Abstract
In sensory neurons (SNs) of adult Aplysia, serotonin (5-HT)-induced spike broadening has long been implicated as important for synaptic facilitation [spike duration–dependent (SDD) facilitation], particularly at nondepressed synapses. At depressed synapses, spike broadening has less impact on synaptic facilitation; under these conditions, 5-HT induces a spike duration–independent (SDI) form of facilitation (Byrne and Kandel, 1996). It has been difficult to dissociate clearly the cellular mechanisms underlying these two forms of facilitation. However, the observation that a major form of spike broadening emerges late in juvenile development (Marcus and Carew, 1998) provides a unique opportunity to examine the relationship between spike broadening and synaptic facilitation in juvenile Aplysia. We have identified three forms of synaptic plasticity in juvenile Aplysia: homosynaptic depression, SDD facilitation, and SDI facilitation. We show that homosynaptic depression is fully developed in the juvenile and that 5-HT reliably induces synaptic facilitation at depressed synapses. However, in nondepressed synapses, 5-HT–induced facilitation is not reliable. Further analysis revealed that the relationship between spike broadening and synaptic facilitation for nondepressed synapses is the inverse of that in adults. Surprisingly, in juveniles, minor spike broadening induced by 5-HT results in significant synaptic facilitation, whereas major spike broadening, when it occurs, does not. These results suggest a model in which juvenile synapses predominantly use SDI facilitation, and with the emergence of major spike broadening, a developmentally transient inhibitory process emerges. This inhibitory process seems to be independent of major spike broadening induced by 5-HT because directly broadening the spike with 4-aminopyridine induces adult-like SDD synaptic facilitation. Finally, in the adult, the inhibitory process is either lost or masked, and SDD facilitation predominates at nondepressed synapses.
Keywords: spike duration–independent facilitation, spike duration–dependent facilitation, synaptic transmission, serotonin, developmental plasticity, tail withdrawal reflex
A hallmark of virtually all chemical synapses is the capacity for modulation of neurotransmitter release. Most mature synapses exhibit a large repertoire of regulatory mechanisms subserving different forms of synaptic plasticity, the underlying mechanisms of which have been extensively studied (Levitan and Kaczmarek, 1996). The functional maturation of synaptic modulation has received much less experimental attention. Studying the developmental assembly of neuromodulatory processes may provide unique insights into mechanisms underlying synaptic plasticity in both developing and mature systems.
The nervous system of Aplysia has proven to be advantageous in examining the ontogeny of several different forms of neuromodulation. The monosynaptic connections between tail sensory neurons (SNs) and tail motor neurons (MNs) are amenable to developmental analysis because they are easily identifiable in both juveniles and adults (Stark, 1997; Marcus and Carew, 1998), and in the adult, the subcellular signaling mechanisms underlying synaptic plasticity in these cells have been extensively studied (Byrne and Kandel, 1996).
In the adult, serotonin (5-HT)-induced SN spike broadening plays an important role in one form of synaptic facilitation, spike duration–dependent (SDD) facilitation. It is now thought that SDD facilitation is the predominant facilitatory mechanism in SN synapses that have not previously undergone homosynaptic depression (Ghirardi et al., 1992; Sugita et al., 1997; for review, see Byrne and Kandel, 1996). After homosynaptic depression, spike broadening has less impact on synaptic facilitation, but 5-HT can still facilitate synaptic transmission (Hochner et al., 1986b; Sugita et al., 1997). Thus, at depressed synapses, spike duration–independent (SDI) facilitation becomes the predominant mechanism of facilitation. SDI facilitation is thought to involve an alteration in vesicle mobilization at the synaptic terminal (Gingrich and Byrne, 1985; Hochner et al., 1986b;Gingrich et al., 1988; Pieroni and Byrne, 1992), although the precise mechanisms underlying SDI facilitation are still a focus of considerable research.
In a developmental analysis, Marcus and Carew (1998) found that different forms of serotonergic modulation in Aplysia tail SNs emerge sequentially during the final juvenile stage (late stage 12). At the beginning of this stage, 5-HT induces adult-like increases in SN excitability but has only a minor effect on spike duration (≤15% broadening). Only later in late stage 12 can 5-HT induce adult-like spike broadening (>15%).
In the present paper we have investigated the development of serotonergic modulation of synaptic transmission from tail SNs, specifically addressing the relationship between spike broadening and synaptic facilitation. We describe the developmental emergence of three forms of synaptic plasticity: homosynaptic depression, SDD facilitation, and SDI facilitation. Confirming developmental studies of other synapses in Aplysia (Rayport and Camardo, 1984; Nolen et al., 1987; Rankin and Carew, 1987), we have found that homosynaptic depression at the SN synapse is present in its adult form in late stage 12 juveniles. The development of synaptic facilitation is more complex. In juveniles, like adults, 5-HT consistently enhanced synaptic transmission from previously depressed synapses. However, unlike adult synapses, nondepressed synapses were not consistently enhanced by 5-HT. A closer examination of facilitation at these synapses revealed a surprising inverse relationship between spike broadening and synaptic facilitation; immature (≤15%) broadening (Marcus and Carew, 1998) was associated with significant synaptic facilitation, whereas adult-like broadening (>15%), when it occurred, was not associated with significant facilitation. The juvenile pattern is exactly the opposite of that observed at SN-MN synapses in mature animals, where modest broadening produces no facilitation, but adult broadening produces significant facilitation.
The preferential facilitation of depressed synapses and the inverse relationship between spike broadening and synaptic facilitation have led us to propose that juvenile sensorimotor connections predominantly use SDI facilitation and later in development establish SDD facilitation. In addition, we have uncovered a novel inhibitory process in juveniles that may be lost or masked in adults.
Parts of this paper have been published previously (Stark and Carew, 1994, 1996).
MATERIALS AND METHODS
Animals and experimental preparation. JuvenileAplysia californica were obtained from theAplysia Resource Facility (Miami, FL) and were staged according to the criteria of Kriegstein (1977). Late stage 12 animals (0.5–1.0 gm and 9–10 weeks after metamorphosis) were used. Wild-caught adult Aplysia (200–350 gm) were obtained from various commercial suppliers: Marinus (Long Beach, CA), Marine Specimens Unlimited (Pacific Palisades, CA), and Pacific Biomarine (Venice, CA). Juvenile and adult animals were housed in separate tanks, each containing constantly circulating, aerated artificial seawater (ASW; Instant Ocean; Aquarium Systems, Mentor, OH) at 15°C.
Animals were anesthetized with an injection of a volume of isotonic MgCl2 equal to approximately one-half of their body weight. Left or right pleural and pedal ganglia were surgically removed. Preparations were treated with glutaraldehyde diluted in ASW (0.1% for juveniles and 0.4% for adults) for 30–45 sec to prevent sheath contractions. Preparations were then briefly rinsed in ASW (460 mm NaCl, 55 mm MgCl2, 11 mm CaCl2, and 10 mm Trizma, pH 7.6) and pinned in a Sylgard-coated (Dow Corning) recording dish containing 50% isotonic MgCl2 and 50% ASW. For juvenile preparations, the connective tissue sheath was gently teased away from the pleural and pedal ganglia with borosilicate micropipettes. For adult preparations, the sheath was surgically removed with iridectomy scissors. Desheathed preparations were superfused with ASW (20–22°C; 3 ml/min) for at least 30 min before recording was commenced. In some experiments (see Fig. 1) the concentrations of CaCl2or MgCl2 in the ASW were increased to 44 mm(4× normal) and 165 mm (3× normal), respectively.
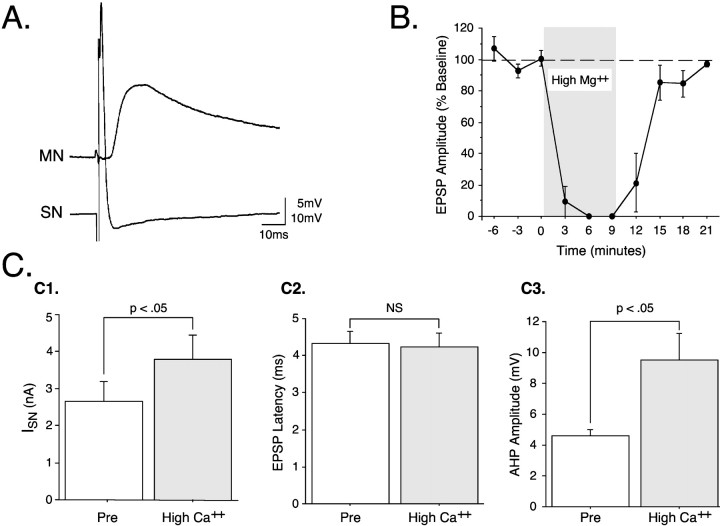
Fig. 1.
Juvenile sensorimotor synapses satisfy physiological criteria for monosynapticity. A, A brief (1.5 msec) depolarizing constant-current pulse injected into a juvenile pleural SN soma elicits a single action potential in theSN and results in a short-latency EPSP in the follower cell, a pedal MN. In this and other figures, capacitive transients caused by the current pulse in theSN have been electronically clipped for clarity.B, High Mg2+ ASW (shaded area) reversibly blocked synaptic transmission.C, High Ca2+ ASW (shaded bars) raised the SN firing threshold (C1) but did not shift EPSP latency (C2). High Ca2+ ASW also increased the afterhyperpolarization (AHP) amplitude of theSN action potential compared with normal Ca2+ (C3).
