Abstract
Although striatal neurons receive continuous dopamine (DA) input, little information is available on the role of such input in regulating normal striatal functions. To clarify this issue, we assessed how systemic administration of selective D1 and D2 receptor blockers or their combination alters striatal neuronal processing in freely moving rats. Single-unit recording was combined with iontophoresis to monitor basal impulse activity of dorsal and ventral striatal neurons and their responses to glutamate (GLU), a major source of excitatory striatal drive, and DA. SCH-23390 (0.2 mg/kg), a D1 antagonist, strongly elevated basal activity and attenuated neuronal responses to DA compared with control conditions, but GLU-induced excitations were enhanced relative to control as indicated by a reduction in response threshold, an increase in response magnitude, and a more frequent appearance of apparent depolarization inactivation. In contrast, the D2 antagonist eticlopride (0.2 mg/kg) had a weak depressing effect on basal activity and was completely ineffective in blocking the neuronal response to DA. Although eticlopride reduced the magnitude of the GLU response, the response threshold was lower, and depolarization inactivation occurred more often relative to control. The combined administration of these drugs resembled the effects of SCH-23390, but whereas the change in basal activity and the GLU response was weaker, the DA blocking effect was stronger than SCH-23390 alone. Our data support evidence for DA as a modulator of striatal function and suggest that under behaviorally relevant conditions tonically released DA acts mainly via D1 receptors to provide a continuous inhibiting or restraining effect on both basal activity and responsiveness of striatal neurons to GLU-mediated excitatory input.
Keywords: striatum, dopamine, glutamate, single-unit activity, iontophoresis, SCH-23390, eticlopride
Striatal neurons integrate cognitive, motivational, and sensorimotor information for behavioral output (Graybiel, 1995). Glutamate (GLU), which is released from both corticostriatal and thalamostriatal fibers, plays a crucial role in this integrative process by controlling striatal neuronal excitability (Groves, 1983; Parent and Hazrati, 1995; Wilson and Kawaguchi, 1996). Striatal neurons also receive dense input from dopamine (DA)-containing afferents, which arise from midbrain nuclei and synapse in close relation to GLU terminals (Smith and Bolam, 1990). Although DA is known to be important in regulating various physiological and behavioral functions (Fibiger and Phillips, 1986; Grace, 1991; Le Moal and Simon, 1991), its essential role has been difficult to characterize. Ample in vitro and in vivoelectrophysiological data suggest that DA acts as a modulator altering the efficiency of neuronal responses to other inputs, particularly to GLU (Calabresi et al., 1997; Grenhoff and Johnson, 1997; Cepeda and Levine, 1998). Further support for this modulatory role has emerged from work with awake, freely moving animals, in which iontophoretic DA has been shown to attenuate neuronal excitations occurring during somatosensory stimulation or behavior (Rolls et al., 1984; Kiyatkin and Rebec, 1996) but to have bidirectional effects on the GLU response (Pierce and Rebec, 1995; Kiyatkin and Rebec, 1996). Although these data suggest that DA modulates the processing of behavior-related information, the pattern of such modulation, its mechanisms, and its role in regulating physiological and behavioral processes remain unclear.
An interesting feature of striatal DA transmission is that apart from phasic fluctuations in release associated with specific environmental and behavioral events, there appears to be a relatively stable level of continuous DA release (Grace, 1991; Le Moal and Simon, 1991). Consistent with this view, midbrain DA neurons discharge in vitro without any excitatory input (Bunney et al., 1991) and most, if not all, are tonically active in vivo (Dai and Tepper, 1998; Kiyatkin and Rebec, 1998). In fact, although the activity of most DA neurons is phasically modulated by various somatosensory stimuli (Chiodo et al., 1980; Strecker and Jacobs, 1985; Kiyatkin, 1988) and during behavior (Schultz, 1986), the mean rate of activity remains relatively stable (Miller et al., 1981; Steinfels et al., 1983;Strecker and Jacobs, 1985; Trulson, 1985). Although a deficit in DA transmission results in profound changes in striatal functioning implicated in various cognitive and motor diseases (Grace, 1991;Zigmond, 1994), the role of tonic DA input in regulating normal striatal functions remains unclear.
The present study was designed to shed light on the role of endogenously released DA in regulating striatal neuronal functions and the receptor mechanisms involved in mediating this action. Single-unit recording was combined with iontophoresis to examine changes in spontaneous impulse activity and the responses of dorsal and ventral striatal neurons to DA and GLU induced by DA receptor blockade. Because the cellular effects of DA are mediated via two distinct families of receptors (Cooper et al., 1991; Jackson and Westlind-Danielsson, 1994), we used systemic administration of selective D1 (SCH-23390) and D2 (eticlopride) antagonists (Neve and Neve, 1997) or their combination. To ensure behaviorally relevant conditions, all recordings were performed in awake, unrestrained rats.
MATERIALS AND METHODS
Animals and surgery. Data were obtained from male, Sprague Dawley rats, weighing ∼400 gm and bred in our animal colony from source animals supplied by Harlan Industries (Indianapolis, IN). All animals were housed individually under standard laboratory conditions (12 hr light/dark cycle beginning at 7:00 A.M.) withad libitum access to food and water. Experiments were performed in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Publication 865–23) and were approved by the Indiana University Bloomington Institutional Animal Care and Use Committee.
Rats were anesthetized with chloropent (0.33 ml/100 gm, i.p.), mounted in a stereotaxic apparatus, and prepared for subsequent single-unit recording as described in detail previously (Kiyatkin and Rebec, 1996). For recording in the striatum, a hole was drilled unilaterally through the skull (1.2–1.4 mm anterior and 2.0 mm lateral to bregma), and the dura was carefully retracted. A plastic, cylindrical hub, designed to mate with the microelectrode holder on the recording day, was centered over the hole and secured with dental cement to three stainless steel screws threaded into the skull. One screw served as both electrical ground and attachment for the head-mounted preamplifier. The hub was sealed with silicone rubber to prevent drying of the brain surface. After a 3–4 d recovery period, during which each animal was habituated to the recording chamber for a total of 4–6 hr, the recording session began and resumed on each of the next 2–5 d. During the recovery period, most animals were also habituated to the injection procedure, in which saline was injected subcutaneously once daily to the lower back area.
Single-unit recording and iontophoresis. Four-barrel, microfilament-filled, glass pipettes (Omega Dot 50744; Stoelting, Wood Dale, IL), pulled and broken to a diameter of between 4 and 5 μm, were used for single-unit recording and iontophoresis. The recording and balance barrels contained 3 m and 0.25 mNaCl, respectively, and the remaining barrels were filled with 0.25m solutions of l-GLU monosodium salt and DA hydrochloride (Sigma, St. Louis, MO) dissolved in distilled water, pH 7.5 and 4.5, respectively. Retaining (approximately ±8 nA) and ejecting (±5–60 nA) currents were applied with a constant current generator (Dagan 6400; Dagan, Minneapolis, MN). The in vivo resistance of the drug-containing barrels ranged between 20 and 40 MΩ (measured at constant current), whereas the recording channel had an impedance of ∼4–10 MΩ (measured at 1 kHz). To prevent electric cross-talk between channels, the microfilaments were removed from the upper part of the pulled pipettes, and the opening of each barrel was separated by 2–3 mm and covered with paraffin. The multibarreled pipette was filled with fresh drug solutions <1 hr before the beginning of each recording session and fixed in a microdrive assembly, which allowed for 11 mm of dorsoventral travel (Rebec et al., 1993). The microdrive assembly was inserted into the skull-mounted hub, and the electrode was advanced 4.0 mm below the skull surface to the starting point of unit recording.
Neuronal discharges were passed through a head-mounted preamplifier (LF 441CN; National Semiconductor), and all electrical connections from the microdrive assembly were fed outside the recording chamber via shielded cable through an electric swivel. Electrophysiological signals were amplified, filtered (bandpass, 100–3000 Hz), and stored on an audio channel of a videocassette recorder (VCR). Spike activity was monitored on-line with a digital oscilloscope and audioamplifier and counted in 2 sec bin widths by computer in conjunction with an amplitude-sensitive spike discriminator. A second audio channel of the VCR was used to mark iontophoretic applications and behavioral events. Behavioral activity was recorded on the video channel.
All recordings took place during the day (10:00 A.M.-6:00 P.M.) in a Plexiglas cage (35 × 35 × 40 cm) housed inside a sound-attenuating, electrically shielded chamber within view of a video camera. After the isolation of single-unit discharges (signal-to-noise ratio of at least 3:1), data collection for each neuron usually lasted for 20–30 min. In most experiments, our protocol included several brief (20 sec) applications of GLU (0 to −40 nA) and DA (0 to +40 nA), performed at 1–2 min intervals with the same or increasing currents. To detect silent cells, some electrodes were advanced during continuous or pulsatile ejections of GLU at low currents. These units were tested with DA during continuous GLU application.
All iontophoretic applications used for statistical analysis were performed when the animals rested quietly with no sign of overt movement. Data obtained during spontaneous movements were analyzed separately.
Pharmacological treatments. Approximately 2–3 hr after recording began, animals received one of three treatments: 0.2 mg/kg SCH-23390 [R(+)7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride]; 0.2 mg/kg S(−)-eticlopride HCl (ETI); or their combination, 0.2 mg/kg SCH plus 0.2 mg/kg ETI. Both drugs have high antagonistic activity at either D1 or D2 DA receptor types (relative D1:D2 affinity, SCH = 2500:1 and ETI = 1:514,000; Neve and Neve, 1997) and, at these doses, interactions with other receptors, particularly 5-HT2 receptors in the case of SCH, appear to be negligible (Bischoff et al., 1986; Hjorth and Carlsson, 1988). SCH and ETI (Research Biochemicals International, Natick, MA) were dissolved in saline immediately before use and injected subcutaneously. Control animals received saline. In most cases, the injections were performed during neuronal recording to permit predrug and postdrug comparisons of drug-induced changes in impulse activity and responsiveness to DA and GLU. Further recordings were made from stochastically sampled units tested at different times (up to 180 min) after drug injection. The relatively long period of postinjection neuronal sampling permitted a rough estimation of the time course of drug action.
Histology. After completion of the last recording session, rats were deeply anesthetized with chloropent, and an epoxy-insulated tungsten electrode, inserted into one barrel of the pipette, was lowered into the recording area. Current was passed through the electrode (50 μA for 15–20 sec) to produce microlesions at depths of 5.0 and 7.0 mm below the skull surface. Rats were then perfused transcardially with formosaline, and the brain was removed and stored for subsequent histological processing. Brain tissue was frozen, cut (50 μm sections), mounted on slides, and stained with cresyl violet. The location of each recording site was determined from histological data on the electrode track, and depth information was noted on the microdrive assembly at the time of each recording. The atlas of Paxinos and Watson (1986) served as the basis for both electrode placement and histological analysis.
