Abstract
Neostriatal cholinergic interneurons fire irregularly but tonicallyin vivo. The summation of relatively few depolarizing potentials and their temporal sequence are thought to underlie spike triggering and the irregularity of action potential timing, respectively. In these experiments we used whole-cell, cell-attached, and extracellular recording techniques to investigate the role of spontaneous synaptic inputs in the generation and patterning of action potentials in cholinergic interneurons in vitro. Cholinergic cells were spontaneously active in vitro at 25 ± 1°C during whole-cell recording from 2 to 3 week postnatal slices and at 35 ± 2°C during cell-attached and extracellular recording from 3 to 4 week postnatal slices. A range of firing frequencies and patterns was observed including regular, irregular, and burst firing. Blockade of AMPA and NMDA receptors altered neither the firing rate nor the pattern, and accordingly, voltage-clamp data revealed a very low incidence of spontaneous EPSCs. GABAAreceptor antagonists were also ineffective in altering the spiking frequency or pattern owing to minimal inhibitory input in vitro. Functional excitatory and inhibitory inputs to cholinergic cells were disclosed after application of 4-aminopyridine (100 μm), indicating that these synapses are present but not active in vitro. Blockade of D1 or D2 dopamine receptors or muscarinic receptors also failed to influence tonic activity in cholinergic cells. Together these data indicate that cholinergic interneurons are endogenously active and generate action potentials in the absence of any synaptic input. Interspike interval histograms and autocorrelograms generated from unit recordings of cholinergic cells in vitro were indistinguishable from those of tonically active neurons recorded in vivo. Irregular spiking is therefore embedded in the mechanism responsible for endogenous activity.
Keywords: neostriatum, basal ganglia, AMPA, GABAA, NMDA, tonic firing, bursting TANs, D1, D2, muscarinic
Unlike the spiny cells of the neostriatum that fire phasically in relation to the motor aspects of a behavior, the cholinergic interneurons respond to stimuli that serve to trigger a learned and rewarded motor task, exhibiting a prolonged pause in their tonic, irregular firing pattern (Crutcher and DeLong, 1984; Kimura et al., 1984, 1996; Liles, 1985; Schultz and Romo, 1988; Hikosaka et al., 1989; Apicella et al., 1991; Aosaki et al., 1994a,b, 1995; Watanabe and Kimura, 1998). The phasic responses of spiny cells have an absolute requirement for a large number of coincident excitatory inputs to initiate a two-step process involving state transition and subsequent spike triggering from the up state (Wilson and Groves, 1981; Wilson et al., 1983; Wilson, 1993; Wilson and Kawaguchi, 1996). By contrast, spikes in cholinergic interneurons are readily triggered by the summation of a very few discrete depolarizing potentials (Wilson et al., 1990), primarily because of the relatively depolarized membrane potential and large input resistance of these cells (Wilson et al., 1990; Jiang and North, 1991; Kawaguchi, 1992,1993). Thus the tonic, irregular firing pattern of cholinergic interneurons observed during extracellular (e.g., Kimura et al., 1984;Aosaki et al., 1995; Raz et al., 1996) and intracellular (Wilson et al., 1990) recording in vivo is thought to reflect primarily irregularities in the temporal structure of the synaptic barrage (Wilson et al., 1990; Wilson, 1993).
That synaptic inputs might not be solely responsible for the triggering of spikes is suggested by data that have shown that cholinergic cells are still tonically active even under conditions of reduced excitatory drive both in vivo (Wilson et al., 1990) and in vitro (Bennett and Wilson, 1998a; Calabresi et al., 1998; Lee et al., 1998). Thus, cholinergic interneurons may be endogenously active. Furthermore, variability in the interspike interval (ISI) during in vitro recordings (Bennett and Wilson, 1998a) indicates either that there is sufficient spontaneous synaptic input in a slice preparation to cause the observed fluctuations in the ISI or that the variability in spike timing is intrinsic in origin. Alterations in the firing pattern of tonically active neurons (TANs) observed with extracellular electrodes in vivo are currently interpreted within the framework of the cholinergic cell behaving as an integrate-and-fire device (Wilson, 1993). Hence, both the tonic, irregular firing and the prolonged pause in the spiking of TANs, which occurs after sensory stimuli that trigger learned and rewarded motor tasks, are considered to arise primarily from moment-to-moment alterations in synaptic input (Wilson, 1993;Aosaki et al., 1995; Yan and Surmeier, 1997; Bennett and Wilson, 1998a;Watanabe and Kimura, 1998). Demonstration of spontaneous activity and, in particular, an intrinsic origin for irregular spiking would suggest a reevaluation of the current interpretation of action potential timing in cholinergic cells. This study was therefore designed to determine whether neostriatal cholinergic interneurons are endogenously active (i.e., generate action potentials in the absence of any synaptic input) and to compare the firing patterns observed in vitro under conditions of minimal extrinsic perturbation with those seen in vivo.
MATERIALS AND METHODS
Slice preparation. The procedure used for preparing slices has been described previously (Bennett and Wilson, 1998a). Briefly, 2–4 week postnatal Sprague Dawley rats of either sex were deeply anesthetized and perfused transcardially with 10–30 ml of ice-cold modified (see Aghajanian and Rasmussen, 1989) artificial CSF (ACSF) containing (in mm): sucrose, 230; KCl, 2.5; NaH2PO4, 1.25; CaCl2, 0.5; MgSO4, 10; and glucose, 10. Coronal or parasagittal slices of 300 μm thickness were then cut through the neostriatum using a vibroslicer (World Precision Instruments, Sarasota, FL), transferred to a holding chamber, and completely submerged in ACSF (25 ± 1°C) containing (in mm): NaCl, 126; KCl, 2.5; NaH2PO4, 1.25; CaCl2, 2; MgSO4, 2; and glucose, 10. The ACSF was continuously oxygenated, and slices were allowed to equilibrate for at least 1 hr before recording.
Visualized recording. Individual slices were transferred to the recording chamber and were continuously perfused (2–3 ml/min) with oxygenated ACSF at 35 ± 2°C for all experiments except whole-cell current-clamp recordings that were conducted at 25 ± 1°C. Infrared differential interference videomicroscopy (IR-DIC) (Dodt and Zieglgansberger, 1990; Stuart et al., 1993) was used to locate giant neostriatal neurons in the slice. Patch pipettes were prepared from thin-walled borosilicate glass (Warner Instrument Company, Hamden, CT) on a P-87 Flaming–Brown electrode puller (Sutter Instrument Company, Novato, CA) and were filled with a solution containing (in mm): K-MeSO4, 119; KCl, 12; MgCl2, 1; CaCl2, 0.1; HEPES, 10; EGTA, 1; Na-GTP, 0.4; Mg-ATP, 2; and biocytin, 5, pH 7.3 and 280–300 mOsm. Voltage-clamp mode was used for all cell-attached recordings. Data collection was terminated if the seal resistance fell below 1 GΩ. Extracellular unit recordings were made using low-resistance (0.5–2 MΩ) patch pipettes filled with ACSF in either current-clamp or voltage-clamp mode. After all extracellular and cell-attached recordings, the whole-cell configuration was obtained to confirm that the recorded neuron possessed the electrical characteristics ascribed to cholinergic interneurons (e.g., Kawaguchi, 1992). During whole-cell recording, series resistance (15–30 MΩ) was monitored throughout, and neurons exhibiting >25% change were rejected. It should be noted that when the series resistance was less than ∼15 MΩ during whole-cell recordings with a K+-based solution, the neurons tended to hyperpolarize and ceased to be spontaneously active. The changes were irreversible and further characterized by a profound reduction in the input resistance, attenuation of the hyperpolarization-induced sag, and a reduction in the amplitude of the afterhyperpolarization. For whole-cell voltage-clamp recordings, the electrode was filled with a solution containing (in mm): Cs-MeSO4, 111; CsCl, 12.5; MgCl2, 1; CaCl2, 0.1; HEPES, 10; EGTA, 1; Na-GTP, 0.4; Mg-ATP, 2; QX-314, 5; and biocytin, 5, pH 7.3 and 280–300 mOsm. Series resistance (8–20 MΩ) was measured periodically, and neurons exhibiting >25% change were rejected. Data were collected using an Axopatch 200A or 200B amplifier and pClamp 6.0 (Axon Instruments, Foster City, CA). Signals were filtered at 2 or 5 KHz and digitized at 10 or 20 KHz, respectively (Digidata 1200; Axon Instruments). Voltage errors attributable to the liquid junction potential were subtracted off-line.
Drug application. All drugs were obtained from Research Biochemicals (Natick, MA) and were bath applied. Application of 50 μm (±)-2-amino-5-phosphonopentanoic acid (APV) or 50 μm (±)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP) was used to block NMDA receptors. Either 20 or 40 μm 6,7-dinitroquinoxaline-2,3-dione (DNQX) or 20 μm 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX) was used for AMPA receptor blockade. During whole-cell voltage-clamp recordings, GABAA receptors were blocked with 30 μm (−)-bicuculline methiodide (BMI). However, during cell-attached recordings, BMI was avoided because it induced burst firing by reducing the amplitude of the afterhyperpolarization (seeJohnson and Seutin, 1997; Seutin et al., 1997; Debarbieux et al., 1998). Consequently, either 30 μm (−)-bicuculline free base (BIC) or 30 μm2-(3-carboxypropyl)-3-amino-6-(4-methoxyphenyl)-pyridazinium bromide [SR-95531 or gabazine (Hamann et al., 1988; Ueno et al., 1997)] was used during cell-attached recordings. Both 10 μmR(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH-23390) and 10 μm sulpiride were used for blocking D1 and D2 dopamine receptors, respectively. Muscarinic receptors were blocked with 10 μm atropine sulfate. During whole-cell voltage-clamp recordings, application of 100 μm 4-aminopyridine (4-AP) was used to increase the frequency of synaptic currents (Flores-Hernández et al., 1994).
