Abstract
Hormones and neurotransmitters have both short-term and long-term modulatory effects on the activity of voltage-gated Ca2+ channels. Although much is known about the signal transduction underlying short-term modulation, there is far less information on mechanisms that produce long-term effects. Here, the molecular basis of long-lasting suppression of Ca2+channel current in pituitary melanotropes by chronic dopamine exposure is examined. Experiments involving in vivo and in vitro treatments with the dopaminergic drugs haloperidol, bromocriptine, and quinpirole show that D2 receptors persistently decrease α1D L-type Ca2+ channel mRNA and L-type Ca2+ channel current without altering channel gating properties. In contrast, another L-channel (α1C) mRNA and P/Q-channel (α1A) mRNA are unaffected. The downregulation of α1D mRNA does not require decreases in cAMP levels or P/Q-channel activity. However, it is mimicked and occluded by inhibition of L-type channels. Thus, interruption of the positive feedback between L-type Ca2+ channel activity and α1D gene expression can account for the long-lasting regulation of L-current produced by chronic activation of D2 dopamine receptors.
Keywords: L-type Ca2+ channel, dopamine, D2 receptor, melanotrope, nimodipine, haloperidol, quinpirole
The activity of voltage-gated Ca2+ channels in neurons can be regulated on a variety of time scales. Much is known about short-term (seconds to minutes) regulation in which acute application of hormones or neurotransmitters triggers transient modulation of Ca2+ channels via G-proteins or phosphorylation (Catterall, 1997). In contrast, little is known about mechanisms by which chronic exposure to hormones or neurotransmitters may produce long-term (hours to days) changes in Ca2+ channel activity. This latter type of regulation is likely to play a role in long-lasting forms of neuroplasticity in physiological and pathological processes. For example, in schizophrenics, chronic therapy with drugs that alter dopaminergic neurotransmission is required to alleviate psychotic symptoms. This chronic therapy leads to changes in the electrical activity of midbrain dopamine neurons of animal models (Grace et al., 1997). These changes in electrical activity are likely the result of long-term regulation of ion channels. Cell heterogeneity and synaptic complexity in the brain complicate the study of long-term Ca2+ channel regulation. However, a long-term effect of dopamine on Ca2+ channels has been identified in the relatively simple system of the pituitary intermediate lobe (IL).
Rat IL is ideal for the study of regulation of Ca2+channels by a dopaminergic synaptic input. The IL contains only one type of excitable cell, the melanotrope (Millington and Chronwall, 1989). Melanotropes secrete peptides derived from the precursor pro-opiomelanocortin (POMC). They are predominantly controlled by direct synapses from hypothalamic neurons that tonically inhibit peptide secretion by activating D2-like dopamine (D2) receptors (for review, see Millington and Chronwall, 1989). One of the effects of chronic D2 receptor activation is a long-lasting (i.e., for days) suppression of total high-voltage-activated (HVA) Ca2+ current in melanotropes (Cota and Hiriart, 1989). To date, in vivo studies of this current suppression have been performed using neonatal, but not adult, melanotropes (Gomora et al., 1996). A similar phenomenon is observed in lactotrophs in vitro (Lledo et al., 1991). Long-lasting suppression of Ca2+ current likely plays a significant role in dopamine inhibition of hormone release because exocytosis is dependent on Ca2+ influx raised to the third power in both of these pituitary cell types (Thomas et al., 1990; Fomina and Levitan, 1995).
The mechanism of suppression of melanotrope HVA Ca2+current by chronic D2 receptor activation is unknown. However, the effect is mimicked by transcription and translation inhibitors (Cota and Hiriart, 1989; Gomora et al., 1996) or application of antisense oligonucleotides directed against c-fos mRNA (Chronwall et al., 1995). These observations suggest the involvement of gene expression regulation. Melanotrope D2 receptors cause a decrease in adenylyl cyclase activity, leading to a reduction in cAMP levels (Meunier and Labrie, 1982). The cAMP pathway has been shown to be involved in regulation of numerous genes (e.g., c-fos, neurotensin, POMC, prolactin) by dopaminergic drugs (Maurer, 1981; Cote et al., 1986; Adams et al., 1997). In addition, D2 receptors induce inhibition of spontaneous action potential firing in melanotropes (Douglas and Taraskevich, 1978), leading to a reduction in Ca2+ influx through voltage-gated Ca2+ channels. A decrease in Ca2+influx contributes to D2 receptor-induced downregulation of prolactin (Elsholtz et al., 1991) and likely also POMC (Loeffler et al., 1988). Thus, D2 receptors may regulate melanotrope gene expression by lowering both cAMP and Ca2+ influx.
These data suggested that the rat IL would provide a unique opportunity to study the hypothesis that ongoing physiological release of a neurotransmitter at the synapse regulates Ca2+channel gene expression. At one time, it was assumed that dopamine targets L-channels (e.g., Chronwall et al., 1995). However, Ciranna et al. (1996) recently found that melanotropes express an HVA Ca2+ current that consists of P/Q-type Ca2+ currents (∼60%), as well as L-type Ca2+ current (∼40%). The pore-forming and voltage-sensing α1 subunits of these HVA Ca2+ channels are encoded by the genes α1C and α1D (L-channels) and α1A (P/Q-channels) (Birnbaumer et al., 1994). Thus, the present study tests (1) whether chronic D2 receptor activation downregulates α1A, α1C, or α1D mRNA in adult rat melanotropes both in vivo and in vitro; (2) whether the corresponding type of HVA current is subject to long-lasting suppression; and (3) whether the D2 receptor effect is mediated by decreases in cAMP and Ca2+ influx. Our results indicate that dopamine acts via inhibition of L-channel activity (but not a reduction in P/Q-channel activity or cAMP levels) to specifically downregulate the α1D L-channel gene.
MATERIALS AND METHODS
In vivo drug treatments. Drugs (Research Biochemicals, Natick, MA) or vehicle were injected intraperitoneally into female Sprague Dawley rats (200–225 gm; Charles River Laboratories, Wilmington, MA). Haloperidol (5 mg/ml) or bromocriptine (2 mg/ml) were dissolved in a vehicle of 20 mm tartaric acid and 10% EtOH and injected at 5 mg/kg. Each treatment group included three to four animals. Animals were killed by metofane inhalation anesthesia or CO2 exposure, followed by decapitation. Neurointermediate lobes (NILs) were dissected out and immediately frozen on dry ice. The NILs from all animals within a treatment group were pooled. Thus, n refers to the number of independent experiments performed, not the number of animals used.
