Abstract
It is demonstrated that acetylcholine released from cholinergic interneurons modulates the excitability of neostriatal projection neurons. Physostigmine and neostigmine increase input resistance (RN) and enhance evoked discharge of spiny projection neurons in a manner similar to muscarine. Muscarinic RN increase occurs in the whole subthreshold voltage range (−100 to −45 mV), remains in the presence of TTX and Cd2+, and can be blocked by the relatively selective M1,4 muscarinic receptor antagonist pirenzepine but not by M2 or M3 selective antagonists. Cs+ occludes muscarinic effects at potentials more negative than −80 mV. A Na+ reduction in the bath occludes muscarinic effects at potentials more positive than −70 mV. Thus, muscarinic effects involve different ionic conductances: inward rectifying and cationic. The relatively selective M2receptor antagonist AF-DX 116 does not block muscarinic effects on the projection neuron but, surprisingly, has the ability to mimic agonistic actions increasing RN and firing. Both effects are blocked by pirenzepine. HPLC measurements of acetylcholine demonstrate that AF-DX 116 but not pirenzepine greatly increases endogenous acetylcholine release in brain slices. Therefore, the effects of the M2 antagonist on the projection neurons were attributable to autoreceptor block on cholinergic interneurons. These experiments show distinct opposite functions of muscarinic M1- and M2-type receptors in neostriatal output, i.e., the firing of projection neurons. The results suggest that the use of more selective antimuscarinics may be more profitable for the treatment of motor deficits.
Keywords: muscarinic receptors, neuromodulation, firing patterns, neostriatum, acetylcholine, Parkinson’s disease, basal ganglia
Neostriatal cholinergic innervation participates in motor control by the basal ganglia (Wang and McGinty 1997; Wilson, 1998). Muscarinic M1 receptor activation facilitates spiny neurons (Dodt and Misgeld, 1986; Misgeld et al., 1986; Pineda et al., 1995; Harsing and Zigmond, 1998) by inhibiting calcium and potassium currents that participate in firing, afterhyperpolarization, and inward rectification (Misgeld et al., 1986;Bargas and Galarraga, 1995; Howe and Surmeier, 1995; Pineda et al., 1995; Hsu et al., 1996). Moreover, muscarinic receptors of the M2 type may work as presynaptic autoreceptors that modify the release of ACh from spontaneously firing cholinergic interneurons (Consolo et al., 1987; Weiler, 1989; Wilson et al., 1990; Kawaguchi, 1992; Hersch et al., 1994).
Despite these physiological actions, the effects of muscarinic receptor antagonists are not as reproducible as those of 3,4-dihydroxy-l-phenylalanine (l-DOPA) at the systemic level. Therefore, they do not constitute the treatment of choice for basal ganglia dysfunctions such as Parkinson’s disease (Kopin, 1993; Riederer et al., 1993). Nevertheless, several muscarinic receptors have been cloned (M1–M5), and relatively selective ligands are now accessible (Potter and Purkerson, 1995; Caulfield and Birdsall, 1998). These ligands have not been thoroughly tested on striatal function or disease models. One reason for this is that antagonist selectivity is still weak (Caulfield and Birdsall, 1998).
However, available evidence points toward an important functional segregation between M1- and M2-type receptors in the neostriatum; receptors of the M2 type are abundant on large interneurons (Yan and Surmeier, 1996), whereas those of the M1 type are abundant on medium-sized projection neurons (Hersch et al., 1994; Howe and Surmeier, 1995; Yan and Surmeier, 1996). In contrast, receptors of the M4 type are located on both large interneurons and projection neurons (Yan and Surmeier, 1996). Therefore, a simple hypothesis would state that activation or blockade of M1- or M2-type receptors should lead to very different results on the output of the neostriatal circuitry, that is, the firing of the spiny projection neuron. In other words, if the segregation of M1- and M2-type receptors has a global physiological importance, a difference in the output firing of the spiny neuron should be readily seen when either of these receptors is blocked, despite the weak selectivity of the muscarinic antagonists.
To test this hypothesis, the present work compares the actions of two reputed and relatively selective muscarinic antagonists, pirenzepine (M1) and AF-DX 116 (M2), on the excitability of the spiny projection neuron.
A preliminary report of this study has been presented in abstract form (Galarraga et al., 1998).
MATERIALS AND METHODS
The present experiments were performed on dissected rat dorsal neostriatal slices maintained in vitro as previously reported (Hernandez-López et al., 1997). Briefly, Wistar rats (100–200 gm) were deeply anesthetized and perfused transcardially with 50 ml of an iced-cold (4°C) solution containing (in mm): 125 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 25 NaHCO3, 10d-glucose, 0.0002 thiourea, and 0.0002l-ascorbic acid (saturated with 95% O2 and 5% CO2; 300 mOsm/l; pH = 7.4). The brain was rapidly removed and placed in this solution before slicing. Saggital slices (350 μm thick) of the neostriatum were obtained in a vibratome and incubated 30 min at 25°C before recording. The slices were then transferred to a submerged recording chamber and superfused with saline of the same composition (34–36°C). Intracellular recordings were performed with microelectrodes filled with 3 m K-acetate and 1% biocytin (80–120 MΩ). Records were obtained with an active bridge electrometer, digitized (Neuro Data, Cygnus Technologies) and saved on VHS tapes (40 KHz) to be analyzed off-line with a PC clone computer. After recordings, neurons were injected with biocytin as previously described (Horikawa and Armstrong, 1988;Flores-Hernández et al., 1994). All neurons analyzed are medium spiny projection neurons.
The stimulus used was a current ramp (0.5–1 nA/sec, 1 mV/msec) (Jahnsen and Llinás, 1984; Uchimura and North, 1990; Bargas et al., 1994; Galarraga et al., 1994; Lee and Heckman, 1998). In current-clamp conditions a ramp response allows ready evaluation of the action of a transmitter both at the subthreshold voltage range (from approximately −100 to approximately −40 mV) and during firing (Pineda et al., 1995; Pacheco-Cano et al., 1996; Lee and Heckman, 1998). Changes in the slope of the current–voltage relationship (I–V plot) can be interpreted as input resistance (RN) changes induced by the transmitter (Uchimura and North, 1990; Galarraga et al., 1994; Pacheco-Cano et al., 1996). The slope of the I–V function used for quantitative comparisons was its derivative at resting membrane potential or zero applied current (Galarraga et al., 1994). Experiments were paired so that records in the presence and absence of drugs were compared in the same neuron and in the same sample, with a nonparametric test (Wilcoxon’s t test). Although not shown for the sake of figure clarity, all transmitter responses described were reversible. Means ± SEM of RN changes are reported.
TTX, cesium chloride (Cs+), cadmiun chloride (Cd2+), physostigmine, neostigmine bromide, atropine sulfate, N-methyl-d-glucamine (Sigma, St. Louis, MO), muscarine, pirenzepine, 4-diphenylacetoxy-N-[2-chloroethyl]-piperidine (4-DAMP) (Research Biochemicals, Natick, MA) and AF-DX 116 (as a generous gift from Karl Thomae, Boehringer-Ingelheim, Ingelheim, Germany) were added from freshly prepared stock solutions to the bath saline. The selective M4-type receptor antagonist MT-3 was obtained from Alomone Labs (Jerusalem, Israel).
