Abstract
Bipolar cells are retinal interneurons that receive synaptic input from photoreceptors. Glutamate, the photoreceptor transmitter, hyperpolarizes On bipolar cells by closing nonselective cation channels, an effect mediated by the metabotropic receptor mGluR6. Previous studies of mGluR6 transduction have suggested that the receptor couples to a phosphodiesterase (PDE) that preferentially hydrolyzes cGMP, and that cGMP directly gates the nonselective cation channel. This hypothesis was tested by dialyzing On bipolar cells with nonhydrolyzable analogs of cGMP. Whole-cell recordings were obtained from On bipolar cells in slices of larval tiger salamander retina. Surprisingly, On bipolar cells dialyzed with 8-(4-chlorophenylthio)-cyclic GMP (8-pCPT-cGMP), or 8-bromo-cyclic GMP (8-Br-cGMP) responded normally to glutamate orl-2-amino-4-phosphonobutyrate (l-APB). Response amplitudes and kinetics were not significantly altered compared with cells dialyzed with cGMP alone. Comparable results were obtained with the PDE inhibitor 3-isobutyl-1-methyl-xanthine (IBMX) or with 8-pCPT-cGMP and IBMX together, indicating that PDE is not required for mGluR6 signal transduction. Addition of the G-protein subunit Goα to the pipette solution suppressed the cation current and occluded the glutamate response, whereas dialysis with Giα or with transducin Gβγ had no significant effect on either the cation current or the response. Dialysis of an antibody directed against Goα also reduced the glutamate response, indicating a functional role for endogenous Goα. These results indicate that mGluR6 may signal through Go, rather than a transducin-like G-protein.
Keywords: cGMP, 8-pCPT-cGMP, mGluR6, phosphodiesterase, retinal bipolar cell, Go, phosphodiesterase
The synapse between photoreceptors and On bipolar cells is the first in the visual system. Glutamate, the photoreceptor transmitter, hyperpolarizes On bipolar cells via activation of a G-protein-coupled receptor (Nawy and Jahr, 1990;Shiells and Falk, 1990). This receptor was first cloned in rat and classified as mGluR6 (Nakajima et al., 1993), one of the group III metabotropic receptors that are distinguished by their high affinity for l-2-amino-4-phosphonobutyrate (l-APB) (Nakanishi, 1994; Pin and Duvoisin, 1995), confirming results of pharmacological studies in retina (Shiells et al., 1981; Slaughter and Miller, 1981, 1985). Receptor activation by l-APB, or the photoreceptor transmitter, suppresses a cation current (Shiells et al., 1981; Attwell et al., 1987; Nawy and Copenhagen, 1987). Introduction of cGMP into the cell through a patch pipette increases the amplitude of this current, leading to the suggestion that the current is generated by a cyclic nucleotide-gated channel (Nawy and Jahr, 1990; Shiells and Falk, 1990; de la Villa et al., 1995), although this has yet to be confirmed by molecular cloning or direct activation of the channel in isolated patches. The channel may additionally be regulated by Ca2+/calmodulin-dependent kinase (Walters et al., 1998).
The mGluR6 receptor is thought to signal through a G-protein to a cGMP-preferring phosphodiesterase (PDE) (Nawy and Jahr, 1990; Shiells and Falk, 1990; Thoreson and Miller, 1994). Activation of PDE would lead to hydrolysis of cGMP and closure of the channel, reminiscent of the phototransduction cascade. Support for this model comes from the observation that the G-protein linking the receptor to the effector enzyme is sensitive to both pertussis and cholera toxin (Shiells and Falk, 1992a), as is rod transducin, but other studies instead suggest a role for Go in the mGluR6 cascade. Antibodies directed against Goα, but not transducin, label dendrites of On bipolar cells (Vardi et al., 1993; Vardi, 1998), and mGluR6 couples more efficiently to Go than to transducin in transfected cells (Weng et al., 1997). If Go is part of the cascade, then there might be an additional step linking Go to PDE, because Go is not known to couple directly to phosphodiesterases. Alternatively, PDE may not have any role in the cascade. The present study was undertaken to distinguish between these possibilities.
One prediction of the PDE model is that analogs of cGMP that are resistant to hydrolysis should be able to support the cation current but should prevent glutamate from shutting off this current. Here it is shown that dialysis with 8-bromo-cyclic GMP (8-Br-cGMP) or 8-(4-chlorophenylthio)-cyclic GMP (8-pCPT-cGMP), or with the phosphodiesterase inhibitor 3-isobutyl-1-methyl-xanthine (IBMX), does not significantly inhibit the response to glutamate, compared with cells dialyzed with cGMP alone. Furthermore, introduction into the cell of either Goα or an antibody directed against Goα interferes with mGluR6 transduction. Thus neither PDE nor a transducin-like G-protein appear to be essential elements in this transduction pathway.
