Abstract
Tumor necrosis factor α (TNF) is a cytokine that is involved in the inflammatory process after CNS injury and is implicated in neuroregeneration. A saturable transport system for TNF located at the blood–brain barrier (BBB) is responsible for the limited entry of TNF from blood to the CNS in normal mice.
After partial disruption of the BBB by compression of the lumbar spinal cord, permeability to TNF was increased not only in the lumbar spinal cord but also in brain and distal spinal cord segments, where the BBB remained intact. The increase in the entry of TNF to the CNS followed a biphasic temporal pattern, with a first peak immediately after injury and a second peak starting on day 3; these changes lasted longer than the mere disruption of the BBB. The increased entry of TNF was abolished by addition of excess unlabeled TNF, showing that the transport system for TNF remained saturable after spinal cord injury (SCI) and providing evidence that the enhanced entry of TNF could not be explained by diffusion or leakage.
This study adds strong support for our concept that the saturable transport system for TNF across the BBB can be upregulated in the diseased state, and it suggests that the BBB is actively involved in the modulation of the processes of degeneration and regeneration after SCI.
Keywords: TNFα, SCI, BBB, transport system, CNS, neuroregeneration, upregulation
Spinal cord injury (SCI) in mammals is associated with disruption of the blood–brain barrier (BBB), increased cytokine production, and degeneration of CNS tissue with poor functional recovery (McKenzie et al., 1995; Popovich et al., 1996;Jaeger and Blight, 1997; Pan et al., 1997b). Production of tumor necrosis factor α (TNF) is increased after SCI (Wang et al., 1996). The CNS effects of this cytokine can be either detrimental or beneficial. Detrimental effects include apoptosis and direct cell toxicity (Gelbard et al., 1993; Chao and Hu, 1994; Miura et al., 1995;Vandenabeele et al., 1995). Beneficial effects include stimulation of the synthesis of neurotrophic proteins and facilitation of the permissiveness of the substrate for neurite outgrowth after CNS injury (Schwartz et al., 1991; Genestier et al., 1995; Mattson, 1997). Thus, TNF can be involved in neuroregeneration.
TNF also can exert different effects on the BBB, including changes in permeability (Camussi et al., 1991; Kim et al., 1992; Duchini et al., 1996; Descamps et al., 1997; Pan et al., 1997c). It is not known whether TNF is involved in the biphasic opening of the BBB that has been observed in some pathological states, including cortical contusion (at 4–6 hr and again 3 d after injury) (Baskaya et al., 1997), transient unilateral middle cerebral artery occlusion (immediately after recirculation and again 5–72 hr later) (Kuroiwa et al., 1985), and lumbar SCI by transection (an immediate increase in adjacent areas and a latent increase in more distal regions) (Pan et al., 1997b). The production of TNF also may be biphasic, such as in traumatic brain injury (Fan et al., 1994). Whether the increase in TNF after trauma is related to disruption of the BBB, however, is not clear.
The interaction between TNF and the BBB in normal animals involves a saturable transport system at the BBB, which regulates the entry of TNF in peripheral blood to the brain and spinal cord unidirectionally (Gutierrez et al., 1993; Pan et al., 1997a). In animals with SCI, one possibility is that the resulting disruption of the BBB might cause greater entry of TNF from the periphery to the CNS secondary to leakage. However, after acute lumbar SCI by transection in mice, we have found that an enhanced transport system for TNF rather than leakage is mainly responsible for the increased entry of TNF (Pan et al., 1997b). Upregulation of the TNF transport system also occurs in experimental autoimmune encephalomyelitis (EAE), a nontraumatic model in which the autoimmune attack is directed primarily against central myelin components, resulting in an ascending but generalized disruption of the BBB that correlates with the functional deficit (Pan et al., 1996). Because TNF is produced in the CNS by both neurons and astrocytes (Pan et al., 1997c), the increased entry of TNF from the periphery may be indicative of an autoregulatory function restricting further excessive production in the CNS by a negative feedback mechanism.
Having found that the TNF transport system across the BBB is increased in two different pathological states (SCI by complete transection and EAE), we investigated the possibility that this upregulation is a more generalized phenomenon. In the study reported here, we applied a compression model of SCI to further study the alterations of the saturable transport system for TNF. The time course and spatial pattern of TNF entry at various times after SCI were measured and compared with entry of radiolabeled albumin, which was indicative of disruption of the BBB. The results were designed to answer the following questions. (1) How does the disruption of the BBB differ in the compressive model of SCI? (2) Does TNF enter the injured CNS by leakage only? (3) If not, where is the transport system for TNF preserved and how does it function? In general, knowledge about the regulation of the availability of TNF to the injured CNS should provide a novel approach, with possible therapeutic implications to understanding how inflammatory processes limit degeneration in the CNS.
