Abstract
The spinal nucleus of the bulbocavernosus (SNB) and its target muscles, bulbocavernosus and levator ani (BC/LA), form a sexually dimorphic neuromuscular circuit whose development and maintenance are androgen-dependent. The mechanisms whereby androgen regulates gene expression in the SNB of adult rats are largely unknown, although a retrograde influence from the BC/LA muscles has been suggested to underlie the suppression of calcitonin gene-related peptide (CGRP) expression observed in SNB motoneurons after systemic androgen treatment. A mosaic paradigm was used to determine the site of action of androgen in the regulation of CGRP expression in SNB motoneurons. As a consequence of random X chromosome inactivation, androgenized female rats heterozygous for the tfm androgen receptor (AR) mutation (XwtXtfm-mosaics) express a mosaic of androgen-sensitive and androgen-insensitive motoneurons in the SNB, whereas the BC/LA target musculature appears to be uniformly sensitive to androgens. In adult mosaics, testosterone administration resulted in a reduction in the proportion of androgen-sensitive cells expressing CGRP, whereas no such reduction was observed in the androgen-insensitive population, indicating that neuronal AR plays an essential role in the neuromuscular regulation of CGRP expression in these motoneurons. This provides the first in vivo demonstration of AR regulation of gene expression unambiguously localized to a neuronal population.
Keywords: mosaic, androgen receptor, spinal nucleus of the bulbocavernosus, bulbocavernosus, levator ani, sexual dimorphism, androgen, tfm mutation, penile reflexes, calcitonin gene-related peptide, cell autonomous
Current opinion holds that target muscles regulate many aspects of motoneuron physiology (Vrbova et al., 1994). The spinal nucleus of the bulbocavernosus (SNB) is a sexually dimorphic neuromuscular system whose survival and development are androgen-dependent (Breedlove and Arnold 1981; Freeman et al., 1996), as is the functional and morphological maintenance of the SNB in adulthood (Hart, 1979; Tanaka and Arnold 1993; Watson et al., 1996). However, it has proven difficult to establish the site of action of androgens in the adult, because both the SNB motoneurons and their targets, the perineal muscles bulbocavernosus and levator ani (BC/LA), are androgen-sensitive (Dube et al., 1975; Breedlove and Arnold, 1981;Jordan et al., 1997).
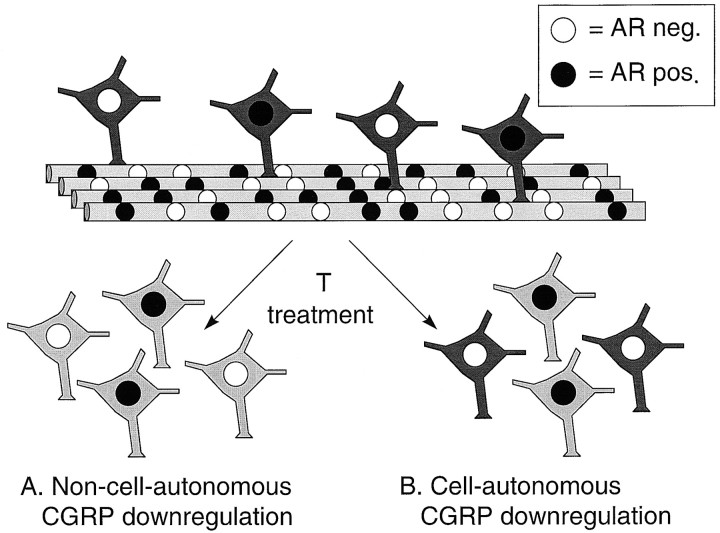
The phenotypic mosaic resulting from the mammalian process of embryonic random X chromosome inactivation (Lyon, 1961; Monk and Harper, 1979) has recently been exploited as a method of localizing androgenic action in the SNB (Freeman et al., 1996; Watson et al., 1996). Specifically, androgenized female rats heterozygous for the tfm mutation (XwtXtfm), which codes a nonfunctional androgen receptor (AR), have been generated to study AR-mediated events in the SNB (Fig. 1). Because early androgen treatment rescues SNB motoneurons indirectly through its actions on the BC/LA muscles (for review, see Forger et al., 1992), these animals exhibit a mosaic of androgen-sensitive and androgen-insensitive motoneurons within the SNB in adulthood (Freeman et al., 1996). This mosaic paradigm has been used to demonstrate both direct and indirect effects of androgen on SNB motoneuron survival and morphology (Freeman et al., 1996; Watson et al., 1996).
Fig. 1.
