Abstract
Difficulty in recording from single neurons in vivohas precluded functional analyses of transmission at central synapses in Drosophila, where the neurotransmitters and receptors mediating fast synaptic transmission have yet to be identified. Here we demonstrate that spontaneously active synaptic connections form between cultured neurons prepared from wild-type embryos and provide the first direct evidence that both acetylcholine and GABA mediate fast interneuronal synaptic transmission in Drosophila. The predominant type of fast excitatory transmission between cultured neurons is mediated by nicotinic acetylcholine receptors (nAChRs). Detailed analysis of cholinergic transmission reveals that spontaneous EPSCs (sEPSCs) are composed of both evoked and action potential-independent [miniature EPSC (mEPSC)] components. The mEPSCs are characterized by a broad, positively skewed amplitude histogram in which the variance is likely to reflect differences in the currents induced by single quanta. Biophysical characteristics of the cholinergic mEPSCs include a rapid rise time (0.6 msec) and decay (τ = 2 msec). Regulation of mEPSC frequency by external calcium and cobalt suggests that calcium influx through voltage-gated channels influences the probability of ACh release. In addition, brief depolarization of the cultures with KCl can induce a calcium-dependent increase in sEPSC frequency that persists for up to 3 hr after termination of the stimulus, illustrating one form of plasticity at these cholinergic synapses. These data demonstrate that cultured embryonic neurons, amenable to both genetic and biochemical manipulations, present a unique opportunity to define genes/signal transduction cascades involved in functional regulation of fast excitatory transmission at interneuronal cholinergic synapses in Drosophila.
Keywords: Drosophila, nAChRs, interneuronal synapses, mEPSC, EPSC, fast excitatory cholinergic transmission
The ability to combine behavioral and biochemical approaches with sophisticated molecular genetics has made Drosophila an important organism for identification of genes involved in the development and functioning of the nervous system. Furthermore, the electrophysiological studies feasible at the larval and embryonic neuromuscular junction (NMJ) have made it possible to assess the phenotypic consequences of mutating specific genes, providing important insights into the molecular mechanisms underlying transmission at peripheral glutamatergic synapses (Wu, 1996; Wu and Bellen, 1997). The NMJ in Drosophila also exhibits features of activity-dependent synaptic plasticity, including paired-pulse facilitation and post-tetanic potentiation. Alterations in these properties in a number of mutant and transgenic strains have demonstrated that cAMP–cAMP response element-binding protein (Zhong and Wu, 1991; Davis et al., 1996) and CaM kinase II signaling (Griffith et al., 1994; Wang et al., 1994) are involved in regulating plasticity at these glutamatergic synapses. Because some of these animals exhibit learning and memory defects, it seems likely that these signaling cascades are also important in regulating transmission and plasticity at central synapses.
Prerequisite to testing this hypothesis is the ability to examine the functional properties of fast transmission at central synapses in mutant and wild-type flies. However, to date, the technical challenge of recording from single neurons in the CNS has precluded electrophysiological analyses of central synaptic transmission inDrosophila. Although a recently described intact embryonic nerve cord preparation suggests that it will be possible to examine some aspects of fast synaptic transmission in vivo (Baines and Bate, 1998), an alternate approach that has been used successfully to study the functional properties of transmission at central synapses in other animals focuses on connections that form between neurons grown in cell culture (Banker and Goslin, 1991). Drosophilaneurons, from both embryos (Seecof et al., 1973; Wu et al., 1990;O’Dowd, 1995) and larvae (Wu et al., 1983), differentiate in dissociated cell culture under various growth conditions. Standard whole-cell recording techniques have documented the expression of voltage-gated ion channels (Solc et al., 1987; O’Dowd and Aldrich, 1988; O’Dowd et al., 1989; Leung and Byerly, 1991; O’Dowd et al., 1995; Tsunoda and Salkoff, 1995) and the development of electrical excitability in cultured neurons (Saito and Wu, 1991; O’Dowd, 1995;Zhao and Wu, 1997). Development of the electrical membrane properties of neurons in embryonic cultures appears to be similar to maturation occurring in vivo based on data obtained from neurons in an intact embryonic nerve cord preparation (Baines and Bate, 1998).
Here we demonstrate that embryonic Drosophila neurons are also capable of forming functional synaptic connections with each other in culture, providing a unique opportunity to examine the electrophysiological properties of interneuronal synaptic transmission. In these neurons the predominant form of fast excitatory synaptic transmission is cholinergic and is mediated by nicotinic acetylcholine receptors (nAChRs). Spontaneous synaptic currents mediated by GABA receptors were also observed in a subpopulation of neurons. Comparison of the synaptic currents in neurons cultured from mutant and transgenic animals with the wild-type data presented in this paper will enable identification of specific genes involved in the function/regulation of central synaptic transmission.
MATERIALS AND METHODS
Fly strains. Two wild-type populations were used in this study. The first strain (wt1) refers to a Canton-S stock maintained in the laboratory for 10 years. The second stock (wt2) is a “Cantonized” white stock (w1118:CS10) generated by outcrossing between w1118 and a Canton-S stock for 10 generations (Boynton and Tully, 1992). wt2 was kindly provided by Dr. T. Tully (Cold Spring Harbor Laboratory).
Cultures. Each culture was prepared from two midgastrula stage embryos, plated on uncoated glass coverslips, and grown in a defined medium (DDM1) as described previously (O’Dowd, 1995). Cultures were maintained in an incubator supplied with 5% CO2 at 23–25°C for up to 9 d.
Immunostaining. Choline acetyltransferase (ChAT) and GABA expression were examined using the Vectastain avidin–biotin–peroxidase complex method (Vector, Burlingame, CA). Neurons at 3–9 d in vitro (DIV) were fixed with 4% paraformaldehyde for 30 min at room temperature. For ChAT staining the primary antibody, monoclonal antibody 4B1 (Takagawa and Salvaterra, 1996), was used at a dilution of 2 μg/ml of IgG in a 4°C overnight incubation. This antibody was kindly provided by Dr. P. Salvaterra (City of Hope). The secondary antibody (biotinylated anti-mouse IgG; Sigma, St. Louis, MO) was used at a 1:500 dilution in a 2 hr room temperature incubation. For GABA staining the primary antibody (polyclonal anti-GABA antibody; Sigma) was used at a dilution of 1:1000 in a 4°C overnight incubation. The secondary antibody (biotinylated anti-rabbit IgG; Sigma) was used at a 1:200 dilution in a 3 hr room temperature incubation. For both ChAT and GABA staining the streptavidin–peroxidase complex solution was applied at a 1:50 dilution for 1 hr at room temperature. The DAB reaction was processed for 1–2 min, a time chosen such that no background staining was observed in control cultures processed in parallel in the absence of primary antibody. In each experiment the total number of neurons and the number of immunopositive neurons were counted in four randomly chosen fields of view on four different coverslips, and the percentage of positive neurons was calculated. The mean percentage of positive neurons was determined from the average percentage of labeled neurons in four or five independent experiments for ChAT and GABA, respectively. The classification of cells as neuronal, based on their morphology, is justified by a previous study demonstrating that >90% of the differentiated cells in these cultures are stained with anti-HRP antibodies (O’Dowd, 1995) that specifically bind toDrosophila neurons (Jan and Jan, 1982).
Electrophysiology. The whole-cell recording technique was used to monitor EPSCs and EPSPs from cultured neurons between 1 and 9 DIV. EPSCs were recorded with whole-cell pipettes (3–6 MΩ) filled with internal solution containing (in mm): 120 CsOH, 120d-gluconic acid, 0.1 CaCl2, 2 MgCl2, 20 NaCl, 1.1 EGTA, and 10 HEPES, pH 7.2. For EPSP and action potential (AP) recordings, potassium gluconate (120 mm) was substituted for CsOH and d-gluconic acid. The external solution contained (in mm): 140 NaCl, 1 CaCl2, 4 MgCl2, 3 KCl, 5 HEPES, pH 7.2. All data shown are corrected for the 5 mV liquid junction potentials generated in these solutions. Resting potentials were measured as the voltage drop observed in current-clamp mode after establishment of the whole-cell configuration. EPSCs recorded in the absence of TTX were defined as sEPSCs, and those recorded in the presence of 1 μm TTX were defined as mEPSCs. The following additional drugs were bath-applied:d-tubocurarine (curare, 100 nm; Aldrich Chemicals), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 5 μm), d(−)-2-amino-5-phosphonopentanoic acid (APV, 50 μm), and picrotoxin (PTX, 10 μm). Data were acquired with an Axopatch 1D (Axon Instruments) amplifier, a Digidata D-A converter (Axon Instruments), a pentium-equipped Gateway 2000 computer, and either pCLAMP6 (Axon Instruments) or SCAN (SES, University of Strathclyde, Scotland) software. All recordings were made at room temperature.
