Abstract
Recent imaging and clinical studies have challenged the concept that the functional role of the cerebellum is exclusively in the motor domain. We present evidence of slowed covert orienting of visuospatial attention in patients with developmental cerebellar abnormality (patients with autism, a disorder in which at least 90% of all postmortem cases reported to date have Purkinje neuron loss), and in patients with cerebellar damage acquired from tumor or stroke. In spatial cuing tasks, normal control subjects across a wide age range were able to orient attention within 100 msec of an attention-directing cue. Patients with cerebellar damage showed little evidence of having oriented attention after 100 msec but did show the effects of attention orienting after 800–1200 msec. These effects were demonstrated in a task in which results were independent of the motor response. In this task, smaller cerebellar vermal lobules VI–VII (from magnetic resonance imaging) were associated with greater attention-orienting deficits.
Although eye movements may also be disrupted in patients with cerebellar damage, abnormal gaze shifting cannot explain the timing and nature of the attention-orienting deficits reported here. These data may be consistent with evidence from animal models that suggest damage to the cerebellum disrupts both the spatial encoding of a location for an attentional shift and the subsequent gaze shift. These data are also consistent with a model of cerebellar function in which the cerebellum supports a broad spectrum of brain systems involved in both nonmotor and motor function.
Keywords: cerebellum, visual attention, orienting attention, spatial attention, autism, lesion
Evidence from brain imaging and clinical studies has aroused considerable new interest in the function of the cerebellum. Although cognitive impairment has been reported in patients with cerebellar disorders since the nineteenth century, the dominant view remains that the cerebellum is associated exclusively with motor function (for review, see Schmahmann, 1997). Now that view is challenged by functional imaging [positron emission tomography and functional magnetic resonance imaging (fMRI)] studies reporting activation in the cerebellum that is associated with nonmotor cognitive processes including attention, perception, language, and working memory (for review, see Cabeza and Nyberg, 1997; Courchesne and Allen, 1997). For example, fMRI studies have reported activation of the posterior cerebellum in normal subjects during a visual selective attention task with no motor component (Allen et al., 1997), an attention-shifting task (Le et al., 1998), and spatial or temporal cuing tasks (Coull and Nobre, 1998).
Because the cerebellum has primary or higher order connections to brain systems controlling motor, social, and cognitive function (for review, see Akshoomoff et al., 1997; Courchesne and Allen, 1997; Schmahmann, 1997), it is well placed to affect a variety of motor and nonmotor behaviors. Cerebellar abnormalities have been identified as an important component of the pathology of autism, a neurological disorder in which social and cognitive development is severely affected. Autopsy studies have reported substantial developmental reduction in Purkinje cell numbers (20–95% across individuals) in the cerebellar vermis and hemispheres in 17 of 19 autism cases examined (Williams et al., 1980;Bauman and Kemper, 1985, 1986, 1990, 1994; Ritvo et al., 1986; Arin et al., 1991; Fehlow et al., 1993; Bailey et al., 1998) (for the two cases in which Purkinje cell loss was not found, in one the Purkinje cells were abnormal, and in the other the diagnosis may have been Rett syndrome, not autism). Quantitative magnetic resonance imaging (MRI) studies from eight independent research groups have also found abnormally small vermal lobules VI–VII in the majority of the several hundred total autistic subjects studied (for review, see Courchesne et al., 1994b; Courchesne, 1997a). Inadequate or uncontrolled signals from the cerebellum could disrupt many if not all of the brain systems that contribute to dysfunctional behaviors in autism (Courchesne, 1995;Yeung-Courchesne and Courchesne, 1997). Among the most salient of these is impaired manipulation of attentional resources. For example, autistic individuals and patients with acquired cerebellar damage are slow to shift attention between and within sensory modalities (Courchesne et al., 1994c; Akshoomoff and Courchesne, 1992, 1994).
Patients with damage to the cerebellum are also slow to orient visual attention in space (Townsend et al., 1992, 1996a,b). Using a spatial cuing task (Posner et al., 1984) in which attentional function is indexed by response time and a task in which attention is indexed by accuracy of performance, we have demonstrated that subjects with autism who have developmental cerebellar abnormality require nearly a second to benefit from a spatial cue. Our data from small samples have suggested that those with greater hypoplasia of cerebellar vermal lobules VI–VII have more severe attention-orienting deficits.
Here we compare spatial attention orienting in patients with autism to those with acquired cerebellar lesions. Evidence from two different tasks suggests slowed spatial attention orienting is associated with structural cerebellar abnormality.
MATERIALS AND METHODS
Research participants
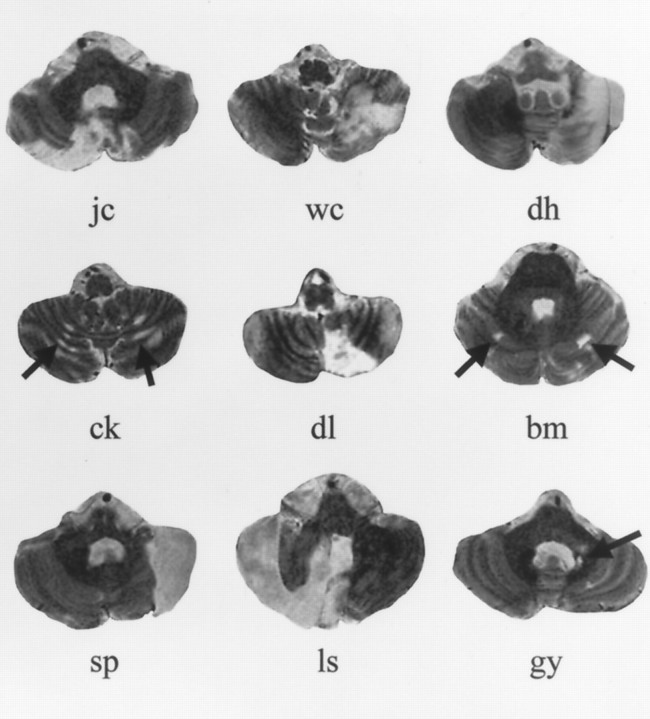
Study participants were 18 nonretarded individuals with autism, 62 normal control subjects, and nine patients with cerebellar lesions from tumor or stroke (five left, one right, and three asymmetric bilateral). Individual lesions are shown in Figure1, and regions of overlap are shown in Figure 2. Thirty-five of the control subjects (mean age, 41.5 ± 24; range, 16–82), seven of the autism subjects (mean age, 27.9 ± 12; range, 13–42), and nine of the lesion subjects (mean age, 45.8 ± 21; range, 15–75) participated in the spatial detection task. Thirty-one of the control subjects (mean age, 34.3 ± 20; range, 15–75), 13 of the autism subjects (mean age, 27.7 ± 8; range, 13–42), and seven of the lesion subjects (mean age, 42.1 ± 22; range, 15–75) participated in the spatial discrimination task. Four of the autism subjects were part of a sample whose data from the spatial detection task were previously reported (Townsend et al., 1996a). One of these and three other of the 18 autism subjects were part of a sample whose data from the spatial discrimination task were previously reported (Townsend et al., 1996b).
Fig. 1.
MRI images showing the most extensive section of lesion for each cerebellar stroke or tumor patient. Displayed MRI images for subjects J.C. and B.M. illustrate bilaterality of the lesions and do not necessarily show the largest lesion area. Images are T2-weighted 3-mm-thick slices acquired in the axial plane. The cerebellar slice for subject D.L. is from the film of a clinical MRI study using gadolinium contrast to enhance the lesion site.
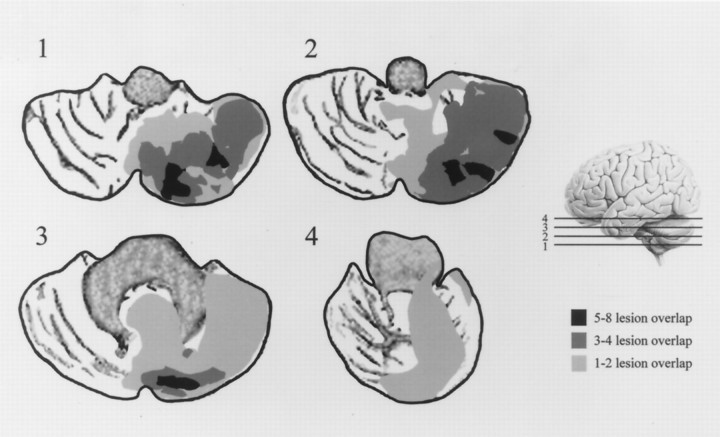
Fig. 2.
Tracings show the degree of lesion overlap among all nine cerebellar lesion patients. Lesion tracings used the selected cerebellar slice (relative position indicated in the figure as1, 2, 3, and 4) from each patient. Right side lesions were transposed to the left side to better illustrate the degree of lesion overlap in the cerebellar hemispheres and vermis. Lesion tracings were overlaid on cerebellar slices traced from an MRI film of a nonlesioned normal control subject.
