Abstract
The bilaterally symmetrical suprachiasmatic nuclei (SCN) of the hypothalamus are the loci of the mammalian clock controlling circadian rhythms. Previous studies suggested that all regions of the SCN are equipotential as circadian rhythmicity is sustained after partial ablation, as long as ∼25% of the nuclei are spared. In contrast to these results, we found that animals bearing partial lesions of the SCN that spared the subregion delimited by cells containing the calcium-binding protein calbindin-D28K (CaBP), sustained circadian locomotor rhythms. Furthermore, there was a correlation between the strength of the rhythm and the number of spared CaBP cells. Partial lesions that destroyed this region but spared other compartments of the SCN resulted in loss of rhythmicity. The next study indicates that transplants of half-SCN grafts that contain CaBP cells restore locomotor rhythms in SCN-lesioned host animals, whereas transplants containing SCN tissue but lacking cells of this subnucleus fail to restore rhythmicity. Finally, there was a correlation between the number of CaBP-positive cells in the graft and the strength of the restored rhythm. Taken together, the results indicate that pacemakers in the region of the CaBP subnucleus are necessary and sufficient for the control of locomotor rhythmicity and that the SCN is functionally heterogeneous.
Keywords: calbindin-D28K, suprachiasmatic nucleus, hamster, rodent, vasopressin, vasoactive intestinal peptide, circadian rhythms, locomotor activity, pacemaker, oscillator
Circadian clocks are ubiquitous endogenous biological oscillators that measure elapsed and local time, thereby regulating the temporal organization of organisms. Rapid progress in the cloning and characterization of clock genes and clock-controlled genes in several species has brought the promise of finding a general mechanism for circadian clocks at the molecular level (Dunlap, 1998). To relate molecular and genetic mechanisms to the physiology of clocks in multicellular organisms, it must be determined which cells are pacemakers. Properties of pacemakers thought to be necessary for producing circadian rhythms include endogenous oscillation, resetting by environmental cues, especially light, and production of an efferent signal(s) that reaches the rest of the brain. It is not clear in mammals which combination of these properties reside within individual cells.
It is well established that in mammals circadian pacemakers that control the phase of circadian rhythms in the rest of the body lie in the paired suprachiasmatic nucleus (SCN) of the hypothalamus (Moore and Silver, 1998). Photic cues from the daily cycle of light and darkness reach the SCN by means of the retinohypothalamic tract, serving to keep the organism in phase with its environment. Output signals from the SCN reach the rest of the brain to ensure the synchronization of circadian organization throughout the body.
In the rodent the SCN contains ∼10,000 cells in each nucleus. These cells are not homogenous and express a large number of different peptides and enzymes (van den Pol and Tsujimoto, 1985). Studies using tract-tracing and fos induction in response to light indicate that the nucleus is also differentiated on the afferent side, with retinal and photic input reaching a subset of SCN cells largely restricted to the ventrolateral aspect in rats and extending more dorsally in hamsters (Johnson et al., 1988; Rea, 1989; Kornhauser et al., 1990; Rusak et al., 1990; Morin, 1994). Although it seems that SCN cells are heterogeneous, lesion studies suggest that many SCN cells function as pacemakers (see Discussion in van den Pol and Powley, 1979), and recording of electrical activity in individual SCN neurons (Welsh et al., 1995) indicates that several SCN cell types are capable of sustaining circadian rhythms. The present report represents a multipronged strategy to localize SCN pacemakers that control locomotor rhythmicity.
There is a compact subnucleus of calcium-binding protein calbindin-D28K (CaBP)-positive cells in the core of the hamster SCN (Fig. 1; Silver et al., 1996). Within this region is a population of substance P cells (Morin et al., 1992), forming a subset of the CaBP cells with most substance P cells expressing CaBP (78%) and only 8% of the CaBP cells expressing substance P (Silver et al., 1996). Approximately 79% of the CaBP cells are fos-positive in response to a light pulse (Silver et al., 1996). We now report that circadian rhythmicity is retained after partial lesions of the SCN if this region is spared but not if this region is ablated. Furthermore, transplantation of parts of the SCN into SCN-lesioned animals rescues circadian rhythms if the CaBP subnucleus is present but not when it is absent.
Fig. 1.
Photomicrograph of a sagittal brain section showing the location of the CaBP subnucleus (arrow). This subregion is highly localized in the caudal SCN, making it possible to ablate this part of the nucleus but to leave other compartments intact. Scale bar, 200 μm.
MATERIALS AND METHODS
Subjects and housing. Subjects were wild-type (free-running period of ∼24 hr) adult male or female LVG hamsters (Mesocricetus auratus) and male mutant hamsters heterozygous and homozygous for the tau mutation (free-running periods of ∼22 and 20 hr, respectively). Animals were housed individually in translucent polypropylene cages (48 × 27 × 20 cm) and provided with access to food and water ad libitum. The room was kept at ∼23°C and was equipped with a white noise generator (91 dB sound pressure level) to mask environmental noise. At ∼7 weeks of age, animals were transferred from a light/dark cycle (14 hr light, 10 hr dark) to constant darkness (DD) in cages equipped with a running wheel (17 cm diameter). A dim red light (<1 lux; Delta 1, Dallas, TX) allowed for maintenance. All experimental protocols conformed to the Institutional Animal Care and Use Committee guidelines of Columbia University.
Experiment 1: lesion study. After 1–2 weeks in DD, hamsters (110–120 gm) were anesthetized (100 mg/kg sodium pentobarbital) and placed in a stereotaxic instrument, and bilateral electrolytic lesions were made using a Grass Instruments (Quincy, MA) LM-5 lesion maker and stainless steel 00 electrodes, insulated with Epoxylite (The Epoxylite Corp., Irvine, CA) except at the tip (0.25 mm). Current was passed for either 10 (n = 24) or 20 (n = 45) sec at 0.55 mA. Lesions were placed 0.8 mm anterior to bregma, 0.1 mm lateral to the midline, and 7.9 mm below the dura. Activity was monitored for 4 weeks in animals that sustained rhythmicity and for 12 weeks in animals that became arrhythmic after surgery.
Experiment 2: transplantation study. Locomotor activity of tau mutant hamsters was monitored for 1–3 weeks. Animals were then given SCN lesions as described above, using a 20 sec current (see above). Hamsters (n = 36) that had become arrhythmic after SCN lesions were used as host animals for transplants.
Timed pregnant female hamsters (Charles River Laboratories, Wilmington, MA) were received on gestation day 12 and housed in DD. Hypothalamic grafts were taken from pups on the day of birth. Brains were dissected out and placed on a sterile Petri dish. Vibratome slices were cut at 500 μm in either the coronal or sagittal plane. The sections were placed in sterile saline on a cold sterile microscope slide for visualization of the SCN under an inverted light microscope (LeSauter et al., 1996). On coronal sections, the SCN was cut with a scalpel blade at an angle of ∼45° (right side; 315° left side) to separate the dorsomedial and ventrolateral parts of the nucleus. Next, two additional cuts were made at the outer limit of the SCN. On sagittal slices, the SCN was cut in half, separating the rostral and caudal SCN. Then, the other cuts were made to separate the SCN from the rest of the brain. Tissue from three donors was pooled in a drop of saline and implanted via a modified 20 gauge needle, which was lowered 7.6 mm below dura, using the opening in the skull made during the lesion.
