Abstract
It is well established that the basolateral amygdala is critically involved in the association between an unconditioned stimulus (US), such as a foot shock, and a conditioned stimulus (CS), such as a light, during classic fear conditioning. However, little is known about how the US (pain) inputs are relayed to the basolateral amygdala. The present studies were designed to define potential US pathways to the amygdala using lesion methods. Electrolytic lesions before or after training were placed in caudal granular/dysgranular insular cortex (IC) alone or in conjunction with the posterior intralaminar nuclei of the thalamus (PoT/PIL), and the effects on fear conditioning were examined. Pretraining lesions of both IC and PoT/PIL, but not lesions of IC alone, blocked the acquisition of fear-potentiated startle. However, post-training combined lesions of IC and PoT/PIL did not prevent expression of conditioned fear. Given that previous studies have shown that lesions of PoT/PIL alone had no effect on acquisition of conditioned fear, these results suggest that two parallel cortical (insula–amygdala) and subcortical (PoT/PIL–amygdala) pathways are involved in relaying shock information to the basolateral amygdala during fear conditioning.
Keywords: fear conditioning, amygdala, insular cortex, posterior intralaminar nuclei, nociception, startle
Classic fear conditioning is one of the most widely used models for studying the neural mechanisms of learning and memory. In this paradigm, an innocuous conditioned stimulus (CS), usually a light or tone, is paired with an aversive unconditioned stimulus (US), such as a foot shock. After pairing, the CS now produces a constellation of behavioral and autonomic responses (conditioned fear responses) formerly produced only by the US. Converging evidence now indicates that the amygdala plays a crucial role in fear conditioning and that different subnuclei within the amygdala may play different roles in the development and expression of conditioned fear. The central nucleus of the amygdala has widespread connections with autonomic, neuroendocrine, and motor-related structures in the hypothalamus and brainstem and is required for the expression of various fear responses via these differential efferents (Davis, 1992; Kapp et al., 1992; LeDoux, 1992a,b). The basolateral amygdala, mainly the lateral and basolateral nuclei, integrates different sensory inputs that are passed on to the dorsal, ventral, and allostriatum to execute different emotional responses (LeDoux et al., 1988, 1990b, 1992a,b; Everitt et al., 1991; Davis et al., 1994; Shi, 1995).
Although anatomical tracing studies have defined various afferents from different sensory cortices, as well as thalamic structures, to the basolateral amygdala (McDonald and Jackson, 1987; LeDoux et al., 1990a;Turner and Herkenham, 1991; Yasui et al., 1991; Mascagni et al., 1993;Romanski and LeDoux, 1993; Shi and Cassell, 1997), the exact functional roles of these pathways in emotional learning remains unclear. Recent lesion studies suggest that there are parallel cortical and thalamic routes through which a simple auditory CS (i.e., a tone) can transmit information to the amygdala in fear conditioning (Romanski and LeDoux, 1992b; Campeau and Davis, 1995). However, it is unknown whether this parallel processing is a general rule in transmitting other CSs, such as light, and USs, such as a foot shock, the most common US used in fear conditioning.
The goal of the present study was to investigate the somatic pain pathways that transmit foot shock information to the basolateral amygdala during fear conditioning in rats. Based on the available literature, two brain areas (areas medial to the medial geniculate nucleus and the insular cortex) were singled out for study. The former includes the posterior triangular nucleus (PoT), the posterior intralaminar nucleus (PIL), the suprageniculate nucleus (SG), parvocellular part of the subparafascicular nucleus (SPFPC), and possibly the medial subdivision of the medial geniculate complex (MGM), which constitute a posterior extension of the intralaminar complex (Winer et al., 1988). Besides receiving acoustic inputs from the inferior colliculus (LeDoux et al., 1987), the posterior intralaminar nuclei also receive somatic pain inputs from the spinal cord (LeDoux et al., 1987, 1990a; Dado and Giesler, 1990; Cliffer et al., 1991) and in turn project to the amygdala, particularly the lateral amygdaloid nucleus (LeDoux et al., 1985, 1987, 1990a; Yasui et al., 1991; Shi and Davis, 1997). Electrical stimulation of this area is an effective unconditioned stimulus for fear conditioning similar to foot shock (Cruikshank et al., 1992). Thus, this thalamoamygdaloid pathway may serve as a US pathway during emotional learning. However, pretraining lesions of the posterior intralaminar nuclei alone did not prevent the acquisition of fear conditioning (Romanski and LeDoux, 1992b; Campeau and Davis, 1995), indicating that an additional pathway or pathways must contribute foot shock information to the amygdala.
Recent anatomical studies suggest that the insular cortex, besides being critical in gustatory and visceral sensation, may also be involved in somatosensory perception, particularly aversive pain sensation. The caudal part of insular cortex, the so-called “parietal insula” (Shi and Cassell, 1998a,b), receives convergent inputs from somatosensory cortices, ventroposterior and posterior thalamic nuclei, posterior intralaminar nuclei, and the midbrain parabrachial nucleus (Yasui et al., 1989; Fabri and Burton, 1991; Craig et al., 1994;Barnett et al., 1995; Shi and Cassell, 1998a). Furthermore, this portion of the insular cortex is probably a primary source in providing cortical somatosensory information to the amygdala (Friedman and Murray, 1986; Shi and Cassell, 1998a). Thus, it is possible that the caudal insula together with posterior intralaminar nuclei may compose parallel cortical and thalamic routes to relay somatic pain information activated by a shock US to the amygdala during fear conditioning.
To test this hypothesis, a series of lesion experiments were designed. Electrolytic lesions of posterior parietal insula alone or combined with posterior intralaminar nuclei of the thalamus (PoT/PIL) were performed either before or after training. Fear conditioning was conducted by pairing a foot shock with a light or a tone. The acquisition of conditioning was tested by measuring fear-potentiated startle, a well defined paradigm for measuring fear conditioning (Davis et al., 1993). We reasoned that if parallel corticoamygdala and thalamoamygdala pain pathways exist, combined cortical and thalamic lesions performed before, but not after, training would be necessary to block fear conditioning.
MATERIALS AND METHODS
Subjects
A total of 71 adult male albino Sprague Dawley rats (Charles River, Portage, MI) weighing 320–400 gm were used. Animals were housed in groups of two or three in wire cases (17 × 35 × 45 cm) with water and laboratory chow continuously available. They were maintained on a 12 hr light/dark cycle (lights on at 7:00 A.M.), and behavioral procedures occurred during the light period. Rats were acclimated to the colony rooms for 2–3 weeks before experimental manipulation.
Experimental design
Three experiments were performed. Experiment 1 tested the effects of pretraining electrolytic lesions of posterior parietal insular cortex alone on fear-potentiated startle. Experiment 2 tested the effects of combined pretraining lesions of posterior parietal insular cortex and posterior intralaminar nuclei of the thalamus on acquisition of fear-potentiated startle. Experiment 3 tested the effects of the same combined lesions performed after training to determine the specificity of the pretraining combined lesion effect that was observed in Experiment 2.
Apparatus
Animals were trained and tested in five identical stabilimeter devices that have been described previously (Cassella and Davis, 1986). Briefly, each stabilimeter consisted of an 8 × 15 × 15 cm Plexiglas and wire mesh cage suspended within a steel frame. The floor of each stabilimeter consisted of four 6-mm-diameter stainless steel bars spaced 18 mm apart through which shock could be delivered. Within the steel frame, the cage was compressed between four springs above and a 5 × 5 cm rubber cylinder below, with an accelerometer (Endevco 2217E) located between the cage and the rubber cylinder. Cage movement resulted in displacement of an accelerometer where the resultant voltage was proportional to the velocity of the cage displacement. Startle amplitude was defined as the maximum accelerometer voltage that occurred during the first 0.2 sec after the startle stimulus was delivered. The analog output of the accelerometer was amplified (Endevco model 104), digitized on a scale of 0–4096 units by a MacADIOS II board (GW Instruments, Somerville, MA), and stored on a Macintosh II microcomputer.
Each stabilimeter was enclosed in a ventilated, light-, and sound-attenuating box (68.5 × 35.5 × 42 cm). This inner isolation box was located within an additional outer-ventilated plywood-isolated box (76 × 47 × 51 cm). The five wooden boxes, in turn, were housed in a larger ventilated, light- and sound-attenuating chamber [2.5 × 2.5 × 2.0 m (Industrial Acoustic, Bronx, NY)]. A TV camera (Ikegami) for observing the behavior of the rats during the experiment was positioned behind the stabilimeter within the inner isolation box and connected to a TV monitor located outside of the chamber. A red light bulb (7.5 W) was located on the floor of the inner isolation box to provide illumination for the cameras in the otherwise dark box.