Data analysis. Spontaneous impulse activity was characterized by mean rate (X), SD, and coefficient of variation (CV), all of which were calculated for each recorded unit based on a 30 sec period of quiet rest (bin width, 2 sec). Although the striatum in awake, unrestrained rats contains a large amount of silent units, which generate discharges during GLU application and become inactive again after the GLU ejection current is turned off (Kiyatkin and Rebec, 1999), such units were not included in our sample of spontaneously active cells.
Individual iontophoretic responses were considered significant (i.e., excitation or inhibition), when mean rates during iontophoresis and the immediately preceding equivalent period were statistically different (p < 0.01; two-tailed Student’s ttest). These responses were also assessed in terms of onset and offset latencies, absolute and relative magnitudes, and the effect of ejection current (dose–response relationships). To detect the effect of DA and GLU on neuronal activity in a group, we used a one-way, repeated-measures ANOVA and made between-group comparisons on the number of units sensitive to the ejected substances and the ratio of excitation and inhibitions for each ejection current. In units tested with multiple GLU applications at different currents, we also assessed the response threshold (the minimum current necessary for inducing a significant and consistent change in discharge rate during a series of tests). Because the duration of each recording ranged from 6 to 90 min, which sometimes included periods of spontaneous movement, not all units were tested equally with DA or GLU. Thus, not all recorded units were included in assessments of response thresholds and dose–response relationships. Our results are reported as numbers of both recorded units and iontophoretic tests.
Various relationships between impulse activity and iontophoretic responses were evaluated with standard statistical techniques, including natural log (ln) transformations, ANOVAs, and correlation and regression analyses. Off-line analysis of videotape records was used to assess changes in impulse activity during bouts of spontaneous behavior (e.g., grooming, locomotion, and rearing) and presentation of somatosensory stimuli; the iontophoretic responses affected by movements or stimuli were considered separately.
RESULTS
Four groups of neuronal data were analyzed: control, SCH, ETI, and SCH plus ETI. Drug data were obtained from 18 rats (six per group); 32, 33, and 31 units were recorded after administration of SCH, ETI, and their combination, respectively. Most units were stochastically sampled and tested at different times after drug administration, providing data for group analysis of drug-induced neuronal changes. Our paradigm also allowed some units to be tested both before and after drug administration to permit an analysis of drug-induced neuronal changes in individual units. Postdrug data were analyzed for time-dependent effects on discharge rate and responses to GLU and DA. Because some units were recorded for a relatively long time (>20 min), the number of data points (impulse activity values and iontophoretic responses) in each sample was larger than the number of recorded units. Control data were obtained from the same 18 rats before drug administration (57 units) as well as from 20 drug-naive rats (150 units). Because neurons in dorsal (caudate putamen) and ventral striatum (accumbens core) have similar impulse activity and comparable responses to GLU and DA in awake, unrestrained rats (Kiyatkin and Rebec, 1996, 1999), these units were combined for most statistical analyses.
Changes in behavior induced by DA antagonists
Compared to the control state (quiet rest with periodic episodes of locomotion, head and body movements, grooming, rearing, etc.), each of the drug treatments had similar inhibitory effects on behavior. Within 5–15 min after injection, the rats appeared sedated and sat quietly in the same place; they slowly returned to predrug activity 60–90 min after drug administration. Apart from these common sedative effects, some differences also appeared. After SCH, for example, rats frequently vocalized or engaged in vacuous chewing in response to touch or air puff, whereas ETI often elicited defecation and urination. The SCH plus ETI combination elicited all these effects, which were more prolonged and more pronounced than after either drug alone.
Spontaneous impulse activity
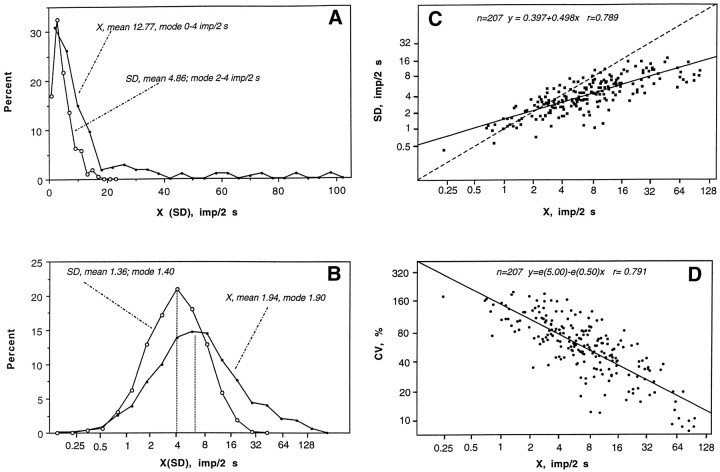
Striatal neurons in the control state (n = 207) had highly variable rates and patterns of spontaneous discharges (Table1). As shown in Figure1A, the X and SD were distributed asymmetrically with modal values shifted toward the low end. The majority of recorded units had a low rate of activity: 31.0% at 0.1–2 impulses/sec, 26.1% at 2–4 impulses/sec, and 15.0% at 4–6 impulses/sec. Only 27.9% of our recorded units had mean rates >6 impulses/sec. An ln transformation of X and SD values (Fig.1B) revealed that they were distributed according to ln normal law with a close matching of mean and modal values calculated for ln derivatives of X and SD. The results of this transformation also indicate that in terms of impulse activity our neuronal population is homogenous and that ln derivatives of X and SD are the best parameters for statistical characterization of neuronal activity. These parameters and their distributions were used for further statistical evaluations of between-group differences in impulse activity. As shown in Figure1C, mean and SD are closely interrelated (r= 0.789; p < 0.0001), i.e., absolute variability of impulse flow depends positively on rate. The same strong correlation was found between X and CV (Fig. 1D;r = 0.791; p < 0.001) but in the opposite direction, i.e., mean rate is related negatively to relative variability (irregularity) of discharges. Although neurons in both striatal divisions had similar parameters of impulse activity, ventral striatal units (n = 66) had a significantly higher X (10.35 impulses/sec) and a lower CV (56.51%) than dorsal striatal cells (n = 141; 4.53 impulses/sec and 75.31%;p < 0.01).
Table 1.
Impulse activity of striatal neurons after DA receptor blockade in awake rats
| Parameters | Control | SCH | ETI | SCH and ETI |
|---|---|---|---|---|
| Number of units | 207 | 31 | 32 | 30 |
| Number of data points | 207 | 53 | 74 | 56 |
| X, impulse/sec | ||||
| Mean | 6.39 | 14.91 | 3.53 | 8.08 |
| Range | 0.1–49.5 | 1.7–69.8 | 0.2–11.2 | 0.2–42.8 |
| Mean ln(X) ± SE | 1.95 ± 0.07 | 3.04 ± 0.12*** | 1.59 ± 0.11** | 2.41 ± 0.13* |
| SD, impulses/2 sec | ||||
| Mean | 4.86 | 9.36 | 3.78 | 6.05 |
| Range | 0.5–17.3 | 1.7–19.4 | 0.7–8.5 | 0.7–15.2 |
| Mean ln(SD) ± SE | 1.37 ± 0.05 | 2.08 ± 0.08*** | 1.14 ± 0.08* | 1.64 ± 0.08** |
| CV, % | ||||
| Mean | 69.31 | 42.65 | 71.88 | 55.32 |
| Range | 7.99–196.92 | 9.01–101.42 | 5.51–197.00 | 11.60–222.30 |
| Mean ln(CV) | 4.03 ± 0.05 | 3.63 ± 0.07*** | 4.12 ± 0.07 | 3.83 ± 0.07* |
| r(lnX-lnSD) | 0.779 | 0.763 | 0.857 | 0.867 |
| r(lnX-lnCV) | 0.790 | 0.819 | 0.806 | 0.854 |
Calculations were based on 2 sec rate values; means and ranges of X are shown as impulses per second.
Asterisks indicate differences from control (*p < 0.05; **p < 0.01; ***p < 0.0001). X, Mean rate; SD, standard deviation; and CV, coefficient of variation of impulse activity.
Fig. 1.
Spontaneous impulse activity of striatal neurons in awake, unrestrained rats under control conditions. Percent distributions of X rates (X, impulses/2 sec) and SDs (impulses/2 sec) (A) and their natural logarithmic transformation (B). Relationships between X and SD (C) and X and CV (percentage) (D). Vertical hatched lines inB indicate modal values. Regression lines and coefficients of correlation (r) are shown inC and D.
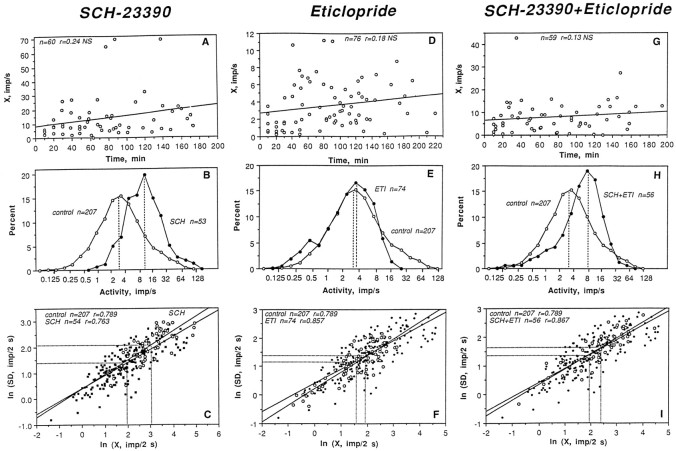
Because units in each treatment group were recorded at different times after drug administration, we first examined the relationships between this variable and discharge rate. As shown in Figure2 (top graphs), neuronal activity in each sample varied considerably in rate, but was independent of recording time within each sample. Thus, the period from 10 to 180 min after drug administration was chosen to characterize spontaneous impulse activity in each treatment group (Table 1; Fig. 2,middle, bottom).
Fig. 2.
Impulse activity of striatal neurons after DA receptor blockade. The top rows indicate dependence of discharge rate (impulses per second) on time after drug administration (minutes). The middle rows depict percent distributions of discharge rate in drug-treated (closed circle) and control (open circle) conditions. Hatched lines show distribution modes. The bottom rowsdepict relationships between discharge rate (X, impulses/2 sec) and SD (impulses/2 sec) of impulse activity shown in ln form for treatment (open symbols) and control (closed symbols) groups. n, Numbers of data points;r, coefficients of correlation.
As shown in Table 1 and Figure 2, B and C, all parameters of impulse activity in the SCH sample were significantly different from control. Although units with highly variable rates were recorded after SCH, most had moderate to high rates of activity (see modal values of rate distributions shown in Fig. 2B). The X in this group (14.91 impulses/sec) was more than two times that of control (6.39 impulses/sec; t = 7.84;p < 0.0001). In addition, the SCH group had a significantly higher SD (9.36 vs 4.86 impulses/2 sec; t= 7.55; p < 0.0001) and lower CV (42.65 vs 69.31%;t = 4.65; p < 0.001) than the control group. Both groups, however, showed similar relationships between mean and SD (compare coefficients of correlation and regression lines shown in Fig. 2C and Table 1).