MATERIALS AND METHODS
Materials. Slices of retina from larval tiger salamanders (Kons Scientific, Germantown, WI) were prepared as described previously (Nawy and Jahr, 1990; Walters et al., 1998). Briefly, salamanders were anesthetized with 3-aminobenzoic acid ethyl ester and decapitated, and the eyes were enucleated. Whole retinas were isolated and placed on a 0.65 μm cellulose acetate/nitrate membrane filter (Millipore, Bedford, MA) that was secured with vacuum grease to a glass slide adjacent to the recording chamber. Slices were then cut to a thickness of 150–200 μm with a tissue slicer (Stoelting, Wood Lane, IL), transferred to the recording chamber while remaining submerged, and viewed with a Zeiss (Thornwood, NY) Axioskop equipped with a water-immersion 40× objective with Hoffman modulation contrast (Modulation Contrast, Greenvale, NY). The extracellular solution contained (in mm): 108 NaCl, 2 CaCl2, 2.5 KCl, 10 HEPES, 10 glucose, 0.1 picrotoxin, pH 7.6, and was perfused continuously through the chamber at ∼1 ml/min. The internal solution was composed of 75 KH2PO4, 10 KCl, 10 HEPES, 10 EGTA, 4 MgATP, 1 cGMP, and 0.5 NaGTP, pH 7.4 with KOH. In the experiments summarized in Figure 6, K+-gluconate (90 mm), was used instead of KH2PO4. To block K+ currents during measurements ofI–V plots, 20 mm tetraethylammonium (TEA) Cl was substituted for NaCl in the extracellular solution, and for KH2PO4 in the pipette solution on an equimolar basis. ATP, GTP, cGMP, 8-Br-cGMP, and 8-pCPT-cGMP were dissolved in water as 100× stocks, aliquoted for single experiments, and stored frozen. A concentrated stock (200–500 mm in DMSO) of IBMX was stored frozen and added to the internal or external solution on the day of the experiment. Cyclosporin A was stored at 4°C in ethanol as a 2 mm stock. Goα and Giα were aliquoted and stored in buffer at −80°C. Gβγ was stored at −20°C in 10 mm HEPES, pH 7.0, 2 mmMgCl2, 1 mm β-mercaptoethanol, and 50% glycerol, and was added to the pipette solution at a dilution of 1:400. Anti-Goα (1 mg/ml) was stored at 4°C and diluted to a final concentration of 30 μg/ml in pipette solution immediately before use. All compounds listed above were obtained from Sigma (St. Louis, MO) except 8-Br-cGMP and 8-pCPT-cGMP (Biolog, La Jolla, CA), Goα and Giα (Calbiochem, San Diego, CA), transducin Gβγ (a gift of Dr. Thomas Sakmar, Rockefeller University), and anti-Goα (Chemicon, Temecula CA).
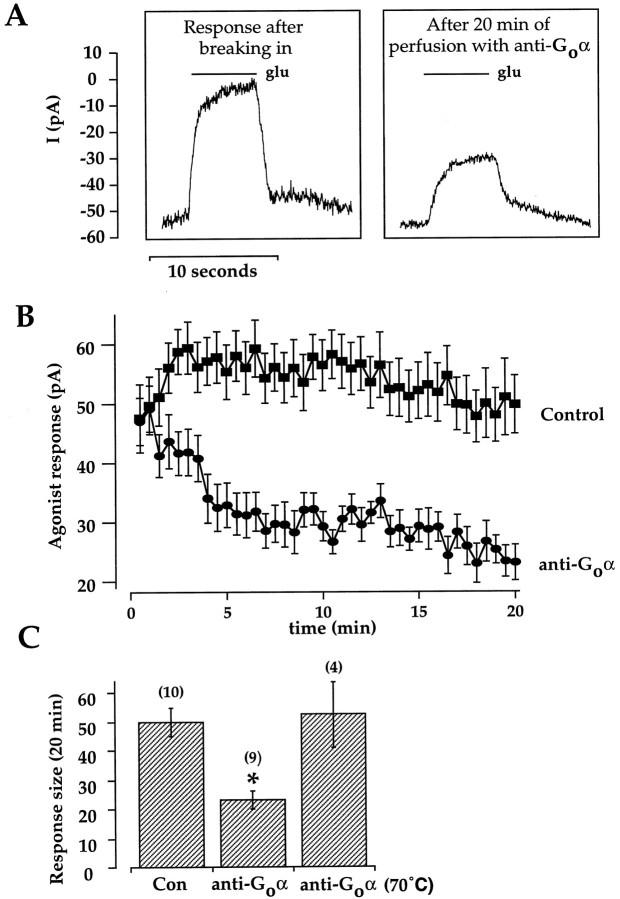
Fig. 6.
An antibody to Goα reduces function of the endogenous G-protein. All data in this figure were obtained using the gluconate-based solution (see Materials and Methods) supplemented with 1 μm Cyclosporin A, which slowed rundown of the response. A, This cell was dialyzed with 30 μg/ml anti-Goα (clone 2A, obtained from Chemicon). Each panel is the average of four consecutive trials. Thecontinuous line indicates the timing of the application of 1 mm glutamate. Left panel, The first of the four averaged traces was obtained ∼30 sec after establishing whole-cell access. Right panel, The first trace was obtained after 19 min of recording. B, Mean and SE of responses to glutamate in control cells (n = 10) and cells dialyzed with antibody (n = 9).C, Summary of the mean responses after 20 min of recording in control (internal solution alone) cells, cells dialyzed with antibody, and cells dialyzed with antibody that had been inactivated by heating at 70°C for 4 min. Asteriskindicates significance of p < 0.01 compared with control.