Stimuli
All sound level measurements were made with a Precision Sound Level Meter (A scale, Model 2235; Bruel & Kjaer). Background noise (0–20 kHz, 55 dB) was produced by a white noise generator (Lafayette, Model 15800, Lafayette, ID) and delivered through high-frequency speakers (Radio Shack Supertweeters; range, 5–40 kHz) located 2 cm from the front of each stabilimeter. This plus the noise of the ventilating fan attached to the side wall of each wooden box produced an overall background noise level of 64 dB. The startle stimulus was a 50 msec burst of white noise (5 msec rise–decay time) of various intensities generated by a white noise generator (Lafayette, Model 15800) and delivered through the same speakers as the background noise.
The visual CS was a 3.7 sec light produced by an 8 W fluorescent bulb (100 μsec rise time, 700 foot lamberts) attached to the back of each stabilimeter. The auditory CS was produced by a white noise generator and bandpass-filtered, with both the low and high passes set at 2 kHz (24 dB/octave attenuation), at an intensity of 70 dB (SPL). Relatively low-frequency auditory CSs were previously found to produce reliable fear-potentiated startle (Campeau and Davis, 1992). The auditory CS was delivered by a speaker located ∼70 cm in front of each cage. The unconditioned stimulus was a 0.6 mA foot shock with a duration of 0.5 sec, generated by five Lehigh Valley constant-current shockers (SGS-004, Beltsville, MD) located outside the chamber. Shock intensity was measured with a 1 kΩ resistor across a differential channel of an oscilloscope in series with a 100 kΩ resistor connected between adjacent floor bars within each stabilimeter. Current was defined as the root mean square voltage across the 1 kΩ resistor, where mA = 0.707 × 0.5 × peak-to-peak voltage. The presentation and sequencing of all stimuli were under the control of the Macintosh II microcomputer.
Behavioral procedures
Matching. On the first 2 d of all experiments, rats were placed in the stabilimeter cages and 5 min later presented with 30 startle stimuli at a 30 sec interstimulus interval. Intensities of 90, 95, and 105 dB were used with 10 startle stimuli at each intensity. Startle stimuli were presented in a balanced, irregular sequence, with the restriction that each of the three intensities had to occur in every three trial blocks. The mean startle amplitude across the 30 startle stimuli on the last matching day was used to assign rats into sham or lesion groups with similar means before training or surgery.
Training. On each of 2 consecutive d, rats were placed in the stabilimeter cages and 5 min later received the first of 10 visual CS-shock or auditory CS-shock pairings. The shock was delivered during the last 0.5 sec of the 3.7 sec light or noise CSs at an average intertrial interval of 4 min (range, 3–5 min).
Shock-induced activity. To obtain a measure of how the lesions might have affected reactivity to foot shock during training, stabilimeter output during the 10 CS-shock pairings was sampled for 0.2 sec periods after shock onset. Shock activity was defined as the mean cage output across the 10 shock presentations.
Testing. Rats were placed in the same startle cages where they were trained and after 5 min presented with 18 startle-eliciting stimuli in the dark (six at each of three intensities: 90, 95, or 105 dB). These initial startle stimuli (hereafter called “leaders”) were used to habituate the rats to the acoustic startle stimuli. Thirty seconds after the last leader stimulus, each animal received 60 startle stimuli (20 at each of three intensities: 90, 95, or 105 dB) with half of the stimuli presented alone (startle alone trials) and the other half presented 3.2 sec after the onset of the 3.7 sec CS (CS-startle trials). The six trial types were presented in randomized order. All startle stimuli were presented at a 30 sec interstimulus interval.
Shock sensitization. One week after potentiated startle testing, the animals were returned to the test cages and presented with a total of 40 startle stimuli (105 dB) at a 30 sec interstimulus interval. Fifteen seconds after the last noise burst, 10 shocks of 0.6 mA each were presented at a rate of one shock per second. Fifteen seconds after the last shock, a final series of 40 startle stimuli (105 dB) were presented at a 30 sec interstimulus interval.
Electrolytic lesions
Stereotaxic surgical procedures were performed under anesthesia with sodium pentobarbital (50 mg/kg, i.p.). Lesion coordinates were based on the rat atlas of Paxinos and Watson (1986). The coronal plane was referenced, and a flat-skull position was achieved by adjusting the incisor bar accordingly. Electrolytic lesions were made with stainless steel electrodes (0.25 mm in diameter) insulated except for 0.5 mm of the tip. A constant current source generated DC anodal current for all electrolytic lesions at an intensity of 1.0 mA. Lesions of insular cortex were performed by applying current for 10 sec at each at the following coordinates: anterioposterior (AP) = −2.0 mm, mediolateral (ML) = ±6.0 mm, dorsoventral (DV) = −7.0 mm; and AP = −3.3 mm, ML= ±6.0 mm, DV = −7.0 mm. Lesions of posterior thalamus were performed by applying current for 5 sec at each at the following coordinates: AP = −4.8 mm, ML= ±2.6 mm, DV = −6.5 mm; and AP = −5.8 mm, ML= ±2.6 mm, DV = −6.5 mm. Sham electrolytic lesions consisted of lowering the electrode 1.0 mm above the ventral lesion coordinate without passing current. All subjects were allowed 7–10 d recovery from surgery before training or testing.
Histology
At the end of experiments, lesioned rats were anesthetized with an overdose of chloral hydrate and perfused with physiological saline followed by 10% buffered formalin phosphate. Brains were removed and stored in a solution of 30% sucrose in formalin for at least 2 d. Sections (50 μm thick) were cut through lesion sites on a frozen microtome and mounted on gelatin-coated slides. After drying, the slides were stained with cresyl violet, and the extent of lesion sites was evaluated under a microscope.
Data analysis
Inclusion of rats in statistical analyses was based strictly on the adequacy of lesions, without knowledge of the behavioral data of individual rats. Criteria for adequate lesions included bilateral damage of the area investigated throughout most of its extent, with incomplete and inconsistent damage of surrounding areas. In the case of the insular cortex, the lesions had to include the granular and dysgranular cortices of caudal insula bilaterally, extending approximately from 1.8 to 3.8 mm behind bregma in Paxinos and Watson (1986). This portion of insular cortex has been shown to connect with limb regions of primary and secondary somatosensory cortices and in turn projects to the amygdala in rats (Fabri and Burton, 1991; Shi and Cassell, 1998a). However, lesions had to be limited to the dorsal bank of the rhinal sulcus. Rats with substantial damage to the ventral bank, which composes the agranular insular cortex, were excluded. For the thalamus, the lesions had to include the PoT, PIL, and MGM (Paxinos and Watson, 1986). These areas receive direct spinothalamic inputs and project to the amygdala.
Mean startle amplitudes were computed for the startle stimulus alone trials and the visual or auditory CS trials, respectively, for each rat. In addition, the mean startle amplitude on the startle stimulus alone trials was subtracted from the mean startle amplitude on the respective visual and auditory CS trials, providing a difference score for each CS modality for each rat. Repeated-measures ANOVAs were first conducted on the mean startle amplitude data, to detect significant levels of fear-potentiated startle (trial type effect) and possible interactions with treatment (sham or lesion). These analyses were complemented, when required, by t tests.
For the shock-induced activity test, mean activity was computed by averaging activity across the 20 shock presentations (i.e., 10 shocks on each of 2 d). A one-way ANOVA was conducted to detect possible interactions with treatment.
Shock sensitization was computed by comparing the mean startle amplitude across the 40 startle stimuli before shocks with the 40 startle stimuli after shocks. Repeated-measures ANOVAs were conducted on the mean startle amplitude data to detect significant levels of shock sensitization of startle reflex by foot shock and possible interactions with treatment. Complementary t tests were also performed, if necessary.
EXPERIMENT 1: PRETRAINING LESIONS OF POSTERIOR PARIETAL INSULA
Materials and methods
The purpose of Experiment 1 was to evaluate the effects of lesions of posterior parietal insula on the acquisition of fear-potentiated startle in which animals were lesioned before training. A total of 18 rats were matched into two groups of 8–10 rats each. Two to 3 d later, rats received electrolytic lesions or sham lesions aimed at the dorsal bank (granular and dysgranular portions) of posterior parietal insular cortex. One week after surgery all animals received conditioned fear training using a visual CS and were tested for fear-potentiated startle 2 d after training. A week later, animals had another 2 d training session in which an auditory CS was paired with foot shock. They were tested with the auditory CS 24 hr later.
Results and discussion
Histology
Two rats in the sham group died as a result of anesthesia during surgery, which left n = 6. From the 10 rats that received bilateral electrolytic lesions aimed at posterior parietal insula, two animals were excluded because of sparing of a significant portion of caudal insular cortex. In the other rats (n= 8), there was also some damage to the ventral portion of secondary somatosensory cortices immediately above the parietal insula. Some animals had inconsistent damage to the adjacent caudate-putamen and most rostral portion of temporal cortex. However, no case had significant damage of agranular insular cortex. Histological reconstructions of the smallest and largest electrolytically induced insular cortex lesions are shown in Figure1.