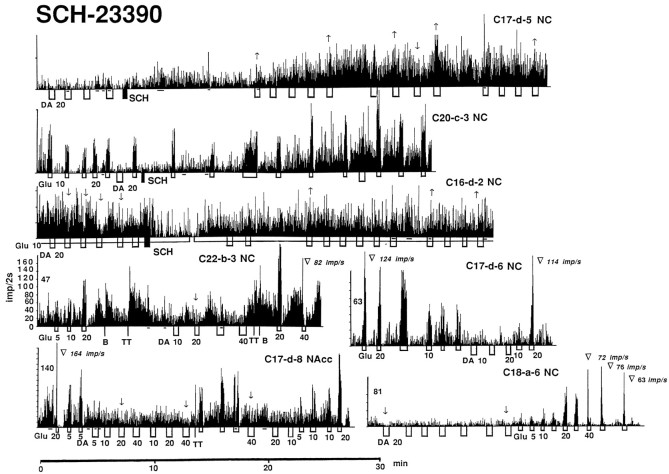
In four of five spontaneously active units recorded both before and after SCH administration, neuronal activity significantly increased (Fig. 3,C17-d-5,C20-c-3) within 10–15 min after injection (p < 0.01; Student’s ttest). The one remaining unit showed no change in activity.
Fig. 3.
Rate-meter histograms showing impulse activity of striatal neurons and their responses to GLU (short open box) and DA (long open box) after administration of SCH-23390 (SCH). DA and GLU were tested both on spontaneously active and GLU-stimulated units (line below histogram). Numbers below iontophoretic applications indicate ejection current in nanoamperes, no numbers in subsequent applications indicate the same current as the last indicated application. In all cases, neuronal activity is presented as impulses/2 sec, and each division on the ordinate of all histograms represents 20 impulses/2 sec. Solid lines below each histogram indicate periods of overt movement; at all other times, the animals were at quiet rest. The histological location of individual recordings in dorsal (NC) and ventral striatum (NAcc) and their numbers (rat, session, and unit, respectively) are identified in the top right corner of each histogram. Arrows above DA tests indicate significant DA-induced decreases (↓) and increases (↑) in activity;no arrows indicate no response. Trianglesshow cases of GLU-induced depolarization inactivation with rate values (impulses per second) immediately preceding the cessation of firing. In the top three examples, SCH was administered in the middle of the recording, but in all other cases, the antagonist was injected before the recording began (numbers above these histograms to the left indicate time in minutes after SCH).
Although the behavioral effects of SCH peaked at 30–40 min and usually disappeared by 80–120 min after drug administration, a time-dependent trend in rate failed to emerge (Fig. 2A). The group mean at 120–170 min was similar to that at 20–70 min, and both were equally higher than control.
Significant differences in neuronal activity also were found between ETI and control (Table 1, Fig.2E,F), but the effect of ETI was opposite in direction and less pronounced than that of SCH. As shown in Figure 2E, the X distributions of the ETI and control samples were largely superimposable, and the majority of units in both groups had relatively low to moderate activity rates (1–8 impulses/sec). In the ETI group, however, there were no units with high rates (maximal value 11.2 impulses/sec vs 49.5 impulses/sec in control; see right sides of distributions) and more units with very low rates (0.22–1.0 impulses/sec) compared with control. The X in the ETI group (3.53 impulses/sec) was significantly lower than that in control (6.39 impulses/sec), but this difference was smaller (t = 2.77; p < 0.01) than that for the SCH–control comparison (compare t values). Although the SD in the ETI group was higher than control (t = 2.44;p < 0.05), these two groups did not differ in CV (69.31 vs 71.88%; t = 1.13; NS). The close similarity in neuronal activity of the ETI and control samples is shown in Figure2F. The regression lines for mean, SD, and the coefficients of correlation in both groups were very similar (Fig.2C).
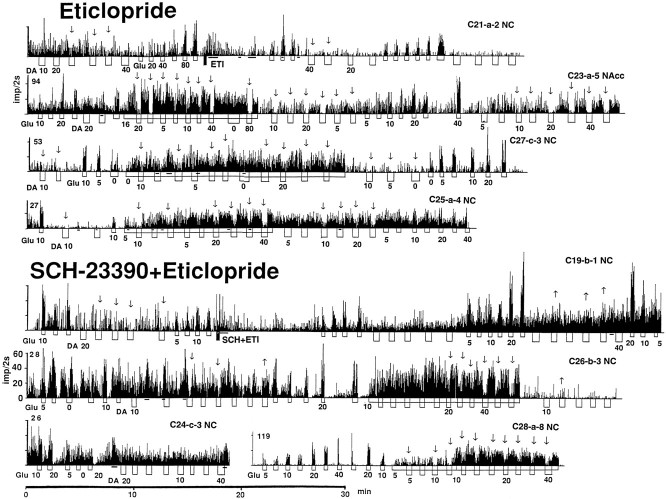
Weak and inconsistent effects of ETI on impulse activity were found in five spontaneously active units recorded before and after ETI injection. Discharge rate significantly decreased in three and did not change in two units (Fig. 4,21-a-2).
Fig. 4.
Rate-meter histograms showing impulse activity of striatal neurons and their responses to GLU and DA after administration of eticlopride and SCH-23390 plus eticlopride. All abbreviations are as in Figure 3.
Similar to SCH, no evident time-dependent trend in discharge rate was seen for the entire recording period after ETI injection (Fig.2D). It appears, however, that the effect of ETI was maximal at 20–40 min and became less evident at 140–190 min, when the proportion of units with a higher discharge rate increased to the level typical of the control state.
Although the SCH–ETI combination had a significant stimulatory effect on striatal neurons (Table 1, Fig.2H,I), this effect differed from that induced by SCH alone. As shown in Figure2H, the range of X values in the sample (0.23–42.83 impulses/sec) was similar to control (0.10–49.5 impulses/sec), but the distribution mode was shifted to higher values. The mean rate in this group (8.08 impulses/sec) was significantly higher than control (t = 2.43; p < 0.05), but significantly lower (t = 3.56; p < 0.01) than SCH. The same intermediate values were found for mean SD and CV, which had significant differences from both the control and SCH groups. The regression line for mean and SD in the SCH plus ETI group was almost identical to that for control (Fig. 2I), and the coefficients of correlation were also similar (Table 1).
Different effects were found in five units successfully recorded both before and after injection of the SCH plus ETI combination. Activity increased in two (Fig. 4, C19-b-1) and did not clearly change in three others. Although the behavioral effects of the SCH plus ETI combination disappeared by the end of the second hour, neuronal activity remained increased without a time-dependent trend over the entire 3 hr recording period (Fig. 2G).
DA responses
Table 2 summarizes DA responses (20–30 sec; 5–40 nA) obtained from 122 spontaneously active (83 dorsal and 39 ventral) and 35 GLU-stimulated (31 dorsal and 4 ventral) striatal neurons in the control state. Under basal conditions, the GLU-stimulated units had no or very slow sporadic activity (<1 impulses/sec), but maintained an enhanced, relatively stable discharge rate (2–41 impulses/sec) during continuous, low-current GLU application (10–20 nA).
Table 2.
DA responses of spontaneously active and GLU-stimulated striatal neurons in awake, drug-naive rats
| Parameters | Dopamine current (nA) | |||
|---|---|---|---|---|
| 5 | 10–15 | 20–25 | 30–40 | |
| Spontaneously active | ||||
| Number of units (tests) | 31 (41) | 84 (153) | 75 (130) | 33 (48) |
| Number of inhibitions (%) | 14 (34.2) | 79 (51.6) | 69 (53.1) | 28 (58.3) |
| Number of excitations | 0 | 2 | 3 | 3 |
| F | 24.03*** | 44.28*** | 87.46*** | 24.72*** |
| Mean changes in rate, % | 84.28 ± 6.18 | 69.40 ± 3.21 | 69.97 ± 5.18 | 90.48 ± 14.01 |
| Inhibition | ||||
| Range | 42.0–88.2 | 16.9–87.7 | 10.6–87.7 | 16.2–93.5 |
| Relative magnitude | 58.31 ± 4.45 | 48.27 ± 2.14 | 45.76 ± 2.22 | 50.15 ± 3.95 |
| Glu-stimulated | ||||
| Number of cells (tests) | 16 (36) | 24 (67) | 32 (97) | 22 (83) |
| Number of inhibitions (%) | 10 (27.8) | 27 (40.3) | 74 (76.3) | 65 (78.3) |
| Number of excitations | 3 | 0 | 2 | 0 |
| F | 9.40** | 60.60*** | 108.00*** | 102.31*** |
| Mean changes in rate, % | 85.01 ± 5.38 | 73.06 ± 3.33 | 62.49 ± 3.01 | 56.45 ± 2.53 |
| Inhibition | ||||
| Range | 19.3–62.2 | 10.9–74.67 | 9.1–80.9 | 7.0–93.4 |
| Relative magnitude | 47.85 ± 5.18 | 48.94 ± 3.49 | 51.74 ± 2.00 | 49.60 ± 2.13 |
F values show the strength of the DA effect on impulse activity estimated by a one-way ANOVA with repeated measures. Asterisks indicate statistical significance of the effect (*p < 0.05; **p < 0.01; ***p < 0.0001). ± values refer to SEM.
Although some units failed to change discharge rate at any current, and some showed significant increases in activity, DA at any tested current had a significant inhibitory effect on impulse activity in both spontaneously active and GLU-stimulated units. The relative magnitude of inhibition varied between 10 and 80% of baseline rate, but mean values were comparable at different currents in both spontaneously active and GLU-stimulated cells (∼50%). DA-induced inhibitions occurred with a variable onset latency (4–12 sec), were relatively small in magnitude (∼50 ± 20% of baseline), and disappeared slowly after the current was turned off (offset latency of at least 4–12 sec). The pattern of the DA-induced inhibition varied widely in different cells and at different levels of discharge rate and often varied in the same unit during repeated DA ejections at the same current. Representative examples are shown in Figures 3 and 4.
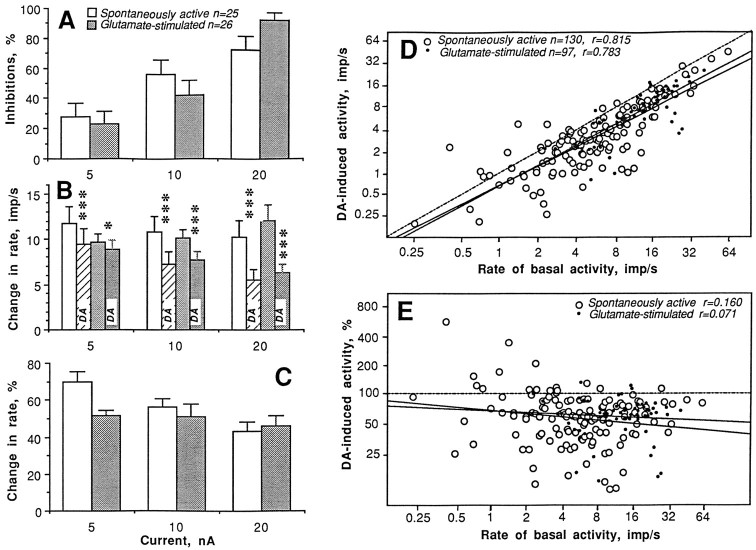
Data obtained from 25 spontaneously active and 26 GLU-stimulated units tested with progressively doubled currents (5, 10, and 20 nA) revealed that the inhibitory effect of DA strongly depends on ejection current (Fig. 5A–C). In both groups of units, all parameters of the response gradually increased with increases in DA current. Although ∼72% of spontaneously active and ∼92% of GLU-stimulated units were inhibited by DA at 20 nA, the relative response magnitude was relatively small in both groups (∼45%). Dose–response relationships for each unit, however, varied in terms of the first appearance of the response and the extent of the rate decrease with the next higher current.