Data analysis. The firing rate = 1/mean ISI. The coefficient of variation (CV) equals the SD of the interspike intervals per mean ISI and was only calculated if the firing rate was >0.33 Hz. For the purposes of generating group data from whole-cell and cell-attached recordings, the CV was determined from 60 sec of continuous recording. For the generation of individual ISI histograms, the duration of the recording is stated in the figure legend. Before the examination of the effects of receptor blockade, the Kolmogorov–Smirnov statistic for intrinsic hypotheses was first used to ensure that the data points in each data group were normally distributed and that in all instances p > 0.01. Consequently, the paired Student’s t test was applied, and significance was assigned when p < 0.05. All values are given as mean ± SD. Spontaneous EPSCs and IPSCs were detected and analyzed using the Mini Analysis Program version 3.0.1 (J. Lee). The threshold was set at 8 pA, and the reversal potential of the spontaneous events was determined by calculating the mean amplitude of all detected events at each holding potential and then plotting this value against membrane voltage.
Histochemical processing of filled cells. At the end of each recording, slices were fixed by immersion in 2.5% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4, at 4°C. After at least 24 hr in fixative, each slice was processed using standard histochemical techniques (Horikawa and Armstrong, 1988), and the biocytin-containing neurons were then post-fixed with osmium, dehydrated, and mounted on slides. Synthetic projection micrographs of filled neurons were prepared using the method of Agard et al. (1989).
RESULTS
Morphological and electrophysiological identification of neostriatal cholinergic interneurons
Neostriatal cholinergic interneurons were initially identified on the basis of their somatodendritic morphology observed using IR-DIC visualization (Kawaguchi, 1992; Götz et al., 1997; Bennett and Wilson, 1998a; Lee et al., 1998). Cholinergic cells were readily identified in the slice by their large somata and thick primary dendrites. After histochemical processing, primary dendrites were found to branch, giving rise to fine-diameter secondary and higher order structures (Fig. 1A). In some fortuitous cases the axonal arborization was also contained within the thickness of the slice and ramified extensively (Fig.1A). Further confirmation that the targeted neurons were cholinergic cells was provided by their electrical response to current injection. Negative current pulses produced an initial hyperpolarization followed by a depolarizing sag in the membrane potential (Fig. 1B), which is caused by activation of the cesium-sensitive, mixed-cation conductanceIh (Jiang and North, 1991; Kawaguchi, 1993). Depolarizing current pulses resulted in nonadapting, regular spiking (Fig. 1B), and at threshold each spike was followed by a large-amplitude, long-duration afterhyperpolarization. These morphological and physiological features are characteristic of cholinergic interneurons (Bolam et al., 1984; Wainer et al., 1984;Phelps et al., 1985; DiFiglia, 1987; Kawaguchi, 1992; Plenz and Aertsen, 1996; Götz et al., 1997), and only neurons in which both were confirmed were used in this study. Many cells also fired spontaneously in the absence of current injection (Fig. 1C), and over the course of this study, this proved to be an additional defining characteristic of cholinergic interneurons.
Fig. 1.
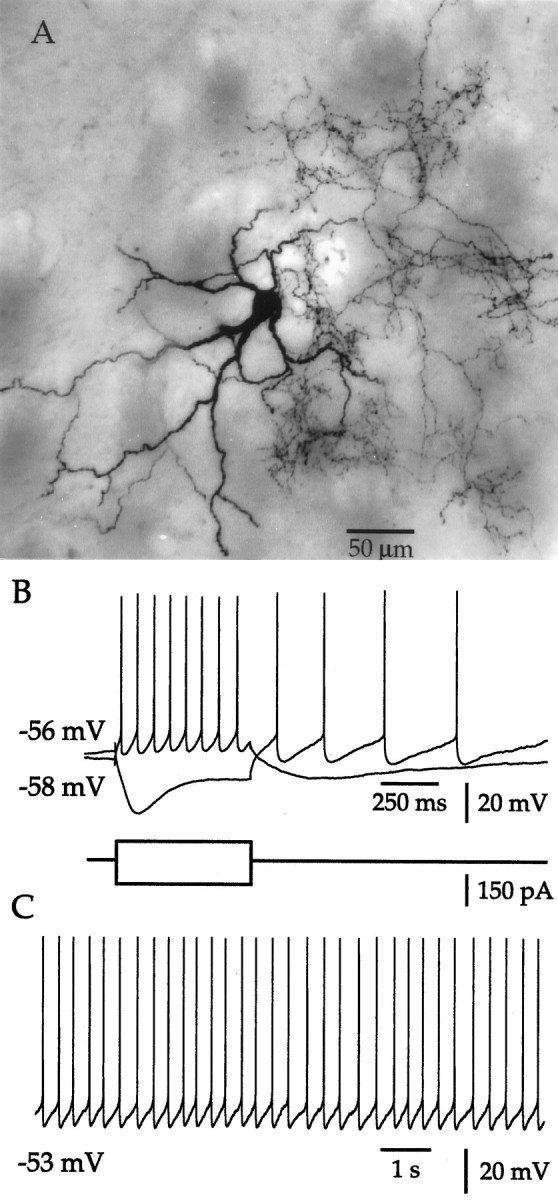
Morphological and physiological identification of neostriatal cholinergic interneurons. A, Synthetic projection micrograph of a cholinergic neuron prepared from a 300-μm-thick whole mount is shown. The large soma and thick primary dendrites that branch to form fine-diameter secondary and higher order processes are characteristic of cholinergic interneurons. In this particular example, the axonal arborization gives rise to a dense plexus that innervates the area surrounding the soma and dendrites.B, During whole-cell recording, cholinergic cells are readily identified by their response to intracellular current injection. Injection of a negative current pulse produces an initial hyperpolarization followed by anIh-dependent sag in the membrane potential. Depolarizing current induces regular spiking and results in a long-lasting afterhyperpolarization after cessation of current injection. C, In the absence of applied current, spontaneous regular spiking (rate = 2.87 Hz; CV = 0.157) was observed in this particular neuron. The membrane potential is indicated for the initial point of each trace in Band C, and the recording was made at 35 ± 2°C.
Spontaneous firing observed during whole-cell recording
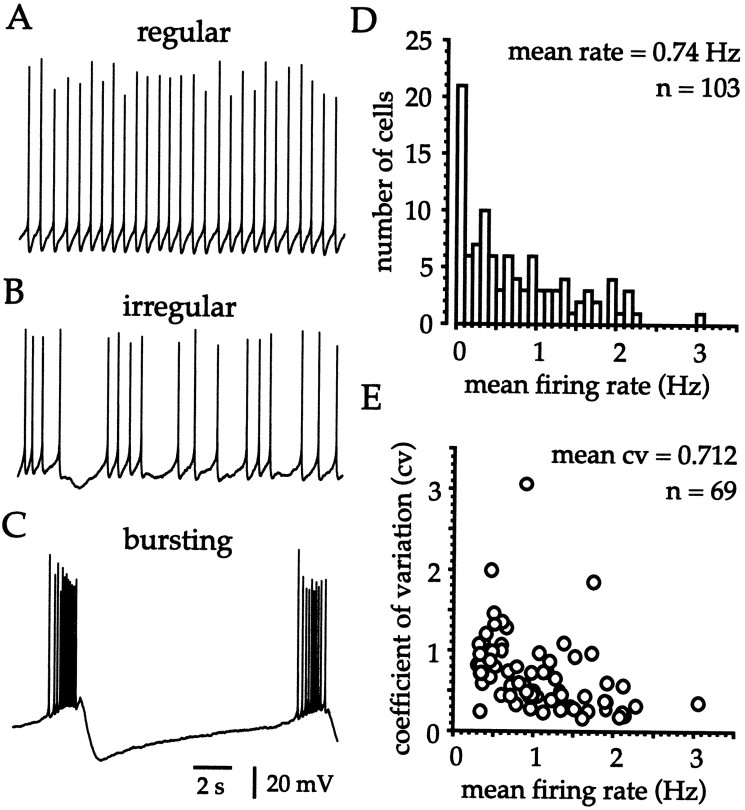
Whole-cell recordings were obtained from 103 cholinergic interneurons at 25 ± 1°C in slices prepared from 2 to 3 week postnatal rats. The majority of cholinergic cells (85%) were spontaneously active (75% fired at >0.2 Hz) with neurons exhibiting regular, irregular, and rhythmic burst firing (Fig.2A–C). Cholinergic interneurons displayed a range of firing frequencies (0–3.06 Hz) with mean and median firing rates of 0.74 ± 0.68 and 0.52 Hz (n = 103), respectively (Fig. 2D). The CV of the ISI was calculated for all neurons that fired at >0.33 Hz and was then plotted against the mean firing rate (Fig.2E). The CV (0.159–3.059) was a function of the firing rate, and neurons that fired more rapidly tended to exhibit more regular spike trains (Fig. 2E). Overall the mean and median CVs were 0.712 ± 0.481 and 0.600 (n = 69), respectively. That the spontaneous activity of cholinergic interneurons arose from normal cellular functioning and was not a result of intracellular dialysis of the neuron with the electrode solution was confirmed using a noninvasive technique.