MATERIALS AND METHODS
Spinal cord compression. Adult male ICR mice received deep anesthesia by intramuscular ketamine and xylazine (Sigma, St. Louis, MO). The skin was incised longitudinally on the lower back, the supraspinal muscles were separated, and laminectomy was performed on L1 and L2 vertebral bones with a pair of iridectomy scissors (Fine Science Tools, Foster City, CA). The spinal cord at this level was compressed by initially flanking the spinal cord with a pair of modified cover glass forceps at a length of 6 mm (Fine Science Tools). SCI involved steady compression of the 3 mm spinal cord to a width of 1 mm for 5 sec. After the injury, the subcutaneous tissue and skin were sutured in layers. The mice were placed on a heating pad until recovery from anesthesia within 1 hr and then were returned to their cages containing soft bedding and easy access to food and water. Manual compression of the bladder was performed for those with bladder distension. The extent of injury was quantified daily with the locomotor rating scale shown in Table 1 (n = 14–18 for daily scoring).
Table 1.
Scoring scales for mice after mild to moderate compressive spinal cord injury
| Locomotor activity | |
| 0 | No movement in hind limbs, extended paralysis bilaterally, limp tail |
| 1 | One hind limb paralyzed and extended, one hind limb paretic and flexed. Tail partially weak |
| 2 | One hind limb paralyzed and extended, one hind limb has weight bearing, or bilateral hind limbs have flexed paresis |
| 3 | Bilateral hind limbs flexed with minimal weight bearing, or one hind limb relatively normal with weight bearing, one hind limb has flexed paresis |
| 4 | Walks with mild deficit, bears weight in bilateral hind limbs |
| 5 | Normal walking |
| Extension withdrawal (reflexion of the hind limb when manually extended) | |
| 0 | No withdrawal |
| 1 | Weak, slow withdrawal on one side, or with normal withdrawal on one side, no withdrawal on the other |
| 2 | Weak, slow withdrawal on both sides |
| 3 | Normal withdrawal bilaterally |
| Parachuting reflex (posturing of the body and limbs when held by tail with head downwards) | |
| 0 | Total body flexed with tremor, no toe spread |
| 1 | Moderately impaired body and hind limb positioning, limited toe spread on either side |
| 2 | Mildly impaired body and hind limb positioning, nearly full toe spread |
| 3 | Normal |
| Righting reflex (return to prone position after being placed supine on a smooth surface) | |
| 0 | No righting |
| 1 | Prolonged return to prone position |
| 3 | Normal righting |
| Inclined plane (the angle of the test plane to the horizontal plane when the mouse is able to hold for >5 sec while facing downward) | |
| 0 | No grabbing on hind limbs |
| 1 | Angle <30° |
| 2 | Angle ≥30°, but <45° |
| 3 | Angle ≥45°, but 60° |
| 4 | Angle ≥60° |
Measurement of BBB permeability. Twelve groups of mice were studied (n = 8–12 per group). Experimental groups were tested immediately after SCI (5 min) at 0.5, 1, 2, 4, and 12 hr and then 1, 2, 3, 4, and 5 d after SCI. A nonsurgical control group was included in each experiment. The mice were anesthetized by intraperitoneal injection of 40% urethane (Sigma). The neck of the mouse was exposed, and the jugular veins and the right carotid artery were separated. Approximately 0.9 μCi/mouse 125I-TNF (R & D Systems, Minneapolis, MN), together with 1.8 μCi/mouse99mTc-albumin (Amersham Health Care, San Antonio, TX), was injected in a total volume of 200 μl into the left jugular vein. The radioactive solution was mixed in lactated Ringer’s solution containing 1% bovine serum albumin. At 10 min after intravenous injection, blood was collected from a cut in the carotid artery, and the mouse was decapitated immediately. Samples of brain and spinal cord (cervical, thoracic, and lumbar regions) were collected and weighed. The tissue sample and 50 μl of serum were counted in a dual channel γ-counter (Wallac, Gaithersburg, MD). The tissue/serum ratio was calculated and expressed as microliters per grams. The entry of99mTc-albumin was used as a measurement of BBB permeability in general, whereas the entry of 125I-TNF may be a combination of leakage and transport across the BBB.
Inhibition study to test saturability. Four groups of mice were studied (n = 8–9 per group): (1) control (non-SCI) injected with 125I-TNF and99mTc-albumin only; (2) control with addition of 1 μg/mouse (∼40 μg/kg body weight) unlabeled TNF in the injection solution of 125I-TNF and 99mTc-albumin; (3) mice at 3 d after SCI injected with 125I-TNF and99mTc-albumin only; and (4) mice at 3 d after SCI with addition of 1 μg/mouse unlabeled TNF in the injection solution with the radiolabeled compounds. BBB permeability was studied as mentioned above.
Statistical analysis. Statistical Program for the Social Sciences software was used. Means were expressed with their SEs, ANOVA was performed for all groups, and, subsequently, Tukey’s range test was performed if ANOVA showed statistical significance (p < 0.05). Sigma Plot was used to generate the figures.