Fig. 5.
Dopamine responses of striatal neurons in awake, unrestrained rats under control conditions. Three parameters of the DA response (A, number of units with inhibitions, percentage; B, discharge rate before and during DA application, impulses per second; C, relative magnitude of DA-induced inhibition, percentage) evaluated in 25 spontaneously active (open bars) and 26 GLU-stimulated (closed bars) units are shown at different DA ejection currents (in nanoamperes). Relationships between basal discharge rate (impulses per second) and absolute (impulses per second; D) or relative (percentage; E) changes in rate induced by DA at the same current (20–25 nA) shown separately for spontaneously active and GLU-stimulated units. Each graph depicts lines of no effect (hatched) and regression lines (solid) and shows number of analyzed tests (n) and coefficients of correlation (r).Asterisks indicate significant DA-induced decrease in discharge rate (*p < 0.05; ***p < 0.001).
Figure 5, D and E, depicts the relationships between discharge rate and DA response shown separately for spontaneously active and GLU-stimulated units tested at the same currents (20 and 25 nA; 130 and 97 DA tests, respectively). The results of all tests, except those with significant excitations (n = 3 and 2, respectively) are shown in ln form. DA-induced activity strongly correlates with basal activity; the regression lines in both groups are located below and almost parallel to the lines (hatched) of no effect. The relative magnitude of DA-induced activity, in contrast, is independent of discharge rate in both groups (all coefficients of correlation were not significant). DA in some cases slightly increased discharge rate (18 of 130 or 13.8%); most of these increases occurred at low rates of spontaneous impulse activity (0.4–4 impulses/sec), and in most cases these increases were not significantly different from pre-DA baseline activity. In GLU-stimulated units, such DA-induced increases in activity were less frequent (5 of 97 or 5.2%; p < 0.05 vs spontaneously active cells).
Table 3 summarizes the DA responses of spontaneously active neurons in each treatment group. Maximal changes versus control were found in the SCH group, in which the majority of units did not show significant DA responses at any tested current (see Fig. 3 for original examples). DA-induced inhibitions, for example, were seen in only 16.2, 18.5, and 33.3% of applications at currents of 10, 20, and 40 nA, respectively, and the mean change in rate in each current group was close to 100% of the basal rate (no effect). In the SCH group, we also observed DA-induced increases in activity, which were especially frequent at 20 nA current (28 of 81 or 34.6 vs 13.8% in control); the number of significant excitations (7 of 81 or 8.6%) was also higher than in control (2.3%). Although an ANOVA revealed that DA after SCH had a significant inhibiting effect on striatal activity, this effect was ∼8–10 times weaker than in control (compare F values with Table 2, especially those for the 20 nA groups).
Table 3.
DA responses of spontaneously active striatal neurons after DA receptor blockade
| Parameters | Dopamine current (nA) | |||
|---|---|---|---|---|
| 5 | 10 | 20 | 40 | |
| SCH-23390 | ||||
| Number of units (tests) | 3 (5) | 16 (37) | 27 (81) | 12 (24) |
| Number of inhibitions (%) | 2 | 6 (16.2) | 15 (18.5) | 8 (33.3) |
| Number of excitations | 0 | 0 | 7 | 1 |
| F | 6.74* | 6.27* | 7.65* | |
| Mean changes in rate, % | 105.88 ± 20.29 | 94.17 ± 3.71 | 93.98 ± 5.87 | |
| Inhibition | ||||
| Range | 35.5–79.3 | 15.4–70.6 | 46.3–87.85 | |
| Relative magnitude | 56.82 ± 6.36 | 52.65 ± 4.19 | 70.32 ± 4.78 | |
| Eticlopride | ||||
| Number of units (tests) | 7 (16) | 15 (37) | 24 (74) | 7 (17) |
| Number of inhibitions (%) | 7 (43.8) | 23 (62.2) | 35 (47.3) | 8 (47.1) |
| Number of excitations | 0 | 1 | 3 | 0 |
| F | 18.05** | 50.32*** | 40.79*** | 7.39* |
| Mean changes in rate, % | 72.22 ± 6.11 | 60.22 ± 4.86 | 77.73 ± 4.73 | 69.98 ± 7.18 |
| Inhibition | ||||
| Range | 34.1–67.2 | 21.2–64.34 | 4.4–78.6 | 26.8–74.4 |
| Relative magnitude | 48.14 ± 5.12 | 43.97 ± 3.52 | 47.97 ± 3.10 | 50.47 ± 5.31 |
| SCH-23390 + Eticlopride | ||||
| Number of units (tests) | 3 (6) | 20 (68) | 12 (36) | 5 (7) |
| Number of inhibitions (%) | 3 (50.0) | 19 (27.9) | 4 (11.1) | 1 (14.3) |
| Number of excitations | 0 | 5 | 4 | 0 |
| n no responses | 3 | 44 | 28 | 6 |
| F | 4.16 | 9.05** | 0.41 | 1.33 |
| Mean changes in rate, % | 64.37 ± 11.71 | 98.08 ± 6.68 | 104.31 ± 6.21 | 97.33 ± 3.91 |
| Inhibition | ||||
| Range | 8.2–69.4 | 37.5–74.3 | ||
| Relative magnitude | 48.91 ± 4.29 | 59.77 ± 8.20 | ||
F values show the strength of the DA effect on impulse activity estimated by a one-way ANOVA with repeated measures. Asterisks indicate statistical significance of the effect (*p < 0.05; **p < 0.01; ***p < 0.0001). ± values refer to SEM.
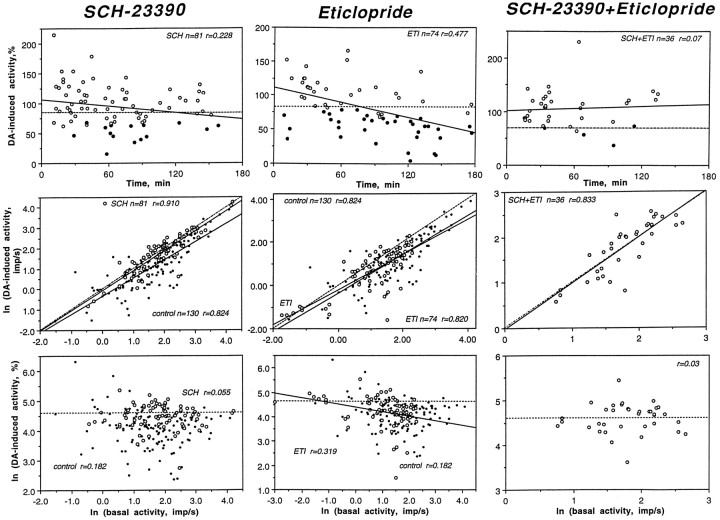
The attenuating effect of SCH on DA responses is evident in Figure6 (left column), which depicts the results of statistical analysis of DA-induced changes in activity with SCH and control at the same current (20 nA). Note that impulse activity during DA application in the SCH group was slightly increased or decreased, and the regression line was almost superimposed on the line of no effect. Although DA-induced inhibitions were rare at any time after SCH administration, the regression analysis (top graph; intersection of the regression line with the line of mean effect in drug-naive rats; ∼70%) suggests that the effect tended (r = 0.228; p < 0.05) to disappear after ∼120 min. In units recorded at later periods, DA-induced inhibitions were seen more frequently; thus, these later data were not included in the SCH sample used for statistical analysis of DA responses.
Fig. 6.
Dopamine responses of striatal neurons (20–25 nA ejection current) after DA receptor blockade. The top rows indicate DA-induced activity (percentage) in units recorded at various times after drug administration [filled symbols, inhibitions; open symbols, nonsignificant changes; solid lines, trends (regression); and hatched lines, mean DA-induced change in activity in control]. The middle rows depict relationships between absolute magnitude of DA-induced activity and basal rate for control and drug-treated groups (large open circles, drug-treated state; small closed circles, control). The bottom rows indicate relationships between the relative magnitude of DA-induced (percentage) activity and basal discharge rate for control and drug-treated groups (symbols as in middle row).n, Numbers of data points; r, coefficients of correlation.
Although the number of GLU-stimulated units was small (five cells, 19 tests at 5, 10, and 20 nA currents), a powerful blocking effect of SCH on DA responses was also evident in this group. In 16 of 19 tests, there were no significant changes in activity during DA iontophoresis, and in three cases activity actually increased (Fig. 3,C16-d-5).
Unlike SCH, DA responses in the ETI group were barely distinguishable from control (see Table 3; Fig. 6, middle row, and Fig. 4 for original examples). Despite significant differences in impulse activity, most units tested for up to 180 min after ETI administration showed DA-induced inhibitions, which occurred in approximately the same proportions as control. This similarity of DA responses is shown in Figure 6 (middle column). The regression lines for both the control and ETI groups were located below the line of no effect, and they were largely superimposable. It is important to note that after ETI, DA appears to be even more effective at inhibiting fast-firing units compared with control conditions; the regression line in this group was lower than the line of no effect in the control group. This difference is especially evident in the bottom graph, indicating that the relative magnitude of DA-induced changes in activity was significantly (r = 0.319;p < 0.05) stronger in units with a higher discharge rate. Although such fast-firing units were rare in the ETI group, these same units appear to be more sensitive to DA compared with control units with the same rates. Consistent with this correlation, we found that GLU-stimulated units (n = 7), which have much higher discharge rates (range, 2.3–20.1; mean, 10.29 impulses/sec) compared with spontaneously active units (mean, 3.53 impulses/sec;p < 0.001), remained highly sensitive to DA after ETI treatment. DA dose-dependently inhibited GLU-evoked activity in all tested units (5 nA, 4 of 6 or 66.7%; 10 nA, 12 of 14 or 85.7%; 20 nA, 18 of 23 or 78.3%; and 40 nA, 4 of 4 or 100%) with a potency slightly higher than that in the control group (Table 2).
