bursts mediated disturbance of appropriate synapse formation of the mossy fibers.
Table 2.
L-type Ca2+ channel blockers prevent epileptiform activity-dependent inhibition of mossy fiber formation
| Drugs | n | Maximal response amplitude | |
|---|---|---|---|
| (mV) | (%) | ||
| Control | 6 | 3.30 ± 0.29 | 100.0 ± 8.7 |
| PTX 50 μm | 6 | 1.70 ± 0.15* | 51.5 ± 4.5 |
| Nic 10 μm | 3 | 2.87 ± 0.57 | 86.9 ± 17.2 |
| PTX 50 μm | |||
| + Nic 0.3 μm | 5 | 1.70 ± 0.30 | 51.5 ± 9.1 |
| + 1 μm | 6 | 2.51 ± 0.18 | 76.1 ± 5.5 |
| + 3 μm | 7 | 3.38 ± 0.26*** | 102.4 ± 7.9 |
| + 10 μm | 6 | 3.31 ± 0.30*** | 100.3 ± 9.1 |
| + (4–8 DiV) 10 μm | 7 | 3.01 ± 0.42** | 91.2 ± 12.7 |
| Nifedipine 10 μm | 3 | 3.07 ± 0.19 | 93.0 ± 5.7 |
| PTX 50 μm + Nifedipine 10 μm | 5 | 3.02 ± 0.29** | 91.5 ± 8.8 |
| NiCl2 50 μm | 5 | 2.94 ± 0.25 | 89.1 ± 7.7 |
| PTX 50 μm + NiCl2 50 μm | 6 | 0.99 ± 0.15 | 30.1 ± 4.6 |
| AP5 50 μm | 5 | 3.10 ± 0.50 | 93.8 ± 15.0 |
| PTX 50 μm + AP5 50 μm | 6 | 1.63 ± 0.17 | 49.4 ± 5.0 |
The maximal amplitude of mossy fiber synaptic responses was measured in hippocampal slice cultures that were fostered in the continuous presence of drugs for 8 d. Data represent means ± SEM of n cases. Values in the right column are expressed as percentage of the amplitude in control slices. PTX, Picrotoxin; Nic, nicardipine. *p < 0.01 vs control, **p< 0.05, ***p < 0.01 vs PTX 50 μm; ANOVA followed by Tukey’s test.
Abnormal targeting of the mossy fibers in epileptiform hippocampus
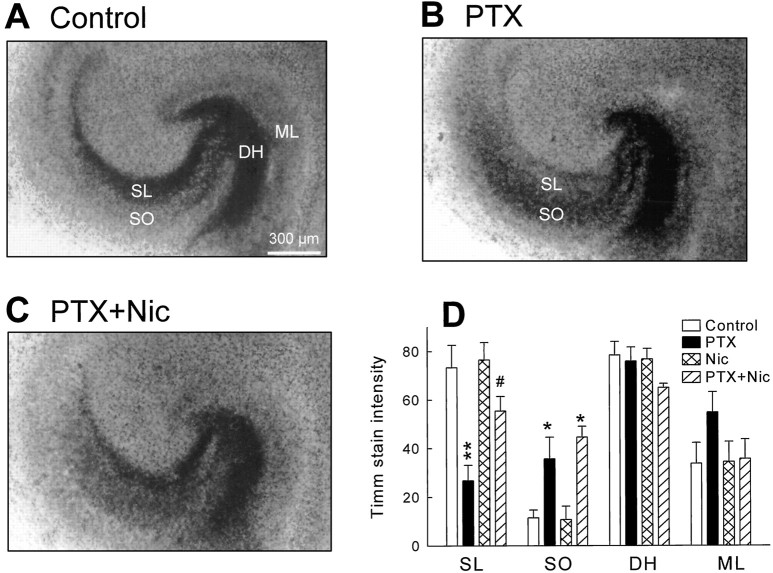
Because cultures were stained with the Timm method, a histochemical technique that selectively labels synaptic terminals of the mossy fibers because of their high Zn2+ content (Danscher and Zimmer, 1978), the stratum lucidum and the dentate hilus were black-lacquered in naïve hippocampal slices (Fig.7A). However, Timm intensity in the stratum lucidum was significantly diminished in the slices that received treatment with picrotoxin, implying that mossy fiber synapses were not sufficiently formed under epileptiform conditions (Fig.7B). This result was compatible with the electrophysiological analysis revealing that mossy fiber responses in picrotoxin-treated slices were reduced. Timm staining provided further interesting findings. In picrotoxin-treated slices, synaptic terminals of the mossy fibers were detected in the CA3 stratum oriens and the dentate molecular layer, with which the mossy fibers rarely make contact under normal physiological conditions. The altered Timm intensity reverted in the stratum lucidum and the molecular layer but not in the stratum oriens of the slices co-treated with nicardipine (Fig. 7C). Nicardipine alone did not alter the appearances of Timm-stained cultures (Fig. 7D).