RESULTS
Functional deficit after SCI
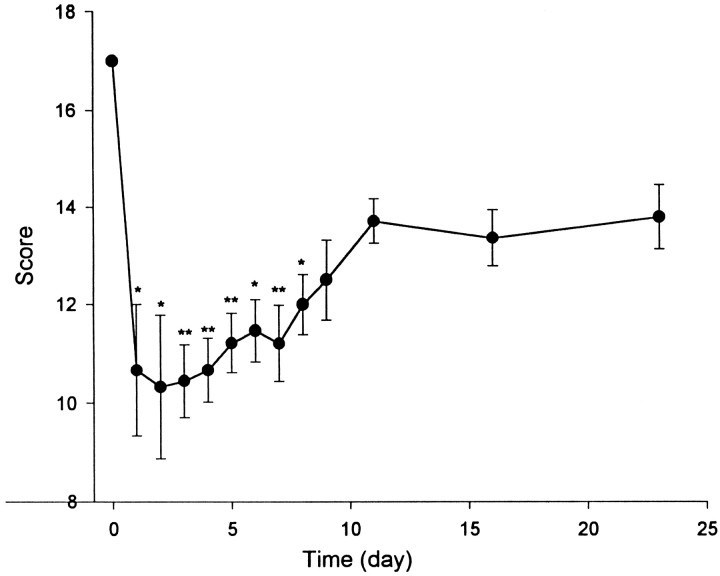
Figure 1 shows that SCI produced a deficit of locomotor activity in the mice that began immediately after their awakening from anesthesia (n = 14–18). Eighteen mice underwent SCI on the same day and were evaluated subsequently for 23 d; four of them died of complications during the first week. The reduction in the combined scores was statistically significant during the first 8 d after injury (p < 0.01 for days 3, 4, 5, and 6; p < 0.05 for the rest of the 8 d).
Fig. 1.
Functional deficit after acute compression of the lumbar spinal cord on day 1. Time 0, Control without SCI with a full score of 17. The decrease in scores was statistically significant from day 1 to day 8. *p < 0.05; **p < 0.01 versus control (n = 14–18 per group).
Time course of BBB disruption after SCI as measured by99mTc-albumin
The extent of BBB disruption at different time intervals after SCI was reflected in the tissue/serum ratios of 99mTc-albumin injected intravenously 10 min before each determination. In the baseline (time 0) control group (no SCI), the cervical area had the greatest permeability among the four regions. After SCI, statistically significant changes occurred in the thoracic and especially the lumbar spinal cord but not in the brain or the cervical spinal cord. The significant change in the thoracic spinal cord consisted of decreased permeability at day 5 after injury (p < 0.05).
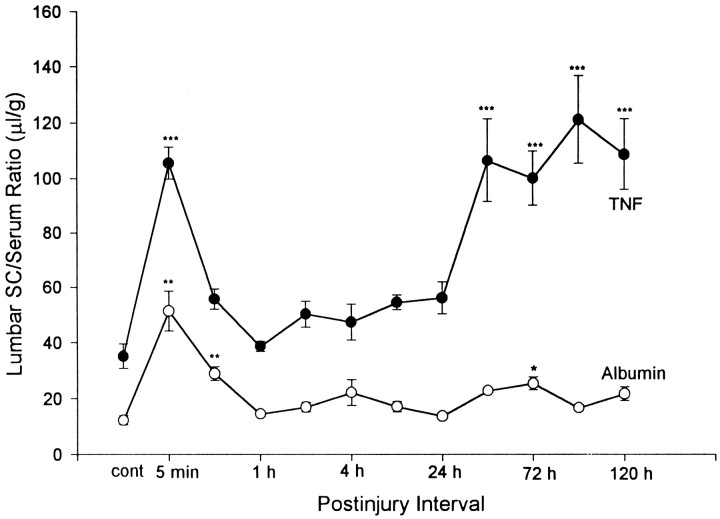
In the lumbar spinal cord, where most of the disruption was evident, a brief increase in entry occurred immediately (5 min after injury) (p < 0.01) after SCI and remained elevated 0.5 hr later (p < 0.01). Permeability to99mTc-albumin in the lumbar area returned to basal levels 1 hr after SCI and remained there, except for a smaller second increase (p < 0.05) on day 3 (Fig.2).
Fig. 2.
BBB permeation of99mTc-albumin and 125I-TNF in the lumbar spinal cord after acute lumbar SCI. Postinjury intervals signify the different time points after injury when the entry of the two compounds was studied. The intervals are as follows: (1) control without injury; (2) immediately (5 min) after injury; (3) 0.5 hr; (4) 1 hr; (5) 2 hr; (6) 4 hr; (7) 12 hr; (8) 24 hr; (9) 48 hr; (10) 72 hr; (11) 96 hr; and (12) 120 hr after injury. *p < 0.05; **p < 0.01; ***p < 0.001 versus control group. SC, Spinal cord.