Electrophysiology. Recording electrodes were made from borosilicate capillary pipettes (1.2 mm outer diameter; 0.6 mm inner diameter; Sutter Instrument Company, Novato, CA) and were filled with 3 m KCl. For juvenile experiments, pipettes were initially pulled to a resistance of 40–60 MΩ and were beveled to a final resistance of 30–50 MΩ. For adult experiments, unbeveled electrodes of 10–15 MΩ were used.
Standard intracellular recording techniques were used. A single sharp electrode was used, in bridge mode, both to record membrane potential and to inject current. Electrical potentials were amplified by an Axoclamp 2-A (Axon Instruments, Foster City, CA), filtered at 10 kHz through a low-pass filter, and digitized by a Universal Signal Manifold (World Precision Instruments, Sarasota, FL) for computer storage and analysis by the customized software program Spike (Hilal Associates, Englewood Cliffs, NJ).
Just before a juvenile pleural SN was impaled, 1 nA of hyperpolarizing current was injected through the electrode to prevent the SN from firing action potentials. Immediately after impalement, the membrane potential was slowly repolarized to the resting potential of the cell. This hyperpolarization was not necessary for the more robust adult cells or for the juvenile MNs.
SNs and MNs were not used if resting potential was less than −30 mV or if input resistance dropped below 30 MΩ (for juvenile cells) or 15 MΩ (for adult cells).
Experimental procedures. To monitor spike duration in a pleural SN, we triggered a single action potential by injecting a very brief (1.5 msec) depolarizing constant-current pulse into the SN soma so that the peak and falling phase of the spike were not contaminated by the depolarizing pulse. Spike duration (in milliseconds) was measured as the time from the peak of the action potential to 33% of the peak. Peak amplitude (in millivolts) of the resultant monosynaptic EPSP was measured in an MN in the pedal ganglion. A synaptic connection was considered monosynaptic if it displayed a short and constant latency over several stimuli and had a smooth rising phase (see, e.g., Walters et al., 1983a; Emptage et al., 1996; Stopfer and Carew, 1996). Occasionally, a second spontaneous EPSP interfered with measurement of the peak of the monosynaptic EPSP. In these cases, the amplitude of the monosynaptic EPSP was taken as the point at which the second EPSP began; thus, if there was any measurement error, it would lead to an underestimation of the actual amplitude of the monosynaptic EPSP. The resting potential of MNs was held at −70 mV with direct injection of hyperpolarizing current to prevent the firing of action potentials. No compensation was made for fluctuations in SN membrane potential.
A range of interstimulus intervals (ISIs) was used to examine the effect of the state of synaptic depression on synaptic modulation in normal ASW. A 1 min ISI was used to specifically depress the synapse, whereas a 10 min ISI was used to ensure a nondepressed baseline (see Fig. 2). Depression was defined as a >20% decrease from the initial EPSP amplitude. Depending on the ISI, a stable baseline of two (for a 10 min ISI) or three (for a 1 min ISI) consecutive measurements was established. The mean of the baseline measurements was compared with the mean of two or three consecutive measurements in the drug application, and data are expressed either as raw difference scores or as normalized percentage change scores.
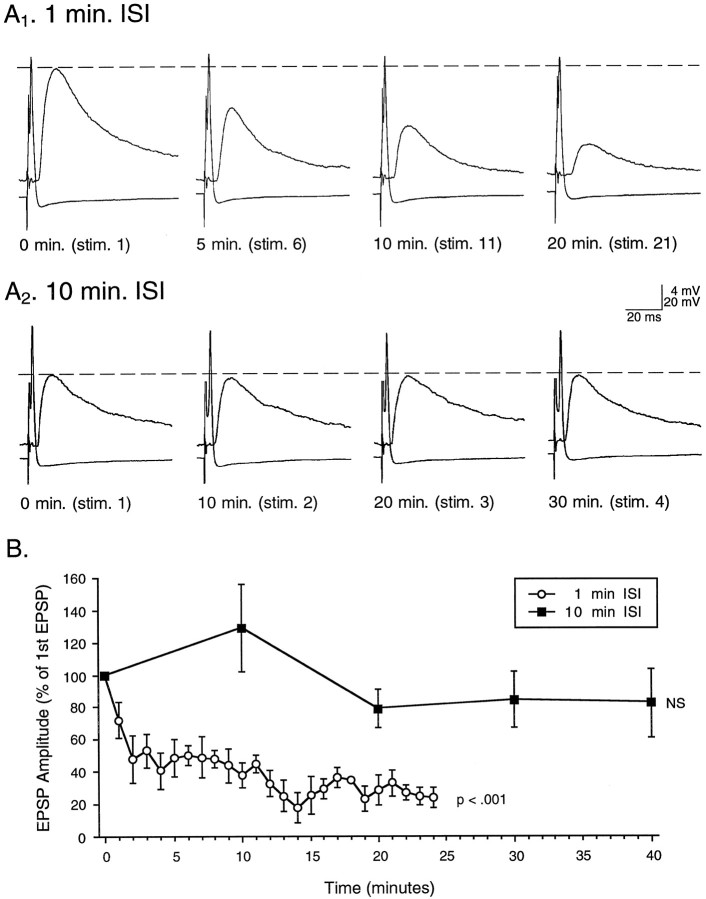
Fig. 2.
Juvenile synapses exhibit adult-like homosynaptic depression. Single action potentials were repetitively elicited in juvenile SNs with a 1 or 10 min ISI.A1, An example of a juvenile synapse that depressed 87% from its initial amplitude over the course of 20 min with a 1 min ISI.A2, An example of a juvenile synapse that did not depress (decreasing only 6% from initial amplitude) over the course of 30 min with a 10 min ISI. Dashed lines indicate initial EPSP amplitudes.B, Group data showing the time course of homosynaptic depression with a 1 min ISI (○; n= 6) compared with no significant depression with a 10 minISI (▪; n = 4).stim., Stimulus.
After the baseline period, either 50 μm 5-HT (creatinine-sulfate complex; Sigma, St. Louis, MO) or 25 μm 4-aminopyridine (4-AP; Sigma) dissolved in ASW was superfused into the recording chamber for 6–9 min. All pharmacological agents were prepared within 1 hr of application. Each preparation was used for a single application of only one drug to preclude the possibility of additive effects.
Data analysis. For single pairwise comparisons, two-tailedt tests were used to assess statistical significance. For multiple comparisons, overall statistical significance was determined by one- or two-way ANOVA. Subsequent planned post hocanalyses were performed using the Fisher’s PLSD test. A pvalue of <0.05 was considered statistically significant; NS indicates not significant. All probability values reported are two-tailed. Data are expressed as mean ± SEM.
RESULTS
Characterization of the juvenile monosynaptic connection
As a first step in understanding the development of synaptic modulation in juvenile Aplysia, it was necessary to confirm that late stage 12 juveniles have functional monosynaptic connections between pleural SNs and pedal MNs. An example of a monosynaptic EPSP from a juvenile preparation is shown in Figure1A. This monosynaptic connection has been extensively characterized in adultAplysia (e.g., Walters et al., 1983a,b). Juvenile SNs and their follower cells are easily identified because the pleural and pedal clusters are located in the same regions in adult ganglia and because these cells exhibit physiological properties similar to those of adult cells (Walters et al., 1983a,b; Marcus and Carew, 1998). This synapse has been shown to fulfill electrophysiological criteria for monosynapticity and chemical synaptic transmission in adults (Walters et al., 1983a). Because no monosynaptic connections have been described previously in developing Aplysia, we performed two classical tests on the juvenile preparation. First, as expected for a chemical synapse (Katz and Miledi, 1967), elevated [Mg2+]o reversibly blocked synaptic transmission (Fig. 1B). A repeated-measures one-way ANOVA indicated a significant effect of high [Mg2+]o on EPSP amplitude [F(4,8) = 25.70; p < 0.0001]. Second, elevated [Ca2+]o raised the SN firing threshold (mean difference, 1.15 ± 0.32 nA;t = 3.53; p < 0.05; n= 4; Fig. 1C1) but did not cause a shift in EPSP latency (mean difference, 0.10 ± 0.18 msec; t = 0.56; NS; n = 4; Fig. 1C2). These synapses are therefore very likely to be monosynaptic because the higher firing threshold for any intercalated interneurons (as was observed in the SNs) would be expected to alter the EPSP latency. Although high [Ca2+]o did not shift EPSP latency, it did significantly increase the amplitude of the SN afterhyperpolarization (mean difference, 4.89 ± 1.32 mV;t = 3.69; p < 0.05; n= 4; Fig. 1C3), probably via activation of Ca2+-dependent K+ channels (Barrett and Barrett, 1976). Although monosynapticity has not been confirmed at the ultrastructural level in juveniles, our physiological results support the conclusion that chemical transmission at the monosynaptic SN–MN connection is fully developed at least by late stage 12.
