Abstract
Brain-derived neurotrophic factor (BDNF) is a survival factor for different classes of neurons, including gustatory neurons. We have studied innervation and development of the gustatory system in transgenic mice overexpressing BDNF under the control of regulatory sequences from the nestin gene, an intermediate filament gene expressed in precursor cells of the developing nervous system and muscle. In transgenic mice, the number and size of gustatory papillae were decreased, circumvallate papillae had a deranged morphology, and there was also a severe loss of lingual taste buds. Paradoxically, similar deficits have been found in BDNF knock-out mice, which lack gustatory neurons. However, the number of neurons in gustatory ganglia was increased in BDNF-overproducing mice. Although gustatory fibers reached the tongue in normal numbers, the amount and density of nerve fibers in gustatory papillae were reduced in transgenic mice compared with wild-type littermates. Gustatory fibers appeared stalled at the base of the tongue, a site of ectopicBDNF expression, where they formed abnormal branches and sprouts. Interestingly, palatal taste buds, which are innervated by gustatory neurons whose afferents do not traverse sites of ectopic BDNF expression, appeared unaffected. We suggest that lingual gustatory deficits in BDNF overexpressing mice are a consequence of the failure of their BDNF-dependent afferents to reach their targets because of the effects of ectopically expressed BDNF on fiber growth. Our findings suggest that mammalian taste buds and gustatory papillae require proper BDNF-dependent gustatory innervation for development and that the correct spatial expression of BDNF in the tongue epithelium is crucial for appropriate target invasion and innervation.
Keywords: taste buds, gustatory, neurotrophins, gustation, transgenic, innervation
The peripheral gustatory system offers an interesting model for the study of target innervation and interaction between ingrowing nerves and neurotrophins. Developmental and experimental studies of gustatory papillae and taste buds have also offered information about the mechanisms underlying sensory organ development and maintenance in the tongue. Lingual papillae cover the dorsal surface of the tongue in mammals. Lingual gustatory papillae, namely fungiform, circumvallate, and foliate papillae contain taste buds that are specialized peripheral sensory organs involved in perceiving chemical stimuli and in taste transduction. Fungiform papillae are located on the anterior dorsal surface of the tongue, and a single circumvallate papilla is located in the midline at the posterior part of the tongue in rodents. Lingual taste buds are innervated by nerve cells residing in the geniculate and petrosal ganglia. The somatosensory innervation of the posterior part of the tongue is derived from the petrosal ganglion, whereas trigeminal neurons provide somatosensory innervation to the anterior part of the tongue. Gustatory nerves innervate taste receptor cells in taste buds, whereas the surrounding epithelium and the remaining lingual epithelium, including filiform papillae, are innervated by somatosensory fibers. Ingrowth and branching of nerve fibers commence during early stages of tongue formation (Farbman and Mbiene, 1991;Mbiene and Mistretta, 1997; Rochlin and Farbman, 1998). The axonal ingrowth and selectivity for either the gustatory or somatosensory innervation start and proceed in a precise and orderly manner, suggesting that this process might be in part regulated by target-derived soluble signals.
The neurotrophins brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) (Lewin and Barde, 1996) play roles in the innervation of sensory organs (Ernfors et al., 1994b, 1995) and are expressed in the respective target areas (Copray and Brouwer, 1994;Pirvola et al., 1992). The gustatory ganglia and cranial ganglia related to general sensory innervation of the tongue, all show neuronal losses in BDNF and NT-3 null-mutated mice (Ernfors et al., 1994a,b;Farinas et al., 1994; Jones et al., 1994), indicating the dependence of these neurons on the survival-promoting actions of these neurotrophins.
Developing gustatory epithelium and adult taste buds in rodents express BDNF mRNA, which might selectively support the gustatory innervation, whereas the surrounding epithelium that receives somatosensory innervation expresses NT-3 mRNA (Nosrat, 1998). Studies in mutant mice have shown that BDNF and NT-3 are necessary for proper gustatory and somatosensory innervation and for survival of gustatory and somatosensory neurons, respectively (Nosrat et al., 1997a; Zhang et al., 1997).
Because of their crucial importance for neuronal survival, the roles of these neurotrophins in target invasion and innervation could not be investigated in mice lacking BDNF or NT-3. To further elucidate the function of BDNF in the tongue and to investigate the role of gustatory innervation in the development of taste buds and gustatory papillae, we examined structures and innervation patterns in the tongues of mice overexpressing BDNF. BDNF-transgenic mice exhibited innervational, anatomical, and histological deficits in their gustatory system, despite having a larger number of neurons in gustatory ganglia.
MATERIALS AND METHODS
Generation of NesPIXpBDNF mice. The generation of transgenic mice has been described earlier (Ringstedt et al., 1998). Briefly, the NesPIXpBDNF construct consisted of a region extending 5.8 kb upstream from the initiation codon of the mouse nestin gene (Zimmerman et al., 1994) followed by a 1 kb fragment from the fifth exon of the mouse BDNF gene containing the complete BDNF protein coding sequence, a 300 bp long SV40 polyadenylation signal, and 5.4 kb of nestin gene downstream sequence, including introns 1, 2, and 3 (Zimmerman et al., 1994). The construct was injected into fertilized mouse oocytes that were subsequently transplanted into pseudopregnant females. The offspring was screened for founders by PCR.
In situ hybridization. Transgene expression was studied by in situ hybridization, performed on fresh frozen 14 μm cryostat sections. Animals of different ages were taken. Sections were fixed for 5 min in 4% paraformaldehyde, rinsed twice in PBS and twice in distilled water, delipidated with 0.2 m HCl for 10 min, acetylated for 20 min with 0.25% acetic anhydride in 0.1 methanolamine, and dehydrated with ethanol. After drying, the sections were incubated overnight in a humidified chamber with 180 μl of hybridization buffer per slide (hybridization buffer is 50% formamide, 20 mm Tris-HCl, pH 7.6, 1 mm EDTA, pH 8.0, 0.3m NaCl, 0.1 m dithiothreitol, 0.5 mg/ml yeast tRNA, 0.1 mg/ml poly(A) RNA (Sigma, St. Louis, MO), 1× Denhardt’s solution, and 10% dextran sulfate containing 2.5 × 106 cpm/μl of BDNF antisense riboprobe. After hybridization, the sections were washed once in 1× SSC at 48°C for 40 min, treated with RNase (10 mg/ml) in 0.5 m NaCl, 20 mm Tris-HCl, pH 7.5, 2 mm EDTA at 37°C for 30 min, and washed twice with 0.5× SSC and twice with 0.1× SSC for 10 min each at 60°C. Finally, sections were dehydrated with ethanol, dried, and then dipped in Kodak (Eastman Kodak, Rochester, NY) NT-B2 emulsion. After a 2–3 week exposure at −20°C, the slides were developed, stained lightly with cresyl violet, and mounted in Permount. Probes (antisense and sense) were labeled with [35S]UTP by in vitro transcription with T3 and T7 RNA polymerases from a 340 bp fragment, corresponding to the DNA sequence of the mature mouse BDNF protein, subcloned into pBS-KS (Strategene, La Jolla, CA).
