Abstract
The inhibition of hippocampal pyramidal cells occurs via inhibitory interneurons making GABAergic synapses on distinct segments of the postsynaptic membrane. In area CA1 of the hippocampus, the activation of mu- and delta-opioid receptors inhibits these interneurons, thereby increasing the excitability of the pyramidal cells. Through the use of selective opioid agonists and biocytin-filled whole-cell electrodes, interneurons possessing somata located within stratum oriens of hippocampal slices were classified according to the location of their primary axon termination and the expression of mu- or delta-opioid receptors. Activation of these opioid receptor subtypes resulted in outward currents in the majority of interneurons, which is consistent with their inhibition. Post hoc morphological analysis revealed that those interneurons heavily innervating the pyramidal cell body layer were much more likely to express mu-opioid receptors, whereas cells with axons ramifying in the pyramidal neuron dendritic layers were more likely to express delta-opioid receptors, as defined by the generation of outward currents. This morphological segregation of interneuron projections suggests that mu receptor activation would diminish GABA release onto pyramidal neuron somata, thereby increasing their excitability and output. Conversely, inhibition of interneurons via delta receptor activation would amplify afferent signaling to pyramidal neuron dendrites by reducing GABAergic inhibition of these structures.
Keywords: delta receptor, electrophysiology, enkephalin, GABA, hippocampus, inhibition, morphology, mu receptor, nonselective cation current, opioid receptor, oriens/alveus interneurons, potassium current
The interaction among principal cells and GABAergic inhibitory interneurons is one means through which computational processes occur in mammalian cortical areas. In the hippocampus, the diverse group of cells classified as inhibitory interneurons constitutes between 10 and 20% of the total neuronal population. The heterogeneity within this group of neurons is attributed to the differential expression of calcium-binding proteins, peptide cotransmitters, and ion channels (Zhang and McBain, 1995;Chikwendu and McBain, 1996; Maccaferri and McBain, 1996; Svoboda and Lupica, 1998) (for review, see Freund and Buzsáki, 1996). In addition, a high degree of anatomical variability exists in the extent and location of the inhibitory axon termination on the principal (pyramidal) neuron membrane. Thus, inhibitory cells providing perisomatic inhibition form synapses on pyramidal neuron somata (basket cells) and axon initial segments (axo-axonic cells), whereas others display circumscribed innervation of pyramidal neuron dendrites (Buhl et al., 1994a; Cobb et al., 1995, 1997; Miles et al., 1996). Because of this axonal segregation, these classes of interneurons exert distinct functional effects on pyramidal neurons in the hippocampus. For example, perisomatic inhibition has been shown to globally limit pyramidal neuron excitability by blocking Na+-dependent action potentials (Miles et al., 1996). Also, somatic inhibition can remove the inactivation of voltage-dependent inward currents, thus permitting the synchronization of groups of pyramidal neurons and the generation of γ oscillations (Cobb et al., 1995; Whittington et al., 1995). In contrast, GABAergic IPSPs impinging on pyramidal neuron dendrites can shunt EPSPs, diminish dendritically generated Ca2+ action potentials (Masukawa and Prince, 1984; Miles et al., 1996; Tsubokawa and Ross, 1996), and remove the inactivation of dendritic K+conductances (Hoffman et al., 1997). These physiological data, together with the differing anatomical profiles of the interneurons strongly suggest that perisomatic and dendritic inhibition serve distinct functional roles in the hippocampus.