Participants with autism all met DSM-III-R (American Psychiatric Association, 1987) criteria for autistic disorder. Thirteen subjects also received the Autism Diagnostic Interviews (ADI) (Le Couteur et al., 1989), the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 1989), and 15 subjects received the Childhood Autism Rating Scale (CARS) (Schopler et al., 1980). Scores for these diagnostic tests are presented in Table 1.
Table 1.
Scores from diagnostic tests for subjects with autism
| Subject | Age | CARS | ADI Social | ADI V com | ADI Nv com | ADI RepBeh |
|---|---|---|---|---|---|---|
| 1 | 13 | 41 | 27 | 22 | 14 | 12 |
| 2 | 17 | 64 | 26 | 20 | 17 | |
| 3 | 18 | 35.5 | 45 | 20 | 14 | 10 |
| 4 | 19 | 44.5 | 22 | 20 | 12 | 8 |
| 5 | 20 | 39.5 | 23 | 12 | 8 | 15 |
| 6 | 20 | |||||
| 7 | 25 | 41 | 23 | 14 | 11 | 12 |
| 8 | 26 | 36.5 | ||||
| 9 | 28 | 35.5 | 12 | 24 | 10 | 14 |
| 10 | 28 | 42.5 | 61 | 32 | 17 | 12 |
| 11 | 29 | 36 | 38 | 15 | 12 | 8 |
| 12 | 30 | 36 | 21 | 22 | 12 | 10 |
| 13 | 31 | |||||
| 14 | 35 | 30 | ||||
| 15 | 37 | 37.5 | 26 | 21 | 14 | 6 |
| 16 | 38 | 32.5 | 39 | 14 | 10 | 10 |
| 17 | 42 | 33.5 | 27 | 21 | 13 | 12 |
| 18 | 43 | 21 | ||||
| Mean | 27.7 | 36.2 | 32.9 | 20.2 | 12.8 | 11.2 |
Criterion scores to meet autism diagnosis for ADI subscales are: social, 10; verbal communication, 6; nonverbal communication, 7; repetitive behaviors, 3. Individuals with CARS scores in the range of 30–36 are considered mildly to moderately autistic, and those with scores greater than 37 are considered to be severely autistic.
None of the autistic subjects had additional psychiatric or neurological diagnoses. All participants with autism were screened for the presence of fragile X syndrome, and all were found to be negative. Normal control participants were volunteers recruited from the community. Controls had no history of substance abuse, special education, major medical or psychiatric illness, or developmental or neurological disorder. Ages and intelligence quotient (IQ) scores for the subject groups are presented in Table2. We obtained IQ data for all of the autism and lesion subjects, and for 35 of the 62 control subjects.
Table 2.
Age and IQ scores for subject groups, from WAIS-III, WAIS-R, or WISC-R
| Normal controls | Autism | Cerebellar lesion | |
|---|---|---|---|
| n | 35 | 18 | 9 |
| Age (SD, Range) | 36.33 (23.7, 9–82) | 26.99 (7.7, 13–42) | 45.80 (21.2, 15–75) |
| VIQ (SD) | 113.77 (11.7) | 77.83 (22.9) | 101.89 (10.0) |
| Vocabulary (SD) | 12.9 (3) | 4.8 (3) | 11.1 (2) |
| Comprehension (SD) | 12.6 (3) | 4.3 (4) | 11.2 (2) |
| PIQ (SD) | 116.34 (12.5) | 85.33 (14.7) | 103.33 (15.4) |
| Block Design (SD) | 12.5 (3) | 10.3 (4) | 10.4 (3) |
| Object Assembly (SD) | 12.6 (2) | 8.7 (3) | 10.9 (3) |
Brain measures from MRI
Participants had MRI according to previously described protocols (Courchesne et al., 1988, 1994b). All subjects were cooperative and were imaged without sedation. Images were acquired on a 1.5 T GE magnet. Protocols used for measurement of cerebellar vermis and for brain volume are described below. In addition to these protocols, subjects received high resolution scans (1.2–1.6 mm slice thickness) in sagittal and coronal planes from which three-dimensional reconstructions of the whole brain were done.
Individuals with autism are from a group with bilateral cerebellar abnormalities that have been previously reported (Courchesne et al., 1988, 1994b). Vermal area and brain volume measures for autism, lesion, and normal control groups are shown in Table3. For all brain measures, images were coded, and normal and non-normal images were randomly intermixed so that area and volume estimates were calculated by researchers who were blinded to the subject’s group. MRI films from all lesion patients were read by a neuroradiologist (G.A. Press) who identified lesion sites and examined the images for any additional structural damage or abnormalities. We were unable to image one lesion subject (D.L.), but the lesion site for this subject was verified from previous MRIs and is included in those displayed in Figure 1. Because we had films only, we were unable to complete quantitative analysis for brain structures on this lesion subject. We obtained complete brain data for 43 normal control subjects and for 15 autism subjects. We were unable to image one autism subject and were able to obtain only partial sets for two others (vermis measures only).
Table 3.
Means and (standard deviations) from magnetic resonance imaging (MRI) quantitative estimates
| Normal control | Autism | Cerebellar lesion | |
|---|---|---|---|
| n | 43 | 15 | 8 |
| Age (SD, Range) | 36.28 (21.2, 12–79) | 26.57 (10.1, 13–47) | 45.71 (22.8, 15–75) |
| Cerebellum (mm2) | |||
| Vermal lobules I–V | 447.15 (63.6) | 451.00 (67.3) | 367.78 (158.8) |
| Vermal lobules VI–VII | 270.82 (45.2) | 251.56 (23.0) | 221.55 (103.2) |
| Brain volume (cc) | |||
| Intracranial | 1419.4 (155) | 1462.7 (112) | 1493.1 (190) |
| Total brain | 1201.0 (157) | 1264.9 (103) | 1184.7 (162) |
| Total gray | 764.2 (111) | 808.3 (71) | 762.7 (147) |
| Total white | 436.8 (67) | 456.6 (61) | 422.0 (53) |
| Total CSF | 218.4 (90) | 197.9 (45) | 308.4 (82) |
Cerebellar vermal area
Vermal lobules I–V (lingula, centralis, culmen) and VI–VII (declive, folium, tuber) were traced by hand from midsagittal T1-weighted spin-echo magnetic resonance images [repetition time (TR), 600 msec; echo time (TE), 12–25 msec; 256 × 256 matrix; field of view (FOV), 16; 5 mm slice thickness]. To obtain midsagittal sections that were comparable across subjects, axial localizers were used to determine a plane of section that passed through the rostral and caudal convexities at the levels of the superior, middle, and inferior positions of the vermis. If a line drawn through the two convexities differed by more than 1 mm in the right/left position between the three transverse levels or was rotated or torqued relative to the sagittal plane, the subject’s head was repositioned, and the process was repeated. Precise alignment is critical for accurate vermis measurement and cannot be determined using extracerebellar structures such as the corpus callosum (Courchesne et al., 1994d).
Vermal lobules were traced on images magnified to provide adequate resolution to determine anatomic landmarks. Tracings were done at Silicon Graphics workstations using software that computed the area in traced regions. The boundary between vermal lobules I–V and lobules VI–VII was defined as the line joining the anterior aspect of the primary fissure to the apex of the fourth ventricle. The boundary between lobules VI–VII and lobule VIII was defined as the line joining the anterior aspect of the prepyramidal fissure to the apex of the fourth ventricle. For additional details of imaging protocol, alignment of vermis, and anatomic landmarks, see Courchesne et al. 1988, 1989, and 1994a–d.
For correlational analyses, vermal measures were divided by intracranial brain volume (see below) to control for overall brain size.
Brain and intracranial volumes
These volumes were measured using 3 mm axial interleaved T2- and PD-weighted images (TR, 3000 msec; TE, 25 and 90 msec; 1 number of excitations; FOV, 20 cm). First, skull and extracranial matter were removed from the T2-weighted images using a semiautomated procedure that used both thresholding and manual tracing for each slice. The lowest slice matching the external morphology of the inferior medulla was chosen as the inferior boundary of the brain. All brain tissue and CSF spaces at and above the lowest slice level as well as the pituitary and infundibulum were included in the brain volume. Excluded from the intracranial measures were: skull, fat, mastoid, and nasal sinuses, venous sinuses, blood vessels, and vessel artifact beyond the brain surface, bony protuberances, and the cranial nerve roots as they extend beyond the brain surface.
Next, gray matter, white matter, and CSF pixels were classified using an automatic segmentation algorithm developed in our laboratory (E. Courchesne, H. J. Chisum, J. Townsend, A. Cowles, J. Covington, B. Egaas, S. Hinds, T. Lowry, and G. A. Press, unpublished observations). This approach was analogous to previous approaches using feature space in the semiautomated segmentation of PD/T2 protocols nearly identical to our image protocol (Jackson et al., 1994; Matsumae et al., 1996). The principle difference is that our algorithm is fully automated and does not require the user to choose pixel clusters in PD/T2 feature space. Our algorithm used all pixels in the image set to form a global histogram in PD versus T2 “feature space”.
