Abstract
Recent studies have shown that release of mitochondrial cytochrome c is a critical step in the apoptosis process. We have reported that cytosolic redistribution of cytochrome c in vivooccurred after transient focal cerebral ischemia (FCI) in rats and preceded the peak of DNA fragmentation. Although the involvement of reactive oxygen species in the cytosolic redistribution of cytochrome cin vitro has been suggested, the detailed mechanism by which cytochrome c release is mediated in vivo has not yet been established. Also, the role of mitochondrial oxidative stress in cytochrome c release is unknown. These issues can be addressed using knock-out mutants that are deficient in the level of the mitochondrial antioxidant manganese superoxide dismutase (Mn-SOD). In this study we examined the subcellular distribution of the cytochrome c protein in both wild-type mice and heterozygous knock-outs of the Mn-SOD gene (Sod2 −/+) after permanent FCI, in which apoptosis is assumed to participate. Cytosolic cytochrome c was detected as early as 1 hr after ischemia, and correspondingly, mitochondrial cytochrome c showed a significant reduction 2 hr after ischemia (p< 0.01). Cytosolic accumulation of cytochrome c was significantly higher in Sod2 −/+ mice compared with wild-type animals (p < 0.05).N-benzyloxycarbonyl-val-ala-asp-fluoromethyl ketone (z-VAD.FMK), a nonselective caspase inhibitor, did not affect cytochrome c release after ischemia. A significant amount of DNA laddering was detected 24 hr after ischemia and increased in Sod2 −/+ mice. These data suggest that Mn-SOD blocks cytosolic release of cytochrome c and could thereby reduce apoptosis after permanent FCI.
Keywords: cerebral ischemia, cytochrome c, manganese superoxide dismutase, apoptosis, mitochondrial injury, reactive oxygen species, caspase
Cytochrome c, a water-soluble peripheral membrane protein of the mitochondria, is known to be an essential component of the mitochondrial respiratory chain (Boyer et al., 1977). Its function is to transport electrons from the coenzyme QH2–cytochrome c reductase complex to the cytochrome c oxidase complex in the electron transport chain. Growing evidence suggests that cytochrome c participates in apoptosis in intact cells (Kluck et al., 1997; Yang et al., 1997) as well as in cell-free systems (Liu et al., 1996). Mitochondria are assumed to be involved in apoptosis by releasing cytochrome c to the cytoplasm where it activates caspase 3 (CPP32), a cysteine protease of the interleukin 1β-converting enzyme family, that has been shown then to trigger apoptosis (Liu et al., 1996; Green and Reed, 1998). In agreement with this finding, microinjection of cytochrome c has been shown to result in apoptosis (Li et al., 1997). It has also been shown that the expression of Bcl-2 in the mitochondrial outer membrane acts to inhibit cytochrome c translocation, thereby blocking CPP32 activation and the apoptotic process (Kluck et al., 1997; Yang et al., 1997; Rosse et al., 1998). Although the mechanism by which Bcl-2 prevents cytosolic redistribution of cytochrome c has not been established, a recentin vitro study showed that overexpression of Bcl-2 prevents superoxide production and then blocks cytochrome c release and apoptosis (Cai and Jones, 1998), suggesting that the antioxidant function of Bcl-2 contributes to the inhibition of cytochrome c release and subsequent apoptosis. It has also been reported that thioredoxin peroxidase, another antioxidant that functions as a peroxidase, was able to inhibit the release of cytochrome c from mitochondria to cytosol during apoptosis (Zhang et al., 1997).
The antioxidant enzyme is thought to be one of the major mechanisms by which cells counteract the deleterious effects of reactive oxygen species (ROS) after focal cerebral ischemia (FCI). We have shown evidence that superoxide dismutase (SOD) plays a protective role against FCI (Kinouchi et al., 1991; Chan, 1996; Kondo et al., 1997;Murakami et al., 1998) as well as against global ischemia (Kawase et al., 1997). Our recent study demonstrated that the mitochondrial overproduction of superoxide exacerbated cerebral infarction after permanent FCI in mutant mice with a heterozygous knock-out gene (Sod2 −/+) encoding mitochondrial manganese superoxide dismutase (Mn-SOD) (Murakami et al., 1998). This study suggested the involvement of mitochondrial ROS production and the protective role of mitochondrial Mn-SOD after permanent ischemia. However, it has not yet been determined whether Mn-SOD, which is also an endogenous mitochondrial antioxidant like Bcl-2, could affect mitochondrial cytochrome c release to cytosol, thereby preventing apoptosis after permanent ischemia. The present study is designed to clarify this critical issue by examining the early release of mitochondrial cytochrome c to cytosol and DNA fragmentation using both wild-type and Sod2 −/+ mice (Li et al., 1995b) after permanent FCI, in which apoptosis, as well as necrosis, participates (Linnik et al., 1993; Tominaga et al., 1993; Gillardon et al., 1996; Asahi et al., 1997).