Fig. 7.
Ectopic distribution of Timm-positive synapses in a picrotoxin-treated slice was prevented by co-treatment with nicardipine. A–C, Mossy fiber terminals were detected by the Timm method in slices cultured in normal medium (A), medium containing 50 μmpicrotoxin (PTX) (B), or medium containing a combination of 50 μm picrotoxin and 10 μm nicardipine (C).D, Timm intensity in the stratum lucidum (SL), the CA3 stratum oriens (SO), the dentate hilus (DH), or the molecular layer (ML) was calculated with a densitometer in slices cultured in normal medium (open column), medium containing 50 μm picrotoxin (closed column), 10 μm nicardipine (cross-hatched column), or a combination of picrotoxin and nicardipine (hatched column). Timm intensity in the stratum lucidum was decreased and that in the stratum oriens was increased in picrotoxin-treated slices. The former was ameliorated by nicardipine. *p < 0.05, **p < 0.01 versusControl, #p < 0.05 versusPTX; ANOVA followed by Tukey’s test. Data represent means ± SEM of each six cases.
Because Zn2+ is released from mossy fiber terminals by seizure activities (Sloviter, 1985), the altered Timm intensity may reflect changes in the amount of axonal Zn2+. Therefore, I tried multipoint recording of field potentials from the stratum lucidum, the stratum oriens, and the stratum pyramidale (Fig.8). The amplitude of negative field potential evoked in the stratum lucidum was significantly decreased in picrotoxin-treated slices and was entirely rescued by co-treatment with nicardipine. Although no conspicuous field potentials were recorded from the stratum oriens of intact slices, apparent negative potentials were evoked in the picrotoxin-treated slices. This strongly suggests that the mossy fibers formed their synaptic contacts within the stratum oriens as a result of prolonged hyperexcitability. This negative potential did not disappear in nicardipine-treated cultures.
Fig. 8.

Aberrant synaptic responses in picrotoxin-treated slices were improved by co-treatment with nicardipine.A, Multipoint recording of field potentials was conducted at 8 DIV to evaluate where the mossy fibers formed their synapses. Top diagram indicates positions of recording (rec.) and stimulating (stim.) electrodes in slice preparation. DG, Dentate gyrus. Bottom traces show typical field potentials recorded from the stratum lucidum (S. lucidum), the CA3 stratum pyramidale (S. pyramidale), or the CA3 stratum oriens (S. oriens) in slices cultured in normal medium (Control), medium containing 50 μmpicrotoxin (PTX), or medium containing a combination of 50 μm picrotoxin and 10 μmnicardipine (PTX+Nic). B, Maximal amplitude of synaptic responses was measured for each region. Note a decrease in response amplitude in the stratum lucidum and a manifestation of electrophysiological dendritic responses from the stratum oriens in picrotoxin-treated group. **p < 0.01 versus Control, #p < 0.05 versus PTX; ANOVA followed by Tukey’s test. Data represent means ± SEM of each five cases.
Aberrant neurotransmission in picrotoxin-treated hippocampal slices
It is feasible that hippocampal slices that contain abnormal synaptic circuitry display aberrant neurotransmission. Spatial and temporal propagation of neuron activities were monitored with a real-time optical recording of membrane potential that was visualized with RH482, a voltage-sensitive dye (Fig.9A). In all seven control slices tested, stimulation of the stratum granulosum distinctly induced sequential neuron excitation along the hippocampal trisynaptic pathway: the dentate gyrus, the CA3 region, and then the CA1 region. However, six of seven slices treated with picrotoxin showed defective propagation from the dentate gyrus to the CA3 region, suggesting a malfunction of mossy fiber transmission. Trisynaptic transmission reappeared in five of seven slices that received co-treatment with nicardipine. Furthermore, analysis of the time courses of optical signals revealed that duration of dentate excitation was prominently prolonged when cultured in picrotoxin (Fig. 9B). The duration was 17.3 ± 3.2 msec in control slices, 58.6 ± 8.7 msec in the picrotoxin-treated group, and 20.1 ± 3.9 msec in the slices cultured in a combination of picrotoxin and nicardipine (mean ± SEM, each n = 7). The duration was significantly extended by picrotoxin (ANOVA,F(2,18) = 15.80, p < 0.01; Tukey’s test, Q(3,18) = 7.11, p< 0.01) and recovered by co-treatment with nicardipine (Q(3,18) = 6.63, p < 0.01).