Using a maximum likelihood criteria algorithm (Vannier et al., 1985), pixel clusters were classed as parenchyma (gray and white), CSF, nonbrain, and partially volumed nonbrain and CSF. All parenchyma pixels were then automatically separated into gray and white matter pixels using a three-dimensional local contrast algorithm. In this three-dimensional local contrast algorithm, the local threshold for gray and white matter pixels was computed from pixel statistics within a cube 2 cm on a side surrounding the pixel being classified (cube = 29 pixels × 29 pixels × 7 slices = 5887 surrounding voxels). The use of this local contrast makes the segmentation relatively insensitive to the signal inhomogeneities intrinsic to scanning that plague simpler methods of segmentation, such as uniform thresholding over an entire slice.
This automated classification has been validated using in vivo magnetic resonance brain images from nine subjects (Courchesne, Chisum, Townsend, Cowles, Covington, Egaas, Hinds, Lowry, and Press, unpublished observations). For eight of these brain datasets, all gray matter, white matter, and CSF spaces within the right hemibrain were manually traced from the T2 and PD images at three different slice locations: the level of the centrum semiovale, the level of the thalamus and basal ganglia, and the level of the pons. The areas designated by this expert-based manual tracing as gray matter, white matter, and CSF within each slice were calculated. Statistical analyses showed that correlations between manual and automatic classification were 0.982 for gray matter, 0.961 for white matter, and 0.887 for CSF. For the ninth brain in the validation dataset, the expert neuroanatomist manually traced cortical gray matter on 20 slices using the PD and T2 images; measures from those manual tracings and from the automated classification algorithm correlated >99%.
Each two-dimensional pixel in the images represents a three-dimensional volume (a voxel), and the intracranial volume (ICV) was determined by summing volumes of all voxels designated as gray, white, and CSF, plus half of the volume of all voxels designated as CSF partially volumed with skull. Total brain volume was determined in a similar manner using gray and white matter voxels only. Total CSF volume was constructed from fully volumed CSF voxels plus half the volume of all voxels designated as CSF partially volumed with skull.
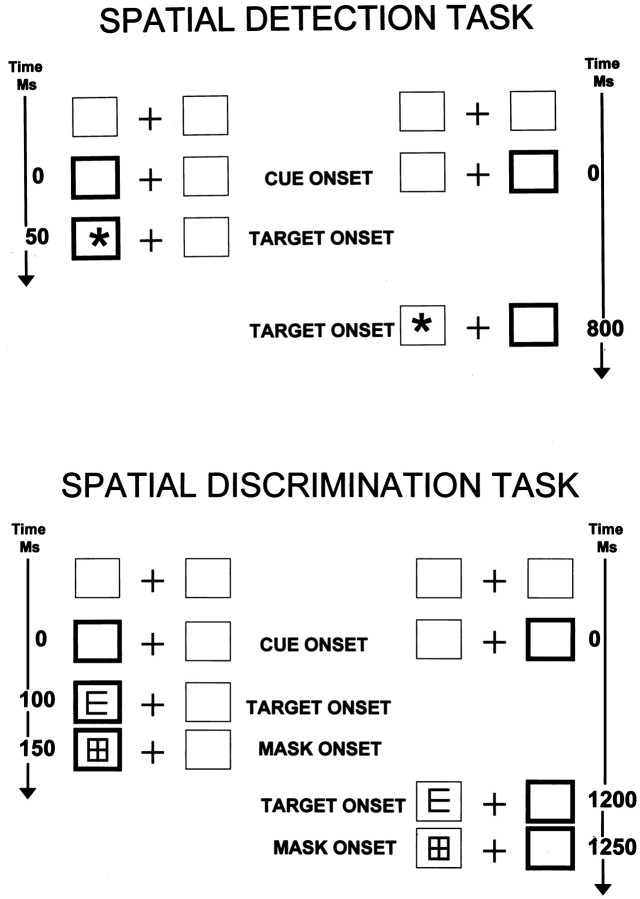
Spatial target detection task (Townsend et al., 1996a)
This spatial task is patterned after a widely used spatial cuing task (Posner et al., 1984). Subjects were seated in a testing room, 90 cm from a 36 cm monitor. The basic visual display was a central fixation cross flanked on the left and right by 4 cm square boxes at 6° of visual angle (Fig.3,top). An asterisk 2.5 cm in diameter was the target stimulus. Each trial began with a stimulus-directing cue (one of the boxes brightened), or by a null cue in which no information was provided about the subsequent target location (i.e., both boxes brightened or neither box brightened). After a delay of 50, 100, or 800 msec, the target was presented in either the left or the right box. The subject’s task was to maintain fixation on the central cross and to press the button when the target was detected. The target was displayed until the subject responded or for a maximum of 2 sec. If a subject failed to respond within the allotted time or responded <100 msec after the target onset, the trial was repeated. The intertrial interval was 1 sec. Targets were preceded by valid cues in two thirds of the trials, by invalid cues in one sixth, and by null cues in one sixth. Within a cue condition, cues and targets occurred at random and with equal probability on the right or the left and with equal probability in each of the three delay intervals. The total number of trials was 432 (there were 48 valid trials in each of the six conditions, and there were 12 invalid and 12 null trials in each of the six conditions). Response time was evaluated as a function of cue-to-target delay and cue type (valid, invalid, or null).
Fig. 3.
Spatial target detection and discrimination task diagrams.
Spatial target discrimination task (Townsend et al., 1996b)
This task was similar in design to the spatial detection task, but required a target discrimination rather than simple target detection (Fig. 3, bottom). The basic visual display was as described above. The target was a block figure “E” that could be oriented up, down, left, or right. As in the detection task, each trial began with an attention-directing cue (the box on either the left or the right was brightened). After a delay of 100, 800, or 1200 msec the target was presented in either the cued location (80% probability) or the uncued location (20% probability). Within a cue condition, cues and targets occurred at random and with equal probability on the right or the left and with equal probability in each of the three delay intervals. The total number of trials was 288 (there were 36 valid trials in each of the six conditions; there were 12 invalid in each of the six conditions). Chance performance for a trial was 25%.
After 50 msec duration, the target was masked by a figure that included all features of the target in any orientation. Three lesion patients (L.S., J.C., and C.K., ages 17, 53, and 75, respectively) and five older control subjects (ages 71, 73, 73, 75, and 78) received a version of the task in which target duration was 100 msec, because they were unable to perform the task at greater than chance (25% accuracy) with 50 msec target duration, regardless of the attentional cue-to-target delay. This difficulty may reflect reduced visual acuity and/or slowed visual processing in these subjects. Although it is certainly not ideal to have differences in this experimental parameter, it was unavoidable. It is, of course, the case that increasing the target duration also increases the time to orient attention. Because, however, that increase was constant across the orienting delay conditions, it did not affect the primary results or conclusions based on comparisons between delay conditions.
The subject’s task was to move a joystick lever to indicate the direction of the target orientation (up, down, left, or right). Both response times and accuracy of response were recorded. Accurate performance in this task depended on speed of processing the target, not motor speed of the response. That is, subjects had only the brief delay between target onset and mask in which to process the target information but had several seconds in which to execute a motor response. Subjects were instructed to respond accurately, not quickly. This design effectively separates the speed of attention orienting (indexed by the cue-to-target delay) from the speed of perceptual processing (the target-to-mask duration) and speed of the motor response.
Data analysis
Repeated measures ANOVA and t tests were used to evaluate statistical significance of effects (BMDP statistical software; Dixon et al., 1990). These analyses are relatively insensitive to heterogeneity of variance, even with small samples (Edwards, 1985). If variances and sample sizes are unequal, however, the statistical test may be either too conservative or too liberal (i.e., too conservative if larger variance is associated with larger sample, see Glass et al., 1972; Kirk, 1982). For this reason,t tests were computed using separate rather than pooled estimates of variability (Welch Test) if Levene F-tests for differences in variability in groups were significant. Separate variance estimates also use computed degrees of freedom (Dixon et al., 1990; appendix B.6). In repeated measures ANOVAs, Greenhouse–Geisser corrections were used if sphericity assumptions were violated. To avoid confusion, the standard degrees of freedom are reported rather than the adjusted degrees of freedom associated with the Welch test and the Greenhouse–Geisser test.
Response time measures. Median response times (RTs) were computed for each subject for each condition and then averaged across groups.
Index of attention orienting. In these tasks, the more quickly attention is directed to a cued location, the faster (detection task) or more accurate (discrimination task) will be the response. Short cue-to-target delays provide little time to orient attention; longer delays provide more. An index of the speed with which attention can be oriented to the cued location is the difference in RT or accuracy at the validly cued location as a function of the cue-to-target delay (orienting deficit detection task = RT at 800 msec delay − RT at 100 msec delay; orienting deficit discrimination task = percent correct at 100 msec delay − percent correct at 1200 msec delay).