Analysis of mEPSCs. mEPSC frequency in a single neuron was determined from 20–100 current traces, each 500 msec in duration, all obtained between 2–7 min after breaking into the whole-cell configuration. During this time there was no significant rundown in frequency. Individual events were detected using an automated mini detection software (Mini Analysis Program, Jaejin Software) with threshold criteria of 10 pA (fourfold greater than the RMS noise level of 2.5 pA) and a charge transfer of 7 fC. Single-channel activity (observed only rarely) was manually rejected during visual inspection of each record. Current traces were filtered at 2 kHz and digitized at 4 kHz using PClamp 6 software. mEPSC biophysical properties were determined from records filtered at 2 kHz and digitized at 20 kHz using SCAN, a trigger-based detection program with an amplitude threshold of 10 pA. The mean amplitude and rise time were determined by averaging the values obtained from 25 or more single events in each neuron. Decay time constants were determined by fitting a single exponential distribution to the falling phase of the averaged mEPSC from each neuron. A small number of mEPSCs with rise times >2 msec were excluded from this analysis.
KCl stimulation. Cultures between 3 and 6 DIV were depolarized by brief exposure to high potassium using the following procedures. (1) 1x KCl: Coverslips were transferred into Petri dishes containing DDM1 growth medium to which 50 mm KCl had been added. After a 5 min incubation the coverslips were briefly washed three times in fresh DDM1. Coverslips were transferred into dishes containing fresh DDM1 and returned to the CO2 incubator for 1 hr before electrophysiological analysis. (2) 3x KCl: KCl exposure and wash were repeated three times, separated by 5 min intervals in DDM1. After the last wash, coverslips were transferred into dishes containing fresh DDM1 and returned to the CO2 incubator for 1 hr before electrophysiological analysis. (3) 3x KCl + 3 mmCo2+: The same protocol was used as for 3x KCl, with the modification that the high potassium solution also contained 3 mm cobalt. The sEPSC frequency in mock-treated cultures, processed in parallel with treated cultures where normal DDM1 replaced the DDM1 supplemented with KCl, was not significantly different from untreated cultures, and therefore the data in these two groups were pooled for statistical comparisons. Two-thirds of the whole-cell recordings were performed blind with respect to the treatment conditions.
RESULTS
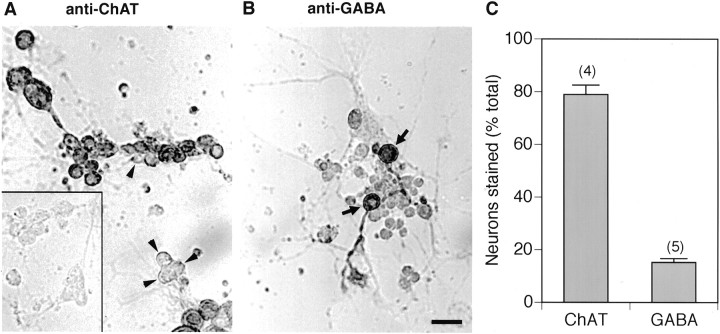
Previous studies from our laboratory documenting the differentiation of electrically excitable neurons in dissociated cell cultures prepared from midgastrula stage Drosophila embryos (O’Dowd, 1995) did not report evidence of spontaneous synaptic activity in these cells. This is perhaps not surprising in that these experiments focused primarily on the properties of large isolated neurons at 1–2 DIV. However, many of the neurons in these cultures were physically contacted by the neurites of a number of neighboring cells, and this led us to ask whether these neurons could form functional synaptic connections with each other. Initial experiments to examine this question were directed at determining the pattern of neurotransmitter expression in the cultured Drosophilaneurons. Immunocytochemical studies revealed that many of the cultured neurons, when plated at a moderately high density, were stained with a monoclonal antibody to ChAT, the synthesizing enzyme for the neurotransmitter acetylcholine (Fig.1A). A smaller population of neurons was stained with a polyclonal antibody for the neurotransmitter GABA (Fig. 1B). Quantitative analysis revealed that ∼80% of the cultured neurons (3–6 DIV) were ChAT positive and 15% were GABA positive (Fig. 1C). These findings suggest that most of the neurons use acetylcholine as a neurotransmitter, whereas a much smaller subset of the cells uses GABA.
Fig. 1.
ChAT and GABA expression in culturedDrosophila neurons. A, Bright-field photomicrograph of a culture at 6 DIV stained with a monoclonal antibody to choline acetyltransferase (ChAT). Although the majority of neurons are positive in this field of view, unstained neurons are indicated by arrowheads.Inset, Background level of staining in the absence of primary antibody. B, Bright-field photomicrograph of a culture at 4 DIV stained with a polyclonal antibody to GABA. Two positive neurons are indicated by arrows. Scale bar, 20 μm. C, Quantitative analysis of the percentage of neurons positive for ChAT or GABA assessed in cultures between 3 and 6 DIV. Bars indicate SEM, and number of experiments is indicated inparentheses.
Fast synaptic transmission mediated by nAChR and GABA receptors in cultured Drosophila neurons
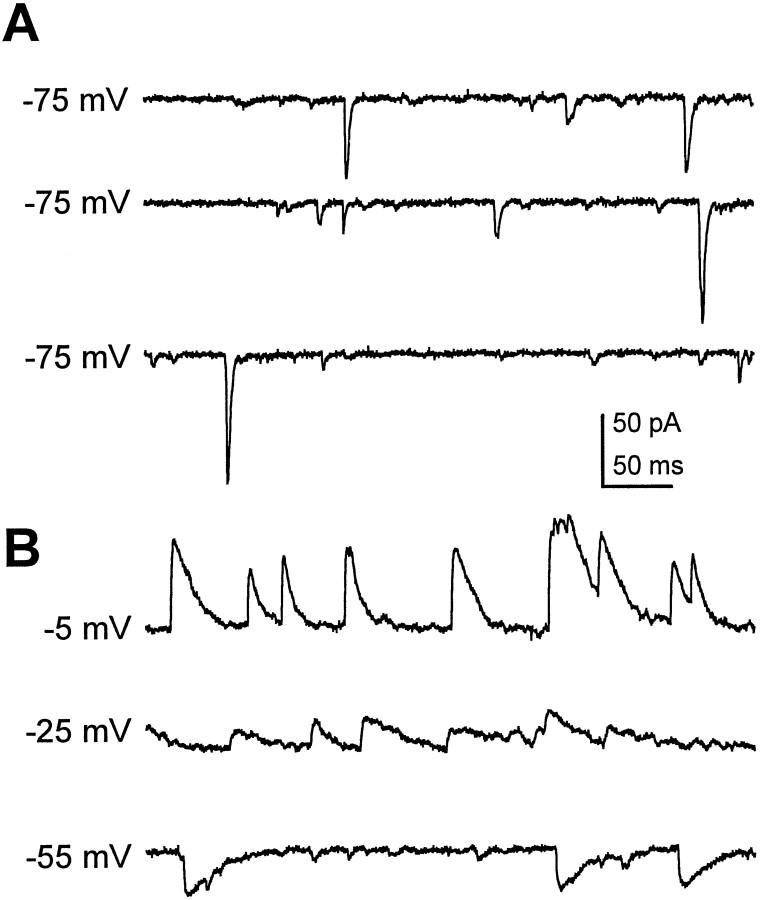
Whole-cell recordings, from neurons between 3 and 9 DIV, revealed that in addition to expressing both GABA and ChAT, these neurons are capable of forming spontaneously active functional synaptic connections with each other. Spontaneous PSCs (sPSCs) (Fig.2A), characterized by a rapid rise and decay, were observed in ∼50% of the cultured wild-type neurons that exhibited physical contacts with neurites from neighboring cells. Initial experiments demonstrated the presence of a second kinetically distinct classes of sPSCs in a subpopulation of neurons (Fig. 2B). In our standard recording solutions, these slowly decaying events were inward at −55 mV but were clearly outward at potentials more depolarized than −30 mV (Fig.2B). This reversal, near the theoretical chloride equilibrium potential in these conditions (ECl = −46 mV), suggested that these events were mediated by GABA-gated chloride channels. This was confirmed by the blockade of these currents with PTX, a specific GABA receptor antagonist (data not shown). In some neurons both the fast transient currents and slow GABAergic currents were observed. Therefore, to examine the properties of rapid transient sPSCs in isolation, all of the subsequent recordings were obtained in the presence of PTX (10 μm).
Fig. 2.
Two classes of kinetically distinct spontaneous synaptic currents recorded from cultured Drosophilaneurons. A, Fast sPSCs recorded from a neuron at 3 DIV, typical of the currents recorded in ∼50% of the wild-type neurons. Holding potential was −75 mV. B, An example of a kinetically distinct class of sPSCs, recorded in a second wild-type neuron at 3 DIV, is characterized by a slow decay time constant. These slow sPSCs are inward when the cell is held at −55 mV and outward at −25 and −5 mV, reversing near the calculated chloride equilibrium potential (−46 mV).