Intrinsic synaptic plasticity: homosynaptic depression
Having identified the tail sensorimotor monosynaptic connection in juveniles, we next examined the capacity of juvenile synapses to express an intrinsic form of plasticity, homosynaptic depression. In the adult, synaptic transmission is rapidly depressed when single action potentials are elicited in an SN with a 30–60 sec ISI; however, stable synaptic transmission is maintained with a 10–15 min ISI (Castellucci et al., 1970; Emptage et al., 1996; Mauelshagen et al., 1996).
We examined the amount of depression in juvenile synapses resulting from SN stimulation with 1 and 10 min ISIs. Juvenile synapses exhibited adult-like profiles with both ISIs. Two examples are shown in Figure2. In Figure2A1, with a 1 min ISI, the synapse has depressed 87% from the initial EPSP amplitude over the course of 20 min. In Figure 2A2, with a 10 min ISI, a different synapse has decreased by only 6% from the initial EPSP amplitude over the course of 30 min. Group data for 1 and 10 min ISI experiments are shown in Figure 2B. Two separate repeated-measures one-way ANOVAs were performed on the 1 and 10 min ISI data. A 1 min ISI significantly depressed the synapse [F(2,24) = 9.68; p < 0.0001], whereas a 10 min ISI maintained a nondepressed state [F(3,4) = 2.63; NS]. This point is further illustrated with a repeated-measures two-way ANOVA. When EPSP amplitude at 0, 10, and 20 min (T0,T10, andT20) is compared between the 1 and 10 min ISI groups, there is a significant interaction between the two main effects of ISI and stimulus time [F(1,2) = 6.77; p < 0.02]. Taken together, these analyses show that, as in the adult, juvenile synapses exhibit synaptic depression with a short 1 min ISI but do not depress with a 10 min ISI. In this respect, it seems that the cellular mechanisms underlying homosynaptic depression of this synapse are likely to be fully developed by late stage 12.
Heterosynaptic facilitation: depressed versus nondepressed synapses
In the adult, it is known that the recent history of synaptic activation alters the relative contributions of two different processes that contribute to 5-HT–induced facilitation in the SNs. Facilitation of nondepressed synapses predominantly uses the SDD process, whereas facilitation of depressed synapses predominantly uses the SDI process (for review, see Byrne and Kandel, 1996; Gingrich and Byrne, 1985;Hochner et al., 1986b; Gingrich et al., 1988; Braha et al., 1990;Ghirardi et al., 1992). On the basis of the observation that juvenile synapses show homosynaptic depression profiles similar to those of adults, we examined in the juvenile the effects of 5-HT on synaptic transmission from both depressed and nondepressed baselines.
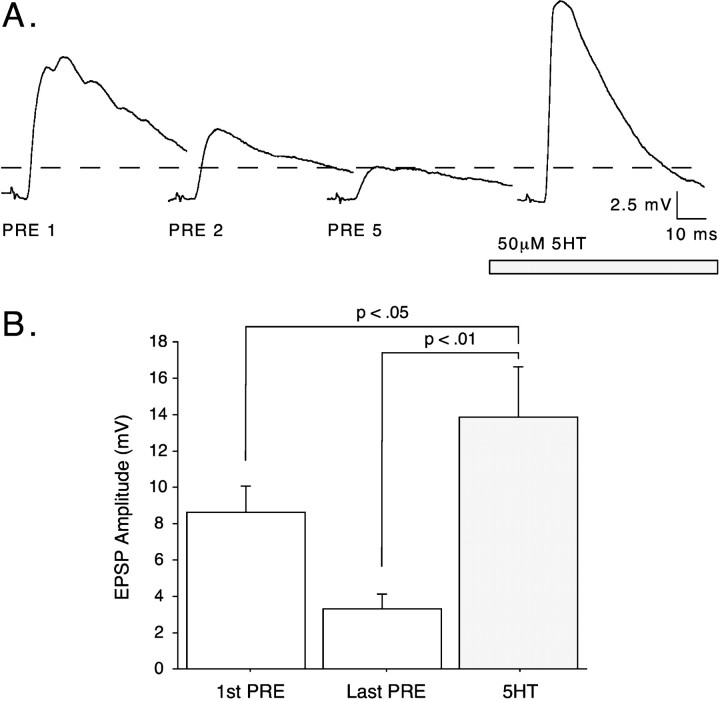
Examining depressed synapses first, we elicited single action potentials from the SN for at least 5 min with a 1 min ISI, depressing the EPSP amplitude to a mean value of 37.9 ± 7.7% of the initial amplitude (t = 4.56; p < 0.01;n = 6). The addition of 50 μm 5-HT to the superfusate facilitated synaptic transmission in all cases (mean difference, 10.57 ± 2.58 mV; t = 4.09;p < 0.01; n = 6), illustrating the reliability of synaptic facilitation from a depressed baseline. Figure3A shows an example of serotonergic facilitation from a depressed baseline, and summary data are shown in Figure 3B. Interestingly, the facilitated EPSP was significantly enhanced above the baseline level before synaptic depression (mean difference, 5.24 ± 1.93 mV; t = 2.72; p < 0.05; n = 6). These results show that the initial (baseline) EPSP does not reflect a “ceiling” above which further enhancement cannot occur (see below).
Fig. 3.
In juveniles, 5-HT significantly facilitates synaptic transmission at depressed synapses. Single action potentials were elicited in juvenile SNs with a 1 min ISI, resulting in homosynaptic depression over the course of at least 5 min.A, An example showing synaptic facilitation induced with 50 μm5-HT (horizontal bar) after depression of the EPSP. Only EPSPs are shown; a small capacitive transient attributable to firing an action potential in the SN precedes eachEPSP. The dashed line indicates the amplitude of the final pre–5-HT EPSP.B, Summary data from the 1 min ISI experiments.5-HT (50 μm) significantly facilitated synaptic transmission from the depressed baseline (Last PRE) and also from the initial nondepressed EPSP(1st PRE; n = 6).
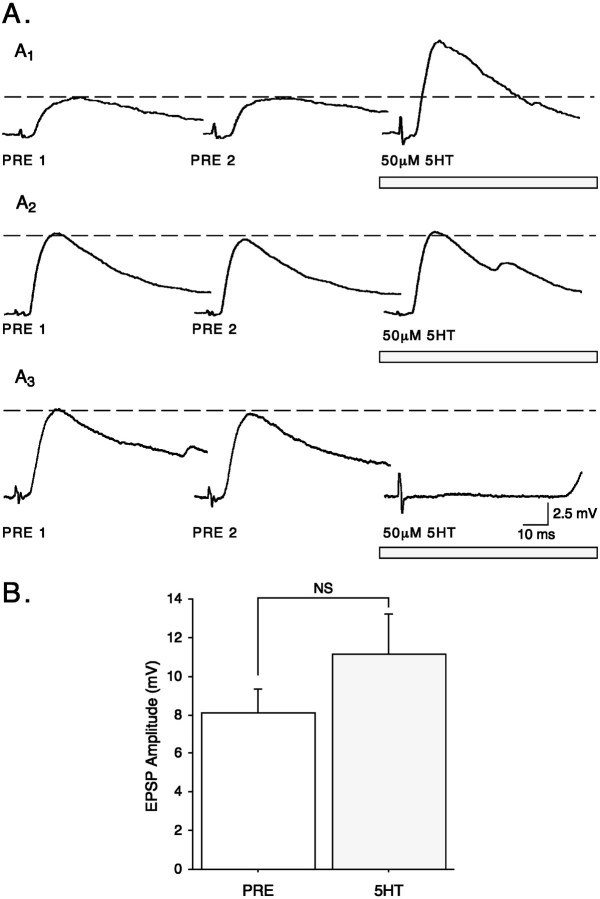
Having established that 5-HT facilitates depressed juvenile synapses, we next examined the effect of 5-HT on nondepressed synapses. At least two baseline measures were taken with a 10 min ISI. A synapse was considered nondepressed if there was no more than a 20% difference between the two baseline EPSPs (see Materials and Methods). In those preparations that satisfied this criterion and that were successfully carried through the complete protocol, there was no significant difference between the two baseline EPSPs (mean difference, 0.06 ± 0.21 mV; t = 0.30; NS; n = 14). The effect of 50 μm 5-HT on nondepressed synapses was extremely variable, ranging from clear facilitation (Fig.4A1) to little or no change in EPSP amplitude (Fig.4A2) to occasional complete depression (Fig. 4A3). The overall effect of 5-HT on nondepressed EPSPs was not significant (mean difference, 3.05 ± 1.59 mV; t = 1.91; NS;n = 14; Fig. 4B). Thus, although significant facilitation is consistently produced by 5-HT from a depressed baseline (Fig. 3), it is not consistently produced from a nondepressed baseline. The lack of significant facilitation from a nondepressed baseline is caused by a large variability in response to 5-HT. Potential reasons for the lack of consistent facilitation of nondepressed synapses are discussed below.
Fig. 4.
In juveniles, 5-HT does not significantly facilitate synaptic transmission in nondepressed synapses. Single action potentials were elicited in juvenile SNs with a 10 min ISI, and the mean of two consecutive stable EPSPsestablished the baseline value (indicated by the dashed lines). A, Three examples illustrating the wide range of effects of 50 μm5-HT(horizontal bars) on synaptic transmission.A1, 5-HT facilitated synaptic transmission. A2,5-HT had no effect.A3, 5-HT depressed synaptic transmission. Only EPSPs are shown; a small capacitive transient attributable to firing an action potential in the SN precedes each EPSP. B, Summary data showing that no significant facilitation is produced with 50 μm5-HT in nondepressed synapses (n = 14). In this and subsequent figures,PRE refers to the EPSP before 5-HT (see Materials and Methods).