In addition to the sources of heterogeneity described above, there is good evidence that different groups of interneurons are selectively innervated by distinct neurotransmitter-containing afferents (Freund and Antal, 1988; Freund et al., 1990; Fuzesi et al., 1997). Furthermore, different interneurons express distinct sets of neurotransmitter receptors (McBain and Dingledine, 1993; McBain et al., 1994; Bergles et al., 1996; McMahon and Kauer, 1997; Miller et al., 1997; Morales and Bloom, 1997; Frazier et al., 1998; Svoboda and Lupica, 1998), suggesting further segregation of function. Among the receptors expressed on inhibitory interneurons in the CA1 region of the hippocampus are μ- and δ-opioid receptors (Mansour et al., 1995;Commons and Milner, 1997). Physiological studies have shown that activation of these receptors can lead to the inhibition of interneuron activity (Madison and Nicoll, 1988; Wimpey and Chavkin, 1991; Svoboda and Lupica, 1998), resulting in diminished GABA release and the disinhibition of pyramidal neurons (Zieglgansberger et al., 1979; Lee et al., 1980; Nicoll et al., 1980; Siggins and Zieglgansberger, 1981;Cohen et al., 1992; Lupica et al., 1992; Lupica, 1995). It was also demonstrated that μ-opioid receptor activation resulted in a more profound increase in pyramidal neuron excitability, the generation of epileptiform activity, and the disruption of γ oscillations when compared with δ receptor activation (Wimpey et al., 1989; Lupica and Dunwiddie, 1991; Lupica, 1995; Whittington et al., 1998). These data suggested that μ- and δ-opioid receptors may be expressed on different subsets of interneurons providing inputs to distinct regions of the pyramidal neuron membrane. To test this hypothesis, we have examined the morphological differences between interneurons expressing either μ- or δ-opioid receptors in the CA1 region of the hippocampus, using biocytin as a cellular marker. Opioid receptor subtype expression was determined through the application of highly selective μ- or δ-opioid receptor agonists and the measurement of membrane currents in voltage-clamped neurons. We then attempted to classify interneurons based on opioid receptor subtype expression, as defined by the generation of these outward currents, and the axonal and dendritic profiles. We observed that interneurons innervating the pyramidal neuron perisomatic region were more likely to express μ-, rather than δ-opioid receptors. In contrast, interneurons projecting to pyramidal neuron dendritic regions more frequently expressed δ, rather than μ-opioid receptors.
MATERIALS AND METHODS
Electrophysiology. Hippocampal slices were prepared and maintained as previously described (Miller et al., 1997). Briefly, 14–30-d-old male Sprague Dawley rats (Sasco, Omaha, NE) were killed by rapid decapitation. Their brains were removed and placed in ice-cold, oxygenated artificial CSF (aCSF; see below). Brain slices containing the hippocampus were cut transverse to the anteroposterior axis at 300 μm nominal thickness using a vibrating tissue slicer (Technical Products International, St. Louis, MO). The slices were then stored suspended on netting in a beaker containing aCSF, aerated continuously with 95% O2 and 5% CO2 at room temperature. Control aCSF consisted of (in mm): NaCl, 126; KCl, 3.0; MgCl2, 1.5; CaCl2, 2.4; NaH2PO4, 1.2; glucose, 11.0; and NaHCO3, 26, and was saturated with 95% O2 and 5% CO2. Interneurons were visualized in stratum oriens of area CA1 using a fixed stage upright microscope equipped with differential interference contrast optics and infrared illumination, as previously described in detail (Dodt and Zieglgansberger, 1990; Miller et al., 1997). Whole-cell recordings were obtained at room temperature (20–23°C) using an Axopatch-200A amplifier (Axon Instruments, Burlingame, CA), and electrodes pulled from thick-walled borosilicate capillary tubing (inner diameter, 0.75 mm; outer diameter, 1.5 mm; Sutter Instrument Company, Novato, CA). The electrodes had resistances of 4–7 MΩ, when filled with (in mm): K+-gluconate, 125.0; KCl, 10.0; HEPES, 10.0; EGTA, 1.0; CaCl2, 0.1; Mg2+-ATP, 2.0; and Na+-GTP, 0.2 (adjusted to pH 7.2–7.4 with 1 m KOH, and brought to 270–280 mOsm with deionized water). All interneurons were voltage-clamped at −66 mV, unless otherwise stated, after correcting for a liquid junction potential. Series resistance was <15 MΩ and was monitored throughout the experiments using the capacitative currents generated by small (−5 to −10 mV; 250 msec) voltage steps. Cells were rejected from analysis if the series resistance changed by ≥10–15%. Voltage-clamp protocols were delivered using a pulse generator (AMPI Master 8; Jerusalem, Israel), and signals were acquired using a personal computer-based data acquisition system (Strathclyde Electrophysiology Software; courtesy of John Dempster, Strathclyde University, UK).
