Abstract
Olfactory receptor neurons in the lobster express a nonselective cation channel that is activated by intracellular Na+ and carries a substantial part of the depolarizing receptor current. Here, we show that phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] and phosphatidylinositol 4-phosphate [PI(4)P] applied to the intracellular face of cell-free patches activate the channel in the absence of Na+and that antibodies against the respective phospholipids irreversibly inhibit the evoked activity. Further, we show that applying PI(4,5)P2 or PI(4)P in the presence of Na+ decreases the concentration of Na+ required to activate the channel from an EC50 of 74 to 22 mm for PI(4,5)P2 and to 29 mm for PI(4)P, respectively. Na+-gated channel activity was irreversibly inhibited by monoclonal antibodies against PI(4,5)P2 and PI(4)P in patches never exposed to exogenous phosphatidylinositols, suggesting that endogenous inositol phospholipids are required for the activation of the channel by intracellular Na+. Our findings suggest that PI(4,5)P2 and/or PI(4)P may serve as intracellular signaling molecules in these primary sensory neurons and provide a general mechanism to explain how the sensitivity of Na+-gated channels to Na+ could be much greater in intact cells than in excised membrane patches.
Keywords: lobster, olfaction, patch clamp, single-channel recording, modulation, inositol phospholipids
Stimulation of various membrane receptors leads to hydrolysis of the inositol-containing phospholipid phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) (Berridge, 1993). Both IP3 and DAG are established second messengers in intracellular signaling; IP3 releases calcium from intracellular calcium stores (Berridge and Irvine, 1984), whereas DAG activates protein kinase C (Nishizuka, 1986). It is becoming clear, however, that PI(4,5)P2 and possibly other membrane phospholipids mediate cellular processes in addition to their role as precursors for the synthesis of IP3 and DAG (Toker, 1998). PI(4,5)P2 has been implicated in rearranging the actin cytoskeleton (Janmey, 1994), modulating the activity of phospholipase D (Liscovitch et al., 1994), and regulating intracellular vesicle trafficking (De Camilli et al., 1996). PI(4,5)P2 also regulates plasma membrane ion channels and transporters, including the ryanodine-sensitive Ca2+ release channel (Chu and Stefani, 1991), ATP-sensitive K+ channels (Hilgemann and Ball, 1996; Fan and Makielski, 1997; Baukrowitz et al., 1998; Shyng and Nichols, 1998), the muscarinic K+ channel (Sui et al., 1998), IP3 receptors (Lupu et al., 1998), the inward rectifier K+ channel (Huang et al., 1998), and the sodium–calcium exchanger (Hilgemann and Ball, 1996). Thus, membrane phosphoinositides, such as PI(4,5)P2, may also serve as intracellular second messengers.
Some nonselective cation channels are either directly activated by, or sensitive to, intracellular Na+. These channels occur in crab neurosecretory terminals (Stuenkel et al., 1990), lobster olfactory receptor neurons (ORNs) (Zhainazarov and Ache, 1995,1997), guinea pig intestinal myocytes (Nouailhetas et al., 1994), frog tectal neurons (Zaykin and Nistri, 1996), and lobster olfactory projection neurons (Zhainazarov and Ache, 1998). The physiological role(s) of these channels, as well as the larger family of Na+-gated cation channels selective to K+ (for review, see Dryer, 1994), is not known. Channels of both families studied in isolated membrane patches typically require ∼60 mm Na+ for activation. Such concentrations are much greater than those believed to occur intracellularly, raising doubt whether sufficiently high amounts of Na+ can enter cells through voltage-activated Na+ channels or neurotransmitter-activated nonselective cation channels to activate them (Dryer, 1994). On the other hand, it is possible that the sensitivity of Na+-gated channels to Na+ is much greater in intact cells than in excised membrane patches.
The channel activated by intracellular Na+ in lobster ORNs carries a substantial part of the depolarizing receptor current (Zhainazarov et al., 1998), suggesting that intracellular Na+ may reach sufficiently high levels to influence the channel, but other factors may also be involved. Because phosphoinositide signaling has been implicated in activation of lobster ORNs by odors (Fadool and Ache, 1992), we propose that membrane phosphoinositides may also influence the lobster olfactory Na+-gated channel. Here, we report that both PI(4,5)P2 and phosphatidylinositol 4-phosphate [PI(4)P] have a profound effect on the channel. Both phospholipids activate the channel in micromolar concentrations in the absence of intracellular Na+, and when either is applied in the presence of Na+, they substantially increase the Na+ sensitivity of the channel. These findings suggest that these membrane phospholipids, together with intracellular Na+, are important in regulating activity of the channel and, in turn, may be involved in olfactory transduction. They also provide a general mechanism to explain how the sensitivity of Na+-gated channels to Na+ could be much greater in intact cells than in excised membrane patches.
MATERIALS AND METHODS
Neuron culture. Primary cultures of lobster ORNs were prepared as described previously (Fadool et al., 1991). Briefly, clusters of ORNs were dissected from the lateral antennular filament (olfactory organ) of adult specimens of the Caribbean spiny lobsterPanulirus argus and transferred to 10 ml of 0.2 μm filter-sterilized Panulirus saline (SP) (see below). The clusters were then transferred to 10 ml of SP containing papain (2.5 mg), l-cysteine (12 mg), penicillin (1%), streptomycin sulfate (1%), and amphotericin (1%) for 50 min at 21°C, and agitated on a orbital shaker (80 rpm). Enzymatic digestion was stopped by replacing the enzyme-containing solution with low-glucose L-15 culture medium supplemented with l-glutamine, dextrose, fetal calf serum, and basic minimal essential vitamins. The clusters were then triturated mechanically, plated on poly-d-lysine-coated glass coverslips, cultured in humidity-saturated chambers at 24°C, and used within 1–7 d after plating. Every third day, cells were given fresh medium.
Electrical recording. Patches were pulled from the soma of the cultured ORNs and recorded from using the inside-out configuration of the patch-clamp technique as described previously (Zhainazarov and Ache, 1995). Briefly, a coverslip containing the cells was transferred to an SP-filled 25 mm culture dish mounted on the stage of an inverted microscope (Axiovert 100; Zeiss, Oberkochen, Germany) and viewed with phase-contrast optics at 320×. Patch pipettes were fabricated from borosilicate glass tubes (BF150-86-10; Sutter Instruments, Novato, CA) and fire polished to a final tip diameter of <1 μm. The pipettes, when filled with SP, had resistances of 5–10 MΩ and easily formed seals on the soma membrane with resistances of 10–15 GΩ. Single-channel currents were recorded with an Axopatch 200A patch-clamp amplifier, low-pass filtered at 1 kHz (−3 dB; four-pole Bessel filter), digitized at 10 kHz (analog-to-digital, digital-to-analog interface, TL-1; software, pClamp 6.0; Axon Instruments, Foster City, CA), and stored on a computer hard disk for later analysis. A rotatory perfusion system (RSC-100; Biologic, Claix, France) was used to perfuse isolated membrane patches with up to nine different solutions. After forming the patch in Panulirussaline, the pipette was moved immediately into sodium-free solution (see below) that continuously flowed from one of nine 100 μm inner diameter tubes and that completely engulfed the membrane patch. Switching between immediately adjacent tubes required <10 msec. Unless stated otherwise, recordings were performed at a holding potential of −60 mV. The recordings were referenced to an Ag-AgCl wire electrode connected to the bath solution through a 3 m KCl–agar bridge. All recordings were made at room temperature (20–22°C).