Fig. 2.
The spectrum of firing patterns observed during whole-cell recording at 25 ± 1°C in slices from 2 to 3 week postnatal rats. A–C, Cholinergic interneurons exhibited a continuum of firing patterns including regular (CV = 0.159), irregular (CV = 0.715), and burst (CV = 3.059) firing.D, Histogram of the mean firing rate illustrates that the majority of cholinergic cells are spontaneously active at 25 ± 1°C and exhibit a range (0.00–3.06 Hz) of firing rates.E, Plot of the relationship between the mean firing rate and the CV of the interspike intervals reveals that, in general, neurons firing with higher rates exhibit more regular spiking patterns. The CV was only calculated in neurons that fired at >0.33 Hz and was generated from a 1 min sample period.
Spontaneous firing observed during cell-attached recording
Cell-attached voltage-clamp recordings were made from 99 cholinergic interneurons at 35 ± 2°C in slices prepared from 3 to 4 week postnatal rats. The majority of cholinergic cells (80%) exhibited spontaneous spiking (69% fired at >0.2 Hz) under these conditions, displaying a range of firing frequencies (0–9.52 Hz) and patterns including regular, irregular, and burst firing (Fig.3). Regularly spiking cells (Fig.3A) were characterized by a narrow, unimodal Gaussian ISI distribution (Fig. 3B). Irregularly firing neurons (Fig.3C) exhibited a much larger variance of interspike intervals (Fig. 3D). By contrast, burst-firing cells (Fig.3E) exhibited a very skewed ISI distribution (Fig.3F), with the modal ISI value corresponding to the predominant intraburst interval and large ISI values (more than ∼1 sec in this example) representing the interburst intervals. Overall cholinergic cells recorded at 35 ± 2°C in the cell-attached configuration exhibited mean and median firing rates of 1.97 ± 2.12 and 1.46 Hz, respectively (Fig. 3G). The CV was calculated for cholinergic cells firing at >0.33 Hz and was plotted against the mean firing rate (Fig. 3H). Similar to that in cells recorded in the whole-cell configuration, the CV (range, 0.050–2.321) varied with firing rate, and in general more rapidly firing neurons were more regular (Fig. 3H). Overall, the mean and median values for the CV were 0.385 ± 0.356 and 0.276, respectively (n = 65). The observation that spontaneous firing of cholinergic cells occurred at 25 ± 1°C and at 35 ± 2°C in slices prepared from rats of two different age groups using both whole-cell and cell-attached configurations indicates that tonic firing is a normal and robust cellular process that is apparently relatively age and temperature independent.
Fig. 3.
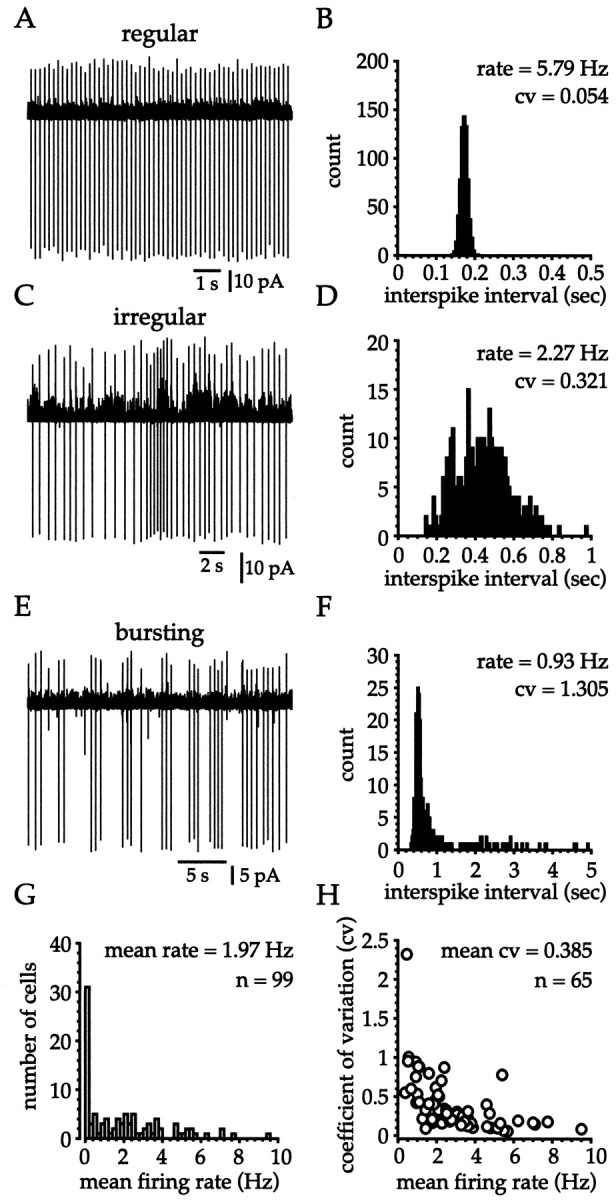
Cholinergic cells exhibit a range of firing rates and patterns during cell-attached recordings at 35 ± 2°C from 3 to 4 week postnatal rats. A, B, Regularly spiking cells were readily identified during cell-attached recording and exhibited a narrow unimodal Gaussian distribution in the ISI histogram (bin width = 5 msec; 2 min sample). C,D, Irregularly spiking cells were recognized by the large variability in the ISI and gave rise to unimodal ISI histograms with a large variance (bin width = 10 msec; 2.5 min sample). The fluctuations in the baseline of the cell-attached voltage-clamptrace result from the opening of large conductance channels. E, F, Burst firing was characterized by the clustering of spikes and produced a very skewed ISI histogram, with the peak corresponding to the modal intraburst interval and variable, long-duration ISIs corresponding to the interburst intervals (bin = 25 msec; 4.5 min sample).G, Cholinergic cells exhibited a range (0.00–9.52 Hz) of firing rates with the majority of cells (69%) spiking at >0.2 Hz.H, The relationship between CV and firing rate shows a general trend for more rapidly firing cells to be more regular.
Spontaneous excitatory synaptic inputs are not responsible for tonic firing
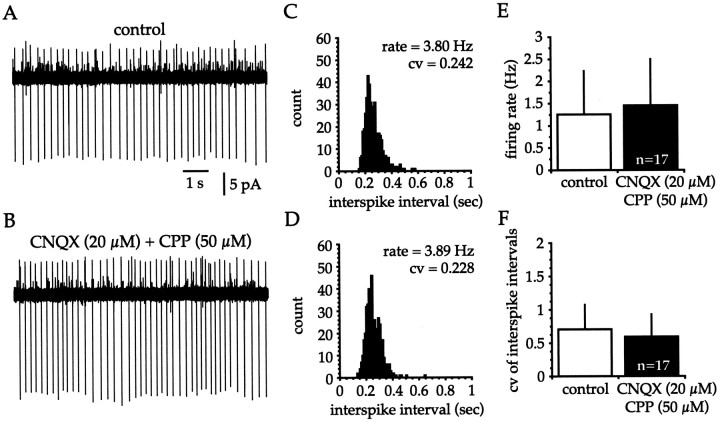
Pharmacological blockade of AMPA and NMDA receptors was used during cell-attached recordings to determine whether spontaneous excitatory synaptic inputs were involved in the triggering and patterning of spikes in cholinergic cells in vitro (Fig.4). Application of CNQX (20 μm) and CPP (50 μm) had no discernible effect on the firing rate or pattern of cholinergic cells (Fig.4A,B). Examination of the corresponding ISI histograms revealed that blocking AMPA and NMDA receptors did not alter the firing frequency or CV (Fig.4C,D), indicating that spontaneous excitatory synaptic inputs played little if any role in spike triggering under these conditions. Statistical examination of group data (Fig.4E,F) revealed that AMPA and NMDA receptor blockade altered neither the firing rate (control = 1.25 ± 0.98 Hz; range, 0.35–3.65 Hz; CNQX + CPP = 1.46 ± 1.04 Hz; range, 0.26–3.95 Hz; n = 17;p > 0.2) nor ISI variability (control CV = 0.703 ± 0.373; range, 0.270–1.454; CNQX + CPP CV = 0.595 ± 0.334; range, 0.194–1.467; n = 17;p > 0.2), suggesting that cholinergic interneurons receive minimal spontaneous excitatory synaptic input in vitro.
Fig. 4.