A possible ceiling effect
The observation that 5-HT reliably facilitated depressed synapses but not nondepressed synapses could possibly be attributable to a ceiling effect. Having been purposely depressed, the baseline EPSP amplitudes of the depressed synapses (3.32 ± 0.81 mV;n = 6) were significantly smaller than were the baseline amplitudes of the nondepressed synapses (8.08 ± 1.25 mV;n = 14; unpaired t test, t = 2.37; p < 0.05; n = 20). If there were an absolute ceiling above which no EPSP amplitude could facilitate, the nondepressed synapses would be closer to this hypothetical ceiling value than would the depressed synapses and therefore would be expected to show little or no facilitation with 5-HT. Two lines of evidence argue against this possibility. First, a regression analysis combining the depressed and nondepressed groups showed no correlation between the baseline EPSP amplitude (pre–5-HT) and the amount of synaptic facilitation (i.e., the difference in amplitude between pre–5-HT EPSPs and EPSPs measured in 5-HT). This lack of correlation (R2 = 0.047; F = 0.88; NS; n = 20) showed that the amount of facilitation was not dependent on the initial EPSP amplitude and that larger EPSPs could facilitate as much as smaller EPSPs. Second, the baseline EPSP amplitudes in the nondepressed group were not significantly different from the initial EPSP amplitudes in the depressed group before homosynaptic depression; compare Figures 4B,PRE, with 3B, 1st PRE (unpairedt test, t = 0.27; NS; n = 20). If nondepressed EPSPs could not be facilitated simply because they were previously at the ceiling level, then it would be expected that facilitation of depressed synapses could restore EPSP amplitude only to the initial nondepressed baseline. However, as seen in Figure 3, 5-HT facilitated depressed EPSPs over and above the initial nondepressed baseline, thereby surpassing the hypothetical ceiling.
An unexpected relationship between spike broadening and facilitation of nondepressed synapses
Spike broadening has long been implicated as an important factor contributing to synaptic facilitation in monosynaptic sensorimotor connections of Aplysia (Klein and Kandel, 1978; Hochner et al., 1986a; Baxter and Byrne, 1990; Goldsmith and Abrams, 1992; Hochner and Kandel, 1992; Sugita et al., 1992). However, as discussed previously, in adults spike broadening contributes more to facilitation of nondepressed synapses than to facilitation of depressed synapses (Klein and Kandel, 1978; Hochner et al., 1986a,b; Gingrich et al., 1988; Sugita et al., 1994, 1997; but see Klein, 1994). In the simplest case, it would be expected that 5-HT–induced synaptic facilitation of nondepressed synapses would emerge at the same developmental stage as adult-like (major) spike broadening and that, as in adults, there would be little or no facilitation associated with the modest (minor) spike broadening observed in juveniles (Marcus and Carew, 1998). The distinction between minor (≤15%) and major (>15%) spike broadening in juveniles is based on several empirical criteria: (1) developmental status (Marcus and Carew, 1998), (2) 5-HT concentration dependency (Stark et al., 1996), and (3) sensitivity to the 5-HT receptor antagonist cyproheptadine (Mercer et al., 1991) (see the Discussion for further details).
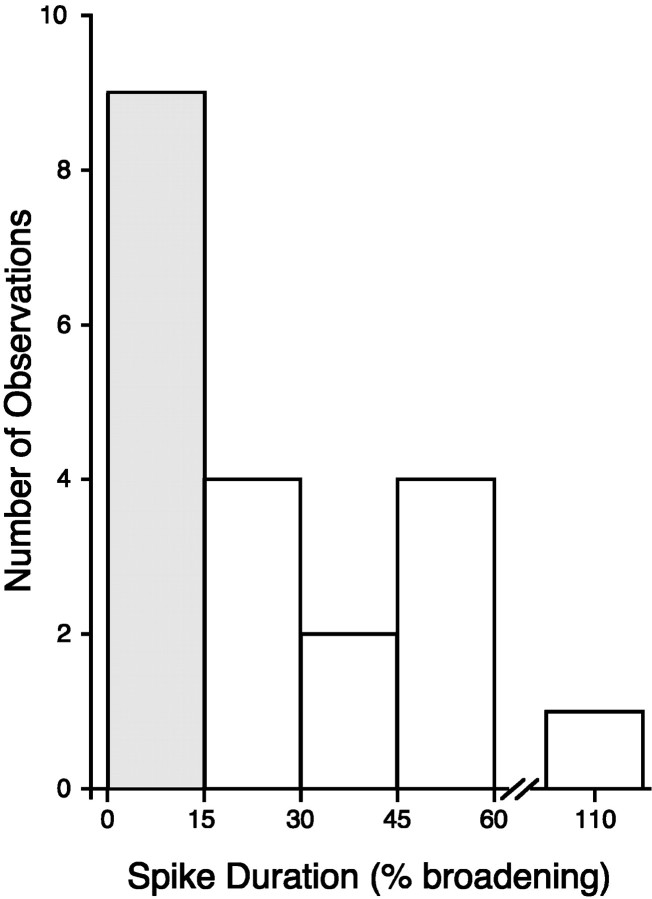
When we examined the amount of SN spike broadening induced by 5-HT (in the same experiments described above), we found a distribution of broadening qualitatively consistent with earlier studies of spike broadening at this developmental stage (Marcus and Carew, 1998). A histogram showing this distribution is shown in Figure5. We found a distinct cluster of SNs exhibiting minor spike broadening (≤15%; n = 9) and a more diffuse distribution of SNs exhibiting major spike broadening (>15%; n = 11). On average, we observed more spike broadening with 50 μm 5-HT in late stage 12 juveniles (0.5–1 gm) than did Marcus and Carew (1998). The difference in the distribution of spike duration may simply be attributable to a slight difference in the relationship between weight and developmental status between the two populations of animals contributing to these studies. Nevertheless, 45% of juvenile SNs described in the present study exhibited only minor spike broadening with 50 μm 5-HT (Fig. 5, shaded bar). When we examined adult SNs under identical conditions, none exhibited minor spike broadening with 50 μm 5-HT; adult broadening was 32.4 ± 4.4% (n = 6; data not shown).
Fig. 5.
Distribution of 5-HT–induced spike broadening in juvenile SNs. The percentage of spike broadening in juvenile SNs produced by 50 μm 5-HT was separated into incremental bins of 15%. The shaded bar indicates the minor spike-broadening group, i.e., those SNs with spike broadening of ≤15% (n = 9). The open bars represent the major spike-broadening group, which is more diffusely distributed (n = 11).
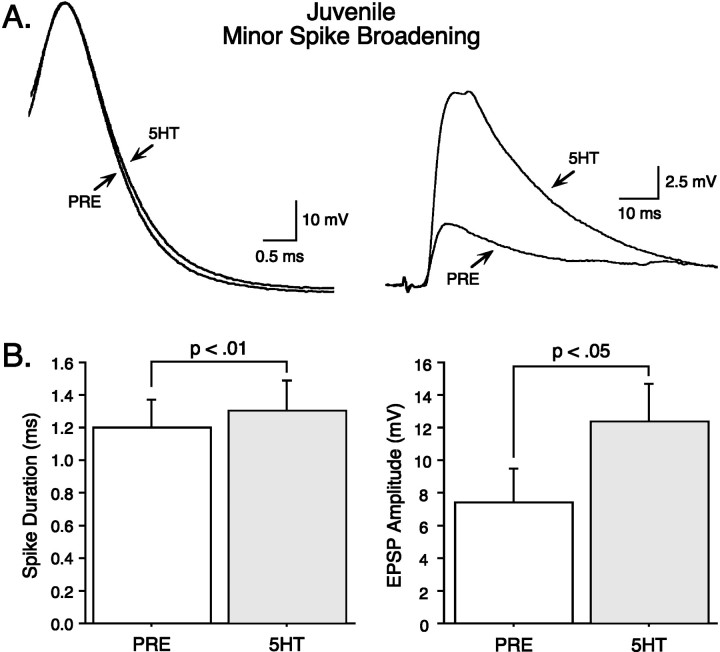
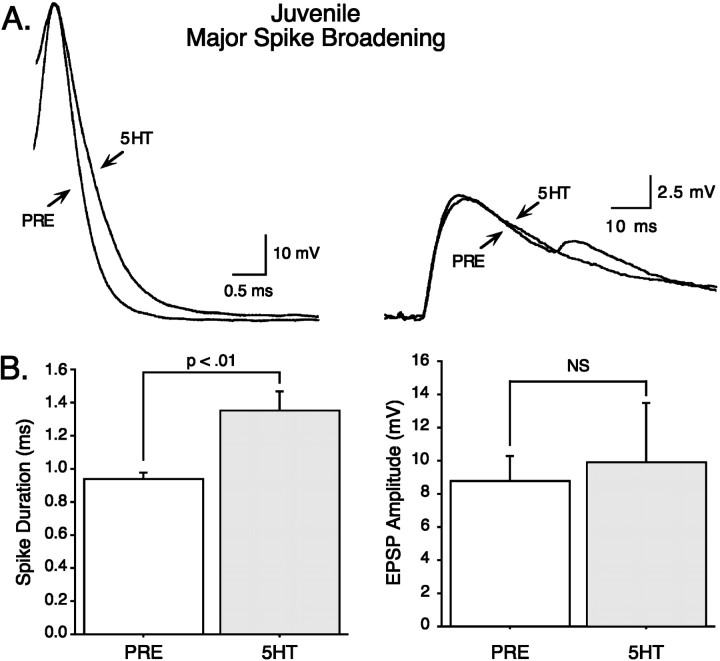
We next evaluated the relationship between spike broadening and synaptic facilitation in juvenile nondepressed synapses, analyzing the minor and major spike-broadening groups separately. Quite unexpectedly, we found that minor spike broadening in the SN is accompanied by substantial synaptic facilitation (example in Fig.6A). An analysis of group data from nondepressed synapses confirms this observation; minor spike broadening (mean difference, 0.10 ± 0.02 msec;t = 4.31; p < 0.01; n= 7) was associated with large and significant synaptic facilitation (mean difference, 5.02 ± 1.88 mV; t = 2.67;p < 0.05; n = 7; Fig.6B). Equally unexpectedly, the opposite result was seen in the major spike-broadening group (example in Fig.7A). Major spike broadening (mean difference, 0.41 ± 0.11 msec; t = 3.79;p < 0.01; n = 7) did not produce significant synaptic facilitation (mean difference, 1.08 ± 2.50 mV; t = 0.44; NS; n = 7, Fig.7B). Thus, unlike in adults (see below), in juveniles, there is a surprising inverse relationship between serotonin-induced spike broadening and synaptic facilitation.