Monitoring ACh outflow in striatal slices. Changes in endogenous ACh release were quantified in brain slices similar to those used for electrophysiology (350 μm thick), except that they were cut with a hollow punch of fixed diameter, so that all the slices used for release experiments had approximately the same size and wet weight. All slices were taken from the dorsal neostriatum and were introduced in small glass holders placed into tubes of 200 μl volume where they were incubated in the same superfusion saline (bubbled with 95% O2 and 5% CO2 at 34–36°C) in the presence of physostigmine or neostigmine (5 μm). The concentration of the ACh esterase inhibitor was the same as that used in electrophysiological experiments (see Results).
The slices were then transferred to several 200 μl tubes in succession for 20 min periods, either in the absence (basal outflow) or the presence of the muscarinic receptor antagonists pirenzepine and AF-DX 116. Control ACh concentration was measured in parallel chambers in the absence of any antagonist. After transferring the tissue to a new tube, the previous one was placed on ice for later processing. The amount of ACh found in the control condition (incubations in the absence of the antagonists) was compared with the incubations in the presence of the antagonists. Data from whole samples are represented with box plots (Tukey, 1977), but mean ± SEM are reported in the text along with nonparametric comparisons (Mann–Whitney Utest).
ACh assay. ACh levels were measured in the superfusion saline from 20 min incubated samples. Twenty microliter samples were taken from each period and measured with an HPLC system with electrochemical detection added (BAS, West Lafayette, IA). Up to 10 samples could be measured from a single tube. Each sample was injected into a polymeric reversed phase column (BAS); ACh and choline were then converted into hydrogen peroxide and betaine in a postcolumn enzyme reactor containing immobilized acetylcholinesterase and choline oxidase. The hydrogen peroxide was detected electrochemically by a platinum electrode set at 500 mV (vs Ag/AgCl) and 5 mA range. The mobile phase consisted of 50 mm sodium phosphate buffer, pH 8.5, and 0.5% kathon reagent (BAS). The smallest extracellular ACh concentration ([ACh]O) detected was ∼0.1 pm in a sample of 20 μl or [ACh]O = 5 nm/l. [ACh]O was quantified by comparison with peak areas of standard solutions that were assayed in parallel (Gutierrez et al., 1997). Finally, [ACh]O is expressed as pm/20 μl (pm/sample). Note that mean [ACh]O in control conditions is 10 times the detection limit.
RESULTS
Actions of muscarine and endogenous ACh
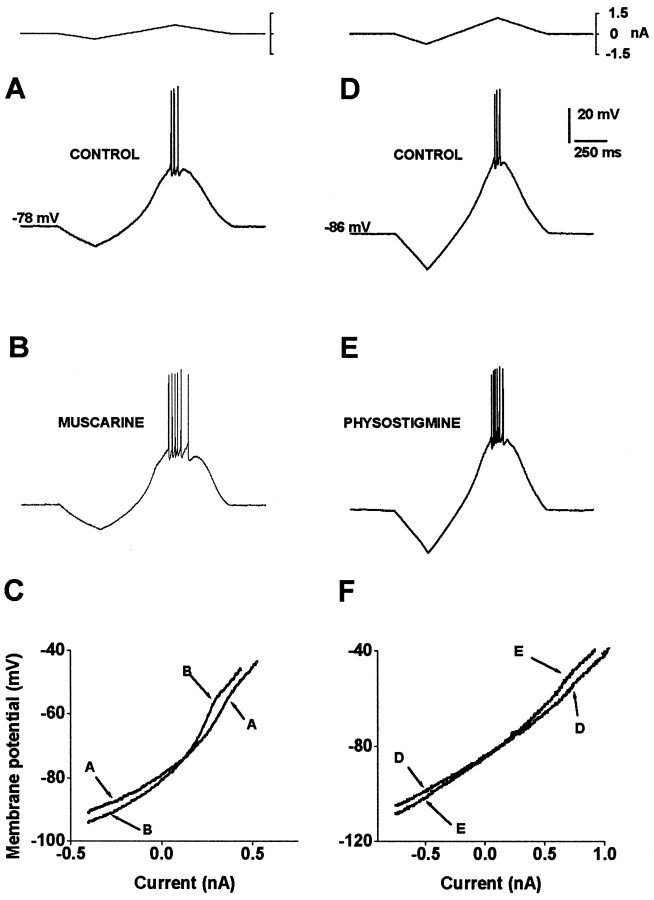
Muscarinic actions were confirmed in medium spiny neurons of the dorsal neostriatum. As previously described, a linear current ramp injection (Fig. 1A, top) evokes a nonlinear subthreshold membrane potential response attributable, in part, to Cs+-sensitive inward rectification (Galarraga et al., 1994; Nisenbaum and Wilson, 1995;Mermelstein et al., 1998) (Fig. 1A,bottom). In all neurons tested (n = 77), muscarine (0.5–1 μm) changed subthreshold response and enhanced evoked firing significantly (Fig. 1B) (Dodt and Misgeld, 1986; Pineda et al., 1995). The ascending portion of the voltage trajectory can be used to build current–voltage relationships (I–V plots), which show a change in subthreshold membrane conductance (Fig. 1C; Galarraga et al., 1994).I–V plots show that muscarine produces an apparent increase in RN as measured by a change in slope (Fig. 1C; see Materials and methods) (Dodt and Misgeld, 1986; Uchimura and North, 1990; Pineda et al., 1995; Hsu et al., 1996). In eight neurons analyzed quantitatively (0.5–1 μm muscarine), mean RNincreased from 42.4 ± 3 to 52.4 ± 4.5 MΩ (p < 0.02, Wilcoxon’s t test). This is compatible with the closing of inward rectifying K+ conductance, because I–V plots cross around −80 mV, that is, near the K+ equilibrium potential (Fig. 1C). This action of muscarine remains in the presence of the selective M4-type receptor antagonist MT-3 (10 nm) (data not shown), suggesting that the receptor type involved in this response is the M1 (Olianas et al., 1996;Adem and Karlsson, 1997; Purkerson and Potter, 1998). The cholinergic actions described are reversible with washing (Pineda et al., 1995). Other concentrations of muscarine (5 and 10 μm) were tested with similar results, indicating that 1 μm is a near-saturating concentration.
Fig. 1.
Cholinergic muscarinic agonists enhance firing and change subthreshold membrane properties of neostriatal medium spiny projection neurons. A, Firing is evoked with a depolarizing and linear current ramp. Note that the subthreshold voltage response to the ramp is not linear. B, The cholinergic muscarinic receptor agonist muscarine (1 μm) enhances firing. C, When voltage trajectories toward firing in A and B are used to build current–voltage relationships (I–V plots), it is seen that muscarine increases the slope of the I–V plot; that is, it increases input resistance (RN) and thus favors the depolarization toward firing. D–F, Similar result using the cholinesterase inhibitor physostigmine (5 μm).
Increasing the endogenous ACh concentration ([ACh]O) in the slice by applying the cholinesterase inhibitor physostigmine (5 μm) had effects similar to those of muscarine: RN and evoked discharge were enhanced (Fig. 1D–F). A significant increase in RN was quantified in a sample of five neurons tested. Mean varied from 33.4 ± 3.9 to 39.7 ± 4.5 MΩ (p < 0.05, Wilcoxon’s t test). Neostigmine had similar effects (n = 2). Thus, increases in endogenous [ACh]O in slices of isolated dorsal neostriatum can be reflected in the subthreshold response and evoked discharge of the medium spiny neuron. Moreover, the effects of muscarine and endogenous ACh were the same.