MATERIALS AND METHODS
Focal cerebral ischemia. Because homozygous knock-outs (Sod2 −/−) showed neonatal lethality because of the dilated cardiomyopathy (Li et al., 1995b), heterozygous knock-outs (Sod2 −/+) were used in the present study. The Sod2 −/+ mice with a CD1/SV129 background were backcrossed with CD1 mice for five generations. These mice and their wild-type littermates with a genetic background identical to that of the Sod2 −/+ mice (3-month-old males; 35–40 gm) were then subjected to permanent focal cerebral ischemia. There were no differences in the anatomy of the cerebral vasculature and cerebral blood flow after ischemia between the Sod2 −/+ mice and wild-type littermates (Murakami et al., 1998). Focal ischemia was induced by intraluminal middle cerebral artery (MCA) occlusion with a nylon monofilament suture as described previously (Yang et al., 1994). The mice were anesthetized with 2.0% isoflurane in 30% oxygen and 70% nitrous oxide using a face mask. The rectal temperature was controlled at 37°C with a homeothermic blanket. Cannulation of a femoral artery allowed the monitoring of blood pressure and arterial blood gases, samples for analysis being taken immediately after cannulation and 5 min after occlusion. After the midline skin was incised, the left external carotid artery was exposed, and its branches were electrocoagulated. An 11.0 mm 5-0 surgical monofilament nylon suture, blunted at the end, was introduced into the left internal carotid artery through the external carotid artery stump. At the end of surgery, the suture was tightly fixed at the final position. To examine the effect of the caspase inhibitor on cytochrome c release, we injected a nonselective caspase inhibitor,N-benzyloxycarbonyl-val-ala-asp-fluoromethyl ketone (z-VAD.FMK) (100 ng in 0.25% dimethylsulfoxide in PBS), and the vehicle (0.25% dimethylsulfoxide in PBS) intracerebroventricularly (2 μl; from bregma, 1.0 mm lateral, 0.2 mm posterior, and 3.1 mm deep) into three wild-type animals in each group 15 min before MCA occlusion.
In situ detection of superoxide anion production. The early production of superoxide anion (O2−) in cerebral ischemia was investigated using hydroethidine (HEt) by the previously described method (Murakami et al., 1998). HEt is diffusible into the CNS parenchyma after an intravenous injection and is selectively oxidized to ethidium (Et) by O2−but not by other ROS such as hydrogen peroxide, hydroxyl radical, or peroxynitrite (Bindokas et al., 1996; Murakami et al., 1998). HEt solution [200 μl; stock solution of HEt (100 mg/ml in dimethylsulfoxide) diluted to 1 mg/ml with PBS] was administered intravenously 15 min before ischemia induction as described (Murakami et al., 1998). In the brains of animals intravenously injected with HEt, fluorescence was assessed microscopically at excitation (Ex) = 355 nm and emission (Em) > 415 nm for HEt detection or at Ex = 510–550 nm and Em > 580 nm for Et detection. Animals were killed 2 hr after ischemia induction by transcardial perfusion as described (Fujimura et al., 1998). After fixation with 3.7% formaldehyde for 2 hr, 50-μm-thick brain sections at the level of the anterior commissure were placed on glass slides using a vibratome. These sections were observed with a microscope under fluorescent light. Photomicrographs of the fluorescent microscopy were taken in both the ischemic and nonischemic hemispheres, and the intensity and expression patterns of the oxidized HEt were observed and compared between the wild-type and knock-out mice. To analyze the fluorescence signal of HEt, we scanned photomicrographs (×630) with a GS-700 imaging densitometer (Bio-Rad, Hercules, CA) and then measured the signal intensity in 12 individual cells in each group using Multi-Analyst software (Bio-Rad).
Western blot analysis of cytochrome c. Protein extraction of both the mitochondrial and cytosolic fractions was performed as described (Fujimura et al., 1998). Approximately 50 mg of ischemic brain and nonischemic brain from the corresponding contralateral brain was cut into pieces after 1, 2, 4, and 24 hr of permanent ischemia and gently homogenized by douncing 30 times in a glass tissue grinder (Wheaton, Millville, NJ) in 7 vol of cold suspension buffer [20 mm HEPES-KOH, pH 7.5, 250 mm sucrose, 10 mm KCl, 1.5 mm MgCl2, 1 mm EDTA, 1 mm EGTA, 1 mm DTT, 0.1 mm PMSF, 2 μg/ml aprotinin, 10 μg/ml leupeptin, 5 μg/ml pepstatin, and 12.5 μg/mlN-acetyl-leu-leu-norleucinal (ALLN)]. The homogenates were centrifuged at 750 × g at 4°C and then at 8000 × g for 20 min at 4°C. The 8000 ×g pellets were used to obtain the mitochondrial fraction. The supernatant was further centrifuged at 100,000 × gfor 60 min at 4°C. Protein concentrations were determined by the Bradford method (Bio-Rad), and exactly 3.6 μg of protein from the cytosolic fraction and 2.2 μg from the mitochondrial fraction were loaded per lane. The primary antibodies were either a 1:1000 dilution of rabbit cytochrome c polyclonal (Santa Cruz Biotechnology, Santa Cruz, CA) or 1 μg/ml 20E8C12 cytochrome oxidase (COX) subunit IV monoclonal (Molecular Probes, Eugene, OR). Western blots were performed with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG using enhanced chemiluminescence Western blotting detection reagents (Amersham, Buckinghamshire, England). A densitometric analysis was made of the results of the mitochondrial fraction from both ischemic and nonischemic brain (n = 6 each) and of the cytosolic fraction of the ischemic brain from both wild-type and Sod2 −/+ mice (n = 5). The film was scanned by a GS-700 imaging densitometer (Bio-Rad), and the results were quantified using Multi-Analyst software (Bio-Rad). To avoid the conditional inconsistency between the results obtained from different membranes, we used the optical density ratio (ODR), defined as the nonischemic/postischemic ratio of the optical density (OD) in each animal, to analyze the amount of the reduction of mitochondrial cytochrome c and/or COX after ischemia. Western blot analysis of β-actin was performed with horseradish peroxidase-conjugated anti-mouse IgG reagents (Amersham).
Immunohistochemistry. Anesthetized animals were perfused with 10 U/ml heparin and subsequently with 4% formaldehyde in 0.1m PBS, pH 7.4, at 1, 2, 4, and 24 hr after permanent ischemia. Brains were removed, post-fixed for 36 hr, sectioned at 50 μm on a vibratome, and processed for immunohistochemistry. The sections were incubated with blocking solution as described (Fujimura et al., 1998) and reacted with anti-cytochrome c polyclonal antibody (Santa Cruz Biotechnology) at a dilution of 1:100. Immunohistochemistry was performed using the avidin–biotin technique, and then the nuclei were counterstained with methyl green solution for 10 min. As a negative control, sections were incubated without primary antibodies. For histological assessment, alternate slices from each brain section were stained with cresyl violet.