After recovery from anesthesia, the animals were returned to their cages. Activity was monitored for 16–20 weeks after transplantation.
Perfusion and histology. Hamsters were heavily anesthetized (pentobarbital, 200 mg/kg) and perfused intracardially with 150 ml of 0.9% saline followed by 300–400 ml of 4% paraformaldehyde in 0.1m phosphate buffer, pH 7.3. Brains were post-fixed for 18–24 hr at 4°C and cryoprotected in 20% sucrose in 0.1m phosphate buffer overnight. Coronal sections were cut on a freezing microtome and processed as free-floating sections.
For the lesion and transplant studies, every fourth brain section was immunostained for neurophysin-associated vasopressin (NP), as a marker for vasopressin in the SCN, and vasoactive intestinal polypeptide (VIP), and every other section was immunostained for CaBP. Polyclonal antisera against NP and VIP (Incstar, Stillwater, MN) were used at dilutions of 1:10,000. Preabsorption of each milliliter of diluted antiserum for 24 hr at 4°C with the purified peptides NP and VIP (Peninsula Laboratories, Belmont, CA) completely eliminated all immunoreaction product. Monoclonal CaBP antibody (Sigma, St. Louis, MO) was used at 1:20,000. Antisera were detected using appropriate biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA) and an avidin–biotin–HRP conjugate (Vector Laboratories) with 3,3′-diaminobenzidine (Polysciences, Warrington, PA) as the chromogen. Sections were mounted on glass slides, dehydrated, cleared, and coverslipped.
Analysis of the SCN. To assess the SCN in lesions and grafts, we took advantage of the fact that the SCN has several unique features when compared with the adjacent hypothalamus. VIP and VP cells are present in a characteristic topographical arrangement (also seeLeSauter et al., 1996; Moore and Silver, 1998) and are absent in the adjacent hypothalamus. Densely packed CaBP-IR cells with very fine efferents in the SCN lie within the NP–VIP region, in an “island” surrounded by a region lacking this peptide (Fig. 1;Silver et al., 1996). Dispersed CaBP-IR cells are seen in the adjacent hypothalamus. CaBP cells of SCN and extra-SCN origin were distinguished by their size: it is known that “… SCN cells are the smallest neurons in the hypothalamus, and among the smallest in the brain” (van den Pol, 1991). CaBP cells of the SCN were significantly smaller than those of adjacent hypothalamic regions (overallF(4,195) = 107.5; p < 0.0001). The perimeter of CaBP cells of the SCN was 33.6 ± 0.4 μm (mean ± SEM; n = 40 cells). CaBP cells in extra-SCN sites in adjacent hypothalamus were significantly smaller, as follows: rostral (42.1 ± 5.5 μm;n = 40; p < 0.0001), lateral (42.4 ± 0.5 μm; n = 40; p < 0.0001), and caudal (47.8 ± 5.6 μm; n = 40;p < 0.0001). The SCN CaBP cells within the grafts were slightly smaller than in the intact SCN (31.0 ± 0.5 μm,n = 40; p = 0.005).
Based on these features, we used the following criteria to identify the SCN in transplants and at the lesion site. The presence of overlapping regions of VIP and VP-IR in adjacent sections definitively denoted the presence of SCN tissue at the lesion site or in grafts. The determination of whether CaBP cells originated in the SCN or adjacent hypothalamus was determined by measuring cell size. Thus, the presence of these peptides in their characteristic distribution and distinguishing cell size, taken together, were used to analyze the SCN lesion and graft.
Brain sections were captured using a CCD video camera (XC77; Sony, Tokyo, Japan) attached to an Olympus (Tokyo, Japan) BH2 microscope using NIH Image (version 1.61). In grafted animals, the size of the transplanted SCN was measured by taking the overall area of the section with the largest NP- or VIP-IR plexus (used for Fig. 5). This system was also used to measure size of CaBP cells (described above) and SCN volume after partial ablation. The size of the cells in these different regions was compared by ANOVA with post hoc Fisher’s PLSD for comparison between groups.
Fig. 5.
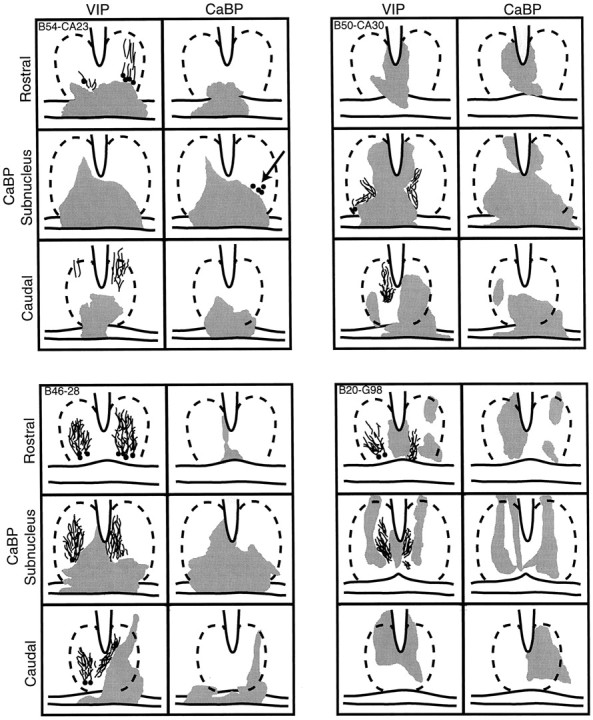
Schematics of the caudal aspect of the SCN depicting the area of damage (gray) in hamsters with partial SCN lesions in one animal with sparing and three animals with ablation of the CaBP subregion. The approximate outline of the SCN is indicated by broken lines. For each animal, the drawings show sections stained for VIP and CaBP. For each peptide, the schematics are shown at three levels, separated by 200 μm through areas rostral to, centered in, and caudal to the CaBP subnucleus. Animal B54-CA23 (top left panel) had a few CaBP cells remaining on one side (arrow). This animal was rhythmic after the lesion. For the other three animals, much of the SCN is spared, and VIP cells and fibers were seen, but CaBP-IR cells were not detected. These three animals were arrhythmic after the lesion.
To count the number of CaBP profiles (what is seen of a cell in a histological section; Coggeshall and Lekan, 1996) after a partial SCN lesion or a transplant cells were counted within the area stained for VIP and VP on adjacent sections. The presence or absence of a rhythm and the absolute power of the restored rhythm were correlated with the number of SCN CaBP profiles, the number of SCN clusters, and the size of the clusters.
Acquisition of behavioral data. Locomotor activity was monitored continuously using a computer-based data acquisition system (Dataquest; Data Sciences, St. Paul, MN). The power of the rhythm was assessed using Fourier analysis (Dataquest). An animal was considered rhythmic when the highest peak occurred at ∼1 cycle/d, with absolute power of at least 0.005 mV/Hz.