RNA isolation and analysis. Total RNA was isolated from frozen NILs or cultured cells by the acid guanidinium thiocyanate–phenol–chloroform extraction method of Chomczynski and Sacchi (1987). Yeast RNA (50 μg) was added during the isolation procedure to serve as a carrier. Frozen NILs were homogenized by repeated passes through an 18 gauge needle. mRNA levels were analyzed by RNase protection assay as described previously (Takimoto et al., 1993). Samples were subject to overnight solution hybridization at 50°C with 105 (β-actin) or 106 (all others) cpm of 32P-labeled RNA probes. Antisense RNA probes were made by in vitrotranscription of the following templates: α1D, plasmid rbDHE470 (Fomina et al., 1996), linearized withHindIII, transcribed with T7 polymerase; α1C, plasmid rbCES337 [equivalent to plasmid “5′ rbC” (Lievano et al., 1994)], linearized with DraI, transcribed with T7 polymerase; α1A, plasmid rbANP550 [consisting of nucleotides 178–684 of the rat α1A gene subcloned into pBluescript KS (Stratagene, La Jolla, CA)], linearized with XbaI, transcribed with T3 polymerase; β-actin, plasmid pTRI-β-actin-125-rat (Ambion, Austin, TX), transcribed with T7 polymerase; and cyclophilin, plasmid CycPA100 (Fomina et al., 1996), linearized with HindIII, transcribed with T7 polymerase. For presentation, the air-dried gels were exposed to x-ray film with an intensifying screen for ∼15 hr at −80°C. For quantitation, the gels were exposed to phosphor screens for 1–3 hr, and the density of bands corresponding to target mRNAs was measured by analysis in a phosphorimager (Molecular Dynamics, Sunnyvale, CA). To control for variation in the amount of sample loaded into each gel lane, Ca2+ channel mRNA levels have been normalized to β-actin or cyclophilin mRNA, except in the case in which comparisons are made between control and 8-Bromo-cAMP (Br-cAMP)-treated melanotropes (see Fig. 4), because it was clear that Br-cAMP regulated β-actin mRNA levels. It is unlikely that Ca2+ channel mRNAs found in our samples came from fibroblasts and glial-like cells of the NIL, because these cells do not express L-, P-, and Q-type voltage-gated Ca2+channels (Beatty et al., 1996).
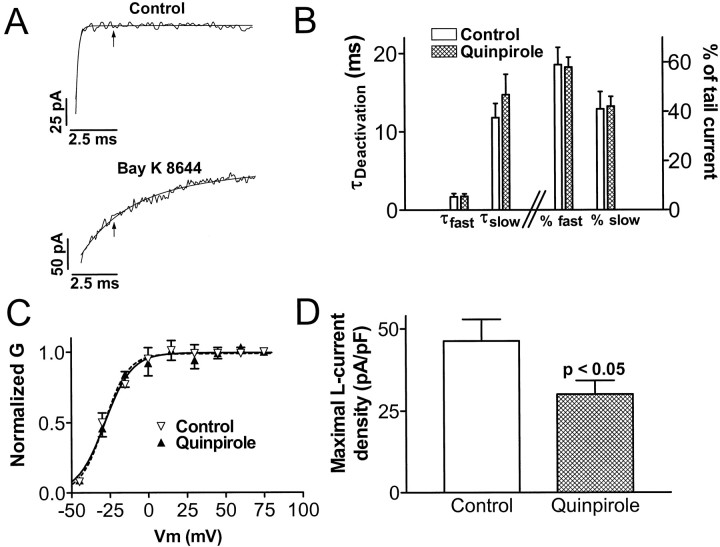
Fig. 4.
Chronic quinpirole treatment in vitro induces a long-lasting suppression of L-type Ca2+ channel current density without changing its functional properties. A, Illustration of the method of isolating L-channel tail current from total Ca2+channel current in melanotropes (see Results for details). Noisy traces are 10 msec portions of tail currents recorded at −50 mV after a step depolarization to +75 mV. The smooth traces are exponential curves fit to the currents. The time constants are 0.16 msec (monoexponential curve in top) and 2.5 and 21.9 msec (biexponential curve in bottom). Thearrows are placed at 2.4 msec after repolarization to −50 mV. B, Chronic quinpirole treatment does not alter L-channel deactivation properties. Data were obtained with biexponential curve-fitting analysis of Bay K 8644-slowed tail currents in control and quinpirole-treated cells. Bars on theleft and right halves of the graph correspond to the left and right y-axes, respectively.C, Chronic quinpirole treatment does not alter the voltage-dependence of activation of L-channels. Normalized conductance (G) versus step depolarization potential (Vm) data for control and quinpirole-treated cells are fitted with Boltzmann equations (smooth curves). D, Maximal L-current density in melanotropes cultured for 6–10 d in control or quinpirole-containing media. Maximal L-current density values were 46.3 ± 6.6 pA/pF in control cells and 30.1 ± 4.1 pA/pF in quinpirole-treated cells. Data in B–D come from 9 control cells and 13 quinpirole-treated cells.
Primary culture of rat melanotropes. NILs were dissected out of male or female Sprague Dawley rats (200–225 gm, from Hilltop or Charles River) and dissociated into individual cells by either sequential digestion with trypsin and viokase (for current recordings only; Fomina and Levitan, 1995) or collagenase and trypsin (for current recordings or RNA isolation; Mains and Eipper, 1979). For current recordings, cells were plated onto poly-lysine (Sigma, St. Louis, MO)-coated glass coverslips in 35 mm culture dishes or protamine (Sigma)- and Nu-Serum IV (Becton Dickinson Labware, Bedford, MA)-treated 35 mm culture dishes at a density of 0.5 NILs per dish in Roswell Park Memorial Institute 1640 medium with 10% FBS or DMEM with 10% FBS (Life Technologies, Gaithersburg, MD). For RNA isolation, cells were plated onto protamine- and Nu-Serum IV-coated four-well plates (15 mm well; Nunc, Naperville, IL) at a density of 3.5 NILs per well in DMEM with 10% FBS. The dishes were kept in a 5% CO2 incubator at 37°C. In both cases, the medium was changed every 2 d. Quinpirole (Research Biochemicals) was added to medium from aliquoted 5 mm stock solutions in H2O or PBS. Other drug stock solutions were as follows: nimodipine (5 mm in EtOH; Research Biochemicals); ω-agatoxin IVA (100 μm in H2O; generous gift from Dr. Nicholas A. Saccomano, Pfizer, Groton, CT); and ω-conotoxin MVIIC (100 μm in H2O; Peptides International, Louisville, KY).