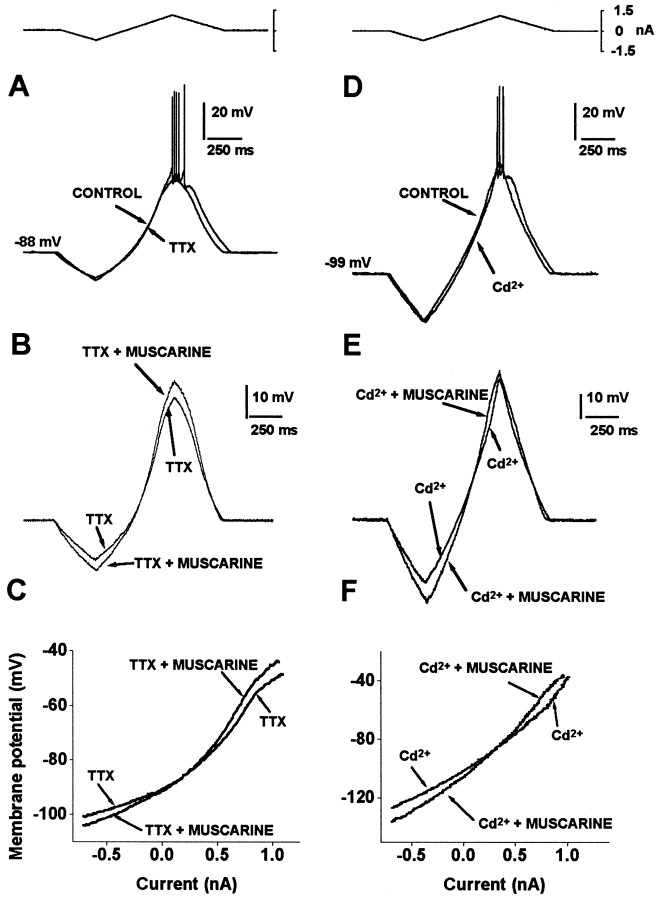
Next, we explored the possibility that the effects of muscarinic receptor activation were direct on spiny neurons and not attributable to the activation of other elements in the slice. One micromolar muscarine was applied in the presence of 1 μm TTX (Fig.2A,B) to abolish neuronal activity or 400 μm Cd2+ (Fig.2D,E) to reduce spontaneous synaptic actions (Flores-Hernandez et al., 1994; Bargas et al., 1998). As previously reported (Galarraga et al., 1994), TTX abolishes firing without changing the subthreshold membrane response for the ascending ramp (Fig. 2A; >30 min with TTX). Nevertheless, muscarine kept changing the subthreshold trajectory and enhancing RNin the presence of TTX in each neuron tested (Fig.2B,C; mean RN, 41 ± 9–52.5 ± 13 MΩ; n = 4). Ca2+ blockade also abolishes repetitive firing in these neurons (Galarraga et al., 1989). However, the ascending portion of the ramp response is not significantly changed (Fig.2D). In Cd2+, muscarine kept enhancing the slope of the I–V plot (RN) in all neurons tested (Fig. 2D,F; 33 ± 5.5–42 ± 8.4 MΩ;n = 4). Pooling together all experiments in which indirect circuitry actions were blocked (TTX or Cd2+), the increase in RN attributable to muscarine was significant (36.7 ± 3.9 MΩ before and 46.4 ± 5.5 MΩ during muscarine; n = 8;p < 0.02, Wilcoxon’s t test). Because spontaneous firing and synaptic activity have been abolished in these experiments, they suggest that muscarinic actions are direct on spiny projection neurons. However, muscarine caused a change in theI–V plot trajectory in the whole subthreshold range, from −120 to −40 mV, whereas inward rectifying conductance should be seen at more negative potentials only (more negative than approximately −80 mV in [K+]O = 3 mm;Mermelstein et al., 1998; Reyes et al., 1998).
Fig. 2.
Muscarinic facilitatory actions are direct. A, Firing is evoked with a current ramp (top). One micromolar TTX blocks the evoked firing but does not change the ascending voltage trajectory. B,Muscarine changes the ascending voltage trajectory in the presence of TTX. C, I–V plots show that 1 μmmuscarine increases RN in the presence of TTX.D–F, Similar experiment shows that 1 μmmuscarine increases RN in the presence of 400 μm Cd2+, departing from a more negative membrane potential.
To test whether muscarinic actions at or below resting potential were attributable to inward rectifying conductances, 2 mmCs+, a blocker of these conductances, was used to block inward rectification (Mermelstein et al., 1998). The subthreshold voltage response to a current ramp is greatly altered by Cs+; the hyperpolarizing portion of the ramp response shows a larger and more sudden change for the same stimulus, caused by Cs+ blockade of an inward current that normally opposes membrane hyperpolarization (Fig.3, compare A, B). This is reflected in the I–V plot as an increase in RN(I–V slope) at potentials more negative than −80 mV (Galarraga et al., 1994; Reyes et al., 1998). The result was that the increase in RN induced by muscarine at potentials more negative than −80 mV was occluded in the presence of Cs+ (n = 3), suggesting that muscarine acts through Cs+-sensitive channels at polarized potentials. However, Cs+ changes very little the membrane potential trajectory, or RN, at more depolarized subthreshold potentials. Accordingly, muscarine was still able to induce an increase in RN at the more depolarized subthreshold potentials (Fig. 3B,C). Note that this action is evident even in the presence of 1 μm TTX, suggesting both that apparent RN enhancement induced by muscarine at this depolarized range is not attributable to inward TTX-sensitive Na+ inward currents and that the effect shown is direct (postsynaptic).
Fig. 3.
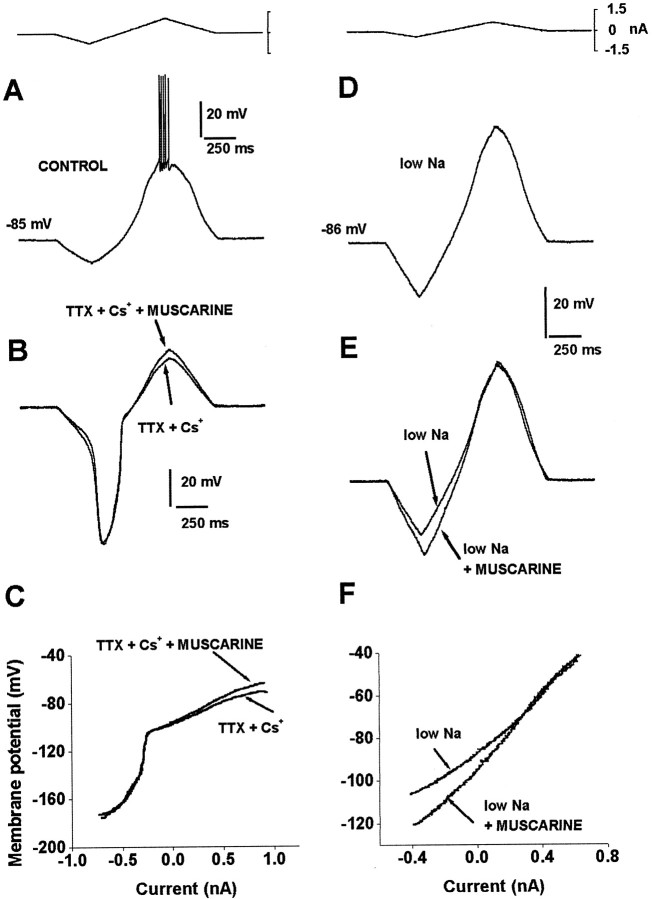
Different conductances are affected by muscarine at different membrane potentials in the subthreshold range.A, Firing is evoked by a current ramp. B,In the presence of 2 mm Cs+, inward rectification produced at polarized potentials is blocked. However, a great increase in the rate of change of the voltage trajectory at this potential range is evident (Galarraga et al., 1994). One micromolar muscarine is no longer able to change the ascending voltage trajectory at polarized potentials in the presence of Cs+. However, muscarine still changes the voltage trajectory at potentials nearer the firing threshold. One micromolar TTX abolished firing in this experiment. C, Muscarine is no longer able to change RN (I–V slope) at polarized potentials in the presence of Cs+, but it is still able to change RN at potentials nearer the firing threshold. D, Subthreshold voltage response to a current ramp in a low-Na+ saline. E, In this condition, muscarine is still able to change the ascending voltage trajectory in the most polarized voltage range, but it is no longer able to change the voltage trajectory near the firing threshold.F, I–V plots show that in low Na+, muscarine changes RN only in the most polarized voltage range (more negative than −80 mV).