Increased permeability to 125I-TNF in comparison with BBB disruption
The BBB permeability to 125I-TNF, also measured 10 min after intravenous injection, was higher than that to99mTc-albumin in each CNS region in both control and SCI mice. In comparison with the control group, entry of125I-TNF was significantly increased in the brain on days 3 (p < 0.01), 4 (p < 0.001), and 5 (p < 0.001). The spinal cord had a greater increase in the entry of 125I-TNF than the brain. The cervical spinal cord showed increases at 12 hr (p < 0.05) and day 5 (p< 0.001), whereas the thoracic spinal cord showed an increase on day 2 (p < 0.001). In the lumbar spinal cord, there was a transient peak immediately (5 min) after SCI (p < 0.001), which paralleled the increase of99mTc-albumin temporally as shown in Figure 2; permeability returned to almost normal between 0.5 and 24 hr after injury. A second, more prolonged, peak of increased permeability to 125I-TNF in the lumbar spinal cord occurred at 48 hr (day 2, p< 0.001), earlier than the second increase in99mTc-albumin, and remained elevated (p < 0.001) up to the end of the study at 120 hr (day 5 after injury).
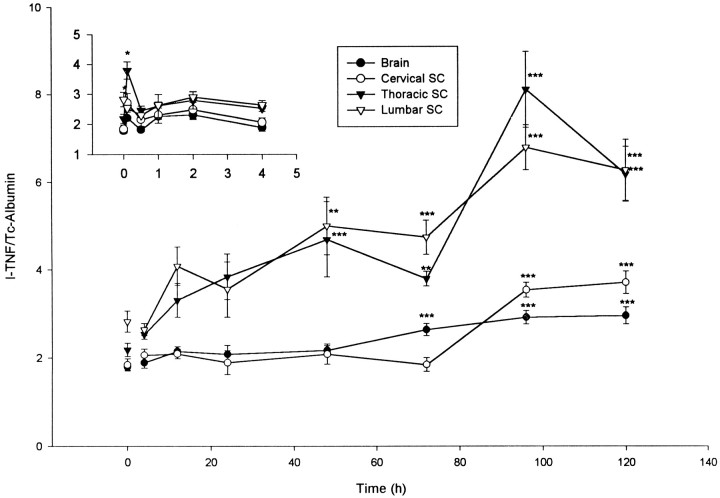
When the ratio of the entry of 125I-TNF to that of the entry of 99mTc-albumin was plotted against time to clearly illustrate differential permeability (Fig.3), it was seen that the immediate increase was significant (p < 0.05) in the cervical and thoracic areas, as well as the lumbar spinal cord. Furthermore, the prolonged second increase was significant in the thoracic (p < 0.001) and lumbar spinal cord (p < 0.005) from day 2 after injury, remaining elevated (p < 0.001) for the 5 d of the study. Increases also were present in brain (day 3–5,p < 0.001) and cervical spinal cord (day 4–5,p < 0.001).
Fig. 3.
Differential permeability of the BBB after acute compression of the lumbar spinal cord. The ratio of the entry of125I-TNF to 99mTc-albumin is plotted against time after SCI performed at time 0. *p < 0.05; **p < 0.01; ***p < 0.001 versus control. The inset shows the earlier times (in hours). SC, Spinal cord.
When the entry of 99mTc-albumin was subtracted from the entry of 125I-TNF as a correction for vascular volume and leakage secondary to BBB disruption, the pattern was somewhat different from that of the differential permeability. The initial increase was transient and appeared only in the lumbar spinal cord (p < 0.005). For brain and lumbar spinal cord, the second peak started on day 2 after injury (p< 0.001) and lasted until day 5. For the thoracic spinal cord, the elevation was significant on day 2–4 (p < 0.05). The cervical spinal cord showed a significant increase at day 5 (p < 0.005).
Inhibition of increased permeability to 125I-TNF
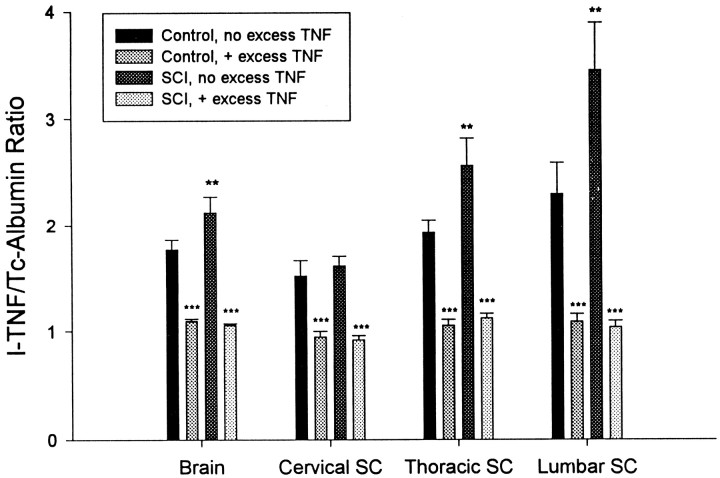
Figure 4 shows the effects of unlabeled TNF on the differential permeability of TNF/albumin 3 d after SCI. After SCI, the increase in the differential permeability was statistically significant (p < 0.01) in the brain, thoracic, and lumbar spinal cord but not in the cervical region. Just as saturability in the control groups was demonstrated by the self-inhibition seen after addition of unlabeled TNF (p < 0.001), a similar pattern of inhibition of125I-TNF entry also was found in the SCI groups. Not only was inhibition of entry of 125I-TNF found with unlabeled TNF in the SCI groups in which entry was already increased by the injury, but saturability occurred in all four of the CNS regions (p < 0.001).