Fig. 9.
Nicardipine improved aberrant neurotransmission in picrotoxin-treated group. Propagation of neuron activities was monitored as changes in optical signals of RH482 at 8 DIV.A, Optical signal propagation after a stimulation of the stratum granulosum in slices cultured in normal medium (Control), medium containing 50 μmpicrotoxin (PTX), or medium containing a combination of 50 μm picrotoxin and 10 μmnicardipine (PTX+Nic) was shown in time sequence.B, Time course of optical signal in the dentate gyrus (DG) or the CA3 or CA1 region was eluted from the same subjects as shown in A. Picrotoxin-treated cultures demonstrated faint neurotransmission from the dentate gyrus to the CA3 region but a prolonged excitation of the dentate gyrus after the stimulation. DG, Dentate gyrus.
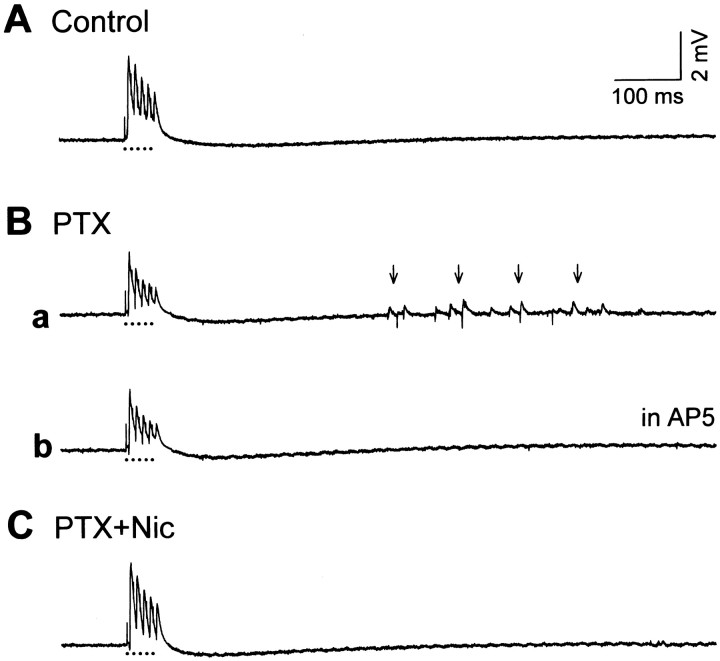
Because the dentate gyrus displayed hyperexcitation, the CA3 field potentials evoked by repetitive stimulation of the stratum granulosum (5 pulses at 100 Hz) were recorded. A typical response in naïve culture is shown in Figure10A. However, in 8 of 17 cultures treated with picrotoxin, diminutive recurrent responses were elicited ∼250–1000 msec after the stimulation (Fig.10Ba). Considering that the epileptiform activities were more severely evoked in picrotoxin-treated slices (Fig.2Bb), the prolonged exposure to picrotoxin may have produced hyperexcitability in the hippocampus. The delayed epileptiform discharges were not evoked in the presence of 50 μm AP5 (Fig. 10Bb). No aberrations were seen in the 15 slices cultured in a combination of picrotoxin and nicardipine (Fig.10C).
Fig. 10.
Diminutive recurrent positive field potentials in picrotoxin-treated hippocampal slices. Responses evoked by brief repetitive stimulation of the stratum granulosum were recorded from the CA3 stratum pyramidale at 8 DIV in slices cultured in normal medium (A), medium containing 50 μmpicrotoxin (PTX) (Ba), or medium containing a combination of 50 μm picrotoxin and 10 μm nicardipine (PTX+Nic) (C). In picrotoxin-treated slices, continuous tiny epileptiform responses were recorded ∼250–1000 msec after the stimulation (arrows), which was blocked by a superfusion of 50 μm AP5. The stratum granulosum was stimulated at the time indicated by each of five dots under a trace.