Electrophysiology. Patch pipettes were fabricated from borosilicate glass (WPI, Sarasota, FL) using a two-stage vertical puller (Narishige, Sea Cliff, NY) and were fire-polished to resistances of 2–3 MΩ. Whole-cell recordings were obtained with an Axopatch 200A amplifier (Axon Instruments, Foster City, CA), and had input and series resistance values of ∼1 GΩ and 10–19 MΩ, respectively. Cells were discarded if the series resistance exceeded 20 MΩ, if the holding current changed suddenly, or if the holding current during the first application of agonist exceeded −20 pA (i.e., current measured while the sustained inward current was suppressed). Drugs were applied via two polymer-coated fused silica tubes (outer diameter 350 μm, inner diameter 250 μm; Polymicro Technologies, Phoenix, AZ) containing external control solution, or agonist, usually glutamate (1 mm), or l-APB (2 μm). The tubes were mounted to a computer-controlled piezo-bimorph (Morgan-Matroc, Bedford, OH). Both barrels of the apparatus were supplied by two separate reservoirs, each with its own control valve, allowing application of IBMX with a delay of ∼15 sec, without repositioning the barrels. After establishment of whole-cell recording, cells were typically voltage clamped at −40 or −30 mV and immediately perfused with control solution. Agonist was applied every 30 sec for a duration of 5 sec beginning 30 sec after breaking into the cell. Data were acquired with Axobasic software and the Digidata 1200 interface (Axon Instruments) and analyzed with Kaleidagraph (Synergy Software, Reading, PA).
RESULTS
Both cGMP and nonhydrolyzable analogs of cGMP potentiate the response to glutamate
On bipolar cells, identified by their characteristic outward responses to glutamate and l-APB, were recorded in solutions that contained ATP and GTP but no cyclic nucleotides. Under these conditions, there was a small outward response to glutamate that did not change appreciably during the first several minutes after the starting of the recording (Fig.1A). The glutamate response results from the suppression of a sustained cation current (Nawy and Jahr, 1990; Shiells and Falk, 1990; de la Villa et al., 1995) and is mediated by mGluR6 (Nomura et al., 1994). Addition of cGMP to the pipette solution enhanced the cation current and increased the amplitude of the glutamate response (Fig. 1B), suggesting that cGMP either modulates or gates the underlying channels directly. Glutamate may suppress the cation current by decreasing cGMP levels through the activation of a cGMP-hydrolyzing PDE (Nawy and Jahr, 1990, 1991; Shiells and Falk, 1990; Shiells and Falk, 1992a; Thoreson and Miller, 1994; de la Villa et al., 1995). Substitution of cGMP with nonhydrolyzable cGMP analogs would be expected to eliminate or reduce suppression by agonist. Accordingly, two 8-substituted cGMP analogs, 8-Br-cGMP and 8-pCPT-cGMP, were added to the pipette in separate experiments. 8-Br-cGMP is poorly hydrolyzed by PDE and has a higher affinity for cGMP-gated channels in rods than cGMP (Zimmerman et al., 1985), whereas 8-pCPT-cGMP is virtually nonhydrolyzable and has an even higher affinity for the rod channel (Kramer and Tibbs, 1996). Neither 8-pCPT-cGMP (Fig. 1C) nor 8-Br-cGMP (Fig.1D) prevented suppression by glutamate. Instead, both increased the glutamate response by potentiating the cation current, as would be expected if the analogs mimic the effect of cGMP.
Fig. 1.
The glutamate response is potentiated by cGMP even when hydrolysis of cGMP is inhibited. A, Whole-cell recording of an On bipolar cell in the tiger salamander retinal slice. The pipette solution contains ATP and GTP but not cGMP. The amplitude of the response to 1 mm glutamate is unchanged during the first 3 min of recording. Thick trace is 30 sec response; thin trace is 3 min response.B, Recording of another On bipolar cell in which the pipette solution contains 1 mm cGMP. The amplitude of the glutamate response is potentiated. C, D, The glutamate response was similarly potentiated when the pipette contained either 250 μm 8-pCPT-cGMP or 1 mm 8-Br-cGMP. InB and D the 3 min traces were shifted slightly for comparison with the 30 sec traces. Holding potential for all cells was −40 mV. E, Summary of the effects of cGMP analogs on the agonist response 30 sec after breaking into the cell (black bars) and after 3 min of dialysis (striped bars). Error bars represent SEM in this and the following figures. Asterisks indicate significance between each condition and control at p < 0.01 level after 3 min (unpaired t test). Numbers in parentheses indicate the number of cells in each group. Data obtained with 1 mm glutamate and with l-APB (2–5 μm) have been pooled because no differences were observed.
These data are summarized in Figure 1E. The black bars are the mean amplitudes of the glutamate response soon after beginning of the recording with the indicated solution. As early as 30 sec there was a slight potentiation of the response with all three analogs, compared with control (no cGMP). After ∼3 min (striped bars), the responses in cGMP or its analogs were not significantly different from each other, but all three differed significantly from control (p < 0.01).
It is possible that the concentrations of the analogs, which are both membrane-permeant, are insufficient to reach the dendrites where the channels are presumably located, although it is clear that the levels are sufficient to enhance the cation current. To rule this out, 8-pCPT-cGMP (100 μm) was added to the control and glutamate flowpipes as well as the pipette solution (250 μm). No inhibition of the agonist response was observed (n = 3 cells; data not shown).