Fig. 4.
Inhibition of the differential permeability of the BBB after acute compression of the lumbar spinal cord. Groups are as follows: (1) control with 125I-TNF and99mTc-albumin; (2) control with 125I-TNF and99mTc-albumin and 1 μg/mouse unlabeled TNF; (3) 72 hr after injury with 125I-TNF and 99mTc-albumin; and (4) 72 hr after injury with 125I-TNF and99mTc-albumin and 1 μg/mouse unlabeled TNF. SCI significantly (**p < 0.01) increased the TNF/albumin ratio in brain and in thoracic and lumbar spinal cord (group 3 vs group 1). Unlabeled TNF significantly (***p < 0.001) decreased the TNF/albumin ratio compared with both control group 1 and SCI group 3. SC, Spinal cord.
DISCUSSION
In the acute stage after lumbar SCI, disruption of the BBB (indicated by dynamic changes in the entry of albumin) was biphasic and restricted to the lumbar spinal cord. In contrast, the entry of TNF was more pronounced in several ways: its magnitude was greater; it occurred earlier; it lasted for a longer time; it involved distal regions, as well as the lumbar spinal cord; and it retained the self-inhibition characteristic of a saturable transport system, which would not be seen with diffusion or leakage.
SCI by compression to a set thickness provides a reproducible model of functional deficit with correlated histological findings that have been well characterized in guinea pigs (Blight, 1991). In our study, the deficit was mild but significant, and the mice showed functional improvement within 10 d. Meanwhile, leakage of the BBB, measured by the entry of radiolabeled albumin (which essentially does not cross the intact BBB and reflects the vascular space), showed an increase confined to the lumbar spinal cord. This disruption of the BBB appeared to be biphasic, peaking immediately after injury and again at day 3 after SCI, although both peaks were transient. This is different from the results of previous subacute or chronic studies (Jaeger and Blight, 1997; Banks et al., 1998). In general, disruption of the BBB in the acute stage of SCI did not correlate with the observed functional deficit. Because BBB disruption is related to the activation and invasion of macrophages from the periphery (Fitch and Silver, 1997), a major cytokine product of activated macrophages (TNF) may play an important role in the recurrence of BBB opening.
The entry of TNF into the CNS, in comparison with albumin, initially followed a similar pattern in that there was an early peak of BBB opening in the lumbar spinal cord. However, the second increase in the entry of TNF preceded that of albumin, was much greater, and lasted a longer time. In addition, in regions that are distal to the site of injury and that did not show the disruption of the BBB characterized by albumin, the entry of TNF also was increased. Because TNF circulates in the blood as a trimer (Smith and Baglioni, 1987) and is comparable in molecular size with albumin (60 kDa), the sensitivity of TNF as a detector of BBB leakage should be similar to that of albumin. Because its entry was much greater than that of albumin and was saturable, the increased entry of TNF after SCI was not solely attributable to leakage of the BBB. Moreover, if TNF caused further disruption of the BBB and thereby facilitated its own penetration into the CNS, the coadministered albumin should have shown similar changes. In our studies, disruption of the BBB is not observed after intravenous bolus administration of up to 100 μg/kg TNF or other cytokines (Gutierrez et al., 1993; Pan et al., 1997a).
Thus, the more widespread and persistent increase in the entry of TNF from the periphery is not related to disruption of the BBB but rather to alternative mechanisms of BBB transport. To illustrate this point further, we performed two conversions. The first showed the differential permeability of TNF and albumin by expression of the results as the ratio of 125I-TNF to99mTc-albumin. The differential permeability best reflects dynamic changes during partial disruption of the BBB in which molecules with different sizes cross at different rates (Ziylan et al., 1983), and it also has been shown to be a good indicator when two substances use different mechanisms for passage (Pan et al., 1996, 1997b). The second conversion corrected for nonspecific leakage by subtraction of albumin from the entry of TNF (125I-TNF −99mTc-albumin). Both results clearly demonstrate that the entry of TNF differs from that caused by disruption of the BBB.
In normal mice, TNF has a unidirectional transport across the BBB from blood to the CNS, with an influx rate of 0.622 ± 0.092 μl · g−1 · min−1in the lumbar region and a very low initial volume of distribution that indicates little nonspecific association with the cerebral vasculature (Pan et al., 1997a). Most 125I-TNF in the brain is present in parenchyma as demonstrated by capillary depletion studies. In addition, the radioactivity represents intact TNF rather than a degradation product as shown by reversed-phase HPLC (Gutierrez et al., 1993). The self-inhibition of 125I-TNF entry by unlabeled TNF proves the saturability of the transport system in all regions of spinal cord, as well as in the brain (Pan et al., 1997a). Because the increased entry of TNF after SCI involved regions and time intervals for which BBB disruption was not detected, the evidence indicates that an enhanced transport system was responsible for the increased entry of TNF. As would be expected for any transport system across the BBB, even an enhanced one such as seen with TNF in this study, there was significant self-inhibition. Together, the results indicate that upregulation of the saturable transport system for TNF is responsible for the increased entry of TNF after SCI. This transport system is likely to be a carrier protein at the BBB that is specific for TNF without interaction with the transport systems of other cytokines, such as interleukins (Gutierrez et al., 1993).