Diagram of the SNB mosaic model. Because the androgen receptor gene is situated on the X chromosome and SNB motoneuronal survival is not dependent on intraneuronal androgen receptor status (Freeman et al., 1996), androgenized females carrying the tfm androgen receptor mutation (mosaic animals) have ∼50:50 blend of androgen-sensitive wild-type neurons and androgen-insensitive tfm neurons in the SNB, with muscle fibers being multinucleate mosaics (Freeman et al., 1996). For simplicity, SNB motoneurons are represented as synapsing on one muscle fiber, whereas a typical SNB motoneuron will synapse on ∼200 LA muscle fibers, and each fiber may be innervated by more than one motoneuron (Jordan et al., 1992). This SNB mosaic allows for localization of androgenic action as effects of androgen mediated locally within SNB motoneurons (B) should be present only in cells expressing functional androgen receptors and only in the presence of androgen, whereas effects arising indirectly from actions on other target tissues (A) should be present in all SNB motoneurons, whether or not they express functional androgen receptors.
Calcitonin gene-related peptide (CGRP) is an example of a peptide downregulated by androgen in the SNB (Popper and Micevych, 1989). CGRP has been implicated in potentiation of cholinergic transmission (Lu et al., 1993) by upregulating muscle acetylcholine receptor (New and Mudge, 1986) and acetylcholinesterase expression (Hodges-Savola and Fernandez, 1995), as well as in preventing disuse-induced sproutingin vivo (Tsujimoto and Kuno, 1988).
Previous work has suggested that androgenic downregulation of CGRP mRNA expression in SNB motoneurons may be mediated indirectly via the BC/LA target musculature. In rats, target muscle paralysis or intramuscular injection of BC/LA muscle extracts from castrated or intact males regulates the proportion of SNB motoneurons expressing CGRP in adult males (Popper et al., 1992a,b). Furthermore, the proportion of motoneurons expressing CGRP in various motoneuron pools, including the SNB, correlates with muscle activity (Blanco et al., 1997).
Using tfm mosaic animals, we evaluated the alternative possibility that androgens act directly on SNB motoneurons to regulate CGRP synthesis. The approach taken was to compare the effects of chronic systemic testosterone on neighboring SNB cells intfm mosaic animals. If androgen acts directly on SNB motoneurons to regulate CGRP expression, androgen treatment should attenuate CGRP expression primarily in neurons containing functional AR. If, however, androgen acts indirectly via its effects on the BC/LA musculature, it would be expected that androgen treatment would attenuate CGRP expression in both tfm and wild-type SNB motoneurons.
MATERIALS AND METHODS
Generation of mosaic animals. All animals were obtained from breeding colonies at Simon Fraser University (Burnaby, British Columbia, Canada) or the University of California, Berkeley (Berkeley, CA). Mosaic animals were generated by the breeding of known carriers of the tfm mutation. Carriers (XwtXtfm) of the tfmmutation, identified by the presence of pups with internal testes in previous litters, were placed with a sexually vigorous male until copulatory plugs were seen, and the appearance of plugs was taken as the day of conception, embryonic day 0 (E0). Pregnant carriers received daily subcutaneous injections of 2.0 mg of testosterone propionate (TP) (Steraloids, Wilton, NH) in oil from E16 through E21. The prenatal injections of TP serve to maximize SNB motoneuron survival in the pups (Ward et al., 1996), as well as to distinguish between wild-type females, whose nipple lines are completely masculinized by prenatal testosterone (Goldman et al., 1976), and tfm mosaic females, who form a partial nipple line because of the presence of androgen-insensitive nipple tissue. Exogenous perinatal testosterone administration occasionally inhibits vaginal delivery; consequently, litters not delivered by E22 were removed by cesarean section under ether anesthesia. Pups delivered by cesarean section were cross-fostered to a recently parturient lactating wild-type female. Carriers undergoing cesarean section were killed immediately after surgery via a lethal dose of sodium pentobarbitol. Pups were further treated with 1.0 mg of TP subcutaneously on postnatal days 1 (P1) and P3 to maximally masculinize the SNB system (Ward et al., 1996).
The genotype of these androgenized animals was determined at P30 according to the system of phenotypic markers used by Freeman et al. (1996). Briefly, (1) pups with inguinal testes but expressing a full nipple line were identified as tfm-affected males; (2) pups with inguinal or external testes but not expressing a nipple line were identified as wild-type males; (3) pups without inguinal testes but not expressing a nipple line were identified as masculinized wild-type females; and (4) pups without inguinal testes and expressing a partial nipple line were identified as masculinized mosaic females. For all experimental animals, identification was verified through dissection of the reproductive tract at the conclusion of the experiment. This process revealed the presence of three tfm-affected males misidentified as masculinized mosaic females. These animals were excluded from further analysis.