Single-channel current was analyzed using the pClamp 6.0 software. Patches typically contained more than one channel, so the open probability of a channel was calculated using the equationPo = <I>/(N · i), where <I> is the mean current over the interval of interest, N is the number of channels in the patch, andi is the single-channel current amplitude. Current amplitude histograms were used to measure single-channel current amplitudes. When the number of channels in a patch was difficult to determine reliably, NPo was used as a measure of the channel activity. Unless noted otherwise, the baseline of single-channel current traces are depicted by dashed lines in figure legends, and the data presented as mean ± SD of n observations.
Solutions. SP contained (in mm): 458 NaCl, 13.4 KCl, 13.4 Na2SO4, 13.6 CaCl2, 9.8 MgCl2, 2 glucose, and 10 HEPES, pH 7.4 adjusted with 1 m NaOH. Sodium-free solution consisted of (in mm): 210 KCl, 11 EGTA, 1 CaCl2, 696 glucose, and 10 HEPES, pH 7.4 adjusted with 1 m Tris. The calculated free calcium concentration in the sodium-free solution was 10 nm(software, chelator; Schoenmakers et al., 1992). In some experiments, part of the KCl in the sodium-free solution was substituted by an equivalent concentration of NaCl as described below and in figure legends. PI, PI(4)P, and PI(4,5)P2 stock solutions (1 mm) were prepared by dispersing the phosphoinositides in distilled water with 30 min sonication on ice, aliquoted, and stored at −20°C for use within 3 d. Stock solutions were diluted to working solutions of the stated concentration and sonicated for an additional 30 min on ice before use. Monoclonal antibodies against PI(4)P and PI(4,5)P2 were obtained commercially (Perceptive Biosystems, Framingham, MA) and diluted 250-fold into the working solution as described below and in figure legends.
All inorganic salts were purchased from Fisher Scientific (Houston, TX), except for AlCl3 and NaF, which were purchased from Sigma (St. Louis, MO). Mouse serum, IP3, arachidonic acid, 1-steroyl-2-arachidonoyl-sn-glycerol, phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylethanolamine (PE),N-(6-aminohexyl)-5-chloro-1-naphthalenesulfon-amide (W7), trifluoperazine, and all general organic chemicals were obtained from Sigma, except for HEPES, which was obtained from Research Organics (Cleveland, OH). PI-specific PLC (PLC-PI) (from Bacillus cereus), PI, PI(4)P, and PI(4,5)P2 were obtained from Boehringer Mannheim (Indianapolis, IN). The cytosolic fraction of the lysate of H5 insect cells expressing rPLC-β2 was a gift from the laboratory of Dr. R. Iyengar (Mount Sinai School of Medicine, New York, NY). The rPLC-β2 lysate was not purified and was applied at a 1:50 dilution.
RESULTS
Exogenous PI(4,5)P2 and PI(4)P directly activate the lobster Na+-gated nonselective cation channel
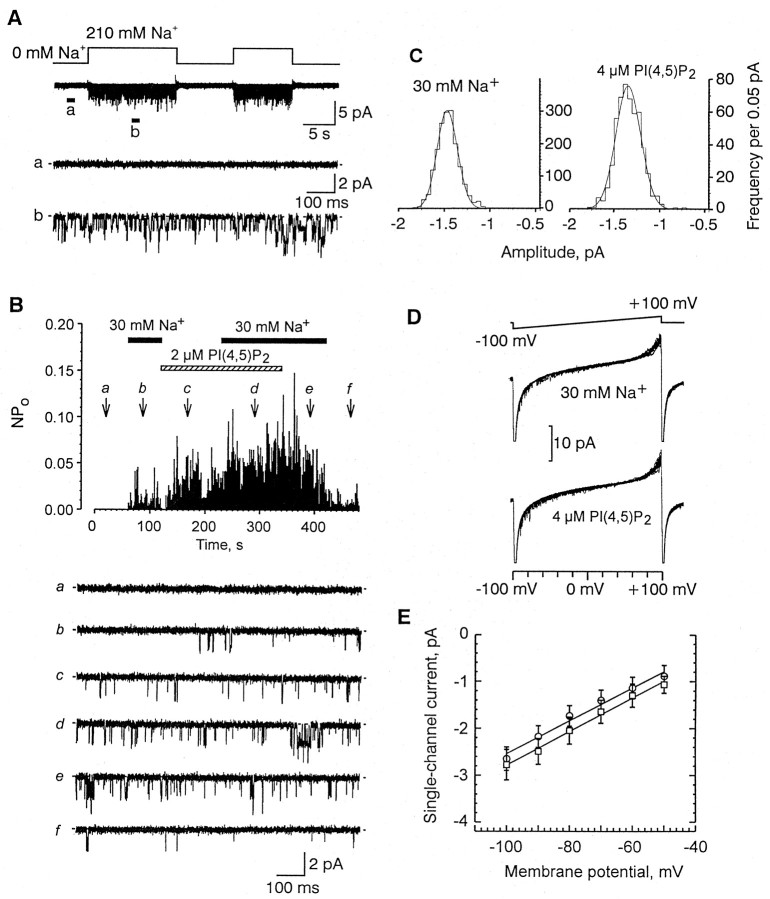
Na+ (10–210 mm) directly and reversibly activated a nonselective cation channel in inside-out patches excised from the soma of cultured lobster ORNs as described previously (Zhainazarov and Ache, 1995) (Fig.1A). Such activity occurred in 142 of 178 (80%) patches tested. Exposing a patch containing Na+-gated channel activity to 2 μm PI(4,5)P2 triggered channel openings, even in the absence of Na+ (n = 46) (Fig.1B). The effect of PI(4,5)P2 was rapid, although typically required ∼1 min for the activity to reach a stable level. On washout, the effect decayed over 15–30 min (Fig.1B). Coapplication of Na+ with PI(4,5)P2 always evoked a greater level of channel activity in the same patch than did application of Na+ alone (Fig. 1B). Na+-gated and PI(4,5)P2-activated channels always colocalized. PI(4,5)P2 (0.5–20 μm) failed to evoke channel openings in patches not exhibiting Na+-gated channel activity (n = 11; data not shown). Similarly, Na+ (10–210 mm) failed to have any effect on patches not possessing PI(4,5)P2-evoked channel activity (n = 3).
Fig. 1.