Histological analysis. Transgenic mice were taken before birth by decapitation of staged pregnant mothers. Embryonic day 15 (E15)–E19 embryos were decapitated and fixed by immersion for 7–14 hr in 4% paraformaldehyde, cryoprotected by overnight immersion in 10% sucrose in PBS (0.5 m NaCl, 0.1 mphosphate buffer, pH 7.3). Heads were rapidly frozen, and 14 μm transverse sections were cut on a cryostat, air-dried and stained with cresyl violet, and mounted in Permount.
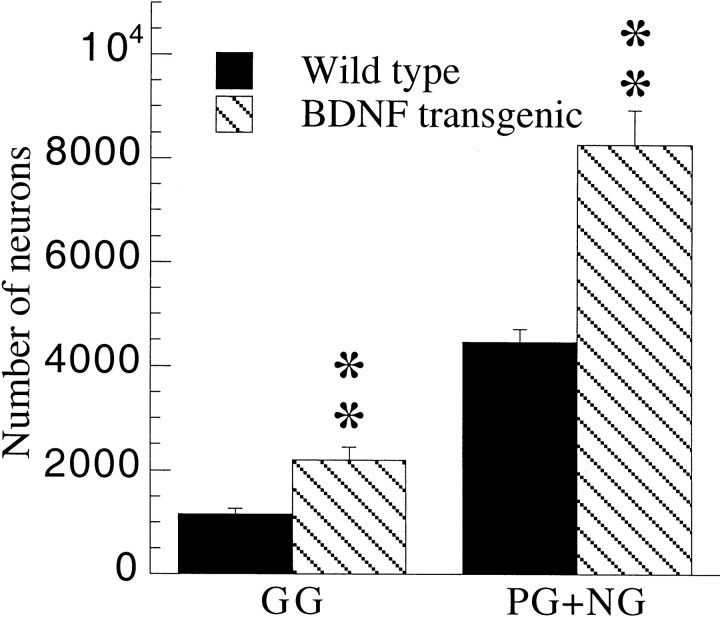
Counts of cranial ganglion neurons. We counted neurons with distinct nucleoli from geniculate (four transgenic and four wild-type mice) and petrosal-nodose (three transgenic and three wild-type mice) ganglia on every seventh tissue section from E19 animals, using light microscopy. To calculate an estimated total number of neurons, the counts were multiplied by seven. The narrowest bridge between adjacent ganglia was regarded as the boundary between ganglia. However, a boundary could not be defined between the petrosal and nodose ganglia, and we therefore counted these ganglia together. Data collected in quantitative analysis were statistically evaluated using unpaired Student’s t test for comparison of the means (see Fig.3).
Fig. 3.
Quantitative analysis of estimated total number of neurons in geniculate ganglion (GG, four transgenic and four wild-type mice) and combined petrosal–nodose ganglia (PG + NG, three transgenic and three wild-type mice). Data are expressed as mean ± SEM (**p < 0.01, unpaired Student’s ttest).
Immunohistochemistry. Animals (E19; n = 10; five controls and five BDNF-transgenic mice) were taken as above, and cryostat sections were preincubated in dilution buffer (PBS, 3% goat serum, and 0.3% Triton X-100) for 1 hr, followed by overnight incubation with different antisera in dilution buffer. Antibodies against protein gene product 9.5 (PGP; 1:400 dilution; Biogenesis), growth-associated protein 43 (GAP; 1:500 dilution; Chemicon, Temecula, CA), calcitonin gene-related peptide (CGRP; 1:400 dilution; Peninsula Laboratories Europe, Merseyside, UK), and gustducin (Santa Cruz Biotechnology, Santa Cruz, CA) were used to visualize the innervation apparatus of the tongue and to study taste cells. Antibodies to PGP (Wilson et al., 1988) and GAP are good neuronal markers and have been used to study tongue innervation (Mbiene and Mistretta, 1997; Wakisaka et al., 1996, 1998). Many adult perigemmal fibers (somatosensory fibers) in the gustatory papillae are CGRP-positive (Finger, 1986). α-gustducin is a taste cell-specific G-protein subunit (McLaughlin et al., 1992). Antibodies to α-gustducin have been characterized in earlier reports (Boughter et al., 1997). Sections were subsequently washed four times in PBS, incubated for 2 hr with rhodamine or FITC-conjugated secondary antiserum, washed three times in PBS, and covered by mounting medium containing 0.1% phenylenediamine to minimize fading (Hökfelt et al., 1973).
DiI tracing. E19 and newborn mice (n = 12; nine controls and three BDNF-transgenic mice) were fixed in 4% paraformaldehyde in PBS. Animals were decapitated, and the brains were removed and the facial nerves were localized bilaterally. Heads were pinned down on Sylgard gel Petri dishes and DiI crystals (Molecular Probes, Eugene, OR) were applied to the facial nerve. Chorda tympani is a gustatory branch of the facial nerve and innervates the taste buds in the anterior part of the tongue. It enters the facial canal as does the rest of the facial nerve. The heads were submerged in 4% paraformaldehyde in PBS and incubated at 42–50°C for 10–12 weeks. After incubation, the heads and tongues were rinsed in PBS containing 10% sucrose and embedded in OCT compound. Sagittal and transversal sections (30 μm) were cut on a cryostat and thawed onto slides (SuperFrost Plus; Menzel-Gläser). They were viewed by fluorescence microscopy.
Scanning electron microscopy. Transgenic and control mice (E19–P0; n = 6; three controls and three BDNF-transgenic mice) were immersion-fixed with 4% paraformaldehyde in PBS. Tongues were dissected out, rinsed in PBS, and dehydrated in a graded series of ethanol, acetone, and tetramethyl silane as final steps. They were then mounted on aluminum stubs, platinum-coated with the sputter technique, and examined in a scanning electron microscope (JEOL JSM 820).
RESULTS
Ectopic BDNF expression in the brain, cranial ganglia, and tongue of nestin-BDNF-transgenic mice
Nestin is an intermediate filament protein expressed by neuronal precursors in both the CNS and PNS and in developing muscle cells (Dahlstrand et al., 1995). Enhancer sequences in intron 1 of thenestin gene are required for the correct spatiotemporal pattern of nestin production in developing muscle and in intron 2 for correct nestin expression in the developing nervous system (Zimmerman et al., 1994). We have generated transgenic mice overexpressingBDNF under the control of promoter and enhancer regions of the nestin gene, including those located in introns 1 and 2 (Ringstedt et al., 1998). These mice die shortly before or after birth probably because of cardiac malformations (T. Ringstedt, C. F. Ibáñez, and B. Hempstead, unpublished observations). Transgenic embryos arising from independent injections of the construct expressed high levels of BDNF mRNA in the ventricular zone of the brain (Fig. 1A–D) (Ringstedt et al., 1998) and moderate levels in cranial ganglia, including gustatory ganglia (Fig. 1B). In addition, high levels of BDNF mRNA were also seen in the developing tongue (Fig.1D,F). Expression in the tongue was very prominent throughout the entire anteroposterior axis in the central musculature (Fig. 1D). BDNF mRNA labeling was not found in the palate and suprapalatal regions (Fig.1D). We did not observe distinct areas ofBDNF overexpression within the path of gustatory fibers toward their lingual target tissues before they reached the tongue (Fig. 1B,D). Quantification of BDNF protein by ELISA in brain extracts indicated a twofold to sixfold increase in transgenic mice (Ringstedt et al., 1998).