The SCH plus ETI combination also had an attenuating effect on DA responses (Table 3, Figs. 4, 6). At low currents, this effect was approximately as strong as with SCH alone and even stronger at higher currents. As with SCH alone, we observed DA-induced excitations (9 of 104 or 8.7% vs 7 of 118 or 5.6%; 10–20 nA currents), which were very rare for control recordings (5 of 283 or 1.8%). The effect of DA was significant only at one current (10 nA), but it was 5 times lower than control (compare F values), and such an effect was completely absent at all other currents. The blocking effect on DA responses is evident in Figure 6 (middle graph), where the regression line for DA-induced changes in impulse activity is completely superimposed on the line (hatched) of no effect. In contrast to SCH and ETI alone, the effect of SCH plus ETI was prolonged, blocking DA-induced responses for up to 130 min after drug administration (top graph).
Although DA was equally ineffective at inhibiting units with different rates of spontaneous activity (Fig. 6, bottom graph), we unexpectedly found that DA maintains an inhibiting action on GLU-evoked activity (54 tests in 10 units) similar to that in the control state (Table 2). Significant DA-induced inhibitions of GLU-evoked activity were found in the SCH plus ETI group in 2 of 6 (33.3%), 8 of 25 (44%), 10 of 14 (71.4%), and 9 of 9 (100%) tests at currents of 5, 10, 20, and 40, respectively.
GLU responses
Table 4 summarizes responses to brief applications of GLU (20 sec; 5–40 nA) obtained from 140 spontaneously active (98 dorsal and 42 ventral) striatal neurons in the control state. In contrast to DA, all striatal neurons were sensitive to GLU and showed a sustained excitation (onset and offset latency <2–6 sec), which was relatively stable during repeated GLU applications; the effect of GLU was significant at each tested current. The GLU response appeared in different cells at different currents (from 2.5 to 35 nA; mean threshold, 19.7 ± 1.75 nA;n = 25) and was frequently accompanied by a decrease in spike magnitude (up to 2–3 times). An apparent depolarization inactivation, i.e., a complete disappearance of discharges caused by overexcitation, however, rarely occurred at the currents used. In some fast-firing units, the GLU-induced excitation was followed by a short-term (4–16 sec) rebound-like decrease in activity; this postexcitatory inhibition was directly related to the magnitude of the preceding excitation.
Table 4.
GLU responses of striatal neurons in control and after DA receptor blockade
| Parameters | GLU current (nA) | |||
|---|---|---|---|---|
| 5 | 10 | 20 | 40 | |
| Control | ||||
| Number of units (tests) | 16 (22) | 34 (61) | 78 (153) | 21 (31) |
| Units with excitations, % | 87.54 | 82.35 | 89.74 | 100 |
| Tests with excitations, % | 90.91 | 78.79 | 89.54 | 96.77 |
| F | 17.89** | 72.16*** | 285.78*** | 42.96*** |
| Mean basal activity, impulses/sec | 5.77 | 4.44 | 6.84 | 5.03 |
| GLU-induced activity | ||||
| Range, impulses/sec | 1.8–67.9 | 0.8–59.7 | 1.3–58.17 | 2.1–61.2 |
| Mean, impulses/sec | 17.19 | 14.81 | 11.74 | 15.01 |
| ln(mean) | 2.41 ± 0.22 | 2.26 ± 0.13 | 2.15 ± 0.07 | 2.38 ± 0.14 |
| SCH-23390 | ||||
| Number of units (tests) | 11 (18) | 16 (27) | 20 (24) | 13 (22) |
| Units with excitations, % | 100 | 87.5 | 100 | 100 |
| Tests with excitations, % | 94.44 | 88.89 | 100 | 100 |
| F | 20.58** | 36.02*** | 49.58*** | 25.85*** |
| Mean basal activity, impulses/sec | 17.82 | 14.48 | 13.01 | 13.24 |
| GLU-induced activity | ||||
| Range, impulses/sec | 7.4–94.8 | 3.1–102.3 | 8.2–115.2 | 7.5–119.4 |
| Mean, impulses/sec | 32.75 | 26.57 | 41.89 | 40.86 |
| ln(mean) | 3.12 ± 0.214-167 | 2.98 ± 0.154-169 | 3.49 ± 0.154-169 | 3.48 ± 0.184-169 |
| GLU-induced inactivity | ||||
| Number of units | 0 (0) | 0 (0) | 1 (2) | 5 (13) |
| Range in rate, impulses/sec | 60–110 | 60–165 | ||
| Mean rate, impulses/sec | 85 | 94 | ||
| Eticlopride | ||||
| Number of units (tests) | 9 (11) | 16 (24) | 16 (27) | 14 (21) |
| Units with excitations, % | 77.78 | 87.50 | 100 | 100 |
| Tests with excitations, % | 81.82 | 83.33 | 96.30 | 100 |
| F | 36.44*** | 36.05*** | 34.69*** | 37.58*** |
| Mean basal activity, impulses/sec | 4.20 | 3.67 | 3.35 | 3.08 |
| GLU-induced activity | ||||
| Range, impulses/sec | 0.28–15.2 | 2.12–18.75 | 2.41–26.04 | 2.66–24.35 |
| Mean, impulses/sec | 7.28 | 8.31 | 9.93 | 13.63 |
| ln(mean) | 1.69 ± 0.324-159 | 1.98 ± 0.11 | 2.15 ± 0.11 | 2.47 ± 0.13 |
| SCH-23390 + Eticlopride | ||||
| Number of units (tests) | 18 (33) | 20 (37) | 21 (35) | 9 (12) |
| Units with excitations, % | 66.67 | 80.00 | 100 | 100 |
| Tests with excitations, % | 94.44 | 88.89 | 100 | 100 |
| F | 38.85*** | 40.19*** | 36.77*** | 7.60* |
| Mean basal activity, impulses/sec | 6.48 | 6.78 | 5.32 | 2.68 |
| GLU-induced activity | ||||
| Range, impulses/sec | 0.6–30.3 | 3.0–34.7 | 1.87–76.7 | 3.82–44 |
| Mean, impulses/sec | 11.44 | 13.04 | 19.55 | 21.34 |
| ln(mean) | 2.23 ± 0.13 | 2.45 ± 0.09 | 2.72 ± 0.134-169 | 2.67 ± 0.26 |
| GLU-induced inactivity | ||||
| Number of units | 0 (0) | 0 (0) | 2 (3) | 3 (5) |
Symbols as in Table 3;
indicates significant differences (
F4-159: p < 0.05;
F4-167: p < 0.01; and
F4-169: p < 0.001) compared with control.
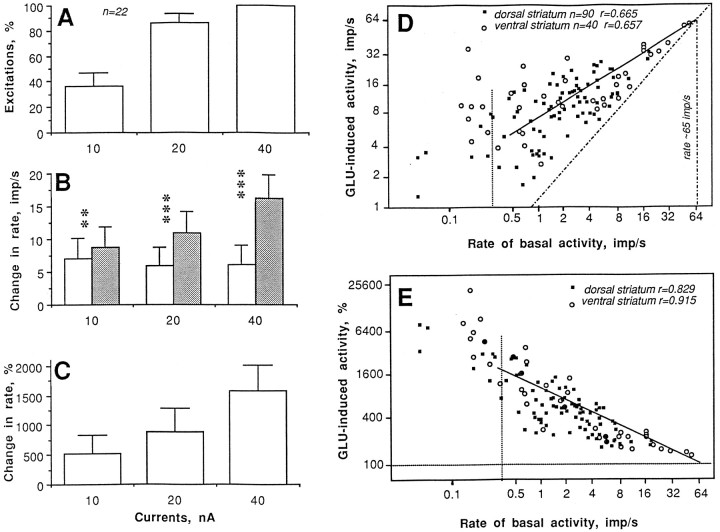
Although both the number of GLU-sensitive units and the magnitude of GLU responses were relatively stable at each current (Table 4), an individual analysis of units tested with progressively doubled currents revealed that the GLU response was clearly dose-dependent (Fig.7A–C). The magnitude of the GLU-induced excitation, moreover, depended on the level of basal activity (Fig.7D,E), whereas absolute magnitude increased (dorsal striatum, r = 0.665; ventral striatum, r = 0.657; one regression line is shown for all spontaneously active units except the most silent), relative magnitude decreased (dorsal striatum, r = 0.829; ventral striatum, r = 0.915) with increases in discharge rate. In both cases, the regression lines intersected with the line of no effect at ∼65 impulses/sec, which approximates the upper level of spontaneous impulse activity. Units with very low, sporadic baseline activity had very high relative magnitudes of the GLU response (1000–25,000%); these data are shown separately (left of the hatched lines in both graphs). Although dorsal and ventral striatal units had similar relationships between basal discharge rate and the parameters of the GLU response, both the basal discharge rate (range, 0.13–50.22; mean, 7.36 impulses/sec) and the absolute magnitude of the GLU response (range, 2.63–58.17; mean, 17.89 impulses/sec) were higher in ventral striatal than in dorsal striatal units (range, 0–17.11; mean, 2.50 impulses/sec and range, 1.28–31.17; mean, 10.34 impulses/sec, respectively).
Fig. 7.
Glutamate responses of striatal neurons in awake, unrestrained rats under control conditions. Parameters of the GLU response (A, number of units with excitation, percentage; B, discharge rate before and during GLU application, impulses per second; C, relative magnitude of GLU-induced excitation, percentage) evaluated in 22 units at different GLU ejection currents (in nanoamperes). Relationships between basal discharge rate (impulses per second) and absolute (impulses per second; D) or relative changes (percentage;E) induced by GLU shown separately for units recorded from dorsal (caudoputamen) and ventral striatum (accumbens). Regression lines (common for all striatal units) are solid, and lines of no effect are hatched. n, Number of tests; r, coefficients of correlation.Vertical hatched line indicates the border between spontaneously active and sporadically active units.
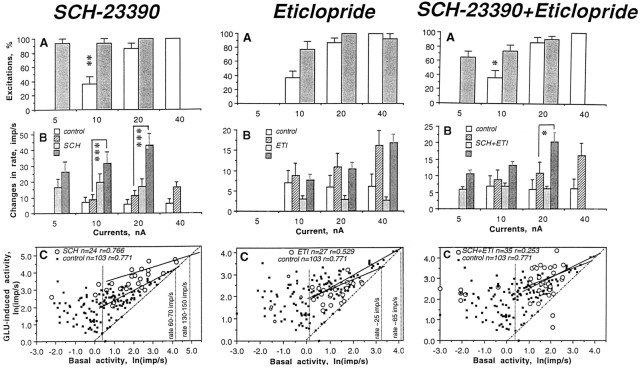
As shown in Table 4 and Figure 8, the most pronounced effect on GLU responses was found in the SCH sample (Fig. 3). Although similar numbers of excitations and an equally strong effect of GLU were found in both the SCH and control groups, the absolute magnitude of the GLU-induced excitation in the former group was significantly higher at each current (Fig. 8B). After SCH, GLU-induced excitations occurred at much lower currents (from 0 to 10 nA) than control with a significant decrease in mean threshold (4.17 ± 0.83 nA; n = 16 vs 19.7 ± 1.75 nA; n = 25 in control; p < 0.01). In addition, 5 of 17 units tested after SCH responded to GLU with partial or total cessation of firing (Fig. 3,C22-b-3,C17-d-8,C18-a-6) at currents (30–40 nA, but in one cell at 20 nA; mean, 31.43 nA; n = 7 units) that did not have this effect under control conditions. This presumed depolarization inactivation always occurred during GLU ejection at the peak of excitation when rates reached 60–145 impulses/sec (mean, 93.62 impulses/sec; n = 7). This range of GLU-induced depolarization inactivation approached the upper limits of spontaneous activity (Fig. 8C; 120–140 impulses/sec), which was much higher than control (60–70 impulses/sec). This cessation of firing was relatively short-lived (4–20 sec), reversible, and repeatable during subsequent tests (Fig.3). The GLU-induced excitation was frequently followed by a pronounced postexcitatory inhibition (Fig. 3,C20-c-3,C22-b-3). This rebound effect greatly varied in magnitude, but occurred in most tested units (11 of 17), especially those with high activity rates and high-magnitude GLU responses.