Suppression of the cation current is not prevented by the PDE inhibitor IBMX
Further evidence that PDE is not required for current suppression was obtained by dialyzing cells with IBMX along with cGMP. IBMX did not prevent cells from responding to glutamate or prevent cGMP from increasing the amplitude of the response (Fig.2A). In separate experiments, IBMX was applied to the outside of On bipolar cells so that its effect on cation current and agonist responses could be compared within a single cell. An example of the effects of extracellular application of IBMX is illustrated in Figure2B, which is a composite of nine trials, each lasting 15 sec and separated by an additional 15 sec period. At the time indicated by the solid line, IBMX was applied by switching the flowpipes from reservoirs containing control and glutamate solution to reservoirs containing IBMX and IBMX with glutamate. Rather than inhibiting the glutamate response, IBMX increased its amplitude as it augmented the cation current, as indicated by the dashed line. The effects of IBMX on inward current and on the glutamate response in four cells are summarized in the right panel of Figure 2B. These findings indicate the presence of basal PDE activity, whose inhibition increases the effective cGMP concentration but whose activity is not required for suppression of the inward current.
Fig. 2.
Inhibition of PDE does not prevent suppression of the cation current by glutamate. A, Left, Glutamate responses at 30 sec (thick trace) and 3 min (thin trace) after breaking into an On bipolar cell dialyzed with 500 μm GTP, 1 mm cGMP, and 250 μm IBMX. IBMX did not inhibit the response to glutamate.Right, Summary of the effect of dialysis of IBMX (250 or 500 μm) on the agonist response 30 sec after breaking into the cell (black bars) and after 3 min of dialysis (striped bars). Control and cGMP data are from Figure 1.B, The glutamate response persists during extracellular application. Left, Response of an On bipolar cell to external application of 1 mm IBMX. At the time indicated by the solid line, the flowpipes were switched to reservoirs containing IBMX, and IBMX + glutamate. Each record is 15 sec long and is separated from the next record by an additional 15 sec.Right, Summary of the increase in holding current and the agonist response produced by IBMX (n = 4 cells). C, Recording of an On bipolar cell dialyzed with 500 mm IBMX and externally perfused with 1 mmIBMX for the duration of the recording. The response to glutamate is unaffected. Similar results were obtained in two other cells. Holding potential for all three cells illustrated in A–C was −40 mV.
To insure that IBMX reached the distal dendrites where the transduction machinery is presumably located, IBMX was added to the pipette and the extracellular solution. In three of three cells tested, one of which is illustrated in Figure 2C, IBMX did not inhibit the response to glutamate.
After 10–15 min of recording under a number of conditions that are highly unfavorable for the hydrolysis of cGMP, responses were still not diminished compared with cells dialyzed in cGMP (summarized in Fig.3A). Furthermore, at the level of resolution afforded by the drug application system, there was essentially no change in the response rise time throughout the duration of the recording in cells dialyzed with either 8-pCPT-cGMP or IBMX (Fig. 3B). Taken together, these results provide strong evidence that hydrolysis of cGMP is not an obligatory step in the mGluR6 transduction pathway.
Fig. 3.

Prolonged inhibition of phosphodiesterases does not block or alter the kinetics of the agonist response.A, Comparison of the amplitude of the agonist (l-APB or glutamate) after 10 min of dialysis with no cGMP, with cGMP, or with solutions designed to inhibit cGMP phosphodiesterase. Responses under all conditions continued to be significantly greater than control (p < 0.01, unpaired t test). B, Glutamate responses after 30 sec and 15 min of recording in On bipolar cells dialyzed with 500 μm GTP, 1 mm cGMP, and 250 μm IBMX (left) and in another cell dialyzed with 500 μm GTP and 250 μm8-pCPT-cGMP (right). Glutamate was applied, after a 2 sec delay, for a period of 5 sec (indicated by the continuous line). In each panel the amplitude of the response after 15 min (thin line traces) was scaled to the response after 30 sec of dialysis (thick line trace). The kinetics of the agonist response, at this degree of temporal resolution, are unchanged by prolonged dialysis with either IBMX or 8-pCPT-cGMP.
Evidence that Goα is involved in the transduction pathway
Antibodies directed against Go label On bipolar cell dendrites, and the labeling is colocalized with mGluR6 antibody labeling (Vardi et al., 1993; Vardi, 1998). Furthermore, mGluR6 couples much more efficiently to Go than to rod transducin in transfected cells (Weng et al., 1997). To test for a functional role of Go in mGluR6 transduction, cells were dialyzed with cGMP and the Goα subunit. After 3 min of whole-cell recording, the agonist response had increased, as expected in the presence of cGMP. Soon after, the response began to decline, and after 10 min, the response was smaller than it was initially (Fig.4A). The averaged time course of the response of cells dialyzed with cGMP alone (control) and those dialyzed with cGMP and Goα is summarized in Figure4B. Initially the average response size in both groups is nearly the same. After a delay of several minutes, the response in cells dialyzed with the Goα was significantly depressed compared with control, although the control cells did run down slightly as well. This delay is presumably caused by the relatively slow rate of diffusion of the 35 kDa α subunit out into the distal dendrites.
Fig. 4.