Hormone manipulation in adulthood. At 60 d of age, 11 animals identified as mosaics received 2 × 20 mm SILASTIC (Dow Corning, Midland, MI) implants (1.57 mm inner diameter, 3.18 mm outer diameter) packed with crystalline testosterone (Steraloids). Implants of this size yield plasma testosterone levels in the high physiological range (Damassa et al., 1977). Twelve 60-d-old animals identified as mosaics received 2 × 20 mm empty SILASTIC implants. SILASTIC implants were placed subcutaneously between the scapulae under ether anesthesia.
After 4–6 weeks, the implants were removed under ether anesthesia. After an additional 24–36 hr, animals received subcutaneously either 0.2 or 2.0 mg of hydroxyflutamide (OH-F) (generous gift of R. Neri, Schering-Plough Research Institute, Kenilworth, NJ) in 0.1 ml of propylene glycol. OH-F binds to the AR and induces nuclear translocation of the ligand-receptor complex, facilitating immunocytochemical analysis without itself inducing AR-mediated gene transcription (Kemppainen et al., 1992). The two doses of OH-F that were administered served as a control to ensure this anti-androgen was not itself altering CGRP expression. No effect of OH-F dose on the proportion of SNB cells identified as expressing CGRP or AR was observed. All animals were perfused 4–6 hr after OH-F injection.
Perfusion and tissue preparation. Animals received an overdose of sodium pentobarbital (∼50 mg, i.p.), and, on achievement of surgical anesthesia as measured by the disappearance of deep reflexes, were perfused transcardially with 200 ml of ice-cold PBS, pH 7.4, over 20 min, followed by 200 ml of ice-cold 4% paraformaldehyde–PBS, pH 7.4, over 20 min. Spinal cords were post-fixed in 4% paraformaldehyde for 2 hr at 4°C. Tissue was then transferred to 20% sucrose–PBS at 4°C until the spinal cords sank, after which tissue was sectioned coronally at a thickness of 50 μm on a freezing microtome. Spinal sections corresponding to L5–L6 and S1 were sequentially collected into de Olmos solution (a propylene glycol-based antigen-conserving cryopreservent; Watson et al., 1986) and stored at −20°C until immunocytochemistry was performed. Every third SNB section collected from each animal was used for immunocytochemistry and data collection.
Immunocytochemistry. All reactions were performed at room temperature unless otherwise indicated. Tissue was placed in tissue wells and rinsed five times for 10 min in PBS solution containing 0.1% gelatin and 0.3% Triton X-100 (Sigma, Oakville, Ontario, Canada). Sequential immunocytochemical double-labeling of AR and CGRP was then performed as follows. Endogenous peroxidase activity was quenched by immersion in 0.3% H2O2 for 30 min. After rinsing, the free-floating sections were incubated with PBS–gelatin–Triton (PBS–GT) containing 10% normal goat serum (NGS) (Vector Laboratories, Burlingame, CA) for 1 hr to prevent nonspecific secondary antibody binding. AR immunoreactivity was assessed using the rabbit polyclonal primary antibody PG21, directed against the 21 amino acid C-terminal epitope of the AR (generous gift of Gail Prins, University of Chicago, Chicago, IL). PG21 has been characterized previously in the rat SNB, and SNB AR-IR has been shown to discriminate between motoneurons expressing wild-type AR, which exhibit dense nuclear labeling, and motoneurons expressing nonfunctional tfm mutant AR, which exhibit light, diffuse cytoplasmic staining and clear nuclei (Freeman et al., 1995). Tissue was incubated at 4°C for 36–48 hr with PG21 primary antiserum diluted 1:3000 in PBS–GT containing 1% NGS, under constant agitation on a mixing platform. After rinsing, tissue was incubated with biotin-conjugated goat anti-rabbit antiserum (Vector Laboratories) at 1:250 dilution in PBS–GT for 1 hr. Tissue was rinsed and then incubated with avidin–biotin peroxidase complex (Vectastain Elite; Vector Laboratories) for 1 hr. AR labeling was subsequently visualized using 3,3′ diaminobenzidine (DAB) (Sigma) in the presence of hydrogen peroxide and nickel chloride in 0.1 m Tris buffer, pH 7.2, resulting in a blue–black label. Tissue was thoroughly rinsed of DAB solution, and CGRP immunocytochemistry was begun.