Fig. 8.
Glutamate responses of striatal neurons after DA receptor blockade. Three graphs in each vertical column show, respectively, the number of excitations, percentage (A), changes in rate induced by GLU, impulses per second (B), and the results of regression analysis of GLU responses in control (filled symbols) and drug-treated (open symbols) states.
Although GLU responses in the ETI group resembled the control state (Table 4, Fig. 8), the mean threshold of the GLU-induced excitation (8.47 ± 1.27 nA; range, 0–20 nA; n = 18) was significantly lower. The excitations also grew with an increase in current, but their absolute magnitudes were smaller than control (Fig.8B) and frequently decreased (5 of 21 units) with a further increase in current. Moreover, regression analysis (Fig.8C) revealed that the maximum rate of GLU-induced excitation under ETI is more than two times smaller (∼25 impulses/sec) than control. Although most units recorded after ETI had slow discharge rates (0.1–13 impulses/sec) and low-magnitude GLU excitations (2.4–26 impulses/sec), we observed postexcitatory inhibitions in 8 of 21 units (some were very pronounced, see examples in Fig. 4) and in three, a presumed depolarization inactivation. In contrast to SCH, cessation of activity occurred at much lower rates (∼15–20 impulses/sec), often resulting in a biphasic GLU response (excitation followed by inhibition).
Most units in the SCH plus ETI group showed more powerful GLU responses than control (Table 4, Fig. 8; see Fig. 4 for original examples), but this effect was less pronounced than that induced by SCH alone. The GLU-induced excitation occurred at low currents (range, 0–15; mean, 6.72 ± 1.42 nA; n = 15; higher than SCH but significantly lower than control) and was manifest as a strong, dose-dependent increase in discharge rate, which was larger than control (Fig. 8B). Although many GLU responses were indistinguishable from those in control conditions, 6 of 19 cells showed a rebound inhibition of varying degrees, and three showed a partial or complete cessation of firing during GLU iontophoresis at high currents (40 nA). Regression analysis of GLU responses in the SCH plus ETI group (Fig. 8C) revealed that, although the discharge rate of tested units was faster than control, the upper limit of GLU-induced activity was close (∼45–50 impulses/sec) to that in the control state.
DISCUSSION
Most information about the role of DA in regulating striatal function is based on electrophysiological changes induced by applications of DA or receptor-selective DA agonists. This approach, commonly used in anesthetized and in vitro preparations, has been highly productive in determining the basic ionic and receptor mechanisms of DA action on single units and the pattern of DA interactions with other neurochemical signals (for review, see White and Hu, 1993; Grenhoff and Johnson, 1997), but such an approach largely ignores the fact that under natural conditions DA is continuously released and interacts with other, more phasic inputs, particularly GLU. The present study used selective DA antagonists to prevent endogenous DA from interacting with its receptors, which allowed us to assess its role in regulating the spontaneous activity of striatal neurons and their responsiveness to iontophoretic GLU. Our units also were tested with iontophoretic DA not only to confirm DA antagonism but also to clarify the receptor mechanisms of DA action. Finally, we used freely moving rats to ensure a normal level of endogenous DA activity on which to base our pharmacological and iontophoretic data.
Responses to iontophoretic DA before and after DA receptor blockade
Consistent with our previous results (Kiyatkin and Rebec, 1996), iontophoretic DA weakly decreased the activity of most dorsal and ventral striatal neurons. Although dose-dependent, the response increased only slightly to each doubling of current and was independent of discharge rate. Comparable results were obtained from silent or sporadically active units tonically stimulated by continuous GLU application. Thus, to the extent that a brief DA application mimics phasic DA release, it appears that under behaviorally relevant conditions endogenously released DA has weak inhibitory effects on spontaneously active and GLU-stimulated neurons. These effects, moreover, were attenuated by SCH but not ETI, strongly suggesting an inhibitory role for D1 receptors. This conclusion also agrees with results obtained from both anesthetized (Johnson et al., 1986; Ohno et al., 1987; Hu and Wang, 1988; White and Hu, 1993) and in vitro preparations (Mercuri et al., 1985; Calabresi et al., 1987;Pennartz et al., 1992; Surmeier et al., 1992; Nicola et al., 1996;O’Donnell and Grace, 1996). Interestingly, however, the SCH plus ETI combination revealed a difference in the DA response between spontaneously active and GLU-stimulated units. Among spontaneously active units, the combination had a stronger attenuating effect than SCH alone. This finding suggests that D2 blockade enhances the effect of D1 antagonism, arguing for a cooperative interaction between these receptors in mediating DA effects (White and Hu, 1993). GLU-stimulated units, on the other hand, showed almost no attenuation of the DA response after the SCH plus ETI combination. This outcome is difficult to explain but it is noteworthy that haloperidol, a D2 antagonist with some D1 affinity, frequently fails to block DA-induced inhibitions of striatal (Ben-Ari and Kelly, 1976; Skirboll and Bunney, 1979; Johnson et al., 1986) and prefrontal cortical neurons (Thierry et al., 1986;Godbout et al., 1991), especially those activated by GLU. We have reported similar failures of haloperidol to reverse amphetamine-induced inhibitions of striatal neurons (Haracz et al., 1993). Of course, haloperidol itself may have unusual actions, some of which may derive from its affinity for sigma receptors (Taylor and Dekleva, 1987), but the failure of D1 plus D2 blockade to prevent DA-induced inhibitions of GLU-stimulated units requires further investigation.
Interestingly, Ohno et al. (1987) reported DA-induced increases in striatal activity during local SCH application. These data have been interpreted as evidence that D1 blockade unmasks an excitatory action of DA at D2 receptors. Although we found that SCH increased the frequency of DA-induced excitations, this effect was still quite rare, occurring in <10% of our tests. In fact, similarly rare excitations were seen after the SCH plus ETI combination. Thus, rather than suggest an excitatory role for D2 receptors, our data indicate instead that by removing an inhibitory DA influence, blockade of either D1 or D1 plus D2 receptors simply makes striatal neurons increasingly sensitive to extraneous stimuli. This conclusion is supported by our results with GLU in which both SCH alone and in combination with ETI enhanced the excitatory effects of iontophoretic GLU.
The role of tonic DA input in regulating spontaneous neuronal activity
The changes in spontaneous activity observed after our treatments were consistent with our data on DA iontophoresis. Relative to control, SCH, which attenuated the inhibiting action of DA, strongly increased spontaneous neuronal activity, whereas ETI, which did not affect or slightly enhanced the DA-induced inhibition, caused spontaneous activity to decline. Thus, the increase in basal activity found during D1 receptor blockade may result from neuronal disinhibition caused by a removal of tonic D1 receptor-mediated inhibitory action of endogenous DA. In contrast, an inhibiting effect of ETI on basal activity may reflect an enhancement of DA-induced inhibition caused by a drug-induced increase in striatal DA release (Imperato and Di Chiara, 1988; Gainetdinov et al., 1994), owing to a preferential action on release-modulating D2 receptors (Bunney et al., 1991). These effects of D1 and D2 blockade were combined after SCH plus ETI, which increased striatal activity, albeit to a lesser extent than after D1 blockade alone. Thus, it appears that under behaviorally relevant conditions endogenously released DA, interacting with D1 receptors, exerts a strong restraining influence on the spontaneous activity of striatal neurons. Removal of this tonic influence may be responsible, at least in part, for the increase in neuronal excitability found after DA denervation (Schultz, 1982; Orr et al., 1986; Calabresi et al., 1993;Mulder et al., 1996). Interestingly, systemic quinpirole, a D2 agonist, also inhibits striatal neurons in behaving rats, suggesting an inhibitory function for D2 receptors as well (Hooper et al., 1997). Our failure to reverse DA-induced inhibitions after ETI as well as the inconsistent effects of iontophoretic quinpirole on striatal and accumbal neurons (White and Hu, 1993) suggest, however, that at least some of the inhibitory action of systemic quinpirole may reflect extrastriatal influences.
Because both SCH and ETI have a high selectivity for DA receptors (see Materials and Methods), an interaction of these drugs with other receptors is unlikely to contribute significantly to our results. SCH, for example, has a limited affinity for 5-HT2 receptors (Bischoff et al., 1986), but 5-HT2 antagonistic activity is simply absent in vivo at the dose used in our study (Hjorth and Carlsson, 1988). Moreover, ritanserin, a relatively selective 5-HT2 antagonist, has no consistent effect on spontaneously active striatal neurons in behaving animals (Rosa-Kenig et al., 1993).
Although we are confident that specific alterations in DA transmission are a primary cause for the observed changes in striatal activity, it is more difficult to explain their underlying mechanisms. Because DA receptors are widely distributed within the brain, and systemic DA antagonists influence DA transmission at multiple sites, the changes in striatal activity may reflect both local alterations in DA input and a modification of other inputs associated with drug actions at extrastriatal sites. The ability of SCH and SCH plus ETI to attenuate striatal DA responses confirms that DA transmission is effectively blocked within the striatum, arguing for the role of local alterations in DA input in mediating the neuronal changes. We cannot exclude the possibility, however, that modified activity in striatal, particularly GLU afferents, may contribute to these changes. In fact, an increased GLU input has been implicated in the hyperactivity and hyperexcitability of striatal neurons after chemical DA denervation (Calabresi et al., 1993).