Goα inhibits the glutamate response.A, Example of the effect of dialysis of Goα on an On bipolar cell. Cell was dialyzed with 64 nm Goα along with 1 mm cGMP added to the normal internal solution. After 3 min the response is enhanced, because of the presence of cGMP (compare left andcenter panels). Later, after allowing for diffusion of Goα into the cell, the response is significantly depressed (right panel). B, Comparison of the time course of inhibition in cells dialyzed with cGMP (▪) or with cGMP and 42 to 62 nm Goα (●).C, Summary of responses in cells dialyzed for 10 min with Goα, Gβγ (60 nm), or Giα (61 nm) along with cGMP. Neither Gβγ nor Giα had any significant effect compared with cGMP alone.
Studies of mGluR6 in expression systems have suggested that mGluR6 can couple negatively to adenylate cyclase through Gi (Nakajima et al., 1993; Nakanishi, 1994; Laurie et al., 1997). However, dialysis of Giα had no significant effect on the response to glutamate (Fig. 4C), suggesting that mGluR6 does not signal through this pathway in the native cell.
It is possible that mGluR6 signals through Goα, but it is Gβγ that provides the relevant signal in this cascade. Addition of exogenous Goα might prevent signaling by buffering endogenous Gβγ subunits. To test this possibility, cells were dialyzed with transducin βγ (kindly provided by Dr. Thomas Sakmar). At three times the concentration necessary to modulate Ca2+ currents (Diverse-Pierluissi et al., 1995;Zamponi et al., 1997), Gβγ had no effect on the glutamate response (Fig. 4C). However, other βγ isoforms will need to be tested to rule out a role for transduction in this pathway.
Inhibition of the glutamate response by Goα would result if the subunit either prevented suppression of the cation current or shut off the current, essentially occluding the glutamate response. To distinguish between these two possibilities, current–voltage (I–V) measurements were obtained immediately after starting the whole-cell recording, and 10 min later, when the glutamate response was diminished and the subunit presumably had reached the dendrites. In this experiment, the pipette and bath solutions contained 20 mm TEA to reduce K+ currents. The holding potential was stepped from −70 to +20 mV for 500 msec, as shown in Figure5A, and the holding current obtained at the end of the step was plotted (Fig. 5C). Glutamate was applied to the cell, and the procedure was repeated. Glutamate dramatically reduced membrane conductance, the small residual current most likely consisting of a mixture of Cl−and nonspecific leak. The reversal potential for the current suppressed by glutamate was −5.4 mV in this cell, in good agreement with previous measurements (Attwell et al., 1987; Nawy and Jahr, 1991; Yamashita and Wassle, 1991; de la Villa et al., 1995). After 10 min of dialysis, membrane conductance was dramatically reduced. Application of glutamate further decreased membrane conductance, but only minimally (Fig.5B,D). The currents suppressed by dialysis with Goα (Fig. 5E, closed symbols) and by glutamate (open triangles) had similar reversal potentials. In all cells, the mean reversal potentials of the current suppressed by Goα (mean: −6.2 ± 2) and by glutamate (mean: −4.3 ± 2.5 mV; n = 10) were nearly identical, providing additional evidence that both acted downstream to close the same population of channels.
Fig. 5.
Goα and glutamate may close the same population of channels. A, Internal and external solutions throughout this figure contained 20 mm TEA to reduce K+ conductances. Immediately after breaking into an On bipolar cell with a pipette solution containing 64 nm Goα and 1 mm cGMP, the holding potential was stepped from −70 to +20 mV in 10 mV increments for 500 msec. Each step was separated by a period of 2 sec, during which the cell was held at −30 mV. Glutamate was then applied continuously to the cell, and the step protocol was repeated. B, After 10 min of dialysis, the procedure was repeated, except that the cell was held at −40 between steps. C, Plot of the data shown in A. Continuous lines are the least-squares fit to the data with an inverse slope of 268 MΩ (30 sec control) and 826 MΩ (glutamate). D, Plot of the data shown in B. Continuous lines are the least-squares fit to the data with an inverse slope of 690 MΩ (10 min perfusion with Goα) and 847 MΩ (glutamate).E, Plots of the current suppressed by glutamate after 30 sec (▵) and after dialysis with Goα for 10 min (⋄). Plots were obtained by taking the difference between the glutamate and control data points in C and D. Dialysis with Goα decreased the slope conductance of the glutamate-suppressed current from 2.52 to 0.27 nS. The current suppressed by Goα is also shown (▴). Plot is the subtraction of the 10 min from the 30 sec controlI–V.
To examine the possibility that the endogenous G-protein is related to Go, cells were dialyzed with a monoclonal antibody that has previously been shown to inhibit Goα function (Kim et al., 1998). This antibody (clone 2A), raised against partially purified bovine brain Goα, reacts with Goα but not with Giα or Gsα (Li et al., 1995). Figure 6 illustrates the effect of dialysis with 30 μg/ml anti-Goα on the agonist response. The left panel is the average of four trials recorded immediately after breaking into an On bipolar cell. The right panel is the average of four traces acquired after 20 min of dialysis with the antibody. The amplitude of the response was reduced, although the baseline current was relatively unchanged, indicating that dialysis of the antibody inhibited G-protein-mediated suppression of the cation current but alone did not directly alter or occlude the current.
In 9 of 10 cells dialyzed with anti-Goα, there was a pronounced time-dependent depression of the agonist response compared with cells that were recorded with the same gluconate-based internal solution but with no antibody (Fig. 6B). On average, the antibody reduced the responses to ∼50% of control. As a further control, cells were dialyzed with antibody that had been heat-inactivated (70°C for 4 min). In these cells, the size of the response after 20 min was not significantly different from control cells (Fig. 6C). These results support the idea that an endogenous Goα-like G-protein mediates suppression of cation current by mGluR6.