Fig. 1.
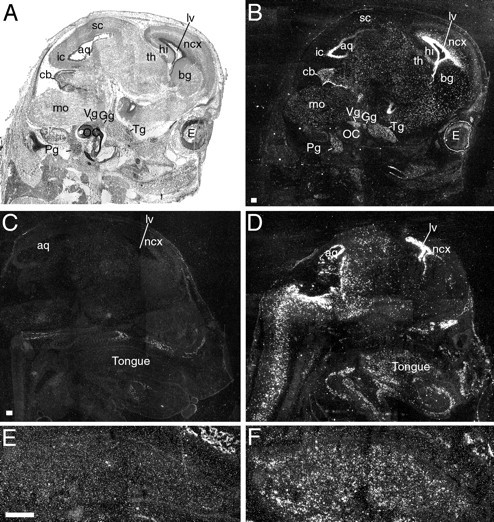
Sagittal sections through heads of wild-type and BDNF-transgenic mice at E15, labeled by radioactive in situ hybridization with a BDNF-specific riboprobe. Sections were exposed for a short period to photographic emulsion; i.e., endogenous BDNF labeling is thus not observed, and only the strong labeling in transgenic animals is seen. Sections were photographed under bright-field (A) and dark-field (B–F) illumination.A, Sagittal section from a BDNF-transgenic mouse, about halfway between the external ear and midline. B, The same section as in A viewed in dark-field illumination. BDNF labeling is found in several regions of the brain. Strong BDNF labeling is found particularly in the ventricular zones, as well as in proliferative zones of the developing cerebellum (cb). Also note that moderate BDNF labeling is found above cranial ganglia (trigeminal ganglion, Tg; geniculate ganglion,Gg; vestibular ganglion, Vg; petrosal ganglion. Pg), including gustatory ganglia (Gg, Pg). C, Sagittal section of a wild-type mouse. Specific labeling is below detection level. Developing bone tissue appears bright in dark-field photomicrographs because of unspecific light scattering.D, Sagittal section of the head of a BDNF-transgenic mouse. BDNF labeling is observed in different areas of the brain and spinal cord, as well as in the tongue. Note that the palate is devoid from BDNF labeling. E, Higher magnification of the tongue in C. F, Higher magnification of the tongue in D. Specific labeling is found in the midportion of the tongue, consisting of muscle and connective tissue, but not in the lingual epithelium. Scale bar, 200 μm.lv, Lateral ventricle; aq, aqueduct;ncx, neocortex; bg, developing basal ganglia; hi, hippocampus; sc, superior colliculus; ic, inferior colliculus; cb, cerebellum; mo, medulla oblongata; OC, otic capsule; E, eye; th, thalamus.
Increased number of neurons in cranial ganglia innervating the tongue of transgenic mice overexpressing BDNF
Cranial ganglia in nestin–BDNF-transgenic mice were significantly larger than in wild-type mice. The geniculate and trigeminal ganglia (as well as petrosal and nodose ganglia, data not shown) appeared fused in transgenic animals, and no distinct boundary could be observed between them (Figs.1A,B,2). An estimated 100% increase in the number of neurons in geniculate, nodose, and petrosal ganglia was observed (Figs. 2, 3).
Fig. 2.

Photomicrographs of transverse sections of the geniculate ganglion and the facial nerve in E19 wild-type (wt, left panels) and E19 BDNF-transgenic (tg, right panels) mice, visualizing the increase in size of both the ganglion and the facial nerve. Photomicrographs are taken at an ∼100 μm interval. The geniculate ganglion and the nerve are delineated. There is not a distinct boundary between geniculate and trigeminal ganglia in BDNF-overproducing mice, therefore the ganglion in B is not delineated and is marked instead by an arrow. Thearrowheads in A–C,E, and G point at a distinct bone structure (the cartilage model) which subsequently surrounds the ganglion and the nerve in the wild-type mouse but not in the transgenic mouse. The arrows in F, H, and J are pointing at the nerve in BDNF-transgenic mice, indicating that there are both peripheral and central projections from the ganglion. The inner ear compartments are located in the lower and brain tissue (hi, posterior parts of the hippocampal formation) in the top parts of the photomicrographs. Scale bar, 400 μm.
Decreased innervation of gustatory papillae and loss of lingual taste buds in BDNF-transgenic mice
The ectopic expression of BDNF in the tongue and the increased number of neurons in the cranial ganglia of transgenic mice prompted us to examine the gustatory innervation of the tongue in these animals. To this purpose, we used antibodies to PGP and GAP, which react with most neuronal fibers irrespective of their neurotransmitter or peptide content. PGP and GAP antibodies are good markers for fine peripheral nerve fibers and have been extensively used for the studies of lingual and gustatory papillae innervation (Mbiene and Mistretta, 1997; Wakisaka et al., 1996, 1998). PGP immunoreactivity was found in a fraction of taste cells, but GAP immunoreactivity was not (see also Wakisaka et al., 1998). We also used antibodies to CGRP, which distinguish a subset of peptidergic perigemmal fibers in the adult gustatory papillae. Although wild-type tongue and gustatory papillae were adequately innervated at early developmental stages (Figs.4A,C,E,5A,C–E), gustatory papillae in BDNF-transgenic animals were less innervated (Figs. 4D,F,5A,F–H). The midportion of the tongues was richly innervated both in wild-type animals as well as in transgenic mice (Fig.4A,B). Although the lingual dorsal surface subepithelial nerve plexus was present in both wild-type and BDNF mice (Fig. 4A–D, open arrows, evaluated by PGP, GAP, and CGRP immunoreactivity) the amount of nerve fibers entering and ramifying in fungiform papillae was decreased in BDNF-transgenic mice (Fig.4C–F). Filiform papillae, on the other hand, appeared to be properly innervated in transgenic mice (Fig.4A–D, open arrows). This was in agreement with the fact that this innervation is somatosensory in character and is dependent on NT-3 but not BDNF (Nosrat et al., 1997a). Circumvallate papillae also showed decreased innervation in BDNF-transgenic mice compared with wild-type littermates (Fig.5A–H) and generally no PGP-positive taste buds were observed. In addition, the subepithelial nerve plexus underlying the gustatory epithelium of circumvallate papillae was diminished in transgenic mice (Fig.5B,F–H). PGP- (Fig. 5A–C,F), GAP- (Fig. 5D,G), and CGRP-positive (Fig. 5E,H) fibers were observed in the area of circumvallate papillae and circumvallate plates (CPs) in transgenic and wild-type mice. The innervation of CPs is, however, NT-3-dependent and is not affected in mice lacking BDNF (Nosrat et al., 1997a) or in BDNF-transgenic animals (Fig.5B,F–H). Together, these data indicate that the gustatory but not the somatosensory innervation is affected in mice overexpressingBDNF. CGRP-positive fibers were generally thin (and contained varicosities) and were observed in the core part of the tongue (among the muscle tissue and close to lingual blood vessels), and in association with the lingual epithelium. Gustducin immunoreactivity was seen within the vallate epithelium (vallate taste buds) of wild-type, but not in that of BDNF-transgenic mice (Fig. 5, comparfe I, J). Posterior palatal taste cells were, however, the most gustducin-immunoreactive taste cells at E19 in wild-type and transgenic mice. We did not observe gustducin-immunoreactive taste cells in fungiform or nasopalatal taste buds (data not shown) in wild-type or transgenic mice at E19.