Brain sections were analyzed by an investigator blind to the experimental groups and to the behavioral aspects of the results.
RESULTS
Experiment 1: partial SCN lesions
In 51 of 69 subjects, the SCN were partially (n = 47) or fully (n = 4) ablated on both sides (summarized in Table 1). The remaining 18 animals were not used in the study, because the lesion either missed the SCN completely (n = 14) or ablated the SCN on one side partially (n = 3) or totally (n = 1), leaving the other side completely intact. These animals remained rhythmic.
Table 1.
The peptidergic analysis of animals with partial lesions of the SCN shows that the presence of locomotor rhythmicity is dependent on the presence of the CaBP subnucleus
| n | NP | VIP | CaBP | Locomotor rhythmicity |
|---|---|---|---|---|
| 24 | + | + | + | + |
| 21 | + | + | − | − |
| 1 | + | − | − | − |
| 1 | + | + | + | − |
| 4 | − | − | − | − |
n, Number of animals; +, presence of the peptide or behavior; −, absence of the peptide or behavior.
Twenty-four animals with sparing of one (n = 18) or both CaBP (n = 6) subnuclei remained rhythmic. In marked contrast, animals with lesions that bilaterally destroyed the subregion of CaBP cells were arrhythmic, although sparing of other SCN tissue occurred (n = 23).
The extent of SCN damage is shown for six animals with partial SCN lesions. Animals with a lesion ablating the CaBP cells and sparing of NP and VIP cells and fibers are shown in Figures2 (B26-V34) and 3 (B56-CA29). The behavioral analysis for these animals (Fig. 4) shows that both are arrhythmic after the lesion. Figure 5shows schematics from images captured on the computer of the area of SCN damage in adjacent sections stained for VIP and CaBP for four animals. The first animal (top left panel, B54-CA23) has sparing of VIP cells and fibers. A few CaBP cells are seen unilaterally in the CaBP subnucleus. This animal retained locomotor rhythmicity. The other three animals depicted in Figure 5 all had sparing of VIP cells, but no CaBP immunoreactivity could be detected in the core of the SCN. These three animals were arrhythmic.
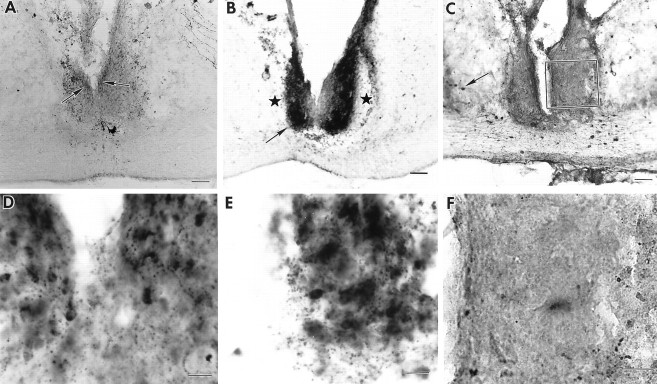
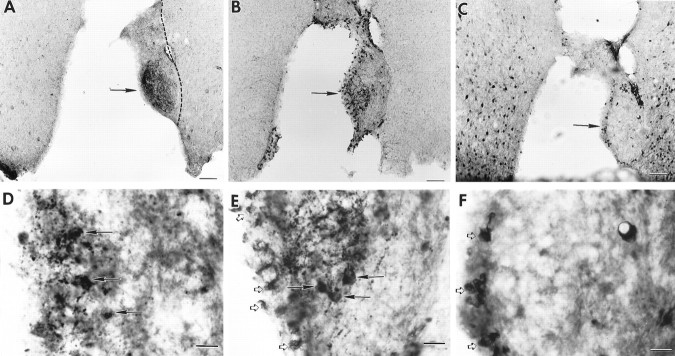
Fig. 3.
Photomicrographs of coronal brain sections showing a partial SCN lesion in animal B56-CA29 (behavior shown in Fig.4B). A–C, Adjacent low-power sections stained for NP, VIP, and CaBP, respectively.A, B, Asterisks indicates partial SCN ablation at the ventral aspect. The arrowsin A and B point to the region shown in high power in D and E, respectively.C. No CaBP cells could be detected in the SCN. Thebox denotes the region that is shown in high power inF. D–F, High-power photomicrographs. The animal lost locomotor rhythmicity (Fig. 4B) after the lesion, although NP (D) and VIP (E) cells (arrows) and fibers were spared. F, No CaBP cells could be detected in the SCN. Scale bars: A–C, 200 μm; D, F, 20 μm; E, 10 μm.
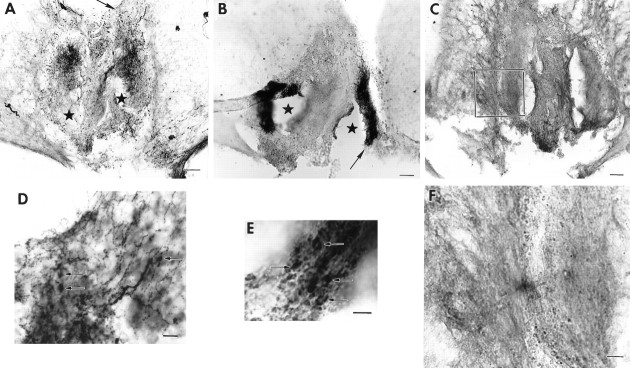
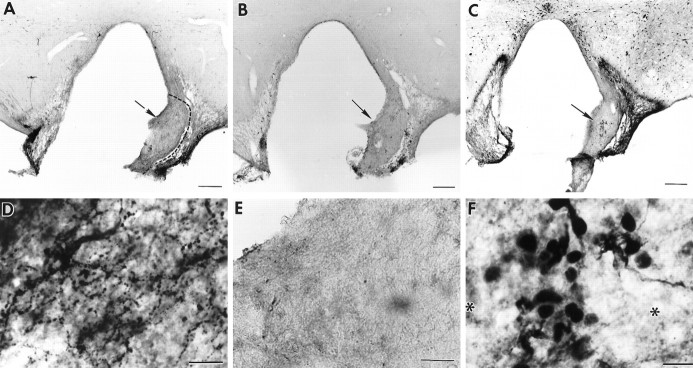
Fig. 2.
Photomicrographs of coronal brain sections showing a partial SCN lesion in animal B26-V34. Much of the SCN is spared in this animal, although circadian rhythmicity is lost after the lesion (behavior shown in Fig. 4A).A–C, Adjacent low-power sections stained for NP, VIP, and CaBP, respectively. A. Low-power view of a section through the SCN stained for NP (arrowsindicate NP cells in the dorsomedial SCN). B. The lateral aspect of the SCN is damaged (asterisks). Thearrow points to the region shown in high power inE. C, No immunostained cells could be detected in the SCN, although sparse CaBP cells can be seen in the adjacent hypothalamus (arrow). The boxdenotes the region that is shown in high power in F.D–F, High-power photomicrographs of sections stained for NP, VIP, and CaBP, respectively. No CaBP cells could be detected in the SCN. Scale bars:A–C, 100 μm; D,E, 10 μm; F, 20 μm.