Index of attention cost. The effect of precuing when the cue is invalid is expressed as the difference in RT or accuracy to targets appearing at the validly cued compared with the invalidly cued location (validity deficit detection task = RT at the validly cued location − RT at the invalidly cued location; validity deficit discrimination task = percent correct at the invalidly cued location − percent correct at the validly cued location).
RESULTS
IQ Measures
Table 2 presents verbal and performance IQ measures for all subject groups. The verbal and performance subscales (vocabulary, comprehension, block design, and object assembly) represent relative strengths (block design and object assembly) and weaknesses (vocabulary and comprehension) in the autism cognitive profile (Dawson, 1983;Lincoln et al., 1988, 1995). Autism subjects had significantly lower scores than control subjects on all IQ measures (p < 0.05 for all) and lower scores than cerebellar lesion patients on overall verbal IQ (VIQ), performance IQ (PIQ), and on the verbal subscales (p < 0.05 for all). For autism subjects, VIQ and verbal subscales also fall in the below normal range based on Wechsler normative data. However, PIQ and performance subscales are within the range of normal performance (VIQ and PIQ mean of 100, SD of 15; all subscales mean of 10, SD of 3;Wechsler, 1981). Cerebellar lesion subjects had significantly lower scores than control subjects on overall verbal and performance IQ (p < 0.05). The overall IQ scores for the lesion subjects fall within the range of normal performance based on the Wechsler normative data.
MRI measures
Table 3 presents cerebellar vermal and brain volume measurements for all subject groups. In groups of subjects for whom complete MRI data were available, subjects with autism were significantly younger than normal control subjects (t(56) = 2.34;p < 0.025). The area measures of vermal lobules VI–VII were smaller in autism subjects than in control subjects (t(56) = 2.12; p < 0.04). Area measures of vermal lobules VI–VII adjusted for intracranial volume were also significantly smaller in autism subjects than in normal control subjects (t(56) = 2.77;p < 0.008). CSF volume was greater in cerebellar lesion subjects than in normal control subjects (t(49) = 2.62; p < 0.015) and greater in lesion subjects than in subjects with autism (t(21) = 3.53; p < 0.0065). There were no other significant differences in brain measure comparisons between subject groups.
Performance at the validly cued location (attention orienting/performance facilitation)
Spatial target detection task
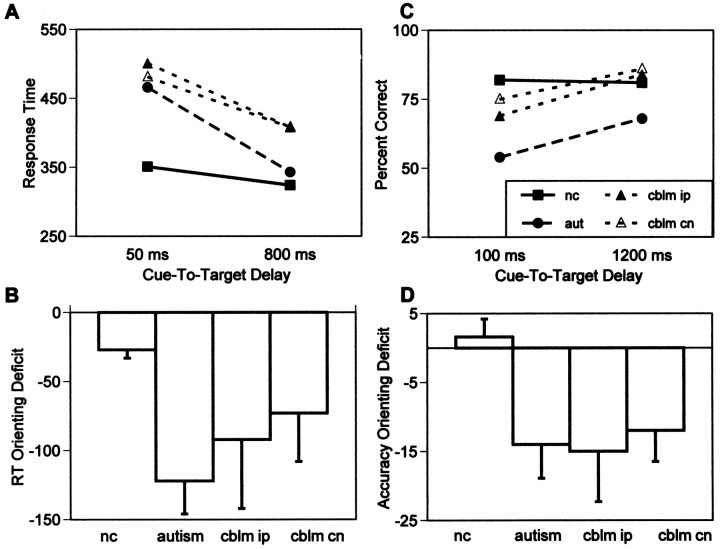
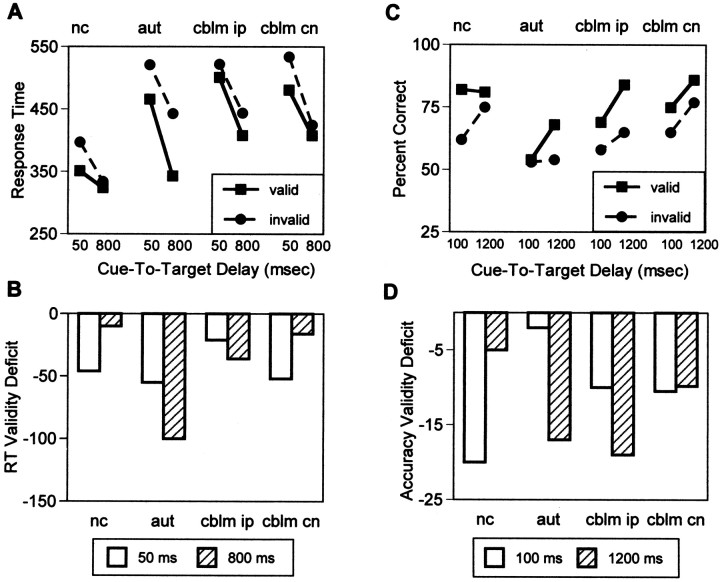
The following ANOVAs are group × cue-to-target delay repeated measures analyses comparing RT to targets at the validly cued location at the three delay intervals (50, 100, or 800 msec) for the three groups (control, autism, or cerebellar lesion). Overall, normal control subjects had faster response times at the validly cued location than did cerebellar lesion subjects (F(1,48) = 9.35; p < 0.004). All subjects were faster with longer cue-to-target delays (F(4,96) = 50.44; p < 0.0001). The rate of change was greater for subjects with autism (F(2,96) = 13.12; p < 0.0004) and for cerebellar lesion subjects (F(2,96) = 4.68; p < 0.03) than for normal control subjects. Follow-up analyses showed that compared with control subjects, lesion subjects’ rate of change was greater to both ipsilesional targets (F(2,96) = 5.12; p < 0.025) and to contralesional targets (F(2,96) = 3.79; p < 0.05; Fig. 4A). At 50 msec compared with 800 msec cue-to-target delay, normal control subjects’ responses were 27 msec slower, and autism subjects’ responses were 122 msec slower. Cerebellar lesion patients’ responses were 92 msec slower to ipsilesional targets and 73 msec slower to contralesional targets. These orienting deficits were significantly larger for autism subjects than for normal control subjects (t(40) = 5.50; p < 0.0001; Fig. 4B).
Fig. 4.
A, RT at the validly cued location for normal control subjects (filled square symbols, solid line), autism subjects (filled circle, long dashed line), and cerebellar lesion subjects for targets in the ipsilesional (filled triangles, short dashed line) and contralesional (open triangles,short dashed line) visual fields as a function of the amount of time to orient attention to that location (50 and 800 msec cue-to-target delays). Normal control subjects achieved the fastest response speed with only 50 msec to orient attention and did not improve performance significantly with longer cue-to-target intervals. Autism and cerebellar lesion subjects improved significantly with longer cue-to-target intervals (i.e., more time to orient attention).B, RT-orienting deficits (RT at validly cued location at 800 msec cue-to-target delay − RT at validly cued location at 50 msec cue-to-target delay) for all groups. Autism and cerebellar lesion subjects showed significantly faster RT with more time to orient attention. C, Accuracy (percent correct) at the validly cued location for normal control subjects (filled square symbols, solid line), autism subjects (filled circle, long dashed line), and cerebellar lesion subjects for targets in the ipsilesional (filled triangles, short dashed line) and contralesional (open triangles,short dashed line) visual fields as a function of the amount of time to orient attention to that location (100 and 1200 msec cue-to-target delays). Normal control subjects achieved the most accurate performance with only 100 msec to orient attention and did not improve performance significantly with longer cue-to-target intervals. Autism and cerebellar lesion subjects improved significantly with longer cue-to-target intervals (i.e., more time to orient attention). Chance performance accuracy was 25%. D, Accuracy-orienting deficits (percent correct at validly cued location at 100 msec cue-to-target delay − percent correct at validly cued location at 1200 msec cue-to-target delay) for all groups. Autism and cerebellar lesion subjects showed significantly greater accuracy with more time to orient attention.
Differences in RT to targets after null cues as a function of the cue-to-target delay were smaller for the patient groups than differences after valid cues, but the patterns were similar. At 50 msec compared with 800 msec cue-to-target delay, normal control subjects’ responses after null cues were 32 msec slower, and autism subjects’ were 89 msec slower. Cerebellar lesion patients’ responses were 73 msec slower to ipsilesional targets and 44 msec slower to contralesional targets. These orienting deficits after null cues were only marginally significantly larger in patient groups than in controls (autism vs controls, p < 0.13; lesion vs controls,p < 0.08). However, the magnitude of the improvement in RT with increasing delays in the absence of an attentional cue suggests the possibility that some general factor such as motor preparation may also contribute to attention-orienting effects at the validly cued location.