Electrophysiology. Recordings were made by standard whole-cell patch-clamp methodology (Hamill et al., 1981) using an Axopatch 200A amplifier with PCLAMP6 software (Axon Instruments, Foster City, CA) or an EPC9 amplifier with PULSE software (Heka Elektronik, Lambrecht/Pfalz, Germany). Leak subtraction was performed by p/5 protocols contained in the software. Sixty percent series resistance compensation was used. Electrodes (model 7052; Garner Glass, Claremont, CA) were filled with a solution containing (in mm): 130 CsCl, 2 MgCl2, 1 CaCl2, 10 EGTA, 10 HEPES, 2.5 Na2ATP, and 0.05 GTP, pH 7.4. The bath solution used during recordings contained (in mm): 10 BaCl2, 140 TEACl, and 10 HEPES, pH 7.4. Except where indicated, both solutions contained the specific L-channel activator Bay K 8644 (Research Biochemicals) at 1 μm (from a 5 mm stock in EtOH). Currents were recorded within 1.5 min after achieving the whole-cell configuration of the patch-clamp electrode to avoid errors in estimation of current density caused by rundown. In experiments involving melanotropes that had been cultured in the presence of quinpirole, recordings were made from cells that had been washed five times in agonist-free saline 1–3 hr earlier to allow reversal of the acute effects of D2 receptor activation (Cota and Hiriart, 1989).
Data analysis. Comparisons between two groups were performed using Student’s t tests. The layered Bonferroni test was applied when multiple comparisons were required. Data are expressed as mean ± SEM.
RESULTS
Dopamine tonically downregulates α1D L-channel mRNA in pituitary NIL in vivo
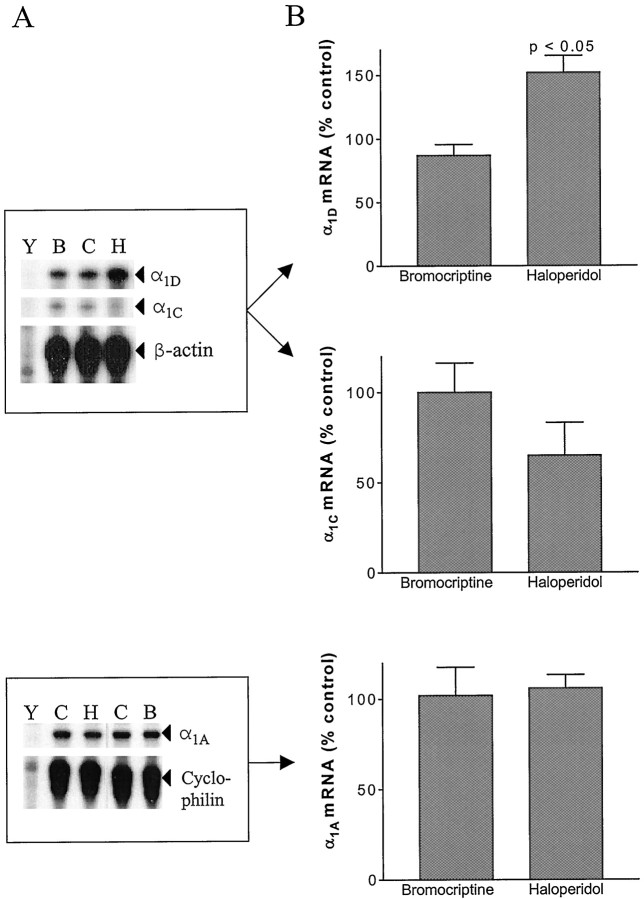
We initially sought to determine whether tonic D2 receptor activation in vivo regulates expression of α1C, α1D, and α1A mRNAs in the rat pituitary NIL. RNase protection assays were used to quantitate NIL Ca2+ channel mRNA levels. Consistent with the greater relative size of P/Q-current versus L-current in melanotropes (Ciranna et al., 1996), the intensity of the mRNA signals on our autoradiographs followed the order of α1A > α1D > α1C. Because normal dopamine regulation of the IL is tonic, we hypothesized that chronic treatment with D2 receptor antagonists would produce an elevation in Ca2+ channel mRNA levels by eliminating a tonic downregulation. Indeed, Figure1A shows that 6 hr treatment with haloperidol (an antagonist used clinically as an antipsychotic agent) produced an ∼50% elevation in α1DmRNA. In contrast, 6 hr treatment with bromocriptine (an agonist) produced no statistically significant change. α1C mRNA levels were not changed by these dopaminergic drugs (Fig.1B), indicating that the two L-channel mRNAs may be regulated by different mechanisms. Moreover, α1A mRNA levels were also unchanged (Fig. 1C). Longer treatments (30 hr or 7 d) with haloperidol produced similar increases in α1D mRNA (data not shown), indicating that the maximal long-term effect of haloperidol treatment on L-channel mRNA is achieved by 6 hr. These data suggest that α1D mRNA in melanotropes is continuously suppressed in vivo by the tonic activation of D2 receptors by endogenous dopamine.
Fig. 1.
Haloperidol treatment in vivo elevates NIL α1D mRNA but not α1C or α1A mRNAs. A, Representative autoradiographs of RNase protection assays designed to measure α1D and α1C (top) or α1A (bottom) mRNAs, along with β-actin or cyclophilin mRNA for normalization. Gel lanes contain the following:Y, yeast RNA (50 μg); B,C, and H, total NIL RNA from bromocriptine, vehicle, and haloperidol treatment groups, respectively.B, Effect of 6 hr in vivo dopaminergic drug treatment on α1D (top;n = 6), α1C (middle;n = 3), and α1A(bottom; n = 4) mRNA levels. Quantitation was performed by phosphorimager analysis using short (1–3 hr) gel exposures. Note that only the effect of haloperidol on α1D mRNA was statistically significant.