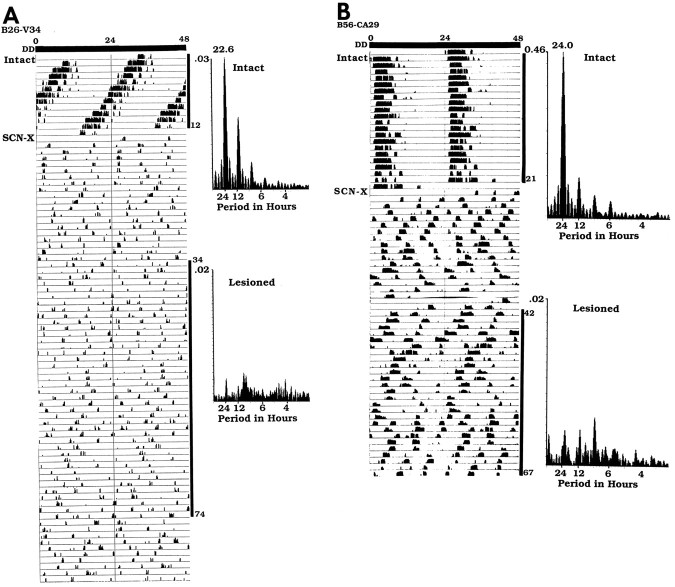
Fig. 4.
Wheel running rhythm of the animals for which anatomy are presented in Figures 2 and 3. To facilitate inspection of the rhythms, the daily activity is plotted twice on a 48 hr time scale. The animals received an SCN lesion (SCN-X) at the point indicated on theleft of the actogram. Spectral analyses of the data are shown on the right of the actograms. The black vertical bars on the right of the actograms indicate the days (intact and lesioned) for which the analyses were done. A, The intact heterozygote tau mutant hamster B26-V34 had a period of 22.6 hr (days 1–12). After the partial SCN lesion, it became arrhythmic (analyses shown for days 34–74).B, The intact wild-type hamster B56-CA29 had a period of 24 hr (days 1–12). The animal became arrhythmic after a partial lesion of the SCN ablating the CaBP subnucleus (analysis shown for days 42–67).
In 21 of 23 arrhythmic animals, both VIP and NP could be seen in the SCN. Dramatically, one animal (B26-V34; Fig. 2) with sparing of ∼67% of the SCN but with no detectable CaBP cells within the SCN was arrhythmic. In 3 of the foregoing 21 animals, CaBP cells overlapped with sparse NP fibers but not with VIP. These CaBP cells were from the outside of the SCN, because they were much larger than the SCN CaBP cells (B52-Z27: 39.4 ± 1.2 μm; n = 20 cells;p < 0.0001; B41–27T: 41.5 ± 1.7 μm;n = 20 cells; p < 0.0001). Additionally, 1 animal had NP, but no VIP cells or fibers could be detected in the SCN, although VIP-IR could be seen in other parts of the brain. Finally, one animal with sparing of a few CaBP cells was arrhythmic. No area of the SCN other than the CaBP subnucleus was consistently damaged in arrhythmic animals. Not surprisingly, animals (n = 4) with complete SCN lesions expressed no circadian rhythms. All animals lacking SCN CaBP-positive cells remained arrhythmic.
Experiment 2: SCN transplant
Transplants were scored in two ways. The first analysis was based on staining for VIP, NP, and CaBP peptides in the graft (Table2). The results reveal that nine half-SCN grafts restored donor-specific rhythmicity. Of these, five contained NP, VIP, and CaBP, whereas VIP was not detected in four animals. In summary, of the 10 animals with CaBP-positive cells in the graft, 9 expressed circadian rhythmicity, whereas of the six animals lacking CaBP cells, none was rhythmic.
Table 2.
Transplant of half-SCN punches indicates that the restoration of rhythmicity is dependent on the presence of tissue from the CaBP subregion
| NP | VIP | CaBP | Recovered | Nonrecovered |
|---|---|---|---|---|
| + | + | + | 5 | 0 |
| + | − | + | 4 | 1 |
| + | + | − | 0 | 3 |
| + | − | − | 0 | 3 |
+, Presence of the peptide; −, absence of the peptide.
For the second analysis, grafts were analyzed in terms of type of donor tissue (for coronal sections, dorsomedial vs ventrolateral SCN; for sagittal sections, rostral or caudal SCN). Although some grafts within each of these categories restored rhythmicity, this analysis was not predictive of recovery of function (Table3). (Note: for five animals used in the analysis CaBP-IR was not examined, and these animals are absent from Table 2.)
Table 3.
Transplant of half-SCN punches indicates that the restoration of rhythmicity cannot be predicted by the SCN region dissected for implantation
| Graft type | Recovered | Nonrecovered |
|---|---|---|
| Dorsomedial | 5 | 2 |
| Ventrolateral | 5 | 3 |
| Rostral | 2 | 1 |
| Caudal | 1 | 2 |
Fifteen grafted animals were not used in the analysis of transplants for the following reasons: the host SCN was not totally ablated (n = 9), and the animal expressed the host rhythm; no graft was present (n = 5); and one animal had a graft that did not express any peptide characteristic of the SCN. The latter six animals were arrhythmic.
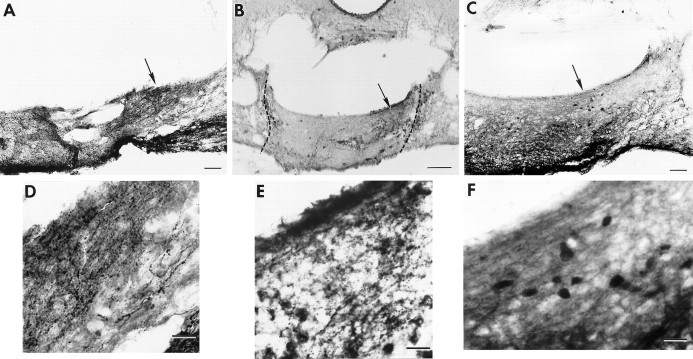
Two examples of recovered animals are shown in Figures6-8. The animal (B28-Q66) shown in Figure 6 has a graft (situated slightly caudal to the lesioned SCN site) that contains a plexus of NP and VIP cells and fibers and, at the same level, a small cluster of CaBP cells surrounded by an area without CaBP cells. Behavioral analysis (Fig. 8A) shows that before lesion, this animal had a free-running period of 21.9 hr, became arrhythmic after lesion placement, and then recovered 21 d after transplantation with the donor period of 24.8 hr. The animal (B33-Q67) shown in Figure 7 has a graft situated caudal to the lesioned SCN site; the graft contains a plexus of NP fibers with a few cells but no VIP and, slightly ventral to the NP fibers, a large cluster of CaBP cells surrounded by an area without CaBP cells. Behavioral analysis (Fig.8B) shows that the animal was rhythmic with a free-running period of 22.6 hr, was lesioned and became arrhythmic, and then recovered ∼28 d after transplantation with the donor period of 24.0 hr.