Effect of PI(4,5)P2 on a lobster olfactory Na+-gated channel. A,Second trace, Original recording of multiple channel activity evoked by 210 mm Na+ applied to the intracellular face of an inside-out patch taken from the soma of cultured lobster olfactory receptor neurons. Top trace, Time course of Na+ application. Bottom two traces, Segments taken where noted from the second trace and shown on an expanded time scale. Membrane potential, −60 mV. B, Top, Plot of the open probability (ordinate) as a function of time (abscissa) of a multiple channel recording after treatment with 2 μm PI(4,5)P2 (dashed horizontal bar) in the presence (solid horizontal bars) and absence of intracellular Na+. Each data point represents the NPo calculated over 1 sec. Note that PI(4,5)P2 directly activates the channel in the absence of Na+ and also increases channel activity in the presence of Na+.Bottom, Representative segments of the actual single-channel current traces taken at time points indicated by thearrows (a–f). C, Amplitude histograms of channel openings evoked by either 30 mm Na+ (left) or 4 μm PI(4,5)P2 (right).Solid lines represent fit of Gaussian functions with a mean value of −1.47 pA for Na+ and −1.35 pA for PI(4,5)P2. Membrane potential, −60 mV. D, Thirty superimposed single-channel current recordings evoked by a voltage ramp (top trace) in the presence of either 30 mm Na+ (middle trace) or 4 μm PI(4,5)P2 (bottom trace). Pipette, SP. Bath, 180 mm KCl plus 30 mm NaCl for the middle trace and 210 mm KCl plus 4 μm PI(4,5)P2 for the bottom trace. E, Plot of the current–voltage relationship of channel openings evoked by either Na+ (open circles) or PI(4,5)P2 (open squares), conditions similar to D. Solid lines, Linear regressions through data points (n = 3).
PI(4,5)P2 (2 μm) and Na+(30 mm) evoked single-channel currents of a similar amplitude at −60 mV (Fig. 1B). Amplitude histograms in both instances could be fit by a single Gaussian function with means of −1.46 ± 0.13 (n = 20) and −1.51 ± 0.11 (n = 21) pA for 30 mmNa+ and 2 μmPI(4,5)P2, respectively (Fig. 1C). Coapplication of Na+ (210 mm) and PI(4,5)P2 (2 μm) induced channel openings to a single level with a mean current amplitude of −1.63 ± 0.10 pA (n = 4) at −60 mV. Figure 1D shows 30 superimposed current traces recorded from the same patch in response to a voltage ramp from −100 to +100 mV in the presence of either 30 mm Na+ (middle trace) or 4 μm PI(4,5)P2 (bottom trace). The single-channel conductance, estimated from the slope of the current–voltage relationship between −100 and −50 mV, was 35.9 ± 0.2 pS (mean ± SEM; n = 4) in the presence of 4 μm PI(4,5)P2, and 35.1 ± 0.8 pS (mean ± SEM; n = 8) in the presence of 30 mm Na+ (Fig.1E). Coapplying 210 mmNa+ and 4 μm PI(4,5)P2evoked 35.1 ± 0.9 pS (mean ± SEM; n = 11) channel openings. PI(4,5)P2-evoked channel activity was reversibly inhibited by intracellular application of Ca2+ (100 μm to 1 mm), Mg2+ (100 μm to 1 mm), W7 (10–100 μm), and trifluoperazine (10–100 μm), all substances previously shown to block the lobster Na+-gated channel (n = 3; data not shown) (Zhainazarov and Ache, 1995; Zhainazarov et al., 1998). These data suggest that Na+ and PI(4,5)P2 are activating the same population of ion channels.
Aluminum (50 μm), which forms a tight complex with PI(4,5)P2 (McDonald and Mamrack, 1995), completely inhibited channel activity induced by 2 μmPI(4,5)P2 (n = 3) (Fig.2). The effect of aluminum was partly reversed by 0.5 mm sodium fluoride (n = 3), which binds aluminum with high affinity (Martin, 1988). The channel activity evoked by 4 μm PI(4,5)P2 was completely and irreversibly inhibited by a monoclonal antibody against PI(4,5)P2 (n = 3; data not shown). Other membrane phospholipids, including PI (0.5–4.0 μm;n = 5), PC (4.0 μm; n = 3), PE (4.0 μm; n = 3), and PS (4.0 μm; n = 3), as well as IP3 (5 μm; n = 3) and the diacylglycerol analog 1-steroyl-2-arachidonoyl-sn-glycerol (10 μm;n = 3), had no appreciable effect on the channel when applied in either the absence or presence of Na+(data not shown). Arachidonic acid (10 μm) reversibly inhibited channel activity in three patches tested, but we did not attempt further to investigate the inhibitory effect of arachidonic acid on the channel (data not shown). The lack of any effect of IP3 on the Na+-gated channel suggests that the channel is distinct from the IP3-activated channels reported by Fadool and Ache (1992).
Fig. 2.
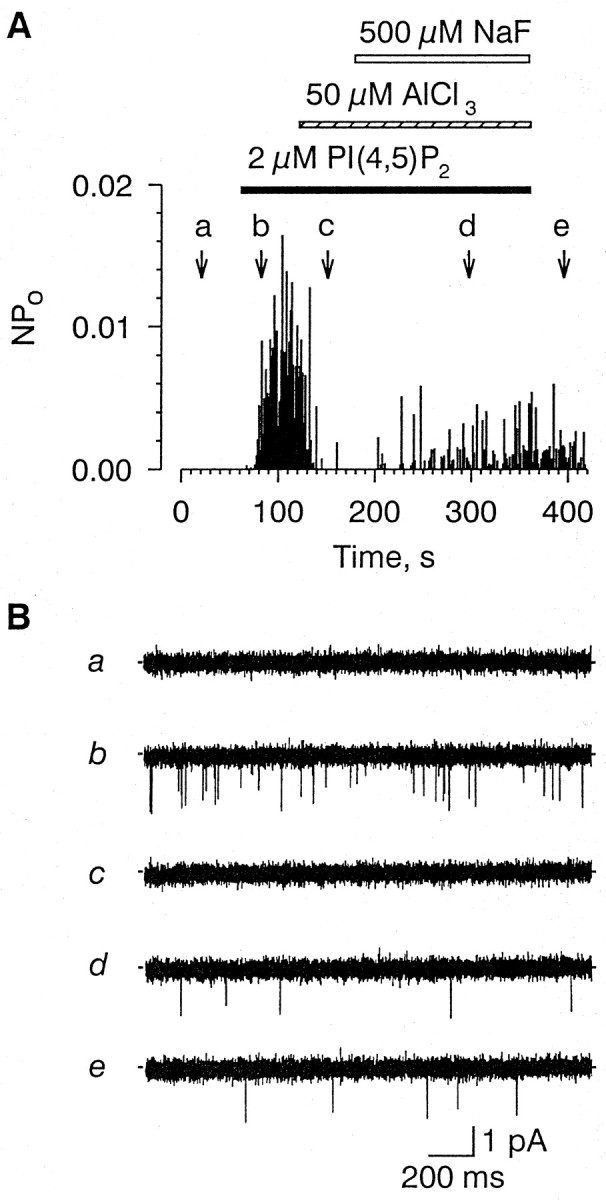
Effect of 50 μm AlCl3and 500 μm NaF on single-channel activity evoked by 2 μm PI(4,5)P2 in the absence of Na+. Top, Plot of the open probability (ordinate) as a function of time of PI(4,5)P2-induced single-channel activity evoked during treatment with 50 μm AlCl3 (dashed horizontal bar) and, subsequently, NaF (open horizontal bar). [Na+]i, 0 mm. Time course of PI(4,5)P2 application is indicated by the solid horizontal bar.Bottom, Representative segments of the actual single-channel current traces taken at time points indicated by thearrows (a–e).