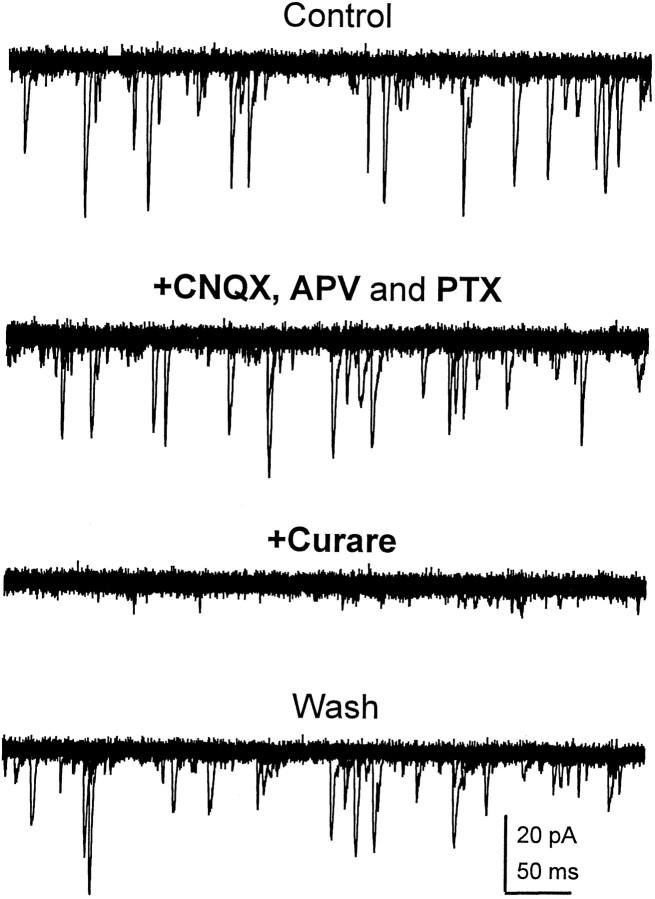
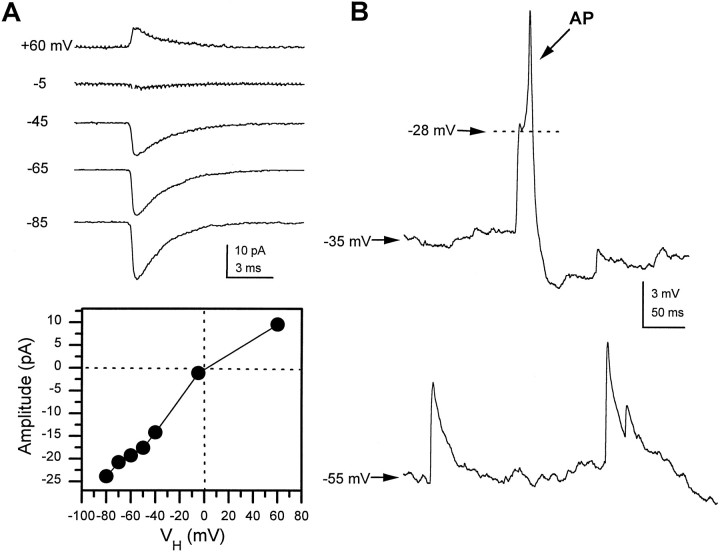
The reversible blockade of the rapid transient sPSCs by bath application of 100 nm curare demonstrates that they are mediated by nAChRs (Fig. 3). These currents were not affected by either glutamate (CNQX, APV) or GABA receptor (PTX) antagonists. To determine whether the cholinergic PSCs were excitatory, we examined their reversal potential. In a single neuron at 7 DIV the averaged PSC (20–50 individual events) was inward at voltages below −45 and outward at +60 mV (Fig.4A). A current–voltage relationship generated by measuring the peak amplitude at each voltage shows a reversal potential near 0 mV (Fig. 4A). A similar depolarized reversal potential was observed in all the neurons examined (n > 10), suggesting that these fast cholinergic PSCs are excitatory. This was confirmed by current-clamp recordings, where spontaneous cholinergic postsynaptic potentials were able to trigger APs in the recipient neuron (Fig.4B). These data demonstrate that the predominant form of fast excitatory synaptic transmission in the culturedDrosophila neurons is cholinergic and mediated by nAChRs.
Fig. 3.
Fast transient synaptic currents are mediated by nAChRs. Superimposed current traces (20 × 500 msec sweeps) at a holding potential of −75 mV during bath perfusion with indicated drugs at the following concentrations: APV, 50 μm; CNQX, 5 μm; PTX, 10 μm; and curare, 100 nm. Records obtained from a single neuron at 6 DIV. Fast sPSCs were reversibly blocked by bath application of curare but were not affected by CNQX, APV, or PTX.
Fig. 4.
Cholinergic synaptic currents are excitatory. A, Averaged mEPSCs (>20 traces at each potential) recorded in the presence of 1 μm TTX from a single neuron at 6 DIV at indicated holding potentials. The peak current–voltage relationship indicates a reversal potential near 0 mV. B, In current-clamp mode, nAChR-mediated sEPSPs were recorded from a single neuron (6 DIV) at two different holding potentials. At rest, −35 mV in this neuron, an sEPSP triggers a spontaneous AP with a threshold at −28 mV. When the cell is held at −55 mV by injection of hyperpolarizing current, the sEPSPs are depolarizing, but they do not give rise to regenerative events.
AP-dependent and AP-independent components of fast excitatory transmission
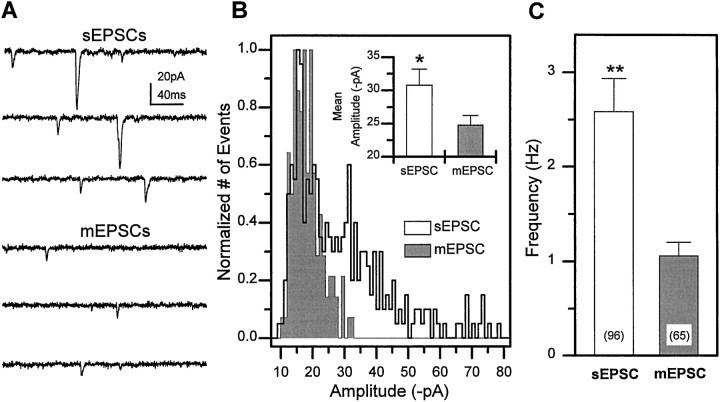
Fast cholinergic sEPSCs recorded from the cultured neurons have both an AP-dependent and an AP-independent component as illustrated in Figure 5. Recordings of sEPSCs from a single neuron at 5 DIV reveals a large variability in the amplitudes of individual events (Fig. 5A,C). Bath application of 1 μm TTX, to block generation of spontaneous APs, reduced the mean current amplitude (Fig. 5A,B). These events are AP independent and are defined as cholinergic mEPSCs. In the population of neurons examined, the mean amplitude of the sEPSCs was significantly larger than that of the mEPSCs (30.7 ± 2.3, n = 16 and 24.8 ± 1.4, n = 24, respectively;p < 0.05, Student’s t test). The frequency of sEPSCs and mEPSCs in the population of neurons examined was quite variable (0.1 to ≥10 Hz), but the average sEPSC frequency (2.6 Hz) was also significantly higher than the average mEPSCs frequency (1.1 Hz) (Fig. 5C). These data demonstrate that evoked currents driven by spontaneous Na-dependent action potentials comprise a significant component of the sEPSCs in the population of neurons examined.
Fig. 5.
sEPSCs have both TTX-sensitive and TTX-insensitive components. A, sEPSCs recorded from a single wild-type neuron (5 DIV) at −75 mV in normal external solution (top three traces). mEPSCs recorded from the same neuron after perfusion of the bathing media with 1 μm TTX to block spontaneous APs (bottom three traces).B, Amplitude distribution of sEPSCs versus mEPSCs recorded from the same neuron. Inset, The mean sEPSC amplitude was significantly larger than the mEPSC amplitude in the population of neurons examined (*p < 0.05, Student’s t test). C, The average sEPSC frequency was also significantly higher than mEPSC frequency in wt1 neurons (**p < 0.01, Student’s ttest). Bars indicate SEM, and number of neurons is indicatedparentheses.