The role of tonic DA input in regulating GLU responsiveness
GLU provides the major excitatory input to striatal neurons (for review, see Parent and Hazrati, 1995), and GLU release appears to be responsible for the excitations of these cells that occur in response to somatosensory stimuli and during behavior (Wilson and Kawaguchi, 1996; Calabresi et al., 1997). Thus, the enhanced striatal GLU release that occurs as a result of DA receptor blockade may contribute to the increased neuronal activity after SCH and, to a lesser extent, after SCH plus ETI, whereas the decreased neuronal activity after ETI may reflect diminished GLU input. Possible changes in GLU release, however, cannot explain the dramatic changes in GLU responsiveness found after our treatments. After SCH, for example, our units became more sensitive to GLU, as indicated by a decrease in response threshold, an increase in the absolute magnitude of excitation, and more frequent occurrence of an apparent depolarization inactivation at high GLU currents. It appears, therefore, that endogenously released DA provides, via D1 receptors, a tonic inhibitory influence on GLU-induced excitations of striatal neurons. The enhanced GLU responsiveness after SCH, therefore, may result from removal of this tonic inhibitory action, whereas decreased GLU responsiveness after ETI may reflect the strengthening of this D1 receptor-mediated action caused by increased striatal DA release. These two opposing influences appear to be combined after joint blockade of D1 and D2 receptors. In this case, the GLU-induced excitations occurred at lower ejection currents, had a higher magnitude, and were more often inactivated by high GLU currents than control, but all these changes were less pronounced than with D1 receptor blockade alone. Thus, our data support the idea that endogenously released DA is involved in modulating the GLU responsiveness of striatal neurons. These data may explain the enhanced afferent responsiveness of these cells after D1 and D1 plus D2 blockade. Interestingly, strong increases in the absolute magnitude of the GLU response were found on accumbal neurons during local DA depletion (Mulder et al., 1996), arguing for local changes in DA input as a primary cause for changes in neuronal activity.
DA modulation of striatal activity under behavioral conditions
In agreement with early ideas on the functional role of DA (Siggins, 1977; Moore and Bloom, 1978; Bloom et al., 1989), our results suggest that tonically released DA provides a continuous restraining influence on striatal neurons, modulating both their activity and responsiveness to other inputs, particularly GLU. To the extent that a brief DA application mimics phasic DA release, our data also support the idea that phasically released DA shares the same, inhibiting pattern of action on both basal and GLU-evoked activity. Such inhibiting effects have been found in most studies using DA applications both in anesthetized and in vitro preparations (Mercuri et al., 1985; Chiodo and Berger, 1986; Johnson et al., 1986;Ohno et al., 1987; Nicola et al., 1996), and these effects were typical of the majority of striatal units in freely moving rats. DA, however, has also been shown to enhance GLU-induced excitations (Chiodo and Berger, 1986; Cepeda et al., 1993; Levine et al., 1996; Hu and White, 1997). This enhancing effect was reported to be relatively weak, occurred only at very low ejection currents (when high-affinity D2 receptors are presumably activated, but low-affinity D1 receptors are unaffected), and consistently changed into a more profound inhibition of the GLU response with further increases in current (when D1 receptors are coactivated). Although these latter results are consistent with the view that DA at different doses activates different types of DA receptors, recent observations that both D1 and D2 agonists may weakly potentiate the GLU response (Hu and White, 1997) argue against this view. DA also has been shown to potentiate GLU-induced excitations in behaving animals (Pierce and Rebec, 1995), but in this case the activating effect of DA appears to involve a D2 mechanism. DA effects on the GLU response also may depend on the type of GLU receptor activated (Cepeda et al., 1993; Levine et al., 1996). Both in striatum and neocortex, for example, DA enhanced neuronal responses induced by NMDA receptor agonists, but it attenuated non-NMDA-mediated responses.
The actual effects of DA released under behavioral conditions, however, may be much more complex than currently believed based on experimental models. Although we found that continuously applied DA in most cases decreased the absolute magnitude of GLU-induced excitations, their relative magnitude (signal-to-noise ratio) could either increase or decrease depending on the basal rate of neuronal activity, the dose of applied DA, the initial magnitude of GLU-induced excitation, as well as on DA-induced changes in discharge rate (Kiyatkin and Rebec, 1996). Thus, it appears that the effect of DA on the neuronal change elicited by phasic GLU input ultimately depends on how DA influences ongoing basal activity, which itself depends on tonic GLU input. This effect, moreover, is modulated concomitantly by other inputs. Such continuous modulation of afferent effectiveness provides a mechanism by which fluctuations in DA release can regulate the transmission of behaviorally relevant information.
Conclusions
Our data support the view of DA as a modulator of striatal function and suggest that endogenously released DA provides a strong restraining influence on basal activity of striatal neurons and their responsiveness to phasic changes in GLU input. These data support a key role of D1 receptors in mediating the effects of DA on striatal neurons. These same receptors are critical for learning, memory, and regulating various forms of adaptive behavior (Miller et al., 1990;Beninger, 1992). The role of D2 receptors is more difficult to characterize in that they are found both presynaptically and postsynaptically, allowing for modulation of both DA and GLU release as well as neuronal responses to these inputs. Despite the opposing effects of D1 and D2 stimulation on striatal activity and GLU responsiveness, our data support the existence of cooperative relationships between D1 and D2 receptors, which act jointly in mediating the cellular effects of DA, regulating DA and GLU release, and integrating DA with other neurochemical inputs. Finally, our data indicate that GLU mechanisms are essentially involved in mediating the effects of DA input on striatal neurons.
Footnotes
This work was supported by the National Institute on Drug Abuse (Grants DA 02451 and DA 00335). We greatly appreciate Paul Langley for technical assistance and Faye Caylor for help in preparing this manuscript.
Correspondence should be addressed to Eugene A. Kiyatkin at the above address.
REFERENCES
- 1.Ben-Ari Y, Kelly J. Dopamine-evoked inhibition of single cells of the feline putamen and basolateral amygdala. J Physiol (Lond) 1976;256:1–22. doi: 10.1113/jphysiol.1976.sp011308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beninger RJ. D-1 receptor involvement in reward-related learning. J Psychopharmacol. 1992;6:34–42. doi: 10.1177/026988119200600109. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff S, Heinrich M, Sonntag HM, Krauss J. The D-1 dopamine receptor antagonist SCH 23390 also interacts potently with brain serotonin (5-HT2) receptors. Eur J Pharmacol. 1986;129:367–370. doi: 10.1016/0014-2999(86)90449-8. [DOI] [PubMed] [Google Scholar]
- 4.Bloom FE, Schulman JA, Koob GF. Catecholamines and behavior. In: Trendelenburg U, Weiner N, editors. Handbook of experimental pharmacology. Vol 90/II. Springer; Berlin: 1989. pp. 27–88. [Google Scholar]
- 5.Bunney BS, Chiodo LA, Grace AA. Midbrain dopamine system electrophysiological functioning: a review and new hypothesis. Synapse. 1991;9:79–94. doi: 10.1002/syn.890090202. [DOI] [PubMed] [Google Scholar]
- 6.Calabresi P, Mercuri N, Stanzione P, Stefani A, Bernardi G. Intracellular studies of dopamine-induced inhibition of neostriatal neurons in vitro: evidence for D1 receptor involvement. Neuroscience. 1987;20:757–771. doi: 10.1016/0306-4522(87)90239-9. [DOI] [PubMed] [Google Scholar]
- 7.Calabresi P, Mercuri NB, Sanscessario G, Bernardi G. Electrophysiology of dopamine-denervated striatal neurons. Implications for Parkinson’s disease. Brain. 1993;116:433–452. [PubMed] [Google Scholar]
- 8.Calabresi P, Pisani A, Centonze D, Bernardi G. Synaptic plasticity and physiological interactions between dopamine and glutamate in the striatum. Neurosci Biobehav Rev. 1997;21:519–523. doi: 10.1016/s0149-7634(96)00029-2. [DOI] [PubMed] [Google Scholar]
- 9.Cepeda C, Levine MS. Dopamine and N-methyl-d-aspartate receptor interactions in the neostriatum. Dev Neurosci. 1998;20:1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- 10.Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid subtype activated. Proc Natl Acad Sci USA. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiodo LA, Berger TW. Interactions between dopamine and amino acid-induced excitation and inhibition in the striatum. Brain Res. 1986;375:198–203. doi: 10.1016/0006-8993(86)90976-5. [DOI] [PubMed] [Google Scholar]
- 12.Chiodo LA, Antelman SM, Caggiula AR, Lineberry CG. Sensory stimuli alter the discharge rate of dopamine (DA) neurons: evidence for two functional types of DA cells in the substantia nigra. Brain Res. 1980;189:544–549. doi: 10.1016/0006-8993(80)90366-2. [DOI] [PubMed] [Google Scholar]
- 13.Cooper JR, Bloom FE, Roth RH. The biochemical basis of neuropharmacology. Oxford UP; New York: 1991. [Google Scholar]
- 14.Dai M, Tepper JM. Do silent dopaminergic neurons exist in rat substantia nigra in vivo? Neuroscience. 1998;85:1089–1099. doi: 10.1016/s0306-4522(97)00615-5. [DOI] [PubMed] [Google Scholar]
- 15.Fibiger HC, Phillips AG. Handbook of physiology, the nervous system, Vol 4, intrinsic regulatory systems of the brain, pp 647–674. American Physiological Society; Bethesda: 1986. Reward, motivation and cognition: psychobiology of the mesotelencephalic dopamine systems. [Google Scholar]
- 16.Gainetdinov RR, Grekhova TV, Sotnikova TD, Rayevsky KS. Dopamine D2 and D3 receptor preferring antagonists differentially affect striatal dopamine release and metabolism in conscious rats. Eur J Pharmacol. 1994;261:327–331. doi: 10.1016/0014-2999(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 17.Godbout R, Mantz J, Rivot S, Glowinski J, Thierry A-M. Inhibitory influence of the mesocortical dopaminergic neurons on their targets. Electrophysiological and pharmacological characterization. J Pharmacol Exp Ther. 1991;258:728–738. [PubMed] [Google Scholar]
- 18.Graybiel AM. The basal ganglia. Trends Neurosci. 1995;18:60–62. [PubMed] [Google Scholar]