Fig. 1.
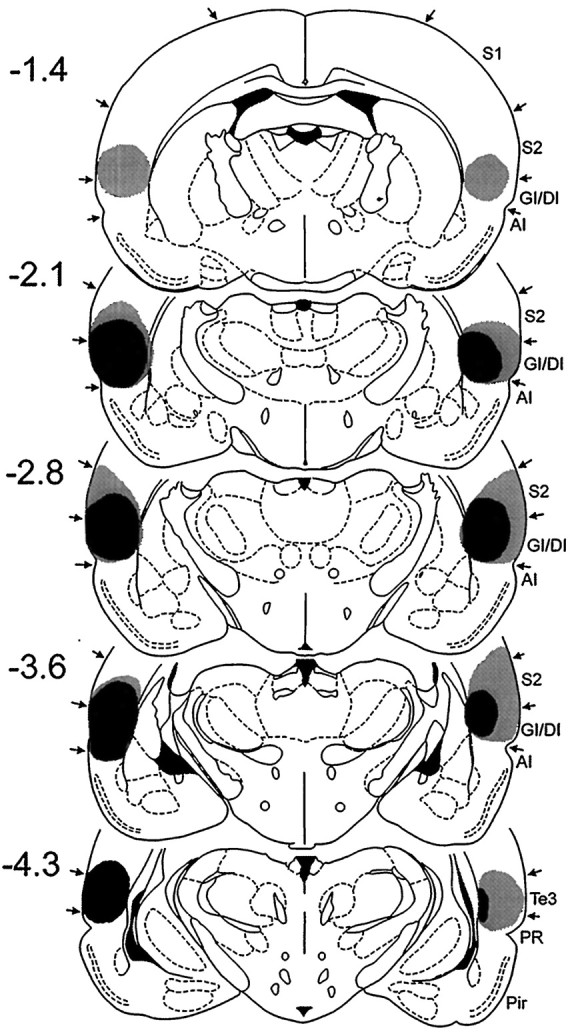
Histological reconstructions of the smallest (black) and largest (gray) electrolytic lesions of posterior parietal insular cortex in Experiment 1 on coronal plates from the atlas of Paxinos and Watson (1986). Thenumbers to the left indicate rostrocaudal levels relative to bregma. S1, Primary somatosensory cortex;S2, secondary somatosensory cortex;GI/DI, granular and dysgranular insular cortex;AI, agranular insular cortex.
Fear-potentiated startle
Pretraining electrolytic lesions of the posterior parietal insular cortex did not prevent the acquisition of fear-potentiated startle to either a visual or auditory CS. Figure2A shows the mean amplitude startle responses on the startle stimulus alone trials and the visual CS + startle stimulus trials, and the difference scores between these two trial types for sham (n = 6) and insular lesioned (n = 8) groups. Figure2B shows the mean amplitude startle of same group of animals on the startle stimulus alone and the auditory CS + startle stimulus trials. Figure 2 indicates that animals in both sham and IC lesioned groups exhibited fear-potentiated startle to both CS modalities.
Fig. 2.
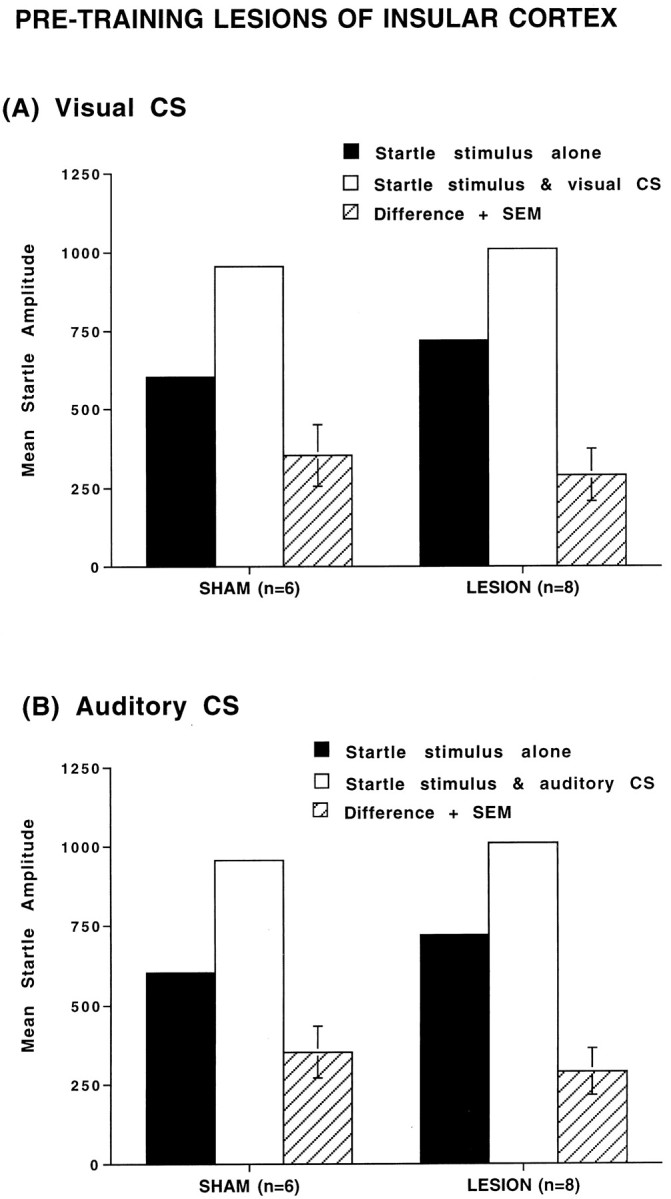
Mean amplitude startle response on startle alone trials (solid bars), startle + CS trials (open bars), and difference (+SEM) between startle alone and startle + CS trials (hatched bars) in sham-operated and lesioned animals. A, Tested with visual CS; B, tested with auditory CS. The pretraining lesion of posterior parietal insular cortex alone had no significant effect on fear-potentiated startle.
A repeated-measures ANOVA showed a significant overall difference between the visual CS + startle stimulus and startle stimulus alone trials (F(1,12) = 21.66, p < 0.001) and the auditory CS + startle stimulus and startle stimulus alone trials (F(1,12) = 36.57, p< 0.001), indicative of fear-potentiated startle to visual and auditory CSs. However, there was no treatment × trial type interaction (F(1,12) = 1.03 using a visual CS;F(1,12) = 0.35 using an auditory CS,p > 0.05) and no other significant differences between the two groups. These data indicate that pretraining lesions of the caudal insular cortex did not significantly alter the magnitude of fear-potentiated startle.
Shock-induced activity
Lesions of IC before training had no effect on an animal’s reaction to foot shock during training. The mean level of reactivity to foot shock in visual CS training was 1255 ± 196 and 1411 ± 169 and in tone CS training it was 1615 ± 281 and 1563 ± 243, for sham and IC lesion groups, respectively. There were no statistically significant differences between the two groups. These data indicate that lesions of posterior parietal insular cortex did not alter the magnitude of shock-induced activity.
EXPERIMENT 2: PRETRAINING COMBINED LESIONS OF IC AND PoT/PIL OF THALAMUS
Experiment 1 showed that electrolytic lesions of the somatosensory part of insular cortex alone had no effect on acquisition of fear conditioning or reactivity to foot shock during training. These data indicate that the insular cortex is either not involved or other structures are capable of providing somatic pain information to the amygdala during fear conditioning after removal of insular cortex. As pointed out in the introductory remarks, the PoT/PIL may also provide pain inputs to the amygdala, despite the finding that pretraining lesions of these structures alone did not affect acquisition of fear conditioning (Romanski and LeDoux, 1992b; Campeau and Davis, 1995). In Experiment 2, we evaluated the effects of combined lesions of both posterior parietal insula and PoT/PIN performed before training on the acquisition of conditioned fear measured with fear-potentiated startle.
Materials and methods
Two groups of animals were used. The first 20 rats were matched into two subgroups of 10 rats each. The rats received bilateral electrolytic lesions 2–3 d later that were aimed at granular and dysgranular portions of posterior parietal insular cortex and caudal posterior thalamic areas (PoT/PIL) or sham lesions. After 9–11 d recovery, all animals were trained by pairing foot shock with a visual CS and tested for fear-potentiated startle 2 d after training. Two weeks later, animals had another 2 d training session in which foot shock was paired with an auditory CS. They were then tested with the auditory CS the next day. The effects of these lesions on shock sensitization were also measured on the same group of animals one week later. The second 20 animals were also matched into two groups of 10 rats each. The same experimental procedures described above were followed, except that testing with the visual CS occurred 2 d after initial training and then retraining with the auditory CS occurred 2 d after testing fear-potentiated startle with the visual CS. Because there were no statistically significant differences between these two procedures, the data were combined and presented together.