Fig. 6.
Minor spike broadening is associated with significant synaptic facilitation in juveniles. The magnitude of synaptic facilitation of nondepressed synapses was analyzed for those SNs that exhibited only minor spike broadening with 50 μm5-HT. A, An example of minor spike broadening in a SN (left) associated with substantial synaptic facilitation of the EPSP in the MN (right). In this and subsequent figures, the time base of action potentials has been expanded to illustrate more clearly changes in spike duration; in addition, tracesin ASW and 5-HT have been superimposed.B, Summary data showing that although minor spike broadening is modest, it is significant (left) and is associated with significant synaptic facilitation (right) (n = 7).
Fig. 7.
Major spike broadening is not associated with significant synaptic facilitation in juveniles. The magnitude of synaptic facilitation of nondepressed synapses was analyzed for the subset of SNs that exhibited major spike broadening with 50 μm5-HT. A, An example of major spike broadening in an SN (left) associated with little or no synaptic facilitation of the EPSP(right). B, Summary data showing that no significant synaptic facilitation (right) is associated with major spike broadening (left) (n = 7).
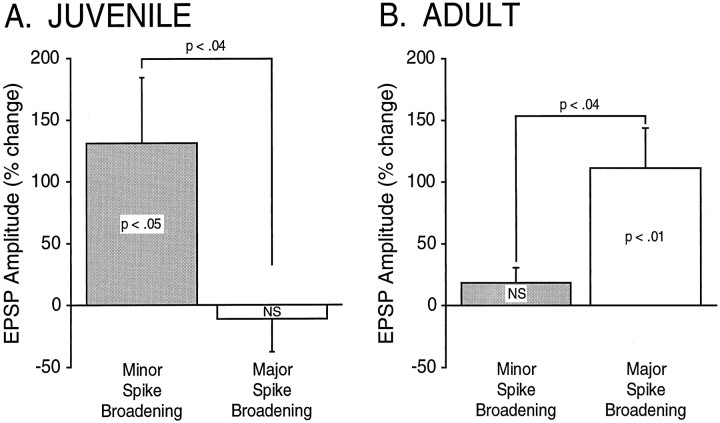
A comparison between nondepressed synapses of juveniles and adults serves to highlight the inverse relationship described above (Fig.8). In juveniles (Fig.8A), minor spike broadening is associated with significant synaptic facilitation (EPSP % change, 130.71 ± 53.36; t = 2.45; p < 0.05;n = 7), whereas major spike broadening is not (EPSP % change, −10.79 ± 27.43; t = −.39; NS;n = 7). An unpaired t test shows a significant difference in the amount of synaptic facilitation between these two groups (t = −2.36; p < 0.04; n = 14). In adults (Fig. 8B), the opposite relationship is seen; major spike broadening is associated with significant synaptic facilitation (EPSP % change, 111.09 ± 32.81; t = 3.39; p < 0.01;n = 10), whereas minor spike broadening is not (EPSP % change, 18.39 ± 12.15; t = 1.51; NS;n = 7). An unpaired t test shows a significant difference in adults as well (t = 2.27;p < 0.04; n = 16) (see also Mercer et al., 1991; Stark et al., 1996). Overall, there is a clear developmental switch in the relationship between 5-HT–induced spike broadening and synaptic facilitation between late stage 12 and adulthood.
Fig. 8.
There is an inverse relationship between spike broadening and synaptic facilitation in juveniles compared with adults. Summary data illustrate a developmental transition between juveniles (A) and adults (B). In juveniles (n = 14), significant synaptic facilitation is associated with minor (and not major) spike broadening, whereas in adults (n = 16), significant facilitation is associated with major (and not minor) spike broadening. In adult SNs, minor spike broadening was produced with low concentrations of 5-HT (0.5–1 μm), whereas higher concentrations of 5-HT (5–50 μm) produced major spike broadening, consistent with previous observations (see Stark et al., 1996).
5-HT exerts presynaptic modulation of SN membrane properties
To discern whether 5-HT exerts its effects pre- or postsynaptically, we monitored the input resistance of SNs and MNs throughout the course of each experiment. In adults, 5-HT increases the input resistance of SNs by closing the S-channel (IKS) (Klein et al., 1982; Siegelbaum et al., 1982; Shuster and Siegelbaum, 1987; Baxter and Byrne, 1989). As in adults, 5-HT increased the input resistance of juvenile SNs (baseline, 67.75 ± 10.22 MΩ; % change, 20.77 ± 6.08;t = 3.42; p < 0.01; n= 20; data not shown). Additionally, 5-HT increased the excitability of the juvenile SNs, measured as an increase in the number of spikes fired with a 150 msec depolarizing pulse (mean difference, 2.43 ± 0.19 spikes; t = 12.9; p < 0.001;n = 7; data not shown) (see also Marcus and Carew, 1998). However, 5-HT did not change the input resistance of the juvenile MNs (baseline, 50.50 ± 8.58 MΩ; % change, 5.11 ± 4.50; t = 1.14; NS; n = 10). Although postsynaptic effects (such as alterations in receptor number or sensitivity) or interneuronal effects cannot be discounted by these results, the data provide preliminary evidence that 5-HT exerts at least some of its modulatory effects presynaptically.
Blocking gKV produces synaptic facilitation in both juvenile and adult SNs
As a first step in trying to pinpoint why juvenile SNs do not exhibit significant synaptic facilitation associated with major spike broadening, we directly induced spike broadening with a blocker of gKV, 4-AP (Baxter and Byrne, 1989;Sugita et al., 1997), to bypass the 5-HT–induced second messenger system(s) mediating major spike broadening in the SNs. Confirming the data of Sugita et al. (1997), we found that, in adults, 25 μm 4-AP substantially broadened the SN spike (mean difference, 1.08 ± 0.19 msec; t = 5.71;p < 0.01; n = 7) and facilitated the nondepressed EPSP (mean difference, 5.93 ± 0.69 mV;t = 8.60; p < 0.001; n= 7; Fig. 9). Interestingly, comparable results were observed in juvenile SNs (Fig.10); 4-AP significantly broadened the juvenile action potential (mean difference, 1.54 ± 0.13 msec;t = 11.57; p < 0.0001;n = 8) and significantly facilitated the nondepressed EPSP (mean difference, 3.16 ± 1.00 mV; t = 3.16;p < 0.02; n = 8). These data show that juvenile nondepressed sensorimotor synapses are indeed capable of exhibiting adult-like synaptic facilitation when the delayed-rectifier K+ channel is blocked with 4-AP, giving rise to substantial spike broadening. Therefore, the lack of 5-HT–induced facilitation associated with major spike broadening in juveniles is not caused by an inability of spike broadening to enhance transmitter release.
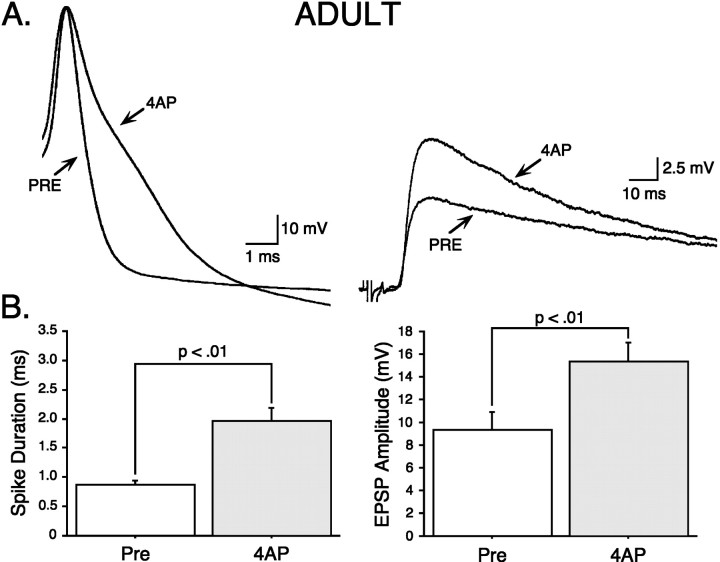
Fig. 9.