DISCUSSION
Using organotypic cultures of hippocampal slices, I have shown here that mossy fiber development was severely disturbed after epileptiform activities and that the hippocampus consequently displayed abnormal neurotransmissions. These phenomena were prevented by chronic pharmacological blockade of L-type Ca2+channels.
Inhibition of mossy fiber development in epileptiform hippocampus
Incremental mossy fiber synaptic responses evoked in the CA3 region were depressed in the hippocampal slices cultured in the presence of picrotoxin. The effect of picrotoxin was dose dependent, which was in accordance with the dose–response profile of its burst-inducing effect. Also, the effect of picrotoxin was completely blocked by tetrodotoxin. Furthermore, all other convulsants used in this study hindered mossy fiber development. Therefore, it is likely that epileptiform activities inhibited mossy fiber growth. Picrotoxin had no influence on mossy fiber development when applied after 8 DIV, when the mossy fibers were almost established, suggesting that paroxysmal activities exert their inhibitory effects on developing mossy fibers but not on mature fibers. This idea is further supported by the result that picrotoxin did not affect synaptic responses of the Schaffer collaterals, which were fully generated at the time of hippocampal slice preparation.
Intracellular Ca2+ dynamics is assumed to be of functional importance for neuronal injury associated with epilepsy (Wasterlain et al., 1993). In the present study, pharmacological investigations revealed that Ca2+ influx through L-type Ca2+ channels may mediate the inhibition of mossy fiber formation under epileptiform conditions. Additionally, inefficiency of T-type Ca2+ channel blockers or NMDA receptor antagonists indicates a pivotal role for L-type Ca2+ channels. Although Ca2+ is generally essential for synaptogenesis (Basarsky et al., 1994), this finding suggests that Ca2+ can serve as a suppressant against synapse formation. This finding hence predicts the existence of an optimal range of [Ca2+]i for synaptogenesis, and the disorders seen under epileptiform conditions are probably accounted for by excessive elevation of [Ca2+]i.
Abnormal targeting of the mossy fibers in epileptiform hippocampus
On the basis of the results of Timm staining, the possible scheme for mossy fiber development in the epileptiform hippocampus is represented in Figure 11. The mossy fibers normally make synaptic contacts with the CA3 pyramidal cells in the stratum lucidum and with hilar neurons in the dentate hilus (Fig.11A). Under epileptiform conditions, however, the number of synapses in the stratum lucidum decreases, and the mossy fibers alternatively come into contact with their targets within the CA3 stratum oriens and the dentate molecular layer (Fig.11B). Administration of an L-type Ca2+ channel blocker improves the inhibited synapse formation in the stratum lucidum and prevents the aberrant synaptogenesis in the dentate molecular layer but not in the stratum oriens (Fig. 11C).
Fig. 11.

Schematic illustration of mossy fiber development in the epileptiform hippocampus. Thick black orgray lines indicate the normal mossy fibers or the aberrantly targeted fibers, respectively.A, B, Epileptiform activities suppress the formation of projections to the stratum lucidum that is the typical mossy fiber recipient layer (dashed line) and drive the mossy fibers ectopically to the CA3 stratum oriens and the molecular layer. C, These phenomena are partially prevented by L-type Ca2+ channel blockade.
Picrotoxin did not completely block the development of synaptic responses even at high concentrations of up to 150 μm. If epileptiform activities directly suppressed synaptogenesis, synapse formation would be entirely inhibited by severe seizure activities. The partial effect of picrotoxin implies a loss of mossy fiber targeting. Actually, in the picrotoxin-treated slices, Timm intensity decreased in the stratum lucidum and increased in the stratum oriens, and thus Timm values in the hippocampal subregion tended to be uniform. Therefore, my findings can be explained by lack of specificity for particular targets of the mossy fibers under paroxysmal conditions. The requirement of appropriate firing patterns for precise neural circuit formation has been argued extensively (Goodman and Shatz, 1993; Haydon and Drapeau, 1995). Gomez-Di Cesare et al. (1997) observed a unique pattern of firings from the CA3 pyramidal cells during the second postnatal week, when the mossy fibers predominantly develop. Such specific patterns of neuron activity are probably necessary for the mossy fibers to target their accurate destinations and to form synaptic contacts with them. Therefore, it is plausible that epileptiform activities distorted appropriate firing patterns and caused the mossy fibers to fail to innervate their accurate targets.