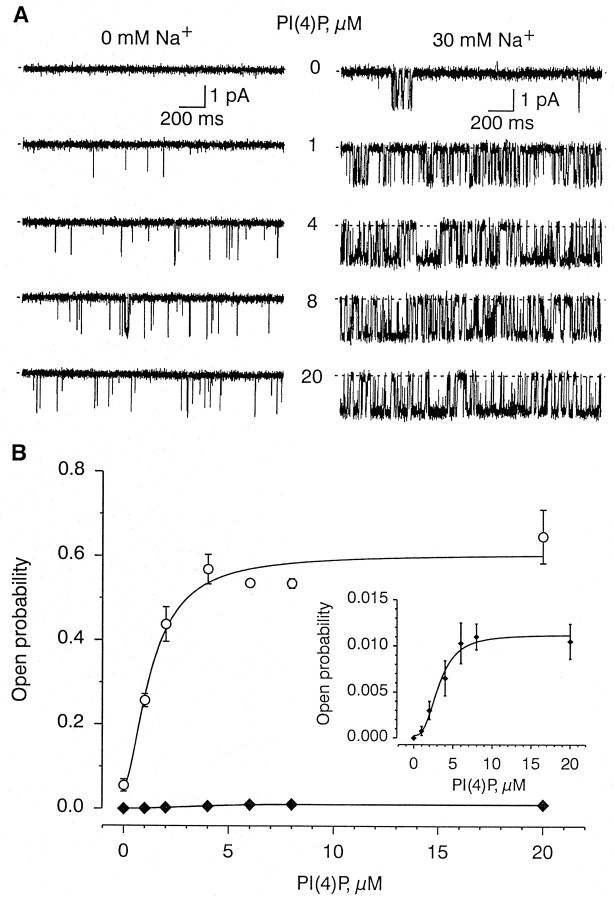
The channel was also activated by PI(4)P (n = 34). As with PI(4,5)P2, PI(4)P (4 μm) activated the channel in the absence of Na+ (Fig.3A–D). The effects of PI(4)P also reversed slowly on washout (Fig.3B,C). The channel activity evoked by 4 μm PI(4)P was completely and irreversibly inhibited by a monoclonal antibody against PI(4)P (n = 3; data not shown).
Fig. 3.
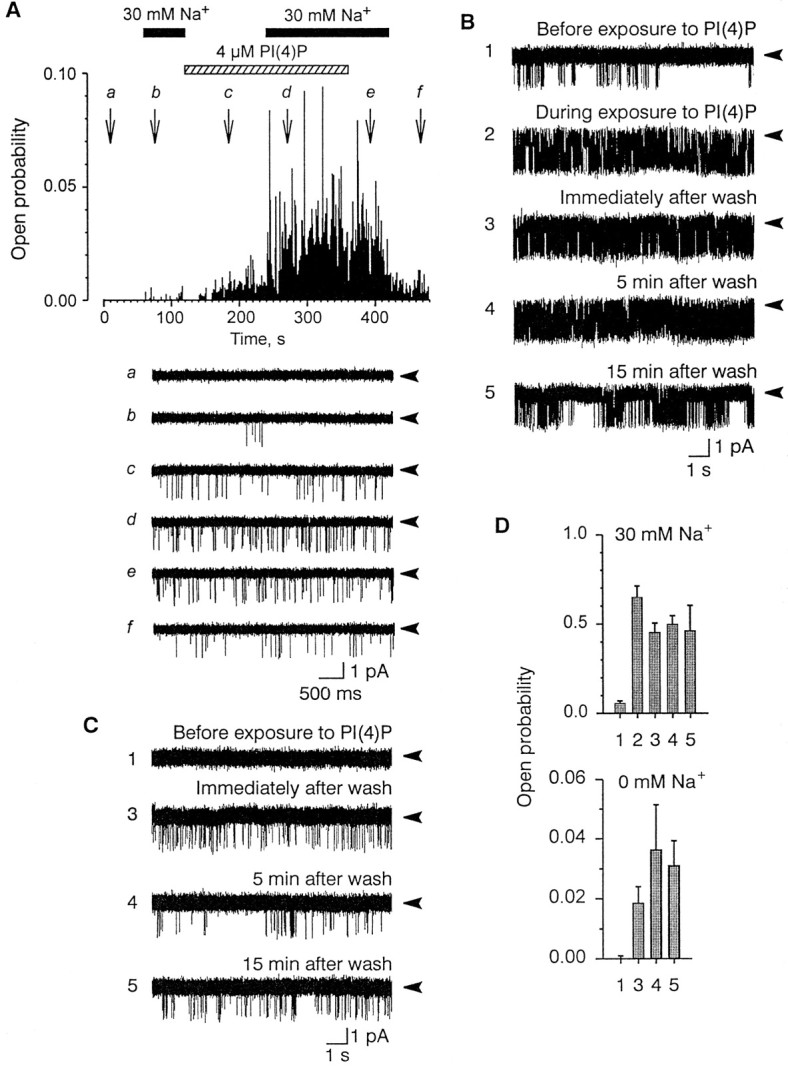
Effect of PI(4)P on single-channel activity.A, Top, Plot of the open probability (ordinate) as a function of time (abscissa) during treatment with 4 μmPI(4)P (dashed horizontal bar) in the presence (solid horizontal bars) and absence of intracellular Na+. Each data point is the open probability calculated over 1 sec. Note that PI(4)P directly activated the channel in the absence of Na+ and also increased the open probability in the presence of 30 mm Na.Bottom, Representative segments of the actual single-channel current traces taken at time points indicated by thearrows (a–f). B,C, Single-channel records before (1), during (2), and after (3–5) exposure to 20 μm PI(4)P at the indicated time points after removal of PI(4)P in the presence (B) and absence (C) of 30 mm Na+. Baselines depicted bysolid arrow heads. D, Plots of the corresponding open probabilities of the traces shown inB and C, calculated from 30 sec records.