CGRP immunoreactivity was assessed using a rabbit polyclonal antiserum directed against synthetic rat CGRP (Peninsula Laboratories, Belmont, CA). Sections were again incubated in 10% NGS for 1 hr, followed by incubation for 48 hr in CGRP antiserum diluted 1:16000, at 4°C under constant agitation on a mixing platform. Sections were rinsed and incubated with biotin-conjugated goat anti-rabbit secondary antiserum in PBS–GT (1:250 dilution) for 1 hr. After rinsing, sections were incubated in avidin-biotin complex for 1 hr, rinsed, and visualized using DAB without nickel enhancement, yielding a red–brown label. Sections were thoroughly rinsed of DAB solution, mounted on gelatin-coated slides, and coverslipped with Permount (Fisher Scientific, Springfield, NJ) after dehydration through graded alcohols and clearing in xylene.
Identification of CGRP-IR and AR-IR cells. Sections were analyzed under a light microscope (Optiphot-2; Nikon, Tokyo, Japan) at 200× magnification by an experimenter blind to experimental condition (see Fig. 2 for representative photomicrographs). A second observer scored six randomly selected animals as a reliability check.
Fig. 2.
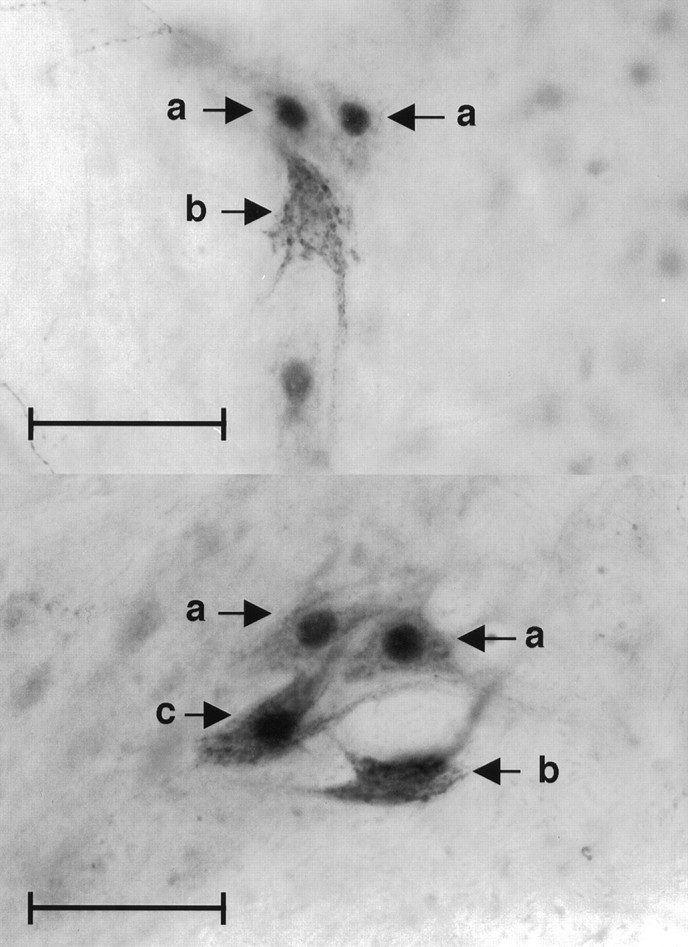
Photomicrographs of tfm-mosaic SNB motoneurons. a, Wild-type (androgen receptor-immunoreactive) cells weakly CGRP-immunoreactive.b, tfm cells strongly CGRP-immunoreactive. c, Wild-type (androgen receptor-immunoreactive) cell strongly CGRP-immunoreactive. Original magnification, 200×. Scale bar, 50 μm.
Wild-type SNB cells were identified as those exhibiting a black nuclear label, whereas tfm SNB cells were identified as those with unlabeled nuclei. SNB cells exhibiting a dark red–brown cytoplasmic label were identified as CGRP-positive, whereas cells exhibiting very light or no cytoplasmic label were identified as CGRP-negative. Cells rated as immunoreactive for either or both proteins were mapped and labeled using a camera lucida attachment.
After identification of immunoreactive SNB cells, coverslips were soaked off in xylene and counterstained with Neutral Red (Sigma). SNB cells unlabeled by either antiserum were identified and added to the cell maps generated in the preceding step. As a result of this procedure, every motoneuron in each mosaic animal’s SNB was assigned to one of the four possible categories: (1) cells containing both labels (AR+/CGRP+), consisting of wild-type neurons expressing CGRP; (2) wild-type cells showing the nuclear AR label but not expressing CGRP (AR+/CGRP−); (3) cells containing neither label (AR−/CGRP−), consisting of tfm cells not expressing CGRP; and (4) tfm cells containing the CGRP label but not the nuclear AR label (AR−/CGRP+). To arrive at a conservative estimate of AR and CGRP expression, cells containing only very light AR or CGRP labeling were considered to be unlabeled. After classification, the number of SNB motoneurons within each category was calculated for each animal.