Increase in sEPSC incidence and frequency during early developmentin vitro
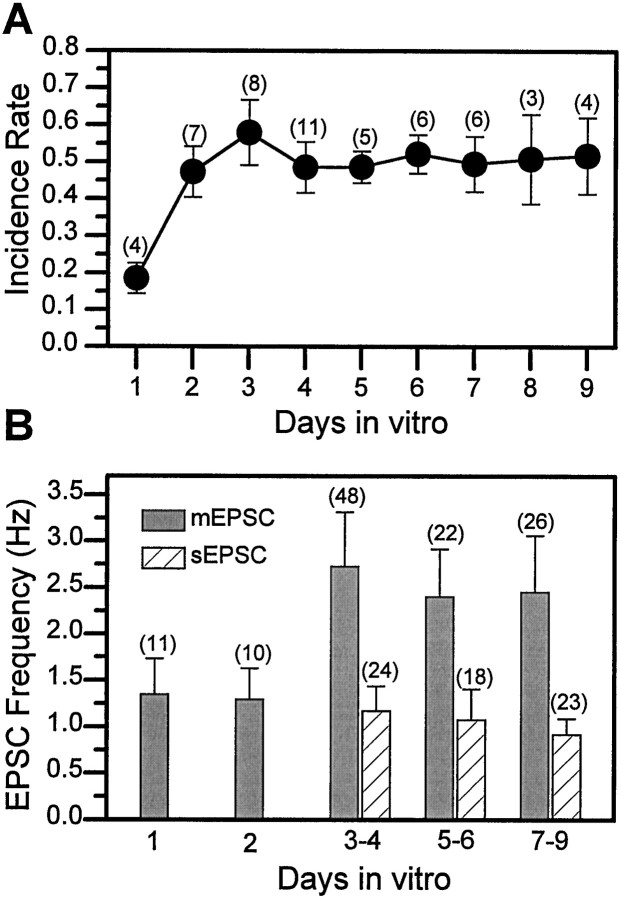
Initial experiments at 3–4 DIV, where many neurons are contacted by processes from one or more neighboring cells, revealed that sEPSCs were observed in ∼50% of the neurons examined. However, neurons in cultures prepared from midgastrula stage embryos undergo extensive neurite outgrowth during the first 24 hr, and by 1 DIV the processes of some neurons are already in contact with those of others. sEPSCs were recorded in ∼18% of the neurons exhibiting physical contacts with other cells at 1 DIV (Fig.6A). This incidence rate was significantly lower than the 50% characteristic of neurons in cultures examined between 2 and 9 DIV. In the cells with currents, the mean sEPSC frequency observed in neurons at both 1 and 2 DIV was also lower than that seen at 3 DIV. There was no further change in these parameters between 3 and 9 DIV (Fig. 6B). Therefore, although the neurites continue to elaborate between 3 and 9 DIV, the sEPSC incidence and frequency are stable under the standard growth conditions used.
Fig. 6.
Incidence and frequency of EPSCs are stable in neurons at ≥ 3 d in vitro (DIV).A, The average percentage of neurons in which sEPSCs were recorded was determined on a daily basis from multiple experiments (n) with total of 17–69 wild-type neurons examined at each day. B, In the cells in which synaptic currents were recorded, the sEPSC/mEPSC frequency was determined and plotted as a function of days in culture. All sEPSCs were recorded in control saline, and all mEPSCs were recorded in the presence of 1 μm TTX. Bars indicate SEM, and number of neurons is indicated in parentheses.
Broad, positively skewed mEPSC amplitude distribution is independent of release probability
Amplitude histograms of mEPSP/mEPSCs at peripheral cholinergic synapses in vertebrates are characterized by a single Gaussian distribution or the sum of multiple Gaussians with distinct modes (Katz, 1966). This suggests little variance in the response induced by release of a single quantum (vesicle) of ACh at these synapses. In contrast, the range of mEPSC amplitudes at cholinergic synapses in a single Drosophila neuron was broad (Fig.7A), and the amplitude histograms were positively skewed, rather than showing a single Gaussian distribution or multiple peaks at defined modes (Fig.7B). Theoretically, the relatively large variance in mEPSC amplitude could be caused by activation of currents at a number of different locations that were variably attenuated by electrotonic decay. However, we found no evidence of a correlation between amplitude and decay time constant of the individual mEPSCs (Fig. 7C), suggesting that electrotonic filtering is not responsible for the broad amplitude distribution.
Fig. 7.
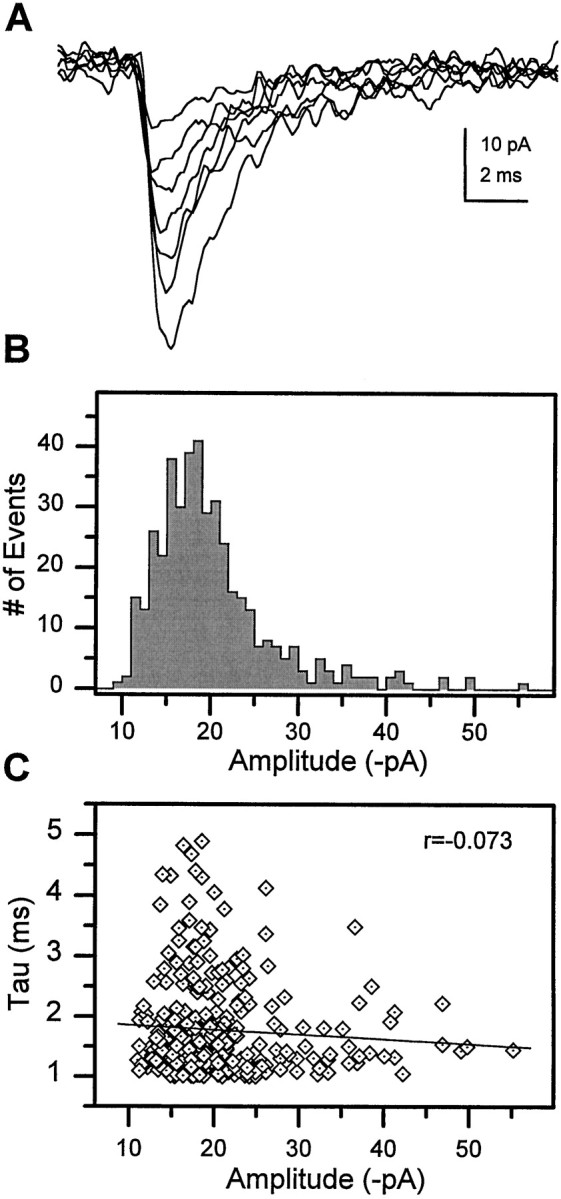
Broad, skewed mEPSC amplitude histogram in single cells. A, Variability in the amplitude of individual mEPSCs in a single neuron at 7 DIV. B, The mEPSC amplitude histogram constructed from 408 individual events recorded from this neuron reveals a broad distribution that is positively skewed. C, Scatter plot of amplitude versus decay time constant for the individual events. A linear regression fit to the data demonstrates little correlation (r = −0.073), indicating that the amplitude variability is not caused by electrotonic filtering.
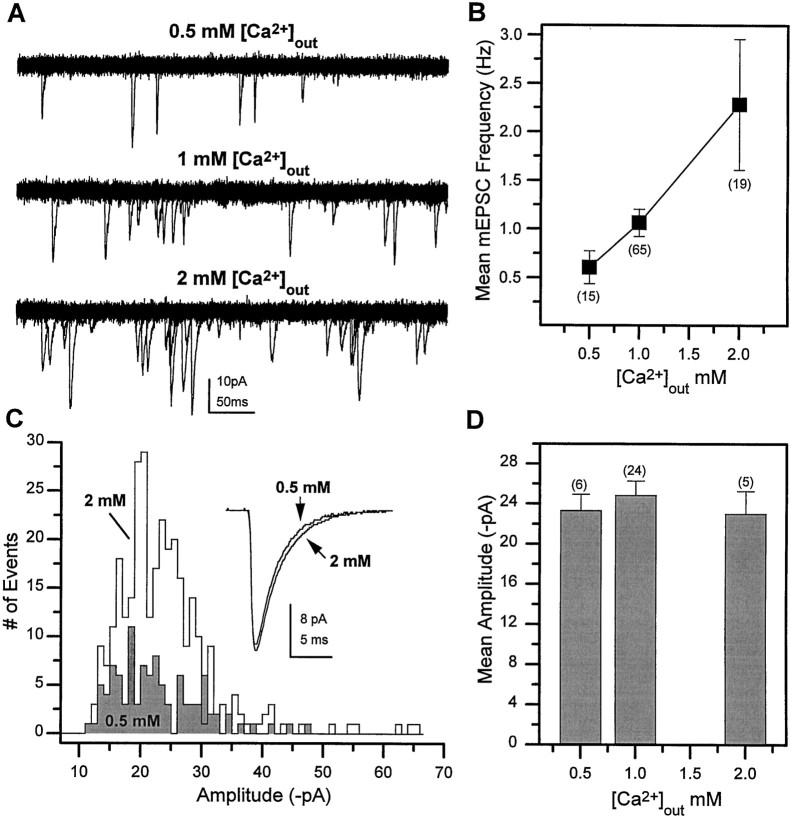
To determine whether mEPSCs generated by release of multiple quanta might contribute to the variation in amplitude, we investigated the relationship between release probability and mEPSC amplitude. If mEPSCs represent currents resulting from release of a mixture of single and multiple quanta, the mEPSC amplitude distribution should co-vary with release probability. However, if each mEPSC represents the response to a single quantum, then the amplitude should be independent of release probability. Similar to the findings in several other preparations, mEPSC frequency (release probability) was influenced by extracellular calcium concentrations at these cholinergic synapses inDrosophila. Under the standard recording conditions of 1 mm Ca2+, the mEPSC frequency was 1.06 ± 0.14 Hz. Increasing extracellular calcium to 2 mm increased the mEPSC frequency, whereas a frequency lower than rest was observed at 0.5 mm Ca2+(Fig. 8A). A threefold change in frequency was observed between 0.5 and 2 mm in the population of wild-type neurons examined (Fig.8B). When no exogenous calcium was added to the recording solution, mEPSCs were observed in only a small percentage of the neurons examined (3/34). As predicted for currents generated by release of a single vesicle, the mEPSC amplitude was independent of release probability, as illustrated by the overlapping mEPSC amplitude histograms generated from a single cell, at two different calcium concentrations (0.5 and 2 mm Ca2+) (Fig.8C). Furthermore, there was no change in the mean amplitude as a function of calcium concentration in the population of neurons examined (Fig. 8D). These data support the conclusion that the broad, skewed distribution of mEPSC amplitudes represents the variability in the currents induced by single quanta.