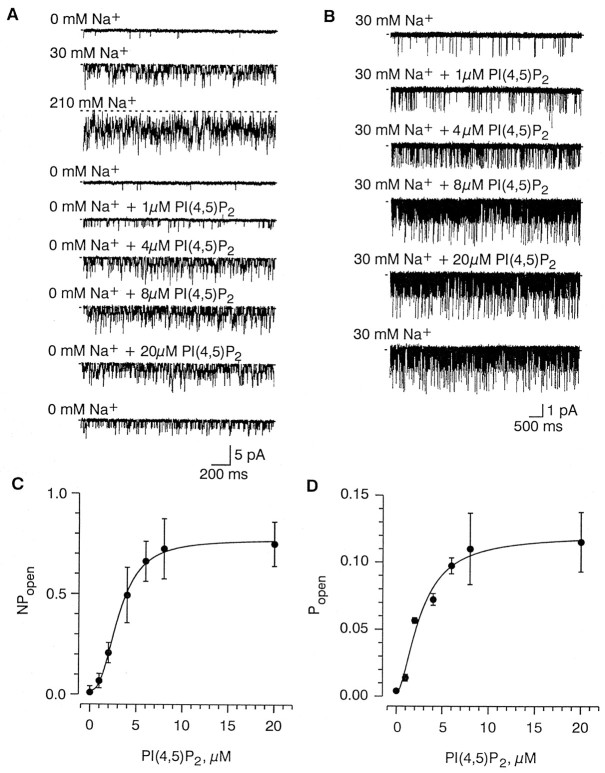
Concentration dependence of the effect of PI(4,5)P2 and PI(4)P on channel open probability
Channel activity increased in a graded manner with PI(4,5)P2 concentration in both the absence and presence of Na+ (Fig.4A,C, 0 mm Na+; Fig.4B,D, 30 mmNa+) (n = 4). Increasing PI(4,5)P2 from 1 to 20 μm at 0 mmNa+ increased the NPo from 0.07 ± 0.03 to a plateau level of 0.75 ± 0.11 (Fig.4C). The number of channels in the patch (N), determined by counting multiple openings at 210 mm Na+, was not affected by PI(4,5)P2, indicating that the observed increase inNPo was caused by an increase in the open probability (Po) of the channel (subsequently, we refer to both NPo and Po as the open probability for convenience). The dose–response relationship of the open probability on PI(4,5)P2 concentration could be fit by the Hill equation with an EC50 of 3.1 μm for 0 mm Na+ and 2.7 μm for 30 mm Na+ (Fig. 4), and a Hill coefficient of 2.5 for 0 mmNa+ and 1.7 for 30 mmNa+. Varying the PI(4,5)P2 concentration had no effect on the single-channel current amplitude. Channel current measured in the presence of 30 mm Na+ at −60 mV was −1.60 ± 0.06 pA for 0 μmPI(4,5)P2 (n = 5), −1.59 ± 0.07 pA for 1 μm PI(4,5)P2 (n = 5), and −1.59 ± 0.01 pA for 20 μmPI(4,5)P2 (n = 3).
Fig. 4.
Concentration dependency of PI(4,5)P2on channel activity. A, B, Representative traces evoked by the indicated concentration of PI(4,5)P2 in the absence (A) and presence (B) of 30 mmNa+. Traces were obtained at least 1 min after the change in PI(4,5)P2 concentration. The last trace in both A and B was recorded immediately after washout of PI(4,5)P2.C, D, Plots of the open probability (ordinate) as a function of PI(4,5)P2concentration (1–20 μm; abscissa) in the absence (C) and presence (D) of 30 mm Na+. Points shown obtained from 1 min recordings (n = 4). In C and D, solid lines through the data points represent the fits of the Hill equation y = y0 +y1[C]k/([C]k+ EC50k) with the following parameters: half-effect concentration (EC50), 3.1 ± 0.1 μm for 0 mmNa+ and 2.7 ± 0.6 μm for 30 mm Na+; y0,NPo,0 = 0.02 ± 0.02 for 0 mmNa+ and Po,0 = 0.00 ± 0.01 for 30 mm Na+;y1, NPo,1 = 0.75 ± 0.03 for 0 mm Na+ andPo,1 = 0.11 ± 0.02 for 30 mmNa+; and Hill coefficient (k), 2.5 ± 0.2 for 0 mm Na+ and 1.7 ± 0.5 for 30 mm Na+. The parameters are given as mean ± SE.
Similarly, varying the concentrations of PI(4)P from 1 to 20 μm increased the open probability of the channel without any appreciable change in either the single-channel current amplitude or the number of active channels in the patch (Fig.5A). The channel activity evoked by PI(4)P was much higher in the presence of Na+ than in its absence (Fig. 5A).Po as a function of PI(4)P concentration was sigmoidal, with an EC50 of 3.2 μm at 0 mm Na+ and 1.3 μm at 30 mm Na+, and a Hill coefficient of 2.8 at 0 mm Na+ and 1.7 at 30 mmNa+ (Fig. 5B).
Fig. 5.
Concentration dependency of PI(4)P on channel activity. A, Representative recordings of single-channel activity evoked by the concentration of PI(4)P in the absence (left) and presence (right) of 30 mm Na+. Traces were recorded at least 1 min after the change in PI(4)P concentration. B, Plot of the open probability (ordinate) as a function of the concentration of PI(4)P (abscissa) in the absence (filled diamonds) and presence (open circles) of 30 mm Na+.Inset, Same plot on an expanded ordinate. Points obtained from 1 min recordings (n = 4).Solid lines through the data points in Brepresent fits by Hill equations with the following parameters: EC50, 3.2 ± 0.4 μm for 0 mm Na+ and 1.3 ± 0.3 μm for 30 mm Na+;y0, 0.0003 ± 0.0007 for 0 mmNa+ and 0.05 ± 0.04 for 30 mmNa+; y1, 0.0109 ± 0.0012 for 0 mm Na+ and 0.56 ± 0.07 for 30 mm Na+; andk, 2.8 ± 0.8 for 0 mmNa+ and 1.7 ± 0.7 for 30 mmNa+. The parameters are given as mean ± SE.
Exogenous PI(4,5)P2 and PI(4)P enhance the sodium sensitivity of the Na+-gated channel
PI(4,5)P2 and PI(4)P also enhanced channel activity in the presence of Na+, and it did so by increasing the channel open probability without affecting its conductance (Figs. 1,3). The open probability of the channel increased with increasing concentrations of Na+ (10–210 mm) in the absence of the phosphatidylinositol phosphates (Fig.6A). The dose–response relationship under these conditions had an EC50 of 74 ± 6 mm Na+ (mean ± SEM) and a Hill coefficient of 2.8 ± 0.1 (n = 4) (Fig.6D, filled triangles), in good agreement with our earlier findings (Zhainazarov and Ache, 1995). Four micromolar PI(4,5)P2 (Fig. 6B) and 4 μm PI(4)P (Fig. 6C) by themselves increased channel activity as described above, but they also substantially increased the Na+ sensitivity of the channel. The EC50 shifted to 22 ± 3 mmNa+ (mean ± SEM) for 4 μmPI(4,5)P2 and to 29 ± 11 mmNa+ for 4 μm PI(4)P (n= 3) (Fig. 6D), with Hill coefficients of 0.9 ± 0.1 (mean ± SEM) for 4 μm PI(4,5)P2 and 1.2 ± 0.1 for 4 μm PI(4)P (n = 3). PI(4)P, however, did not alter the open probability at saturating concentrations of Na+ (210 mm). Although 2 μm PI(4)P increased the open probability from 0.06 ± 0.01 to 0.44 ± 0.04 at 30 mmNa+, it did not affect Po,max at 210 mm Na+ (0.47 ± 0.02 in the absence vs 0.49 ± 0.05 in the presence of 2 μmPI(4)P; n = 4). Two micromolar PI(4,5)P2had a similar effect on Po,max (n = 3). Thus, exogenous PI(4,5)P2 and PI(4)P increase the apparent affinity of the channel for Na+.
Fig. 6.