Results and discussion
Histology
Two rats in the sham group and one in the lesion group died as a result of anesthesia during surgery. Eight animals in the lesion group were excluded from the study because of inadequate damage of caudal insular cortex and PoT/PIL. Some of these animals that had complete damage of caudal insular cortex but no damage to a crucial region of thalamus exhibited normal fear-potentiated startle. Thus, a total of 11 lesioned rats and 18 sham rats were used for the final data analysis. In these lesioned rats, cortical and thalamic damage covered all targeted areas, i.e., dorsal bank of caudal insular cortex, PoT, PIL, and MGM. There was also some limited and inconsistent damage to the neighboring structures, such as secondary somatosensory cortex, caudate-putamen, temporal cortex, posterior thalamic nucleus, dorsal and ventral medical geniculate nuclei, peripeduncular nucleus, lateral posterior nucleus, and anterior pretectal nucleus. However, no case had any significant damage of agranular insular cortex. The thalamic lesion sites did not impinge on amygdalofugal pathways that run ventromedially to the PoT/PIL. Histological reconstructions of the smallest and largest electrolytically induced insular cortex lesions are presented in Figure 3.
Fig. 3.
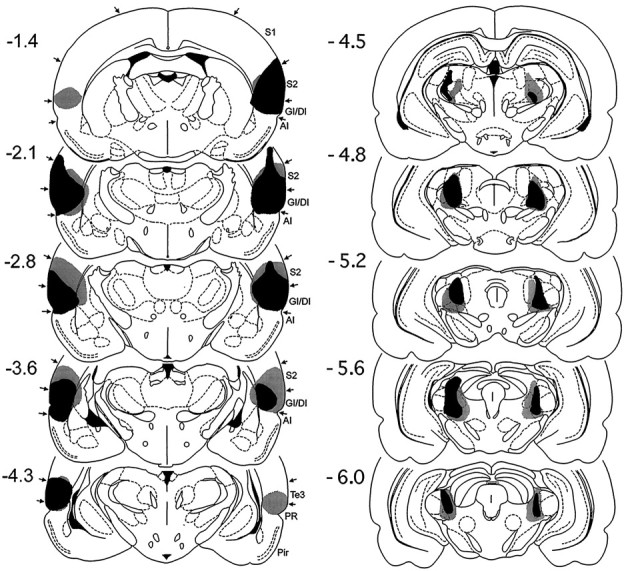
Histological reconstructions of the smallest (black) and largest (gray) combined lesions of posterior parietal insular cortex and posterior intralaminar nuclei in Experiment 2 on coronal plates from the atlas ofPaxinos and Watson (1986). The numbers to the leftindicate rostrocaudal levels relative to bregma.
Fear-potentiated startle
Combined electrolytic lesions of the posterior parietal insular cortex and PoT/PIL made before training severely disrupted the acquisition of fear-potentiated startle. Figure4A shows the mean amplitude startle responses on the startle stimulus alone and visual CS + startle stimulus trials and the difference scores between these two trial types (+SEM) for sham (n = 18) and insular lesioned (n = 11) groups. Figure 4Bshows the mean amplitude startle of same animals on the startle stimulus alone versus auditory CS + startle stimulus trials. Figure 4shows that the lesioned animals, in comparison with shams, showed a blockade of fear-potentiated startle to both visual and auditory CSs.
Fig. 4.
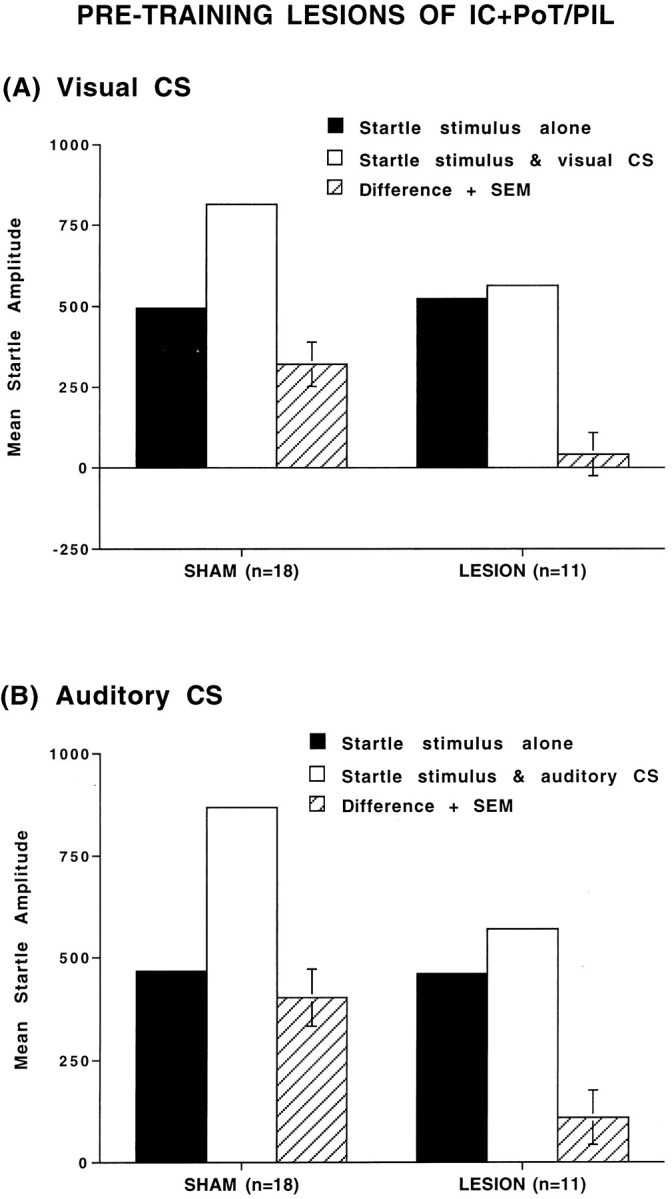
Mean amplitude startle response on startle alone trials (solid bars), startle + CS trials (open bars), and difference (+SEM) between startle alone and startle + CS trials (hatched bars) in sham-operated and lesioned animals. A, Tested with visual CS; B, tested with auditory CS. The combined pretraining lesion of posterior parietal insular cortex (IC) and posterior intralaminar nuclei (PoT/PIL) blocked acquisition of fear-potentiated startle to both visual and auditory CSs.
A repeated-measures ANOVA found a significant overall difference between the visual CS + startle stimulus and startle stimulus alone trials (F(1,25) = 50.94, p < 0.001) and the auditory CS + startle stimulus and startle stimulus alone trials (F(1,25) = 24.12, p< 0.001), indicative of fear-potentiated startle to the visual and auditory CSs. More importantly, there was a significant treatment × trial type interaction (F(1,25) = 29.53,p < 0.01 on visual CS trials;F(1,25) = 7.87, p < 0.01 on auditory CS trials), indicating different levels of fear-potentiated startle in the sham versus lesioned groups. Subsequent ttests on the difference scores demonstrated significant potentiation to either the visual or the auditory CSs in the sham animals (t(17) = 9.38, p < 0.001 andt(17) = 5.99, p < 0.001, respectively), but no significant potentiation in the lesioned groups to either visual or auditory CSs (t(10) = 1.18 and 1.73, respectively; p > 0.05). An ANOVA that used only the startle stimulus alone scores showed no significant differences in baseline startle. These data indicate that combined lesions significantly altered the magnitude of fear-potentiated startle.
Shock-induced activity
As expected of a lesion that disrupts a US pathway, lesions of both IC and PoT/PIL before training also had an effect on animals’ reaction to foot shock during training. The mean level of reactivity to foot shock was 1586 ± 104 and 811 ± 134 on visual CS trials and 1527 ± 134 and 1068 ± 171 in auditory CS training for sham and combined lesion groups, respectively. There were no statistically significant differences of shock activities between visual and auditory CS trials, but there were significant differences between sham and lesion groups in both visual CS (p < 0.01) and auditory CS (p < 0.05) training sessions. These data indicate that lesions of insular cortex along with PoT/PIL significantly attenuated the magnitude of shock-induced activity.