Fig. 4.
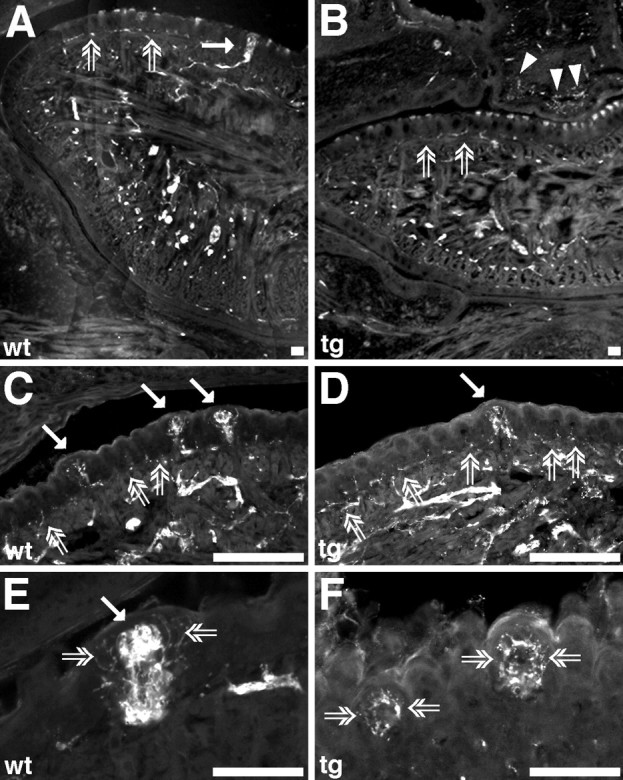
PGP immunoreactivity in the tongue of wild-type (wt) and BDNF-transgenic (tg) mice at E19. A, Tongue, fungiform (filled arrow), and filiform papillae are well innervated in wild-type mice. Open arrows point at the subepithelial nerve plexus and filiform papillae. B, Tongue in BDNF-overexpressing mice is also well innervated. Filiform papillae are adequately innervated, and the subepithelial nerve plexus is clearly visible (open arrows). Arrowheads point at PGP-positive fibers in the area of the nasoincisor duct.C, Higher magnification of the lingual epithelium (containing three fungiform papillae, filled arrows and numerous filiform papillae, open arrows). Note the rich innervation of the papillae. The round positive structures in the papillae are fungiform taste buds. Filiform papillae are innervated, and the subepithelial nerve plexus is present (open arrows). D, Higher magnification of the lingual epithelium from BDNF-transgenic mice (containing one fungiform papilla, filled arrow and numerous filiform papillae,open arrows). Note that the papillae (both fungiform and filiform) are innervated. Fungiform papilla is, however, less innervated in BDNF-transgenic mice compared with wild-type fungiform papillae. E, Higher magnification of a wild-type fungiform papilla. The taste bud (filled arrow) is richly innervated. There are also perigemmal fibers (open arrows) surrounding the taste bud. F, Fungiform papillae (i.e., the remaining papillae) have a rich perigemmal innervation (open arrows) in transgenic mice. Scale bars: A, B, E,F, 100 μm; C, D, 200 μm.
Fig. 5.

PGP, GAP, CGRP, and gustducin (Gust.) immunoreactivity in the tongue and palate of wild-type (wt) and BDNF-transgenic (tg) mice at E19. A, Circumvallate papillae are well developed and well innervated in wild-type mice, and an extensive nerve plexus is observed within the papillae and surrounding the lateral trench wall epithelium. Superior surface taste buds are well developed and differentiated, as well as richly innervated (filled arrow). CPs are also well developed and well innervated (asterisks). Nerve bundles are directed toward the CPs and innervate them. B, Circumvallate papillae in BDNF-transgenic mice have distorted morphology and are underdeveloped. The (gustatory) nerve plexus within the papillae as well as the superior surface taste buds are missing. Nerve bundles (open arrows) are however directed toward the CPs (asterisks). C–H, Consecutive sections of circumvallate papillae from wild-type and BDNF-transgenic mice reacted with antibodies to PGP, GAP, and CGRP.C, D, Superior surface taste buds are well innervated (filled arrows). Nerve fibers are also observed in trench wall epithelium (open arrows).E, CGRP-positive fibers (i.e., somatosensory) in wild-type mice are thin and contain varicosities, and are found in association with the papilla and CPs.F–G, In transgenic mice, PGP- and GAP-positive fibers (open arrows) are innervating CPs. There is a severe reduction of subepithelial nerve plexus underlying the gustatory epithelium, corresponding to the loss of vallate taste buds. H, CGRP-positive fibers (open arrows) are found in association with CPs, and a few fibers are found in the midportion of the papilla. I, In wild-type mice, gustducin-positive palatal taste buds (filled arrow) are found posteriorly at the level of circumvallate papillae. Gustducin immunoreactivity is more intense in the palatal taste cells (which might represent the maturity of palatal taste cells) than in vallate taste buds. Nevertheless, positive taste cells are also found in the circumvallate papillae (open arrows). Theinset is a higher magnification of the area marked witharrowheads. J, Only palatal (and laryngeal, data not shown) taste buds are gustducin-positive in BDNF-transgenic mice (filled arrow). No gustducin immunoreactivity is observed in the circumvallate papillae.K, Higher magnification of the gustducin-immunoreactive taste cells marked with a filled arrow inI. L, Higher magnification of a gustducin-immunoreactive taste bud from BDNF-transgenic mice. Cells are clearly elongated. M, N, Palatal taste buds (open arrows) at “geschmacks-streifen” in BDNF-transgenic mice are well innervated, and taste cells are PGP-positive. The elongated shape of the taste cells is clearly visible. Scale bars: A, B, 50 μm;C–J, 100 μm; M,N, 200 μm.
Palatal taste buds appear to be unaffected in BDNF-transgenic mice
In addition to lingual sites, taste buds are also found in the palate. Palatal taste buds, as well as fungiform taste buds, are innervated by gustatory fibers emanating from the geniculate ganglion. Palatal taste buds normally express BDNF mRNA (Nosrat and Olson, 1995) and show deficits in BDNF-knock-out mice (Cooper and Oakley, 1998). The transgene was not expressed in the palate of BDNF-transgenic mice (Fig.1D) or was it found in the suprapalatal regions through which greater petrosal nerve passes toward its final targets, the palatal taste buds. Lingual gustatory innervation showed deficits in BDNF-transgenic mice, including the innervation of the fungiform papillae. Because the geniculate ganglion is the common source of gustatory innervation for palatal and fungiform taste buds, we examined the innervation of palatal taste buds. Palatal taste buds were well innervated, as evaluated by using antibodies to PGP and GAP. Palate epithelium at the region of the nasoincisor duct, where nasopalatal taste buds are located, was well innervated in transgenic (Fig.4B, arrowheads) and wild-type mice. Many fusiform PGP-positive taste cells were observed in the posterior parts of the palate in BDNF-transgenic mice (Fig.5M,N; taste buds in the area of “geschmacks-streifen”). We studied palatal taste buds in more detail using antibodies to gustducin. Posteriorly located palatal taste cells in both wild-type and transgenic mice expressed gustducin, and distinct positive taste cells could be seen (Fig.5I–L). We did not observe nasopalatal gustducin-positive taste cells, although, laryngeal taste buds were also gustducin-positive in both wild-type and BDNF-transgenic mice.