DISCUSSION
Evaluation of the existing model of mGluR6 transduction
According to the existing model of mGluR6 transduction, the postsynaptic channel is gated by cGMP, and mGluR6 activation lowers cGMP levels by activating a cGMP-hydrolyzing PDE, closing the synaptic channel and hyperpolarizing the dendrites. Evidence presented in this study strongly suggests that PDE does not have an obligatory role in the mGluR6 transduction cascade of On bipolar cells. Bipolar cells continued to respond to glutamate when dialyzed with nonhydrolyzable analogs of cGMP, with the PDE inhibitor IBMX, or with both. When compared with cells dialyzed only with cGMP, there was no difference in the amplitude or the kinetics of the response throughout the recording. In contrast, dialysis of these and similar compounds into photoreceptors profoundly alters the amplitude and kinetics of light responses (Zimmerman et al., 1985; Sather and Detwiler, 1987).
Because bipolar cells continue to respond to glutamate when intracellular cGMP levels are fixed, it seems clear that a change in cGMP is not the signal for channel closure. Does this mean that cGMP does not gate the channel? One possibility is that the channel may require cGMP to open but is closed through a separate, cGMP-independent process, such as a direct interaction with Go. Alternatively, the channel may be modulated, but not gated by cGMP, and might more closely resemble the recently cloned family of hyperpolarization-activated cation channels, which also show modulation by cyclic nucleotides (Gauss et al., 1998; Ludwig et al., 1998; Santoro et al., 1998), than cyclic nucleotide-gated (CNG) channels. It is clear that this cation channel has properties that are functionally distinct from known CNG channels. The On bipolar cell cation channel is not blocked by physiological concentrations of Ca2+(Shiells and Falk, 1992b) as CNG channels are (Frings et al., 1995). As a result, the open probability of the On bipolar cell cation channel is thought to exceed 90% in the presence of cGMP and physiological concentrations of Ca2+ (de la Villa et al., 1995), whereas CNG channels in rods spend ∼1% of the time in the open state (Yau and Baylor, 1989). Furthermore, antibodies against known CNG channels do not label On bipolar cells (Wassle et al., 1992). However, none of these studies directly address the issue of channel gating, and questions about the relative homology of this channel with CNG or hyperpolarization-activated channels can only be answered when more detailed and quantitative information about the properties of the channel becomes available.
The evidence for PDE as the effector enzyme in the On bipolar cell pathway was based primarily on the observation that IBMX increases the size of the cation current (Nawy and Jahr, 1990; Shiells and Falk, 1990), and that IBMX, when applied together with agonist, appeared to inhibit responses to low but not high concentrations of agonist (Nawy and Jahr, 1990). An alternative hypothesis is that basal PDE activity is present, that inhibition of this PDE augments the size of the current, and that a saturating concentration of agonist can suppress this added current, as was demonstrated in this study. Application of subsaturating concentrations of agonist, followed by agonist and IBMX, would increase the inward current. Superimposed on the agonist response, this might be interpreted as a decrease in the agonist response.
An alternative model: transduction via Go
Two lines of evidence suggest that mGluR6 may couple through Go. First, comparison of the I–V relations obtained at the start of recording, during application of glutamate, and after 10 min of dialysis suggests that Go may close the same population of cation channels that are closed by the endogenous G-protein during the agonist response. No effect on these channels was observed during dialysis of Giα or transducin Gβγ. Second, dialysis of an antibody that blocks function of Goα inhibits the agonist response. The simplest model of mGluR6 transduction that can account for these observations is that mGluR6 couples to Go, a finding that is consistent with recent studies showing that antibodies directed against Goα label On bipolar cell dendrites, and the labeling is colocalized with mGluR6 antibody labeling (Vardi et al., 1993; Vardi, 1998). Recently, mGluR6 has been shown to couple much more efficiently to Go than to rod transducin in transfected cells (Weng et al., 1997). Cation currents in various cells are modulated by activation of metabotropic (Crepel et al., 1994; Guerineau et al., 1995; Batchelor and Garthwaite, 1997; Congar et al., 1997; Tempia et al., 1998) and muscarinic (Inoue and Isenberg, 1990; Kim et al., 1998) receptors. In many cases there is evidence for the involvement of a pertussis-sensitive G-protein.
The mechanism by which Go closes the cation channel remains to be elucidated. It is well established that Go can inhibit ion channels via a membrane-delimited pathway (Hille, 1994;Dolphin, 1998). Recent studies of G-protein regulation of N-type Ca2+ channels (Herlitze et al., 1996; Ikeda, 1996) and rectifying K+ channels (Logothetis et al., 1987;Wickman et al., 1994) indicate that many, if not all, of these membrane-delimited effects of Go are mediated by Gβγ rather than Goα. In the present study, injection of transducin βγ was unable to mimic the effect of glutamate. It might have been anticipated that exogenous Gβγ would have buffered endogenous Goα and prevented signaling, but this was not observed. The reason for this is unclear but may be caused by a surplus of endogenous Goα. It will be necessary to test other β subunits before a role for Gβγ in mGluR6 signaling can be defined. The simplest model that can account for the present results is a direct interaction of the channel with Goα or perhaps Gβγ, but these results do not rule out the possibility that there are additional steps in the cascade.