Gel electrophoresis. Animals were killed at 4 and 24 hr after permanent ischemia. Thirty to fifty milligrams wet weight of ischemic tissue were taken from the third 2 mm section along with homologous tissue from the contralateral side after the brain was cut coronally. Samples were incubated overnight in 0.6 ml of lysis buffer (0.5% SDS, 10 mm Tris-HCl, and 0.1 m EDTA) with 0.6 mg of proteinase K (Boehringer Mannheim, Indianapolis, IN) at 55°C. The DNA was extracted with equal volumes of phenol and phenol–chloroform–isoamyl alcohol (25:24:1) and precipitated overnight in 0.2 m sodium chloride in 100% ethanol at −80°C. The DNA was washed twice with 75% ethanol, air dried, and resuspended in DNase-free water (Sigma, St. Louis, MO). The DNA concentration was measured using To-Pro-1 dye (Molecular Probes). Gel electrophoresis for detecting DNA laddering was performed according to the manufacturer’s instructions (Trevigen, Gaithersburg, MD). Before electrophoresis, 1 μg of DNA was incubated with 50 μg/ml DNase-free RNase (Boehringer Mannheim) for 30 min at 37°C. Then the samples were reacted with Klenow enzyme (Trevigen) and dNTP (Trevigen) in 1× Klenow buffer (Trevigen) for 10 min at room temperature. Samples were mixed with loading buffer and subjected to electrophoresis on a 1.5% agarose gel. Then the gel was washed with 0.25 m HCl, 0.4m NaOH or 0.8 m NaCl, and 0.5 mTris buffer, pH 7.5. DNA was transferred to a nylon membrane overnight in 10× SSC. The membrane was first blocked by 5% powdered milk (Bio-Rad) in PBS for 30 min and incubated with Strept-HRP conjugate (Trevigen) for 30 min. Finally, the bands were visualized by the chemiluminescence method using PeroxyGlow (Trevigen), and the films were exposed to x-ray film. The bands of both genomic DNA and 200 ladder were scanned by a GS-700 imaging densitometer (Bio-Rad) and were quantified using Multi-Analyst software (Bio-Rad).
RESULTS
Physiological data and cerebral infarction
Physiological parameters showed no significant differences in mean arterial blood pressure and arterial blood gas analysis between Sod2 −/+ mice and wild-type littermates both before and after MCA occlusion. The preischemic physiological variables were as follows (wild type vs Sod2 −/+): mean arterial blood pressure, 77.3 ± 10.0 versus 76.0 ± 6.98 mmHg; PaO2, 147.0 ± 9.9 versus 140.4 ± 17.1 mmHg; PaCO2, 25.6 ± 7.8 versus 26.9 ± 11.2 mmHg; and pH, 7.37 ± 0.06 versus 7.38 ± 0.02 (variables are mean ± SD; n = 4). An ischemic lesion in the core of the caudate putamen was visible as a pale, slightly stained area in the ischemic hemisphere and was seen as early as 1 hr after permanent ischemia and extended to the entire MCA territory at 4 hr by cresyl violet staining (data not shown). The time-dependent increase of infarction in mouse brain using the intraluminal suture blockade is consistent with a previous report that used the same focal stroke model in mice (Murakami et al., 1998).
Production of O2− after permanent cerebral ischemia
Production of O2− was determined using HEt, a fluorescent dye selectively oxidized to Et by O2−, 2 hr after permanent focal cerebral ischemia as described previously (Murakami et al., 1998). Because we administered HEt 15 min before MCA occlusion, this fluorescent probe was sufficiently distributed, even to the brain tissue of the ischemic area where the blood vessels were occluded (Murakami et al., 1998). In the current study, O2− production was shown by oxidized HEt signals as small particles in the cytosol under normal physiological conditions (Fig.1A,B), which is consistent with previous observations (Kondo et al., 1997;Murakami et al., 1998). These vesicular signals were increased in the knock-out mutants (Fig. 1B) compared with the wild-type animals (Fig. 1A). Two hours after ischemia, the cytosolic O2− signal markedly increased in both wild-type and mutant mice (Fig.1C,D). The vesicular O2−signals were masked with diffuse cytosolic signals in the wild-type mice (Fig. 1C), whereas strong vesicular HEt signals, as well as enhanced cytosolic signals, were observed in the mutant mice (Fig. 1D). The mean intensity of the HEt signal was significantly higher in mutant mice (0.300 ± 0.059; mean optical density ± SD) than in wild-type mice (0.213 ± 0.035) (p < 0.001). These results that showed increased superoxide radical signals in mutant mice in ischemic and nonischemic brain are considered to be because of the 50% reduction of Sod2 activity in the mutant mice compared with that in the wild-type mice (Li et al., 1995b).
Fig. 1.
Representative photomicrographs showing the production of O2− by the expression of oxidized HEt in both wild-type and Sod2 −/+ mouse brains.A, B, A slight increase in the basal level of O2− was observed under normal physiological conditions in the mutant mice (B) compared with the wild-type mice (A). Under normal physiological conditions, HEt signals were detected as small particles in the cytosol, indicating mitochondrial production of O2− (Murakami et al., 1998). The size of the particles is apparently larger in the Sod2 −/+ mice compared with the wild-type animals. Two hours after permanent ischemia, the intense and different subcellular pattern of HEt signals was observed in the ischemic brain. C, D, Homogenous cytosolic expression of the HEt signals is shown in the wild-type mice (C), whereas predominant vesicular patterns, in addition to the diffuse cytosolic expression, are observed in the Sod2 −/+ animals (D), suggesting enhanced mitochondrial production of O2− in the Sod2 −/+ mice after permanent ischemia. Scale bar, 20 μm.