Chronic D2 receptor activation downregulates melanotrope α1D mRNA in vitro
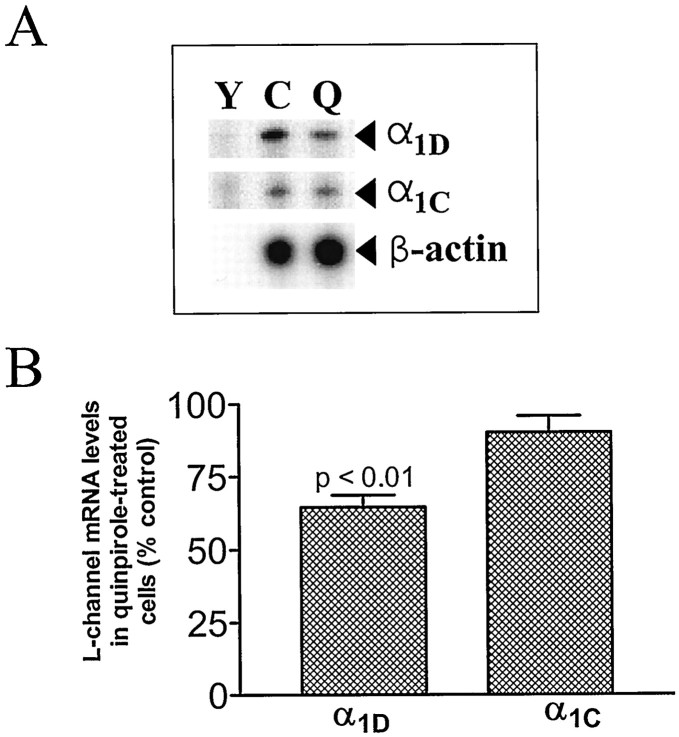
In vivo treatments with haloperidol should alter neurotransmission in all neuronal circuits that use D2 receptors, and so the effect on melanotrope α1D mRNA could, in principle, be caused by a polysynaptic indirect effect. To determine whether the effect of haloperidol is attributable to a direct action on melanotrope D2 receptors, we turned to melanotrope primary cultures. These cultures do not retain the dopaminergic neurons that innervate the IL. Thus, it is necessary to add agonists to test for effects of D2 receptor activation on Ca2+ channel gene expression. We found that 4 d treatment with the D2 receptor agonist quinpirole (1 μm), initiated immediately after plating the cultures, produced an ∼35% decrease in α1D mRNA (Fig. 2). Shorter treatments (such as 24 hr of drug after 3 d in culture) produced smaller decreases (data not shown). As was the case in vivo, α1CmRNA was not regulated. These in vitro data indicate that the specific upregulation of α1D mRNA produced by haloperidol treatment in vivo can be attributed to a direct action on melanotrope D2 receptors.
Fig. 2.
Chronic quinpirole treatment in vitro downregulates melanotrope α1D mRNA but not α1C mRNA. A, Representative autoradiograph of an RNase protection assay measuring α1D and α1C mRNAs. Gel lanes contain the following:Y, yeast RNA (50 μg); C andQ, total RNA from melanotropes cultured in control media or media with quinpirole (1 μm), respectively.B, Effect of 4 d quinpirole treatment on α1D (n = 15) and α1C(n = 14) mRNA levels.
Chronic D2 receptor activation induces a long-lasting suppression of L-type Ca2+ channel current in melanotropes
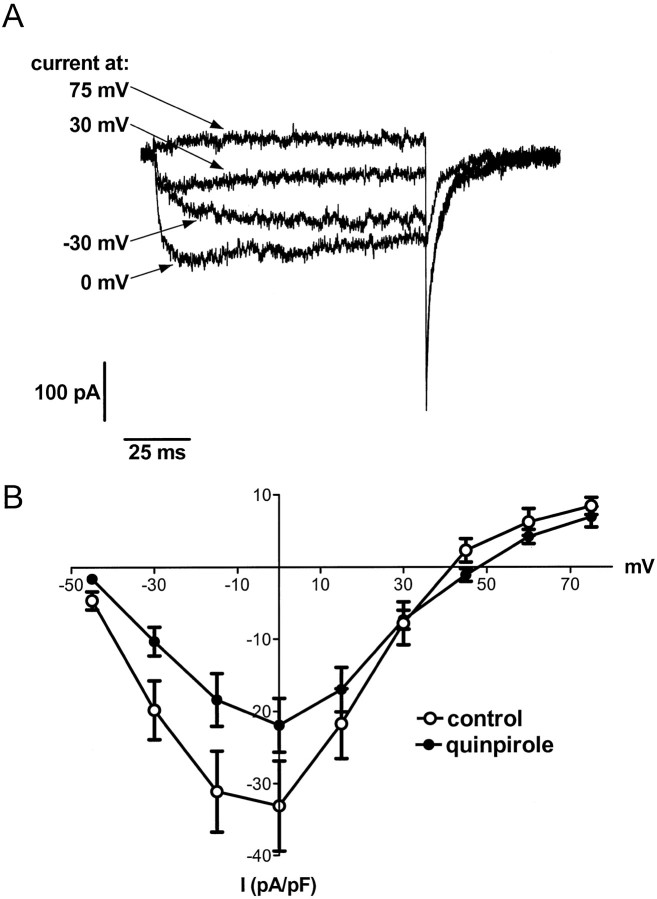
The observation that quinpirole treatment downregulated α1D mRNA suggested that chronic D2 receptor activation would also produce a persistent decrease in the corresponding L-type Ca2+ current in melanotropes. To test this idea, we initially recorded total Ca2+ channel currents from melanotropes cultured for 5–10 d in the absence or presence of quinpirole. Figure 3A shows examples of Ca2+ channel currents evoked by 100 msec depolarizations from a holding potential of −50 mV in a control cell. The peak currents during the depolarization (normalized to membrane capacitance) versus voltage are plotted in Figure 3B. Total Ca2+ channel currents in quinpirole-treated cells were smaller at all voltages, although quinpirole-containing media had been replaced with drug-free saline 1–3 hr before the recordings. This could reflect long-lasting decreases in the levels of any of several types of Ca2+ currents expressed in melanotropes (Cota and Hiriart, 1989; Ciranna et al., 1996).
Fig. 3.
Chronic quinpirole treatment in vitro induces a long-lasting suppression of total Ca2+ channel currents in melanotropes.A, Currents recorded in the presence of 1 μm Bay K 8644 using 100 msec depolarizations from a holding potential of −50 mV to several potentials from a representative control cell. B, Plot of peak currents (normalized to membrane capacitance) recorded during 100 msec depolarizations to various potentials versus voltage in melanotropes cultured in the absence or presence of quinpirole for 5–10 d. Data inA and B were obtained from melanotropes dissociated by trypsin–viokase digestion (see Materials and Methods).