4-AP induces major spike broadening and synaptic facilitation in adult SNs. A, An example of major spike broadening induced by 25 μm4-AP in an adult SN (left) and concomitant synaptic facilitation in the MN (right). B, Summary data showing that 25 μm4-AP significantly broadens the SN action potential (left) and significantly facilitates synaptic transmission (right) (n = 7).
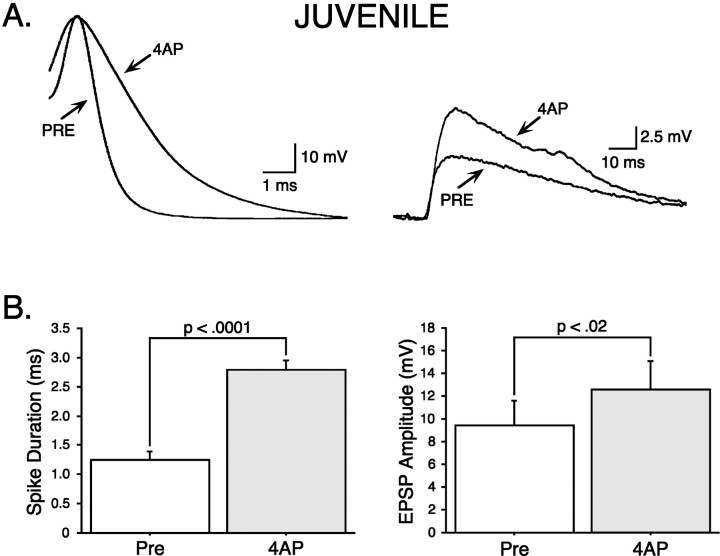
Fig. 10.
4-AP induces major spike broadening and synaptic facilitation in juvenile SNs. A, An example of major spike broadening induced by 25 μm4-AP in a juvenile SN (left) and concomitant synaptic facilitation in the MN (right). B, Summary data showing that 25 μm4-AP significantly broadens the SN action potential (left) and significantly facilitates synaptic transmission (right) (n = 8).
DISCUSSION
Juvenile synapses resemble adult synapses in the expression of homosynaptic depression
In adults, repetitive stimulation of a SN can lead to a progressive decrement in the amplitude of the monosynaptic EPSP (Castellucci et al., 1970). We have found that juvenile sensorimotor synapses reliably decrement with a 1 min ISI but maintain a relatively constant amplitude with a 10 min ISI. The corresponding adult synapses present a similar profile (Castellucci et al., 1970; Emptage et al., 1996; Mauelshagen et al., 1996; Stopfer and Carew, 1996). The early developmental emergence of synaptic depression at the reflex level has been described previously in the abdominal ganglion ofAplysia (Rayport and Camardo, 1984). Monitoring afferent reflex input by recording complex PSPs in the giant neuron R2, Rayport and Camardo (1984) found that synaptic depression of the complex EPSP was present in stage 9, just after metamorphosis. In the tail sensorimotor connection, it has not yet been possible to record from the relevant cells earlier than stage 12; however the decrement of the monosynaptic EPSP we observe indicates that homosynaptic depression is fully developed at least by late stage 12.
Juvenile synapses predominantly express SDI facilitation
In adult SNs, the neuromodulator 5-HT activates at least two processes contributing to synaptic facilitation; these are differentially expressed depending on the state of synaptic depression. Depressed synapses primarily use an SDI process that may involve mobilization of synaptic vesicles from a storage pool into a readily releasable pool (Gingrich and Byrne, 1985; Hochner et al., 1986b;Gingrich et al., 1988; Pieroni and Byrne, 1992). Nondepressed synapses are more sensitive to spike broadening that allows increased Ca2+ influx at the synaptic terminal and greater transmitter release (Blumenfeld et al., 1990; Edmonds et al., 1990;Eliot et al., 1993). To understand more fully the contributions of these two processes to synaptic facilitation, we have examined the effect of 5-HT on synaptic plasticity in depressed and nondepressed juvenile synapses.
In juvenile SNs, serotonergic facilitation is predominantly caused by SDI facilitation. This conclusion is based on two observations.
First, 5-HT significantly facilitates synaptic transmission of depressed, but not of nondepressed, synapses
Because in adults depressed synapses predominantly use SDI facilitation, our results showing that juvenile synapses exhibit significant facilitation only from a depressed baseline led us to infer that juvenile synapses predominantly express SDI facilitation. Facilitation from depressed and nondepressed baselines differed in two ways. (1) Depressed synapses displayed a greater magnitude of facilitation; this difference was not caused by a ceiling effect. (2) Depressed synapses consistently facilitated (in 100% of cases). However, unlike the adult, juvenile nondepressed synapses did not consistently facilitate.
Second, in juvenile nondepressed synapses, minor spike broadening is associated with significant facilitation, but major spike broadening is not
To examine the relationship between spike broadening and synaptic facilitation in juvenile nondepressed synapses, we made use of a previously described distinction between minor (≤15%) and major (>15%) spike broadening. Briefly, this distinction is based on three empirical criteria.
5-HT concentration dependence. In adult SNs, a minor and transient form of spike broadening is induced by low concentrations of 5-HT (0.5–1 μm), whereas major spike broadening requires a higher concentration (5–50 μm) (Stark et al., 1996).
Cyproheptadine sensitivity. The 5-HT receptor antagonist cyproheptadine blocks major spike broadening in adults but spares minor spike broadening (Mercer et al., 1991).
Development. At the onset of late stage 12, 50 μm 5-HT induces only minor spike broadening; later in late stage 12, 50 μm 5-HT induces adult-like major spike broadening (Marcus and Carew, 1998). Because in adults major spike broadening is so strongly implicated in synaptic facilitation, we hypothesized that the two neuromodulatory processes would emerge simultaneously in development. Surprisingly, however, the opposite was observed. In juvenile animals, minor spike broadening was associated with significant synaptic facilitation, whereas major spike broadening was not. This differs from adult nondepressed synapses in which major spike broadening and synaptic facilitation are closely associated but in which minor spike broadening produces virtually no synaptic facilitation (see also Mercer et al., 1991; Stark et al., 1996). Thus, it seems that there is a developmental switch in the relationship between 5-HT–induced spike broadening and synaptic facilitation at nondepressed tail sensorimotor synapses in Aplysia.
Juvenile synapses are capable of SDD facilitation induced by 4-AP
As a first step in exploring why 5-HT is able to induce major spike broadening at a subset of juvenile synapses but does not concomitantly induce SDD synaptic facilitation, we examined the effect of 4-AP on spike duration and synaptic transmission in juvenile and adult synapses. 4-AP (25 μm) is known to be a relatively specific blocker of IKV in Aplysia(Baxter and Byrne, 1989; Sugita et al., 1997). Reduction inIKV results in substantial broadening of the action potential and a concomitant increase in neurotransmitter release (Sugita et al., 1997).
Our experiments show that 4-AP affected juvenile and adult preparations in a similar manner; major spike broadening and synaptic facilitation were induced by 4-AP at both ages. These data suggest that juvenile SNs have intact delayed-rectifier channels that are blocked by 4-AP. Furthermore, the transmitter-release machinery downstream ofIKV seems to be fully intact. In the adult, increased Ca2+ influx through rapidly inactivating, dihydropyridine-insensitive (N-type) Ca2+ channels has been implicated in 5-HT–mediated, spike broadening–associated presynaptic facilitation (Blumenfeld et al., 1990; Edmonds et al., 1990; Eliot et al., 1993). Finally, although there is some indication in other systems that 4-AP may have effects on synaptic release independent of spike broadening (Illes and Thesleff, 1978; Wheeler et al., 1996), at the SN–MN synapse in Aplysia, considerable evidence suggests that the effects of 4-AP on synaptic release and spike duration are closely related (Baxter and Byrne, 1989; Sugita et al., 1997).
Taken together, these data indicate that juvenile synapses are capable of adult-like SDD facilitation when the presynaptic spike is pharmacologically broadened with 4-AP. That is, whenIKV is reduced via a channel blocker and the second messenger systems that normally modulateIKV via a receptor-mediated process are bypassed, then spike broadening can directly influence transmitter release in juveniles. This raises the following question: in juvenile synapses, why does spike broadening induced by 4-AP give rise to synaptic facilitation, whereas comparable spike broadening induced by 5-HT does not?