In addition to TTX, the increase in RN induced by muscarine at the more depolarized subthreshold potentials could not be blocked by Ba2+, Cd2+, or Co2+ (data not shown, but see Fig.2D–F). This excludes Na+, K+, and Ca2+ channels sensitive to these blockers as the main cause of RN increase between −70 and −45 mV. However, the change in RN induced by muscarine at these more depolarized potentials could be blocked by superfusing the slice in a low Na+ saline (Fig.3D–F; N-methyl-d-glucamine-Cl substituted for 125 mm NaCl). In contrast, the change in RN induced by muscarine at polarized potentials (more negative than −80 mV) was still present in low Na+(Fig. 3F; n = 3; Cs+ was absent). These experiments show that although muscarine induces a change in the voltage trajectory and RN in the whole subthreshold range (between −100 and −40 mV), Cs+blocks this action only at polarized potentials (more negative than −80 mV), whereas a low Na+ saline is the procedure that blocks this action at potentials nearer to the firing threshold (between −70 and −40 mV). This suggests that muscarinic action on the subthreshold response is not attributable to a single ion conductance.
Actions of ACh receptor antagonist
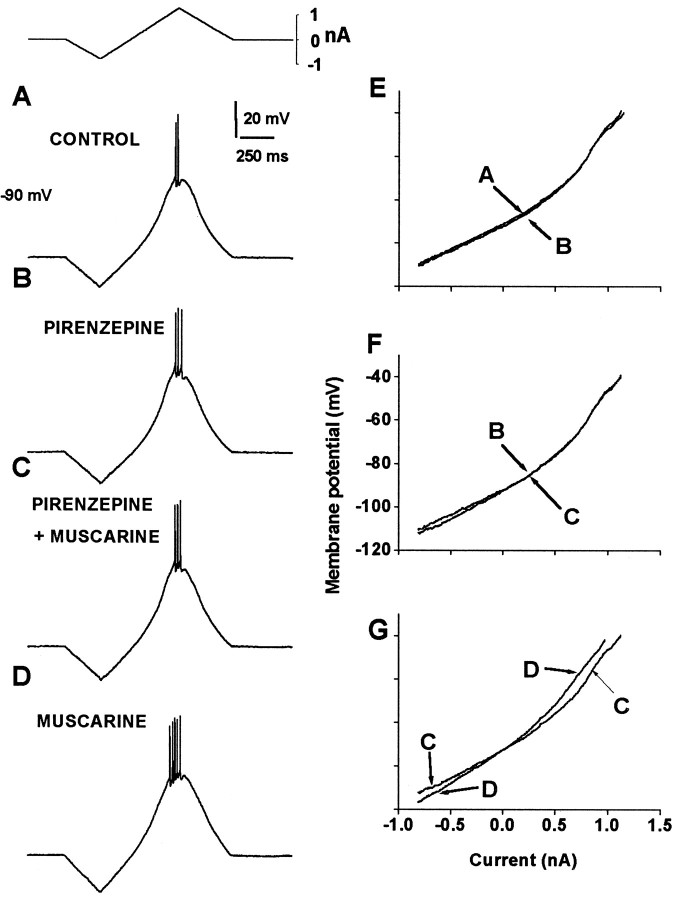
As shown in Figure 4, 25–100 nm pirenzepine, a relatively selective muscarinic M1,4-type receptor antagonist, completely blocked all muscarinic actions on the subthreshold response (Fig.4A–G). Pirenzepine had no action by itself (Figs.4A,E, 5D), but it blocked the postsynaptic effects of 1 μm muscarine (Fig.4B,F), which were recovered when the M1,4 receptor antagonist was removed (Fig.4D,G; n = 4). Neither AF-DX 116 (see Fig. 7) a selective blocker of M2-type receptors, nor 4-DAMP (data not shown), a selective blocker of M3,4-type receptors, could block the muscarinic actions on the spiny neuron. Because the antagonistic action of AF-DX 116 exerts overlapping effects on both M2 and M4 receptors (Caulfield and Birdsall, 1998), and the muscarinic action remains in the presence of MT-3, it was concluded that these muscarinic facilitatory effects are more probably mediated through M1-type receptors, which are abundant on spiny neurons but present in a lesser percentage on the cholinergic interneurons (Hersch et al., 1994; Yan and Surmeier, 1996).
Fig. 4.
Pirenzepine, an M1-like receptor antagonist, blocks the action of muscarine on firing and RN. A, Firing is evoked with a current ramp.B, When 50 nm pirenzepine is added to the superfusion, firing is not significantly changed. RN is not changed by pirenzepine (E), which virtually has no action by itself. C, When 1 μmmuscarine is added in the presence of pirenzepine, no change in firing rate or RN (F) is produced.D, When pirenzepine is washed off, leaving muscarine in the bathing saline, the usual effects of the cholinergic agonist are manifest: enhancement of the firing rate and increase in RN(G). Axes in F(voltage) and G (current) apply to allI–V plots (E–G).
Fig. 5.
AF-DX 116, an M2-like receptor antagonist, mimics the action of muscarine on firing and RN and increases the release of ACh as measured by HPLC.A, Firing is evoked by a current ramp. B,Firing is enhanced when 100 nm AF-DX 116 is added to the superfusion. C, RN is increased during AF-DX 116. D, Using slices similar to those used for intracellular recordings, HPLC determinations of ACh concentrations were done under three conditions: control, in the presence of AF-DX 116, and in the presence of pirenzepine. Box plotsdepict whole sample distributions in the three cases. ACh concentration is significantly higher when AF-DX 116 is present in the bath, showing that AF-DX 116 increases the endogenous ACh release.
Fig. 7.