Interestingly, the allopatric distribution of the mossy fiber synapses found in this study are similar to the mossy fiber sprouting in human or experimental epileptic hippocampi, in which mossy fiber terminals are often detected in the molecular layer (Tauck and Nadler, 1985;Sutula et al., 1988; Babb et al., 1991; Cavazos et al., 1991) or in the CA3 stratum oriens (Van der Zee et al., 1995; Adams et al., 1997). Because the mossy fibers are newly generated even in adult brain, abnormal targeting of developing mossy fibers may contribute, in part, to mossy fiber sprouting in epilepsy.
It is widely accepted that adhesion molecules play a crucial role in axon guidance (Dodd and Jessell, 1988; Goodman, 1996; Klostermann and Bonhoeffer, 1996). Numerous reports indicate that intracellular Ca2+ modulates the expression and activity of adhesion molecules (Renkonen et al., 1990; Covault et al., 1991;Doherty et al., 1991; Saffell et al., 1992; Williams et al., 1992;Hailer et al., 1994). My findings can be discussed in the context of possible contributions from limbic system-associated membrane protein (LAMP) or neural cell adhesion molecule (NCAM). LAMP is an immunoglobulin superfamily member and a 64–68 kDa glycoprotein (Horton and Levitt, 1988; Zacco et al., 1990). Pimenta et al. (1995)reported that intraventricular administration of anti-LAMP antibody to early postnatal rats resulted in aberrant growth of mossy fiber projections. On the other hand, polysialic acid-incorporated NCAM is abundantly expressed in cells displaying developmental plasticity and the potential to change their morphology and migrate. Enzymatic ablation of the polysialic acid portions of NCAM causes abnormal development of the mossy fibers (Rivkin and Malouf, 1997; Seki and Rutishauser, 1998) and reduces the amplitude of mossy fiber synaptic responses recorded from the CA3 region (Muller et al., 1994). These reports are consistent with the details in the present study. Interestingly, Lyles et al. (1993) reported that expression of NCAM is altered by application of L-type Ca2+ channel blockers in chick myotube cultures. Therefore, Ca2+influx via L-type Ca2+ channels may modulate the activities of adhesion molecules involved in mossy fiber guidance.
Aberrant neurotransmission in epileptiform hippocampus
Consistent with the attenuation of CA3 field response in picrotoxin-treated cultures, optical voltage recording indicated that neurotransmission from the dentate gyrus to the CA3 region was severely disturbed in picrotoxin-treated hippocampal slices. However, the ectopic mossy fiber synapses in the stratum oriens had virtually normal physiological function because a typical dendritic response was elicited by dentate stimulation. It is suggested, therefore, that the depolarization induced by EPSP in the stratum oriens is not sufficient to produce nerve impulses in the pyramidal cell.
Picrotoxin-treated slices displayed prolonged excitation of the dentate gyrus and delayed epileptiform activation of CA3 neurons after dentate stimulation. These aberrant electrophysiological properties may be attributed to the direct recurrent excitatory inputs to the granule cells (Fig. 11B). That the CA3 delayed epileptiform activities were canceled by NMDA receptor blockers implies that NMDA receptor-mediated current was enhanced in the excitatory synapse transmission of the dentate gyrus. Indeed, several previous reports showed that the NMDA receptor-mediated component appears in dentate neurotransmission as a result of epilepsy (Mody and Heinmann, 1987; Urban et al., 1990). Therefore, the dentate granule cells that become highly excitable through excessive NMDA receptor activation may send asynchronous signals to the CA3 region and induce delayed activation of the CA3 neurons.
Regardless, it should be noted that the hippocampus exposed to paroxysmal conditions acquires an epileptogenic nature. This may form a cellular basis for chronic epilepsy. Other research indicates that adult complex partial seizures result from prolonged seizures early in life (Falconer, 1970; Sagar and Oxbury, 1987). In addition, my findings predict that the hippocampal physiological function may be altered after epilepsy and thereby may explain the factors that underlie the adverse prognoses of epilepsy. Furthermore, the L-type Ca2+ channel blocker, a conventional and classic medicine, is shown here to possess a novel efficiency to prevent abnormal growth of mossy fibers and the aberrant alteration of hippocampal properties without affecting ictal discharges and hence may ameliorate the problem of medically refractory childhood epilepsy. Thus, the organotypic slice culture used in the present study may provide a useful system for studying developmental cellular dynamics in a mammalian CNS.