Western blot analysis demonstrating the early release of mitochondrial cytochrome c
As shown in Figure 2, cytochrome c immunoreactivity was evident as a single band of molecular mass 15 kDa in the cytosolic fraction in the ischemic brain in the wild-type mice as early as 1 hr after permanent MCA occlusion, whereas it was barely detected in either the normal control brain (lane 1) or the contralateral brain (lane 2). The characteristic single band in the ischemic sample increased in a time-dependent manner after permanent ischemia (Fig. 2). These data not only confirm the specificity of the polyclonal antibody for cytochrome c used in this study but also show that cytosolic localization of cytochrome c was significantly increased after permanent ischemia. The mitochondrial fraction of cytochrome c was also examined in the wild-type animals 2 hr after permanent ischemia. As shown in Figure3, a significant amount of mitochondrial cytochrome c was detected in the nonischemic brain, and this decreased after permanent ischemia. Correspondingly, the cytosolic fraction from the same sample showed a marked increase of cytochrome c in the ischemic brain (Fig. 3). COX was strongly expressed in the mitochondrial fraction but not in the cytosolic fraction in both the ischemic and nonischemic brain (Fig. 3). Decrease of mitochondrial cytochrome c was further confirmed by statistically analyzing the nonischemic/postischemic ratio of the ODR (n = 6 each). The amount of mitochondrial cytochrome c was significantly decreased in the ischemic brain (ODR = 0.477 ± 0.270) compared with the nonischemic brain (p < 0.01). Reduction of COX was also detected after ischemia (ODR = 0.759 ± 0.108). However, ODR was significantly lower in cytochrome c than in COX (p < 0.05), suggesting a lesser amount of reduction in COX 2 hr after ischemia. Finally, the amount of cytosolic cytochrome c was compared between Sod2 −/+ mice and wild-type mice 2 hr after permanent cerebral ischemia (Fig.4). There was no difference in the β-actin level between the wild-type and Sod2 −/+ mice. The mean OD of the characteristic bands from the Sod2 −/+ mice (8.33 ± 4.07) was significantly higher than that from the wild-type mice (2.52 ± 1.69) 2 hr after ischemia (p < 0.05;n = 5), indicating that cytosolic localization of cytochrome c was significantly increased in the mutant mice compared with the wild-type mice.
Fig. 2.
Western blot analysis of cytosolic cytochrome c from wild-type mice. Top, Cytochrome c (Cyt. c) from the cytosolic fraction in the control and nonischemic brains (lane 1 on theleft), 24 hr after ischemia (lane 2), and in the ischemic brains (lanes 3–6), with 3.6 μg of protein loaded per lane. Cytochrome c immunoreactivity was evident as a single band of molecular mass 15 kDa in the cytosolic fraction in the ischemic brain as early as 1 hr after ischemia (lane 3), whereas it was barely detected in both the normal control brain and the contralateral brain. A time-dependent increase of cytosolic cytochrome c was observed.Bottom, The result of the β-actin analysis shown as an internal control. The results shown are representative of two independent studies. C, Control brain.
Fig. 3.
Western blot analysis of the mitochondrial fraction. Top, Cytochrome c from a mitochondrial fraction and from a cytosolic fraction. Bottom, COX from mitochondria and from a cytosolic fraction in ischemic and nonischemic brains from wild-type mice. The results shown are representative of two independent studies. The amount of mitochondrial cytochrome c was significantly decreased in the ischemic brain (optical density ratio = 0.477 ± 0.270) compared with the nonischemic brain (p < 0.01; n = 6).C, Nonischemic brain 2 hr after ischemia;I, ischemic brain 2 hr after ischemia.
Fig. 4.
Top, Western blot analysis of cytosolic cytochrome c in wild-type mice and Sod2 −/+ mice 2 hr after ischemia. The data shown are from three different animals of each group; 3.6 μg of protein was loaded per lane. Bottom, The result of the β-actin analysis as an internal control. The mean optical density of the characteristic bands from the Sod2 −/+ mice (8.33 ± 4.07) was significantly higher than that from the wild-type mice (2.52 ± 1.69) 2 hr after ischemia (p < 0.05; n = 5), indicating that cytosolic redistribution of cytochrome c was significantly increased in mutant mice compared with wild-type mice.Con, Control brain; Wt, wild type.