To isolate L-current from other types of Ca2+current in melanotropes (Fig.4A), tail currents were recorded at −50 mV after 100 msec depolarizations from a holding potential of −50 mV in the absence and presence of Bay K 8644 (1 μm). This relatively depolarized holding potential inactivated low-voltage-activated Ca2+ channels that can produce slowly decaying components of the tail current. The resulting tail current recorded in the absence of Bay K 8644 (Fig.4A, top) is monoexponential, with a rapid deactivation rate. At 2.4 msec after repolarization to −50 mV (Fig. 4A, arrow), the current has returned to baseline, and thus all L-, P-, and Q-type Ca2+channels have deactivated. The presence of Bay K 8644 induces two distinct slow components of the tail current (Fig.4A, bottom). These two components are similar to two Bay K 8644-revealed components of L-current in GH3 clonal pituitary cells that arise from the ability of L-channels to access multiple open states (Fass and Levitan, 1996a,b). Because the two components are induced by the specific slowing of deactivation of L-current, the 2.4 msec time point (Fig.4A, arrow) reflects only the activity of L-channels (i.e., L-channels are active, but P- and Q-channels are completely deactivated at 2.4 msec into the tail current). Therefore, we used the amplitude at 2.4 msec into the tail current recorded in the presence of Bay K 8644 as a specific measure of L-current.
Using this L-current isolation method, we measured L-currents in melanotropes maintained in culture for 6–10 d in the absence or presence of quinpirole (1 μm). Initially, the effect of chronic quinpirole treatment on the functional properties of L-channels was assessed. First, we analyzed the rate of deactivation of melanotrope L-type Ca2+ currents (Fig.4B). The two time constants (“fast” and “slow”) obtained from biexponential curve fits were similar in control and quinpirole-treated melanotropes. Also, the proportion of the tail current made up by each component was not changed. Second, we analyzed the voltage-dependence of activation of L-currents (Fig.4C). L-currents isolated in tail currents recorded after step depolarizations over a range of potentials were normalized to their maximal amplitude to obtain the plot of normalized conductance (G) versus step depolarization voltage (V). Data points were fit with Boltzmann equations to determine activation parameters. In control and quinpirole-treated cells, L-currents were half-maximally activated by −28 ± 1 and −29 ± 1 mV; the slope factors were 8.9 ± 1.0 and 7.8 ± 0.9, respectively. Thus, the voltage-dependence of activation of L-currents was not altered by chronic quinpirole treatment. In contrast, acute application of D2 receptor agonists does induce a 5 mV rightward shift in the voltage-dependence of activation of total Ca2+ currents in melanotropes (Cota and Hiriart, 1989). Thus, by the measures of deactivation kinetics (Fig.4B) and voltage-dependence of activation (Fig.4C), chronic quinpirole treatment does not persistently alter melanotrope L-channel gating properties.
Finally, we measured the effect of chronic quinpirole treatment on maximal L-channel tail current amplitude. Average membrane capacitance was identical in control and quinpirole-treated melanotropes (6.6 ± 1.2 and 6.7 ± 0.9 pF, respectively), suggesting that chronic D2 receptor activation did not alter melanotrope cell surface area. Therefore, we normalized isolated maximal L-channel tail currents to membrane capacitance to obtain maximal L-current density. Figure4D shows that 6–10 d quinpirole treatment suppresses maximal L-current density by ∼35%. Thus, chronic D2 receptor activation induces a decrease in melanotrope L-current density that persists long after agonist removal. This decrease is consistent with the downregulation of α1D mRNA. Together with the lack of change in L-channel gating properties, these data suggest that a decrease in the number of L-channels underlies the suppression of L-current density by chronic D2 receptor activation.
Blockade of L-channel activity mimics and occludes downregulation of α1D mRNA by chronic D2 receptor activation
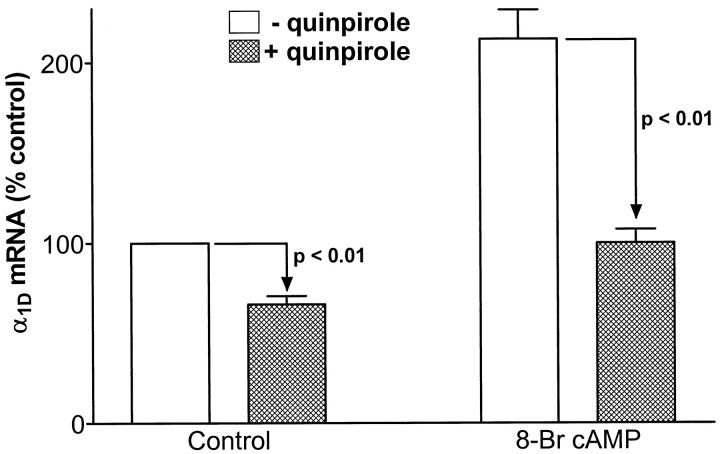
We next sought to determine the signaling mechanism underlying D2 receptor-mediated downregulation of α1D mRNA. Initially, the role of the decrease in cAMP produced by melanotrope D2 receptors (Meunier and Labrie, 1982) was tested. First, cAMP levels were elevated by application of the membrane-permeant nonhydrolyzable analog Br-cAMP. Then, the effectiveness of 4 d quinpirole treatment was assessed in the absence and presence of Br-cAMP. To show that our manipulation of cAMP levels with Br-cAMP was sufficient to alter D2 receptor-mediated gene regulation, we measured POMC mRNA, which is regulated in part via changes in cAMP levels (Loeffler et al., 1988). In confirmation, quinpirole downregulation of POMC was significantly reversed by 1 mm Br-cAMP (n = 3;p < 0.01); quinpirole reduced POMC mRNA levels to 39 ± 2 and 64 ± 2% of control in the absence and presence of Br-cAMP, respectively. Figure 5 shows that Br-cAMP produces a approximately twofold increase in α1D mRNA (p < 0.01). This suggests that cAMP does play some role in the regulation of α1D mRNA. However, quinpirole-induced downregulation of α1D mRNA was actually slightly enhanced by the presence of Br-cAMP (although this was not statistically significant). Thus, the clear failure of Br-cAMP to reduce the effect of quinpirole suggests that decreases in cAMP levels likely do not mediate downregulation of α1D mRNA by chronic activation of D2 receptors.
Fig. 5.