There are pathophysiological considerations for the upregulation of TNF transport system at the BBB. On the one hand, adequate TNF in the damaged CNS is related to increased neurite outgrowth by recruitment and activation of macrophages, counteracting myelin-associated inhibitory molecules so as to provide a permissive substrate for axonal regrowth (Lotan and Schwartz, 1994; Schwartz et al., 1994) and facilitate neuroregeneration (Schwartz et al., 1991; Klusman and Schwab, 1997). After compressive SCI in the rat, axonal regeneration is facilitated by transplantation of macrophages, which secrete TNF but not neurotrophins (Franzen et al., 1998). On the other hand, TNF overproduction may lead to apoptosis after SCI (Li et al., 1996) or result in direct toxicity, as well as hyperactive inflammatory and immune responses (Probert and Selmaj, 1997; Probert et al., 1997;Shohami et al., 1997).
Production of TNF within the CNS is induced after injury (Fan et al., 1994, 1996). The TNF could arise from several CNS cells, such as microglia (Boddeke et al., 1995) and neurons (Liu et al., 1994) after focal ischemia, or astrocytes in various pathological conditions (Pan et al., 1997c). Yet a peripheral source still seems to be prominent, particularly because CNS trauma is usually accompanied by peripheral tissue injury. The second peak of TNF entry preceded the general disruption of the BBB measured by albumin entry and reflected upregulation of TNF transport system in this study. This peak corresponds with the marked increase in macrophage recruitment observed in a mouse model of compressive SCI (Fujiki et al., 1996) and also is similar in time course to infiltration of inflammatory leukocytes after traumatic brain injury (Soares et al., 1995). Thus, the second peak of TNF entry into the CNS provides a direct source of TNF that may be accompanied by an extra indirect source from infiltrating macrophages. The limited entry of TNF from the periphery to regions distant from the site of injury might not only provide a feedback signal to restrict local production but may also serve as a mediator for neuroregeneration. Above all, an adequate concentration of TNF seems to be essential for homeostasis in the CNS, and a saturable transport system regulated by pathological changes may help to control the entry of additional TNF from the periphery and restrict TNF production at the site of SCI.
In summary, although lumbar spinal cord compressive injury caused a restricted biphasic opening of the BBB, it caused a much greater increase in the permeation of TNF from blood to all CNS regions. The prolonged increased entry of TNF during the second phase was related to the upregulation of a saturable transport system rather than to disruption of the BBB. This upregulation showed active involvement of the BBB during SCI in controlling the availability of the blood-borne cytokine. The increased, but still limited, entry of TNF from blood to the CNS may be beneficial in restricting secondary tissue damage and in aiding regeneration of the CNS.
Footnotes
This work was supported by Veterans Affairs, Office of Naval Research, and National Institutes of Health.
Correspondence should be addressed to Dr. Weihong Pan, Veterans Affairs Research 8F 159, 1601 Perdido Street, New Orleans, LA 70112-1262.
REFERENCES
- 1.Banks WA, Kastin AJ, Arimura A. Effect of spinal cord injury on the permeability of the blood–brain and blood–spinal cord barriers to the neurotrophin PACAP. Exp Neurol. 1998;151:116–123. doi: 10.1006/exnr.1998.6786. [DOI] [PubMed] [Google Scholar]
- 2.Baskaya MK, Rao AM, Dogan A, Donaldson D, Dempsey RJ. The biphasic opening of the blood–brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci Lett. 1997;226:33–36. doi: 10.1016/s0304-3940(97)00239-5. [DOI] [PubMed] [Google Scholar]
- 3.Blight AR. Morphometric analysis of a model of spinal cord injury in guinea pigs, with behavioral evidence of delayed secondary pathology. J Neurol Sci. 1991;103:156–171. doi: 10.1016/0022-510x(91)90159-5. [DOI] [PubMed] [Google Scholar]
- 4.Boddeke HWGM, Appel K, Sauter A, Gebicke-Haerter PJ, Buttini M. Expression of TNFα mRNA and protein after focal cerebral ischaemia in the rat. Cytokines in the brain, meeting abstract, Arcachon. 1995.