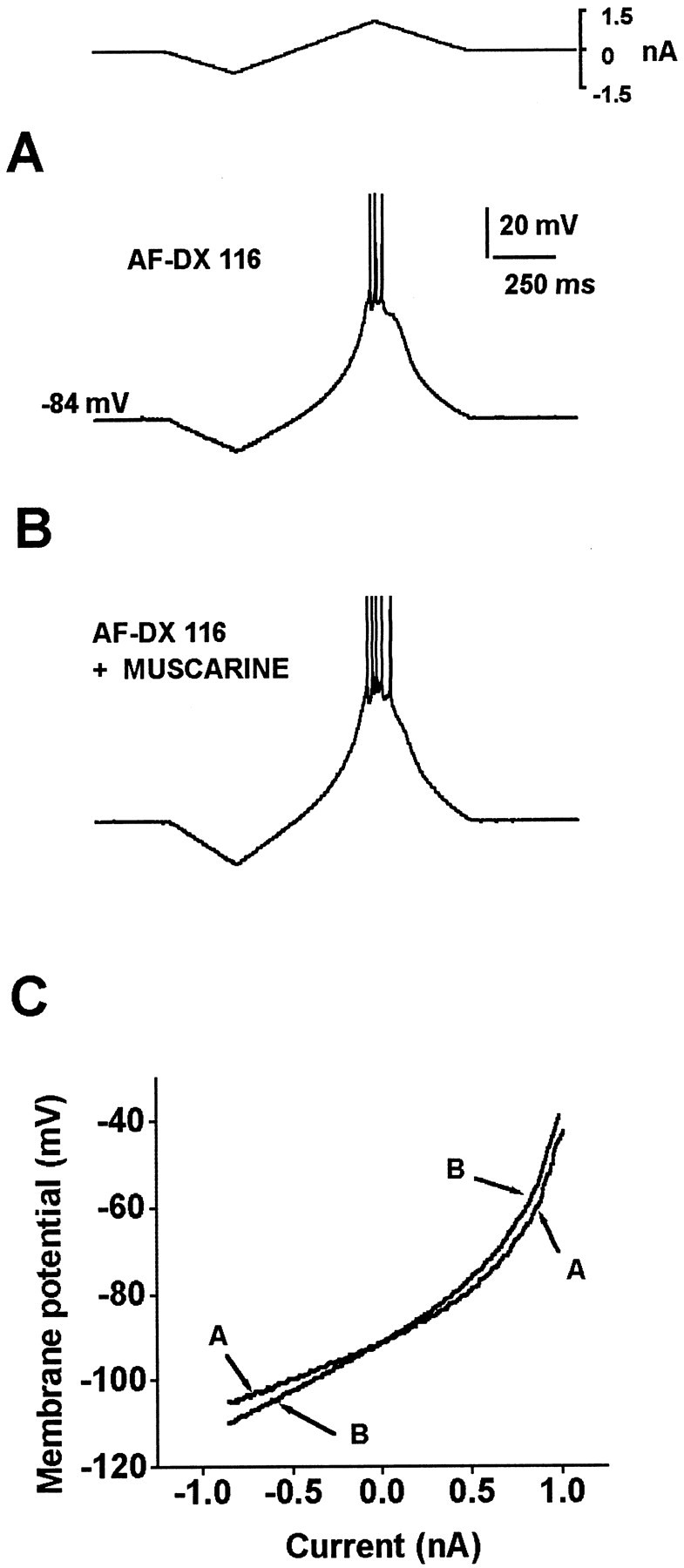
The effects of AF-DX 116 are far from saturation, because the addition of muscarine produces an additional enhancement of the response. A, Firing is evoked with a current ramp in the presence of 100 nm AF-DX 116. B, One micromolar muscarine produces an increase in firing frequency, showing that M2 antagonists do not block this response and that the effects of 100 nm AF-DX 116 are not saturating.C, Muscarine also produces a further increase in RN in the presence of AF-DX 116.
Previous evidence has shown that ACh may modulate (decrease) its own release in the neostriatum (Consolo et al., 1987; Weiler, 1989), probably through the activation of M2-type muscarinic autoreceptors located in the cholinergic interneurons (Hersch et al., 1994; Yan and Surmeier, 1996). And because it has been shown that endogenous ACh is readily detected by the spiny neuron membrane (see above), we tested the idea that the increase in endogenous ACh release induced by M2 receptor blockade is able to modulate the membrane responses of neostriatal output neurons. This would demonstrate a direct action of cholinergic interneurons on spiny projection neurons attributable to muscarinic M2 receptor blockade.
Figure 5 shows that bath application of 50–100 nm concentrations of the relatively selective M2 receptor antagonist AF-DX 116 increases the firing response of spiny neurons to the same stimulus (Fig. 5A,B). This response was always followed by an increase in RN.
Mean RN changed from 31.7 ± 1.7 to 38.8 ± 1.52 MΩ (n = 7; p < 0.02; Wilcoxon’st test). Therefore, the blockade of an M2-type receptor mimicked the activation of an M1-type receptor, revealing a functional antagonism between M1 and M2 types of muscarinic receptors on the neostriatal output. To test whether this action could be imputed to an increase in the slice [ACh]O attributable to M2 autoreceptor blockade, HPLC measurements (with electrochemical detection) were performed in slices similar to those used for electrophysiology. These slices were exposed to concentrations of muscarinic receptor antagonists similar to those used in electrophysiological experiments and compared with those maintained in control saline (see Materials and Methods). Figure 5D shows box plots that demonstrate that the blocking of presynaptic muscarinic autoreceptors by the selective M2 receptor antagonist AF-DX 116 (50–100 nm) leads to an increase in endogenous ACh release in the slice. Mean ACh concentration increased significantly from 1.47 ± 0.25 (n = 22) to 3.72 ± 0.65 pm/20 μl (n = 12) (p < 0.002, Mann–Whitney U test). In contrast, incubation with pirenzepine had no significant effect on ACh efflux: 1.37 ± 0.26 pm/20 μl. These experiments support previous evidence on the existence of a neostriatal cholinergic feedback mediated by autoreceptors (Consolo et al., 1987; Weiler, 1989) and show that this mechanism can be readily demonstrated in slices used for electrophysiology. Therefore, it is sufficient to explain the electrophysiological results; that is, interaction between cholinergic interneurons and projection neurons could be demonstrated in vitro because the released ACh attributable to autoreceptor blockade was able to induce an increase in the evoked discharge of the output neuron. These experiments also show that despite the weak selectivity of the muscarinic receptor antagonists, they can show significant functional differences between the activation of M1- or M2-type receptors when used at low saturating concentrations.
As shown in Figure 6, the indirect action of the relatively selective M2-type receptor antagonist AF-DX 116 (100 nm) on the firing and RN of spiny neurons can be blocked by the relatively selective M1,4 receptor antagonist pirenzepine (100 nm;n = 3). This further shows a functional antagonism between the actions of M1- and M2-type receptors (see Discussion) in the neostriatal microcircuitry and, in particular, on the firing of the output neuron. The action of endogenous ACh is not saturating in these conditions, because the addition of muscarine produces a further increase in firing and RN (Fig. 7). Figure 7 also shows that M2-type receptor antagonists are unable to block the effect of muscarine on the firing and subthreshold RNof the output neuron. On the contrary, the effects of agonist and antagonist are synergistic.
Fig. 6.

Pirenzepine blocks the effects of AF-DX 116.A, Firing is evoked with a current ramp in the presence of 100 nm pirenzepine. B, One hundred nanomolar AF-DX 116 is no longer able to mimic the actions of muscarine in the presence of pirenzepine. C, No change in RN is seen after AF-DX 116 when pirenzepine is present.