Analyses. After normalization of the proportion data using an arcsine transformation (Ferguson and Takane, 1989), a 2 × 2 factorial ANOVA was performed for cell type (wild-type vstfm) by treatment condition (testosterone vs blank implants). Subsequent planned comparisons of group means were performed using t tests. Only animals having at least 20 SNB motoneurons, an index for masculinization in this system, were included in statistical analyses; four animals were excluded under this criterion, yielding a total of six testosterone-treated mosaic animals and 10 mosaic animals not treated with steroid.
RESULTS
Effects of ARs and testosterone exposure on CGRP immunoreactivity
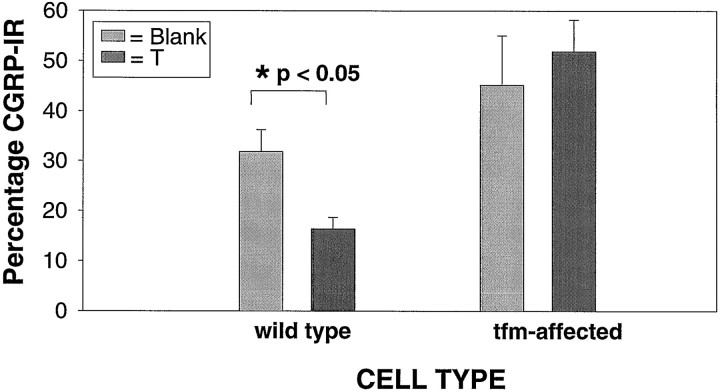
In these mosaic animals, the downregulation of CGRP expression by testosterone was clearly limited to the wild-type SNB neurons, containing functional AR (Figs. 2,3). These data strongly suggest that androgen acts directly via intraneuronal AR to regulate CGRP expression. ANOVA (treatment by cell type) revealed a significant effect for cell type (df = 1,14; F = 8.528; p < 0.05), and subsequent t tests revealed that significantly fewer wild-type than tfm cells expressed CGRP labeling in the testosterone implanted group (df = 5; t = 3.487; p< 0.05) but not in the group receiving blank implants (df = 9; t = 1.146; p> 0.05).
Fig. 3.
Effects of testosterone treatment on proportion of CGRP-IR mosaic SNB motoneurons by cell type. Data are grouped into wild-type cells, which are immunoreactive for androgen receptor and thus expressing functional androgen receptors, andtfm cells, which are not immunoreactive for androgen receptor and thus do not possess functional androgen receptors.Dark bars represent animals receiving testosterone implants in adulthood, and light bars indicate animals receiving blank implants in adulthood. Only the androgen-sensitive wild-type population shows a decrease in the proportion of cells immunoreactive for CGRP in response to androgen treatment. *p < 0.05; t test. Error bars indicate SEM.
Degree of observed mosaicism
As predicted with random X chromosome inactivation, the mean proportion of SNB cells immunoreactive for AR was found to be 49.8%, ranging from 30 to 70% (Table 1). This agrees well with previous descriptions of AR mosaicism in the rat (Freeman et al., 1996). No difference in the degree of mosaicism was observed between the two hormone treatment groups (independent samplest test; df = 14; t = −0.47;p > 0.05).
Table 1.
Characteristics of SNB motoneurons in mosaic rats
| Treatment | n | Number of cells | Proportion AR+ | Proportion CGRP+ |
|---|---|---|---|---|
| Blank | 10 | 58.4 ± 5.6 | 0.49 ± 0.05 | 0.64 ± 0.04 |
| Testosterone | 6 | 52.7 ± 4.6 | 0.52 ± 0.04 | 0.53 ± 0.08 |
All values represent mean ± SEM. AR+ indicates SNB motoneurons identified as being wild type. CGRP+ indicates SNB motoneurons identified as expressing CGRP.
DISCUSSION
It is clear from the present study that the androgenic regulation of CGRP expression in SNB motoneurons is mediated locally. Functional AR within SNB motoneurons are necessary for the androgenic suppression of SNB motoneuronal CGRP expression. This constitutes the firstin vivo demonstration of AR regulation of gene expression unambiguously localized to a specific neural population. The apparent involvement of the intraneuronal AR in the regulation of CGRP expression in the SNB is surprising in light of previous indirect evidence suggesting that the site of action of androgen in modulating SNB CGRP production is the target musculature (Popper et al., 1992a,b).