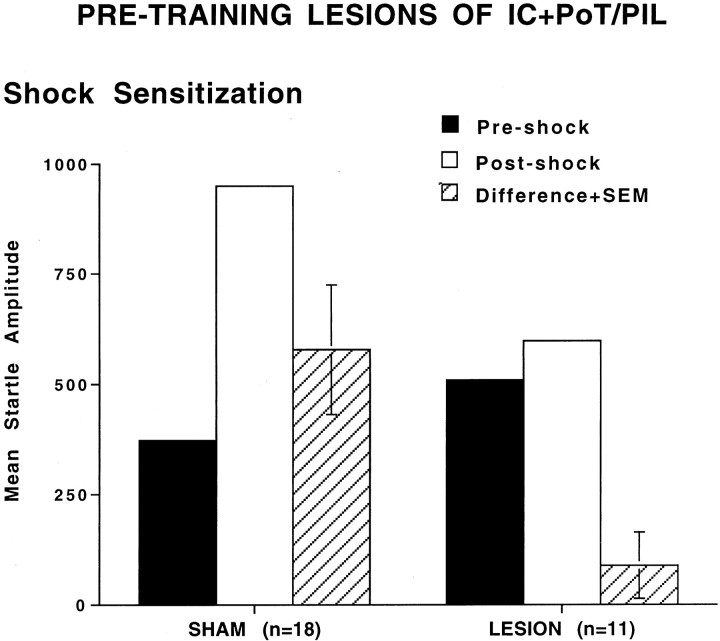
Shock sensitization
Consistent with the results of fear-potentiated startle, combined lesions of cortex and thalamus also had an effect on shock sensitization. Figure 5 shows the mean amplitude startle response before and after shock in sham and lesioned groups. A repeated-measures ANOVA found a significant overall difference between the pre-shock and post-shock trials,F(1,25) = 18.67, p < 0.001, indicative of shock sensitization. More importantly, there was a significant treatment by trial type interaction,F(1,25) = 8.65, p < 0.05, indicating different levels of shock sensitization in the sham versus lesioned groups. Subsequent t tests on the post-shock versus pre-shock scores demonstrated significant shock sensitization in the sham animals (t(17) = 4.05, p < 0.01) but no significant sensitization of startle in the lesioned rats (t(10) = 1.24, p > 0.05). These data indicate that lesioned animals had a blockade of shock sensitization.
Fig. 5.
Mean amplitude startle response on preshock trials (solid bars), post-shock trials (open bars), and difference (+SEM) between pre-shock and post-shock trials (hatched bars) in sham-operated and lesioned animals. The combined cortical and thalamic lesions blocked shock sensitization.
EXPERIMENT 3: POST-TRAINING COMBINED LESIONS OF IC AND PoT/PIL OF THALAMUS
Experiment 2 showed that the animals with pretraining lesions of posterior parietal insular cortex and posterior intralaminar nuclei of thalamus had a blockade of fear-potentiated startle. In Experiment 3, we evaluated whether the same combined lesions performed after training would have an effect on the expression of conditioned fear measured by fear-potentiated startle.
Materials and methods
Thirteen rats were matched into two subgroups of six to seven rats each and trained by pairing foot shock with a visual CS. Two days later, rats received bilateral electrolytic lesions aimed at granular and dysgranular portions of posterior parietal insular cortex and PoT/PIL or sham lesions. One week later, all animals were tested for fear-potentiated startle using a light CS.
Results and discussion
Histology
One rat in the lesion group was excluded from the study because of inadequate damage of caudal insular cortex and PoT/PIL. In the other rats, the cortical and thalamic lesions were essentially identical to those in the Experiment 2. Histological reconstructions of lesions of a representative case are presented in Figure6.
Fig. 6.
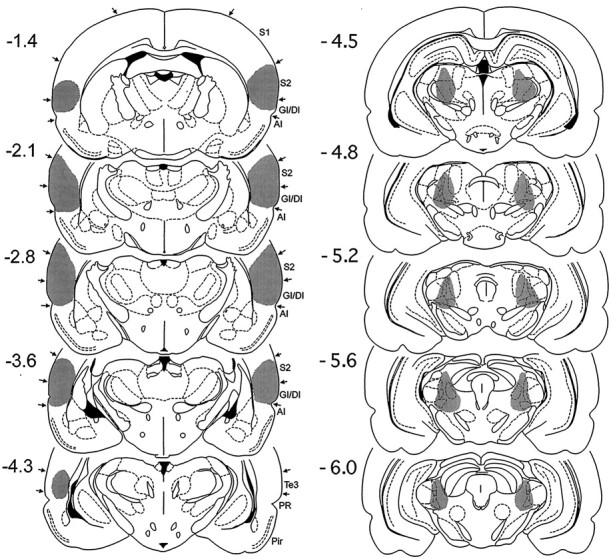
Histological reconstructions of a representative case with combined post-training lesions of posterior parietal insular cortex and posterior intralaminar nuclei in Experiment 3 on coronal plates from the atlas of Paxinos and Watson (1986). The numbers to the left indicate rostrocaudal levels relative to bregma.
Fear-potentiated startle
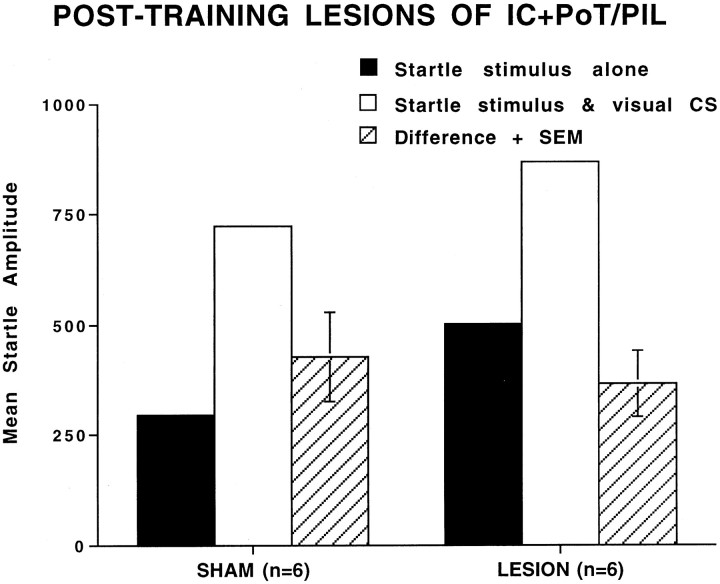
Combined electrolytic lesions of the posterior parietal insular cortex and PoT/PIL performed after training did not block the expression of fear-potentiated startle to a visual CS. Figure7 shows the mean amplitude startle responses on the startle stimulus alone and visual CS + startle stimulus trials and the difference scores between these two trial types for sham (n = 6) and lesioned (n = 6) groups. Figure 7 indicates that post-training lesions only slightly attenuated fear-potentiated startle.
Fig. 7.
Mean amplitude startle response on startle alone trials (solid bars), startle + visual CS trials (open bars), and difference (+SEM) between startle alone and startle + visual CS trials (hatched bars) in sham-operated and lesioned animals. The post-training lesion of posterior parietal insular cortex and posterior intralaminar nuclei had no significant effect on fear-potentiated startle.
A repeated-measures ANOVA found a significant overall difference between the visual CS + startle stimulus and startle stimulus alone trials, F(1,10) = 47.31, p < 0.001, indicative of fear-potentiated startle to a visual CS. However, there was no significant treatment × trial type interaction,F(1,10) = 0.28, p > 0.05, indicating equivalent levels of fear-potentiated startle in the sham versus lesioned groups. An ANOVA that used only the startle stimulus alone scores showed significant differences in baseline startle,F(1,10) = 7.03, p < 0.05. These data indicate that post-training lesions did not significantly alter the magnitude of fear-potentiated startle, despite the fact that lesioned animals had a higher level of baseline startle in comparison with matched sham animals.
DISCUSSION
The present studies showed that combined electrolytic lesions of posterior parietal insular cortex and the posterior intralaminar nuclei of the thalamus applied before training interrupted the acquisition of fear-potentiated startle using foot shock as a US. In contrast, lesions of posterior parietal insular cortex alone had no effect on either acquisition or expression of fear-potentiated startle. Furthermore, the same combined cortical and thalamic lesions performed after training did not prevent expression of conditioned fear to a light CS. In addition, combined pretraining lesions attenuated shock activity during training and blocked shock sensitization of the startle reflex as well. Considered with previous studies, the present results suggest a parallel cortical (insula-amygdala) and subcortical (thalamoamygdala) pathway involved in relaying foot shock information to the basolateral amygdala during fear conditioning.
The role of the insular cortex in relaying somatosensory inputs to the amygdala
The present cortical lesions involved the central portion of the rhinal cortex, ∼1.8–4 mm posterior to bregma in Paxinos and Watson (1986). There is a discrepancy regarding whether this area is part of the perirhinal cortex or the insular cortex. Based on the demarcation of Paxinos and Watson (1986), Rosen et al. (1992) and other investigators (Burwell et al., 1995) have referred to this area as anterior perirhinal cortex. However, a recent comprehensive anatomic tracing study of the rhinal cortex (Shi and Cassell, 1998a,b, 1999) concluded that the perirhinal cortex begins ∼4 mm behind bregma. The present lesion area corresponds to the posterior portion of parietal insular cortex, which extends rostrocaudally from about the level of bregma to the caudal end of the insular cortex (i.e., 4 mm behind bregma).