Spontaneous excitatory synaptic inputs are not responsible for the tonic firing of cholinergic interneurons in vitro. A, B, Cell-attached voltage-clamp recordings show that pharmacological blockade of AMPA and NMDA receptors had no obvious effect on the spike rate or pattern.C, D, Application of CNQX (20 μm) and CPP (50 μm) did not produce any change in the ISI histogram (bin width = 10 msec; 2 min sample), and accordingly the firing frequency and CV were unaltered. Control data are in C. E, F, Group data illustrate that spontaneous excitatory inputs do not have any detectable effect on the firing rate (E; control = 1.25 ± 0.98 Hz; CNQX + CPP = 1.46 ± 1.04 Hz;n = 17; p > 0.2) or CV (F; control = 0.703 ± 0.373; CNQX + CPP = 0.595 ± 0.334; n = 17;p > 0.2).
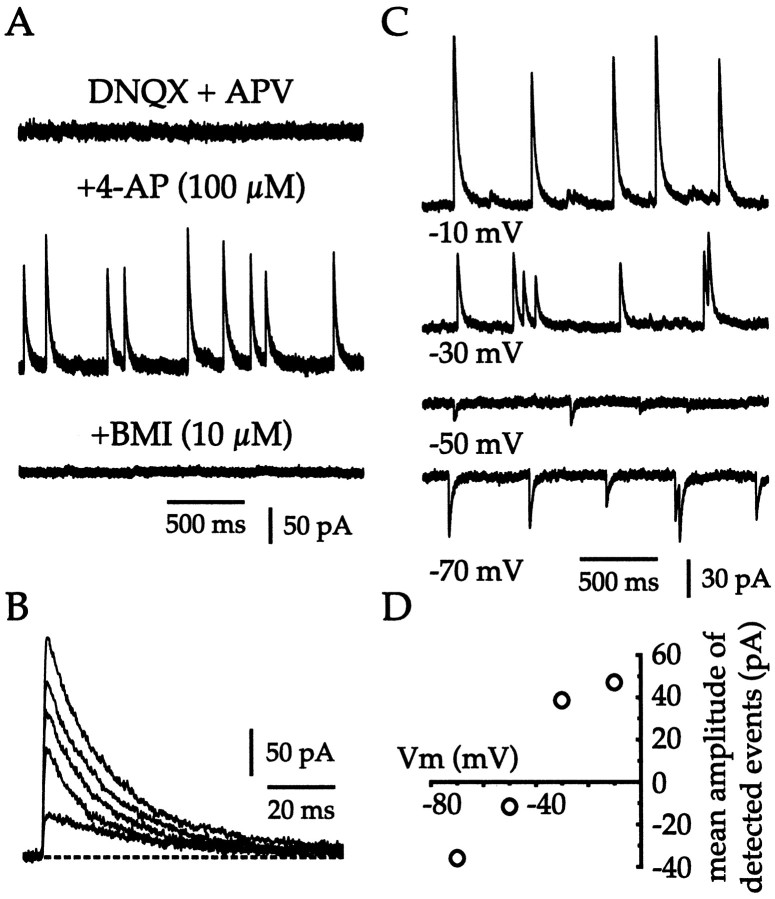
Whole-cell voltage-clamp recordings confirmed that cholinergic neurons are subject to very few spontaneous glutamatergic inputs in vitro (Fig. 5). After blockade of GABAA receptors, a very low frequency of spontaneous inward currents (0.02 ± 0.04 Hz; n = 21) was detected in cholinergic cells clamped at −60 mV. Pharmacological dissociation of AMPA and NMDA receptor–mediated events revealed that at −60 mV all spontaneous EPSCs (sEPSCs) were attributable to activation of AMPA receptors (Fig. 5A). In the presence of BMI (30 μm) and APV (50 μm), the AMPA-mediated sEPSCs were infrequent (0.02 ± 0.02 Hz; n = 6) and of small amplitude (11.95 ± 1.63 pA; n = 6). Application of 4-AP (100 μm) produced a large increase in both the frequency (8.89 ± 3.52 Hz; n = 6) and the amplitude (37.71 ± 17.32 pA; n = 6) of AMPA-mediated sEPSCs (Fig. 5A,B). Further confirmation that sEPSCs triggered by 4-AP application were mediated by AMPA receptors was provided by the rapid kinetics, voltage-dependent reversal (EAMPA = 11 ± 2 mV; n = 6), and sensitivity to DNQX (40 μm; n = 6) of these events (Fig.5A–D).
Fig. 5.
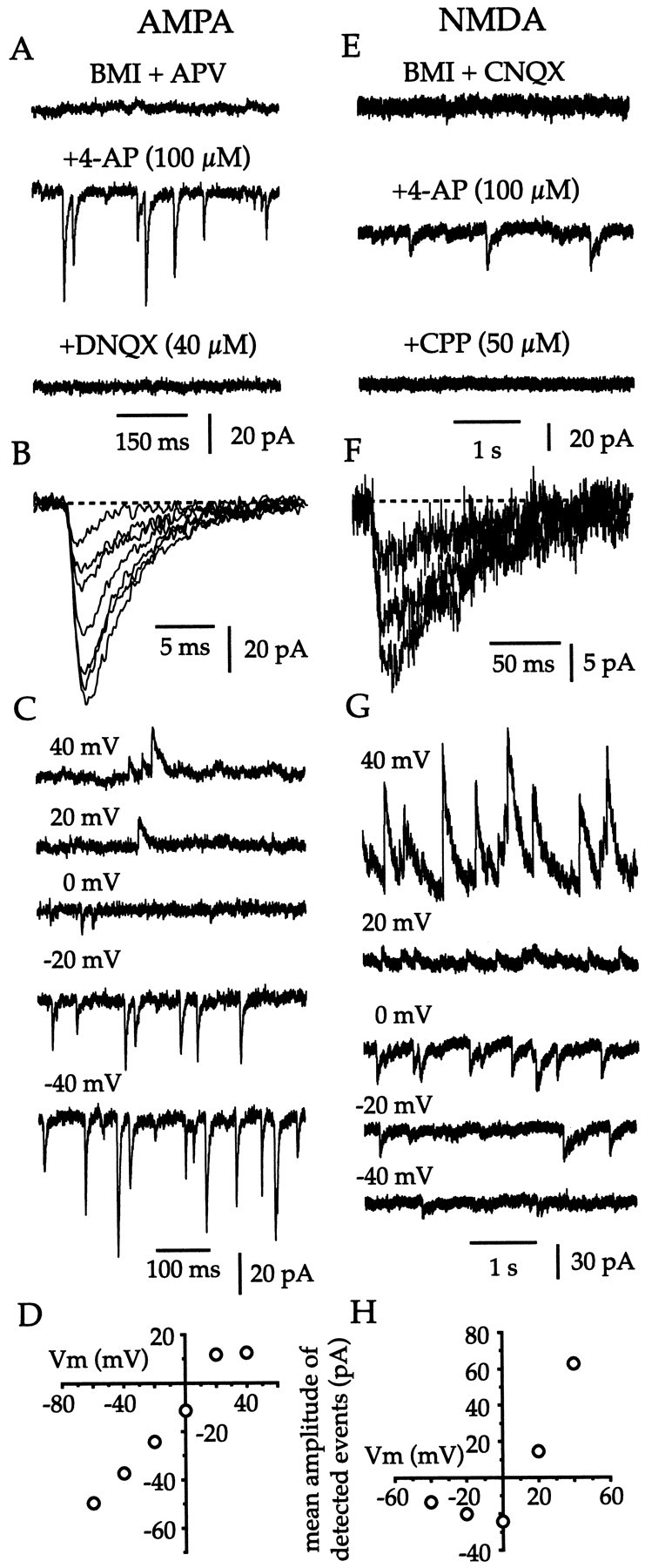
Cholinergic interneurons receive minimal tonic excitatory input in vitro. A, At a holding potential of −60 mV in the presence of BMI (30 μm) and APV (50 μm), very few spontaneous inward currents were detected. Application of 4-AP (100 μm) caused the appearance of many, fast EPSCs that were blocked by DNQX (40 μm). B, The rapid kinetics of the 4-AP–induced AMPA receptor–mediated EPSCs is shown using a faster sweep speed. C, D, Further confirmation that the 4-AP–induced EPSCs are mediated by AMPA receptors is provided by the voltage-dependent reversal of these events. E, Cholinergic cells do not receive detectable spontaneous NMDA receptor–mediated synaptic inputs at 0 mV in the presence of BMI (30 μm) and CNQX (20 μm). Addition of 4-AP (100 μm) produced many slow, inward currents, which were confirmed by blockade with CPP (50 μm) to be caused by activation of NMDA receptors.F, Superimposed 4-AP–induced NMDA receptor–mediated EPSCs are shown at a faster sweep speed to illustrate their slow kinetics. G, H, Further confirmation that the 4-AP–induced events are mediated by NMDA receptors is provided by their voltage dependence.
In the presence of BMI (30 μm) and CNQX (20 μm), sEPSCs mediated by NMDA receptors were undetectable in cholinergic interneurons at −60 or 0 mV (Fig. 5E). Application of 4-AP (100 μm) at a holding potential of 0 mV disclosed the presence of NMDA inputs to cholinergic cells (Fig.5E) and caused an elevation in the frequency (1.64 ± 1.04 Hz; n = 6) and the amplitude (28.11 ± 21.46 pA) of NMDA-dependent sEPSCs (Fig.5E,F). That the 4-AP–induced sEPSCs were mediated via the activation of NMDA receptors was confirmed by the slow kinetics, voltage-dependent reversal (ENMDA = 7 ± 9 mV; n= 6), and CPP sensitivity (50 μm; n = 5) of these events (Fig. 5E–H). These data demonstrate that although synapses that give rise to AMPA and NMDA receptor–mediated inputs to cholinergic cells are physically present and functional, they are basically inactive in vitro. Hence, the tonic firing of cholinergic interneurons in slices cannot be attributed to a barrage of sEPCSs and indicates that these neurons are probably endogenously active.