- 5.Camussi G, Turello E, Bussolino F, Baglioni C. Tumor necrosis factor alters cytoskeletal organization and barrier function of endothelial cells. Int Arch Allergy Appl Immunol. 1991;96:84–91. doi: 10.1159/000235539. [DOI] [PubMed] [Google Scholar]
- 6.Chao CC, Hu S. Tumor necrosis factor-α potentiates glutamate neurotoxicity in human fetal brain cell cultures. Dev Neurosci. 1994;16:172–179. doi: 10.1159/000112104. [DOI] [PubMed] [Google Scholar]
- 7.Descamps L, Cecchelli R, Torpier G. Effects of tumor necrosis factor on receptor-mediated endocytosis and barrier functions of bovine brain capillary endothelial cell monolayers. J Neuroimmunol. 1997;74:173–184. doi: 10.1016/s0165-5728(96)00226-3. [DOI] [PubMed] [Google Scholar]
- 8.Duchini A, Govindarajan S, Santucci M, Zampi G, Hofman FM. Effects of tumor necrosis factor-α and interleukin-6 on fluid-phase permeability and ammonia diffusion in CNS-derived endothelial cells. J Investig Med. 1996;44:474–482. [PubMed] [Google Scholar]
- 9.Fan L, Perlman KP, Young PR, Barone FC, Feuerstein GZ, Gennarelli TA, Smith DH, McIntosh TK. Experimental brain injury induces expression of tumor necrosis factor-α mRNA. J Neurotrauma. 1994;11:108. [Google Scholar]
- 10.Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK. Experimental brain injury induces differential expression of tumor necrosis factor-α mRNA in the CNS. Brain Res Mol Brain Res. 1996;36:287–291. doi: 10.1016/0169-328x(95)00274-v. [DOI] [PubMed] [Google Scholar]
- 11.Fitch MT, Silver J. Activated macrophages and the blood–brain barrier: inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp Neurol. 1997;148:587–603. doi: 10.1006/exnr.1997.6701. [DOI] [PubMed] [Google Scholar]
- 12.Franzen R, Schoenen J, Leprince P, Joosten E, Moonen G, Martin D. Effects of macrophage transplantation in the injured adult rat spinal cord: a combined immunocytochemical and biochemical study. J Neurosci Res. 1998;51:316–327. doi: 10.1002/(SICI)1097-4547(19980201)51:3<316::AID-JNR5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Fujiki M, Zhang Z, Guth L, Steward O. Genetic influences on cellular reactions to spinal cord injury: activation of macrophages/microglia and astrocytes is delayed in mice carrying a mutation (Wlds) that causes delayed wallerian degeneration. J Comp Neurol. 1996;371:469–484. doi: 10.1002/(SICI)1096-9861(19960729)371:3<469::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Gelbard HA, Dzenko KA, DiLoreto D, del Cerro C, del Cerro M, Epstein LG. Neurotoxic effects of tumor necrosis factor α in primary human neuronal cultures are mediated by activation of the glutamate AMPA receptor subtype: implications for AIDS neuropathogenesis. Dev Neurosci. 1993;15:417–422. doi: 10.1159/000111367. [DOI] [PubMed] [Google Scholar]
- 15.Genestier L, Bonnefoy-Berard N, Rouault JP, Flacher M, Revillard JP. Tumor necrosis factor-α up-regulates Bcl-2 expression and decreases calcium-dependent apoptosis in human B cell lines. Int Immunol. 1995;7:533–540. doi: 10.1093/intimm/7.4.533. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor α is transported from blood to brain in the mouse. J Neuroimmunol. 1993;47:169–176. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- 17.Jaeger CB, Blight AR. Spinal cord compression injury in guinea pigs: structural changes of endothelium and its perivascular cell associations after blood–brain barrier breakdown and repair. Exp Neurol. 1997;144:381–399. doi: 10.1006/exnr.1996.6405. [DOI] [PubMed] [Google Scholar]
- 18.Kim KS, Wass CA, Cross AS, Opal SM. Modulation of blood–brain barrier permeability by tumor necrosis factor and antibody to tumor necrosis factor in the rat. Lymphokine Cytokine Res. 1992;11:293–298. [PubMed] [Google Scholar]
- 19.Klusman I, Schwab ME. Effects of pro-inflammatory cytokines in experimental spinal cord injury. Brain Res. 1997;762:173–184. doi: 10.1016/s0006-8993(97)00381-8. [DOI] [PubMed] [Google Scholar]
- 20.Kuroiwa T, Ting P, Martinez H, Klatzo I. The biphasic opening of the blood–brain barrier to proteins following temporary middle cerebral artery occlusion. Acta Neuropathol. 1985;68:122–129. doi: 10.1007/BF00688633. [DOI] [PubMed] [Google Scholar]
- 21.Li GL, Brodin G, Farooque M, Funa K, Holtz A, Wang WL, Olsson Y. Apoptosis and expression of Bcl-2 after compression trauma to rat spinal cord. J Neuropathol Exp Neurol. 1996;55:280–289. doi: 10.1097/00005072-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Tumor necrosis factor-α expression in ischemic neurons. Stroke. 1994;25:1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- 23.Lotan M, Schwartz M. Cross talk between the immune system and the nervous system in response to injury: implications for regeneration. FASEB J. 1994;8:1026–1033. doi: 10.1096/fasebj.8.13.7926367. [DOI] [PubMed] [Google Scholar]