Spatial target discrimination task
The following ANOVAs are group × cue-to-target delay repeated measures analyses comparing RT to targets at the validly cued location at the three delay intervals (100, 800, or 1200 msec) for the three groups (control, autism, and cerebellar lesion). Overall, normal control subjects were more accurate at the validly cued location than were subjects with autism (F(1,48) = 14.10;p < 0.0005). There was no significant difference in overall accuracy of performance at the validly cued location between normal control and cerebellar lesion subjects. All subjects were more accurate with longer cue-to-target delays (F(2,96) = 7.37; p < 0.0015). The rate of improvement in accuracy over the three cue-to-target delays was greater for subjects with autism (F(2,96) = 5.84; p < 0.005) and for cerebellar lesion subjects (F(2,96) = 4.49; p < 0.015) than for normal control subjects. Follow-up analyses showed that compared with control subjects, lesion subjects’ rate of change was greater to both ipsilesional targets (F(2,96) = 4.49; p < 0.015) and to contralesional targets (F(2,96) = 4.37; p < 0.02; Fig. 4C). At 100 msec compared with 1200 msec cue-to-target delay, normal control subjects were 1.6% more accurate, and autism subjects were 14% less accurate. Cerebellar lesion patients were 15% less accurate to ipsilesional targets and 12% less accurate to contralesional targets at 100 msec compared with 1200 msec cue-to-target delay. These orienting deficits were significantly larger for autism subjects (t(42) = 3.14;p < 0.0035) and cerebellar lesion subjects to ipsilesional targets (t(36) = 2.67;p < 0.012) and to contralesional targets (t(36) = 2.24; p < 0.032) than for normal control subjects (Fig. 4D).
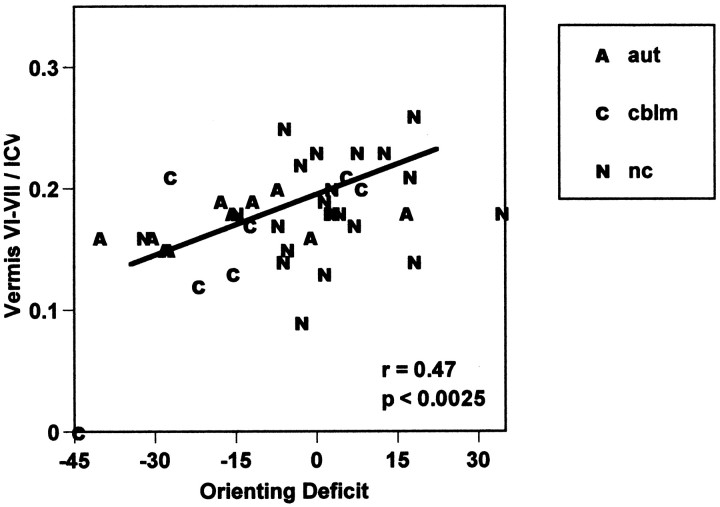
In the spatial discrimination task, larger orienting deficits (difference in accuracy at the validly cued location with 100 and 1200 msec cue-to-target delay using ipsilateral orienting deficits for cerebellar lesion patients) were associated with smaller vermal lobules VI–VII (r = 0.44; F(1,37)= 8.78; p < 0.0054). This correlation is slightly larger when the area measure for vermal lobules VI–VII is divided by intracranial brain volume to control for overall brain size (r = 0.47; F(1,37) = 10.62;p < 0.0025; Fig. 5). Because brain measures in some of the lesion and autism subjects were extreme and may have inflated the magnitude of the correlation coefficient, a Spearman rank order correlation was also computed for the association between orienting deficits and vermal lobules VI–VII. With this nonparametric test, smaller vermal lobules VI–VII were also associated with larger orienting deficits (r(39) = −0.40; p < 0.01).
Fig. 5.
Correlation of orienting with vermal lobules VI–VII in 22 normal control subjects, 10 autism subjects, and 7 cerebellar lesion subjects. Orienting deficit is an index of time to orient attention computed from response at the cued location as follows: (percent correct with 100 msec cue-to-target interval − percent correct with 1200 msec cue-to-target interval). Vermal lobule VI–VII area measures in each subject were divided by that subject’s intracranial brain volume to control for overall size of brain.
Larger orienting deficits (more negative orienting scores) were also associated with larger intracranial brain volume (r = −0.36; F(1,37) = 5.63; p< 0.025) and with lower performance IQ (r = 0.44;F(1,37) = 8.68; p < 0.0056), but not with age or other brain measures, including cerebellar vermal lobules I–V. Performance IQ was also significantly correlated with a measure of overall performance, the average percent correct at the validly cued location (higher performance IQ associated with overall better performance; r = 0.42;F(1,37) = 7.70; p < 0.009). None of the MRI brain measures were correlated with this measure of overall performance accuracy. There were also significant intercorrelations among size of cerebellar vermal lobules and amount of CSF. More CSF was associated with smaller vermal lobules VI–VII (r = −0.66; F(1,37) = 28.33; p < 0.0001) and I–V (r = −0.41; F(1,37) = 7.49; p< 0.0095).
Partial correlations were computed to estimate the unique association with the orienting index of vermal lobules VI–VII, ICV, and PIQ. With the linear effect of the other variables removed, the correlation of vermal lobules VI–VII with the orienting score was 0.39 (i.e., smaller lobules VI–VII associated with greater orienting deficits), whereas correlations for ICV and PIQ were reduced to −0.12 and 0.39, respectively. When the overall measure of performance accuracy was added to the model, partial correlations for vermal lobules VI–VII and ICV changed little (0.35 and −0.14, respectively), and the partial correlation for PIQ was reduced to 0.27.
A subset of the normal control subjects in which there were no significant age or PIQ differences from the cerebellar lesion patients (n = 10; age range, 16–71; mean age, 27 ± 17; PIQ = 106.6 ± 10) were analyzed to further examine the possible effect of PIQ on orienting differences. Results were identical to those from comparisons using the entire control sample. Compared to this PIQ-matched control sample, cerebellar lesion patients had significantly greater orienting deficits (0.03 vs −0.20;t(15) = 2.43; p < 0.029). It was, of course, not possible to form a similarly matched group for the autism subjects because their PIQ scores were below the normal range.
Cerebellar lesion patients with lesions that included the vermis (L.S. and J.C., Fig. 1) had the largest orienting deficits (55 and 37% change, respectively). Altogether, five of the seven cerebellar lesion patients showed orienting deficits that were >3 SEs greater than those of normal control subjects. One lesion subject (S.P.) had orienting deficits that were within the range of those shown by normal control subjects. The remaining lesion subject (G.Y.) had orienting deficits that were >2.5 SEs smaller than those of control subjects. This reversed pattern indicates a failure to sustain attention at the cued location (i.e., better performance at the incorrectly cued location with longer cue-to-target delays). This lesion patient had the smallest of the cerebellar lesions (lacunar white matter infarcts specifically affecting the left dentate nucleus and superior cerebellar peduncle).
Performance at the invalidly cued location (attention orienting/performance cost)
Spatial target detection task
The following ANOVAs are group × cue-to-target delay × cue validity repeated measures analyses comparing RT to targets at the three delay intervals (50, 100, or 800 msec) for the three groups (control, autism, or cerebellar lesion) in the two cue conditions (valid or invalid). Normal control subjects were faster overall at valid and invalid cued locations than were autism subjects (F(1,48) = 5.48; p < 0.025) or cerebellar lesion subjects (F(1,48) = 9.39; p < 0.005). Normal control subjects showed the largest difference in RT at valid compared with invalid locations when the cue-to target delay was short, whereas subjects with autism and cerebellar lesion subjects showed the largest difference in accuracy at valid compared with invalid locations when the cue-to-target delay was long (group × cue-to-target delay × cue validity interaction;F(4,96) = 7.76; p < 0.0002; Fig. 6A).
Fig. 6.
A, RT at the validly (filled squares, solid line) and invalidly (filled circles, long dashed line) cued locations for normal control, autism, and cerebellar lesion (for targets in the ipsilesional and contralesional visual fields) subjects as a function of the amount of time to orient attention to that location (50 and 800 msec cue-to-target delays). Normal control subjects showed maximum cue-related performance facilitation after 50 msec (greatest difference in RT at the cued compared with the uncued location). Autistic subjects showed maximum cue-related performance facilitation after 800 msec. Cerebellar lesion patients showed maximum cue-related performance facilitation to contralesional targets after 50 msec, but showed maximum cue-related facilitation after 800 msec to ipsilesional targets. B, RT validity deficits (RT at validly cued location − RT at invalidly cued location) for all groups after 50 msec (white bars) or 800 msec (striped bars) cue-to-target delays. Normal control subjects and cerebellar lesion subjects (to contralesional targets) showed largest validity deficits (i.e., greatest effect of attentional cue) at the 50 msec delay interval. Autism subjects and cerebellar lesion subjects (to ipsilesional targets) showed largest validity deficits (i.e., greatest effect of attentional cue) at the 800 msec delay interval. C, Accuracy of response at the validly cued (filled squares, solid line) compared with the invalidly cued (filled circles, long dashed line) location as a function of the amount of time to orient attention to that location (cue-to-target delay). Normal control subjects showed greatest cue-related response differences with only 100 msec to orient attention. Autism and cerebellar lesion subjects (to ipsilesional targets) showed the greatest performance facilitation after 1200 msec to orient attention. Chance performance accuracy was 25%. D, Accuracy validity deficits (percent correct at invalidly cued location − percent correct at validly cued location) for all groups at 100 msec (white bars) and 1200 msec (striped bars) cue-to-target delays. Normal control subjects showed largest validity deficits (i.e., greatest effect of attentional cue) at the 100 msec delay interval. Autism and cerebellar lesion subjects (to ipsilesional targets) showed maximum validity deficits after 1200 msec to orient attention.