Spontaneous inhibitory inputs are not responsible for irregular spiking in vitro
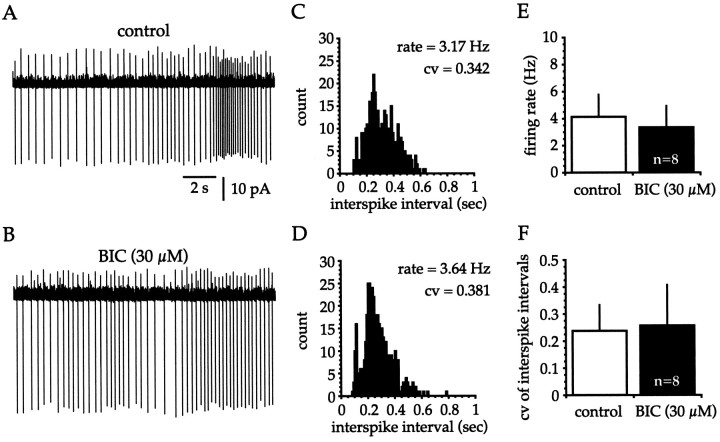
The observation that spike trains recorded from cholinergic cells were irregular raised the possibility that perhaps there were sufficient spontaneous inhibitory synaptic inputs to pattern action potential timing. Blockade of GABAA receptors with BIC (30 μm; n = 4) or SR-95531 (30 μm;n = 4) produced no obvious alteration in the firing rate or pattern of tonically active cholinergic interneurons recorded in the cell-attached configuration (Fig.6A,B). The ISI histogram showed no appreciable change after application of BIC (Fig. 6C,D) or SR-95531, and examination of group data (Fig. 6E,F) revealed that GABAA receptor antagonists failed to produce any significant effect on the firing frequency (control = 4.13 ± 1.64 Hz; range, 1.80–7.13 Hz; BIC or SR-95531 = 3.35 ± 1.57 Hz; range, 1.52–6.80 Hz; n = 8; p > 0.2) or CV (control = 0.237 ± 0.097; range, 0.096–0.392; BIC or SR-95531 = 0.257 ± 0.150; range, 0.101–0.487;n = 8; p > 0.2). These data predict that cholinergic cells receive minimal tonic inhibitory synaptic inputin vitro.
Fig. 6.
Irregular spiking of cholinergic cells is not attributable to spontaneous inhibitory inputs in vitro.A, B, No discernible alteration in the firing rate or pattern of this cholinergic cell occurs after blockade of GABAA receptors with BIC (30 μm).C, D, Examination of the ISI histograms (bin width = 10 msec; 2 min sample) generated from data from which the spike trains were taken (control data in C) reveals that BIC (30 μm) produces no obvious effect on the spiking rate or pattern. E, F, Grouped data reveal that blockade of GABAA receptors with BIC (30 μm; n = 4) or SR-95531 (30 μm; n = 4) does not produce a significant alteration in the firing frequency (E; control = 4.13 ± 1.64 Hz; BIC or SR-95531 = 3.35 ± 1.57 Hz; n = 8; p > 0.2) or CV (F; control = 0.237 ± 0.097; BIC or SR-95531 = 0.257 ± 0.150; n = 8;p > 0.2) of cholinergic cells.
Voltage-clamp recordings at a holding potential of −10 mV after blockade of AMPA and NMDA receptors revealed a low frequency (1.10 ± 0.78 Hz; n = 14) of small-amplitude (12.72 ± 1.42 pA; n = 13) spontaneous IPSCs (sIPSCs) in cholinergic interneurons (Fig.7A). Application of 4-AP (100 μm) produced a large increase in the frequency (3.95 ± 2.97 Hz; n = 14) and the amplitude (31.06 ± 27.03 pA; n = 13) of sIPSCs (Fig.7A,B). That the 4-AP–induced events were mediated via the activation of GABAAreceptors was confirmed by their sensitivity to BMI (30 μm; n = 11) and their voltage-dependent reversal (EGABA = −48 ± 10 mV;n = 5) close to the equilibrium potential for chloride-mediated events calculated from the Nernst equation (ECl = −54 mV) (Fig 7). These findings demonstrate that cholinergic interneurons are subject to minimal inhibitory synaptic input in vitro and that the irregular spiking cannot be attributed to tonic inhibition.
Fig. 7.
Cholinergic interneurons receive minimal spontaneous inhibitory synaptic input in vitro.A, In the presence of DNQX (40 μm) plus APV (50 μm) at a holding potential of −10 mV, a few small-amplitude outward currents are detectable. Application of 4-AP induced a large increase in the amplitude and frequency of spontaneous IPSCs, and their sensitivity to BMI (10 μm) indicates that they are mediated by GABAA receptors.B, Superimposed sweeps at a faster speed are shown to illustrate the kinetics of the 4-AP–induced IPSCs. C,D, The IPSCs are confirmed by their voltage-dependent reversal close to the chloride equilibrium potential to be caused by GABAA receptor activation.
Spike timing is not influenced by tonic dopaminergic or cholinergic inputs in vitro
Recent physiological studies have demonstrated that cholinergic cells are responsive to agonists acting at D1 dopamine receptors (Aosaki et al., 1998). We therefore tested the possibility that these synapses might be spontaneously releasing transmitter and influencing the spiking rate or pattern of cholinergic cells in vitro. Application of SCH-23390 (10 μm; n = 8) or sulpiride (10 μm; n = 7) to block D1 and D2 dopamine receptors, respectively, produced no significant (p > 0.2) alteration of either the firing rate or pattern of cholinergic interneurons. Another recent study has shown stimulus-evoked M2 muscarinic receptor–mediated IPSPs in cholinergic cells (Calabresi et al., 1998). However, the muscarinic receptor antagonist atropine (10 μm; n = 13) also failed to influence significantly (p > 0.2) the spiking rate or pattern. Overall, these data demonstrate that spontaneous synaptic inputs mediated by AMPA, NMDA, GABAA, D1, D2, or muscarinic receptors are either undetectable or minimal in vitro, and the spiking rate and pattern of cholinergic cells are therefore of intrinsic origin.
Cholinergic interneurons generate tonic, irregular firing in the absence of any synaptic input
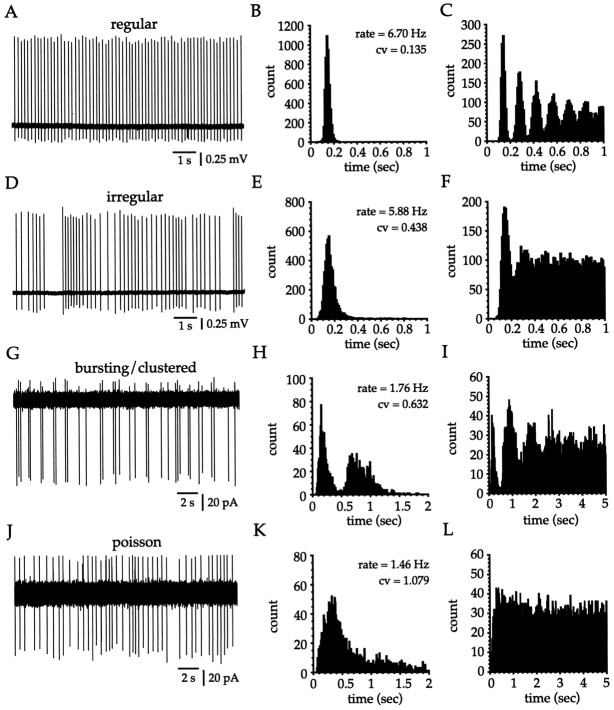
Extracellular single-unit recordings were made for the purposes of comparing the firing rate and pattern of cholinergic cells recordedin vitro with those of TANs recorded from awake behaving monkeys in vivo. Unit recordings from cholinergic interneurons were taken from cells that fired spontaneously at >1 Hz (range, 1.50–6.70 Hz; n = 16). This group of cells exhibited a range of firing patterns (range of CV, 0.14–1.54) and had mean and median firing rates of 3.21 ± 1.54 and 3.02 Hz (n = 16), respectively, with mean and median CVs of 0.471 ± 0.406 and 0.295 (n = 16), respectively. The firing rates and CVs for unit recordings exhibited the same relationship as that seen for whole-cell (Fig. 2E) and cell-attached (Fig. 3H) recordings. Neurons that fired more rapidly fired more regularly. Although cholinergic cells exhibited a continuum of firing patterns on the basis of the CV, examination of the corresponding autocorrelogram generated from the spike times facilitated the discrimination between different spike patterns. In confirmation of the data collected during whole-cell (Fig.2) and cell-attached (Fig. 3) recordings, cholinergic interneurons exhibited regular, irregular, bursty, and seemingly random spike trains during extracellular unit recordings (Fig.8).
Fig. 8.