Footnotes
This work was supported by National Institutes of Health Grant EY10254, and by an unrestricted grant from Research to Prevent Blindness Inc. I thank Drs. Alejandro Peinado and Eric Wexler for helpful discussions.
Correspondence should be addressed to Dr. Scott Nawy, Kennedy Center Room 525, Albert Einstein College of Medicine, 1410 Pelham Parkway South, Bronx, NY 10461.
REFERENCES
- 1.Attwell D, Mobbs P, Tessier-Lavigne M, Wilson M. Neurotransmitter-induced currents in retinal bipolar cells of the axolotl, Ambystoma mexicanum. J Physiol (Lond) 1987;387:125–161. doi: 10.1113/jphysiol.1987.sp016567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batchelor AM, Garthwaite J. Frequency detection and temporally dispersed synaptic signal association through a metabotropic glutamate receptor pathway. Nature. 1997;385:74–77. doi: 10.1038/385074a0. [DOI] [PubMed] [Google Scholar]
- 3.Congar P, Leinekugel X, Ben-Ari Y, Crepel V. A long-lasting calcium-activated nonselective cationic current is generated by synaptic stimulation or exogenous activation of group I metabotropic glutamate receptors in CA1 pyramidal neurons. J Neurosci. 1997;17:5366–5379. doi: 10.1523/JNEUROSCI.17-14-05366.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crepel V, Aniksztejn L, Ben-Ari Y, Hammond C. Glutamate metabotropic receptors increase a Ca(2+)-activated nonspecific cationic current in CA1 hippocampal neurons. J Neurophysiol. 1994;72:1561–1569. doi: 10.1152/jn.1994.72.4.1561. [DOI] [PubMed] [Google Scholar]
- 5.de la Villa P, Kurahashi T, Kaneko A. l-glutamate-induced responses and cGMP-activated channels in three subtypes of retinal bipolar cells dissociated from the cat. J Neurosci. 1995;15:3571–3582. doi: 10.1523/JNEUROSCI.15-05-03571.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diverse-Pierluissi M, Goldsmith PK, Dunlap K. Transmitter-mediated inhibition of N-type calcium channels in sensory neurons involves multiple GTP-binding proteins and subunits. Neuron. 1995;14:191–200. doi: 10.1016/0896-6273(95)90254-6. [DOI] [PubMed] [Google Scholar]
- 7.Dolphin AC. Mechanisms of modulation of voltage-dependent calcium channels by G proteins. J Physiol (Lond) 1998;506:3–11. doi: 10.1111/j.1469-7793.1998.003bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frings S, Seifert R, Godde M, Kaupp UB. Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide-gated channels. Neuron. 1995;15:169–179. doi: 10.1016/0896-6273(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 9.Gauss R, Seifert R, Kaupp UB. Molecular identification of a hyperpolarization-activated channel in sea urchin sperm. Nature. 1998;393:583–587. doi: 10.1038/31248. [DOI] [PubMed] [Google Scholar]
- 10.Guerineau NC, Bossu JL, Gahwiler BH, Gerber U. Activation of a nonselective cationic conductance by metabotropic glutamatergic and muscarinic agonists in CA3 pyramidal neurons of the rat hippocampus. J Neurosci. 1995;15:4395–4407. doi: 10.1523/JNEUROSCI.15-06-04395.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 12.Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 14.Inoue R, Isenberg G. Acetylcholine activates nonselective cation channels in guinea pig ileum through a G protein. Am J Physiol. 1990;258:C1173–1178. doi: 10.1152/ajpcell.1990.258.6.C1173. [DOI] [PubMed] [Google Scholar]
- 15.Kim YC, Kim SJ, Sim JH, Cho CH, Juhnn YS, Suh SH, So I, Kim KW. Suppression of the carbachol-activated nonselective cationic current by antibody against alpha subunit of Go protein in guinea-pig gastric myocytes. Pflügers Arch. 1998;436:494–496. doi: 10.1007/s004240050663. [DOI] [PubMed] [Google Scholar]
- 16.Kramer RH, Tibbs GR. Antagonists of cyclic nucleotide-gated channels and molecular mapping of their site of action. J Neurosci. 1996;16:1285–1293. doi: 10.1523/JNEUROSCI.16-04-01285.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurie DJ, Schoeffter P, Wiederhold KH, Sommer B. Cloning, distribution and functional expression of the human mGlu6 metabotropic glutamate receptor. Neuropharmacology. 1997;36:145–152. doi: 10.1016/s0028-3908(96)00172-4. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Mumby SM, Greenwood A, Jope RS. Pertussis toxin-sensitive G protein alpha-subunits: production of monoclonal antibodies and detection of differential increases on differentiation of PC12 and LA-N-5 cells. J Neurochem. 1995;64:1107–1117. doi: 10.1046/j.1471-4159.1995.64031107.x. [DOI] [PubMed] [Google Scholar]
- 19.Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 20.Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for l-2-amino-4-phosphonobutyrate. J Biol Chem. 1993;268:11868–11873. [PubMed] [Google Scholar]
- 22.Nakanishi S. Metabotropic glutamate receptors: synaptic transmission, modulation, and plasticity. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 23.Nawy S, Copenhagen DR. Multiple classes of glutamate receptor on depolarizing bipolar cells in retina. Nature. 1987;325:56–58. doi: 10.1038/325056a0. [DOI] [PubMed] [Google Scholar]