Footnotes
I thank Dr. N. Matsuki and Dr. N. Nishiyama for their kind comments on the experiments, Mr. Brett Cox for his critical review of this manuscript, and Dr. Akira Nagayoshi and Miss Satoko Nakajima for their assistance with manuscript preparation.
Correspondence should be addressed to Dr. Yuji Ikegaya, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan.
REFERENCES
- 1.Adams B, Sazgar M, Osehobo P, Van der Zee CE, Diamond J, Fahnestock M, Racine RJ. Nerve growth factor accelerates seizure development, enhances mossy fiber sprouting, and attenuates seizure-induced decreases in neuronal density in the kindling model of epilepsy. J Neurosci. 1997;17:5288–5296. doi: 10.1523/JNEUROSCI.17-14-05288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpherts WC, Aldenkamp AP. Computerized neuropsychological assessment of cognitive functioning in children with epilepsy. Epilepsia. 1990;31:S35–40. doi: 10.1111/j.1528-1157.1990.tb05868.x. [DOI] [PubMed] [Google Scholar]
- 3.Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus. I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- 4.Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- 5.Basarsky TA, Parpura V, Haydon PG. Hippocampal synaptogenesis in cell culture: developmental time course of synapse formation, calcium influx, and synaptic protein distribution. J Neurosci. 1994;14:6402–6411. doi: 10.1523/JNEUROSCI.14-11-06402.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavazos JE, Golarai G, Sutula TP. Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J Neurosci. 1991;11:2795–2803. doi: 10.1523/JNEUROSCI.11-09-02795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covault J, Liu QY, el-Deeb S. Calcium-activated proteolysis of intracellular domains in the cell adhesion molecules NCAM and N-cadherin. Mol Brain Res. 1991;11:11–16. doi: 10.1016/0169-328x(91)90015-p. [DOI] [PubMed] [Google Scholar]
- 8.Danscher G, Zimmer J. An improved Timm sulphide silver method for light and electron microscopic localization of heavy metals in biological tissues. Histochemistry. 1978;55:27–40. doi: 10.1007/BF00496691. [DOI] [PubMed] [Google Scholar]
- 9.Dodd J, Jessell TM. Axon guidance and the patterning of neuronal projections in vertebrates. Science. 1988;242:692–699. doi: 10.1126/science.3055291. [DOI] [PubMed] [Google Scholar]
- 10.Doherty P, Ashton SV, Moore SE, Walsh FS. Morphoregulatory activities of NCAM and N-cadherin can be accounted for by G protein-dependent activation of L- and N-type neuronal Ca2+ channels. Cell. 1991;67:21–33. doi: 10.1016/0092-8674(91)90569-k. [DOI] [PubMed] [Google Scholar]
- 11.Eichenbaum H, Otto T, Cohen NJ. The hippocampus—what does it do? Behav Neural Biol. 1992;57:2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- 12.Falconer MA. The pathological substrate of temporal lobe epilepsy. Guy’s Hosp Rep. 1970;119:47–60. [PubMed] [Google Scholar]
- 13.Farwell JR, Dodrill CB, Batzel LW. Neuropsychological abilities of children with epilepsy. Epilepsia. 1985;26:395–400. doi: 10.1111/j.1528-1157.1985.tb05670.x. [DOI] [PubMed] [Google Scholar]
- 14.Gaarskjaer FB. The development of the dentate area and the hippocampal mossy fiber projection of the rat. J Comp Neurol. 1985;241:154–170. doi: 10.1002/cne.902410204. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Di Cesare CM, Smith KL, Rice FL, Swann JW. Axonal remodeling during postnatal maturation of CA3 hippocampal pyramidal. J Comp Neurol. 1997;384:165–180. [PubMed] [Google Scholar]
- 16.Goodman CS. Mechanisms and molecules that control growth cone guidance. Annu Rev Neurosci. 1996;19:341–377. doi: 10.1146/annurev.ne.19.030196.002013. [DOI] [PubMed] [Google Scholar]