Extracellular single-unit recordings of cholinergic cells in vitro. The ISI histogram (B, E, H,K) and autocorrelogram (C,F, I, L) accompanying eachtrace (A, D,G, J) are shown. Bin width is 10 msec. A–C, Regularly spiking cholinergic cells give rise to a narrow, unimodal Gaussian distribution in the ISI histogram (12.5 min sample). The autocorrelogram (2.5 min sample) exhibits multiple, uniformly spaced peaks, owing to the stationarity and regularity of the spike train. D–F, Irregularly firing cholinergic cells exhibit relatively stationary ISIs interspersed with periods of more variable spike timing. The corresponding ISI histogram (15.5 min sample) is unimodal but skewed toward theright. The autocorrelogram (5 min sample) shows a single peak, caused by the increased likelihood of firing at the end of the afterhyperpolarization, and little additional structure.G–I, Cholinergic cells also exhibit bursting or clustered firing that was characterized most clearly by an obvious bimodal distribution in the ISI histogram (10 min sample). The autocorrelogram (10 min sample) exhibits two clear peaks, the first corresponding to the intraburst intervals and the second to the interburst intervals. J–L, Cholinergic cells that fired in a seemingly random manner were also encountered. The ISI histogram (28 min sample) displays a clear modal value but is very skewed, and examination of the autocorrelogram (10 min sample) reveals that, other than the decreased probability of spike generation during the afterhyperpolarization, there is no structure to the spiking pattern.
Regular spiking was readily discerned from the spike train (Fig.8A), giving rise to a unimodal ISI histogram with a Gaussian distribution (Fig. 8B) and an associated autocorrelogram that was multipeaked (Fig. 8C), confirming a high periodicity of firing. Irregularly firing cholinergic cells exhibited spike trains that primarily contained relatively stationary periods of firing interspersed with less frequent longer duration ISIs (Fig. 8D). The corresponding ISI histogram was unimodal, but with a broader distribution than that seen for regularly spiking cells, and was skewed to the right (Fig. 8E). This type of firing pattern gave rise to an autocorrelogram with a single peak and a subsequent trough with little additional structure, indicating the increased probability of spike generation at the end of the afterhyperpolarization (Fig. 8F). Burst firing was characterized by the clustering of action potentials in the spike train (Fig. 8G) and was readily detected in the ISI histogram (Fig. 8H) owing to the extremely platykurtic or bimodal distribution. The first modal value corresponds to the predominant ISI within a burst, and the second mode reflects the interburst interval (Fig. 8H). In this particular example, the CV was <1 because the spike clusters primarily contained only two spikes, but in cases in which individual bursts contained three or more spikes, the CV was >1. The associated autocorrelogram exhibited two clear peaks (Fig. 8I), the first corresponding to the intraburst increased spike probability and the second to the predominant interburst duration. Cholinergic interneurons exhibiting seemingly random spike trains were also encountered (Fig.8J,K), and examination of the autocorrelogram (Fig. 8L) indicated that the spike train displayed little structure other than the initial low probability of firing during the afterhyperpolarization. These data show that even in the absence of any synaptic input, cholinergic interneurons generate a wide variety of spiking patterns and firing frequencies that are on the average indistinguishable from published unit recordings of TANs in vivo (see Discussion).
DISCUSSION
Cholinergic interneurons are tonically active in the absence of synaptic input
Intracellular recordings of cholinergic cells in vivohave demonstrated that action potentials are triggered by summation of a few, apparently unitary depolarizing synaptic potentials but that a ceiling is placed on the firing rate by the spike afterhyperpolarization (Wilson et al., 1990; Wilson, 1993). These observations led to the suggestion that cholinergic cells behave as a sensitive integrate and fire device with spike timing principally determined by the temporal structure of the synaptic barrage (Wilson et al., 1990; Wilson, 1993). That cholinergic interneurons might be endogenously active was initially indicated by the observation that tonic firing persists in vivo after hemidecortication (Wilson et al., 1990). However, the first recordings of cholinergic cells in slices did not describe tonic activity (Jiang and North, 1991;Kawaguchi, 1992), and more recent in vitro data have failed to produce a consensus (Kawaguchi, 1993; Aosaki and Kawaguchi, 1996;Götz et al., 1997; Aosaki et al., 1998; Bennett and Wilson, 1998a; Calabresi et al., 1998; Lee et al., 1998). Data from the present study demonstrate that cholinergic interneurons are tonically activein vitro. The spontaneous firing of these cells was attributable to a normal and robust cellular process because the firing rates and patterns observed during whole-cell recording in slices from 2 to 3 week postnatal animals at ∼25°C were observed with two noninvasive techniques in slices from 3 to 4 week postnatal animals at ∼35°C. Furthermore, blockade of ionotropic glutamate receptors produced no discernible alteration of the firing rate or pattern because glutamatergic synaptic inputs, although physically present, are essentially inactive in vitro. Thus, in contrast to the spiny projection cells of the neostriatum that have an absolute requirement for synaptic excitation in the spike-generating process (Wilson and Groves, 1981; Wilson et al., 1983; Wilson, 1993; Wilson and Kawaguchi, 1996; Stern et al., 1997), excitatory synaptic inputs, although clearly influential (Wilson et al., 1990; Bennett and Wilson, 1998a), are not essential for spike triggering in cholinergic interneurons.
Other classes of spontaneously active neurons, such as Purkinje cells and molecular layer interneurons of the cerebellum (Llinás and Sugimori, 1980; Häusser and Clark, 1997), subthalamic neurons (Nakanishi et al., 1987a; Beurrier et al., 1999), and substantia nigra pars reticulata cells (Nakanishi et al., 1987b; Richards et al., 1997), generate regular spike trains in the absence of synaptic input. However, perturbation of pacemaker activity by synaptic potentials produces irregular firing (Bernard and Axelrad, 1993; Häusser and Clark, 1997; Jaeger et al., 1997). We therefore investigated the possibility that irregular spiking in cholinergic cells arises from disruption of an intrinsic pacemaker mechanism by some form of synaptic input.
Firing patterns of cholinergic cells are generated endogenously
Pharmacological blockade of GABAA receptors did not produce any detectable effect on the firing rate or pattern of cholinergic cells, and accordingly, voltage-clamp recordings revealed a very low incidence of small-amplitude IPSCs. Two other possible extrinsic influences that might give rise to irregular spiking are synaptic inputs mediated by dopaminergic and muscarinic receptors. A tonic D1 dopamine receptor–mediated inward current has been reported in cholinergic interneurons in vitro (Aosaki et al., 1998), which might be expected to produce a steady depolarization and possibly a maintained neuromodulatory influence over the spike afterhyperpolarization (Bennett and Wilson, 1998a). However, both D1 and D2 dopamine receptor antagonists were ineffective in altering the firing rate or pattern of cholinergic cells. Recently, stimulus-evoked, M2 muscarinic receptor–mediated slow IPSPs were described in cholinergic cells (Calabresi et al., 1998). Because the majority of cholinergic neurons are spontaneously active in slices, one would predict that spontaneous muscarinic IPSPs might influence spike timing. However, blockade of muscarinic receptors did not alter the firing rate or pattern. An additional neuromodulatory influence that could potentially regulate the tonic activity of cholinergic cells in vitro is that of substance P (Aosaki and Kawaguchi, 1996). However, no spontaneous synaptic inputs were detected after blockade of GABAergic and glutamatergic synaptic transmission during voltage-clamp recordings. Furthermore, the spiny cells, which provide the substance P–containing input to the cholinergic cells, are electrically quiescent in vitro. Together these data show that both the generation and patterning of spikes in cholinergic cells originate from intrinsic mechanisms and are not the result of spontaneous synaptic inputs in vitro.
Cholinergic cells possess several ionic conductances that are known to be involved in endogenous firing in other classes of neurons. Specifically, a persistent sodium conductance (Chao and Alzheimer, 1995) and the hyperpolarization-activated cation currentIh (Jiang and North, 1991; Kawaguchi, 1993) are found in cholinergic interneurons. Tonic activity of cholinergic cells is therefore likely to arise from the depolarizing action of inward currents available in the subthreshold voltage range. Furthermore, the spike afterhyperpolarization, which is a calcium-dependent potassium current (Kawaguchi, 1993), is known to be of pivotal importance in regulating the firing rate and pattern of cholinergic cells (Bennett and Wilson, 1998b) and is therefore also likely to play a key role in shaping the tonic activity of cholinergic cells.
Modeling studies of the endogenously active pancreatic β-cell (Chay and Rinzel, 1985) and the R15 neuron of Aplysia (Canavier et al., 1990) have demonstrated that irregular firing can occupy a portion of the parameter space between “periodic beating,” i.e., continuous regular spiking, and burst firing. However, experimental studies have shown that in general, extrinsic perturbations of endogenous activity are required to produce irregular spiking (Hayashi et al., 1982; Holden et al., 1982). Cholinergic cells may therefore provide a rare opportunity to investigate the mechanisms underlying endogenous, irregular spiking because although they exhibit both regular spiking and rhythmic bursting, the majority of their time is spent firing irregularly. The endogenous irregular activity has important implications for the interpretation of spike timing in TANs in vivo.