DISCUSSION
Given the weak selectivity of available muscarinic antagonists (Caulfield and Birdsall, 1998) and the failure to clearly impute physiological actions to a single type of muscarinic receptor in many systems (e.g., Hernández-Echeagaray et al., 1999), it is reassuring to find out that a clear-cut distinction between the actions of M1- and M2-type receptors can be observed in the neostriatal output neuron (see below). This finding is important because the activity of the spiny neurons is the final output of the circuit and the basis of all pathophysiological models that are basic for understanding the therapeutics for Parkinson’s disease and other motor deficits.
Given that the muscarinic receptor antagonists that were used are not very selective (Caulfield and Birdsall, 1998), the contrasting actions described in this work support the main hypothesis, i.e., the functional segregation of M1- and M2-type receptors in the neostriatal microcircuitry. The experiments show that two relatively selective antagonists for M1,4- and M2-type receptors (Caulfield and Birdsall, 1998), pirenzepine and AF-DX 116, respectively, have clear opposite actions on the output of the microcircuitry: whereas pirenzepine blocks, AF-DX 116 mimics and augments (but does not block) the enhanced excitability produced by muscarine. Because M4-type receptors are present in both large cholinergic interneurons and medium-sized projection neurons (Weiner et al., 1990; Hersch et al., 1994; Yan and Surmeier, 1996), and AF-DX 116 also has some affinity for M4-type receptors, it is then posited that the sharp opposite actions found between pirenzepine and AF-DX 116 have to be attributed to different locations and actions of M1- and M2-type receptors. Hence, they can hardly be assigned to the actions of the M4-type receptor. This last inference is supported by the fact that the facilitatory muscarinic action persists in the presence of MT-3, a selective peptidic toxin against the M4-type receptor.
It is concluded that the M1-type receptor action, i.e., the increase in discharge and RN of spiny neurons (Dodt and Misgeld, 1986; Pineda et al., 1995), is direct or postsynaptic, because neither Cd2+ nor TTX applied to the bathing saline could abolish the effects on RN, and this last action has been reported on dissociated neurons (Hsu et al., 1996). The above conclusion is supported by previous evidence that indicates that neostriatal M1-type receptors are found abundantly on spiny projection neurons and much less on cholinergic interneurons (Weiner et al., 1990; Hersch et al., 1994; Yan and Surmeier, 1996). In addition, only pirenzepine but not AF-DX 116 or 4-DAMP could block these postsynaptic effects when administered at nanomolar concentrations. Moreover, it has been reported that muscarinic activation preferentially enhances GABA release from neostriatal projection neurons (Kayadjanian et al., 1994; Harsing and Zigmond, 1998).
In contrast, M2-type receptors are abundant in cholinergic interneurons and less on projection neurons (Weiner et al., 1990;Hersch et al., 1994; Yan and Surmeier, 1996). Cholinergic interneurons exhibit spontaneous firing and probably maintain a tonic [ACh]O in the neostriatum (Consolo et al., 1987; Wilson et al., 1990; Kawaguchi, 1992). Thus, the location of the M2-type receptors makes them suitable to function as autoreceptors to regulate firing and ACh release by the interneurons (Weiler, 1989; Hersch et al., 1994). In agreement with this hypothesis, the present experiments show that the preferential blockade of M2-type receptors by AF-DX 116 increases the release of endogenous ACh and that this release augments the excitability of spiny projection neurons. The facilitation was mediated by M1-type receptors because the effects of AF-DX 116 could be blocked by pirenzepine. That is, a receptor antagonist blocked the action of another receptor antagonist, evidencing the mainly presynaptic and mainly postsynaptic actions of M2- and M1-type receptors, respectively.
The experiments demonstrate the interaction between the activity of the cholinergic interneuron and the excitability of the projection neuron in the neostriatal microcircuitry. They show that an autoregulation of the cholinergic tone is continuously modulating the output of the projection neuron. It is known that the firing of the projection neuron releases substance P from axon collaterals and that this peptide increases the firing of the cholinergic interneuron (Aosaki and Kawaguchi, 1996; Galarraga et al., 1999). It is also known that both projection and cholinergic interneurons are activated by neostriatal afferents (Wilson, 1998). Thus, in physiological conditions, an excess of ACh released by an active module (Graybiel et al., 1994) would be autoregulated by M2 autoreceptors. They would shut down the firing of the cholinergic interneuron at the moment of maximal concurrent firing (Graybiel et al., 1994) and may also participate in the presynaptic inhibition of the afferent input (Bargas et al., 1998;Hernandez-Echeagaray et al., 1999). This tuning may be critical for motor control, because the action of antimuscarinic drugs on facilitating dopaminergic activation of neuronal activity, motor behavior, and substance P expression is well known (e.g.,Hernández-López et al., 1997; Wang and McGinty, 1997;Galarraga et al., 1999). We propose that this mechanism would regulate the level of activation of a given output module.
Finally, it is important to emphasize what is not shown by the present experiments. On the one hand, the actions of the M4-type receptor in shaping the firing pattern of the projection neuron remain unknown. In a similar way, the actions of dopaminergic D1receptor activation on the spiny neuron are better known than the actions of D2 receptor activation (Surmeier et al., 1995;Hernández-López et al., 1997). On the other hand, the interaction between dopaminergic and cholinergic receptor activation is far from being understood. What is known may predict both synergistic and antagonistic actions during firing. For example, both dopamine and ACh would inhibit N- and P/Q-type Ca2+ currents (Howe and Surmeier, 1995; Surmeier et al., 1995). Because these currents activate the K+ currents that generate the afterhyperpolarizing potential (AHP) (Vilchis et al., 1998; Bargas et al., 1999), it may be predicted that both dopamine and ACh will be synergistic in reducing the AHP and increasing the firing frequency. However, ACh decreases L-type Ca2+ current and inward rectification, whereas dopamine enhances both (Howe and Surmeier, 1995; Surmeier et al., 1995; Hsu et al., 1996; Pacheco-Cano et al., 1996; Cepeda et al., 1998; Galarraga et al., 1999). It has been shown that the L-type Ca2+ channel maintains sustained firing (Hernández-López et al., 1997). Therefore, in these cases the transmitters may be antagonistic. To conclude, more experimental evidence is needed to make a reliable model on cholinergic–dopaminergic interactions at the cell-firing level.
Physiological consequences
If an M2-type receptor antagonist leads to an increased excitability of the projection neuron, which can be blocked by an M1-type receptor antagonist, a question is raised about the use of nonselective antimuscarinic drugs in therapeutics and animal models of motor deficits.
The antagonists used in therapeutics and many behavioral and physiological studies are nonselective. Thus, it is not surprising that the antimuscarinic treatment has not been very reliable (e.g., Kopin, 1993; Riederer et al., 1993). Nevertheless, a sharp distinction between the actions of available M1- and M2-type receptor antagonists can be readily demonstrated on the neostriatal output. Therefore, based on the present work, we predict that the use of more selective antimuscarinics will be more profitable for the therapy of Parkinson’s disease and other motor deficits (Caulfield and Birdsall, 1998). A functional question originated by the present experiments is which of the two classes of antagonists would act synergistically with L-DOPA or other dopaminergic agonists. It would be hard to answer this question a priori.