The presence of well innervated and gustducin-positive palatal (and laryngeal) taste buds in BDNF-transgenic mice indicates that the transient expression of the transgene in the gustatory ganglia does not affect target innervation, as long as the transgene is not ectopically expressed in the target area.
Abnormal target invasion of gustatory fibers in BDNF-transgenic mice
The decreased innervation of gustatory papillae prompted us to investigate the fate of gustatory nerves entering the tongue. To this end, we performed DiI tracing of the chorda tympani. In wild-type mice, chorda tympani entered the tongue, and the labeled nerve was observed in the ventral part sending out branches as it progressed toward the anterior part of the tongue. These branches traversed through the muscle toward the lingual epithelium (Fig.6A,I) where they innervated fungiform papillae and taste buds (Fig.6A, inset). In nestin–BDNF mice, chorda tympani was also seen entering the tongue (Fig. 6C), and several branches were observed in the ventral side of the tongue, anteriorly to the area of entrance (Fig. 6D). Some branches were observed among the muscle tissue of the tongue, and in the posterior part of the tongue some fibers were found close to the epithelium (Fig. 6E). However, the lingual epithelium appeared not to be innervated (Fig. 6B). Tangles of fine labeled fibers were sometimes observed in the core part of the tongue among the muscle tissue (Fig.6F–H). No such tangles were observed in wild-type animals (Fig. 6I). In transgenic mice, gustatory fibers appeared stalled at the base of the tongue, a site of ectopic BDNF expression, where they formed abnormal branches and sprouts.
Fig. 6.

Sagittal and transverse sections of the tongue in E19 wild-type (wt) and E19 BDNF-transgenic mice (tg), visualizing DiI-labeled gustatory fibers of the chorda tympani nerve (gustatory branch of the seventh cranial nerve).A, In wild-type mice, gustatory fibers enter the tongue, pass through muscle tissue (I), and reach the lingual epithelium and innervate fungiform taste buds (arrows). A, inset, shows a higher magnification of the fungiform papilla located on the left side. B, Chorda tympani fibers generally do not reach the lingual epithelium in BDNF-transgenic mice. In these mice, labeled gustatory nerves enter the tongue (C,arrow), and several branches are found in more anterior locations (D, arrows) than the place of entrance. E, In the midportion part of the tongue, a few labeled chorda tympani fibers are found projecting toward the lingual epithelium (arrow). F–H, Occasional labeled fine chorda tympani fiber tangles are observed in the core part of the tongue, among the muscle tissue. I, In wild-type littermates, labeled gustatory fibers traverse through the muscle tissue toward the lingual epithelium (arrows), and fiber tangles are not observed. Scale bars:A–E, I, 200 μm;F–H, 50 μm.
Abnormal tongue surface morphology
The gross morphology of gustatory papillae appeared abnormal in both light and scanning electron microscopical (SEM) examination of BDNF-transgenic mice (Figs. 7,8). In contrast, nestin NT-3 transgenic mice (Ringstedt et al., 1997) appeared to have a normal appearance of the tongue surface (Fig.7E,F).
Fig. 7.

Transverse sections of the tongue in wild-type (wt), BDNF-transgenic (BDNF tg), and NT-3 transgenic (NT-3 tg) mice.A, Normal appearance of filiform and fungiform papillae (arrow). Note that there is a taste bud within the superior surface epithelium. B, Circumvallate papilla is well developed in wild-type mice and contains many well differentiated taste buds in its superior surface (one taste bud is visible in the midportion of the superior surface epithelium in this photomicrograph).C, In BDNF-transgenic mice there are less fungiform papillae. The fungiform papilla in this photomicrograph is smaller than in wild-type and NT-3 transgenic mice but appears to contain a taste bud. D, Circumvallate papilla has distorted morphology in BDNF-transgenic mice. E, F, Tongue papillae appear normal in NT-3 overproducing Mice. Scale bar:A, represents 100 μm in all panels.
Fig. 8.

Scanning electron photomicrographs of mouse tongue at E19. Anterior side faces to right in all figures. A, A circumvallate papilla with its dome-shaped midportion (arrowhead) in a wild-type (wt) mouse.B, Anterior part of a tongue in a wild-type mouse. Fungiform papillae are well developed, large, and well defined. Many fungiform papillae are observed (arrows).C, A circumvallate papilla with a distorted morphology in a BDNF-transgenic (tg) mouse. The midportion of the papilla (arrowhead) is not observed. D, There is a severe decrease in the number of fungiform papillae (and loss of fungiform taste buds) in BDNF-transgenic mice. In this scanning electron photomicrograph of the anterior part of the tongue, well developed and well defined fungiform papillae are missing.E, Another circumvallate papilla with a distorted morphology in a BDNF-transgenic mouse. The midportion of the papilla (arrowhead) has decreased width. F, A few fungiform papillae (arrows) with normal appearance, but reduced in size, could however be found in the midportion of the dorsal surface of the tongue in some of BDNF-transgenic mice (see also Fig.5A,C). Scale bars, 100 μm.
Fungiform papillae in BDNF-transgenic mice were of smaller size (Fig.7C) and were less in number (Fig. 8D) compared with normal mice (Figs. 7A, 8B) or to NT-3 transgenic mice (Fig. 7E). Although thickened surface areas, indicating developing taste buds, were observed in the fungiform papillae of both wild-type and NT-3 mice, only few developing taste buds were seen in BDNF-transgenic mice. Using SEM, few small-size fungiform papillae were observed in the midportion of the dorsal surface of the tongue (Fig. 8F), whereas the papillae were missing in the anterior part of the tongue (Fig.8D), where the highest number of fungiform papillae is normally observed. Fungiform papillae in the posterior part of the tongue are generally larger than those located anteriorly in wild-type mice (Nosrat et al., 1996, 1997a). Fungiform papillae in BDNF-transgenic mice (Fig. 8F) were, however, smaller than those in the anterior part of the tongue of wild-type mice (Fig.8B). Circumvallate papillae in BDNF-transgenic mice had disturbed morphology (Figs. 7D,8C,E); the trench system was not fully developed, and the trenches were shorter in length in comparison with both wild-type (Figs. 7B, 8A) and NT-3 overexpressing mice (Fig. 7F). The superior surface taste buds, which appear first and are well developed at birth, were missing, as well as the taste buds in the inner and outer wall trench epithelium.
DISCUSSION
We have studied the development of the gustatory system in transgenic mice overexpressing BDNF. BDNF has been shown to have specific roles in the innervation of the gustatory system as well as in the proper development of the peripheral gustatory sensory organs, the taste buds. Nestin–BDNF-transgenic mice exhibited innervational, anatomical, and histological deficits in their lingual gustatory system. Gustatory papillae in BDNF-overexpressing mice were significantly less innervated, were smaller, had deranged morphology, and the number of lingual taste buds was severely reduced, whereas the lingual somatosensory innervation, which has been shown to be dependent on NT-3 (Nosrat et al., 1997a), was not affected. Palatal taste buds in BDNF-transgenic mice appeared unaffected. Peripheral gustatory development and lingual taste buds appeared normal in transgenic mice overexpressing NT-3.