Drug application. The selective δ-opioid receptor agonist DPDPE (d-Pen2, Pen5-enkephalin) and the selective μ-opioid receptor agonist DAMGO (Tyr-d-Ala2,N-CH3-Phe4,Gly-ol-enkephalin) were obtained from the National Institute on Drug Abuse Drug Supply System (Rockville, MD). Both drugs were made at 100 times their final concentration in deionized, purified water and added to the aCSF bathing the slice (flow rate, 2 ml/min) using calibrated syringe pumps (Razel Scientific Instruments, Stamford, CT). Unless otherwise stated, both the μ and the δ agonists were applied at a concentration of 1 μm. This concentration has been shown in previous studies to provide maximal occupation of, and selectivity at, the respective receptor subtype (Mosberg et al., 1983; Goldstein and Naidu, 1989;Lupica, 1995).
Histology. Biocytin (0.25%; Sigma, St. Louis, MO) was added to the internal solution in the recording pipette for post hoc evaluation of neuronal morphology. At the end of the recording session, the pipette was slowly withdrawn, and the slice was transferred to chilled (4°C) 4% paraformaldehyde in PBS solution. Slices were stored in the chilled fixative for 3–7 d and were not resectioned before biocytin development procedures (Ceranik et al., 1997). After fixation, they were rinsed in PBS (3 × 5 min), quenched (0.3% H2O2/PBS for 30 min), rinsed again in PBS (3 × 5 min), and then exposed to avidin–biotin horseradish peroxidase complex containing 0.3% Triton X-100 (ABC kit; Vector Laboratories, Burlingame, CA) for 4 d. On the fourth day, they were rinsed again in PBS and then incubated in 0.04% diaminobenzidine (DAB) and 0.0025% H2O2 for 20–30 min. The DAB reaction was stopped by rinsing again in PBS. The slices were then dehydrated in incrementing concentrations of ethanol (70, 95, 100, and 100%). After dehydration, they were cleared in Hemo-D (3 × 5 min) and then mounted on slides. Cells were reconstructed using a 40× objective with the aid of a camera lucida drawing tube attached to an Olympus microscope.
RESULTS
Morphology of stratum oriens interneurons
Whole-cell recordings were obtained from 94 interneurons with somata located within stratum oriens. Complete axon fills were observed in 75 interneurons that were used in the subsequent morphological and physiological analyses. The photomicrographs in Figure1 illustrate the four major classes of interneuron that were encountered in this study. These cells were classified based on the stratum (pyramidale, lacunosum-moleculare, radiatum, or oriens) that was most heavily innervated by the primary axon (Fig. 1). Twelve interneurons (16%) displayed axonal arborizations restricted, primarily, to the pyramidal cell body layer (stratum pyramidale), and were thus classified as perisomatic cells (Fig. 1A). These cells typically possessed two to eight large, apically oriented dendrites that projected through stratum pyramidale and terminated in stratum radiatum and/or stratum lacunosum-moleculare (Fig. 1A, see Fig. 3). In addition, these neurons usually possessed several basally oriented dendrites within stratum oriens, occasionally making contact with the alveus. Cells displaying these morphological characteristics include the basket and axo-axonic cells that have been described by others (Buhl et al., 1994b, 1995; Miles et al., 1996; Cobb et al., 1997) (for review, see Freund and Buzsáki, 1996).
Fig. 1.