Cytosolic expression of cytochrome c was detected by immunohistochemistry in ischemic brain after permanent cerebral ischemia
Cytochrome c protein expression after permanent focal ischemia was also analyzed by immunohistochemistry in the wild-type mice (Fig.5) and was compared between mutant mice and wild-type animals 2 hr after ischemia (Fig.6). The data on the cytosolic cytochrome c expression in immunohistochemistry are summarized in Table1. Homogenous cytoplasmic immunoreactivity of cytochrome c was visible as early as 1 hr after permanent ischemia in the wild-type mice (Fig. 5B,arrowheads). After 2 hr of ischemia, the cytosolic immunoreactivity was slightly increased; however, immunopositive cells with neuronal morphology were still intact (Fig. 5C,arrowheads). Four hours after ischemia, cytosolic immunoreactivity was further increased (Fig. 5D,arrowheads). At 24 hr, a marked increase in the cytosolic immunoreactivity was observed in the cells with the ischemic morphology (Fig. 5E, arrowheads) as described (Li et al., 1997). Most of the cells were destroyed, and the background immunoreactivity was markedly increased (Fig. 5E). Through all time courses, cytochrome c was predominantly expressed in the MCA territory cortex and the piriform cortex, whereas it was barely expressed in the ischemic core of the caudate putamen, except 24 hr after ischemia. There was no immunoreactivity in the contralateral hemisphere or in the control specimens, which were treated without a primary antibody (Fig. 5F). The absence of immunoreactivity in the nonischemic brain (Fig. 5A), which is consistent with our previous study of transient ischemia in rats (Fujimura et al., 1998), is considered to be because of the thorough fixation of the brain with formaldehyde, which prevented the antibody from reaching the mitochondrial intermembrane space but not the cytosol. In fact, immunohistochemistry with frozen sections resulted in dotted cytosolic immunoreactivity of cytochrome c in the control brain (data not shown). The cytosolic cytochrome c expression was further compared between wild-type mice and Sod2 −/+ mice (Fig.6A–D) and between z-VAD.FMK-treated wild-type mice and vehicle-treated wild-type animals (Fig.6E,F). Cytochrome c immunoreactivity was barely seen in the nonischemic specimens both from wild-type (Fig.6A) and Sod2 −/+ (Fig. 6C) mice. Two hours after ischemia, enhanced cytosolic immunoreactivity of cytochrome c was demonstrated in the Sod2 −/+ mice (Fig. 6D) compared with the wild-type mice (Fig. 6B). At this time point, diffuse cytosolic immunoreactivity was shown in the wild-type mice (Fig. 6B), whereas strong vesicular immunoreactivity, as well as diffuse cytosolic expression, was observed in the Sod2 −/+ mice (Fig. 6D). No remarkable difference in cytochrome c immunoreactivity was observed between the vehicle-treated mice (Fig. 6E, arrowheads) and the z-VAD.FMK-treated animals (Fig. 6F,arrowheads) 2 hr after ischemia. The specificity of the anti-cytochrome c polyclonal antibody was confirmed by Western blot analysis using rat heart cytochrome c (Sigma) (data not shown).
Fig. 5.

Cytochrome c immunostaining with methyl green counterstaining in coronal brain sections from wild-type mice 1, 2, 4, and 24 hr after permanent ischemia. A, The normal control cortex from wild-type mice presenting no immunoreactivity of cytochrome c. B, C, Ischemic cortex from wild-type mice showing cytosolic immunoreactivity of cytochrome c in the morphologically intact cells as early as 1 hr (B,arrowheads) after permanent ischemia, which was increased 2 hr (C, arrowheads) after ischemia. D, Ischemic cortex 4 hr after ischemia (arrowheads) presenting enhanced cytosolic immunoreactivity in the cells that show ischemic morphology.E, Ischemic cortex 24 hr after ischemia (arrowheads) showing increased background immunoreactivity as well as enhanced cytosolic intensity.F, The ischemic cortical section incubated in the absence of primary antibody for cytochrome c. Scale bar, 20 μm.
Fig. 6.
Nonischemic and ischemic cortex from both wild-type mice (A, B) and Sod2 −/+ mice (C, D). A,C, Cytochrome c immunoreactivity was barely seen in the nonischemic specimens from both the wild-type (A) and Sod2 −/+ (C) mice. B,D, Two hours after ischemia, enhanced cytosolic immunoreactivity of cytochrome c was demonstrated in Sod2 −/+ mice (D) compared with wild-type mice (B). At this time point, diffuse cytosolic immunoreactivity was shown in the wild-type mice, whereas a strong vesicular immunoreactivity, as well as diffuse cytosolic expression, was observed in the Sod2 −/+ mice. E, F, Cytosolic cytochrome c was also observed in the z-VAD.FMK-treated mice (F, arrowheads) as well as in the vehicle-treated animals (E, arrowheads) 2 hr after ischemia. Scale bars: A, C,E, F, 20 μm; B,D, 50 μm; small boxes inB, D, 10 μm.
Table 1.
Summary of cytosolic cytochrome c protein expression after permanent MCA occlusion
| 1 hr | 2 hr | 4 hr | 24 hr | |
|---|---|---|---|---|
| MCA territory cortex | ||||
| Ipsilateral | + | ++ | ++ | +++ |
| Contralateral | − | − | − | − |
| Cortical penumbra | ||||
| Ipsilateral | + | ++ | ++ | +++ |
| Contralateral | − | − | − | − |
| Caudate putamen (core) | ||||
| Ipsilateral | − | − | − | ++ |
| Contralateral | − | − | − | − |
| Piriform cortex | ||||
| Ipsilateral | + | ++ | ++ | +++ |
| Contralateral | − | − | − | − |
+, Weak expression; ++, prominent expression; +++, intense expression with background immunoreactivity; −, no expression.