- 24.Mattson MP. Neuroprotective signal transduction: relevance to stroke. Neurosci Biobehav Rev. 1997;21:193–206. doi: 10.1016/s0149-7634(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 25.McKenzie AL, Hall JJ, Aihara N, Fukuda K, Noble LJ. Immunolocalization of endothelin in the traumatized spinal cord: relationship to blood–spinal cord barrier breakdown. J Neurotrauma. 1995;12:257–268. doi: 10.1089/neu.1995.12.257. [DOI] [PubMed] [Google Scholar]
- 26.Miura M, Friedlander RM, Yuan J. Tumor necrosis factor-induced apoptosis is mediated by a CrmA-sensitive cell death pathway. Proc Natl Acad Sci USA. 1995;92:8318–8322. doi: 10.1073/pnas.92.18.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan W, Banks WA, Kennedy MK, Gutierrez EG, Kastin AJ. Differential permeability of the BBB in acute EAE: enhanced transport of TNF-α. Am J Physiol. 1996;271:E636–E642. doi: 10.1152/ajpendo.1996.271.4.E636. [DOI] [PubMed] [Google Scholar]
- 28.Pan W, Banks WA, Kastin AJ. Permeability of the blood–brain and blood–spinal cord barriers to interferons. J Neuroimmunol. 1997a;76:105–111. doi: 10.1016/s0165-5728(97)00034-9. [DOI] [PubMed] [Google Scholar]
- 29.Pan W, Banks WA, Kastin AJ. BBB permeability to ebiratide and TNF in acute spinal cord injury. Exp Neurol. 1997b;146:367–373. doi: 10.1006/exnr.1997.6533. [DOI] [PubMed] [Google Scholar]
- 30.Pan W, Zadina JE, Harlan RE, Weber JT, Banks WA, Kastin AJ. Tumor necrosis factor α: a neuromodulator in the CNS. Neurosci Biobehav Rev. 1997c;21:603–613. doi: 10.1016/s0149-7634(96)00047-4. [DOI] [PubMed] [Google Scholar]
- 31.Popovich PG, Horner PJ, Mullin BB, Stokes BT. A quantitative spatial analysis of the blood–spinal cord barrier. I. Permeability changes after experimental spinal cord contusion injury. Exp Neurol. 1996;142:258–275. doi: 10.1006/exnr.1996.0196. [DOI] [PubMed] [Google Scholar]
- 32.Probert L, Selmaj K. TNF and related molecules: trends in neuroscience and clinical applications. J Neuroimmunol. 1997;72:113–117. doi: 10.1016/s0165-5728(96)00176-2. [DOI] [PubMed] [Google Scholar]
- 33.Probert L, Akassoglou K, Kassiotis G, Pasparakis M, Alexopoulou L, Kollias G. TNF-α transgenic and knockout models of CNS inflammation and degeneration. J Neuroimmunol. 1997;72:137–141. doi: 10.1016/s0165-5728(96)00184-1. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz M, Solomon A, Lavie V, Ben-Bassat S, Belkin M, Cohen A. Tumor necrosis factor facilitates regeneration of injured central nervous system axons. Brain Res. 1991;545:334–338. doi: 10.1016/0006-8993(91)91309-o. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz M, Sivron T, Eitan S, Hirschberg DL, Lotan M, Elman-Faber A. Cytokines and cytokine-related substances regulating glial cell response to injury of the central nervous system. Prog Brain Res. 1994;103:331–341. doi: 10.1016/s0079-6123(08)61147-4. [DOI] [PubMed] [Google Scholar]
- 36.Shohami E, Gallily R, Mechoulam R, Bass R, Ben-Hur T. Cytokine production in the brain following closed head injury: dexanabinol (HU-211) is a novel TNF-α inhibitor and an effective neuroprotectant. J Neuroimmunol. 1997;72:169–177. doi: 10.1016/s0165-5728(96)00181-6. [DOI] [PubMed] [Google Scholar]
- 37.Smith RA, Baglioni C. The active form of tumor necrosis factor is a trimer. J Biol Chem. 1987;262:6951–6954. [PubMed] [Google Scholar]
- 38.Soares HD, Hicks RR, Smith D, McIntosh TK. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J Neurosci. 1995;15:8223–8233. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandenabeele P, Declercq W, Vanhaesebroeck B, Grooten J, Fiers W. Both TNF receptors are required for TNF-mediated induction of apoptosis in PC60 cells. J Immunol. 1995;154:2904–2913. [PubMed] [Google Scholar]
- 40.Wang CX, Nuttin B, Heremans H, Dom R, Gybels J. Production of tumor necrosis factor in spinal cord following traumatic injury in rats. J Neuroimmunol. 1996;69:151–156. doi: 10.1016/0165-5728(96)00080-x. [DOI] [PubMed] [Google Scholar]
- 41.Ziylan YZ, Robinson PJ, Rapoport SI. Differential blood–brain barrier permeabilities to [14C]sucrose and [3H]inulin after osmotic opening in the rat. Exp Neurol. 1983;79:845–857. doi: 10.1016/0014-4886(83)90047-x. [DOI] [PubMed] [Google Scholar]