Unexpectedly, the lingual gustatory deficits seen in BDNF-transgenic mice have a remarkable resemblance to those previously described in BDNF knock-out mice (Nosrat et al., 1997a; Zhang et al., 1997; Oakley et al., 1998). Although mice overexpressing BDNF showed an increased number of neurons in cranial ganglia, BDNF knock-out mice display a distinct loss of neuronal cells, including a subpopulation of gustatory neurons (Ernfors et al., 1994a; Jones et al., 1994; Liu et al., 1995). This neuronal loss explains the gustatory deficits observed in BDNF knock-out mice. However, the abnormalities in the gustatory system of nestin–BDNF-transgenic mice occur in the absence of neuronal loss.
Nestin is expressed in neural stem cells in different parts of the developing peripheral nervous system. Analysis of the cranial ganglia, chorda tympani, and palatal taste buds in nestin–BDNF mice showed bundles of nerve fibers clearly projecting away from the ganglia, reaching the tongue and the palate, but only innervating palatal taste buds. This would indicate that BDNF overexpression in gustatory ganglia did not prevent gustatory neuron outgrowth, nor was target innervation by gustatory fibers affected, as long as the transgene was not ectopically expressed in the target area. Gustatory nerve bundles were increased in size compared with the corresponding nerves of wild-type animals, consistent with the increased size of the ganglion itself. It has been suggested that cranial sensory neurons are independent of neurotrophins during the initial period of axonal outgrowth and become dependent on neurotrophins once the axons reach their targets (Davies and Lumsden, 1984; Vogel and Davies, 1991; Buchman and Davies, 1993). According to this view, gustatory nerves grow into the tongue under the influence of guiding molecules other than BDNF (see Tessier-Lavigne and Goodman, 1996). After they reach the tongue, they become dependent on BDNF for trophic support, survival, and target invasion. At early stages of tongue development, BDNF mRNA is expressed in the core part of the tongue in the area of intermolar eminence (where the gustatory fibers normally enter the tongue), and later, it is exclusively found in the gustatory epithelium (Nosrat and Olson, 1995; Nosrat et al., 1996, 1997b). The core part of the tongue in nestin–BDNF-transgenic mice expresses abnormally high amounts of BDNF. Gustatory fibers have to traverse this region on their way toward the gustatory epithelium. These sites of ectopic BDNF expression supply the gustatory neurons with high amounts of BDNF, which could rescue them from developmental programmed cell death. Our data also suggest that ingrowing gustatory fibers make terminal ramifications at these sites of ectopic BDNF expression, something that might prevent them from reaching the gustatory papillae. Some gustatory fibers manage, nevertheless, to pass through this region in the tongue rich in BDNF and grow toward the normal sites of BDNFexpression. Lingual somatosensory fibers, which have been shown to be NT-3-dependent, grow normally toward their final target destinations in BDNF-transgenic mice. This might explain the presence of a few fungiform papillae in the midportion of the dorsal surface of the tongue and of DiI-labeled fibers in these regions.
Although neurotrophins do not appear to be involved in axon guidance over long distances, they do exhibit tropic influences on axons that have already reached the target area. In NT-3-overproducing mice, central Ia afferents were shown to project toward the midline of the spinal cord, where NT-3 was ectopically expressed under the control of the nestin promoter (Ringstedt et al., 1997). Other reports have also emphasized tropic actions of neurotrophins on growing axons. Neurotrophins elicit turning responses of growth cones toward the neurotrophin source and modulate responses of the axonal growth cone (Gundersen and Barrett, 1980; Ming et al., 1997; Paves and Saarma, 1997; Tuttle and O’Leary, 1998). NT-3 has been proposed to exert tropic effects on spiral neurons of the auditory system (Malgrange et al., 1996) and to increase the expression of the cytoskeletal microtubuline-associated protein 5 in cochleovestibular ganglion neurons in culture (Sanjose et al., 1997).
The data in the present study are also in agreement with studies on transected peripheral nerves. Neonatal chorda tympani– lingual nerve transection in rats leads to a deficit in fungiform papillae development (i.e., fungiform papillae are nondistinguishable from filiform papillae) and loss of taste buds (Nagato et al., 1995). It has also been shown that gustatory fibers are more efficacious in maintaining taste buds and gustatory papillae than other type of nerves (Hård af Segerstad et al., 1989; Oakley et al., 1990). The majority of vallate taste buds develop postnatally (Hosley and Oakley, 1987), and thus when the glossopharyngeal nerve was transected during this period, the majority of vallate taste buds failed to develop (Hosley et al., 1987). When mouse embryos were treated with β-bungarotoxin, disrupting sensory and motor neuron development, no taste buds were observed in the few remaining fungiform papillae present in the midportion of the tongue or associated with the circumvallate papillae (Morris-Wiman et al., 1997).
Taste bud primordial cells require the action of the early arriving gustatory fibers for their differentiation and maturation. In the absence of gustatory nerves, mammalian taste buds do not develop and fail to support and to maintain the developing gustatory papillae (Nosrat, 1998a; Oakley, 1998). This appears to be in contrast to taste buds from axolotl, which are capable of developing in the absence of innervation (Northcutt and Barlow, 1998). Neonatal nerve transection studies and studies of the gustatory system in mice deficient in BDNF or the BDNF receptor trkB (Fritzsch et al., 1997;Zhang et al., 1997), as well as the data in our present study, clearly show that taste bud development and maturation in mammals require reciprocal interactions between nerves and taste bud progenitor cells. However, nerve-independent signals might also contribute to the establishment of mammalian taste cell lineage and taste bud induction. A number of studies have indicated that taste buds and gustatory papillae develop in prespecialized regions of the lingual epithelium. It has been shown that BDNF mRNA is expressed in the gustatory epithelium before the arrival of gustatory nerves (Nosrat and Olson, 1995) and in explanted tongue organ cultures without the presence of nerve fibers, where it shows a temporospatial expression pattern resembling that seen in vivo (Nosrat et al., 1998). The morphogenic protein Sonic Hedgehog and its receptor Patched are also expressed in the same areas as BDNF and NT-3 mRNAs before the arrival of nerves (Bitgood and McMahon, 1995; Hall et al., 1999), where gustatory papillae and taste buds develop. Taste buds arise from the local epithelium (Stone et al., 1995), although not randomly but in specialized regions (Zalewski, 1974). These data clearly demonstrate that taste bud progenitor cells have different properties than the surrounding epithelium and these properties (i.e., prespecialization) precede innervation. Nevertheless, presence of nerve fibers in proximity of developing taste buds and synaptic vesicles in taste bud-progenitor cells are among one of the earliest signs of taste bud development in humans (Witt and Reutter, 1996, 1998). The size of adult fungiform taste buds is also directly related to the number of geniculate neurons innervating the taste buds (Krimm and Hill, 1998). It has also been shown that Mash1 expression in basal taste cells requires gustatory innervation (Seta et al., 1999). Taken together, the evidence available so far supports the contributions from early nerve-independent mechanisms that bring competency to the gustatory epithelium and nerve-dependent mechanisms of taste bud development in mammals (Farbman, 1965; Oakley, 1991), leading to mammalian taste bud development and maturation.