The present data show that pretraining lesions of posterior parietal insular cortex had no effect on the acquisition of fear-potentiated startle. This is consistent with previous results from our laboratory that pretraining electrolytic or chemical lesions of central portions of rhinal cortex did not prevent acquisition of fear-potentiated startle (Campeau and Davis, 1995). Our results are also supported by other studies in which extensive pretraining electrolytic lesions of temporal cortex including caudal insula did not affect fear conditioning using freezing and autonomic responses (blood pressure changes) as measures (Romanski and LeDoux, 1992a,b).
Although the cortical lesions alone had no effect on fear conditioning, combined cortical and thalamic lesions performed before training, but not after training, did interrupt acquisition of fear-potentiated startle. These results suggest that the posterior parietal insular cortex is at least partially involved in the acquisition of fear conditioning. Because the same lesioned animals also showed a blockade of shock sensitization and a significant attenuation of the shock reactivity as well, we would suggest that the role of the insular cortex is probably to relay foot shock information to the amygdala during training. This interpretation is consistent with recent anatomical studies that implicate the parietal insular cortex as a somatosensory-related structure (Fabri and Burton, 1991; Shi and Cassell, 1998a).
In contrast to the posterior insular cortex (from 2 mm anterior to bregma), which is generally accepted as a gustatory cortex, recent anatomic tracing studies found that the dorsal bank of the parietal insular cortex (from bregma to 4 mm behind), i.e., the granular and dysgranular portions, has reciprocal connections with primary and secondary somatosensory cortices and ventroposterior/posterior thalamic nuclei as well (Fabri and Burton, 1991; Barnett et al., 1995; Shi and Cassell, 1998a). Furthermore, these cortical and thalamic connections are topographically organized, indicating a body representation on the dorsal bank of the parietal insula. Although the head is represented rostrally, next to the gustatory area, the forelimbs and hindlimbs are represented more caudally (Shi and Cassell, 1998a). Thus, the present cortical lesions correspond to the limb representation on the insular cortex. Additionally, the parietal insular cortex receives direct inputs from the midbrain parabrachial nucleus as well as posterior intralaminar thalamic nuclei (Yasui et al., 1989; LeDoux et al., 1990a;Shi and Davis, 1997), both of which are targeted by afferents arising from superficial layers of the dorsal horn, and is assumed to transmit somatic pain information (LeDoux et al., 1987; Bernard et al., 1992, 1995; Feil and Herbert, 1995; Jasmin et al., 1997). Studies in humans also indicate that pain stimuli specifically activate the posterior dorsal insula (Casey et al., 1994; Craig et al., 1996;Derbyshire et al., 1997), the area corresponding to the parietal insula in the rat (Shi and Cassell, 1998a). Taken together, the present behavioral results, plus previous anatomic findings, indicate that the parietal insular cortex may represent a US pathway relaying foot shock information to the amygdala during fear conditioning.
Previous studies from this laboratory found that post-training lesions of the same rostrocaudal extent of insular cortex (∼1.8–4 mm posterior to bregma) completely blocked the expression of fear-potentiated startle using either a visual or an auditory CS (Rosen et al., 1992; Campeau and Davis, 1995). In contrast, in the present study, lesions of the same rostrocaudal extent of insular cortex had no significant effect on the expression of fear-potentiated startle. However, in both of the former studies, the lesions also included the ventral bank of the rhinal sulcus, primarily the agranular part of insular cortex, which have completely different connections from those of the granular and dysgranular portions. In fact, Rosen et al. (1992)even noted that when the lesions were restricted to the dysgranular and granular insular cortices there was no effect on the expression of the fear-potentiated startle, consistent with the current results. Although it is not clear whether lesions of the agranular insular cortex alone would be sufficient to block the expression of conditioned fear, the present experiments, as well as previous studies, strongly suggest that the granular/dysgranular versus agranular portions of posterior insula may have differential roles in the acquisition and expression of fear conditioning. Ongoing studies in our laboratory are attempting to lesion only the agranular cortex to examine this issue.
The role of posterior intralaminar nuclei of the thalamus in relaying pain information to the amygdala
A posterior group of intralaminar-like nuclei that surround the medial geniculate nucleus, including PoT, PIL, SG, SPFPC, and possibly MGM, constitutes a posterior extension of the intralaminar complex (Winer et al., 1988). Anatomical tracing studies show that all of these nuclei receive afferents from the spinal cord (LeDoux et al., 1987; Cliffer et al., 1991; Shi and Davis, 1997) and in turn project to the amygdala (LeDoux et al., 1985, 1990a; Yasui et al., 1991; Shi and Davis, 1997). This spinothalamic pathway arises from neurons in both superficial and deep layers of the dorsal horn (LeDoux et al., 1987;Dado and Giesler, 1990). These spinothalamic projection neurons are highly responsive to noxious stimuli and may relay nociceptive inputs to posterior intralaminar nuclei (Dado et al., 1994; Katter et al., 1996). Single-unit recording studies found that pain stimuli applied on the animal’s limbs could activate many units in the posterior intralaminar nuclei, including those that project to the lateral amygdaloid nucleus (Bordi and LeDoux, 1994). Furthermore, microstimulation within the PIL could serve as an effective unconditioned stimulus for fear conditioning, in place of standard foot shock (Cruikshank et al., 1992). In agreement with these anatomic and physiological findings, the present behavioral results further demonstrate that pretraining lesions of the posterior intralaminar nuclei, in conjunction with insular cortex, prevent the acquisition of fear-potentiated startle. All of these data would strongly indicate that this thalamoamygdaloid pathway might be a US pathway that is critically involved in emotional learning by relaying nociceptive inputs to the amygdala.
It has been proposed that the projections from the posterior intralaminar nuclei provide a pathway parallel with the auditory cortex by which auditory stimuli can be transmitted to the amygdala and engage fear and other affective responses (LeDoux et al., 1990b; Romanski and LeDoux, 1992b). However, we believe it is unlikely that the present thalamic lesions blocked fear conditioning by preventing transmission of CS information to the amygdala. First of all, lesions of this area only block fear conditioning to an auditory stimulus in conjunction with lesions of the auditory cortex but not when they are made by themselves (Romanski and LeDoux, 1992b). However, the auditory cortex was not consistently damaged in our combined lesioned animals. Second, the present thalamic lesions interrupted fear conditioning using both acoustic and visual CSs. Despite receiving inputs from the inferior colliculus and being responsive to acoustic stimuli, the posterior intralaminar nuclei probably are not involved in relaying any visual information. Third, the present lesions also significantly attenuated shock-induced activity and shock sensitization in which no CSs were involved. Finally, post-training lesions of these areas did not block the expression of fear-potentiated startle as one would expect of a lesion that disrupted a CS pathway.
In fact, the current results suggest that the conclusion that projections from the posterior intralaminar nuclei provide a CS pathway to the amygdala parallel with the auditory cortex should be considered with some caution. That conclusion is based on the finding that lesions of the posterior intralaminar nuclei, in conjunction with lesions of the auditory cortex, block fear conditioning using an auditory stimulus, whereas lesions of either area alone are not sufficient to block fear conditioning (LeDoux et al., 1984; Romanski and LeDoux, 1992b). However, these studies have always used pretraining lesions. Furthermore, the lesions of the auditory cortex always involved damage to the rhinal cortex, adjacent to the amygdala. Hence, it is possible that the blockade of fear conditioning produced by these combined pretraining lesions of the posterior intralaminar nuclei and the rhinal cortex adjacent to the amygdala resulted from an interruption of parallel US pathways required for fear conditioning rather than an interruption of parallel auditory CS pathways. Indeed, the projections of the inferior colliculus to the posterior intralaminar nuclei, which are assumed to provide auditory inputs, arise from the shell of the inferior colliculus, a multimodal area also targeted by somatosensory inputs from the spinal cord (Aitkin et al., 1978; Coleman and Clerici, 1987; Li and Mizuno, 1997). Moreover, our laboratory found that chemical lesions of the dorsal and ventral medial geniculate nuclei, but not posterior intralaminar nuclei, were enough to interrupt the expression of conditioned fear responses to an auditory CS (Campeau and Davis, 1995). Thus, the role of the thalamoamygdala pathway in auditory CS transmission should be considered cautiously, and further studies must be performed to clarify this issue.
Parallel cortical and thalamic US pathways in fear conditioning
Although the present report did not include thalamic lesion alone experiments, studies by both Romanski and LeDoux (1992b) and Davis (1995) showed clearly that thalamic lesions alone did not prevent acquisition of conditioned fear. In the later study (Campeau and Davis, 1995), large pretraining lesions of posterior thalamus, including medial geniculate as well as posterior intralaminar nuclei, prevented acquisition of fear-potentiated startle to an auditory CS but not to a visual CS, indicating a blockade of transmission to the auditory CS but not to a visual CS or, most important for the present study, foot shock US.