Br-cAMP fails to prevent dopaminergic downregulation of α1D mRNA. Effect of 4 d treatment with 1 μm quinpirole on α1D mRNA levels in melanotropes cultured in the absence (control) or presence of 1 mm Br-cAMP (n = 3). Note that Br-cAMP had no statistically significant effect on quinpirole-induced downregulation of α1D mRNA (downregulation in the absence and presence of Br-cAMP was 34 ± 5 and 47 ± 4%, respectively; p > 0.05 for the comparison between these two percentage downregulation values).
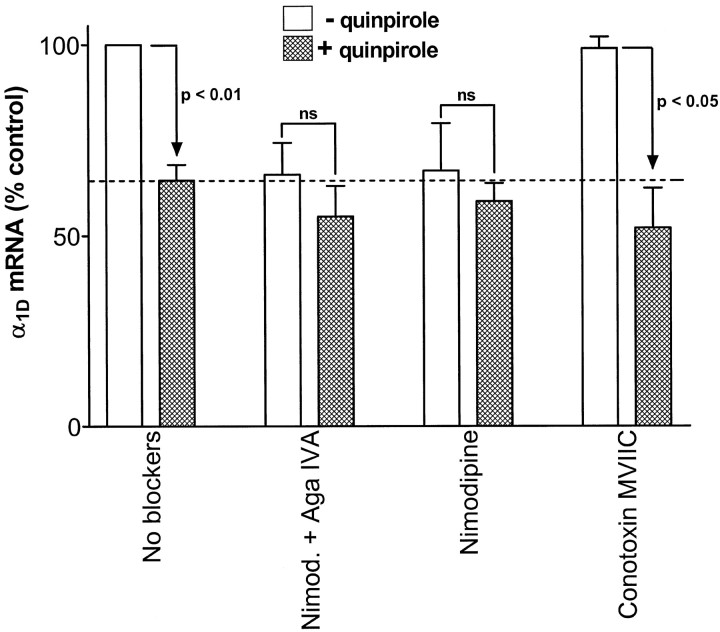
Next, we assessed the role of the inhibition of Ca2+channel activity in quinpirole downregulation of α1DmRNA. Melanotrope HVA Ca2+ channels were blocked with a combination of the P/Q-channel blocker ω-agatoxin IVA (500 nm) and the L-channel blocker nimodipine (1 μm). Also, the effects of individual blockers [1 μm either nimodipine or the N/P/Q-channel blocker ω-conotoxin MVIIC (Hillyard et al., 1992; McDonough et al., 1996)] were tested. Figure 6 shows that 4 d treatments with either the blocker combination or nimodipine alone produced a downregulation of α1D mRNA equal in magnitude to the quinpirole effect. Treatment with ω-conotoxin MVIIC alone had no effect on α1D mRNA. Therefore, the effect of the blocker combination can be attributed, in whole, to nimodipine. Furthermore, nimodipine had no effect on α1C mRNA (data not shown). Thus, inhibition of L-channel activity with nimodipine mimics the specific effect of quinpirole on α1D mRNA.
Fig. 6.
Inhibition of L-channels, but not P/Q-channels, mimics and occludes D2 receptor-induced downregulation of α1D mRNA. α1D mRNA levels in control and quinpirole-treated melanotropes cultured for 4 d in control media (No blockers; n = 15) or in the absence or presence of the indicated Ca2+ channel blockers (n = 3 in all cases). ns, Not statistically significant. The dashed line indicates the level of α1D mRNA in quinpirole-treated cells cultured in the absence of Ca2+ channel blockers. Note that the L-channel inhibitor nimodipine mimics and occludes the effect of the D2-receptor agonist quinpirole. In contrast, P/Q-channel blockers have no effect.
We next examined the effectiveness of 4 d treatments with quinpirole in the presence of the channel blockers. Quinpirole produced no further downregulation of α1D mRNA in the presence of either the blocker combination or nimodipine alone. In contrast, quinpirole was effective at inducing downregulation in the presence of ω-conotoxin MVIIC. Thus, nimodipine both mimics and occludes the effect of quinpirole, whereas the P/Q-channel blockers have no effect. The occlusion effect of nimodipine is not caused by loss of D2 receptor function because quinpirole does produce downregulation of POMC mRNA in the presence of nimodipine (data not shown). Therefore, inhibition of L-type Ca2+ channel activity is sufficient to fully account for downregulation of α1D mRNA by chronic D2 receptor activation.
DISCUSSION
This study examined the mechanism of long-term suppression of voltage-gated Ca2+ channels induced by chronic activation of D2 receptors in adult rat melanotropes. We hypothesized that D2 receptors act by decreasing cAMP levels and Ca2+ influx to downregulate HVA Ca2+ channel mRNAs, leading to a reduction in Ca2+ current. Indeed, the α1D L-type Ca2+ channel mRNA was specifically downregulated both in vivo (Fig. 1) and in vitro (Fig. 2). The corresponding L-type Ca2+ current was also suppressed (Fig. 4). However, elevating cAMP levels with Br-cAMP had no effect on quinpirole downregulation of α1D mRNA (Fig. 5). Thus, a decrease in cAMP is not necessary for the D2 receptor effect. Instead, the effect appears to involve only inhibition of L-channel activity, because nimodipine completely mimics and occludes downregulation of α1D mRNA induced by quinpirole (Fig.6).
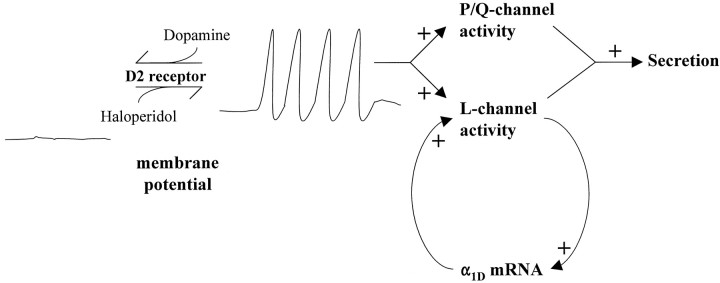
Figure 7 illustrates a mechanism that can explain our data in live adult rats and cultured melanotropes. In the absence of dopamine, melanotropes spontaneously fire action potentials. The depolarization of the spontaneous action potentials activates L-type Ca2+ channels. The fact that nimodipine downregulates α1D mRNA (Fig. 6) suggests that L-channel activity normally stimulates the expression of α1D mRNA. This forms a positive feedback loop, leading to the expression of more L-channels. P/Q-type Ca2+ channels are also activated by action potentials. However, the facts that supplementing nimodipine with ω-agatoxin IVA produces no greater effect on α1D mRNA and that ω-conotoxin MVIIC fails to downregulate α1D mRNA indicate that P/Q-channels do not participate in the positive feedback loop. These results are consistent with previous studies indicating that L-channels (Morgan and Curran, 1986; Murphy et al., 1991; Bading et al., 1993), but not P/Q-channels (Deisseroth et al., 1998), can trigger nuclear events associated with the activation of gene expression. However, the demonstration that the activity of a Ca2+ channel can specifically regulate the expression of its own mRNA is novel. Activation of D2 receptors induces hyperpolarization and inhibition of spontaneous action potential firing (Douglas and Taraskevich, 1978), thus removing the stimulus that activated the L-channel positive feedback loop. Thus, chronic D2 receptor activation can induce a long-lasting suppression of melanotrope L-current by interrupting the positive feedback between L-channel activity and α1D gene expression. This mechanism may also be applicable to lactotrophs because suppression of Ca2+ current in lactotrophs by chronic D2 receptor activation is also insensitive to Br-cAMP (Lledo et al., 1991).