Although not statistically significant, a smaller proportion of androgen-sensitive SNB cells appears to express CGRP relative to the androgen-insensitive population. Because mosaic animals were neither gonadectomized nor adrenalectomized, this may reflect the actions of endogenous steroids on the functional androgen receptors expressed by these cells. If this is indeed the case, the sensitivity of AR-mediated downregulation of CGRP expression in this system argues for the importance of this process in the course of normal physiology. Alternately, this may reflect a permanent attenuation of adult levels of CGRP expression by perinatal androgens.
Because continued androgen exposure is necessary to maintain low levels of CGRP mRNA (Popper and Micevych, 1990), the most parsimonious interpretation of the present result is a genomic effect of the AR in downregulating CGRP gene expression. Nevertheless, the possibility that CGRP expression is downstream from the genomic effects of the AR in SNB motoneurons remains untested. Several factors regulating motoneuronal CGRP expression have been described, both in vivo andin vitro, including the following: upregulation by nerve growth factor after spinal hemisection (Christensen and Hulsebosch, 1997), downregulation by basic fibroblast growth factor after sciatic nerve transection (Piehl et al., 1998), as well as upregulation by ciliary neurotrophic factor (CNTF) and downregulation by basic fibroblast growth factor in primary cultures of rat motoneurons (Piehl et al., 1998). It is unclear whether and to what extent these factors contribute to the suppression of CGRP expression in SNB motoneurons by AR. It is known, for example, that androgen regulates CNTF receptor expression in SNB motoneurons and in BC/LA target muscles (Xu and Forger, 1998).
Two assumptions inherent in the present model warrant further discussion. The method of genotype identification used (Freeman et al., 1996), although imperfect, likely underestimates the magnitude of AR-mediated regulation of CGRP expression in SNB motoneurons, because any misclassifications of SNB motoneuron genotype in mosaic animals (Freeman et al., 1996) would serve to diminish the observed effect size. Similarly, the assumption of uniform postsynaptic androgen sensitivity in mosaic animals is warranted by observations of high levels (similar to wild-type littermates) of AR antigenicity in mosaic LA targets (Freeman et al., 1996), as well as observations that each SNB motoneuron innervates hundreds of LA muscle fibers and that LA fibers are often multiply innervated (Jordan et al., 1992).
The results of this study challenge the view that androgenic action on the BC/LA target musculature is sufficient for the regulation of CGRP levels in the SNB. Previous work reporting that injections of a BC/LA crude muscle extract modulates SNB CGRP mRNA levels (Popper et al., 1992a,b) suggests that the target musculature may be sufficient to increase CGRP expression in the SNB. However, it is not clear that this type of regulation underlies the suppression of CGRP expression in SNB motoneurons by androgen. Muscle may be involved in the regulation of CGRP levels in SNB motoneurons, but the suppression of CGRP expression resulting from systemic androgen in physiological doses requires functional AR expression in SNB motoneurons.
An interpretation that may reconcile both the mosaic data and the extract injection findings is that the AR in SNB motoneurons has a permissive role in a process of androgenic regulation of CGRP, which includes muscular events. For example, androgen may act on muscle to regulate trophic factor secretion and on motoneurons to regulate cognate receptor expression, enabling a synergistic response to androgen exposure. Because the developmental action of androgen on the SNB is mediated through the target musculature (Freeman et al., 1995;Jordan et al., 1997) and paralysis of muscles leads to increased CGRP expression in SNB motoneurons (Popper and Micevych, 1989), such a mechanism is plausible.
Establishing the role of target musculature in the regulation of motoneurons has proven an especially difficult question to address in the SNB, whose maintenance is regulated by androgens that act on both SNB motoneurons and their target muscles. The use of a mosaic model has simplified the problem of localizing androgenic effects on this system. Where elaborate and indirect methodologies have been necessary to approach this question in vivo, the mosaic model offers a more direct methodology, and the present study provides a demonstration of its utility in studies of gene expression. To date, no mosaic studies have been reported in androgen-regulated neural systems other than the SNB, although such studies may prove valuable in establishing the role of AR activity in the development and maintenance of sexual dimorphism in the nervous system and behavior.