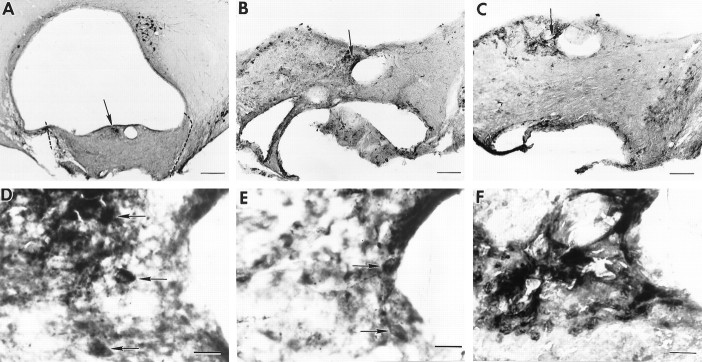
Fig. 6.
Photomicrographs of coronal brain sections showing the transplant containing the SCN in animal B28-Q66 (behavior shown in Fig. 8A). A, The graft lies caudal to the lesion site. There is a plexus of NP staining (arrow) indicating the presence of the donor SCN.B, In a section 50 μm from A, VIP fibers (arrow) overlap the region of the NP fiber plexus; the graft borders are indicated by a dashed line. C, Section adjacent to Bshowing CaBP cells at the same level as the NP and VIP fibers.D–F, Higher magnifications of the areas marked by arrows in A–C, respectively. Scale bars: A–C, 200 μm;D–F, 20 μm.
Fig. 7.
Photomicrographs of coronal brain sections showing the transplant containing the SCN in animal B33-Q67 (behavior shown in Fig. 8B). A, The graft (borders are indicated by a dashed line) lies caudal to the lesion site. There is a plexus of NP staining (arrow) indicating the presence of the donor SCN. B, In a section 50 μm from A, no VIP plexus was seen within the graft. C, Section adjacent to Bshowing a cluster of CaBP cells at the same level as the NP plexus.D–F, Higher magnifications of the areas marked by arrows in A–C, respectively. D, Plexus of NP fibers within the graft.E, Absence of VIP staining. F, CaBP subnucleus within the graft, surrounded by a space devoid of CaBP cells (asterisks). Scale bars: A–C, 200 μm;D–F, 20 μm.
Fig. 8.
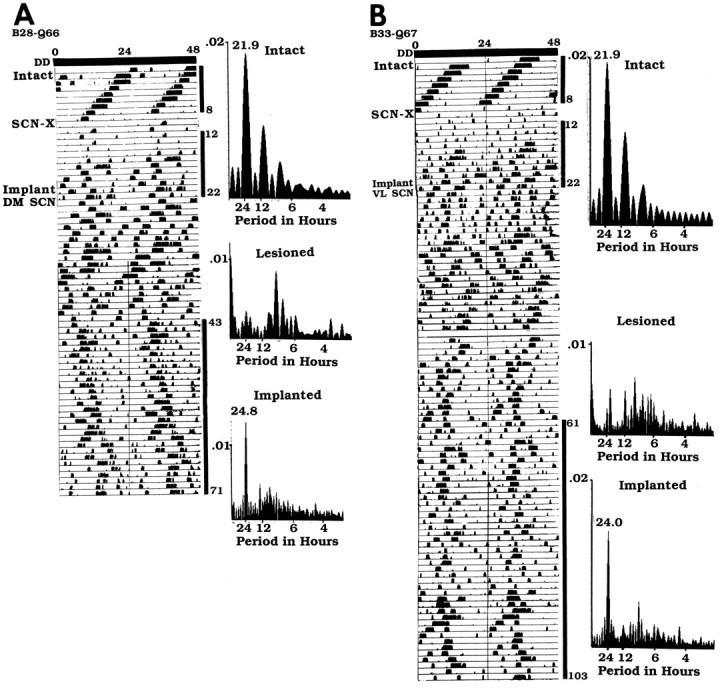
Wheel running rhythm of heterozygote tau mutants for which the anatomical data are presented in Figures 6 and 7. The animals received an SCN lesion (SCN-X) and received a half-SCN transplant at the points indicated on theleft of the actogram (see Fig. 4 legend for further details). A, The intact hamster B28-Q66 had a free-running period of 21.9 hr (days 1–8) and became arrhythmic after an SCN lesion (days 12–22). After transplantation, it recovered with the donor period of 24.8 hr (days 43–71). B, The intact hamster B33-Q67 had a period of 21.9 hr (days 1–8). After an SCN lesion, it became arrhythmic (days 12–22). After transplantation, it recovered with the donor period of 24.0 hr (days 61–103).
Of the seven half-SCN grafts that did not restore rhythmicity, six contained no detectable SCN CaBP cells but had NP and/or VIP. In one arrhythmic animal the graft contained NP and CaBP but no VIP.
Two examples of grafts that did not restore rhythmicity are shown in Figures 9-11. The animal (B46–28T) shown in Figure 9 has a graft (situated caudal to the lesioned SCN site), which contains a large plexus of NP and VIP cells and fibers and, at the same level, no CaBP cells. Behavioral analysis (Fig. 11A) shows that the animal was rhythmic with a free-running period of 22.6 hr, was lesioned and became arrhythmic, and did not recover rhythmicity after transplantation. The animal (B39-Q70) shown in Figure 10 has a graft situated slightly caudal to the lesioned SCN site, which contains a plexus of NP cells and fibers and a few VIP cells and fibers but no CaBP cells. Behavioral analysis (Fig.11B) shows that the animal was rhythmic with a free-running period of 22.6 hr and was lesioned and became arrhythmic, but did not recover a locomotor activity rhythm after transplantation.
Fig. 9.
Photomicrographs of coronal brain sections of the SCN within the grafts in animal B46–28T (behavior shown in Fig.11A). A, The graft (borders are indicated by a dashed line) lies caudal to the lesion site. There is a plexus of NP staining (arrow) indicating the presence of the donor SCN. B, In a section 50 μm from A, a plexus of VIP fibers (arrow) lies at the same level as the NP plexus.C, The graft lacks CaBP cells, although many CaBP cells can be seen in the host’s hypothalamus.D–F, Higher magnifications of the areas marked by arrows in A–C, respectively. D, E, Plexus of NP and VIP cells (arrows) and fibers within the graft (open arrows indicate nonspecific immunoreactivity within the ventricular epithelium in E). F, No CaBP cells can be detected within the brain parenchyma at the level of the NP and VIP plexi (open arrows indicate nonspecific immunoreactivity within the ventricular epithelium). Scale bars:A–C, 200 μm;D–F, 20 μm.
Fig. 10.