- 17.Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;72:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- 18.Greene JG, Greenamyre JT. Bioenergetics and glutamate excitotoxicity. Prog Neurobiol. 1996;48:613–634. doi: 10.1016/0301-0082(96)00006-8. [DOI] [PubMed] [Google Scholar]
- 19.Hailer NP, Blaheta RA, Harder S, Scholz M, Encke A, Markus BH. Modulation of adhesion molecule expression on endothelial cells by verapamil and other Ca++ channel blockers. Immunobiology. 1994;191:38–51. doi: 10.1016/S0171-2985(11)80266-4. [DOI] [PubMed] [Google Scholar]
- 20.Horton HL, Levitt P. A unique membrane protein is expressed on early developing limbic system axons and cortical targets. J Neurosci. 1988;8:4653–4661. doi: 10.1523/JNEUROSCI.08-12-04653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haydon PG, Drapeau P. From contact to connection: early events during synaptogenesis. Trends Neurosci. 1995;18:196–201. doi: 10.1016/0166-2236(95)93901-9. [DOI] [PubMed] [Google Scholar]
- 22.Ikegaya Y, Yoshida M, Saito H, Nishiyama N. Epileptic activity prevents synapse formation of hippocampal mossy fibers via L-type calcium channel activation in vitro. J Pharmacol Exp Ther. 1997;280:471–476. [PubMed] [Google Scholar]
- 23.Ikonomidou C, Turski L. Excitotoxicity and neurodegenerative diseases. Curr Opin Neurol. 1995;8:487–497. doi: 10.1097/00019052-199512000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Jiang M, Lee CL, Smith KL, Swann J. Spine loss and other persistent alternations of hippocampal pyramidal call dendrites in a model of early-onset epilepsy. J Neurosci. 1998;18:8356–8368. doi: 10.1523/JNEUROSCI.18-20-08356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klostermann S, Bonhoeffer F. Investigations of signaling pathways in axon growth and guidance. Perspect Dev Neurobiol. 1996;4:237–252. [PubMed] [Google Scholar]
- 26.Lyles JM, Amin W, Bock E, Weill CL. Regulation of NCAM by growth factors in serum-free myotube cultures. J Neurosci Res. 1993;34:273–286. doi: 10.1002/jnr.490340304. [DOI] [PubMed] [Google Scholar]
- 27.Mizrahi EM. Seizure disorders in children. Curr Opin Pediatr. 1994;6:642–646. doi: 10.1097/00008480-199412000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Mody I, Heinmann U. NMDA receptors of dentate gyrus granule cells participate in synaptic transmission following kindling. Nature. 1987;326:701–704. doi: 10.1038/326701a0. [DOI] [PubMed] [Google Scholar]
- 29.Muller D, Buchs PA, Stoppini L. Time course of synaptic development in hippocampal organotypic cultures. Dev Brain Res. 1993;71:93–100. doi: 10.1016/0165-3806(93)90109-n. [DOI] [PubMed] [Google Scholar]
- 30.Muller D, Stoppini L, Wang C, Kiss JZ. A role for polysialylated neural cell adhesion molecule in lesion-induced sprouting in hippocampal organotypic cultures. Neuroscience. 1994;61:441–445. doi: 10.1016/0306-4522(94)90424-3. [DOI] [PubMed] [Google Scholar]
- 31.Nakagami Y, Saito H, Matsuki N. Basic fibroblast growth factor and brain-derived neurotrophic factor promote survival and neuronal circuit formation in organotypic hippocampus culture. Jpn J Pharmacol. 1997;75:319–326. doi: 10.1254/jjp.75.319. [DOI] [PubMed] [Google Scholar]
- 32.Pimenta AF, Zhukareva V, Barbe MF, Reinoso BS, Grimley C, Henzel W, Fischer I, Levitt P. The limbic system-associated membrane protein is an Ig superfamily member that mediates selective neuronal growth and axon targeting. Neuron. 1995;15:287–297. doi: 10.1016/0896-6273(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 33.Renkonen R, Mennander A, Ustinov J, Mattila P. Activation of protein kinase C is crucial in the regulation of ICAM-1 expression on endothelial cells by interferon-gamma. Int Immunol. 1990;2:719–724. doi: 10.1093/intimm/2.8.719. [DOI] [PubMed] [Google Scholar]
- 34.Rivkin A, Malouf AT. PSA-NCAM and mossy fiber development in hippocampal slice cultures. Soc Neurosci Abstr. 1997;23:767.8. [Google Scholar]