DNA laddering was detected by genomic DNA gel electrophoresis
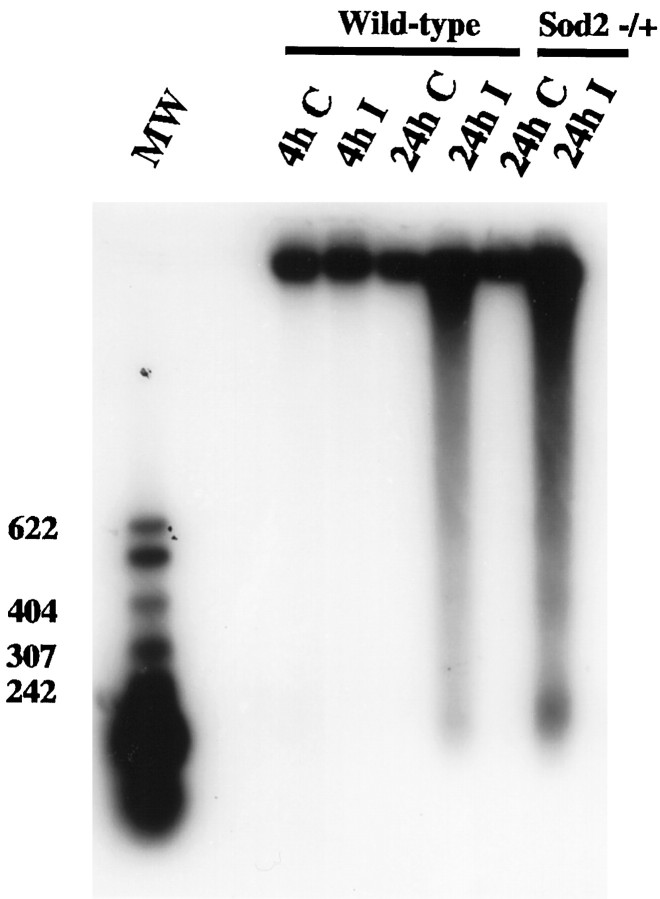
To detect the occurrence of apoptosis as characterized by intranucleosomal DNA fragmentation, we analyzed DNA from both the ischemic brain and the homologous sample on the contralateral side. DNA laddering was absent in both the control and ischemic tissue after 4 hr of permanent ischemia in the wild-type mice (Fig.7, lanes 1–3). A significant amount of DNA laddering was detected 24 hr after ischemia (Fig. 7,lane 4). Twenty-four hours after ischemia, the amount of DNA laddering was markedly increased in the mutant mice (lane 6) compared with the wild-type mice (lane 4). Similar results were obtained in three independent studies.
Fig. 7.
Genomic DNA gel electrophoresis. No DNA laddering is observed in the contralateral brain. In the ischemic brain, DNA laddering is detected at 24 hr but not at 4 hr after ischemia in the wild-type mice. In the Sod2 −/+ mice, DNA laddering was markedly enhanced at 24 hr compared with that in the wild-type mice. DNA was end-labeled with biotinylated dNTP, electrophoresed on a 1.5% agarose gel, transferred to a nylon membrane, and visualized by the chemiluminescent method. The ladders corresponding to 200, 400, and 600 bp are shown. Lanes 1–4, Wild-type; lanes 5, 6, Sod2 −/+ mice. C, Contralateral brain; I, ischemic brain; MW, molecular weight.
DISCUSSION
The current study provides the first evidence that cytochrome c, an essential component of the mitochondrial respiratory chain, is released from mitochondria to cytosol after permanent FCI and that this cytosolic redistribution of cytochrome c and the DNA fragmentation that follows are increased in knock-out mice that are deficient in Mn-SOD, an endogenous mitochondrial antioxidant that has been reported to have a protective effect against permanent ischemia (Murakami et al., 1998). Our findings are that a significant amount of mitochondrial cytochrome c was detected in the control brain and profoundly decreased in the ischemic brain at 2 hr after ischemia and that the cytosolic fraction from the same samples showed a marked increase of cytochrome c in the ischemic brain (Fig. 3). Also, by immunohistochemistry, cytosolic cytochrome c was detected only in the ischemic area as early as 1 hr after ischemia (Fig. 5). Furthermore, the amount of cytosolic cytochrome c was significantly higher in Sod2 −/+ mice compared with wild-type mice 2 hr after ischemia. The amount of DNA laddering 24 hr after ischemia consistently increased in Sod2 −/+ mice compared with wild-type mice (Fig. 7). To compare cytosolic cytochrome c between wild-type and mutant mice, we chose the time point of 2 hr after ischemia for the following reasons. First, as described above, the statistical decrease of mitochondrial cytochrome c and the corresponding increase of cytosolic cytochrome c at this time point are considered to indicate that increased cytosolic cytochrome c was mainly derived from mitochondrial cytochrome c, which is known to be apoptogenic (Liu et al., 1996). Second, immunohistochemistry showed cytosolic cytochrome c in the cells with relatively intact morphology at this time point (Fig. 5C). Third, Namura et al. (1998)have reported on the activation and cleavage of CPP32 in ischemic apoptosis, which was shown to be cleaved by released cytochrome c (Liu et al., 1996) during the short period of reperfusion after 2 hr of ischemia, suggesting the possibility that 2 hr of MCA occlusion with or without minimum reperfusion might induce cytosolic localization of cytochrome c. Taken together, this evidence suggests that Mn-SOD deficiency may contribute to the increase of cytosolic release of cytochrome c and to the DNA fragmentation that follows. On the basis of our observation, it is conceivable that Sod2 −/+ mice could have higher activity of CPP32, which is known to be activated and cleaved by translocated cytochrome c (Liu et al., 1996). The detailed mechanism by which CPP32 leads to DNA fragmentation and apoptosis is unclear except for its link to the DNA repair enzyme poly(ADP)-ribose polymerase (PARP). PARP is known to be cleaved by caspase family proteases, and this proteolytic cleavage results in a dysfunctional PARP that is unable to contribute to the repair of DNA damage (Nicholson et al., 1995). Furthermore, the Ca2+- and Mg2+-dependent endonuclease that generates internucleosomal DNA cleavage characteristic of apoptosis is negatively regulated by poly(ADP)-ribosylation. Therefore, inactivation of PARP by activated CPP32 could increase DNA cleavage and contribute to apoptosis. Further evaluation of downstream events such as activation and cleavage of CPP32 and/or cleavage of PARP in Sod2 −/+ mice would address this issue.