Fig. 7.
Model of long-lasting suppression of L-current induced by D2 receptor-triggered interruption of positive feedback between L-channel activity and α1D gene expression. See Discussion for detailed explanation. Dopamine occupation of D2 receptors induces hyperpolarization and inhibition of spontaneous action potential activity (both indicated by line drawings labeled membrane potential). Haloperidol antagonizes activation of D2 receptors by endogenous dopamine, thus allowing spontaneous action potential firing. L- and P/Q-channels are activated by the depolarization of action potentials. L-channel activity triggers both secretion and α1D gene expression. P/Q-channels may also trigger secretion in melanotropes.
Our finding that haloperidol upregulates α1D mRNA (Fig.1) suggests that the long-lasting suppression is a consequence of ongoing tonic dopaminergic neurotransmission in the adult IL. In our model (Fig. 7), the effect of haloperidol is caused by a disruption of endogenous dopamine occupation of D2 receptors and the resulting hyperpolarization and inhibition of spontaneous action potential firing. This allows L-channels to be activated by the depolarization of action potentials, which in turn stimulates α1D gene expression. Thus, haloperidol prevents the tonic dopaminergic interruption of the positive feedback between L-channel activity and α1D gene expression.
Suppression of L-current is likely to play a significant role in dopamine inhibition of melanotrope secretion. Nimodipine inhibits secretion from melanotropes (Taraskevich and Douglas, 1986), and thus L-channels can trigger exocytosis in these cells. Exocytosis in melanotropes depends on Ca2+ influx to the approximately third power (Thomas et al., 1990), and so a 35% reduction in Ca2+ influx through L-channels might have a much larger effect on secretion. The effect might be further amplified if exocytosis in melanotropes is more tightly coupled to L-channels than to P/Q-channels (Artalejo et al., 1994).
Haloperidol is used clinically as an antipsychotic agent. Interestingly, a reduction in psychotic symptoms generally occurs only with long-term drug use. Such chronic treatment induces hyperexcitability in midbrain dopamine neurons of animal models (Grace et al., 1997). The molecular basis of chronic haloperidol action is thought to involve regulation of neural gene expression (Hyman and Nestler, 1996). Therefore, it is intriguing to speculate that neuronal L-type Ca2+ channel gene expression may be elevated during chronic haloperidol treatment, and the resulting increase in L-current may be an important cellular mechanism of hyperexcitability and the alleviation of psychotic symptoms.
Footnotes
This work was supported by National Institutes of Health Grants NS32385 and HL55312 (E.S.L.), National Institute on Drug Abuse Grant DA-00266 (R.E.M.), and predoctoral fellowships from the American Heart Association and the Andrew Mellon Foundation (D.M.F). E.S.L. is an Established Investigator of the American Heart Association. We thank C. Cheng (University of Pittsburgh) and A. M. Oyarce (Johns Hopkins University) for excellent technical assistance, P. Shepard (University of Maryland) for advice, and T. P. Snutch (University of British Columbia) for plasmids used in the construction of α1subunit probes.
Correspondence should be addressed to Edwin S. Levitan, Department of Pharmacology, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261.
Dr. Fass’s present address: Vollum Institute, Portland, OR 97201.
REFERENCES
- 1.Adams MR, Brandon EP, Chartoff EH, Idzerda RL, Dorsa DM, McKnight GS. Loss of haloperidol induced gene expression and catalepsy in protein kinase A-deficient mice. Proc Natl Acad Sci USA. 1997;94:12157–12161. doi: 10.1073/pnas.94.22.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artalejo CR, Adams ME, Fox AP. Three types of Ca2+ channel trigger secretion with different efficacies in chromaffin cells. Nature. 1994;367:72–76. doi: 10.1038/367072a0. [DOI] [PubMed] [Google Scholar]
- 3.Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- 4.Beatty DM, Sands SA, Morris SJ, Chronwall BM. Types and activities of voltage-operated calcium channels change during development of rat pituitary neurointermediate lobe. Int J Dev Neurosci. 1996;14:597–612. [PubMed] [Google Scholar]
- 5.Birnbaumer L, Campbell KP, Catterall WA, Harpold MM, Hofmann F, Horne WA, Mori Y, Schwartz A, Snutch TP, Tanabe T, Tsien RW. The naming of voltage-gated calcium channels. Neuron. 1994;13:505–506. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 6.Catterall WA. Modulation of sodium and calcium channels by protein phosphorylation and G-proteins. Adv Second Messenger Phosphoprotein Res. 1997;31:159–181. doi: 10.1016/s1040-7952(97)80017-1. [DOI] [PubMed] [Google Scholar]
- 7.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 8.Chronwall BM, Beatty DM, Sharma P, Morris SJ. Dopamine D2 receptors regulate in vitro melanotrope L-type Ca2+ channel activity via c-fos. Endocrinology. 1995;136:614–621. doi: 10.1210/endo.136.2.7835295. [DOI] [PubMed] [Google Scholar]
- 9.Ciranna L, Feltz P, Schlichter R. Selective inhibition of high voltage-activated L-type and Q-type Ca2+ currents by serotonin in rat melanotropes. J Physiol (Lond) 1996;490:595–609. doi: 10.1113/jphysiol.1996.sp021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cota G, Hiriart M. Hormonal and neurotransmitter regulation of Ca channel activity in cultured adenohypophyseal cells. In: Oxford GS, Armstrong CM, editors. Secretion and its control. Rockefeller UP; New York: 1989. pp. 143–165. [PubMed] [Google Scholar]