Fig. 8.
mEPSC frequency but not amplitude is regulated by extracellular Ca2+ concentration.A, Superimposed current traces (20 × 500 msec sweeps in each) obtained at three different extracellular calcium concentrations in the presence of 1 μm TTX.B, Mean mEPSC frequency in the indicated number of neurons at three different calcium concentrations. C, mEPSC amplitude histograms from a single cell at 2 mm and 0.5 mm Ca2+. Inset, Averaged mEPSCs generated from 103 events in 0.5 mm Ca2+and from 310 events in 2 mm Ca2+.D, The mean mEPSC amplitude observed in the indicated number of neurons at three different external calcium concentrations. Bars indicate SEM, and number of neurons is indicated inparentheses.
mEPSC frequency regulated by calcium entry through voltage-gated channels
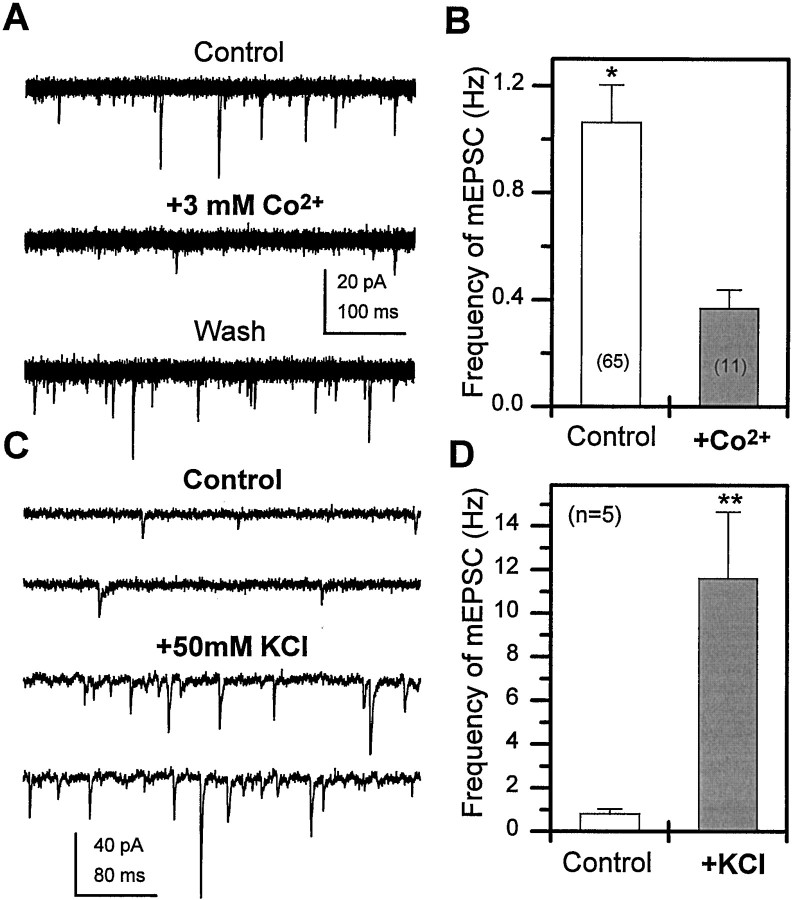
To determine whether the dependence of mEPSC frequency on extracellular calcium concentration was mediated by voltage-gated Ca2+ channels, we examined mEPSCs in the standard external concentration of 1 mm Ca2+ in the presence and absence of a calcium channel blocker. As illustrated in Figure 9A, bath application of 3 mm Co2+ reversibly decreases mEPSC frequency in a single neuron. The average mEPSC frequency recorded in 1 mm Ca2+ + 3 mmCo2+ was significantly lower than that recorded in 1 mm Ca2+ alone, in the population of neurons examined (Fig. 9B). These data demonstrate that AP-independent influx of calcium ions through voltage-gated calcium channels influences the probability of ACh release from the presynaptic terminals at rest. The effects of focal depolarization on mEPSC frequency was also examined by recording from a neuron before and during pressure ejection of 50 mm KCl in the presence of TTX (Fig. 9C). A rapid and dramatic increase in the mEPSC frequency of approximately 11-fold was observed during the depolarizing stimulus (Fig. 9D). This finding suggests that depolarization of nerve terminals, in the absence of sodium-dependent APs, also influences the probability of release.
Fig. 9.
Flux of calcium through voltage-gated ion channels regulates mEPSC frequency at rest and during depolarization.A, Superimposed current traces (20 × 500 msec sweeps in each) reveal a reversible decrease in mEPSC frequency when 3 mm Co2+ is added to the recording solution (1 μm TTX, 1 mmCa2+). B, The mean mEPSC frequency was significantly lower in recordings obtained from neurons in the presence of 3 mm Co2+ when compared with control (*p < 0.05, Student’s ttest). C, Top two traces represent current recordings from a neuron in control solution (1 μm TTX, 1 mm Ca2+). Thebottom two traces, recorded after pressure ejection of 50 mm KCl from a pipette located ∼20 μm from the cell body, represent the average increase in mEPSC frequency seen during the 10 sec KCl puff. D, The mean mEPSC frequency recorded before (Control) and during the 10 sec puff of 50 mm KCl (**p < 0.01, Student’st test). Bars indicate SEM, and number of neurons is indicated in parentheses.
Kinetic properties of cholinergic mEPSCs
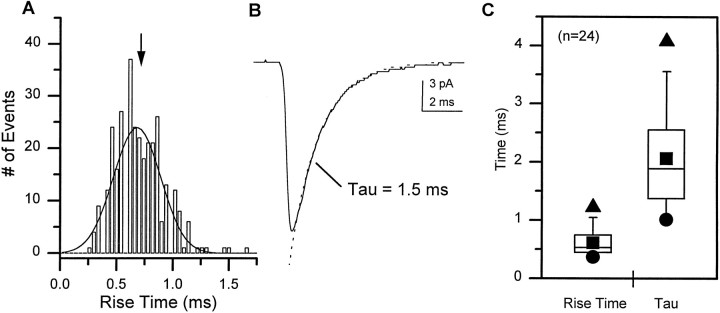
Kinetic parameters of the mEPSCs, reflecting the properties of the nAChRs in the postsynaptic membrane, were assessed in individual neurons. The distribution of the 10–90% rise times of mEPSCs in single cells could be reasonably fit with a single Gaussian distribution (Fig.10A). The mean mEPSC rise time for each neuron was determined by averaging the rise times of all individual events. The decay time constant was determined by fitting a single exponential distribution to the decay phase of the average mEPSC (Fig. 10B). Most neurons exhibited both a rapid rise time and a fast decay time constant. A box plot illustrates the range and variability in these parameters for the whole population of neurons examined (Fig. 10C). There was no indication from these analyses of more than one kinetically distinct population of mEPSCs. Furthermore, analysis of the data as a function of development did not reveal any significant differences in mEPSC properties between 3 and 9 DIV (Table1).
Fig. 10.
Cholinergic mEPSCs are characterized by rapid activation and decay kinetics. A, A histogram of the 10–90% rise time was generated from 314 events recorded from a single neuron at 7 DIV. The data are fit with a single Gaussian curve with the mean indicated by the arrow. B, The averaged mEPSC in this neuron exhibits a typical rapid decay phase that is well fit by a single exponential distribution with a τ of 1.5 msec. C, A box plot illustrates the variability in the 10–90% rise time and decay time constant for the mEPSCs in the population of neurons examined. The 5th and 95th percentiles are indicated by the whiskers, and the 25th, 50th, and 75th percentile boundaries are indicated by thebox for each group. ▴, Maximum values; ●, minimum values; ▪, the mean.
Table 1.
Biophysical properties of cholinergic mEPSCs in wt1 neurons do not change between 3 and 9 DIV
| Age (DIV) | Amplitude (pA) | Rise time (msec) | τ (msec) | n |
|---|---|---|---|---|
| 3–4 | 24.9 ± 2.8 | 0.62 ± 0.12 | 1.89 ± 0.33 | 7 |
| 5–6 | 24.9 ± 3.1 | 0.61 ± 0.14 | 2.18 ± 0.29 | 5 |
| 7–9 | 25.1 ± 2.1 | 0.61 ± 0.20 | 2.11 ± 0.26 | 12 |
The characteristic properties of cholinergic transmission described in this paper are not specific to the particular strain of wild-type flies, as demonstrated by analysis of cholinergic currents in a second wild-type strain, wt2, a Cantonized white stock. There was no difference in the incidence of neurons receiving sEPSCs in the two different strains: 50% in wt1 compared with 49% in wt2. In addition the mean frequencies of the sEPSCs and mEPSCs, 2.5 ± 0.6 Hz (n = 18) and 1.1 ± 0.2 Hz (n = 21), respectively, in wt2 were not different from those seen in the wt1 neurons (Fig. 5C). Finally, the biophysical properties observed in cultured neurons prepared from the two different wild-type populations were very similar (Table2).