- 24.Nawy S, Jahr CE. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990;346:269–271. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- 25.Nawy S, Jahr CE. cGMP-gated conductance in retinal bipolar cells is suppressed by the photoreceptor transmitter. Neuron. 1991;7:677–683. doi: 10.1016/0896-6273(91)90380-i. [DOI] [PubMed] [Google Scholar]
- 26.Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994;77:361–369. doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 27.Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 28.Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 1998;93:717–729. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- 29.Sather WA, Detwiler PB. Intracellular biochemical manipulation of phototransduction in detached rod outer segments. Proc Natl Acad Sci USA. 1987;84:9290–9294. doi: 10.1073/pnas.84.24.9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiells RA, Falk G. Glutamate receptors of rod bipolar cells are linked to a cyclic GMP cascade via a G-protein. Proc R Soc Lond B Biol Sci. 1990;242:91–94. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- 31.Shiells RA, Falk G. The glutamate-receptor linked cGMP cascade of retinal on-bipolar cells is pertussis and cholera toxin-sensitive. Proc R Soc Lond B Biol Sci. 1992a;247:17–20. doi: 10.1098/rspb.1992.0003. [DOI] [PubMed] [Google Scholar]
- 32.Shiells RA, Falk G. Properties of the cGMP-activated channel of retinal on-bipolar cells. Proc R Soc Lond B Biol Sci. 1992b;247:21–25. doi: 10.1098/rspb.1992.0004. [DOI] [PubMed] [Google Scholar]
- 33.Shiells RA, Falk G, Naghshineh S. Action of glutamate and aspartate analogues on rod horizontal and bipolar cells. Nature. 1981;294:592–594. doi: 10.1038/294592a0. [DOI] [PubMed] [Google Scholar]
- 34.Slaughter MM, Miller RF. 2-Amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- 35.Slaughter MM, Miller RF. Characterization of an extended glutamate receptor of the ON Bipolar neuron in the vertebrate retina. J Neurosci. 1985;5:224–233. doi: 10.1523/JNEUROSCI.05-01-00224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tempia F, Miniaci MC, Anchisi D, Strata P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J Neurophysiol. 1998;80:520–528. doi: 10.1152/jn.1998.80.2.520. [DOI] [PubMed] [Google Scholar]
- 37.Thoreson WB, Miller RF. Actions of (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (1S,3R-ACPD) in retinal ON bipolar cells indicate that it is an agonist at L-AP4 receptors. J Gen Physiol. 1994;103:1019–1034. doi: 10.1085/jgp.103.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vardi N. Alpha subunit of Go localizes in the dendritic tips of ON bipolar cells. J Comp Neurol. 1998;395:43–52. [PubMed] [Google Scholar]
- 39.Vardi N, Matesic DF, Manning DR, Liebman PA, Sterling P. Identification of a G-protein in depolarizing rod bipolar cells. Vis Neurosci. 1993;10:473–478. doi: 10.1017/s0952523800004697. [DOI] [PubMed] [Google Scholar]
- 40.Walters RJ, Kramer RH, Nawy S. Regulation of cGMP-dependent current in On bipolar cells by calcium/calmodulin-dependent kinase. Vis Neurosci. 1998;15:257–261. doi: 10.1017/s0952523898152057. [DOI] [PubMed] [Google Scholar]
- 41.Wassle H, Grunert U, Cook NJ, Molday RS. The cGMP-gated channel of rod outer segments is not localized in bipolar cells of the mammalian retina. Neurosci Lett. 1992;134:199–202. doi: 10.1016/0304-3940(92)90516-a. [DOI] [PubMed] [Google Scholar]
- 42.Weng K, Lu C, Daggett LP, Kuhn R, Flor PJ, Johnson EC, Robinson PR. Functional coupling of a human retinal metabotropic glutamate receptor (hmGluR6) to bovine rod transducin and rat Go in an in vitro reconstitution system. J Biol Chem. 1997;272:33100–33104. doi: 10.1074/jbc.272.52.33100. [DOI] [PubMed] [Google Scholar]
- 43.Wickman KD, Iniguez-Lluhl JA, Davenport PA, Taussig R, Krapivinsky GB, Linder ME, Gilman AG, Clapham DE. Recombinant G-protein beta gamma-subunits activate the muscarinic-gated atrial potassium channel. Nature. 1994;368:255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- 44.Yamashita M, Wassle H. Responses of rod bipolar cells isolated from the rat retina to the glutamate agonist 2-amino-4-phosphonobutyric acid (APB). J Neurosci. 1991;11:2372–2382. doi: 10.1523/JNEUROSCI.11-08-02372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yau KW, Baylor DA. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- 46.Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP. Crosstalk between G proteins and protein kinase C mediated by the calcium channel alpha1 subunit. Nature. 1997;385:442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]
- 47.Zimmerman AL, Yamanaka G, Eckstein F, Baylor DA, Stryer L. Interaction of hydrolysis-resistant analogs of cyclic GMP with the phosphodiesterase and light-sensitive channel of retinal rod outer segments. Proc Natl Acad Sci USA. 1985;82:8813–8817. doi: 10.1073/pnas.82.24.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]