Implications for spike timing in TANs
The TANs detected in extracellular single-unit recordings in vivo are believed to be the cholinergic cells of the neostriatum (Kimura et al., 1990; Wilson et al., 1990; Kawaguchi, 1993; Aosaki et al., 1995; Götz et al., 1997). This assumption is supported by the observation that unit recordings of cholinergic cells in vitro and unit data from TANs recorded in vivo (Kimura et al., 1990; Aosaki et al., 1994b, 1995; Raz et al., 1996) are nearly indistinguishable on the basis of firing rate and pattern. This is somewhat surprising considering that cholinergic cells in vivo are subjected to a continuous barrage of depolarizing synaptic input (Wilson et al., 1990). However, superimposing irregular excitatory synaptic input on endogenous irregular spiking would be expected to cause an elevation in firing rate and further disruption of spike timing. Comparison of the mean firing rate (5.52 vs 3.21 Hz) and CV (0.63 vs 0.47) of TANs recorded in vivo (Aosaki et al., 1995) and in vitro confirmed that cholinergic cells fire more rapidly and less regularly in vivo. Thus the firing rate and pattern of TANs in vivo result from a dynamic interaction between the synaptic barrage and the endogenous mechanisms responsible for irregular spiking.
The endogenous irregular activity of cholinergic interneurons suggests that information in the spike train might be encoded as a modulation of firing rate. One of the most striking features of the TANs is the pause in tonic firing triggered by a sensory stimulus in a learned and rewarded motor task (Crutcher and DeLong, 1984; Kimura et al., 1984,1996; Liles, 1985; Schultz and Romo, 1988; Hikosaka et al., 1989;Apicella et al., 1991; Aosaki et al., 1994a,b, 1995; Watanabe and Kimura, 1998). After acquisition of the task, the pause response is exhibited by cholinergic cells over a very widespread area of the neostriatum (Aosaki et al., 1994a,b, 1995; Graybiel et al., 1994). This implies that there is a mechanism for synchronizing the pause (Raz et al., 1996) that might be necessary to produce an appreciable alteration in the degree of muscarinic receptor activation if individual spiny cells are recipients of synaptic input from a considerable number of cholinergic cells. The pause response is unlikely to be mediated by a synchronous GABAergic input because these synapses are ill suited to provide a precisely timed reduction in firing rate (Bennett and Wilson, 1998a), and there is no obvious anatomical substrate that could subserve such a function. An alternative hypothesis is that a reduction in excitatory drive is responsible for the pause. Monosynaptic, excitatory synaptic inputs to the cholinergic cells are provided by neurons of the parafascicular nucleus of the thalamus (Wilson et al., 1990; Lapper and Bolam, 1992). Axons arising from individual parafascicular neurons arborize over very large areas of the neostriatum (Deschênes et al., 1996) and are therefore ideally positioned to produce a synchronized reduction in excitatory input. Furthermore, inactivation of the centromedian-parafascicular nuclei results in the abolition of the pause response in TANs (Matsumoto et al., 1997).
Cholinergic inputs to spiny cells activate muscarinic receptors that reduce N-, P-, and L-type calcium currents (Howe and Surmeier, 1995) and cause a hyperpolarizing shift in the voltage range of activation and inactivation of the fast A-type potassium current (IAf) (Akins et al., 1990). During the synchronous pause in the firing of TANs, the tonic level of muscarinic receptor activation is transiently reduced, which should enhance voltage-dependent calcium currents and cause a state-dependent alteration in the availability of IAf. Both of these changes will influence the electrical response characteristics of the spiny cells and may provide a window for modification of synaptic weights by increasing the size of the voltage deflection and the magnitude of calcium influx produced by excitatory inputs.
In conclusion, our data demonstrate that the firing pattern of TANsin vivo results from the interaction between a barrage of synaptic inputs and the intrinsic mechanisms responsible for endogenous activity. Because irregular spiking is generated in the absence of any extrinsic perturbation, the pause observed in TANs in vivocould arise from a reduction of excitatory drive and subsequent expression of the endogenous spiking pattern of the neuron.
Footnotes
This work was supported by National Institutes of Health Grant NS 37760.
Correspondence should be addressed to Dr. Ben David Bennett, Department of Anatomy and Neurobiology, 875 Monroe Avenue, University of Tennessee, Memphis, TN 38163.
REFERENCES
- 1.Agard DA, Hiraoka Y, Shaw P, Sedat JW. Fluorescence microscopy in three dimensions. Methods Cell Biol. 1989;30:353–377. doi: 10.1016/s0091-679x(08)60986-3. [DOI] [PubMed] [Google Scholar]
- 2.Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- 3.Akins PT, Surmeier DJ, Kitai ST. Muscarinic modulation of a transient K+ conductance in rat neostriatal neurons. Nature. 1990;344:240–242. doi: 10.1038/344240a0. [DOI] [PubMed] [Google Scholar]
- 4.Aosaki T, Kawaguchi Y. Actions of substance P on rat neostriatal neurons in vitro. J Neurosci. 1996;16:5141–5153. doi: 10.1523/JNEUROSCI.16-16-05141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aosaki T, Graybiel AM, Kimura M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994a;265:412–415. doi: 10.1126/science.8023166. [DOI] [PubMed] [Google Scholar]
- 6.Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci. 1994b;14:3969–3984. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J Neurophysiol. 1995;73:1234–1252. doi: 10.1152/jn.1995.73.3.1234. [DOI] [PubMed] [Google Scholar]
- 8.Aosaki T, Kiuchi K, Kawaguchi Y. Dopamine D1-like receptor activation excites rat striatal large aspiny neurons in vitro. J Neurosci. 1998;18:5180–5190. doi: 10.1523/JNEUROSCI.18-14-05180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apicella P, Scarnati E, Schultz W. Tonically discharging neurons of monkey striatum respond to preparatory and rewarding stimuli. Exp Brain Res. 1991;84:672–675. doi: 10.1007/BF00230981. [DOI] [PubMed] [Google Scholar]
- 10.Bennett BD, Wilson CJ. Synaptic regulation of action timing in neostriatal cholinergic interneurons. J Neurosci. 1998a;18:8539–8549. doi: 10.1523/JNEUROSCI.18-20-08539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett BD, Wilson CJ. The ionic origin of tonic activity in neostriatal cholinergic interneurons. Int Bas Gang Soc Abstr. 1998b;6:51. [Google Scholar]
- 12.Bernard C, Axelrad H. Effects of recurrent collateral inhibition on Purkinje cell activity in the immature rat cerebellar cortex—an in vivo electrophysiological study. Brain Res. 1993;626:234–258. doi: 10.1016/0006-8993(93)90584-a. [DOI] [PubMed] [Google Scholar]
- 13.Beurrier C, Congar P, Bioulac B, Hammond C. Subthalamic nucleus neurons switch from single-spike to burst-firing mode. J Neurosci. 1999;19:599–609. doi: 10.1523/JNEUROSCI.19-02-00599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolam JP, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience. 1984;12:711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- 15.Calabresi P, Centonze D, Pisani A, Sancesario G, North RA, Bernardi G. Muscarinic IPSPs in rat striatal cholinergic interneurones. J Physiol (Lond) 1998;510:421–427. doi: 10.1111/j.1469-7793.1998.421bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canavier CC, Clark JW, Byrne JH. Routes to chaos in a model of a bursting neuron. Biophys J. 1990;57:1245–1251. doi: 10.1016/S0006-3495(90)82643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chao TI, Alzheimer C. Do neurons from rat neostriatum express both a TTX-sensitive and a TTX-insensitive slow Na+ current? J Neurophysiol. 1995;74:934–941. doi: 10.1152/jn.1995.74.3.934. [DOI] [PubMed] [Google Scholar]
- 18.Chay TR, Rinzel J. Bursting, beating, and chaos in an excitable membrane model. Biophys J. 1985;47:357–366. doi: 10.1016/S0006-3495(85)83926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crutcher MD, DeLong MR. Single cell studies of the primate putamen. II. Relations to direction of movement and pattern of muscular activity. Exp Brain Res. 1984;53:244–258. doi: 10.1007/BF00238154. [DOI] [PubMed] [Google Scholar]
- 20.Debarbieux F, Brunton J, Charpak S. Effect of bicuculline on thalamic activity: a direct blockade of IAHP in reticularis neurons. J Neurophysiol. 1998;79:2911–2918. doi: 10.1152/jn.1998.79.6.2911. [DOI] [PubMed] [Google Scholar]
- 21.Deschênes M, Bourassa J, Doan VD, Parent A. A single-cell study of the axonal projections arising from the posterior intralaminar thalamic nuclei in the rat. Eur J Neurosci. 1996;8:329–343. doi: 10.1111/j.1460-9568.1996.tb01217.x. [DOI] [PubMed] [Google Scholar]