Figure 6B shows validity deficits (difference in RT at valid compared with invalid locations) at the shortest and longest cue-to-target delays. Normal control subjects showed maximal validity deficits at the 50 msec delay, whereas autism subjects showed maximal validity deficits at the 800 msec delay (group × cue-to-target delay interaction; F(1,40) = 20.80;p < 0.0001). Like autism subjects, cerebellar lesion subjects showed maximal validity effects at the 800 msec delay to ipsilesional targets (lesion subjects vs controls, group × cue-to-target delay interaction; F(1,42) = 5.79; p < 0.025). Like control subjects, lesion subjects showed maximal validity effects at 50 msec to contralesional targets. Overall, autism subjects had larger validity deficits than control subjects (F(1,40) = 16.49;p < 0.0002). There was no difference in the magnitude of validity deficits to contralesional or ipsilesional targets between cerebellar lesion subjects and controls.
Spatial target discrimination task
The following ANOVAs are group × cue-to-target delay × cue validity repeated measures analyses comparing RT to targets at the three delay intervals (100, 800, or 1200 msec) for the three groups (control, autism, or cerebellar lesion) in the two cue conditions (valid or invalid). Normal control subjects were more accurate overall at valid and invalid cued locations than were subjects with autism (F(1,48) = 10.42; p < 0.0025), but not different from cerebellar lesion subjects. Normal control subjects showed the largest difference in accuracy at valid compared with invalid locations when the cue-to target delay was short, whereas subjects with autism and cerebellar lesion subjects showed the largest difference in accuracy at valid compared with invalid locations when the cue-to-target delay was long (group × cue-to-target delay × cue validity interaction;F(4,96) = 3.45; p < 0.02; Fig. 6C).
Figure 6D shows validity deficits (difference in accuracy at valid compared with invalid locations) at the shortest and longest cue-to-target delays. As in the spatial detection task, normal control subjects showed maximal validity deficits at the shortest, 100 msec, delay whereas autism subjects showed maximal validity deficits at the longest, 1200 msec, delay (autism vs controls; group × delay interaction; F(1,42) = 7.60;p < 0.009). Also, as in the spatial detection task, cerebellar lesion subjects showed maximal validity effects at the longest, 1200 msec, delay to ipsilesional targets (lesion subjects vs controls; group × delay interaction;F(1,36) = 3.78; p < 0.06), but maximal validity effects at 100 msec to contralesional targets. There was no difference between normal control subjects and autism or cerebellar lesion subjects in the overall size of validity deficits in this task.
Results from analyses of validity deficits comparing the cerebellar lesion patients to the subset of normal control subjects matched for age and PIQ (described above) were again the same as those from comparisons with the entire control sample. Like the entire control sample, the lower PIQ control subjects showed maximal validity deficits at the shortest cue-to-target delay interval (validity deficit at 100 msec delay = −23, at 1200 msec delay = −3). As in analyses with the entire control sample, the group × delay interaction comparing the PIQ-matched controls to lesion patients was marginally significant for ipsilesional targets (F(1,15) = 4.42; p < 0.053) and not different for contralesional targets.
DISCUSSION
In two different spatial attention tasks, patients with autism and those with acquired lesions of the cerebellum were slow to orient attention in space. In both tasks, with only 50–100 msec to orient attention, control subjects showed optimal performance (shorter RTs or greater accuracy) at a location to which their attention had been cued and maximal costs of that attention shift if the target did not occur at the cued location. Patients with cerebellar abnormality however, showed optimal performance at a cued location and maximal costs of the attentional cue only after 800–1200 msec.
In the target detection task, an attention-directing cue was followed by a target to be detected. The effect of the attentional cue was assessed by differences in response time to targets that were cued correctly or incorrectly. The times to orient attention to the cue, detect and respond to the target were all reflected in response time, but were not separable. Results from this task showing slower RTs with shorter cue-to-target delays in cerebellar patients suggests slowed attention orienting, but could also reflect slowed response preparation.
In the target discrimination task, an attention-directing cue was followed by a stimulus whose orientation was identified. The effect of the attentional cue was assessed by accuracy of response as a function of the cue validity. Masking the stimulus to be discriminated after 50 msec effectively limited the time to process the target. Using accuracy rather than RT as the dependent measure eliminated concerns about slowed response preparation or execution in subjects with neurological disorders. The design of this task separated time to orient attention (time between cue onset and target onset) from target processing (time between target onset and mask onset) and response preparation and execution (variable of interest is accuracy not speed of response). With only 100 msec to orient attention to the cued location, control subjects were as accurate as they were with longer cue-to-target intervals. Moreover, with only 100 msec to orient attention, normal controls showed the largest increases in accuracy at the cued compared with the uncued locations. In contrast, patients with autism and patients with cerebellar lesions improved performance significantly with more time to orient attention and showed maximal performance facilitation after the longest cue-to-target intervals. In this task, slowed orienting cannot be caused by motor preparation or execution.
In the discrimination task, larger attention-orienting deficits were significantly correlated with smaller cerebellar lobules VI–VII (from MRI measures). Although orienting deficits were also correlated with measures of performance IQ and ICV, partial correlations suggested that orienting deficit variance associated with lobules VI–VII was unique, whereas the association with ICV was secondary to a correlation between CSF (a component of ICV) and lobules VI–VII. PIQ was not uniquely associated with orienting deficits, but was associated more generally with overall performance (mean accuracy across all conditions). This is not an unexpected result because processing speed and competence are among the cognitive operations assessed by PIQ scales that could affect overall task performance. Evidence that slowed attention orienting in patients with cerebellar pathology is not secondary to lowered PIQ comes from analyses comparing attention orienting in cerebellar lesion patients to an age- and PIQ-matched normal control group. These analyses yielded results that were identical to those done with the entire control sample.
The size of vermal lobules VI–VII were associated specifically with orienting deficits and not with overall measures of performance competency. Although this correlation with orienting deficits was specific to lobules VI–VII, patients from both clinical groups had cerebellar abnormalities extending beyond this region. Because Purkinje cell loss has been reported throughout the cerebellum in autism (Ritvo et al., 1986; Bailey et al., 1998), it is likely that our autistic subjects have cerebellar abnormalities beyond those measured in the vermis. The cerebellar patients in this sample whose lesions involve the posterior vermis had the largest orienting deficits. However, the greatest overlap in lesions (Fig. 2) for these subjects was paramedial and in the lateral posterior cerebellar hemispheres. Lesions or abnormalities in these regions may involve deep cerebellar nuclei that control cerebral–cerebellar communication. Damage to these nuclei could disrupt vermal function even in the absence of vermal structural impairment.
Because vermal lobules VI–VII may be part of the oculomotor network that controls saccadic eye movement (for review, see Noda, 1991), one final concern about the interpretation of these results is that slowed orienting in patients with cerebellar abnormality could be caused by disruption of eye movements (for discussion, see Akshoomoff et al., 1997). Although eye movements may also be disrupted in the patients with cerebellar damage, the attention-orienting deficits observed in our patients occur too early to be the result of abnormal gaze shifting. Normal control subjects orient attention within 100 msec of the onset of a peripheral stimulus. It is at this short interval that control subjects and cerebellar-damaged patients differ most. That is, normal control subjects are able to use an attentional cue effectively within 100 msec, but those with cerebellar damage are not. Additionally, if cerebellar patients improved use of an attentional cue with longer time intervals by moving their eyes to the attended location, they would be expected to show an associated decrease in performance at the incorrectly cue location. As Figure 6 shows, this is not the case for either task.
Although it is likely that covert attention and gaze shift processes use overlapping brain systems, there is ample evidence that these systems can be manipulated independently (Posner et al., 1980; Goldberg and Segraves, 1987; Corbetta et al., 1993; Ladavas et al., 1997) (for review, see Goldberg and Colby, 1992). Covert attention shifts may in fact be used to direct a gaze shift (Posner and Cohen, 1984; Fischer and Breitmeyer, 1987). Single cell recordings in alert monkeys have demonstrated that activity in parietal cortex precedes an intended eye movement to predict the location of expected visual input (Duchamel et al., 1992). In our patients, covert orienting deficits could precede similar deficits in gaze orienting.