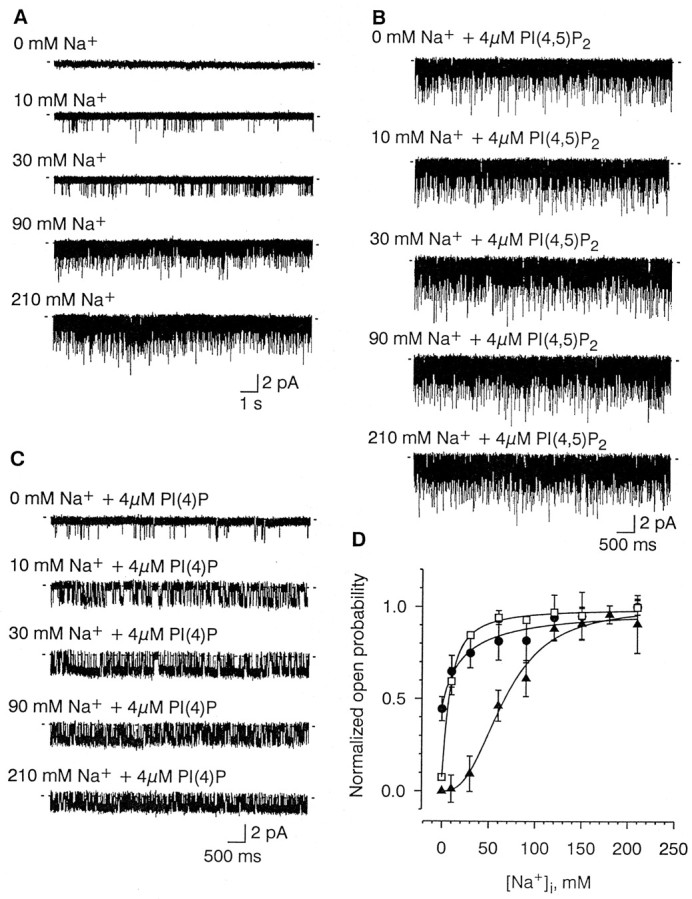
Effect of PI(4)P and PI(4,5)P2 on the Na+ sensitivity of the channel. A–C, Representative samples of single-channel activity induced by the concentration of Na+ indicated before (A) and during exposure to either 4 μm PI(4,5)P2 (B) or 4 μm PI(4)P (C).Traces in A–C were obtained from different patches. Traces in B andC were recorded at least 5 min after the addition of phosphatidylinositols. D, Plot of the open probability (ordinate) as a function of Na+concentration (abscissa) in the absence (filled triangles) and presence of either 4 μm PI(4,5)P2 (filled circles) or 4 μm PI(4)P (open squares). Data points obtained from 1 min recordings (n = 4). Solid lines represent fits of Hill equations (see Fig. 4) with the following parameters: EC50 (in mm), 67.0 ± 5.0 in control, 22.4 ± 16.7 for PI(4,5)P2, and 8.1 ± 0.5 for PI(4)P; y0/(y0 +y1), 0.00 ± 0.06 in control, 0.45 ± 0.04 for PI(4,5)P2, and 0.07 ± 0.01 for PI(4)P; and k, 2.7 ± 0.5 in control, 1.0 ± 0.6 for PI(4,5)P2, and 1.3 ± 0.2 for PI(4)P. The parameters are given as mean ± SE. For illustration clarity, the data points were normalized by the sum of y0and y1 obtained from the fits.
Endogenous PI(4,5)P2 or PI(4)P is necessary for channel activation by Na+
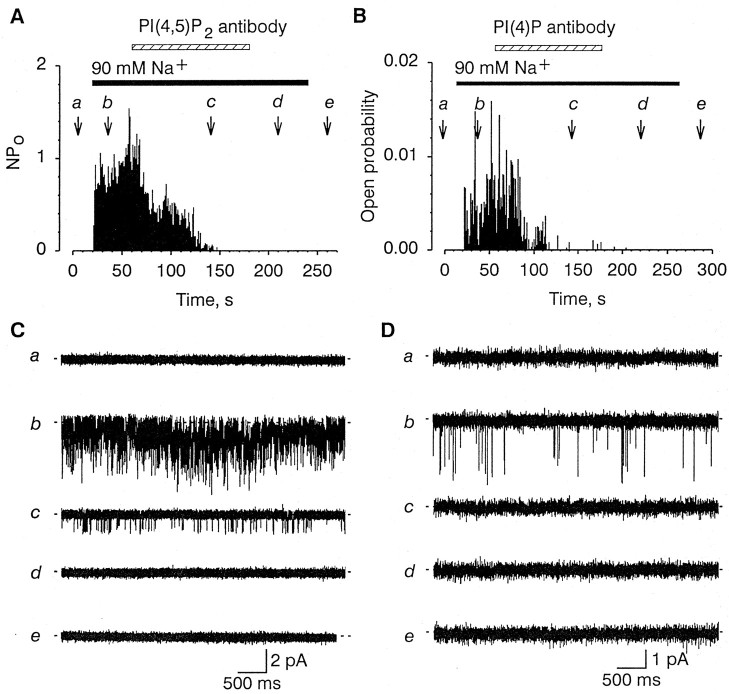
Although there was no noticeable channel activity before application of either Na+ or phosphatidylinositol phosphates in the vast majority of the patches tested, 7 of the 178 patches tested showed rare channel openings in control conditions. The frequency of these spontaneous openings was also increased by Na+ and phosphatidylinositol phosphates (Fig.4A), suggesting that the Na+-gated channel can open spontaneously, even in the absence of Na+, although with very low probability. Fifty micromolar aluminum substantially reduced the channel activity evoked by Na+, even in the absence of PI(4,5)P2(n = 4; data not shown). Aluminum altered the open probability of the channel without affecting the amplitude of the single-channel current. The effect of aluminum was partly reversible by exposing the patch to 0.5 mm NaF (n = 3; data not shown). These results suggest that endogenous phosphatidylinositol phosphates may be crucial for the normal function of the Na+-gated channel. Consistent with this view, monoclonal antibodies raised against either PI(4,5)P2 or PI(4)P altered channel activity when applied to the intracellular side of the patch. The PI(4,5)P2 antibody completely and irreversibly inhibited Na+-gated channel activity within 1–2 min after application (n = 6) (Fig.7A). The single-channel current amplitude measured within the first minute after antibody application was −1.27 ± 0.03 versus −1.25 ± 0.16 pA before application (−60 mV; n = 3), indicating that the antibody affects only the open probability of the channel. The PI(4)P antibody similarly irreversibly inhibited Na+-gated channel activity (n = 4) (Fig. 7B). Neither mouse serum nor antibodies against PI(4,5)P2 or PI(4)P that were boiled for 30 min before application altered activity of the Na+-gated channel (n = 9; data not shown).
Fig. 7.
Effect of antibodies raised against PI(4,5)P2 (A) and PI(4)P (B) on channel activity. A,Top, Plot of the open probability (ordinate) as a function time (abscissa) during treatment with anti-PI(4,5)P2. Time course of Na+ and antibody application indicated bysolid and dashed horizontal bars, respectively. Bottom, Representative segments of the actual single-channel current traces taken at time points indicated by the arrows (a–e). B, Top, Time course of the change in Po of single-channel activity observed during treatment with anti-PI(4)P. Time course of Na+ and antibody application indicated by solid and dashed horizontal bars, respectively. Bottom, Representative segments of the actual single-channel current traces taken at time points indicated by the arrows (a–e). Membrane potential: A, −60 mV; B, −100 mV.