Photomicrographs of coronal brain sections of the SCN within the grafts in animal B39-Q70 (behavior shown in Fig.11B). A, The graft (borders are indicated by a dashed line) lies caudal to the lesion site. A plexus of NP staining (arrow) indicates the location of the donor SCN. B, In a section 50 μm fromA, there are a few VIP cells and fibers (arrow) at the same level as the NP plexus.C, The SCN region lacks the cluster of CaBP cells, although sparse CaBP cells can be seen in other parts of the graft.D–F, Higher magnifications of the areas marked by arrows in A–C, respectively. D, E, Plexus of NP and VIP cells (arrows) and fibers within the graft.F, Although there is some nonspecific staining around the edges of ventricles and blood vessels in the area where NP and VIP are present, no CaBP cells could be detected in the SCN. Scale bars: A, 200 μm; B, C, 100 μm; D–F, 20 μm.
Fig. 11.
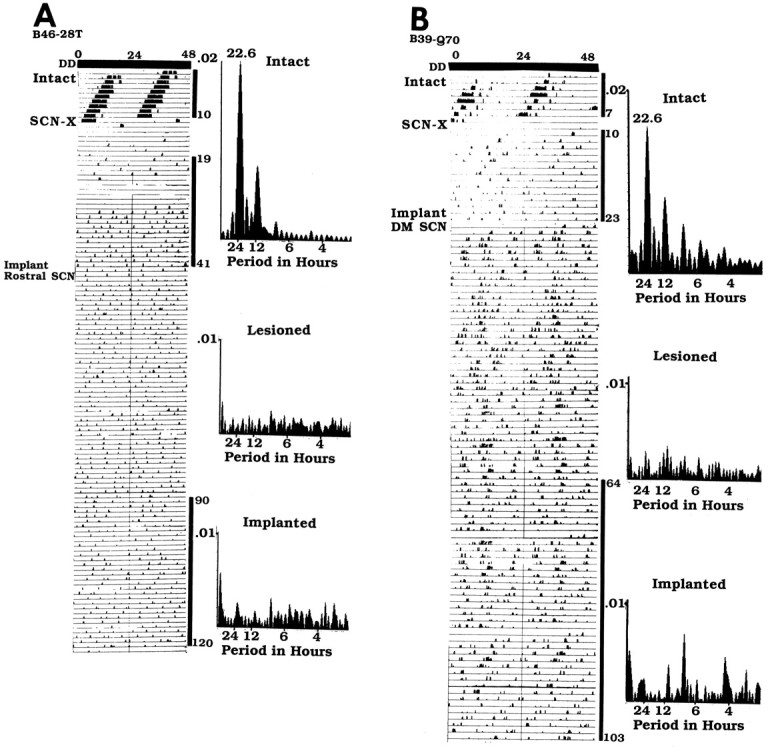
Wheel running rhythm of the heterozygotes that did not recover rhythmicity after transplantation (anatomical data are presented in Figs. 9, 10). The animals were lesioned (SCN-X) and received a half-SCN transplant at the points indicated on the left of the actogram (details of legend and analysis shown in Fig. 4). A, The intact hamster B46–28T had a free-running period of 22.6 hr (days 1–10). After an SCN lesion, it became arrhythmic (days 19–41).B, Intact animal B39-Q70 had a free-running period of 22.6 hr (days 1–7). It became arrhythmic (days 10–23) after SCN ablation.
Correlation between rhythmicity and number of CaBP cells
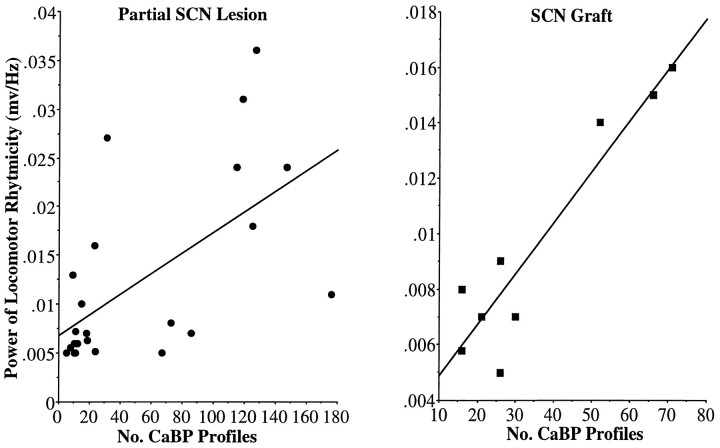
In animals expressing circadian rhythms in which the CaBP subregion of the SCN was present (summarized in Tables 1, 3), the absolute power of the locomotor rhythm was significantly correlated with the number of SCN CaBP cells remaining in the partially lesioned SCN (r = 0.61; n = 23;p = 0.002; in one animal, all the CaBP cells could not be counted, because a section was broken) and with the number of SCN CaBP cells in the graft (r = 0.94; n = 9; p = 0.0002; Fig.12).
Fig. 12.
There is a positive correlation between the number of SCN CaBP profiles and the absolute power of the rhythm in animals with a partial lesion of the SCN (A;r = 0.61; p = 0.002) and in the lesioned-grafted animals (B; r = 0.94; p = 0.0002).
There was no significant correlation between the power of the rhythm and the number of clusters (r = 0.06; n= 9; p = 0.87) or the size of the SCN plexus (r = 0.33; n = 9; p = 0.38) within the graft of animals with a restored rhythm. Nor was there a correlation between restoration of rhythmicity and number of SCN clusters (r = 0.23; n = 16;p = 0.37) or size of the clusters (r = 0.04; n = 16; p = 0.89) within the grafts.
DISCUSSION
Cells in the CaBP subnucleus control locomotor rhythmicity
Previous work indicated that “… the SCN operate in an ‘all-or-none’ manner and suggested that even a small part of the SCN is capable of driving circadian rhythms” (van den Pol and Powley, 1979, page 323). This hypothesis has been supported in numerous studies indicating that as long as ∼25% of the SCN survive ablation, circadian rhythms are sustained (Rusak, 1977; Davis and Gorski, 1988;Harrington et al., 1993). The present results lead us to the conclusion that there is regional specialization of function, suggesting a paradigm shift in our conceptualization of the SCN. Specifically, the present lesion and transplant studies indicate that cells within the CaBP subnucleus of the hamster SCN are necessary and sufficient for the control of circadian locomotor rhythms. Second, other regions are not sufficient to sustain rhythmicity of locomotor activity, even when as much as 67% of SCN tissue remains intact after the lesion, as long as the CaBP subregion is damaged. Finally, the strength of the rhythm is proportional to the amount of CaBP subnucleus tissue remaining after the lesion or present in the grafts. Two animals (one lesioned and one grafted) in which few CaBP cells were detected were arrhythmic. The absence of rhythmicity may be attributable to any number of factors, as has previously been reported in animals bearing whole SCN grafts (Aguilar-Roblero et al., 1994). More importantly, no animals lacking SCN CaBP cells, after lesion or in the graft, expressed locomotor rhythmicity.