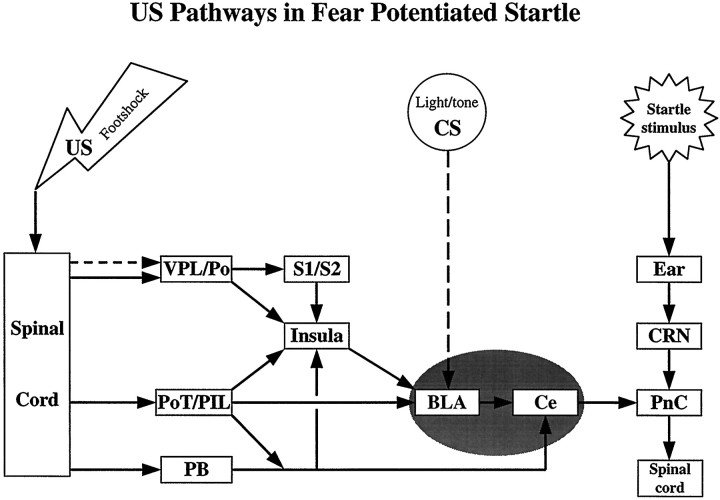
On the basis of a combination of anatomical, physiological, and behavioral studies, we propose that that there are parallel corticoamygdala and thalamoamygdala US pathways that are illustrated in Figure 8. For simplicity, the CS pathways and interconnections with agranular insular cortex are not included and will be discussed elsewhere. During fear conditioning, the US (i.e., foot shock) information is transmitted to the amygdala via either posterior intralaminar nuclei or parietal insular cortex. The posterior intralaminar thalamic nuclei receive shock information directly from the spinal cord. The parietal insular cortex receives convergent information from leminscal inputs that arise from the primary and secondary somatosensory cortices and ventroposterior nuclei of the thalamus, and nonleminscal inputs from posterior and posterior intralaminar thalamic nuclei and the midbrain parabrachial nucleus. Both the parietal insular cortex and posterior intralaminar thalamic nuclei in turn project to the lateral, basolateral, basomedial, and central nuclei of the amygdala. In contrast, the visual and auditory inputs from modality specific areas of thalamus and cortex exclusively or primarily target the lateral amygdaloid nucleus (LeDoux et al., 1990a; Romanski and LeDoux, 1993; McDonald and Mascagni, 1996; Shi and Cassell, 1997). Consequently, only the lateral nucleus of the amygdala receives both foot shock US inputs and auditory/visual CSs inputs. Because of this, it may be the site of plasticity for CS–US associations within the amygdala (LeDoux, 1992a,b). Consistent with this, combined lesions of both parietal insular cortex and posterior intralaminar nuclei of the thalamus were necessary to interrupt the transmission of foot shock information to the amygdala and thus block the acquisition of fear-potentiated startle. Furthermore, lesions restricted to the lateral amygdaloid nucleus were sufficient to block the acquisition of fear conditioning as well (LeDoux et al., 1990b).
Fig. 8.
Schematic diagram summarizing parallel corticoamygdala and thalamoamygdala pain US pathways involved in fear-potentiated startle. BLA, Basolateral amygdala;Ce, central amygdaloid nucleus; CRN, cochlea root neurons; PB, parabrachial nucleus;PnC, pontine reticular nucleus, caudal;PoT/PIL, posterior thalamic nucleus, triangular, and posterior intralaminar thalamic nucleus; VPL/Po, ventroposterior lateral thalamic nucleus and posterior thalamic nucleus.
Besides the insular cortex and posterior intralaminar nuclei of the thalamus, the parabrachial nucleus may also provide direct nociceptive inputs to the amygdala (Bernard and Besson, 1990; Bernard et al., 1993). However, the projection from the parabrachial nucleus targets predominantly the central amygdaloid nucleus but not the lateral amygdaloid nucleus. Although the projections from insular cortex and posterior intralaminar thalamic nuclei transmit foot shock US information to the lateral amygdaloid nucleus for sensory–sensory association, those from the cortex, thalamus, and parabrachial nucleus to the central amygdaloid nucleus may be involved in nonconditioned responses, such as modification of shock activity. In fact, lesions of central nucleus, but not the basolateral amygdala, attenuated shock reactivity (Hitchcock et al., 1989). In the present study, combined lesions of cortex and thalamus also significantly reduced shock reactivity, which might have resulted from interrupting shock inputs to the central nucleus of the amygdala. Finally, because shock sensitization of startle may reflect rapid context conditioning (Borszcz et al., 1989), blockade of shock sensitization by these combined lesions may also have resulted from a blockade of the US pathway required for context conditioning, which we are currently testing using more traditional procedures to produce context conditioning.
Overall, the present studies implicate the parietal insular cortex and the posterior intralaminar nucleus of the thalamus as being parallel US shock pathways necessary for the acquisition of fear conditioning using the fear-potentiated startle paradigm. It should be acknowledged, however, that the present lesions were made by passing electrolytic current, which damages both cell bodies and fibers in the lesion site. Hence it is not yet possible to assess the relative contribution of fibers of passage versus cell bodies in these behavioral effects. Instead, the present results provide a working model for the US pathways involved in fear conditioning, which can now be tested more rigorously with other techniques such as chemical lesioning or local inactivation via transmitter agonists (e.g., muscimol) or antagonists (e.g., excitatory amino antagonists).
Footnotes
This work was supported by National Institute of Mental Health Grants MH-57250 and MH-47840, Research Scientist Development Award MH-00004 to M.D., and a grant from the Air Force Office of Scientific Research and the State of Connecticut.
Correspondence should be addressed to Dr. Changjun Shi, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, 1636 Pierce Drive, Suite 4000, Atlanta, GA 30322.
REFERENCES
- 1.Aitkin L, Dickhaus H, Schultz W, Zimmermann M. External nucleus of inferior colliculus: auditory and spinal somatosensory afferents and their interactions. J Neurophysiol. 1978;41:837–847. doi: 10.1152/jn.1978.41.4.837. [DOI] [PubMed] [Google Scholar]
- 2.Barnett E, Evans G, Sun N, Perlman S, Cassell M. Anterograde tracing of trigeminal afferent pathways from the murine tooth pulp to cortex using herpes simplex virus type 1. J Neurophysiol. 1995;15:2972–2984. doi: 10.1523/JNEUROSCI.15-04-02972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard J, Besson J. The spino(trigemino)pontoamygdaloid pathway: electrophysiologial evidence for an involvement in pain process. J Neurophysiol. 1990;63:473–490. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- 4.Bernard J, Dallel R, Raboisson P, Villanueva L, Le Bars D. Organization of the efferent projections from the spinal cervical enlargement to the parabrachial area and periaqueductal gray: a PHA-L study in the rat. J Comp Neurol. 1995;353:480–505. doi: 10.1002/cne.903530403. [DOI] [PubMed] [Google Scholar]
- 5.Bernard JF, Huang GF, Besson JM. Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1992;68:551–569. doi: 10.1152/jn.1992.68.2.551. [DOI] [PubMed] [Google Scholar]
- 6.Bernard J-F, Alden M, Besson J-M. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- 7.Bordi F, LeDoux J. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. II. Cells receiving convergent auditory and somatosensory inputs and cells antidromically activated by amygdala stimulation. Exp Brain Res. 1994;98:275–286. doi: 10.1007/BF00228415. [DOI] [PubMed] [Google Scholar]
- 8.Borszcz GS, Cranney J, Leaton RN. Influence of long-term sensitization on long-term habituation of the acoustic startle response in rats: central gray lesions, preexposure and extinction. J Exp Psychol. 1989;15:54–64. [PubMed] [Google Scholar]
- 9.Burwell RD, Witter MP, Amaral DG. Perirhinal and postrhinal cortices of the rat: a review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus. 1995;5:390–408. doi: 10.1002/hipo.450050503. [DOI] [PubMed] [Google Scholar]
- 10.Campeau S, Davis M. Fear potentiation of the acoustic startle reflex using noises of various spectral frequencies as conditioned stimuli. Anim Learn Behav. 1992;20:177–186. [Google Scholar]
- 11.Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2312–2327. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casey K, Minoshima S, Berger K, Koeppe R, Morrow T, Frey K. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol. 1994;71:802–807. doi: 10.1152/jn.1994.71.2.802. [DOI] [PubMed] [Google Scholar]
- 13.Cassella JV, Davis M. The design and calibration of a startle measurement systems. Physiol Behav. 1986;36:377–383. doi: 10.1016/0031-9384(86)90032-6. [DOI] [PubMed] [Google Scholar]
- 14.Cliffer KD, Burstein R, Giesler GJ., Jr Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J Neurosci. 1991;11:852–868. doi: 10.1523/JNEUROSCI.11-03-00852.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman J, Clerici W. Sources of projections to subdivisions of the inferior colliculus in the rat. J Comp Neurol. 1987;262:215–226. doi: 10.1002/cne.902620204. [DOI] [PubMed] [Google Scholar]
- 16.Craig A, Bushnell M, Zhang E, Blomqvist A. A thalamic nucleus specific for pain and temperature sensation. Nature. 1994;372:770–773. doi: 10.1038/372770a0. [DOI] [PubMed] [Google Scholar]