- 19.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 20.Grenhoff J, Johnson SW. Electrophysiological effects of dopamine receptor stimulation. In: Neve KA, Neve RL, editors. The dopamine receptors. Humana; Totowa, NJ: 1997. pp. 267–304. [Google Scholar]
- 21.Groves PM. A theory of functional organization of the neostriatum and neostriatal control of voluntary movements. Brain Res Rev. 1983;5:109–132. doi: 10.1016/0165-0173(83)90011-5. [DOI] [PubMed] [Google Scholar]
- 22.Haracz JL, Tsang JT, Wang Z, White IM, Rebec GV. Striatal single-unit responses to amphetamine and neuroleptics in freely moving rats. Neurosci Biobehav Rev. 1993;17:1–12. doi: 10.1016/s0149-7634(05)80226-x. [DOI] [PubMed] [Google Scholar]
- 23.Hjorth S, Carlsson A. In vivo receptor binding, neurochemical and functional studies with the dopamine D-1 receptor antagonist SCH 23390. J Neural Transm. 1988;72:83–97. doi: 10.1007/BF01250232. [DOI] [PubMed] [Google Scholar]
- 24.Hooper KC, Banks DA, Stordahl LJ, White IM, Rebec GV. Quinpirole inhibits striatal and excites pallidal neurons in freely moving rats. Neurosci Lett. 1997;237:69–72. doi: 10.1016/s0304-3940(97)00812-4. [DOI] [PubMed] [Google Scholar]
- 25.Hu X-T, Wang RY. Comparison of effects of D-1 and D-2 dopamine receptor agonists on neurons in the rat caudate putamen. An electrophysiological study. J Neurosci. 1988;8:4340–4348. doi: 10.1523/JNEUROSCI.08-11-04340.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu X-T, White FJ. Dopamine enhances glutamate-induced excitation of rat striatal neurons by cooperative activation of D1 and D2 class receptors. Neurosci Lett. 1997;224:61–65. doi: 10.1016/s0304-3940(97)13443-7. [DOI] [PubMed] [Google Scholar]
- 27.Jackson DM, Westlind-Danielson A. Dopamine receptors: molecular biology, biochemistry and behavioral aspects. Pharmacol Ther. 1994;64:291–269. doi: 10.1016/0163-7258(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 28.Johnson SW, Hoffer BJ, Freedman R. Investigation of the failure of parenterally administered haloperidol to antagonize dopamine released from micropipettes in the caudate. J Neurosci. 1986;6:572–580. doi: 10.1523/JNEUROSCI.06-02-00572.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imperato A, Di Chiara G. Effects of locally applied D-1 and D-2 receptor agonists and antagonists studied with brain dialysis. Eur J Pharmacol. 1988;156:385–393. doi: 10.1016/0014-2999(88)90284-1. [DOI] [PubMed] [Google Scholar]
- 30.Kiyatkin EA. Functional properties of presumed dopamine-containing and other ventral tegmental area neurons in conscious rats. Int J Neurosci. 1988;42:21–43. doi: 10.3109/00207458808985756. [DOI] [PubMed] [Google Scholar]
- 31.Kiyatkin EA, Rebec GV. Dopaminergic modulation of glutamate-induced excitation of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol. 1996;75:142–153. doi: 10.1152/jn.1996.75.1.142. [DOI] [PubMed] [Google Scholar]
- 32.Kiyatkin EA, Rebec GV. Heterogeneity of ventral tegmental area neurons: single-unit recording and iontophoresis in awake, unrestrained rats. Neuroscience. 1998;85:1285–1309. doi: 10.1016/s0306-4522(98)00054-2. [DOI] [PubMed] [Google Scholar]
- 33.Kiyatkin EA, Rebec GV. Modulation of striatal neuronal activity by glutamate and GABA: Iontophoresis in awake, unrestrained rats. Brain Res. 1999;822:88–106. doi: 10.1016/s0006-8993(99)01093-8. [DOI] [PubMed] [Google Scholar]
- 34.Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- 35.Levine MS, Li Z, Cepeda C, Cromwell HC, Alteemus KL. Neuromodulatory actions of dopamine on synaptically-evoked neostriatal responses in slices. Synapse. 1996;24:65–78. doi: 10.1002/syn.890240102. [DOI] [PubMed] [Google Scholar]
- 36.Mercuri N, Bernardi G, Calabresi P, Cotugno A, Levi G, Stanzione P. Dopamine decreases cell excitability in rat striatal neurons by pre- and postsynaptic mechanisms. Brain Res. 1985;358:110–121. doi: 10.1016/0006-8993(85)90954-0. [DOI] [PubMed] [Google Scholar]
- 37.Miller JD, Sanghera MK, German DC. Mesencephalic dopaminergic unit activity in the behaviorally conditioned rat. Life Sci. 1981;29:1255–1263. doi: 10.1016/0024-3205(81)90231-9. [DOI] [PubMed] [Google Scholar]
- 38.Miller R, Wickens JD, Beninger RJ. Dopamine D-1 and D-2 receptors in relation to reward and performance: a case for the D-1 receptor as a primary site of therapeutic action of neuroleptic drugs. Prog Neurobiol. 1990;34:143–183. doi: 10.1016/0301-0082(90)90005-2. [DOI] [PubMed] [Google Scholar]
- 39.Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the dopamine system. Annu Rev Neurosci. 1978;1:129–169. doi: 10.1146/annurev.ne.01.030178.001021. [DOI] [PubMed] [Google Scholar]
- 40.Mulder AB, Manshanden I, Vos E, Wolterink G, Van Ree JM, Lopes de Silva FH. Modification of glutamatergic transmission after dopamine depletion of the nucleus accumbens. A combined in vivo/in vitro electrophysiological study in the rat. Neuroscience. 1996;72:1009–1021. doi: 10.1016/0306-4522(96)00035-8. [DOI] [PubMed] [Google Scholar]
- 41.Neve KA, Neve RL. Molecular biology of dopamine receptors. In: Neve KA, Neve RL, editors. The dopamine receptors. Humana; Totowa, NJ: 1997. pp. 27–76. [Google Scholar]
- 42.Nicola SM, Kombian SB, Malenka RC. Psychostimulants depress excitatory synaptic transmission in the nucleus accumbens via presynaptic D-1 like dopamine receptors. J Neurosci. 1996;16:1591–1604. doi: 10.1523/JNEUROSCI.16-05-01591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Donnell P, Grace AA. Dopaminergic reduction of excitability in nucleus accumbens neurons recorded in vitro. Neuropsychopharmacology. 1996;15:87–97. doi: 10.1016/0893-133X(95)00177-F. [DOI] [PubMed] [Google Scholar]
- 44.Ohno Y, Sasa M, Takaori S. Coexistence of inhibitory dopamine D-1 and excitatory D-2 receptors on the same caudate nucleus neurons. Life Sci. 1987;40:1937–1945. doi: 10.1016/0024-3205(87)90054-3. [DOI] [PubMed] [Google Scholar]
- 45.Orr WP, Gardiner TW, Stricker EM, Zigmond MJ, Berger TW. Short-term effects of dopamine-depleting brain lesions on spontaneous activity of striatal neurons: relation to local dopamine concentration and behavior. Brain Res. 1986;376:20–28. doi: 10.1016/0006-8993(86)90895-4. [DOI] [PubMed] [Google Scholar]
- 46.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortical-basal ganglia-thalamo-cortical loop. Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 47.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Ed 2. Academic; Sydney: 1986. [DOI] [PubMed] [Google Scholar]
- 48.Pennartz CMA, Dolleman-Van der Weel MJ, Kitai ST, Lopes de Silva FH. Presynaptic dopamine D1 receptors attenuate excitatory and inhibitory limbic inputs to the shell region of the rat nucleus accumbens studied in vitro. J Neurophysiol. 1992;67:1325–1334. doi: 10.1152/jn.1992.67.5.1325. [DOI] [PubMed] [Google Scholar]
- 49.Pierce RC, Rebec GV. Iontophoresis in the neostriatum of awake, unrestrained rats: differential effects of dopamine, glutamate and ascorbate on motor- and nonmotor related neurons. Neuroscience. 1995;67:313–324. doi: 10.1016/0306-4522(95)00012-8. [DOI] [PubMed] [Google Scholar]
- 50.Rebec GV, Langley PE, Pierce RC, Wang Z, Heidenreich BA. A simple micromanipulator for multiple uses in freely moving rats: electrophysiology, voltammetry, and simultaneous intracerebral infusions. J Neurosci Methods. 1993;47:53–59. doi: 10.1016/0165-0270(93)90021-i. [DOI] [PubMed] [Google Scholar]
- 51.Rolls ET, Thorpe SJ, Boytim M, Szabo I, Perrett DI. Responses of striatal neurons in the behaving monkey. 3. Effects of iontophoretically applied dopamine on normal responsiveness. Neuroscience. 1984;12:1201–1212. doi: 10.1016/0306-4522(84)90014-9. [DOI] [PubMed] [Google Scholar]
- 52.Rosa-Kenig A, Puotz JK, Rebec GV. The involvement of D1 and D2 dopamine receptors in amphetamine-induced changes in striatal unit activity in behaving rats. Brain Res. 1993;619:347–351. doi: 10.1016/0006-8993(93)91633-4. [DOI] [PubMed] [Google Scholar]
- 53.Schultz W. Depletion of dopamine in the striatum as an experimental model of parkinsonism; direct effects and adaptive mechanisms. Prog Neurobiol. 1982;18:121–166. doi: 10.1016/0301-0082(82)90015-6. [DOI] [PubMed] [Google Scholar]
- 54.Schultz W. Responses of midbrain dopamine neurons to behavioral trigger stimuli in the monkey. J Neurophysiol. 1986;56:1439–1461. doi: 10.1152/jn.1986.56.5.1439. [DOI] [PubMed] [Google Scholar]
- 55.Siggins GR. Electrophysiological role of dopamine in striatum: excitatory or inhibitory? In: Lipton MA, DiMascio A, Killam KF, editors. Psychopharmacology: a generation of progress. Raven; New York: 1977. pp. 143–157. [Google Scholar]
- 56.Skirboll LR, Bunney BS. The effects of acute and chronic haloperidol treatment on spontaneously firing neurons in caudate nucleus of the rat. Life Sci. 1979;25:1419–1343. doi: 10.1016/0024-3205(79)90420-x. [DOI] [PubMed] [Google Scholar]
- 57.Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurons. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- 58.Steinfels GF, Heym J, Strecker RE, Jacobs BL. Behavioral correlates of dopaminergic unit activity in freely moving cats. Brain Res. 1983;258:217–228. doi: 10.1016/0006-8993(83)91145-9. [DOI] [PubMed] [Google Scholar]
- 59.Strecker RE, Jacobs BL. Substantia nigra dopaminergic unit activity in behaving cats: effect of arousal on spontaneous discharge and sensory evoked activity. Brain Res. 1985;361:339–350. doi: 10.1016/0006-8993(85)91304-6. [DOI] [PubMed] [Google Scholar]
- 60.Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, Stefani A, Kitai ST. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc Natl Acad Sci USA. 1992;89:10178–10182. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor DP, Dekleva J. Potential antipsychotic BMY-14802 selectively binds to sigma sites. Drug Dev Res. 1987;11:65–70. [Google Scholar]
- 62.Thierry AM, Le Douarin C, Penit J, Ferron A, Glowinski J. Variation in the ability of neuroleptics to block the inhibitory influence of dopaminergic neurons on the activity of cells in the rat prefrontal cortex. Brain Res Bull. 1986;16:155–160. doi: 10.1016/0361-9230(86)90027-4. [DOI] [PubMed] [Google Scholar]
- 63.Trulson ME. Activity of dopamine-containing substantia nigra neurons in the freely moving cats. Neurosci Biobehav Rev. 1985;9:283–297. doi: 10.1016/0149-7634(85)90051-x. [DOI] [PubMed] [Google Scholar]
- 64.White FJ, Hu XT. Electrophysiological correlates of D1:D2 interactions. In: Waddington J, editor. Dopamine receptor interactions. Academic; London: 1993. pp. 79–114. [Google Scholar]
- 65.Wilson CJ, Kawaguchi Y. The origin of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zigmond MJ. Chemical transmission in the brain: homeostatic regulation and its functional implications. In: Bloom FE, editor. Progress in brain research. Elsevier; New York: 1994. pp. 115–122. [DOI] [PubMed] [Google Scholar]