Depleting the membrane of endogenous PI(4,5)P2 by exposing the patches to the cytosolic fraction of the rPLC-β2 [PI(4,5)P2-specific PLC] lysate from H5 insect reduced the open probability of the channel to 42 ± 7% (n = 2) of its pretreatment value (Fig.8). The effect of the rPLC-β2 lysate was completely reversed with 4 μm PI(4,5)P2(n = 5) (Fig. 8). PLC-PI (0.5 U/ml) did not have an appreciable effect on the channel (n = 5; data not shown). Together, these result suggest that endogenous PI(4,5)P2 and PI(4)P are required for normal activation of the Na+-gated channel by intracellular Na+.
Fig. 8.
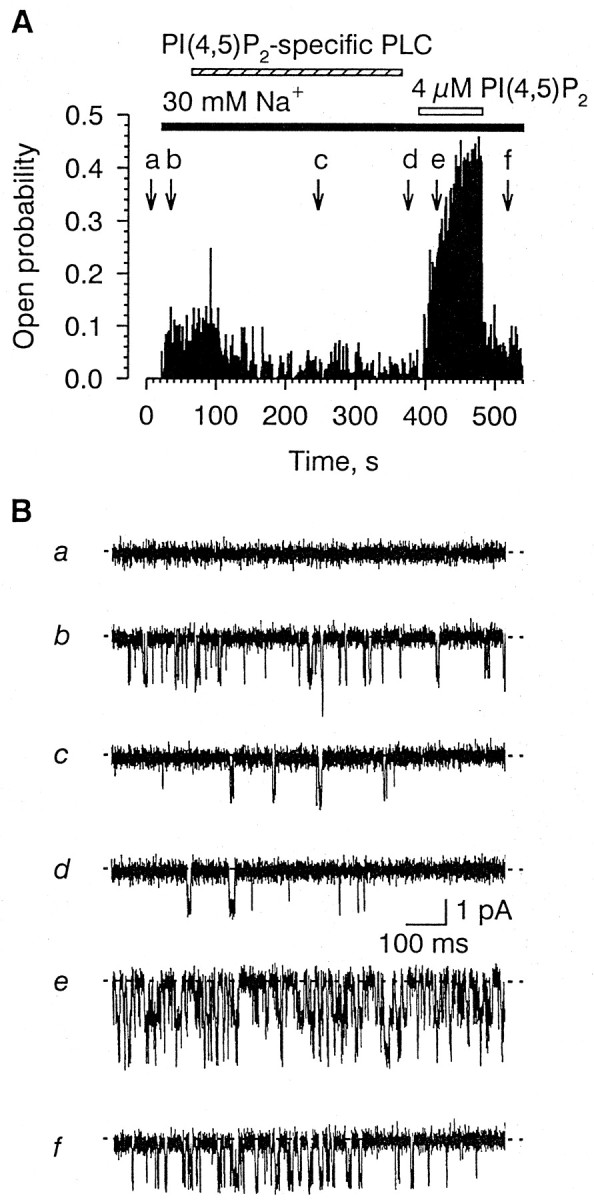
Effect of PLC-β2 and, subsequently, PI(4,5)P2 on channel activity. A,Top, Plot of the open probability (ordinate) as a function time (abscissa). [Na+]i, 90 mm(solid horizontal bar). PLC-β2 (dashed horizontal bar) applied as 1:50 dilution from rPLC-β2 lysate from H5 insect cells. PI(4,5)P2, 4 μm(open horizontal bar). Bottom, Representative segments of the actual single-channel current traces taken at time points indicated by the arrows(a–f).
DISCUSSION
Six lines of evidence are consistent with the interpretation that the lobster olfactory Na+-gated channel is directly activated in a selective manner by PI(4,5)P2 and PI(4)P: (1) application of either PI(4,5)P2 or PI(4)P never induced channel openings in patches not exhibiting Na+-gated channel activity, and similarly, Na+ never evoked channel activity in patches not showing channel openings induced by these two phosphatidylinositols; (2) channel openings evoked by each of these phosphatidylinositols had a single-channel conductance similar to that of the Na+-gated channel and could be blocked from intracellular side by pharmacological agents (Ca2+, Mg2+, W7, and trifluoperazine) that inhibit Na+-gated channel activity (Zhainazarov and Ache, 1995); (3) PI, IP3, and a DAG analog were ineffective in activating the Na+-gated channel; (4) other membrane phospholipids, such as PC, PE, and PS, had no effect on the channel; (5) channel openings evoked by PI(4,5)P2 and PI(4)P were irreversibly inhibited by monoclonal antibodies raised against these phosphatidylinositols; and (6) PI(4,5)P2 and PI(4)P activated the Na+-gated channel in a concentration-dependent manner starting at 0.5 μm.
The lobster olfactory Na+-gated channel, therefore, can be added to the growing list of ion channels and transporters that are directly activated by phosphatidylinositols, PI(4,5)P2in particular. These include the ATP-sensitive K+channel (Hilgemann and Ball, 1996; Fan and Makielski, 1997), the inward rectifier K+ channel (Huang et al., 1998), and the sodium–calcium exchanger (Hilgemann and Ball, 1996).
In addition to their stimulatory effect on the channel in the absence of Na+, both PI(4,5)P2 and PI(4)P enhanced the Na+ sensitivity of the channel when coapplied with Na+. Similar dual trigger–regulatory action occurs in the ATP-sensitive K+ channel, where PI(4,5)P2 directly activates the channel (Hilgemann and Ball, 1996; Fan and Makielski, 1997) and also controls the sensitivity of the channel to ATP-mediated inhibition (Baukrowitz et al., 1998;Shyng and Nichols, 1998). The common mode of action of the phosphatidylinositols on these two different types of ion channels raises the possibility of this being a common mechanism for phospholipid control of ion channel function, even if the specific effects of the ligands (e.g., increased or decreased open probability) vary for different channel types. Phosphatidylinositols, including PI(4,5)P2 and PI(4)P, are ubiquitous in the membranes of eukaryotic cells, and their levels are carefully regulated by a complex system of multiple kinases and phosphatases that interconvert these lipid molecules (Zhang and Majerus,1998).
Finding that Na+-gated channel activity was irreversibly inhibited by monoclonal antibodies against PI(4,5)P2 and PI(4)P in patches never exposed to exogenous phosphatidylinositols suggests that endogenous phospholipids are required for the activation of the channel by intracellular Na+. This view is also supported by the finding that depleting endogenous PI(4,5)P2 by treating the membrane with rPLC-β2 substantially decreases channel activity, an effect that can be reversed by exposing the patch to exogenous PI(4,5)P2. Modulation of channel activity by levels of PI(4,5)P2 and PI(4)P in the cell membrane could therefore provide an intracellular mechanism for regulating the Na+-sensitivity of the lobster olfactory Na+-gated channel.
To determine whether PI(4,5)P2 and PI(4)P are involved in olfactory transduction in lobster ORNs, it must first be established that odors change the concentration of PI(4,5)P2 and/or PI(4)P in the cell membrane and that any such changes occur fast enough to account for odor detection. Odor-induced increases in cAMP and IP3, predicted signaling molecules in lobster ORNs, peak within 50 msec of stimulation in vitro (Boekhoff et al., 1994), fast enough to account for the rapid (20–50 msec) onset of odor-evoked currents (Fadool et al., 1993). If similar rapid odor-induced increases in phosphatidylinositols occur in lobster ORNs, phosphatidylinositols could potentially activate the channel directly, and the Na+-sensitivity of the channel could act as a positive feedback, or they could facilitate Na+-gated activation by lowering the sensitivity of the channel to Na+ (gain control). In the latter case, IP3 -activated nonselective cation channels, which have been shown to be activated by odor stimulation (Fadool and Ache, 1992), could act as a potential route for Na+ influx into the cell. Alternatively, PI(4,5)P2 and/or PI(4)P could mediate longer term, adaptive changes in the odor sensitivity of the cells. Further study is required to address these questions.