In conclusion, our data demonstrate the importance of BDNF for proper structural and functional development of the gustatory innervation. Overexpression of BDNF in the core part of the tongue creates a lingual phenotype similar to that of BDNF knock-out mice, but does not appear to affect palatal taste buds. We propose that loss of proper target innervation underlies these developmental deficits in both BDNF knock-out and in nestin–BDNF-transgenic mice. We suggest that BDNF acts as a target invasion factor for the early arriving gustatory fibers in the tongue and coordinates innervation of the correct targets. If BDNF is expressed incorrectly, the pattern of innervation is changed. This leads to failure in taste bud and gustatory papillae development, which confirms and extends the general notion of the importance of gustatory innervation in mammalian taste bud development and maintenance.
Footnotes
This work was supported by the Swedish Medical Research Council, the Swedish Cancer Society, the Medical and Dental Faculties of the Karolinska Institutet, the Swedish Medical and Dental Associations, and David and Astrid Hageléns Stiftelse. We thank Erik Nilsson and Annika Ahlén for technical assistance, and Kjell Hultenby for the use of scanning electron microscope.
Correspondence should be addressed to Dr. Christopher Nosrat at the above address.
REFERENCES
- 1.Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- 2.Boughter JD, Jr, Pumplin DW, Yu C, Christy RC, Smith DV. Differential expression of α-gustducin in taste bud populations of the rat and hamster. J Neurosci. 1997;17:2852–2858. doi: 10.1523/JNEUROSCI.17-08-02852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchman VL, Davies AM. Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development. 1993;118:989–1001. doi: 10.1242/dev.118.3.989. [DOI] [PubMed] [Google Scholar]
- 4.Cooper D, Oakley B. Functional redundancy and gustatory development in BDNF null mutant mice. Dev Brain Res. 1998;105:79–84. [PubMed] [Google Scholar]
- 5.Copray JC, Brouwer N. Selective expression of neurotrophin-3 messenger RNA in muscle spindles of the rat. Neuroscience. 1994;63:1125–1135. doi: 10.1016/0306-4522(94)90578-9. [DOI] [PubMed] [Google Scholar]
- 6.Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Dev Brain Res. 1995;84:109–129. doi: 10.1016/0165-3806(94)00162-s. [DOI] [PubMed] [Google Scholar]
- 7.Davies A, Lumsden A. Relation of target encounter and neuronal death to nerve growth factor responsiveness in the developing mouse trigeminal ganglion. J Comp Neurol. 1984;223:124–137. doi: 10.1002/cne.902230110. [DOI] [PubMed] [Google Scholar]
- 8.Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994a;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 9.Ernfors P, Lee KF, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994b;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 10.Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- 11.Farbman AI. Electron microscope study of the developing taste bud in rat fungiform papilla. Dev Biol. 1965;11:110–135. doi: 10.1016/0012-1606(65)90040-0. [DOI] [PubMed] [Google Scholar]
- 12.Farbman AI, Mbiene JP. Early development and innervation of taste bud-bearing papillae on the rat tongue. J Comp Neurol. 1991;304:172–186. doi: 10.1002/cne.903040203. [DOI] [PubMed] [Google Scholar]
- 13.Farinas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- 14.Finger TE. Peptide immunohistochemistry demonstrates multiple classes of perigemmal nerve fibers in the circumvallate papillae of the rat. Chem Senses. 1986;11:135–144. [Google Scholar]
- 15.Fritzsch B, Sarai PA, Barbacid M, Silossantiago I. Mice with a targeted disruption of the neurotrophin receptor trkB lose their gustatory ganglion cells early but do develop taste buds. Int J Dev Neurosci. 1997;15:563–576. doi: 10.1016/s0736-5748(96)00111-6. [DOI] [PubMed] [Google Scholar]
- 16.Gundersen RW, Barrett JN. Characterization of the turning response of dorsal root neurites toward nerve growth factor. J Cell Biol. 1980;87:546–554. doi: 10.1083/jcb.87.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall JM, Hooper JE, Finger TE (1999) Expression of sonic hedgehog, patched and gli1 in developing taste papillae of the mouse. J Comp Neurol, in press. [DOI] [PubMed]
- 18.Hosley MA, Oakley B. Postnatal development of the vallate papilla and taste buds in rats. Anat Rec. 1987;218:216–222. doi: 10.1002/ar.1092180217. [DOI] [PubMed] [Google Scholar]
- 19.Hosley MA, Hughes SE, Morton LL, Oakley B. A sensitive period for the neural induction of taste buds. J Neurosci. 1987;7:2075–2080. doi: 10.1523/JNEUROSCI.07-07-02075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hård af Segerstad C, Hellekant G, Farbman AI. Changes in number and morphology of fungiform taste buds in rat after transection of chorda tympani or chorda-lingual nerve. Chem Senses. 1989;14:335–348. [Google Scholar]
- 21.Hökfelt T, Fuxe K, Goldstein M, Joh TH. Immunohistochemical localization of three catecholamine synthesizing enzymes: aspects on methodology. Histochemie. 1973;33:231–254. doi: 10.1007/BF00274236. [DOI] [PubMed] [Google Scholar]
- 22.Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krimm RF, Hill DL. Innervation of single fungiform taste buds during development in rat. J Comp Neurol. 1998;398:13–24. [PubMed] [Google Scholar]
- 24.Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Ernfors P, Wu H, Jaenisch R. Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature. 1995;375:238–241. doi: 10.1038/375238a0. [DOI] [PubMed] [Google Scholar]
- 26.Malgrange B, Lefebvre PP, Martin D, Staecker H, Vandewater TR, Moonen G. NT-3 has a tropic effect on process outgrowth by postnatal auditory neurones in vitro. NeuroReport. 1996;7:2495–2499. doi: 10.1097/00001756-199611040-00019. [DOI] [PubMed] [Google Scholar]
- 27.Mbiene JP, Mistretta CM. Initial innervation of embryonic rat tongue and developing taste papillae. Acta Anat. 1997;160:139–158. doi: 10.1159/000148006. [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 29.Ming GL, Lohof AM, Zheng JQ. Acute morphogenic and chemotropic effects of neurotrophins on cultured embryonic Xenopus spinal neurons. J Neurosci. 1997;17:7860–7871. doi: 10.1523/JNEUROSCI.17-20-07860.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris-Wiman J, Basco E, Du Y. The effects of β-bungarotoxin on the morphogenesis of taste papillae and taste buds. In: XII International Symposium on Olfation and Taste. San Diego, CA; 1997. [Google Scholar]
- 31.Nagato T, Matsumoto K, Tanioka H, Kodama J, Toh H. Effects of denervation on morphogenesis of the rat fungiform papilla. Acta Anat. 1995;153:301–309. doi: 10.1159/000147739. [DOI] [PubMed] [Google Scholar]