These results are consistent with previous findings. We show that analysis of which region of the SCN has been lesioned (dorsal vs ventral or rostral vs caudal) did not suggest regional specialization. However, the use of antibodies to CaBP, NP, and VIP as markers has permitted a detailed analysis of the locus and extent of SCN lesion sites and has led to new conclusions. It is noteworthy that loss of rhythms was previously reported after sparing of part of the SCN (van den Pol and Powley, 1979). Also, small electrolytic lesions altered both the circadian rhythm of locomotor activity and the normal temporal pattern of gonadal regression on short days, whereas other lesions affected one parameter but not the other (Pickard and Turek, 1985). In their study of hamsters, detailed histological analysis of histochemically stained sections examining retinal afferents did not reveal regional specificity of function. However, the detailed description of each animal bearing partial SCN lesions provided by these authors suggests that animals that sustained locomotor rhythms had lesions sparing the caudal aspect of the SCN, the region of CaBP cells. Similar dissociation of various circadian rhythms has been reported in rats (Satinoff and Prosser, 1988).
The possibility that all SCN cells are pacemakers regulating circadian rhythmicity remains controversial
Welsh et al. (1995) cultured dispersed rat SCN cells on fixed microelectrode arrays to record spontaneous action potentials from individual neurons. The presence of prominent circadian rhythms in firing rate suggested that the SCN contains a large population of autonomous single-cell circadian oscillators (or clocks). The high proportion of clock cells in this preparation led to the conclusion that the capacity for generating circadian oscillations could not be restricted to a specific peptidergic cell type. On the other hand,Mirmiran et al. (1995) recorded spontaneous extracellular discharges of single SCN neurons in long-term cultured organotypic SCN slices. In this preparation, not all SCN neurons showed circadian firing rhythms. Furthermore, neurons recorded simultaneously from the same SCN slice were not necessarily in synchronization with each other. These authors concluded that not all SCN neurons are pacemakers. Consistent with this hypothesis is evidence that when the ventrolateral and dorsomedial areas of the rat SCN are separated in vitro, the former maintains a circadian pattern of firing, but the latter does not (Tcheng and Gillette, 1990)
Each SCN contains 8000–10,000 cells in rats (Güdner, 1976; van den Pol and Tsujimoto, 1985). Detailed cell counts indicate the presence of the following peptidergic types: VIP, 2081; vasopressin, 3176; gastrin-releasing peptide, 1140; calretinin, 1370; neurotensin, 283; substance P, 168; and somatostatin, 302 (Speh et al., 1994). In hamsters, the CaBP cells number ∼250 per nucleus (LeSauter and Silver, 1995). If all SCN cells are pacemakers, and the cells of the CaBP region alone are necessary for the regulation of locomotor rhythmicity, the question arises of what function is served by the remaining SCN cells.
Some cells within the SCN may be specialized for intranuclear communication. It has been suggested that distinct cell populations exhibit axonal arbors that are largely confined to the nucleus, because an unusual feature of the rat SCN is the large number of local circuit neurons in the nucleus (Moore and Card, 1985; Buijs et al., 1994). Or intra-SCN cells may be specialized to provide afferent or efferent information to and from pacemaker cells (Moore, 1997). Alternatively, different cells (or cell clusters) subserve different functions. That the SCN has separable populations of oscillators is suggested fromin vitro work. Thus, NMDA differentially shifted the phase of VIP and vasopressin rhythms measured in the efflux, indicating control by separate circadian oscillators (Shinohara et al., 1995). That subsets of SCN cells regulate different rhythms is also suggested by accumulating work characterizing specialized SCN afferents and efferents. Thus, SCN afferent connections are topographically organized (Moga and Moore, 1997). Also, there is a monosynaptic pathway between the SCN and preoptic area gonadotropin-releasing hormone neurons (Huhman and Van der Beek, 1996; van der Beek et al., 1997). The suggestion has recently been made that the SCN has two subdivisions, a “core” and a “shell” (Moore, 1996, 1997). Cells of the SCN shell are small with sparse dendritic arbors, whereas the dendritic arbors are larger and more extensive in the SCN core. Core neurons receive retinal afferent input and project more extensively within the SCN than do shell neurons. Moore (1996) further suggests that species differences in the SCN tend to occur in shell rather than in core regions. Thus, all species have VIP in the ventrolateral region and vasopressin in the dorsomedial region. However, humans have a large population of neurotensin neurons in the dorsolateral area; this is not seen in other species. The CaBP region in hamster, which also contain substance P cells (Morin et al., 1992) and gastrin-releasing peptide cells (our unpublished data), fits the definition of a core area in that it receives direct retinal input (Bryant et al., 1996; Silver et al., 1996), although the projections of these cells are not known. The applicability of our findings to other species cannot be ascertained at this time. Calcium-binding proteins of the EF-hand family form a large group of ∼200 members (Heizmann and Hunziker, 1991); functional properties similar to those of hamster CaBP cells remain to be determined.
Which properties of pacemakers are present in the CaBP subregion?
It is generally agreed that there are three components of a circadian system necessary for regulation of rhythmicity: an input pathway connecting the clock to the external environment, a central pacemaker, and an output pathway. As noted above, we have documented that the CaBP-IR cells receive direct retinal input (Bryant et al., 1996; Silver et al., 1996). The present results indicate that ablation of cells of this region results in loss of locomotor rhythmicity, and their replacement reinstates this response. It remains to be established whether cells of this region are pacemakers regulating all circadian responses and/or whether their projections reach efferent targets in the SCN and/or extra-SCN region to regulate locomotor rhythmicity. It also remains to be determined whether pacemakers regulating circadian rhythmicity are the CaBP cells themselves or other as yet unidentified neurons lying within this subnucleus.
Footnotes
This work was supported by Air Force Office of Scientific Research Grant F49620 and National Institutes of Health Grant NS-37919 (to R.S.). We thank Dr. M. Ralph for the gift of the tau mutant hamsters and Patricia Romero for technical assistance. We also thank Drs. A.-J. Silverman and L. P. Morin for their helpful comments on an earlier version of this manuscript.
Correspondence should be addressed to Dr. Rae Silver, Psychology Department, Mail Code 5501, Columbia University, 1190 Amsterdam Avenue, New York, NY 10027.