Footnotes
This work was supported by National Institute on Deafness and Other Communication Disorders Grant DC01655. We thank A. Hastings for the preparation of the cultured cells, M. Milstead for assistance with the illustrations, and Drs. B-A. Battelle, P. A. V. Anderson, and G. A. Cottrell for helpful comments on this manuscript. We also thank E. Buck and Dr. R. Iyengar (Mount Sinai School of Medicine, New York, NY) for the rPLC-β2 lysates.
Correspondence should be addressed to A. B. Zhainazarov, Whitney Laboratory, University of Florida, 9505 Ocean Shore Boulevard, St. Augustine, FL 32086-8623.
REFERENCES
- 1.Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ. Inositol trisphosphate and calcium signaling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- 4.Boekhoff I, Michel WC, Breer H, Ache BW. Single odors differentially stimulate dual second messenger pathways in lobster olfactory receptor cells. J Neurosci. 1994;14:3304–3309. doi: 10.1523/JNEUROSCI.14-05-03304.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu A, Stefani E. Phosphatidylinositol 4,5-bisphosphate-induced Ca2+ release from skeletal muscle sarcoplasmic reticulum terminal cisternal membranes. Ca2+ flux and single channel studies. J Biol Chem. 1991;266:7699–7705. [PubMed] [Google Scholar]
- 6.De Camilli P, Emr SD, McPherson PS, Novick P. Phosphoinositides as regulators in membrane traffic. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- 7.Dryer SE. Na+-activated K+ channels: a new family of large-conductance ion channels. Trends Neurosci. 1994;17:155–160. doi: 10.1016/0166-2236(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 8.Fadool DA, Ache BW. Plasma membrane inositol 1,4,5-triphosphate-activated channels mediate signal transduction in lobster olfactory receptor neurons. Neuron. 1992;9:907–918. doi: 10.1016/0896-6273(92)90243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fadool DA, Michel WC, Ache BW. Sustained primary culture of lobster (Panulirus argus) olfactory receptor neurons. Tissue Cell. 1991;23:719–732. doi: 10.1016/0040-8166(91)90025-o. [DOI] [PubMed] [Google Scholar]
- 10.Fadool DA, Michel WC, Ache BW. Odor sensitivity of cultured lobster olfactory receptor neurones is not dependent on process formation. J Exp Biol. 1993;174:215–233. doi: 10.1242/jeb.174.1.215. [DOI] [PubMed] [Google Scholar]
- 11.Fan Z, Makielski JC. Anionic phospholipids activate ATP-sensitive potassium channels. J Biol Chem. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- 12.Hilgemann DW, Ball R. Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- 13.Huang C-L, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 14.Janmey P. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu Rev Physiol. 1994;56:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- 15.Liscovitch M, Chalifa V, Pertile P, Chen CS, Cantley LC. Novel function of phosphatidylinositol 4,5-bisphosphate as a cofactor for brain membrane phospholipase D. J Biol Chem. 1994;269:21403–21406. [PubMed] [Google Scholar]
- 16.Lupu VD, Kaznacheyeva E, Krisna UM, Falck JR, Bezprozvanny I. Functional coupling of phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1998;273:14067–14070. doi: 10.1074/jbc.273.23.14067. [DOI] [PubMed] [Google Scholar]
- 17.Martin RB. Ternary hydroxide complexes in neutral solutions of Al3+ and F−. Biochem Biophys Res Commun. 1988;155:1194–2000. doi: 10.1016/s0006-291x(88)81266-x. [DOI] [PubMed] [Google Scholar]
- 18.McDonald LJ, Mamrack MD. Phosphoinositide hydrolysis by phospholipase C modulated by multivalent cations Li3+, Al3+, neomycin, polyamines, and melittin. J Lipid Mediat Cell Signal. 1995;11:81–91. doi: 10.1016/0929-7855(94)00029-c. [DOI] [PubMed] [Google Scholar]
- 19.Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- 20.Nouailhetas VLA, Aboulafia J, Frediani-Neto E, Ferreira AT, Paiva ACM. A Na+-sensitive cation channel modulated by angiotensin II in cultured intestinal myocytes. Am J Physiol. 1994;266:C1538–C1543. doi: 10.1152/ajpcell.1994.266.6.C1538. [DOI] [PubMed] [Google Scholar]
- 21.Schoenmakers TJM, Visser GJ, Filk G, Theuvenet APR. Chelator: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992;12:994–1000. [PubMed] [Google Scholar]
- 22.Shyng S-L, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- 23.Stuenkel EL, Ruben P, Cooke IM, Lemos JR. Sodium-activated cation channels in peptidergic nerve terminals. Brain Res. 1990;517:35–43. doi: 10.1016/0006-8993(90)91004-z. [DOI] [PubMed] [Google Scholar]
- 24.Sui JL, Petit-Jacques J, Logothetis D. Activation of the artrial KACh channel by the βγ subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc Natl Acad Sci USA. 1998;95:1307–1312. doi: 10.1073/pnas.95.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toker A. The synthesis and cellular roles of phosphatidylinositol 4,5-bisphosphate. Curr Opin Cell Biol. 1998;10:254–261. doi: 10.1016/s0955-0674(98)80148-8. [DOI] [PubMed] [Google Scholar]
- 26.Zaykin A, Nistri A. A transient outward cationic current activated by Na+ into frog visual neurones in vitro. Neurosci Lett. 1996;205:5–8. doi: 10.1016/0304-3940(95)12330-x. [DOI] [PubMed] [Google Scholar]
- 27.Zhainazarov AB, Ache BW. Na+-activated nonselective cation channels in primary olfactory neurons. J Neurophysiol. 1995;73:1774–1781. doi: 10.1152/jn.1995.73.5.1774. [DOI] [PubMed] [Google Scholar]
- 28.Zhainazarov AB, Ache BW. Gating and conduction properties of a sodium-activated cation channel from lobster olfactory receptor neurons. J Membr Biol. 1997;156:173–190. doi: 10.1007/s002329900199. [DOI] [PubMed] [Google Scholar]
- 29.Zhainazarov AB, Ache BW. Na+-gated nonselective cation channel from lobster olfactory projection neurons. J Neurophysiol. 1998;80:3387–3391. doi: 10.1152/jn.1998.80.6.3387. [DOI] [PubMed] [Google Scholar]
- 30.Zhainazarov AB, Doolin RE, Ache BW. Sodium-gated cation channel implicated in the activation of lobster olfactory receptor neurons. J Neurophysiol. 1998;79:1349–1359. doi: 10.1152/jn.1998.79.3.1349. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Majerus PW. Phosphatidylinositol signaling reactions. Semin Cell Dev Biol. 1998;9:153–160. doi: 10.1006/scdb.1997.0220. [DOI] [PubMed] [Google Scholar]