- 35.Rodin EA, Schmaltz S, Twitty G. Intellectual functions of patients with childhood-onset epilepsy. Dev Med Child Neurol. 1986;28:25–33. doi: 10.1111/j.1469-8749.1986.tb03826.x. [DOI] [PubMed] [Google Scholar]
- 36.Sagar HJ, Oxbury JM. Hippocampal neuron loss in temporal lobe epilepsy: correlation with early childhood convulsions. Ann Neurol. 1987;22:334–340. doi: 10.1002/ana.410220309. [DOI] [PubMed] [Google Scholar]
- 37.Saffell JL, Walsh FS, Doherty P. Direct activation of second messenger pathways mimics cell adhesion molecule-dependent neurite outgrowth. J Cell Biol. 1992;118:663–670. doi: 10.1083/jcb.118.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartzkroin PA. Role of the hippocampus in epilepsy. Hippocampus. 1994;4:239–242. doi: 10.1002/hipo.450040302. [DOI] [PubMed] [Google Scholar]
- 39.Seki T, Rutishauser U. Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J Neurosci. 1998;18:3757–3766. doi: 10.1523/JNEUROSCI.18-10-03757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sloviter RS. A selective loss of hippocampal mossy fiber Timm stain accompanies granule cell seizure activity induced by perforant path stimulation. Brain Res. 1985;18:150–153. doi: 10.1016/0006-8993(85)90017-4. [DOI] [PubMed] [Google Scholar]
- 41.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 42.Stirling RV, Bliss TV. Hippocampal mossy fiber development at the ultrastructural level. Prog Brain Res. 1978;48:191–198. doi: 10.1016/S0079-6123(08)61023-7. [DOI] [PubMed] [Google Scholar]
- 43.Stores G. Cognitive function in children with epilepsy. Dev Med Child Neurol. 1971;13:390–393. doi: 10.1111/j.1469-8749.1971.tb03280.x. [DOI] [PubMed] [Google Scholar]
- 44.Strasser U, Fischer G. Quantitative measurement of neuronal degeneration in organotypic hippocampal cultures after combined oxygen/glucose deprivation. J Neurosci Methods. 1995;57:177–186. doi: 10.1016/0165-0270(94)00147-9. [DOI] [PubMed] [Google Scholar]
- 45.Sutula T, He XX, Cavazos J, Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239:1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- 46.Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urban L, Aitken PG, Friedman A, Somjen GG. An NMDA-mediated component of excitatory synaptic input to dentate gyrus granule cells in “epileptic” human hippocampus studies in vitro. Brain Res. 1991;515:319–322. doi: 10.1016/0006-8993(90)90615-i. [DOI] [PubMed] [Google Scholar]
- 48.Van der Zee CE, Rashid K, Le K, Moore KA, Stanisz J, Diamond J, Racine RJ, Fahnestock M. Intraventricular administration of antibodies to nerve growth factor retards kindling and blocks mossy fiber sprouting in adult rats. J Neurosci. 1995;15:5316–5323. doi: 10.1523/JNEUROSCI.15-07-05316.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wasterlain CG, Fujikawa DG, Penix L, Sankar R. Pathophysiological mechanisms of brain damage from status epilepticus. Epilepsia. 1993;34:S37–S53. doi: 10.1111/j.1528-1157.1993.tb05905.x. [DOI] [PubMed] [Google Scholar]
- 50.Williams EJ, Doherty P, Turner G, Reid RA, Hemperly JJ, Walsh FS. Calcium influx into neurons can solely account for cell contact-dependent neurite outgrowth stimulated by transfected L1. J Cell Biol. 1992;119:883–892. doi: 10.1083/jcb.119.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zacco A, Cooper V, Chantler PD, Fisher-Hyland S, Horton HL, Levitt P. Isolation, biochemical characterization and ultrastructural analysis of the limbic system-associated membrane protein (LAMP), a protein expressed by neurons comprising functional neural circuits. J Neurosci. 1990;10:73–90. doi: 10.1523/JNEUROSCI.10-01-00073.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zola-Morgan S, Squire LR. Neuroanatomy of memory. Annu Rev Neurosci. 1993;16:547–563. doi: 10.1146/annurev.ne.16.030193.002555. [DOI] [PubMed] [Google Scholar]