Table 2.
Biophysical properties of cholinergic mEPSCs are similar in two wild-type strains
| Genotype | Amplitude (pA) | Rise time (msec) | τ (msec) | n |
|---|---|---|---|---|
| wt1 | 24.8 ± 1.4 | 0.61 ± 0.05 | 2.1 ± 0.2 | 24 |
| wt2 | 21.6 ± 1.3 | 0.70 ± 0.05 | 2.0 ± 0.2 | 5 |
Activity-dependent modulation of cholinergic synaptic transmission
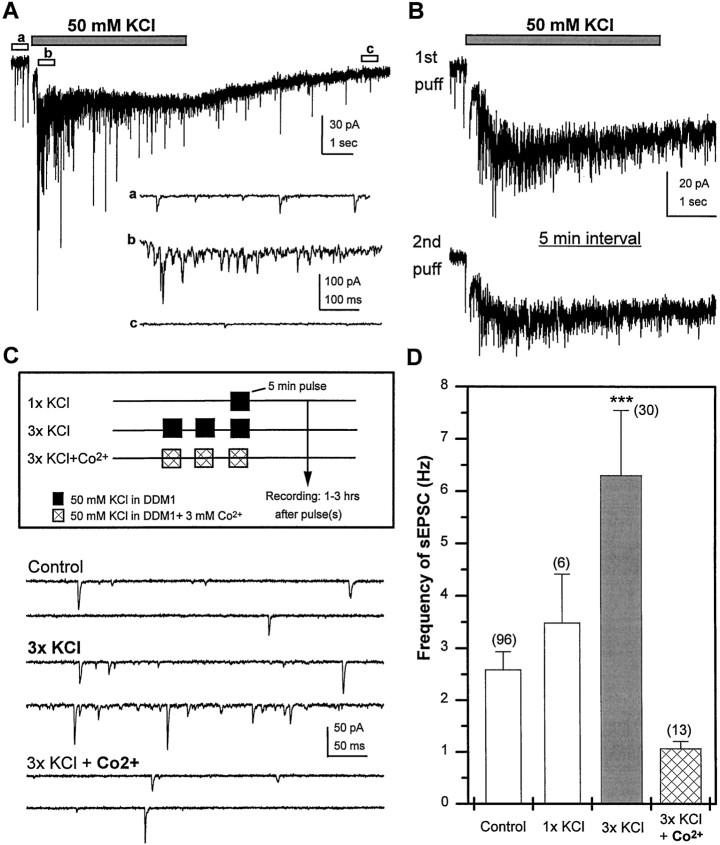
The capacity to exhibit both short-term and long-lasting alterations in the efficacy of transmission is a common feature of chemical synapses mediating fast transmission in the CNS. To investigate the parameters involved in regulating transmission at cholinergic synapses between Drosophila neurons we chose to assess the affects of brief depolarization. Our first experiment examined the acute effects of depolarization on synaptic activity. Focal application of 50 mm KCl for a short period (5 sec), using a micropipette (2–3 μm in diameter), induced a fast and dramatic elevation in sEPSC frequency (Fig.11A). This was followed by a slower depletion phase where the sEPSC frequency declined below base line levels. A second application of KCl within 1–2 min did not significantly increase sEPSC frequency during the pulse (data not shown). However, the ability of neurons to respond to high potassium recovered after a 5 min rest period in normal external solution (Fig.11B). These data suggest that acute depolarization results in a brief increase in neurotransmitter release followed by a refractory period, consistent with a transient depletion of neurotransmitter stores.
Fig. 11.
Brief depolarization of differentiated wild-type neurons induces calcium-dependent persistent increase in sEPSC frequency. A, A 5 sec, focal application of 50 mm KCl, pressure-ejected from a micropipette ∼20 μm from the cell body, induces a rapid and transient increase in sEPSC frequency riding on a sustained inward current, followed by a decline to below baseline levels within 10 sec. Higher-resolution images of sEPSCs recorded before (a), during (b), and after (c) the KCl puff are indicated below.B, In a single neuron, similar responses are observed to two puffs of KCl when separated by a 5 min rest period in normal external solution. C, Three different paradigms used for global depolarization of cultures at 3–6 DIV by exposure to 50 mm KCl in DDM1. Recordings obtained 1–3 hr after stimulus illustrate an increase in sEPSC frequency after repetitive KCl stimulation that is blocked if stimulation is conducted in the presence of Co2+. D, Mean sEPSC frequency, assayed 1–3 hr after treatment, is plotted for each of the depolarization paradigms. The slightly higher mean sEPSC frequency after 1x KCl was not significantly different from control. However, a significant increase in sEPSC frequency was observed in cultures treated with high 3x KCl when compared with control (ANOVA; ***p < 0.0001, Fisher PLSD). This increase was blocked when Co2+ was present during the repeated exposure to high potassium. Bars indicate SEM, and the number of neurons is indicated in parentheses.
To determine whether it was possible to cause a persistent change in transmission at cholinergic synapses, bath exposure of the whole culture to 50 mm KCl was used to induce a global increase in activity. Cultures at 3–6 DIV were transferred from their DDM1 growth medium to DDM1 containing 50 mm KCl for 5 min. Cultures were transferred back into fresh DDM1 and returned to the incubator (Fig. 11C). There was no significant change in the mean sEPSC frequency in a population of neurons within these cultures examined 1–3 hr after the treatment (Fig. 11D). Extending the depolarizing stimulus to 15 min (uninterrupted) resulted in neurons that appeared morphologically unhealthy 1 hr after the treatment, suggesting that this extended exposure to high potassium was detrimental to cell survival. However, when we increased the total time of depolarization to 15 min, using repetitive depolarizations separated by short rest intervals (Fig. 11C), the cultures were morphologically indistinguishable from the untreated controls. Furthermore, this depolarizing stimulus was effective in inducing a significant increase in sEPSC frequency that persisted for at least 1–3 hr after treatment (Fig. 11D). This increase was blocked when Co2+ was present during the depolarizing stimulus (Fig. 11C,D). These data demonstrate that a series of brief depolarizations can induce a persistent calcium-dependent increase in transmission at excitatory cholinergic synapses.
DISCUSSION
A number of classic neurotransmitters and receptors, including acetylcholine, glutamate, and GABA, are expressed in theDrosophila CNS (Restifo and White, 1990). However, in the absence of a preparation in which to monitor the functional properties of transmission at central synapses, their role in mediating fast synaptic transmission remained obscure. This study provides the first direct demonstration that both nAChRs and GABA receptors mediate fast transmission between Drosophila neurons. In conjunction with previous studies documenting widespread expression of both acetylcholine and AChRs in the Drosophila CNS (Salvaterra and McCaman, 1985; Schuster et al., 1993; Yasuyama et al., 1995), our finding that the predominant form of fast excitatory transmission in cultured neurons is mediated by nAChRs is consistent with the hypothesis that acetylcholine is the major excitatory neurotransmitter in the fly CNS.
Spontaneous APs contribute to release of ACh in culturedDrosophila neurons
The presence of an evoked component in the sEPSCs in the majority of neurons examined, indicated by the decrease in mean frequency and amplitude of the synaptic currents after bath application of TTX, suggests that many of the cultured neurons are capable of spontaneously firing sodium-dependent APs. Current-clamp recordings also directly demonstrated that cultured wild-type neurons fire spontaneous action potentials. Interestingly, the resting potentials of the cultured neurons were relatively depolarized (−37 mV, n = 6), and spontaneous APs were rarely observed when hyperpolarizing current was used to hold the cells below −50 mV. We believe that the properties governing spontaneous firing in these cultured neurons are likely to mirror those occurring in vivo based on the recent recordings obtained from embryonic Drosophila neurons in an intact nerve cord preparation. In these cells, spontaneously occurring regenerative inward sodium currents were observed at −40 mV but were absent when the cells were held at more hyperpolarized potentials (Baines and Bate, 1998). In future studies paired recordings will be conducted to clearly define the properties of evoked release at cholinergic synapses formed between cultured embryonic neurons.
Synaptic currents of variable amplitude are induced by release of single quanta of ACh
Action potential-independent release of acetylcholine at peripheral cholinergic synapses in vertebrates results in mEPSCs that show little variation in amplitude, each event representing the current induced by release of a single quantum (del Castillo and Katz, 1954;Katz, 1966) or integer numbers of quanta (Martin and Pilar, 1964;Bornstein, 1974). In contrast, we observed a relatively high variance in the amplitude of the cholinergic mEPSCs in the Drosophilaneurons in which the amplitude histogram was broad and positively skewed without distinct modes. Although the events recorded from a single cell are likely to represent currents generated at multiple release sites, we found no evidence that large events were kinetically faster than the small events, as would be expected if electrotonic filtering were contributing to the large variance in mEPSC amplitude. In light of these data, our finding that the mEPSC amplitude was independent of release probability suggests that the variance represents differential responses to single quanta of neurotransmitter at these interneuronal cholinergic synapses.