- 17.Craig A, Reiman E, Evans A, Bushnell M. Functional imaging of an illusion of pain. Nature. 1996;384:258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- 18.Cruikshank SJ, Edeline JM, Weinberger NM. Stimulation at a site of auditory-somatosensory convergence in the medial geniculate nucleus is an effective unconditioned stimulus for fear conditioning. Behav Neurosci. 1992;106:471–483. doi: 10.1037//0735-7044.106.3.471. [DOI] [PubMed] [Google Scholar]
- 19.Dado R, Giesler GJ. Afferent input to nucleus submedius in rats: retrograde labeling of neurons in the spinal cord and caudal medulla. J Neurosci. 1990;10:2672–2686. doi: 10.1523/JNEUROSCI.10-08-02672.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dado R, Katter J, Giesler GJJ. Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. II. Responses to innocuous and noxious mechanical and thermal stimuli. J Neurophysiol. 1994;71:981–1002. doi: 10.1152/jn.1994.71.3.981. [DOI] [PubMed] [Google Scholar]
- 21.Davis M. The role of the amygdala in conditioned fear. In: Aggleton J, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley; New York: 1992. pp. 255–305. [Google Scholar]
- 22.Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- 23.Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 24.Derbyshire S, Jones A, Gyulai F, Clark S, Townsend D, Firestone L. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- 25.Everitt BJ, Morris KA, O’Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- 26.Fabri M, Burton H. Ipsilateral cortical connections of primary somatic sensory cortex in rats. J Comp Neurol. 1991;311:405–424. doi: 10.1002/cne.903110310. [DOI] [PubMed] [Google Scholar]
- 27.Feil K, Herbert H. Topographic organization of spinal and trigeminal somatosensory pathways to the rat parabrachial and Kollike-Fuse nuclei. J Comp Neurol. 1995;353:506–528. doi: 10.1002/cne.903530404. [DOI] [PubMed] [Google Scholar]
- 28.Friedman D, Murray E. Thalamic connectivity of the second somatosensory area and neighboring somatosensory fields of the lateral sulcus of the macaque. J Comp Neurol. 1986;252:348–373. doi: 10.1002/cne.902520305. [DOI] [PubMed] [Google Scholar]
- 29.Hitchcock JM, Sananes CB, Davis M. Sensitization of the startle reflex by foot shock: blockade by lesions of the central nucleus of the amygdala or its efferent pathway to the brainstem. Behav Neurosci. 1989;103:509–518. doi: 10.1037//0735-7044.103.3.509. [DOI] [PubMed] [Google Scholar]
- 30.Jasmin L, Burkey A, Card J, Basbaum A. Transneuronal labeling of a nociceptive pathway, the spino-(trigemino-)parabrachio-amygdaloid, in the rat. J Neurosci. 1997;17:3751–3765. doi: 10.1523/JNEUROSCI.17-10-03751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapp BS, Whalen PJ, Supple WF, Pascoe JP. Amygdaloid contributions to conditioned arousal and sensory information processing. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley; New York: 1992. pp. 229–254. [Google Scholar]
- 32.Katter J, Dado R, Kostarczyk E, Giesler GJ. Spinothalamic and spinohypothalamic tract neurons in the sacral spinal cord of rats. II. Responses to cutaneous and visceral stimuli. J Neurophysiol. 1996;75:2606–2628. doi: 10.1152/jn.1996.75.6.2606. [DOI] [PubMed] [Google Scholar]
- 33.LeDoux JE. Brain mechanisms of emotion and emotional learning. Curr Opin Neurobiol. 1992a;2:191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- 34.LeDoux JE. Emotion and the amygdala. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley; New York: 1992b. pp. 339–352. [Google Scholar]
- 35.LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci. 1984;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol. 1985;242:182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- 37.LeDoux JE, Ruggiero DA, Forest R, Stornetta R, Reis DJ. Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat. J Comp Neurol. 1987;264:123–146. doi: 10.1002/cne.902640110. [DOI] [PubMed] [Google Scholar]
- 38.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci. 1990a;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus, sensory interface of the amygdala in fear conditioning. J Neurosci. 1990b;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Mizuno N. Collateral projections from single neurons in the dorsal column nuclei to the inferior colliculus and the ventrobasal thalamus: a retrograde double-labeling study in the rat. Neurosci Lett. 1997;225:21–24. doi: 10.1016/s0304-3940(97)00183-3. [DOI] [PubMed] [Google Scholar]
- 42.Mascagni F, McDonald AJ, Coleman JR. Corticoamygdaloid and corticocortical projections of the rat temporal cortex: a phaseolus vulgaris leucoagglutinin study. Neuroscience. 1993;57:697–715. doi: 10.1016/0306-4522(93)90016-9. [DOI] [PubMed] [Google Scholar]
- 43.McDonald AJ, Jackson TR. Amygdaloid connections with posterior insular and temporal cortical areas in the rat. J Comp Neurol. 1987;262:59–77. doi: 10.1002/cne.902620106. [DOI] [PubMed] [Google Scholar]
- 44.McDonald AJ, Mascagni F. Cortico-cortical and cortico-amygdaloid projections of the rat occipital cortex: a phaseolus vulgaris leucoagglutinin study. Neuroscience. 1996;71:37–54. doi: 10.1016/0306-4522(95)00416-5. [DOI] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, ed 2. Academic; Orlando: 1986. [DOI] [PubMed] [Google Scholar]
- 46.Romanski LM, LeDoux JE. Bilateral destruction of neocortical and perirhinal projection targets of the acoustic thalamus does not disrupt auditory fear conditioning. Neurosci Lett. 1992a;142:228–232. doi: 10.1016/0304-3940(92)90379-l. [DOI] [PubMed] [Google Scholar]
- 47.Romanski LM, LeDoux JE. Equipotentiality of thalamo-amygdala and thalamo-cortico amygdala circuits in auditory fear conditioning. J Neurosci. 1992b;12:4501–4509. doi: 10.1523/JNEUROSCI.12-11-04501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex. 1993;3:515–532. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- 49.Rosen JB, Hitchcock JM, Miserendino MJD, Falls WA, Campeau S, Davis M. Lesions of the perirhinal cortex but not of the frontal, medial prefrontal, visual, or insular cortex block fear-potentiated startle using a visual conditioned stimulus. J Neurosci. 1992;12:4624–4633. doi: 10.1523/JNEUROSCI.12-12-04624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi C-J. PhD thesis. University of Iowa; 1995. The anatomical substrates underlying the role of the amygdala in associative learning. . [Google Scholar]
- 51.Shi C-J, Cassell MD. Cortical, thalamic, and amygdaloid projections of rat temporal cortex. J Comp Neurol. 1997;381:1–23. [PubMed] [Google Scholar]
- 52.Shi C-J, Cassell MD. Cascade projections from somatosensory cortex to the rat basolateral amygdala via the posterior parietal insular cortex. J Comp Neurol. 1998a;399:469–491. doi: 10.1002/(sici)1096-9861(19981005)399:4<469::aid-cne3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 53.Shi C-J, Cassell MD. Cortical, thalamic and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998b;399:440–468. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 54.Shi C-J, Cassell MD (1998c) Perirhinal cortex projections to the amygdaloid complex and hippocampal formation in the rat. J Comp Neurol, in press. [DOI] [PubMed]
- 55.Shi C-J, Davis M. Anatomical tracing and lesion studies of pain pathways involved in fear conditioning measured with fear potentiated startle. Soc Neurosci Abstr. 1997;23:1612. doi: 10.1523/JNEUROSCI.19-01-00420.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turner BH, Herkenham M. Thalamoamygdaloid projections in the rat: a test of the amygdala’s role in sensory processing. J Comp Neurol. 1991;313:295–325. doi: 10.1002/cne.903130208. [DOI] [PubMed] [Google Scholar]
- 57.Winer J, Morest D, Diamond I. A cytoarchitectonic atlas of the medial geniculate body of the opossum, Didelphys virginiana, with a comment on the posterior intralaminar nuclei of the thalamus. J Comp Neurol. 1988;272:422–448. doi: 10.1002/cne.902740310. [DOI] [PubMed] [Google Scholar]
- 58.Yasui Y, Saper C, Cechetto D. Calcitonin gene-related peptide immunoreactivity in the visceral sensory cortex, thalamus, and related pathways in the rat. J Comp Neurol. 1989;290:487–501. doi: 10.1002/cne.902900404. [DOI] [PubMed] [Google Scholar]
- 59.Yasui Y, Saper C, Cechetto D. Calcitonin gene-related peptide (CGRP) immunoreactive projections from the thalamus to the striatum and amygdala in the rat. J Comp Neurol. 1991;308:293–310. doi: 10.1002/cne.903080212. [DOI] [PubMed] [Google Scholar]