A novel inhibitory process may be associated with major spike broadening in juvenile SNs
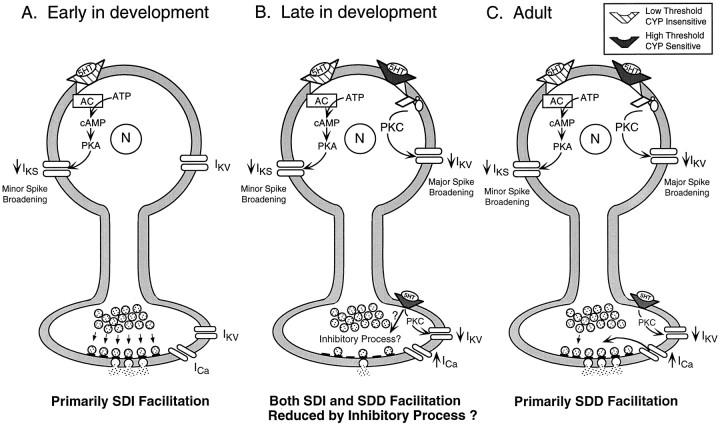
The results of the 4-AP experiments described above suggest that the lack of SDD facilitation in juveniles is not attributable to the inability of the synaptic terminal to be sensitive to changes in presynaptic spike duration and, therefore, to changes in Ca2+ influx. We returned to the original 5-HT data to examine two seemingly paradoxical inverse relationships to evaluate further possibilities to explain the lack of SDD facilitation in juveniles. The data are consistent with the hypothesis that in juveniles a novel inhibitory process may be associated with 5-HT–induced major spike broadening that may reduce the expression of synaptic facilitation (Fig. 11).
Fig. 11.
A schematic model illustrating the development of 5-HT–induced synaptic facilitation in nondepressed sensorimotor synapses of Aplysia. In this three-step model, each structure represents a tail SN, with the soma at the topand the synaptic terminal at the bottom. The pathway on the left of each SN [cAMP/protein kinase A (PKA)–dependent reduction of IKS] is intact as early as has been examined and is responsible for 5-HT–mediated increased excitability, increased input resistance, and minor spike broadening (Marcus and Carew, 1998). The pathway on theright in B and C(PKC-dependent reduction of IKV) contributes to major spike broadening and SDD facilitation. Two 5-HT receptors are shown. The receptor that mediates excitability and minor spike broadening is activated with a low concentration of 5-HT and is not blocked by the 5-HT receptor antagonist cyproheptadine; the 5-HT receptor that mediates major spike broadening and SDD facilitation has a higher threshold for 5-HT activation and is sensitive to cyproheptadine (Mercer et al., 1991; Stark et al., 1996). Development of synaptic facilitation proceeds sequentially in three phases.A, SDI facilitation appears to be the exclusive form of synaptic facilitation early in development, and its mechanism is depicted as a translocation of synaptic vesicles from a storage pool to a releasable pool of vesicles docked in active zones (Gingrich and Byrne, 1985; Hochner et al., 1986b; Gingrich et al., 1988; Pieroni and Byrne, 1992). B, With the emergence of major spike broadening (PKC-dependent reduction ofIKV), SDD facilitation concomitantly emerges but is accompanied by a proposed inhibitory process. This inhibitory process is schematically shown to reduce presynaptically both SDI and SDD facilitation, but its underlying mechanism is unknown.C, In the adult SN, SDD facilitation, shown as increased influx of Ca2+ through voltage-gated Ca2+ channels, is the predominant form of facilitation, whereas SDI facilitation makes a smaller contribution. SNs in A–C are all representative of nondepressed synapses after at least 3 min in 5-HT. AC, Adenylate cyclase; CYP, cyproheptadine; N, nucleus.
One striking inverse relationship we observed is that, in juveniles, minor spike broadening is associated with significant synaptic facilitation and major spike broadening is not. This suggests that, with the advent of major spike broadening, there may be an inhibitory process that decreases the expression of synaptic facilitation. Because juvenile synapses are capable of SDD facilitation induced by 4-AP, the proposed inhibitory process may mask the expression of 5-HT–induced SDD facilitation upstream of gKV, perhaps via activation of the 5-HT receptor that also induces major spike broadening. In juvenile SNs in which major spike broadening has not yet developed, the SDI process seems to be sufficient to facilitate the synapse.
A second inverse relationship we observed is that in juvenile nondepressed synapses, minor spike broadening is associated with synaptic facilitation, whereas in adults, major spike broadening seems to be the primary factor in synaptic facilitation. Again, a developmentally transient inhibitory process could explain this developmental reversal. In adult nondepressed synapses, SDD facilitation may effectively overwhelm any residual inhibitory effects, or the inhibitory mechanism(s) may recede in adulthood. Thus, there seems to be a developmental conversion at nondepressed synapses in which the primary facilitatory mechanism shifts from the SDI to the SDD process, and this may involve a modification of an inhibitory process. Although the present study is consistent with the existence of an inhibitory process, the elucidation of the mechanism(s) underlying this process awaits further investigation. Possible mechanisms include a direct inhibitory effect of 5-HT on synaptic release or an indirect effect via 5-HT activation of inhibitory interneurons that modulate the SNs.
A significant implication of the results discussed above is that the cellular mechanisms underlying SDI facilitation can now be directly studied (in the absence of SDD facilitation) in juvenile SNs that have not yet developed major spike broadening. In addition, the late emergence of SDD facilitation indicates that the functional maturation of these synapses is not complete until almost the end of juvenile development. On a broader level, the approach used in this study illustrates the usefulness of a developmental analysis in revealing complexities of synaptic transmission that are not readily apparent in the adult. By elucidating the developmental assembly of a complex phenomenon such as synaptic facilitation, it is possible to detect processes at an early stage that, in the adult, are either masked by other counteracting processes or may subside with development. This general approach has been used quite effectively in analyzing the developmental assembly of the ionic basis of the action potential (Spitzer, 1991; Gurantz et al., 1996), the ACh receptor subunit composition at the neuromuscular junction (Mishina et al., 1986), and developmental changes in NMDA receptor-mediated synaptic currents underlying plasticity in mammalian and amphibian brain (Carmignoto and Vicini, 1992; Hestrin, 1992; Hofer and Constantine-Paton, 1994; Crair and Malenka, 1995). In the present study and in related work (Marcus and Carew, 1998), we have extended this approach to study the mechanistic assembly of neuromodulation, a cardinal feature of many chemical synapses. This approach may provide insight into mechanisms used to regulate synaptic plasticity in both the developing and adult nervous system.
Footnotes
This work was supported by the National Institute of Mental Health Grant MH-10673 to L.L.S. and by the National Science Foundation Grant IBN-9221117 and the National Institutes of Health Grant R01-MH-14–1083 to T.J.C. We thank Thomas Fischer for helpful criticism of an earlier draft of this manuscript.
Correspondence should be addressed to Dr. Thomas J. Carew, Department of Psychology, Yale University, 2 Hillhouse Avenue, New Haven, CT 06520-8205.
Dr. Stark’s present address: Department of Neuroscience, University of Pennsylvania, 215 Stemmler Hall, Philadelphia, PA 19104-6074.