A similar broad, skewed amplitude distribution of mPSCs has been described at glutamatergic (Bekkers and Stevens, 1989; Jonas et al., 1993; Wyllie et al., 1994) and GABAergic (Edwards et al., 1990; Otis and Mody, 1992) synapses in the vertebrate CNS. It has been suggested that differences in postsynaptic receptor number or distribution at specific release sites (De Konick and Mody, 1994; Nusser et al., 1997) and/or variations in vesicle size or number of neurotransmitter molecules/vesicle (Bekkers et al., 1990; Frerking et al., 1995) may contribute to the large variance in mEPSC amplitudes. A recent report suggests that increased variability in mEPSCs may also be a characteristic of immature central synapses in mammals (Wall and Usowizc, 1998). Additional studies in Drosophila will be needed to determine the cellular features underlying the broad mEPSC amplitude distribution at central cholinergic synapses.
The release probability of ACh influenced by Ca2+ flux through voltage-gated channels at rest and by depolarization
Although in most preparations the mean amplitude of mEPSCs is independent of extracellular calcium concentrations, the frequency (probability of release) appears to be variably influenced by calcium at different synapses. For example, blockade of voltage-gated calcium channels with Cd2+ does not alter the frequency or amplitude of either mEPSCs or mIPSCs in CA3 pyramidal cells in hippocampal slice cultures (Scanziani et al., 1992; Capogna et al., 1995). However, at many peripheral and central synapses in both vertebrates and invertebrates, the mEPSC frequency is influenced by extracellular Ca2+ concentration (Katz, 1969; Hori et al., 1992; Broadie et al., 1997; Wall and Usowizc, 1998). Our data demonstrate that at rest, external calcium concentrations strongly influence the mEPSC frequency in cultured Drosophilaneurons. This is mediated by an influx of calcium through voltage-gated ion channels. In addition, mEPSC frequency is dramatically increased in response to KCl-induced depolarization in the presence of TTX. This suggests that the probability of release of ACh from the presynaptic terminals, responsible for the mEPSCs, is influenced by influx of calcium through voltage-gated calcium channels both at resting membrane potentials and during depolarization. This is consistent with our observations that these neurons express voltage-gated calcium channels that can open at voltages near the average resting potential and are further activated at more depolarized voltages (O’Dowd, 1995).
nAChRs mediating mEPSCs appear kinetically homogenous
To date, three different α-subunits and two β-subunits of the nAChR have been identified in Drosophila (Gundelfinger, 1992; Schulz et al., 1998). Although preliminary RT-PCR analysis suggests that all of these are expressed in the cultured neurons (data not shown), we did not see any evidence of kinetically distinct populations of cholinergic mEPSCs. This suggests that a single subunit combination is responsible for generation of the currents or that the different combinations produce channels with kinetically similar properties. The biophysical properties of receptors composed of different subunit combinations has not yet been explored in expression systems because they do not by themselves give rise to functional receptors. However, coexpression of individual Drosophilaα-subunits in Xenopus oocytes with vertebrate β-subunits did reveal two pharmacologically distinct types of AChRs defined as α-bungarotoxin sensitive and resistant (Bertrand et al., 1994; Schulz et al., 1998). Additional pharmacological and permeability studies, in conjunction with single-cell RT-PCR, will be necessary to determine whether there are functionally and molecularly distinct populations of nAChRs in Drosophila neurons.
Depolarization can induce a persistent increase in transmission at cholinergic synapses
Activity-dependent regulation of transmission at central synapses plays a fundamental role in information processing in all animals. Contributing to this process are long-term facilitation, long-term potentiation, and long-term depression, examples of synapse-specific changes in synaptic strength that persist for hours to days after induction (Bliss and Collingridge, 1993; Linden and Connor, 1995;Nicoll and Malenka, 1995; Milner et al., 1998). More recently, a novel form of synaptic plasticity, regulated by global activity levels, has been identified at excitatory glutamatergic synapses in cultured cortical neurons (Rutherford et al., 1998; Turrigiano et al., 1998). Although we have not yet examined whether cholinergic synapses inDrosophila neurons exhibit synapse-specific changes in synaptic strength, our studies demonstrate that a brief increase in overall level of neuronal activity can induce a calcium-dependent, persistent change in cholinergic transmission. Because the depolarizing stimulus affects both presynaptic and postsynaptic neurons, future studies will be necessary to determine whether the increase in sEPSC frequency reflects alterations in presynaptic excitability, probability of neurotransmitter release, sensitivity of the postsynaptic receptors, or some combination thereof. However, the ability to induce a persistent change in transmission by a brief depolarization suggests that, like glutamatergic synapses in the vertebrate CNS, cholinergic synapses are potential sites of plasticity important in regulating neuronal function in the insect CNS. In a parallel study in our laboratory we have found that cAMP signaling also plays an important role in regulating transmission at these synapses, and it is possible that an increase in presynaptic activity is the physiological stimulus that activates this signal transduction cascade (our unpublished observations).
Neuronal cultures: a model system to study functional aspects of transmission at central synapses in Drosophila
The detailed description of cholinergic transmission in wild-typeDrosophila neurons presented in this manuscript forms the basis for comparisons, with data obtained from mutants or after biochemical manipulation, that will enable identification of specific genes or factors important in function or regulation of central cholinergic synaptic transmission. In conjunction with the well established model of synaptic transmission at the NMJ, identification of both similarities and differences in the role of specific genes in synaptic transmission at distinct synapses (e.g., peripheral vs central synapses, glutamatergic vs cholinergic) will contribute to our knowledge of the fundamental rules governing synaptic function and plasticity. Because fast excitatory transmission mediated by nAChRs has also been reported in the mammalian hippocampus (Frazier et al., 1998) and cortex (Roerig et al., 1997), the studies in this model system may also reveal genes that are important in regulating cholinergic transmission in mammals.
Footnotes
This work was supported by National Institutes of Health Grants NS27501 and NS01854, and a generous gift from Merck. We thank Dr. M. A. Smith for helpful comments on this manuscript.
Correspondence should be addressed to Diane K. O’Dowd, Department of Anatomy and Neurobiology, University of California Irvine, Irvine, CA 92697-1280.
REFERENCES
- 1.Baines RA, Bate M. Electrophysiological development of central neurons in the Drosophila embryo. J Neurosci. 1998;18:4673–4683. doi: 10.1523/JNEUROSCI.18-12-04673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banker G, Goslin K. Culturing nerve cells. Cellular and molecular neuroscience series. MIT; Cambridge, MA: 1991. [Google Scholar]
- 3.Bekkers JM, Stevens CF. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989;341:230–233. doi: 10.1038/341230a0. [DOI] [PubMed] [Google Scholar]
- 4.Bekkers JM, Richerson GB, Stevens CF. Origin of variability in quantal size in cultured hippocampal neurons and hippocampal slices. Proc Natl Acad Sci USA. 1990;87:5359–5362. doi: 10.1073/pnas.87.14.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertrand D, Ballivet M, Gomez M, Bertrand S, Phannavong B, Gundelfinger ED. Physiological properties of neuronal nicotinic receptors reconstituted from the vertebrate β2 subunit and Drosophila α-subunits. Eur J Neurosci. 1994;6:869–875. doi: 10.1111/j.1460-9568.1994.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 6.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 7.Bornstein JC. Mulitquantal release of acetylcholine in mammalian ganglia. Nature. 1974;248:529–531. doi: 10.1038/248529a0. [DOI] [PubMed] [Google Scholar]
- 8.Boynton S, Tully T. latheo, a new gene involved in associative learning and memory in Drosophila melanogaster, identified from P element mutagenesis. Genetics. 1992;131:655–672. doi: 10.1093/genetics/131.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broadie KS, Rushton E, Skoulakis EMC, Davis RL. Leonardo, a Drosophila 14–3-3 protein involved in learning, regulates presynaptic function. Neuron. 1997;19:391–402. doi: 10.1016/s0896-6273(00)80948-4. [DOI] [PubMed] [Google Scholar]
- 10.Capogna M, Gahwiler BH, Thompson SM. Presynaptic enhancement of inhibitory synaptic transmission by protein kinase A and C in the rat hippocampus in vitro. J Neurosci. 1995;15:1249–1260. doi: 10.1523/JNEUROSCI.15-02-01249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis GW, Schuster CM, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. III. CREB is necessary for presynaptic functional plasticity. Neuron. 1996;17:669–679. doi: 10.1016/s0896-6273(00)80199-3. [DOI] [PubMed] [Google Scholar]