Mosaic studies of gene expression extend the resolution of in vivo genetic analysis from tissue events to cellular events, and in the case of mosaic SNB studies, further allows for the discrimination of developmental effects and those occurring in adulthood, a limitation of gene “knock-out” approaches (Nelson, 1997). With advances in recombinant technologies, the possibility of experimentally inducing mosaic expression of genes other than those normally found on the X chromosome is emerging (Kedzierski et al., 1998).
Footnotes
This study was supported by National Institutes of Health Grant NS28421 and Natural Sciences and Engineering Research Council of Canada Grant 0194522. We are grateful to Drs. Marc Breedlove and Cindy Jordan (University of California, Berkeley) for invaluable and innumerable contributions of expert advice and material.
Correspondence should be addressed to Dr. Neil V. Watson, Department of Psychology, Simon Fraser University, 8888 University Drive, Burnaby, British Columbia, V5A 1S6 Canada.
REFERENCES
- 1.Blanco CE, Popper P, Micevych P. α-CGRP mRNA levels in motoneurons innervating specific rat muscles. Mol Brain Res. 1997;44:253–261. doi: 10.1016/s0169-328x(96)00227-6. [DOI] [PubMed] [Google Scholar]
- 2.Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225:297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- 3.Christensen MD, Hulsebosch CE. Spinal cord injury and anti-NGF treatment results in changes in CGRP density and distribution in the dorsal horn in the rat. Exp Neurol. 1997;147:463–475. doi: 10.1006/exnr.1997.6608. [DOI] [PubMed] [Google Scholar]
- 4.Damassa DA, Smith ER, Tennent B, Davidson JM. The relationship between circulating testosterone levels and male sexual behavior in rats. Horm Behav. 1977;8:275–286. doi: 10.1016/0018-506x(77)90002-2. [DOI] [PubMed] [Google Scholar]
- 5.Dube JY, Lesage R, Tremblay RR. Androgen and estrogen binding in rat skeletal and perineal muscles. Can J Biochem. 1975;54:50–55. doi: 10.1139/o76-008. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson GA, Takane Y. Statistical analysis in psychology and education. Ed 6. McGraw-Hill; New York: 1989. [Google Scholar]
- 7.Forger NG, Hodges LL, Roberts SL, Breedlove SM. Regulation of motoneuron death in the spinal nucleus of the bulbocavernosus. J Neurobiol. 1992;23:1192–1203. doi: 10.1002/neu.480230910. [DOI] [PubMed] [Google Scholar]
- 8.Freeman LM, Padgett BA, Prins GS, Breedlove SM. Distribution of androgen receptor immunoreactivity in the spinal cord of wild-type, androgen-insensitive and gonadectomized male rats. J Neurobiol. 1995;27:51–59. doi: 10.1002/neu.480270106. [DOI] [PubMed] [Google Scholar]
- 9.Freeman LM, Watson NV, Breedlove SM. Androgen spares androgen-insensitive motoneurons from apoptosis in the spinal nucleus of the bulbocavernosus in rats. Horm Behav. 1996;30:424–433. doi: 10.1006/hbeh.1996.0047. [DOI] [PubMed] [Google Scholar]
- 10.Goldman AS, Shapiro BH, Neuman F. Role of testosterone and its metabolites in the differentiation of the mammary gland in rats. Endocrinology. 1976;99:1490–1495. doi: 10.1210/endo-99-6-1490. [DOI] [PubMed] [Google Scholar]
- 11.Hart BL. Activation of sexual reflexes by male rats by dihydrotestosterone but not estrogen. Physiol Behav. 1979;23:107–109. doi: 10.1016/0031-9384(79)90129-x. [DOI] [PubMed] [Google Scholar]
- 12.Hodges-Savola CA, Fernandez Hl. A role for calcitonin-gene related peptide in the regulation of rat skeletal muscle G4 acetylcholinesterase. Neurosci Lett. 1995;190:117–120. doi: 10.1016/0304-3940(95)11517-z. [DOI] [PubMed] [Google Scholar]
- 13.Jordan CL, Pawson PA, Arnold AP, Grinnel AD. Hormonal regulation of motor unit size and synaptic strength during synapse elimination in the rat levator ani muscle. J Neurosci. 1992;12:4447–4459. doi: 10.1523/JNEUROSCI.12-11-04447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan CL, Padgett B, Hershey J, Prins G, Arnold A. Ontogeny of androgen receptor immunoreactivity in lumbar motoneurons and in the sexually dimorphic levator ani muscle of male rats. J Comp Neurol. 1997;379:88–98. [PubMed] [Google Scholar]