REFERENCES
- 1.Barrett EF, Barrett JN. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol (Lond) 1976;255:737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baxter DA, Byrne JH. Serotonergic modulation of two potassium currents in the pleural sensory neurons of Aplysia. J Neurophysiol. 1989;62:665–679. doi: 10.1152/jn.1989.62.3.665. [DOI] [PubMed] [Google Scholar]
- 3.Baxter DA, Byrne JH. Differential effects of cAMP and serotonin on membrane current, action potential duration and excitability in somata of pleural sensory neurons of Aplysia. J Neurophysiol. 1990;64:978–990. doi: 10.1152/jn.1990.64.3.978. [DOI] [PubMed] [Google Scholar]
- 4.Blumenfeld H, Spira ME, Kandel ER, Siegelbaum SA. Facilitatory and inhibitory transmitters modulate calcium influx during action potentials in Aplysia sensory neurons. Neuron. 1990;5:487–499. doi: 10.1016/0896-6273(90)90088-w. [DOI] [PubMed] [Google Scholar]
- 5.Braha O, Dale N, Hochner B, Klein M, Abrams TW, Kandel ER. Second messengers involved in the two processes of presynaptic facilitation that contribute to sensitization and dishabituation in Aplysia sensory neurons. Proc Natl Acad Sci USA. 1990;87:2040–2044. doi: 10.1073/pnas.87.5.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- 8.Castellucci VF, Pinsker H, Kupfermann I, Kandel ER. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1745–1748. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- 9.Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- 10.Edmonds B, Klein M, Kandel ER. Contributions of two types of calcium channels to synaptic transmission and plasticity. Science. 1990;250:1142–1147. doi: 10.1126/science.2174573. [DOI] [PubMed] [Google Scholar]
- 11.Eliot LS, Kandel ER, Siegelbaum SA, Blumenfeld H. Imaging terminals of Aplysia sensory neurons demonstrates a role of enhanced Ca2+ influx in presynaptic facilitation. Nature. 1993;361:634–637. doi: 10.1038/361634a0. [DOI] [PubMed] [Google Scholar]
- 12.Emptage NJ, Mauelshagen J, Carew TJ. Threshold serotonin concentration required to produce synaptic facilitation differs for depressed and nondepressed synapses in Aplysia sensory neurons. J Neurophysiol. 1996;75:843–854. doi: 10.1152/jn.1996.75.2.843. [DOI] [PubMed] [Google Scholar]
- 13.Ghirardi M, Braha O, Hochner B, Montarolo PG, Kandel ER, Dale NE. Roles of PKA and PKC in facilitation of evoked and spontaneous transmitter release at depressed and nondepressed synapses in Aplysia sensory neurons. Neuron. 1992;9:479–489. doi: 10.1016/0896-6273(92)90185-g. [DOI] [PubMed] [Google Scholar]
- 14.Gingrich KJ, Byrne JH. Simulation of synaptic depression, posttetanic potentiation, and presynaptic facilitation of synaptic potentials from sensory neurons mediating gill-withdrawal reflex in Aplysia. J Neurophysiol. 1985;53:652–669. doi: 10.1152/jn.1985.53.3.652. [DOI] [PubMed] [Google Scholar]
- 15.Gingrich KJ, Baxter DA, Byrne JH. Mathematical model of cellular mechanisms contributing to presynaptic facilitation. Brain Res Bull. 1988;21:513–520. doi: 10.1016/0361-9230(88)90167-0. [DOI] [PubMed] [Google Scholar]
- 16.Goldsmith BA, Abrams TW. cAMP modulates multiple K+ currents, increasing spike duration and excitability in Aplysia sensory neurons. Proc Natl Acad Sci USA. 1992;89:11481–11485. doi: 10.1073/pnas.89.23.11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurantz D, Ribera AB, Spitzer NC. Temporal regulation of Shaker- and Shab-like potassium channel gene expression in single embryonic spinal neurons during K+ current development. J Neurosci. 1996;16:3287–3295. doi: 10.1523/JNEUROSCI.16-10-03287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992;357:686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- 19.Hochner B, Kandel ER. Modulation of a transient K+ current in the pleural sensory neurons of Aplysia by serotonin and cAMP: implications for spike broadening. Proc Natl Acad Sci USA. 1992;89:11476–11480. doi: 10.1073/pnas.89.23.11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hochner B, Klein M, Schacher S, Kandel ER. Action potential duration and the modulation of transmitter release from sensory neurons of Aplysia in presynaptic facilitation and behavioral sensitization. Proc Natl Acad Sci USA. 1986a;83:8410–8414. doi: 10.1073/pnas.83.21.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochner B, Klein M, Schacher S, Kandel ER. Additional component in the cellular mechanism of presynaptic facilitation contributes to behavioral dishabituation in Aplysia. Proc Natl Acad Sci USA. 1986b;83:8794–8798. doi: 10.1073/pnas.83.22.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofer M, Constantine-Paton M. Regulation of N-methyl-d-aspartate (NMDA) receptor function during the rearrangement of developing neuronal connections. Prog Brain Res. 1994;102:277–285. doi: 10.1016/S0079-6123(08)60546-4. [DOI] [PubMed] [Google Scholar]
- 23.Illes P, Thesleff S. 4-Aminopyridine and evoked transmitter release from motor nerve endings. Br J Pharmacol. 1978;64:623–629. doi: 10.1111/j.1476-5381.1978.tb17325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz B, Miledi R. The timing of calcium action during neuromuscular transmission. J Physiol (Lond) 1967;189:535–544. doi: 10.1113/jphysiol.1967.sp008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein M. Synaptic augmentation by 5-HT at rested Aplysia sensorimotor synapses: independence of action potential prolongation. Neuron. 1994;13:159–166. doi: 10.1016/0896-6273(94)90466-9. [DOI] [PubMed] [Google Scholar]
- 26.Klein M, Kandel ER. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci USA. 1978;75:3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein M, Camardo J, Kandel ER. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci USA. 1982;79:5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kriegstein AR. Stages of post-hatching development of Aplysia californica. J Exp Zool. 1977;199:275–288. doi: 10.1002/jez.1401990212. [DOI] [PubMed] [Google Scholar]
- 29.Levitan IB, Kaczmarek LK. The neuron. Oxford UP; New York: 1996. [Google Scholar]
- 30.Marcus EA, Carew TJ. Developmental emergence of biophysical plasticity in sensory neurons of juvenile Aplysia. Proc Natl Acad Sci USA. 1998;95:4726–4731. doi: 10.1073/pnas.95.8.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauelshagen J, Parker GR, Carew TJ. Dynamics of induction and expression of long-term synaptic facilitation in Aplysia. J Neurosci. 1996;16:7099–7108. doi: 10.1523/JNEUROSCI.16-22-07099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mercer AR, Emptage NJ, Carew TJ. Pharmacological dissociation of modulatory effects of serotonin in Aplysia sensory neurons. Science. 1991;254:1811–1813. doi: 10.1126/science.1662413. [DOI] [PubMed] [Google Scholar]
- 33.Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, Methfessel C, Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986;321:406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- 34.Nolen TG, Marcus EA, Carew TJ. Development of learning and memory in Aplysia. III. Central neuronal correlates. J Neurosci. 1987;7:144–153. doi: 10.1523/JNEUROSCI.07-01-00144.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pieroni JP, Byrne JH. Differential effects of serotonin, FMRFamide, and small cardioactive peptide on multiple, distributed processes modulating sensorimotor synaptic transmission in Aplysia. J Neurosci. 1992;12:2633–2647. doi: 10.1523/JNEUROSCI.12-07-02633.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rankin CH, Carew TJ. Development of learning and memory in Aplysia. II. Habituation and dishabituation. J Neurosci. 1987;7:133–143. doi: 10.1523/JNEUROSCI.07-01-00133.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rayport SG, Camardo JS. Differential emergence of cellular mechanisms mediating habituation and sensitization in the developing Aplysia nervous system. J Neurosci. 1984;4:2528–2532. doi: 10.1523/JNEUROSCI.04-10-02528.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shuster MJ, Siegelbaum SA. Pharmacological characterization of the serotonin-sensitive potassium channel of Aplysia sensory neurons. J Gen Physiol. 1987;90:587–608. doi: 10.1085/jgp.90.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegelbaum SA, Camardo JS, Kandel ER. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982;299:413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- 40.Spitzer NC. A developmental handshake: neuronal control of ionic currents and their control of neuronal differentiation. J Neurobiol. 1991;22:659–673. doi: 10.1002/neu.480220702. [DOI] [PubMed] [Google Scholar]
- 41.Stark LL. PhD thesis. Yale University; 1997. Development of synaptic plasticity in Aplysia californica. . [Google Scholar]
- 42.Stark LL, Carew TJ. Serotonergic facilitation of synaptic transmission in juvenile Aplysia. Soc Neurosci Abstr. 1994;20:814. [Google Scholar]
- 43.Stark LL, Carew TJ. Synaptic facilitation is independent of spike duration in sensory neurons of juvenile Aplysia. Soc Neurosci Abstr. 1996;22:695. [Google Scholar]
- 44.Stark LL, Mercer AR, Emptage NJ, Carew TJ. Pharmacological and kinetic characterization of two functional classes of serotonergic modulation in Aplysia sensory neurons. J Neurophysiol. 1996;75:855–866. doi: 10.1152/jn.1996.75.2.855. [DOI] [PubMed] [Google Scholar]
- 45.Stopfer M, Carew TJ. Heterosynaptic facilitation of tail sensory neuron synaptic transmission during habituation in tail-induced tail and siphon withdrawal reflexes of Aplysia. J Neurosci. 1996;16:4933–4948. doi: 10.1523/JNEUROSCI.16-16-04933.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugita S, Goldsmith JR, Baxter DA, Byrne JH. Involvement of protein kinase C in serotonin-induced spike broadening and synaptic facilitation in sensorimotor connections of Aplysia. J Neurophysiol. 1992;68:643–651. doi: 10.1152/jn.1992.68.2.643. [DOI] [PubMed] [Google Scholar]
- 47.Sugita S, Baxter DA, Byrne JH. Activators of protein kinase C mimic serotonin-induced modulation of a voltage-dependent potassium current in pleural sensory neurons of Aplysia. J Neurophysiol. 1994;72:1240–1249. doi: 10.1152/jn.1994.72.3.1240. [DOI] [PubMed] [Google Scholar]
- 48.Sugita S, Baxter DA, Byrne JH. Differential effects of 4-aminopyridine, serotonin, and phorbol esters on facilitation of sensorimotor connections in Aplysia. J Neurophysiol. 1997;77:177–185. doi: 10.1152/jn.1997.77.1.177. [DOI] [PubMed] [Google Scholar]
- 49.Walters ET, Byrne JH, Carew TJ, Kandel ER. Mechanoafferent neurons innervating tail of Aplysia. I. Response properties and synaptic connections. J Neurophysiol. 1983a;50:1522–1542. doi: 10.1152/jn.1983.50.6.1522. [DOI] [PubMed] [Google Scholar]
- 50.Walters ET, Byrne JH, Carew TJ, Kandel ER. Mechanoafferent neurons innervating tail of Aplysia. II. Modulation by sensitizing stimulation. J Neurophysiol. 1983b;50:1543–1559. doi: 10.1152/jn.1983.50.6.1543. [DOI] [PubMed] [Google Scholar]
- 51.Wheeler DB, Randall A, Tsien RW. Changes in action potential duration alter reliance of excitatory synaptic transmission on multiple types of Ca2+ channels in rat hippocampus. J Neurosci. 1996;16:2226–2237. doi: 10.1523/JNEUROSCI.16-07-02226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]