Studies of orienting gaze shifts in the cat after muscimol inactivation of the cerebellar fastigial nucleus (Goffart and Pelisson, 1994) suggest that cerebellar contribution to spatial orienting may be both motor and nonmotor. Visually triggered gaze shifts in the treated animals were hypermetric (with constant error) when directed ipsilateral to the injected side, and hypometric (with error that increased as a function of the required movement amplitude) when directed contralateral to the injected side. The authors conclude that the adaptive error in the hypometric shifts may reflect an inability to control gain of the movement. The constant hypermetric overshoots, however, suggest that the target location of the saccade is fixed and erroneous, perhaps reflecting a cerebellar-dependent faulty perception of the target location that precedes the decision to execute an eye movement.
Neural connections suggest pathways by which the cerebellum may influence both nonmotor and motor aspects of spatial orienting. Efferent fibers from the fastigial nucleus project to the ventral thalamus. There are reciprocal projections from the ventral thalamus to other brain regions known to be involved in spatial attention, including posterior parietal and precentral frontal cortex (Ito, 1984;Carpenter, 1985; Nieuwenhuys et al., 1988; Middleton and Strick, 1994). These same cortical regions are activated by electrical stimulation of the fastigial nucleus (Steriade, 1995). Additionally, fibers from the fastigial, dentate, and interposed nuclei terminate in deep layers of the superior colliculus via the superior cerebellar peduncle. The thalamus has been suggested to be a critical component of systems that control covert orienting of attention (Rafal and Posner, 1987) (for review, see Desimone and Duncan, 1995), whereas the superior colliculus may be more closely related to the programming and subsequent execution of saccadic movement (Posner and Petersen, 1990; Goldberg and Colby, 1992). Damage to the cerebellum could disrupt spatial encoding and cortical activation via a cerebellothalamocortical circuit and disrupt programming to direct a gaze shift via cerebellocolliculocortical pathways, thus delaying both covert and subsequent overt orienting to a salient location.
A growing number of studies indicate that the traditional model of the cerebellum as a brain structure whose sole purpose is to support motor function needs modification. Building on previous suggestions that the cerebellum plays a role in sensory tracking, prediction, association, and anticipatory learning (Bower and Kassel, 1990; Miall et al., 1993;Paulin, 1993; Coenen and Sejnowski, 1996; Bell et al., 1997), Courchesne has proposed an anticipatory model of cerebellar function. The cerebellum may serve to prepare internal systems for upcoming events based on predictions computed from continuous tracking of and learning from sensory, cognitive, and motor information. In this way, the cerebellum supports a broad spectrum of brain systems involved in both motor and nonmotor function (Courchesne and Allen, 1997;Courchesne, 1997b). Data from the present study are consistent with this model. Continuous tracking of sensory information in space may allow the cerebellum to compute and relay to other brain systems the predictions that guide optimal attentional responses.
Footnotes
This work was supported by National Institute of Mental Health Grant 2RO1-MH36840–11 and National Institute of Neurological Diseases and Stroke Grant 1RO1-NS34155–01. We thank Janet Werner, RN, Clinical Nurse, Department of Neurosciences, University of California, San Diego for her invaluable assistance.
Correspondence should be addressed to Jeanne Townsend, Department of Neurosciences, MC-0217, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0217.
REFERENCES
- 1.Akshoomoff NA, Courchesne E. A new role for the cerebellum in cognitive operations. Behav Neurosci. 1992;106:731–738. doi: 10.1037//0735-7044.106.5.731. [DOI] [PubMed] [Google Scholar]
- 2.Akshoomoff NA, Courchesne E. ERP evidence for a shifting attention deficit in patients with damage to the cerebellum. J Cognit Neurosci. 1994;6:388–399. doi: 10.1162/jocn.1994.6.4.388. [DOI] [PubMed] [Google Scholar]
- 3.Akshoomoff NA, Courchesne E, Townsend J. Attention coordination and anticipatory control. In: Schmahmann JD, editor. International review of Neurobiology, Vol 41, The cerebellum and cognition. Academic; San Diego: 1997. pp. 575–598. [DOI] [PubMed] [Google Scholar]
- 4.Allen G, Buxton RB, Wong EC, Courchesne E. Attentional activation of the cerebellum independent of motor control. Science. 1997;275:1940–1943. doi: 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, Ed 3, revised. American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- 6.Arin DM, Bauman ML, Kemper TL. The distribution of Purkinje cell loss in the cerebellum in autism. Neurology. 1991;41[Suppl 1]:307. [Google Scholar]
- 7.Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- 8.Bauman ML, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- 9.Bauman ML, Kemper TL. Developmental cerebellar abnormalities: A consistent finding in early infantile autism. Neurology. 1986;36[Suppl 1]:190. [Google Scholar]
- 10.Bauman ML, Kemper TL. Limbic and cerebellar abnormalities are also present in an autistic child of normal intelligence. Neurology. 1990;40[Suppl 1]:359. [Google Scholar]
- 11.Bauman ML, Kemper TL. The neurobiology of autism, pp 119–145. John Hopkins UP; Baltimore: 1994. Neuroanatomic observations of the brain in autism. [Google Scholar]
- 12.Bell C, Bodznick D, Montgomery J, Bastian J. The generation and subtraction of sensory expectations within cerebellum-like structures. Brain Behav Evol. 1997;50[Suppl 1]:17–31. doi: 10.1159/000113352. [DOI] [PubMed] [Google Scholar]
- 13.Bower JM, Kassel J. Variability in tactile projection patterns to the cerebellar folia crus IIA of the Norway rat. J Comp Neurol. 1990;302:768–778. doi: 10.1002/cne.903020409. [DOI] [PubMed] [Google Scholar]
- 14.Cabeza R, Nyberg L. Imaging cognition: an empirical review of PET studies with normal subjects. J Cognit Neurosci. 1997;9:1–26. doi: 10.1162/jocn.1997.9.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter MB. Core text of neuroanatomy, Ed 3, p 215. Williams and Wilkins; Baltimore: 1985. [Google Scholar]
- 16.Corbetta M, Miezin MM, Shulman GL, Petersen SE. A PET study of visuospatial attention. J Neurosci. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coenen OJMD, Sejnowski TJ (1996) Learning to make predictions in the cerebellum may explain the anticipatory modulation of the vestibulo-ocular reflex (VOR) gain with vergence3. Proceedings of the Third Joint Symposium on Neural Computation: Institute of Neural Computation, University of California, San Diego, CA and Center for Neuromorphic Systems Engineering, Caltech, Pasadena, CA.