The cholinergic facilitation of the neostriatal projection neuron
An enhancement on the excitability of spiny neurons by muscarinic activation has been well documented (Dodt and Misgeld, 1986; Pineda et al., 1995; Galarraga et al., 1999). A common finding has been an increase in RN. Inward rectification is known to be present at potentials more negative than approximately −70 or −80 mV (depending on [K+]O) (Galarraga et al., 1994; Nisenbaum and Wilson, 1995; Mermelstein et al., 1998;Reyes et al., 1998). This work shows that, in fact, the blockade of this Cs+-sensitive conductance abolishes the muscarinic actions on RN at this voltage range. However, at more positive potentials (more positive than −70 mV), muscarinic activation still produces an increase in I–V slope that is not blocked by Cs+, Cd2+, TTX, or Co2+. These muscarinic effects could only be occluded by reducing [Na+]O. This occlusion was manifest even if the muscarinic action at potentials more negative than −80 mV was still present (in the absence of Cs+). Taken together, the experiments suggest that muscarinic actions on excitability target several ion conductances, and one of them may be cationic (Inoue and Kuriyama, 1991; Shen and North, 1992; Howe and Surmeier, 1995; Pineda et al., 1995; Haj-Dahmane and Andrade, 1996; Hsu et al., 1996; Klink and Alonso, 1997). Note that effects on Na+ currents have not been discarded by TTX blockade. It is only suggested that TTX-sensitive Na+ currents are not necessary for the main subthreshold actions described here. More experiments are necessary to address this issue specifically.
In conclusion, as in the case of dopamine (Cepeda and Levine, 1998), cholinergic activity within the neostriatum involves many targets in the same neuron and several targets in different neurons of the microcircuitry. More experimentation is needed to elucidate these actions completely and to correlate them with the clinical and behavioral levels.
Footnotes
This work was supported in part by Dirección General de Asuntos del Personal Académico–Universidad Nacional Autónoma de México Grants IN201397 and IN201597 and Consejo Nacional de Ciencia y Tecnologâa Grants 400346-5-25812N and 3260PN9608. We thank Dagoberto Tapia for skillful help in anatomical work.
Correspondence should be addressed to Dr. Elvira Galarraga, Instituto de Fisiología Celular, Universidad Nacional Autónoma de México, P.O. Box 70-253, México DF 04510, México.
REFERENCES
- 1.Adem A, Karlsson E. Muscarinic receptor subtype selective toxins. Life Sci. 1997;60:1069–1076. doi: 10.1016/s0024-3205(97)00049-0. [DOI] [PubMed] [Google Scholar]
- 2.Aosaki T, Kawaguchi Y. Actions of substance P on rat neostriatal neurons in vitro. J Neurosci. 1996;16:5141–5153. doi: 10.1523/JNEUROSCI.16-16-05141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bargas J, Galarraga E. Firing response modulation in neostriatal projection neurons by cholinergic and dopaminergic agonists. In: Ariano MA, Surmeier DJ, editors. Molecular and cellular mechanisms of neostriatal neurons. Landes; Austin, TX: 1995. pp. 183–191. [Google Scholar]
- 4.Bargas J, Howe A, Eberwine J, Cao Y, Surmeier DJ. Cellular and molecular characterization of Ca2+ currents in acutely isolated, adult rat neostriatal neurons. J Neurosci. 1994;14:6667–6686. doi: 10.1523/JNEUROSCI.14-11-06667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bargas J, Ayala GX, Hernandez E, Galarraga E. Ca2+-channels involved in neostriatal glutamatergic transmission. Brain Res Bull. 1998;45:521–524. doi: 10.1016/s0361-9230(97)00439-5. [DOI] [PubMed] [Google Scholar]
- 6.Bargas J, Ayala GX, Vilchis C, Pineda JC, Galarraga E. Ca2+-activated outward currents in neostriatal neurons. Neuroscience. 1999;88:479–488. doi: 10.1016/s0306-4522(98)00211-5. [DOI] [PubMed] [Google Scholar]
- 7.Caulfield MP, Birdsall NJM. Classification of muscarine acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 8.Cepeda C, Levine MS. Dopamine and N-methyl-d-aspartate receptor interactions in the neostriatum. Dev Neurosci. 1998;20:1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- 9.Cepeda C, Colwell CS, Itri JN, Chandler SH, Levine MS. Dopaminergic modulation of NMDA-induced whole-cell currents in neostriatal neurons in slice: contribution of calcium conductances. J Neurophysiol. 1998;79:82–94. doi: 10.1152/jn.1998.79.1.82. [DOI] [PubMed] [Google Scholar]
- 10.Consolo S, Ladinsky H, Vinci R, Palazzi E, Wang J. An in vivo pharmacological study on muscarinic receptor subtypes regulating cholinergic neurotransmission in rat striatum. Biochem Pharmacol. 1987;36:3075–3077. doi: 10.1016/0006-2952(87)90226-7. [DOI] [PubMed] [Google Scholar]
- 11.Dodt HU, Misgeld U. Muscarinic excitation and muscarinic inhibition of synaptic transmission in the rat neostriatum. J Physiol (Lond) 1986;380:593–603. doi: 10.1113/jphysiol.1986.sp016304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flores-Hernández J, Galarraga E, Pineda JC, Bargas J. Patterns of excitatory and inhibitory synaptic transmission in the rat neostriatum as revealed by 4-AP. J Neurophysiol. 1994;72:2246–2256. doi: 10.1152/jn.1994.72.5.2246. [DOI] [PubMed] [Google Scholar]
- 13.Galarraga E, Bargas J, Sierra A, Aceves J. The role of calcium in the repetitive firing of neostriatal neurons. Exp Brain Res. 1989;75:157–168. doi: 10.1007/BF00248539. [DOI] [PubMed] [Google Scholar]
- 14.Galarraga E, Pacheco-Cano MT, Flores-Hernández J, Bargas J. Subthreshold rectification in neostriatal spiny projection neurons. Exp Brain Res. 1994;100:239–249. doi: 10.1007/BF00227194. [DOI] [PubMed] [Google Scholar]
- 15.Galarraga E, Hernández-López S, Vilchis C, Miranda MI, Reyes A, Bermudez-Rattoni F, Bargas J. Functional antagonism between muscarinic receptor antagonists in the neostriatum. Soc Neurosci Abstr. 1998;24:1640. doi: 10.1523/JNEUROSCI.19-09-03629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galarraga E, Hernández-López S, Tapia D, Reyes A, Bargas J (1999) Action of substance P (neurokinin-1) receptor activation on rat neostriatal projection neurons. Synapse, in press. [DOI] [PubMed]
- 17.Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez H, Miranda MI, Bermudez-Rattoni F. Learning impairment and cholinergic deafferentation after cortical nerve growth factor deprivation. J Neurosci. 1997;17:3796–3803. doi: 10.1523/JNEUROSCI.17-10-03796.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haj-Dahmane S, Andrade R. Muscarinic activation of a voltage-dependent cation nonselective current in rat association cortex. J Neurosci. 1996;16:3848–3861. doi: 10.1523/JNEUROSCI.16-12-03848.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harsing LG, Zigmond MJ. Postsynaptic integration of cholinergic and dopaminergic signals on medium-sized GABAergic projection neurons in the neostriatum. Brain Res Bull. 1998;45:607–613. doi: 10.1016/s0361-9230(97)00460-7. [DOI] [PubMed] [Google Scholar]