- 32.Northcutt RG, Barlow LA. Amphibians provide new insights into taste-bud development. Trends Neurosci. 1998;21:38–43. doi: 10.1016/s0166-2236(97)01146-6. [DOI] [PubMed] [Google Scholar]
- 33.Nosrat CA. Neurotrophic factors in the tongue; expression patterns, biological activity, relation to innervation and studies of neurotrophin knockout mice. Ann NY Acad Sci. 1998;855:28–49. doi: 10.1111/j.1749-6632.1998.tb10544.x. [DOI] [PubMed] [Google Scholar]
- 34.Nosrat CA, Olson L. Brain-derived neurotrophic factor mRNA is expressed in the developing taste bud-bearing tongue papillae of rat. J Comp Neurol. 1995;360:698–704. doi: 10.1002/cne.903600413. [DOI] [PubMed] [Google Scholar]
- 35.Nosrat CA, Ebendal T, Olson L. Differential expression of brain-derived neurotrophic factor and neurotrophin 3 mRNA in lingual papillae and taste buds indicates roles in gustatory and somatosensory innervation. J Comp Neurol. 1996;376:587–602. doi: 10.1002/(SICI)1096-9861(19961223)376:4<587::AID-CNE7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 36.Nosrat CA, Blomlöf J, Elshamy WM, Ernfors P, Olson L. Lingual deficits in BDNF and NT3 mutant mice leading to gustatory and somatosensory disturbances, respectively. Development. 1997a;124:1333–1342. doi: 10.1242/dev.124.7.1333. [DOI] [PubMed] [Google Scholar]
- 37.Nosrat CA, Fried K, Lindskog S, Olson L. Cellular expression of neurotrophin mRNAs during tooth development. Cell Tissue Res. 1997b;290:569–580. doi: 10.1007/s004410050962. [DOI] [PubMed] [Google Scholar]
- 38.Nosrat CA, MacCallum DK, Mistretta CM. Early molecular events in gustatory papilla development are independent of nerve fibers. Chem Senses. 1998;23:603–604. [Google Scholar]
- 39.Oakley B. Neuronal-epithelial interactions in mammalian gustatory epithelium. Ciba Found Symp. 1991;160:277–287. doi: 10.1002/9780470514122.ch14. [DOI] [PubMed] [Google Scholar]
- 40.Oakley B. Vertebrate taste-bud development. Trends Neurosci. 1998;21:337. doi: 10.1016/s0166-2236(98)01255-7. [DOI] [PubMed] [Google Scholar]
- 41.Oakley B, Wu LH, Lawton A, deSibour C. Neural control of ectopic filiform spines in adult tongue. Neuroscience. 1990;36:831–838. doi: 10.1016/0306-4522(90)90026-z. [DOI] [PubMed] [Google Scholar]
- 42.Oakley B, Brandemihl A, Cooper D, Lau D, Lawton A, Zhang CX. The morphogenesis of mouse vallate gustatory epithelium and taste buds requires BDNF-dependent taste neurons. Dev Brain Res. 1998;105:85–96. [PubMed] [Google Scholar]
- 43.Paves H, Saarma M. Neurotrophins as in vitro growth cone guidance molecules for embryonic sensory neurons. Cell Tissue Res. 1997;290:285–297. doi: 10.1007/s004410050933. [DOI] [PubMed] [Google Scholar]
- 44.Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumae U, Saarma M. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci USA. 1992;89:9915–9919. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ringstedt T, Kucera J, Lendahl U, Ernfors P, Ibáñez CF. Limb proprioceptive deficits without neuronal loss in transgenic mice overexpressing neurotrophin-3 in the developing nervous system. Development. 1997;124:2603–2613. doi: 10.1242/dev.124.13.2603. [DOI] [PubMed] [Google Scholar]
- 46.Ringstedt T, Linnarsson S, Wagner J, Lendahl U, Kokaia Z, Arenas E, Ernfors P, Ibáñez CF. BDNF regulates reelin expression and Cajal-Retzius cell development in the cerebral cortex. Neuron. 1998;21:305–315. doi: 10.1016/s0896-6273(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 47.Rochlin MW, Farbman AI. Trigeminal ganglion axons are repelled by their presumptive targets. J Neurosci. 1998;18:6840–6852. doi: 10.1523/JNEUROSCI.18-17-06840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanjose I, Vazquez E, Garciaatares N, Rodriguez S, Vega JA, Represa J. Expression of the cytoskeletal protein map5 and its regulation by neurotrophin 3 in the inner ear sensory neurons. Anat Embryol. 1997;195:299–310. doi: 10.1007/s004290050049. [DOI] [PubMed] [Google Scholar]
- 49.Seta Y, Toyono T, Takeda S, Toyoshima K. Expression of Mash1 in basal cells of rat circumvallate taste buds is dependent upon gustatory innervation. FEBS Lett. 1999;444:43–46. doi: 10.1016/s0014-5793(99)00023-x. [DOI] [PubMed] [Google Scholar]
- 50.Stone LM, Finger TE, Tam P, Tan SS. Taste receptor cells arise from local epithelium, not neurogenic ectoderm. Proc Natl Acad Sci USA. 1995;92:1916–1920. doi: 10.1073/pnas.92.6.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 52.Tuttle R, O’Leary DDM. Neurotrophins rapidly modulate growth cone response to the axon. Mol Cell Neurosci. 1998;11:1–8. doi: 10.1006/mcne.1998.0671. [DOI] [PubMed] [Google Scholar]
- 53.Vogel KS, Davies AM. The duration of neurotrophic factor independence in early sensory neurons is matched to the time course of target field innervation. Neuron. 1991;7:819–830. doi: 10.1016/0896-6273(91)90284-7. [DOI] [PubMed] [Google Scholar]
- 54.Wakisaka S, Miyawaki Y, Youn SH, Kato J, Kurisu K. Protein gene-product 9.5 in developing mouse circumvallate papilla. Anat Embryol. 1996;194:365–372. doi: 10.1007/BF00198538. [DOI] [PubMed] [Google Scholar]
- 55.Wakisaka S, Daikoku H, Miyawaki Y, Youn SH, Maeda T, Kurisu K. Immunohistochemical observation of growth-associated protein 43 in the developing circumvallate papilla of the rat. Cell Tissue Res. 1998;293:499–507. doi: 10.1007/s004410051142. [DOI] [PubMed] [Google Scholar]
- 56.Wilson PO, Barber PC, Hamid QA, Power BF, Dhillon AP, Rode J, Day IN, Thompson RJ, Polak JM. The immunolocalization of protein gene product 9.5 using rabbit polyclonal and mouse monoclonal antibodies. Br J Exp Pathol. 1988;69:91–104. [PMC free article] [PubMed] [Google Scholar]
- 57.Witt M, Reutter K. Embryonic and early fetal development of human taste buds: a transmission electron microscopical study. Anat Rec. 1996;246:507–523. doi: 10.1002/(SICI)1097-0185(199612)246:4<507::AID-AR10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 58.Witt M, Reutter K. Innervation of developing human taste buds. Histochem Cell Biol. 1998;109:281–291. doi: 10.1007/s004180050228. [DOI] [PubMed] [Google Scholar]
- 59.Zalewski AA. Neuronal and tissue specifications involved in taste bud formation. Ann NY Acad Sci. 1974;228:344–349. doi: 10.1111/j.1749-6632.1974.tb20523.x. [DOI] [PubMed] [Google Scholar]
- 60.Zhang CX, Brandemihl A, Lau D, Lawton A, Oakley B. BDNF is required for the normal development of taste neurons in vivo. NeuroReport. 1997;8:1013–1017. doi: 10.1097/00001756-199703030-00039. [DOI] [PubMed] [Google Scholar]
- 61.Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, Mann J, Vassileva G, McMahon A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]