- 15.Kedzierski W, Bok D, Travis GH. Non-cell-autonomous photoreceptor degeneration in rds mutant mice mosaic for expression of a rescue transgene. J Neurosci. 1998;18:4076–4082. doi: 10.1523/JNEUROSCI.18-11-04076.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kemppainen JA, Lane MV, Sar M, Wilson EM. Androgen receptor phosphorylation, turnover, nuclear transport and transcriptional activation. J Biol Chem. 1992;267:968–974. [PubMed] [Google Scholar]
- 17.Lu B, Fu W-M, Greengard P, Poo M-M. Calcitonin gene related peptide potentiates synaptic responses at developing neuromuscular junction. Nature. 1993;363:76–79. doi: 10.1038/363076a0. [DOI] [PubMed] [Google Scholar]
- 18.Lyon MF. Gene action in the X chromosome of the mouse (Mus musculus L). Nature. 1961;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- 19.Monk M, Harper MI. Sequential X chromosome inactivation coupled with cellular differentiation in early mouse embryos. Nature. 1979;281:311–313. doi: 10.1038/281311a0. [DOI] [PubMed] [Google Scholar]
- 20.Nelson RJ. The use of genetic “knockout” mice in behavioral endocrinology research. Horm Behav. 1997;31:188–196. doi: 10.1006/hbeh.1997.1381. [DOI] [PubMed] [Google Scholar]
- 21.New HV, Mudge AW. Calcitonin gene-related peptide regulates muscle acetylcholine receptor synthesis. Nature. 1986;323:809–811. doi: 10.1038/323809a0. [DOI] [PubMed] [Google Scholar]
- 22.Piehl F, Cullheim S, Hokfelt T, Hammarberg H. Regulatory effects of trophic factors on expression and distribution of CGRP and GAP-43 in rat motoneurons. J Neurosci Res. 1998;51:1–14. doi: 10.1002/(SICI)1097-4547(19980101)51:1<1::AID-JNR1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 23.Popper P, Micevych PE. The effect of castration on calcitonin gene-related peptide in spinal motor neurons. Neuroendocrinology. 1989;50:338–343. doi: 10.1159/000125243. [DOI] [PubMed] [Google Scholar]
- 24.Popper P, Micevych PE. Steroid regulation of calcitonin gene-related peptide mRNA expression in motoneurons of the spinal nucleus of the bulbocavernosus. Mol Brain Res. 1990;8:159–166. doi: 10.1016/0169-328x(90)90060-q. [DOI] [PubMed] [Google Scholar]
- 25.Popper P, Abelson L, Micevych PE. Differential regulation of α-calcitonin gene-related peptide and preprocholcystokinin messenger RNA expression in α motoneurons. Neuroscience. 1992a;51:87–96. doi: 10.1016/0306-4522(92)90473-f. [DOI] [PubMed] [Google Scholar]
- 26.Popper P, Ulbarri C, Micevych PE. Muscle activity regulates the expression of calcitonin gene-related peptide in motoneurons of the spinal nucleus of the bulbocavernosus. Mol Brain Res. 1992b;13:43–51. doi: 10.1016/0169-328x(92)90043-b. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka J, Arnold AP. Androgenic modulation of the activity of lumbar motoneurons involved in the rat bulbocavernosus reflex. Exp Brain Res. 1993;94:301–307. doi: 10.1007/BF00230300. [DOI] [PubMed] [Google Scholar]
- 28.Tsujimoto T, Kuno M. Calcitonin gene-related peptide prevents disuse-induced sprouting of rat motor nerve terminals. J Neurosci. 1988;8:3951–3957. doi: 10.1523/JNEUROSCI.08-10-03951.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vrbova G, Gordon T, Jones R. Nerve-muscle interaction. Chapman & Hall; New York: 1994. [Google Scholar]
- 30.Ward OB, Ward IL, Eckert MA, Carlucci JR, Wexler AM. Critical periods of sensitivity of sexually dimorphic spinal nuclei to prenatal testosterone exposure in female rats. Horm Behav. 1996;30:407–415. doi: 10.1006/hbeh.1996.0045. [DOI] [PubMed] [Google Scholar]
- 31.Watson NV, Freeman LM, Breedlove SM. Neuronal size in the spinal nucleus of the bulbocavernosus: direct modulation by androgen in animals with mosaic genetic androgen insensitivity. Soc Neurosci Abstr. 1996;26:697. doi: 10.1523/JNEUROSCI.21-03-01062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson RE, Weigand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Forger NG. Expression and androgen regulation of the ciliary neurotrophic factor receptor (CNTFRα) in muscles and spinal cord. J Neurobiol. 1998;35:217–225. [PubMed] [Google Scholar]