We have reported that ROS, superoxide in particular, are involved in neuronal cell death, including apoptosis and necrosis, and in the pathogenesis of brain edema after FCI (Kinouchi et al., 1991; Chan, 1996; Kondo et al., 1997; Murakami et al., 1998). The extent of edema formation and of infarct volume after transient FCI is significantly decreased in transgenic mice that overexpress copper, zinc-SOD (Kinouchi et al., 1991), whereas DNA fragmentation and infarction are markedly increased in SOD-1 knock-out mice (Chan, 1996; Kondo et al., 1997), suggesting that superoxide radicals play an important role in the pathogenesis of FCI. As for Mn-SOD, Keller et al. (1998) have reported that neuronal apoptosis and infarction volume were significantly reduced in transgenic mice that overexpress Mn-SOD. We have also shown that infarct volume is markedly increased in Mn-SOD knock-out mice (Murakami et al., 1998). Although it is still unclear how superoxide radicals increase DNA fragmentation and whether superoxide radicals play some role in DNA-damaged neuronal cell death after FCI, an early mitochondrial event is likely to explain such a correlation. Because mitochondria are known as the site where O2− is produced during insults such as FCI (Piantadosi and Zhang, 1996), it is conceivable that overproduction of ROS in mitochondria could cause mitochondrial dysfunction, including the release of intermembrane proteins such as cytochrome c. In fact, we have shown that increased HEt oxidation, a possible index of O2−, is observed as early as 1 hr after ischemia and is increased in the nonischemic area as well as in the ischemic area in Sod2 −/+ mice (Murakami et al., 1998). In our study, a significant induction of increased HEt oxidation was observed 2 hr after ischemia (Fig. 1). At this time point, a diffuse cytosolic expression of Et was seen in wild-type mice (Fig. 1C), whereas a large vesicular expression of Et was predominant in Sod2 −/+ mice, in addition to the enhanced diffuse cytosolic pattern (Fig.1D). These results indicate the increased mitochondrial production of O2− in Sod2 −/+ mice compared with wild-type mice, which is consistent with previous reports (Kondo et al., 1997; Murakami et al., 1998). Furthermore, our results showing the increased cytosolic localization of cytochrome c in mutant mice 2 hr after ischemia (Fig. 4) may suggest that enhanced O2− production could contribute to the increased release of cytochrome c to the cytosol. Because the mechanism by which cytochrome c could exit the mitochondria is thought to be the formation of the mitochondrial transition pore (MTP) that can be prevented by the mitochondrial antioxidant Bcl-2 (Marzo et al., 1998;Reed et al., 1998), it is conceivable that Mn-SOD may prevent cytochrome c release also by blocking the formation of the MTP. In fact, we have demonstrated previously that the mitochondrial transmembrane potential, measured by rhodamine 123, was significantly decreased in Sod2 −/+ mice after permanent FCI (Murakami et al., 1998).
Increasing evidence suggests that an active process similar to programmed cell death contributes to the death of neurons (Linnik et al., 1993; Tominaga et al., 1993; Li et al., 1995a; Gillardon et al., 1996; Asahi et al., 1997; Hara et al., 1997; Namura et al., 1998) and to the expansion of the lesion after FCI (Du et al., 1996). Our most recent study has shown that cytochrome c is being released from mitochondria to cytoplasm after transient FCI (Fujimura et al., 1998). Furthermore, it is also reported that the activated form of CPP32 is detected at the early stage of ischemia/reperfusion injury in the brain (Namura et al., 1998), and the inhibition of the caspase family protease can reduce infarct volume and the extent of apoptosis after transient ischemia (Hara et al., 1997). Although it is unclear whether z-VAD.FMK is protective against permanent FCI in our study, the evidence suggests that attenuated caspase expression could be related to the reduction of infarction volume after permanent FCI (Kitagawa et al., 1998). Therefore, we examined the effect of z-VAD.FMK, a nonselective caspase inhibitor, on cytochrome c release to cytosol. The results showed that z-VAD.FMK did not affect cytosolic accumulation of cytochrome c 2 hr after ischemia as shown by immunohistochemistry (Fig.6E,F), suggesting that cytochrome c release rather than the activation and cleavage of caspases after FCI might be the upper stream event. In fact, a recent in vitro study showed that z-VAD.FMK blocked caspase activity, a reduction of mitochondrial membrane potential, and subsequent DNA fragmentation but did not affect the translocation of cytochrome c from mitochondria to cytosol during apoptosis (Bossy-Wetzel et al., 1998). It has been shown recently that the amount of DNA fragmentation can be quantified by terminal transferase-dependent [32P]ddATP end-labeling (Endres et al., 1998). In the present study, the chemiluminescent method makes it somewhat difficult for us to quantify the DNA laddering. Nevertheless, our preliminary result using imaging revealed that the optical density ratio of the 200 base ladder band/genomic DNA band was much higher in Sod2 −/+ mice compared with wild-type mice 24 hr after ischemia. Further examination for quantifying the amount of DNA fragmentation is warranted in future studies.
In conclusion, our results imply that Mn-SOD contributes to the inhibition of apoptosis induced by FCI by reducing the early formation of superoxide radicals and then by preventing the release of mitochondrial cytochrome c to cytosol. The lack of Mn-SOD in mitochondria exacerbates the biochemical cascade that leads to apoptosis after permanent FCI (Fig.8).
Fig. 8.
Diagrammatic view of our knowledge of apoptosis after focal cerebral ischemia. Superoxide radical production and translocation of cytochrome c release, which could be blocked by mitochondrial Mn-SOD, were observed at the early time point after ischemic insult. The caspase inhibitor did not affect cytochrome c release but blocks caspase activation and subsequent DNA fragmentation.
Footnotes
This study was supported by National Institutes of Health Grants NS14543, NS25372, NS36147, and NS38653 and Contract NO1 NS82386. P.H.C. is a recipient of the Jacob Javits Neuroscience Investigator Award. We thank Liza Reola for her technical assistance and Cheryl Christensen for her editorial assistance.
Correspondence should be addressed to Dr. Pak H. Chan, Neurosurgical Laboratories, Stanford University, 701B Welch Road, 148, Palo Alto, CA 94304.