- 18.Coull JT, Nobre AC. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci. 1998;18:7426–7436. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courchesne E. Infantile autism, part 2: a new neurodevelopmental model. Int Pediat. 1995;10:155–165. [Google Scholar]
- 20.Courchesne E. Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr Opin Neurobiol. 1997a;7:269–278. doi: 10.1016/s0959-4388(97)80016-5. [DOI] [PubMed] [Google Scholar]
- 21.Courchesne E. Prediction and preparation: anticipatory role of the cerebellum in diverse neurobehavioral functions. Behav Brain Sci. 1997b;20:248–249. [Google Scholar]
- 22.Courchesne E, Allen G. Prediction and preparation, fundamental functions of the cerebellum. Learn Mem. 1997;4:1–35. doi: 10.1101/lm.4.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL. Hypoplasia of cerebellar lobules VI and VII in infantile autism. N Engl J Med. 1988;318:1349–1354. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- 24.Courchesne E, Press GA, Murakami J, Berthoyt D, Grafe M, Wiley CA, Hesselink JR. The cerebellum in sagittal plane: anatomic-MR correlation: 1. The vermis. AJNR Am J Neuroradiol. 1989;10:659–665. doi: 10.2214/ajr.153.4.829. [DOI] [PubMed] [Google Scholar]
- 25.Courchesne E, Saitoh O, Yeung-Courchesne R, Press GA, Haas R, Lincoln A, Schreibman L. Abnormality of vermian lobules VI and VII in patients with infantile autism: identification of hypoplastic and hyperplastic subgroups. AJR Am J Roentgenol. 1994a;162:123–130. doi: 10.2214/ajr.162.1.8273650. [DOI] [PubMed] [Google Scholar]
- 26.Courchesne E, Townsend J, Saitoh O. The brain in infantile autism: posterior fossa structures are abnormal. Neurology. 1994b;44:214–223. doi: 10.1212/wnl.44.2.214. [DOI] [PubMed] [Google Scholar]
- 27.Courchesne E, Townsend J, Akshoomoff NA, Saitoh O, Yeung-Courchesne R, Lincoln A, James H, Haas RH, Schreibman L, Lau L. Impairment in shifting attention in autistic and cerebellar patients. Behav Neurosci. 1994c;108:848–865. doi: 10.1037//0735-7044.108.5.848. [DOI] [PubMed] [Google Scholar]
- 28.Courchesne E, Yeung-Courchesne R, Egaas B. Methodology in neuroanatomic measurement. Neurology. 1994d;44:203–208. doi: 10.1212/wnl.44.2.203. [DOI] [PubMed] [Google Scholar]
- 29.Dawson G. Lateralized brain function in autism: evidence from the Halstead-Reitan neuropsychological battery. J Autism Dev Disord. 1983;13:369–386. doi: 10.1007/BF01531566. [DOI] [PubMed] [Google Scholar]
- 30.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 31.Dixon WJ, Brown MB, Engelman L, Jennrich RI. BMDP statistical software manual, Vols 1–2. University of California; Berkeley, CA: 1990. [Google Scholar]
- 32.Duchamel J-R, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255:90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- 33.Edwards AL. Experimental design in psychological research, Ed 5. Harper and Row; New York: 1985. [Google Scholar]
- 34.Fehlow P, Bernstein K, Tennstedt A, Walther F. Autismus infantum und exzessive aerophagie mit symptomatischem megakolon und ileus bei einem fall von ehlers-danslos-syndrom. Padiatr Grenzgeb. 1993;31:259–267. [PubMed] [Google Scholar]
- 35.Fischer B, Breitmeyer B. Mechanisms of visual attention revealed by saccadic eye movements. Neuropsychology. 1987;25:73–84. doi: 10.1016/0028-3932(87)90044-3. [DOI] [PubMed] [Google Scholar]
- 36.Glass GV, Peckham PD, Sanders JR. Consequences of failure to meet assumptions underlying the analysis of variance and covariance. Rev Educ Res. 1972;42:237–288. [Google Scholar]
- 37.Goffart L, Pelisson D. Cerebellar contribution to the spatial encoding of orienting gaze shifts in the head-free cat. J Neurophysiol. 1994;72:2547–2550. doi: 10.1152/jn.1994.72.5.2547. [DOI] [PubMed] [Google Scholar]
- 38.Goffart L, Pelisson D. Changes in initiation of orienting gaze shifts after muscimol inactivation of the caudal fastigial nucleus in the cat. J Physiol (Lond) 1997;503.3:657–671. doi: 10.1111/j.1469-7793.1997.657bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg ME, Colby CL. Oculomotor control and spatial processing. Curr Opin Neurobiol. 1992;2:198–202. doi: 10.1016/0959-4388(92)90012-a. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg ME, Segraves MA. Visuospatial and motor attention in the monkey. Neuropsychologia. 1987;25:107–118. doi: 10.1016/0028-3932(87)90047-9. [DOI] [PubMed] [Google Scholar]
- 41.Ito M. The cerebellum and neural control, pp 142–144. Raven; New York: 1984. [Google Scholar]
- 42.Jackson EF, Narayana PA, Falconer JC. Reproducibility of nonparametric feature map segmentation for determination of normal human intracranial volumes with MR imaging data. J Magn Reson Imaging. 1994;4:692–700. doi: 10.1002/jmri.1880040512. [DOI] [PubMed] [Google Scholar]
- 43.Kirk RE. Experimental design: procedures for the behavioral sciences. Ed 2. Brooks/Cole; Monterey, CA: 1982. [Google Scholar]
- 44.Ladavas E, Zeloni G, Zaccara G, Gangemi P. Eye movements and orienting of attention in patients with visual neglect. J Cognit Neurosci. 1997;9:67–74. doi: 10.1162/jocn.1997.9.1.67. [DOI] [PubMed] [Google Scholar]
- 45.Le TH, Pardo JV, Hu X. 4 T-fMRI study of nonspatial shifting of selective attention: cerebellar and parietal contributions. J Neurophysiol. 1998;79:1535–1548. doi: 10.1152/jn.1998.79.3.1535. [DOI] [PubMed] [Google Scholar]
- 46.Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, McLennan J. Autism diagnostic interview: a standardized investigator-based instrument. J Autism Dev Disord. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- 47.Lincoln AJ, Courchesne E, Kilman BA, Elmasian R, Allen M. A study of intellectual abilities in high-functioning people with autism. J Autism Dev Disord. 1988;8:505–524. doi: 10.1007/BF02211870. [DOI] [PubMed] [Google Scholar]
- 48.Lincoln AJ, Allen MH, Kilman A. The assessment and interpretation of intellectual abilities in people with autism. In: Schopler E, Mesibov GB, editors. Learning and cognition in autism. Plenum; New York: 1995. pp. 89–117. [Google Scholar]
- 49.Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- 50.Matsumae M, Kikinis R, Morocz IA, Lorenzo AV, Sandor T, Albert MS, Black PM, Jolesz F. Age-related changes in intracranial compartment volumes in normal adults assessed by magnetic resonance imaging. J Neurosurg. 1996;84:982–991. doi: 10.3171/jns.1996.84.6.0982. [DOI] [PubMed] [Google Scholar]
- 51.Miall RC, Weir DJ, Wolpert DM, Stein JF. Is the cerebellum a smith predictor? J Mot Behav. 1993;25:203–216. doi: 10.1080/00222895.1993.9942050. [DOI] [PubMed] [Google Scholar]
- 52.Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- 53.Nieuwenhuys R, Voogd J, vanHuijzen C. The human central nervous system, a synopsis and atlas, Ed 3, pp 232–236. Springer; Berlin: 1988. [Google Scholar]
- 54.Noda H. Cerebellar control of saccadic eye movements: its neural mechanisms and pathways. Jpn J Physiol. 1991;41:351–368. doi: 10.2170/jjphysiol.41.351. [DOI] [PubMed] [Google Scholar]
- 55.Paulin MG. The role of the cerebellum in motor control and perception. Brain Behav Evol. 1993;41:39–50. doi: 10.1159/000113822. [DOI] [PubMed] [Google Scholar]
- 56.Posner MI, Cohen Y. Component of performance. In: Bouma H, Bowhuis D, editors. Attention and performance X. Erlbaum; Hillsdale, NJ: 1984. pp. 531–556. [Google Scholar]
- 57.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 58.Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;21:160–174. [PubMed] [Google Scholar]
- 59.Posner MI, Walker JA, Freidrich FA, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rafal RD, Posner MI. Deficits in human visual spatial attention following thalamic lesions. Proc Natl Acad Sci USA. 1987;84:7349–7353. doi: 10.1073/pnas.84.20.7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ritvo ER, Freeman BJ, Scheibel AB, Duong T, Robinson H, Guthrie D, Ritvo A. Lower Purkinje cell counts in the cerebella of four autistic subjects: initial findings of the UCLA-NSAC autopsy research report. Am J Psychiatry. 1986;143:862–866. doi: 10.1176/ajp.143.7.862. [DOI] [PubMed] [Google Scholar]
- 62.Schmahmann JD. Rediscovery of an early concept. In: Schmahmann JD, editor. International review of Neurobiology, Vol 41, The cerebellum and cognition. Academic; San Diego: 1997. pp. 2–35. [DOI] [PubMed] [Google Scholar]
- 63.Schopler E, Reichler RJ, De Velis RF, Daly K. Toward objective classification of childhood autism: childhood autism rating scale (CARS). J Autism Dev Disord. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- 64.Steriade M. Two channels in the cerebellothalamocortical system. J Comp Neurol. 1995;354:57–70. doi: 10.1002/cne.903540106. [DOI] [PubMed] [Google Scholar]
- 65.Townsend J, Courchesne E, Egaas B. Deficits in orienting attention in patients with cerebellar and parietal damage. Soc Neurosci Abstr. 1992;18:332. [Google Scholar]
- 66.Townsend J, Courchesne E, Egaas B. Slowed orienting of covert visual-spatial attention in autism: specific deficits associated with cerebellar and parietal abnormality. Dev Psychopathol. 1996a;8:563–584. [Google Scholar]
- 67.Townsend J, Singer-Harris N, Courchesne E. Visual attention abnormalities in autism: delayed orienting to location. J Int Neuropsychol Soc. 1996b;2:541–550. doi: 10.1017/s1355617700001715. [DOI] [PubMed] [Google Scholar]
- 68.Vannier MW, Butterfield RL, Jordan D, Murphy WA, Levitt RG, Gado M. Multispectral analysis of magnetic resonance images. Radiology. 1985;154:221–224. doi: 10.1148/radiology.154.1.3964938. [DOI] [PubMed] [Google Scholar]
- 69.Wechsler D. Wechsler intelligence scale for children-revised. Psychological Corporation; New York: 1974. [Google Scholar]
- 70.Wechsler D. Wechsler adult intelligence scale-revised. Psychological Corporation; New York: 1981. [Google Scholar]
- 71.Williams RS, Hauser SL, Purpura DP, DeLong R, Swisher CN. Autism and mental retardation: neuropathological studies performed in four retarded persons with autistic behavior. Arch Neurol. 1980;37:749–753. doi: 10.1001/archneur.1980.00500610029003. [DOI] [PubMed] [Google Scholar]
- 72.Yeung-Courchesne R, Courchesne E. From impasse to insight in autism research: from behavioral symptoms to biological explanations. Dev Psychopathol. 1997;9:389–419. doi: 10.1017/s0954579497002101. [DOI] [PubMed] [Google Scholar]