- 21.Hernández-Echeagaray E, Galarraga E, Bargas J. 3-α-Chloro-imperialine, a potent blocker of cholinergic presynaptic modulation of glutamatergic afferents in the rat neostriatum. Neuropharmacology. 1999;37:1493–1502. doi: 10.1016/s0028-3908(98)00131-2. [DOI] [PubMed] [Google Scholar]
- 22.Hernández-López S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hersch SM, Gutekunst C, Rees HD, Heilman CJ, Levey AI. Distribution of m1–m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horikawa A, Armstrong WE. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods. 1988;25:1–11. doi: 10.1016/0165-0270(88)90114-8. [DOI] [PubMed] [Google Scholar]
- 25.Howe A, Surmeier DJ. Muscarinic receptors modulate N-, P-, and L-type Ca2+ currents in rat striatal neurons through parallel pathways. J Neurosci. 1995;15:458–469. doi: 10.1523/JNEUROSCI.15-01-00458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu KS, Yang CH, Huang CC, Gean PW. Carbachol induces inward current in neostriatal neurons through M1-like muscarinic receptors. Neuroscience. 1996;73:751–760. doi: 10.1016/0306-4522(96)00066-8. [DOI] [PubMed] [Google Scholar]
- 27.Inoue M, Kuriyama H. Muscarinic receptor is coupled with a cation channel through a GTP-binding protein in guinea-pig chromaffin cells. J Physiol (Lond) 1991;436:511–529. doi: 10.1113/jphysiol.1991.sp018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jahnsen H, Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol (Lond) 1984;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawaguchi Y. Large aspiny cells in the matrix of the rat neostriatum in vitro: physiological identification, relation to the compartments and excitatory postsynaptic currents. J Neurophysiol. 1992;67:1669–1682. doi: 10.1152/jn.1992.67.6.1669. [DOI] [PubMed] [Google Scholar]
- 30.Kayadjanian N, Gioanni H, Menetrey A, Besson MJ. Musacrinic receptor stimulation increases the spontaneous [3H]GABA release in the rat substantia nigra through muscarinic receptors localized on sitriatonigral terminals. Neuroscience. 1994;63:989–1002. doi: 10.1016/0306-4522(94)90567-3. [DOI] [PubMed] [Google Scholar]
- 31.Klink R, Alonso A. Ionic mechanisms of muscarinic depolarization in entorhinal cortex layer II neurons. J Neurophysiol. 1997;77:1829–1843. doi: 10.1152/jn.1997.77.4.1829. [DOI] [PubMed] [Google Scholar]
- 32.Kopin LJ. Parkinson’s disease: past, present and future. Neuropsychopharmacology. 1993;9:1–12. doi: 10.1038/npp.1993.39. [DOI] [PubMed] [Google Scholar]
- 33.Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol. 1998;80:572–582. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- 34.Mermelstein PG, Song WJ, Tkatch T, Yan Z, Surmeier DJ. Inwardly rectifying potassium (IRK) currents are correlated with IRK subunit expression in rat nucleus accumbens medium spiny neurons. J Neurosci. 1998;18:6650–6661. doi: 10.1523/JNEUROSCI.18-17-06650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misgeld U, Calabresi P, Dodt HU. Muscarinic modulation of calcium dependent plateau potentials in rat neostriatal neurons. Pflügers Arch. 1986;407:482–487. doi: 10.1007/BF00657504. [DOI] [PubMed] [Google Scholar]
- 36.Nisenbaum ES, Wilson CJ. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J Neurosci. 1995;15:4449–4463. doi: 10.1523/JNEUROSCI.15-06-04449.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olianas MC, Adem A, Karlsson E, Onali P. Rat striatal muscarinic receptors coupled to inhibition of adenylyl cyclase activity: potent block by the selective m4 ligand muscarinic toxin 3 (MT3). Br J Pharmacol. 1996;118:283–288. doi: 10.1111/j.1476-5381.1996.tb15400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pacheco-Cano MT, Bargas J, Hernández-López S, Tapia D, Galarraga E. Inhibitory action of dopamine involves a subthreshold Cs+-sensitive conductance in neostriatal neurons. Exp Brain Res. 1996;110:205–211. doi: 10.1007/BF00228552. [DOI] [PubMed] [Google Scholar]
- 39.Pineda JC, Bargas J, Flores-Hernández J, Galarraga E. Muscarinic receptors modulate the afterhyperpolarizing potential in neostriatal neurons. Eur J Pharmacol. 1995;281:271–277. doi: 10.1016/0014-2999(95)00263-k. [DOI] [PubMed] [Google Scholar]
- 40.Potter LT, Purkerson SL. Pharmacology of striatal cholinergic receptors. In: Ariano MA, Surmeier DJ, editors. Molecular and cellular mechanisms of neostriatal function. Landes; Austin, TX: 1995. pp. 241–254. [Google Scholar]
- 41.Purkerson SL, Potter LT. Use of antimuscarinic toxins to facilitate studies of striatal m4 muscarinic receptors. J Phamacol Exp Ther. 1998;284:707–713. [PubMed] [Google Scholar]
- 42.Reyes A, Galarraga E, Flores-Hernández J, Tapia D, Bargas J. Passive properties of neostriatal neurons during potassium conductance blockade. Exp Brain Res. 1998;120:70–84. doi: 10.1007/s002210050379. [DOI] [PubMed] [Google Scholar]
- 43.Riederer P, Lange KW, Youdim MBH. Recent advances in pharmacological therapy of Parkinson’s disease. In: Narabayashi H, Nagatsu T, Yanagisawa N, Mizuno Y, editors. Parkinson’s disease: from basic research to treatment, Advances in Neurology 60. Raven; New York: 1993. pp. 626–635. [PubMed] [Google Scholar]
- 44.Shen KZ, North RA. Muscarine increases cation conductance and decreases potassium conductance in rat locus coeruleus neurons. J Physiol (Lond) 1992;455:471–485. doi: 10.1113/jphysiol.1992.sp019312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Surmeier DJ, Bargas J, Hemmings HC, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 46.Tukey JW. Exploratory data analysis. Addison–Wesley; Menlo Park, CA: 1977. [Google Scholar]
- 47.Uchimura N, North RA. Muscarine reduces inwardly rectifying potassium conductance in rat nucleus accumbens neurones. J Physiol (Lond) 1990;422:369–380. doi: 10.1113/jphysiol.1990.sp017989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vilchis MC, Ayala GX, Galarraga E, Bargas J. Calcium channels that activate Ca2+ activated K+ currents in neostriatal projection neurons. Soc Neurosci Abstr. 1998;24:1640. [Google Scholar]
- 49.Wang JQ, McGinty JF. Intrastriatal injection of a muscarinic receptor agonist and anatgonist regulates striatal neuropeptide mRNA expression in normal and amphetamine-treated rats. Brain Res. 1997;748:62–70. doi: 10.1016/s0006-8993(96)01244-9. [DOI] [PubMed] [Google Scholar]
- 50.Weiler M. Muscarinic modulation of endogenous acetylcholine release in rat neostriatal slices. J Pharmacol Exp Ther. 1989;250:617–623. [PubMed] [Google Scholar]
- 51.Weiner D, Levey A, Brann M. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Neurobiology. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson CJ. Basal ganglia. In: Shepherd GM, editor. The synaptic organization of the brain. Oxford UP; Oxford: 1998. pp. 279–316. [Google Scholar]
- 53.Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan Z, Surmeier DJ. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J Neurosci. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]