- 22.DiFiglia M. Synaptic organization of cholinergic neurons in the monkey neostriatum. J Comp Neurol. 1987;255:245–258. doi: 10.1002/cne.902550208. [DOI] [PubMed] [Google Scholar]
- 23.Dodt HU, Zieglgansberger W. Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Res. 1990;537:333–336. doi: 10.1016/0006-8993(90)90380-t. [DOI] [PubMed] [Google Scholar]
- 24.Flores-Hernández J, Galarraga E, Pineda JC, Bargas J. Patterns of excitatory and inhibitory synaptic transmission in the rat neostriatum as revealed by 4-AP. J Neurophysiol. 1994;72:2246–2256. doi: 10.1152/jn.1994.72.5.2246. [DOI] [PubMed] [Google Scholar]
- 25.Götz T, Kraushaar U, Geiger J, Lubke J, Berger T, Jonas P. Functional properties of AMPA and NMDA receptors expressed in identified types of basal ganglia neurons. J Neurosci. 1997;17:204–215. doi: 10.1523/JNEUROSCI.17-01-00204.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- 27.Hamann M, Desarmenien M, Desaulles E, Bader MF, Feltz P. Quantitative evaluation of the properties of a pyridazinyl GABA derivative (SR 95531) as a GABAA competitive antagonist. An electrophysiological approach. Brain Res. 1988;442:287–296. doi: 10.1016/0006-8993(88)91514-4. [DOI] [PubMed] [Google Scholar]
- 28.Häusser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–678. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi H, Nakao M, Hirakawa K. Chaos in the self-sustained oscillation of an excitable biological membrane under sinusoidal stimulation. Phys Rev Lett. 1982;88A:265–266. [Google Scholar]
- 30.Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. I. Activities related to saccadic eye movements. J Neurophysiol. 1989;61:780–798. doi: 10.1152/jn.1989.61.4.780. [DOI] [PubMed] [Google Scholar]
- 31.Holden AV, Winlow W, Haydon PG. The induction of periodic and chaotic activity in a molluscan neurone. Biol Cybern. 1982;43:169–173. doi: 10.1007/BF00319976. [DOI] [PubMed] [Google Scholar]
- 32.Horikawa K, Armstrong WE. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods. 1988;25:1–11. doi: 10.1016/0165-0270(88)90114-8. [DOI] [PubMed] [Google Scholar]
- 33.Howe AR, Surmeier DJ. Muscarinic receptors modulate N-, P-, and L-type Ca2+ currents in rat striatal neurons through parallel pathways. J Neurosci. 1995;15:458–469. doi: 10.1523/JNEUROSCI.15-01-00458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaeger D, De Schutter E, Bower JM. The role of synaptic and voltage-gated currents in the control of Purkinje cell spiking: a modeling study. J Neurosci. 1997;17:91–106. doi: 10.1523/JNEUROSCI.17-01-00091.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang ZG, North RA. Membrane properties and synaptic responses of rat striatal neurones in vitro. J Physiol (Lond) 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson SW, Seutin V. Bicuculline methiodide potentiates NMDA-dependent burst firing in rat dopamine neurons by blocking apamin-sensitive Ca2+-activated K+ currents. Neurosci Lett. 1997;231:13–16. doi: 10.1016/s0304-3940(97)00508-9. [DOI] [PubMed] [Google Scholar]
- 37.Kawaguchi Y. Large aspiny cells in the matrix of the rat neostriatum in vitro: physiological identification, relation to the compartments and excitatory postsynaptic currents. J Neurophysiol. 1992;67:1669–1682. doi: 10.1152/jn.1992.67.6.1669. [DOI] [PubMed] [Google Scholar]
- 38.Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimura M, Rajkowski J, Evarts E. Tonically discharging putamen neurons exhibit set-dependent responses. Proc Natl Acad Sci USA. 1984;81:4998–5001. doi: 10.1073/pnas.81.15.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura M, Kato M, Shimazaki H. Physiological properties of projection neurons in the monkey striatum to the globus pallidus. Exp Brain Res. 1990;82:672–676. doi: 10.1007/BF00228811. [DOI] [PubMed] [Google Scholar]
- 41.Kimura M, Kato M, Shimazaki H, Watanabe K, Matsumoto N. Neural information transferred from the putamen to the globus pallidus during learned movement in the monkey. J Neurophysiol. 1996;76:3771–3786. doi: 10.1152/jn.1996.76.6.3771. [DOI] [PubMed] [Google Scholar]
- 42.Lapper SR, Bolam JP. Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience. 1992;51:533–545. doi: 10.1016/0306-4522(92)90293-b. [DOI] [PubMed] [Google Scholar]
- 43.Lee K, Dixon AK, Freeman TC, Richardson PJ. Identification of an ATP-sensitive potassium channel current in rat striatal cholinergic interneurones. J Physiol (Lond) 1998;510:441–453. doi: 10.1111/j.1469-7793.1998.441bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liles SL. Activity of neurons in putamen during active and passive movements of wrist. J Neurophysiol. 1985;53:217–236. doi: 10.1152/jn.1985.53.1.217. [DOI] [PubMed] [Google Scholar]
- 45.Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol (Lond) 1980;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumoto N, Minamimoto T, Graybiel AM, Kimura M. Expression of behaviorally conditioned responses of tonically active striatal neurons depends on thalamic input from CM-Pf complex. Soc Neurosci Abstr. 1997;23:464. [Google Scholar]
- 47.Nakanishi H, Kita H, Kitai ST. Electrical membrane properties of rat subthalamic neurons in an in vitro slice preparation. Brain Res. 1987a;437:35–44. doi: 10.1016/0006-8993(87)91524-1. [DOI] [PubMed] [Google Scholar]
- 48.Nakanishi H, Kita H, Kitai ST. Intracellular study of rat substantia nigra pars reticulata neurons in an in vitro slice preparation: electrical membrane properties and response characteristics to subthalamic stimulation. Brain Res. 1987b;437:45–55. doi: 10.1016/0006-8993(87)91525-3. [DOI] [PubMed] [Google Scholar]
- 49.Phelps PE, Houser CR, Vaughn JE. Immunocytochemical localization of choline acetyltransferase within the rat neostriatum: a correlated light and electron microscopic study of cholinergic neurons and synapses. J Comp Neurol. 1985;238:286–307. doi: 10.1002/cne.902380305. [DOI] [PubMed] [Google Scholar]
- 50.Plenz D, Aertsen A. Neural dynamics in cortex-striatum co-cultures—I. anatomy and electrophysiology of neuronal cell types. Neuroscience. 1996;70:861–891. doi: 10.1016/0306-4522(95)00406-8. [DOI] [PubMed] [Google Scholar]
- 51.Raz A, Feingold A, Zelanskaya V, Vaadia E, Bergman H. Neuronal synchronization of tonically active neurons in the striatum of normal and parkinsonian primates. J Neurophysiol. 1996;76:2083–2088. doi: 10.1152/jn.1996.76.3.2083. [DOI] [PubMed] [Google Scholar]
- 52.Richards CD, Shiroyama T, Kitai ST. Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience. 1997;80:545–557. doi: 10.1016/s0306-4522(97)00093-6. [DOI] [PubMed] [Google Scholar]
- 53.Schultz W, Romo R. Neuronal activity in the monkey striatum during the initiation of movements. Exp Brain Res. 1988;71:431–436. doi: 10.1007/BF00247503. [DOI] [PubMed] [Google Scholar]
- 54.Seutin V, Scuvee-Moreau J, Dresse A. Evidence for a non-GABAergic action of quaternary salts of bicuculline on dopaminergic neurones. Neuropharmacology. 1997;36:1653–1657. doi: 10.1016/s0028-3908(97)00147-0. [DOI] [PubMed] [Google Scholar]
- 55.Stern EA, Kincaid AE, Wilson CJ. Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. J Neurophysiol. 1997;77:1697–1715. doi: 10.1152/jn.1997.77.4.1697. [DOI] [PubMed] [Google Scholar]
- 56.Stuart GJ, Dodt HU, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- 57.Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J Neurosci. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wainer BH, Bolam JP, Freund TF, Henderson Z, Totterdell S, Smith AD. Cholinergic synapses in the rat brain: a correlated light and electron microscopic immunohistochemical study employing a monoclonal antibody against choline acetyltransferase. Brain Res. 1984;308:69–76. doi: 10.1016/0006-8993(84)90918-1. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe K, Kimura M. Dopamine receptor-mediated mechanisms involved in the expression of learned activity of primate striatal neurons. J Neurophysiol. 1998;79:2568–2580. doi: 10.1152/jn.1998.79.5.2568. [DOI] [PubMed] [Google Scholar]
- 60.Wilson CJ. Chemical signalling in the basal ganglia, progress in brain research 99, pp 277–297. Elsevier; Oxford: 1993. The generation of natural firing patterns in neostriatal neurons. [DOI] [PubMed] [Google Scholar]
- 61.Wilson CJ, Groves PM. Spontaneous firing patterns of identified spiny neurons in the rat neostriatum. Brain Res. 1981;220:67–80. doi: 10.1016/0006-8993(81)90211-0. [DOI] [PubMed] [Google Scholar]
- 62.Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson CJ, Chang HT, Kitai ST. Disfacilitation and long-lasting inhibition of neostriatal neurons in the rat. Exp Brain Res. 1983;51:227–235. doi: 10.1007/BF00237198. [DOI] [PubMed] [Google Scholar]
- 64.Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan Z, Surmeier DJ. D5 dopamine receptors enhance Zn2+-sensitive GABA(A) currents in striatal cholinergic interneurons through a PKA/PP1 cascade. Neuron. 1997;19:1115–1126. doi: 10.1016/s0896-6273(00)80402-x. [DOI] [PubMed] [Google Scholar]