REFERENCES
- 1.Asahi M, Hoshimaru M, Uemura Y, Tokime T, Kojima M, Ohtsuka T, Matsuura N, Aoki T, Shibahara K, Kikuchi H. Expression of interleukin-1 beta converting enzyme gene family and bcl-2 gene family in the rat brain following permanent occlusion of the middle cerebral artery. J Cereb Blood Flow Metab. 1997;17:11–18. doi: 10.1097/00004647-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer PD, Chance B, Ernster L, Mitchell P, Racker E, Slater EC. Oxidative phosphorylation and photophosphorylation. Annu Rev Biochem. 1977;46:955–1026. doi: 10.1146/annurev.bi.46.070177.004515. [DOI] [PubMed] [Google Scholar]
- 5.Cai J, Jones DP. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem. 1998;273:11401–11404. doi: 10.1074/jbc.273.19.11401. [DOI] [PubMed] [Google Scholar]
- 6.Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 7.Du C, Hu R, Csernansky CA, Hsu CY, Choi DW. Very delayed infarction after mild focal cerebral ischemia: a role for apoptosis? J Cereb Blood Flow Metab. 1996;16:195–201. doi: 10.1097/00004647-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Endres M, Namura S, Shimizu-Sasamata M, Waeber C, Zhang L, Gomez-Isla T, Hyman BT, Moskowitz MA. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J Cereb Blood Flow Metab. 1998;18:238–247. doi: 10.1097/00004647-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Fujimura M, Morita-Fujimura Y, Murakami K, Kawase M, Chan PH. Cytosolic redistribution of cytochrome c after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1998;18:1239–1247. doi: 10.1097/00004647-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Gillardon F, Lenz C, Waschke KF, Krajewski S, Reed JC, Zimmermann M, Kuschinsky W. Altered expression of Bcl-2, Bcl-X, Bax, and c-Fos localizes with DNA fragmentation and ischemic cell damage following middle cerebral artery occlusion in rats. Brain Res Mol Brain Res. 1996;40:254–260. doi: 10.1016/0169-328x(96)00059-9. [DOI] [PubMed] [Google Scholar]
- 11.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 12.Hara H, Friedlander RM, Gagliardini V, Ayata C, Fink K, Huang Z, Shimizu-Sasamata M, Yuan J, Moskowitz MA. Inhibition of interleukin 1 beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci USA. 1997;94:2007–2012. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawase M, Carlson E, Murakami K, Chen SF, Epstein CJ, Chan PH. Neuronal damage is reduced after transient global ischemia in CuZn-superoxide dismutase transgenic rats. Soc Neurosci Abstr. 1997;23:2186. [Google Scholar]
- 14.Keller JN, Kindy MS, Holtsberg FW, St Clair DK, Yen HC, Germeyer A, Steiner SM, Bruce-Keller AJ, Hutchins JB, Mattson MP. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinouchi H, Epstein CJ, Mizui T, Carlson E, Chen SF, Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci USA. 1991;88:11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitagawa H, Hayashi T, Mitsumoto Y, Koga N, Itoyama Y, Abe K. Reduction of ischemic brain injury by topical application of glial cell line-derived neurotrophic factor after permanent middle cerebral artery occlusion in rats. Stroke. 1998;29:1417–1422. doi: 10.1161/01.str.29.7.1417. [DOI] [PubMed] [Google Scholar]
- 17.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 18.Kondo T, Reaume AG, Huang TT, Carlson E, Murakami K, Chen SF, Hoffman EK, Scott RW, Epstein CJ, Chan PH. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997;17:4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Chopp M, Jiang N, Yao F, Zaloga C. Temporal profile of in situ DNA fragmentation after transient middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1995a;15:389–397. doi: 10.1038/jcbfm.1995.49. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995b;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Chopp M, Powers C, Jiang N. Apoptosis and protein expression after focal cerebral ischemia in rat. Brain Res. 1997;765:301–312. doi: 10.1016/s0006-8993(97)00524-6. [DOI] [PubMed] [Google Scholar]
- 22.Linnik MD, Zobrist RH, Hatfield MD. Evidence supporting a role for programmed cell death in focal cerebral ischemia in rats. Stroke. 1993;24:2002–2009. doi: 10.1161/01.str.24.12.2002. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extract: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 24.Marzo I, Brenner C, Zamzami N, Susin SA, Beutner G, Bridiczka D, Remy R, Xie ZH, Reed JC, Kroemer G. The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J Exp Med. 1998;187:1261–1271. doi: 10.1084/jem.187.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 28.Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke. 1996;27:327–332. doi: 10.1161/01.str.27.2.327. [DOI] [PubMed] [Google Scholar]
- 29.Reed JC, Jurgensmeier JM, Matsuyama S. Bcl-2 family proteins and mitochondria. Biochim Biophys Acta. 1998;1366:127–137. doi: 10.1016/s0005-2728(98)00108-x. [DOI] [PubMed] [Google Scholar]
- 30.Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 31.Tominaga T, Kure S, Narisawa K, Yoshimoto T. Endonuclease activation following focal ischemic injury in the rat brain. Brain Res. 1993;608:21–26. doi: 10.1016/0006-8993(93)90768-i. [DOI] [PubMed] [Google Scholar]
- 32.Yang G, Chan PH, Chen J, Carlson E, Chen SF, Weinstein P, Epstein CJ, Kamii H. Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke. 1994;25:165–170. doi: 10.1161/01.str.25.1.165. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 34.Zhang P, Liu B, Kang SW, Seo MS, Rhee SG, Obeid LM. Thioredoxin peroxidase is a novel inhibitor of apoptosis with a mechanism distinct from that of Bcl-2. J Biol Chem. 1997;272:30615–30618. doi: 10.1074/jbc.272.49.30615. [DOI] [PubMed] [Google Scholar]