- 12.De Konick Y, Mody I. Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. J Neurophysiol. 1994;71:1318–1335. doi: 10.1152/jn.1994.71.4.1318. [DOI] [PubMed] [Google Scholar]
- 13.del Castillo J, Katz B. Quantal components of the endplate potential. J Physiol (Lond) 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards FA, Konnerth A, Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol (Lond) 1990;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV. Synaptic potentials mediated via α-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J Neurosci. 1998;18:8228–8235. doi: 10.1523/JNEUROSCI.18-20-08228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frerking M, Borges S, Wilson M. Variation in GABA mini amplitude is the consequence of variation in transmitter concentration. Neuron. 1995;15:885–895. doi: 10.1016/0896-6273(95)90179-5. [DOI] [PubMed] [Google Scholar]
- 17.Griffith LC, Wang J, Zhong Y, Wu C-F, Greenspan R. Calcium/calmodulin-dependent protein kinase II and potassium channel subunit Eag similarly affect plasticity in Drosophila. Proc Natl Acad Sci USA. 1994;91:10044–10048. doi: 10.1073/pnas.91.21.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gundelfinger ED. How complex is the nicotinic receptor system of insects? Trends Neurosci. 1992;15:206–211. doi: 10.1016/0166-2236(92)90035-7. [DOI] [PubMed] [Google Scholar]
- 19.Hori Y, Endo K, Takahashi T. Presynaptic inhibitory action of enkephalin on excitatory transmission in superficial dorsal horn of rat spinal cord. J Physiol (Lond) 1992;450:673–685. doi: 10.1113/jphysiol.1992.sp019149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jan L, Jan Y. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and grasshopper embryos. Proc Natl Acad Sci USA. 1982;79:2700–2704. doi: 10.1073/pnas.79.8.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonas P, Major G, Sakmann B. Quantal components of unitary EPSCs at the mossy fiber synapse on CA3 pyramidal cells of rat hippocampus. J Physiol (Lond) 1993;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz B. Nerve, muscle, and synapse. McGraw-Hill; New York: 1966. [Google Scholar]
- 23.Katz B. The release of neurotransmitter substances. Thomas; Springfield, IL: 1969. [Google Scholar]
- 24.Leung H-T, Byerly L. Characterization of single calcium channels in Drosophila embryonic nerve and muscle cells. J Neurosci. 1991;11:3047–3059. doi: 10.1523/JNEUROSCI.11-10-03047.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linden DJ, Connor JA. Long-term synaptic depression. Annu Rev Neurosci. 1995;18:319–357. doi: 10.1146/annurev.ne.18.030195.001535. [DOI] [PubMed] [Google Scholar]
- 26.Martin AR, Pilar G. Quantal components of the synaptic potential in the ciliary ganglion of the chick. J Physiol (Lond) 1964;175:1–16. doi: 10.1113/jphysiol.1964.sp007499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- 28.Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 29.Nusser A, Cull-Candy S, Farrant M. Differences in synaptic GABAA receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- 30.O’Dowd DK. Voltage-gated currents and firing properties of embryonic Drosophila neurons grown in a chemically defined medium. J Neurobiol. 1995;27:113–126. doi: 10.1002/neu.480270111. [DOI] [PubMed] [Google Scholar]
- 31.O’Dowd DK, Aldrich RW. Voltage-clamp analysis of sodium channels in wild-type and mutant Drosophila neurons. J Neurosci. 1988;8:3633–3643. doi: 10.1523/JNEUROSCI.08-10-03633.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Dowd DK, Germeraad S, Aldrich RW. Alterations in the expression and gating of Drosophila sodium channels by mutations in the para gene. Neuron. 1989;2:1301–1311. doi: 10.1016/0896-6273(89)90068-8. [DOI] [PubMed] [Google Scholar]
- 33.O’Dowd DK, Gee JR, Smith MA. Sodium current density correlates with expression of specific alternatively spliced sodium channel mRNAs in single neurons. J Neurosci. 1995;15:4005–4012. doi: 10.1523/JNEUROSCI.15-05-04005.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otis TS, Mody I. Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents in hippocampal neurons. Neuroscience. 1992;49:13–32. doi: 10.1016/0306-4522(92)90073-b. [DOI] [PubMed] [Google Scholar]
- 35.Restifo LL, White K. Molecular and genetic approaches to neurotransmitter and neuromodulator systems in Drosophila. Adv Insect Physiol. 1990;22:115–219. [Google Scholar]
- 36.Roerig B, Nelson DA, Katz LC. Fast synaptic signaling by nicotinic acetylcholine and serotonin 5-HT3 receptors in developing visual cortex. J Neurosci. 1997;17:8353–8362. doi: 10.1523/JNEUROSCI.17-21-08353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- 38.Saito M, Wu C-F. Expression of ion channels and mutational effects in giant Drosophila neurons differentiated from cell division-arrested embryonic neuroblasts. J Neurosci. 1991;11:2135–2150. doi: 10.1523/JNEUROSCI.11-07-02135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salvaterra PM, McCaman RE. Choline acetyltransferase and acetylcholine levels in Drosophila melanogaster: a study using two temperature-sensitive mutants. J Neurosci. 1985;5:903–910. doi: 10.1523/JNEUROSCI.05-04-00903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scanziani M, Capogna M, Gahwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- 41.Schulz R, Sawruk E, Mulhardt C, Bertrand S, Baumann A, Phannavong B, Betz H, Bertrand D, Gundelfinger ED, Schmitt B. D α-3, a new functional α-subunit of nicotinic acetylcholine receptors from Drosophila. J Neurochem. 1998;71:853–862. doi: 10.1046/j.1471-4159.1998.71020853.x. [DOI] [PubMed] [Google Scholar]
- 42.Schuster R, Phannavong B, Schroder C, Gundelfinger ED. Immunohistochemical localization of ligand-binding and a structural subunit of nicotinic acetylcholine receptors in the central nervous system of Drosophila melanogaster. J Comp Neurol. 1993;335:149–162. doi: 10.1002/cne.903350202. [DOI] [PubMed] [Google Scholar]
- 43.Seecof RL, Donady JJ, Teplitz RL. Differentiation of Drosophila neuroblasts to form ganglion-like clusters of neurons. Cell Differ. 1973;2:143–149. doi: 10.1016/0045-6039(73)90014-6. [DOI] [PubMed] [Google Scholar]
- 44.Solc CK, Zagotta WN, Aldrich RW. Single-channel and genetic analyses reveal two distinct A-type potassium channels in Drosophila. Science. 1987;236:1094–1098. doi: 10.1126/science.2437657. [DOI] [PubMed] [Google Scholar]
- 45.Takagawa K, Salvaterra P. Analysis of choline acetyltransferase protein in temperature sensitive mutant flies using newly generated monoclonal antibody. Neurosci Res. 1996;24:237–243. doi: 10.1016/0168-0102(95)00999-x. [DOI] [PubMed] [Google Scholar]
- 46.Tsunoda S, Salkoff L. Genetic analysis of Drosophila neurons: Shal, Shaw, and Shab encode most embryonic potassium currents. J Neurosci. 1995;15:1741–1754. doi: 10.1523/JNEUROSCI.15-03-01741.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turrigiano GG, Leslie KR, Desai NA, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 48.Wall MJ, Usowizc MM. Development of the quantal properties of evoked and spontaneous synaptic currents at a brain synapse. Nat Neurosci. 1998;1:675–682. doi: 10.1038/3677. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Renger JJ, Griffith LC, Greenspan RJ, Wu C-F. Concomitant alterations of physiological and developmental plasticity in Drosophila CaM kinase II-inhibited synapses. Neuron. 1994;13:1373–1384. doi: 10.1016/0896-6273(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 50.Wu C-F. Neuronal activity and neural plasticity in Drosophila. In: Koike H, Kidokoro Y, Takahashi K, Kanaseki T, editors. Basic neuroscience in invertebrates. Japan Scientific Press; Tokyo: 1996. pp. 267–290. [Google Scholar]
- 51.Wu C-F, Suzuki N, Poo M-M. Dissociated neurons from normal and mutant Drosophila larvae central nervous system in cell culture. J Neurosci. 1983;3:1888–1899. doi: 10.1523/JNEUROSCI.03-09-01888.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu C-F, Sakai K, Saito M, Hotta Y. Giant Drosophila neurons differentiated from cytokinesis-arrested embryonic neuroblasts. J Neurobiol. 1990;21:499–507. doi: 10.1002/neu.480210310. [DOI] [PubMed] [Google Scholar]
- 53.Wu MN, Bellen HJ. Genetic dissection of synaptic transmission in Drosophila. Curr Opin Neurobiol. 1997;7:624–630. doi: 10.1016/s0959-4388(97)80081-5. [DOI] [PubMed] [Google Scholar]
- 54.Wyllie DJA, Manabe T, Nicoll RA. A rise in postsynaptic Ca2+ potentiates miniature excitatory postsynaptic currents and AMPA responses in hippocampal neurons. Neuron. 1994;12:127–138. doi: 10.1016/0896-6273(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 55.Yasuyama K, Kitamoto T, Salvaterra PM. Immunocytochemical study of choline acetyltransferase in Drosophila melanogaster: analysis of cis-regulatory regions controlling expression in the brain of cDNA-transformed flies. J Comp Neurol. 1995;361:25–37. doi: 10.1002/cne.903610103. [DOI] [PubMed] [Google Scholar]
- 56.Zhao M-L, Wu C-F. Alterations in frequency coding and activity dependence of excitability in cultured neurons of Drosophila memory mutants. J Neurosci. 1997;17:2187–2199. doi: 10.1523/JNEUROSCI.17-06-02187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong Y, Wu CF. Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science. 1991;251:198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]