- 11.Cote TE, Felder R, Kebabian JW, Sekura RD, Reisine R, Affolter H-U. D-2 dopamine receptor-mediated inhibition of pro-opiomelanocortin synthesis in rat intermediate lobe. J Biol Chem. 1986;261:4555–4561. [PubMed] [Google Scholar]
- 12.Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- 13.Douglas WW, Taraskevich PS. Action potentials in gland cells of rat pituitary pars intermedia: inhibition by dopamine, an inhibitor of MSH secretion. J Physiol (Lond) 1978;285:171–184. doi: 10.1113/jphysiol.1978.sp012565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsholtz HP, Lew AM, Albert PR, Sundmark VC. Inhibitory control of prolactin and pit-1 gene promoters by dopamine. J Biol Chem. 1991;266:22919–22925. [PubMed] [Google Scholar]
- 15.Fass DM, Levitan ES. Bay K 8644 reveals two components of L-type Ca2+ channel current in clonal rat pituitary cells. J Gen Physiol. 1996a;108:1–11. doi: 10.1085/jgp.108.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fass DM, Levitan ES. L-type Ca2+ channels access multiple open states to produce two components of Bay K 8644-dependent current in GH3 cells. J Gen Physiol. 1996b;108:13–26. doi: 10.1085/jgp.108.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fomina AF, Levitan ES. Three phases of TRH-induced facilitation of exocytosis by single lactotrophs. J Neurosci. 1995;15:4982–4991. doi: 10.1523/JNEUROSCI.15-07-04982.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fomina AF, Levitan ES, Takimoto K. Dexamethasone rapidly increases calcium channel subunit messenger RNA expression and high voltage-activated calcium current in clonal pituitary cells. Neuroscience. 1996;72:857–862. doi: 10.1016/0306-4522(95)00580-3. [DOI] [PubMed] [Google Scholar]
- 19.Gomora JC, Avila G, Cota G. Ca2+ current expression in pituitary melanotropes of neonatal rats and its regulation by D2 dopamine receptors. J Physiol (Lond) 1996;492:763–773. doi: 10.1113/jphysiol.1996.sp021344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grace AA, Bunney BS, Moore H, Todd CL. Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs. Trends Neurosci. 1997;20:31–37. doi: 10.1016/S0166-2236(96)10064-3. [DOI] [PubMed] [Google Scholar]
- 21.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 22.Hillyard DR, Monje VD, Mintz IM, Bean BP, Nadasdi L, Ramachandran J, Miljanich G, Azimi-Zoonooz A, McIntosh JM, Cruz LJ, Imperial JS, Olivera BM. A new conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron. 1992;9:69–77. doi: 10.1016/0896-6273(92)90221-x. [DOI] [PubMed] [Google Scholar]
- 23.Hyman SE, Nestler EJ. Initiation and adaptation: a paradigm for understanding psychotropic drug action. Am J Psychiatry. 1996;153:151–162. doi: 10.1176/ajp.153.2.151. [DOI] [PubMed] [Google Scholar]
- 24.Lievano A, Bolden A, Horn R. Calcium channels in excitable cells: divergent genotypic and phenotypic expression of α1-subunits. Am J Physiol. 1994;267:C411–C424. doi: 10.1152/ajpcell.1994.267.2.C411. [DOI] [PubMed] [Google Scholar]
- 25.Lledo P-M, Israel JM, Vincent J-D. Chronic stimulation of D2 dopamine receptors specifically inhibits calcium but not potassium currents in rat lactotrophs. Brain Res. 1991;558:231–238. doi: 10.1016/0006-8993(91)90773-o. [DOI] [PubMed] [Google Scholar]
- 26.Loeffler J-P, Demeneix BA, Kley NA, Hollt V. Dopamine inhibition of proopiomelanocortin gene expression in the intermediate lobe of the pituitary. Neuroendocrinology. 1988;47:95–101. doi: 10.1159/000124898. [DOI] [PubMed] [Google Scholar]
- 27.Mains RE, Eipper BA. Synthesis and secretion of corticotropins, melanotropins, and endorphins by rat intermediate pituitary cells. J Biol Chem. 1979;254:7885–7894. [PubMed] [Google Scholar]
- 28.Maurer RA. Transcriptional regulation of the prolactin gene by ergocryptine and cyclic AMP. Nature. 1981;294:94–97. doi: 10.1038/294094a0. [DOI] [PubMed] [Google Scholar]
- 29.McDonough SI, Swartz KJ, Mintz IM, Boland LM, Bean BP. Inhibition of calcium channels in rat central and peripheral neurons by ω-conotoxin MVIIC. J Neuroscience. 1996;16:2612–2623. doi: 10.1523/JNEUROSCI.16-08-02612.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meunier H, Labrie F. The dopamine receptor in the intermediate lobe of the rat pituitary gland is negatively coupled to adenylate cyclase. Life Sci. 1982;30:963–968. doi: 10.1016/0024-3205(82)90625-7. [DOI] [PubMed] [Google Scholar]
- 31.Millington WR, Chronwall BM. Dopaminergic regulation of the intermediate pituitary. In: Muller ES, MacLeod RM, editors. Neuroendocrine Perspectives, Vol 7. Springer; New York: 1989. pp. 1–48. [Google Scholar]
- 32.Morgan JI, Curran T. Role of ion flux in the control of c-fos expression. Nature. 1986;322:552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- 33.Murphy TH, Worley PF, Baraban JM. L-type voltage-sensitive calcium channels mediate synaptic activation of immediate early genes. Neuron. 1991;7:625–635. doi: 10.1016/0896-6273(91)90375-a. [DOI] [PubMed] [Google Scholar]
- 34.Takimoto K, Fomina AF, Gealy R, Trimmer JS, Levitan ES. Dexamethasone rapidly induces Kv1.5 K+ channel gene transcription and expression in clonal pituitary cells. Neuron. 1993;11:359–369. doi: 10.1016/0896-6273(93)90191-s. [DOI] [PubMed] [Google Scholar]
- 35.Taraskevich PS, Douglas WW. Effects of Bay K 8644 and other dihydropyridines on basal and potassium-evoked output of MSH from mouse melanotropes in vitro. Neuroendocrinology. 1986;44:384–389. doi: 10.1159/000124673. [DOI] [PubMed] [Google Scholar]
- 36.Thomas P, Surprenant A, Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotropes of the rat pituitary. Neuron. 1990;5:723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]