REFERENCES
- 1.Aguilar-Roblero R, Morin LP, Moore RY. Morphological correlates of circadian rhythm restoration induced by transplantation of the suprachiasmatic nucleus in hamsters. Exp Neurol. 1994;130:250–260. doi: 10.1006/exnr.1994.1203. [DOI] [PubMed] [Google Scholar]
- 2.Buijs RM, Hou YX, Shin S, Renaud LP. Ultrastructural evidence for intra- and extranuclear projections of GABA-ergic neurons of the suprachiasmatic nucleus. J Comp Neurol. 1994;340:381–391. doi: 10.1002/cne.903400308. [DOI] [PubMed] [Google Scholar]
- 3.Bryant DN, LeSauter J, Silver R, Romero M-T. Retinal synapses on calbindin-ir cells of the hamster suprachiasmatic nucleus. Soc Neurosc Abstr. 1996;22:1140. [Google Scholar]
- 4.Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Davis FC, Gorski RA. Development of hamster circadian rhythms: role of the maternal suprachiasmatic nucleus. J Comp Physiol. 1988;162:601–610. doi: 10.1007/BF01342635. [DOI] [PubMed] [Google Scholar]
- 6.Dunlap JC. Molecular basis for circadian clocks. Cell. 1998;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 7.Güldner FH. Synaptology of the rat suprachiasmatic nucleus. Cell Tissue Res. 1976;165:509–544. doi: 10.1007/BF00224478. [DOI] [PubMed] [Google Scholar]
- 8.Harrington ME, Rahmani T, Lee CA. Effects of damage to SCN neurons and efferent pathways on circadian activity rhythms of hamsters. Brain Res Bull. 1993;30:655–669. doi: 10.1016/0361-9230(93)90097-u. [DOI] [PubMed] [Google Scholar]
- 9.Heizmann CW, Hunziker W. Intracellular calcium-binding proteins: more sites than insights. Trends Biochem Sci. 1991;16:98–103. doi: 10.1016/0968-0004(91)90041-s. [DOI] [PubMed] [Google Scholar]
- 10.Huhman KL, van der Beek EM. Peptidergic innervation of gonadotropin releasing hormone (GnRH) neurons in female Syrian hamsters. Soc Neurosci Abstr. 1996;22:1141. [Google Scholar]
- 11.Kornhauser JM, Nelson DE, Mayo KE, Takahashi JS. Photic and circadian regulation of c-fos gene expression in the hamster suprachiasmatic nucleus. Neuron. 1990;5:127–134. doi: 10.1016/0896-6273(90)90303-w. [DOI] [PubMed] [Google Scholar]
- 12.Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988;460:297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- 13.LeSauter J, Silver R. Localization of pacemaker cells in the hamster SCN: I. Studies using fos, ablation and mutants. Soc Neurosc Abstr. 1995;21:178. [Google Scholar]
- 14.LeSauter J, Lehman MN, Silver R. Restoration of circadian rhythmicity by transplants of SCN “micropunches.”. J Biol Rhythms. 1996;11:163–171. doi: 10.1177/074873049601100208. [DOI] [PubMed] [Google Scholar]
- 15.Mirmiran M, Koster-Van Hoffen GC, Bos NPA. Circadian rhythm generation in the cultured suprachiasmatic nucleus. Brain Res Bull. 1995;38:275–283. doi: 10.1016/0361-9230(95)00100-s. [DOI] [PubMed] [Google Scholar]
- 16.Moga MM, Moore RY. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J Comp Neurol. 1997;389:508–534. doi: 10.1002/(sici)1096-9861(19971222)389:3<508::aid-cne11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 17.Moore RY. Entrainment pathways and the functional organization of the circadian system. Prog Brain Res. 1996;111:103–119. doi: 10.1016/s0079-6123(08)60403-3. [DOI] [PubMed] [Google Scholar]
- 18.Moore RY. Chemical neuroanatomy of the mammalian circadian system. In: Redfern PH, Lemmer B, editors. Handbook of experimental pharmacology, Vol 125, Physiology and pharmacology of biological rhythms. Springer; Berlin: 1997. pp. 79–93. [Google Scholar]
- 19.Moore RY, Card JP. Visual pathways and the entrainment of circadian rhythms. Ann NY Acad Sci. 1985;453:123–133. doi: 10.1111/j.1749-6632.1985.tb11805.x. [DOI] [PubMed] [Google Scholar]
- 20.Moore RY, Silver R. Suprachiasmatic nucleus organization. Chronobiol Int. 1998;15:475–487. doi: 10.3109/07420529808998703. [DOI] [PubMed] [Google Scholar]
- 21.Morin LP. The circadian visual system. Brain Res Rev. 1994;67:102–127. doi: 10.1016/0165-0173(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 22.Morin LP, Blanchard J, Moore RY. Intergeniculate leaflet and suprachiasmatic nucleus organization and connections in the golden hamster. Vis Neurosci. 1992;8:219–230. doi: 10.1017/s095252380000287x. [DOI] [PubMed] [Google Scholar]
- 23.Pickard GE, Turek FW. Effects of partial destruction of the suprachiasmatic nuclei on two circadian parameters: wheel-running activity and short-day induced testicular regression. J Comp Physiol. 1985;156:803–815. [Google Scholar]
- 24.Rea MA. Light increases Fos-related protein immunoreactivity in the rat suprachiasmatic nuclei. Brain Res Bull. 1989;23:577–581. doi: 10.1016/0361-9230(89)90204-9. [DOI] [PubMed] [Google Scholar]
- 25.Rusak B. The role of the suprachiasmatic nuclei in the generation of circadian rhythms in the golden hamster, Mesocricetus auratus. J Comp Physiol. 1977;118:145–164. [Google Scholar]
- 26.Rusak B, Robertson HA, Wisden W, Hunt SP. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990;248:1237–1240. doi: 10.1126/science.2112267. [DOI] [PubMed] [Google Scholar]
- 27.Satinoff E, Prosser RA. Suprachiasmatic nuclear lesions eliminate circadian rhythms of drinking and activity, but not of body temperature, in male rats. J Biol Rhythms. 1988;3:1–22. doi: 10.1177/074873048800300101. [DOI] [PubMed] [Google Scholar]
- 28.Shinohara K, Honma S, Katsuno Y, Abe H, Honma K. Two distinct oscillators in the rat suprachiasmatic nucleus in vitro. Proc Natl Acad Sci USA. 1995;92:7396–7400. doi: 10.1073/pnas.92.16.7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silver R, Romero MT, Besmer HR, Leak R, Nunez JM, LeSauter J. Calbindin-D28K cells in the hamster SCN express light-induced Fos. NeuroReport. 1996;7:1224–1228. doi: 10.1097/00001756-199604260-00026. [DOI] [PubMed] [Google Scholar]
- 30.Speh JC, Suhan N, Moore RY. Peptide phenotypes in the suprachiasmatic nucleus of the rat. Soc Neurosci Abstr. 1994;20:1601. [Google Scholar]
- 31.Tcheng TK, Gillette MU. Electrical characterization of the ventrolateral and dorsomedial regions of the suprachiasmatic nucleus. Soc Neurosci Abstr. 1990;16:770. [Google Scholar]
- 32.van den Pol AN. The suprachiasmatic nucleus: morphological and cytochemical substrates for cellular interaction. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic nucleus: the mind’s clock. Oxford UP; New York: 1991. pp. 17–50. [Google Scholar]
- 33.van den Pol AN, Powley T. A fine-grained anatomical analysis of the role of the rat suprachiasmatic nucleus in circadian rhythms of feeding and drinking. Brain Res. 1979;160:307–320. doi: 10.1016/0006-8993(79)90427-x. [DOI] [PubMed] [Google Scholar]
- 34.van den Pol AN, Tsujimoto KL. Neurotransmitters of the hypothalamic suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience. 1985;15:1049–1086. doi: 10.1016/0306-4522(85)90254-4. [DOI] [PubMed] [Google Scholar]
- 35.van der Beek EM, Horvath TL, Wiegand VM, van den Hurk R, Buijs RM. Evidence for a monosynaptic pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopical studies. J Comp Neurol. 1997;384:569–579. doi: 10.1002/(sici)1096-9861(19970811)384:4<569::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]