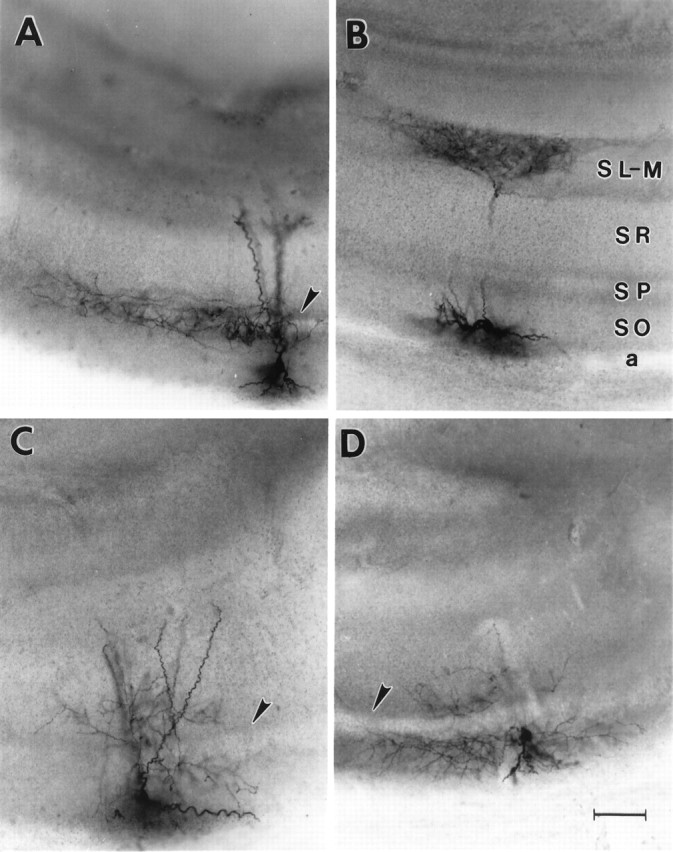
Morphology of stratum oriens interneurons. Photomicrographs of the four subtypes of biocytin-filled stratum oriens interneurons analyzed in this study. A, An interneuron with its axon almost exclusively innervating stratum pyramidale, in which the CA1 pyramidal cell bodies are located.B, An OLM or horizontal cell. Note the dense axon termination in stratum-lacunosum moleculare and the horizontally oriented dendrites in stratum oriens. These are the defining anatomical characteristics of this cell type. C, An interneuron with its primary axonal ramification in stratum radiatum.D, An interneuron with its primary axonal projection confined to stratum oriens. The arrowheads denote stratum pyramidale in A, C, andD. Scale bar, 100 μm. a, Alveus;SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum;SL-M, stratum lacunosum-moleculare.
Fig. 3.
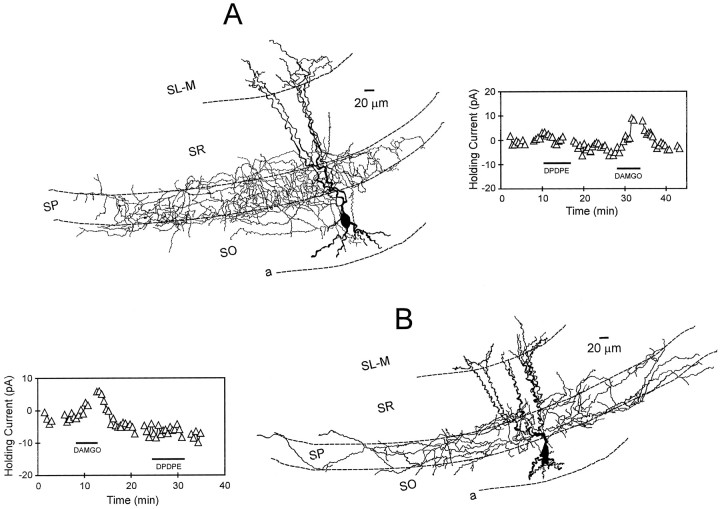
Interneurons innervating stratum pyramidale: morphology and opioid pharmacology. A, Camera lucida reconstruction of an interneuron with its axonal projection restricted almost exclusively to stratum pyramidale (same cell as Fig.1A). The apical dendrites of this cell (darker structures) extend across stratum radiatum and into stratum lacunosum-moleculare. The inset at theright illustrates time course of the DAMGO-induced outward current. This cell was presumed to express μ and not δ receptors because an outward current was only generated with DAMGO.B, Another interneuron with similar morphology to that shown in A. The inset at theleft shows the time course of the DAMGO-generated outward current. The lateral extent of the axon in this cell was 1133 μm. Note that this neuron did not respond to the δ agonist DPDPE.
Seventeen neurons (23%) exhibited axons that arborized extensively within stratum lacunosum-moleculare (Fig.1B, see Figs. 4, 5). This region is known to contain the apical dendrites of CA1 pyramidal neurons that receive excitatory input from afferents arising in the entorhinal cortex and thalamus (Gulyas et al., 1993a; Desmond et al., 1994; Sik et al., 1995; Yanovsky et al., 1997). Typically, these neurons possessed a single axon that crossed stratum pyramidale, although some neurons had minor axon collaterals that terminated either in stratum radiatum and/or stratum oriens (see Fig. 4B). The dendrites of these cells were oriented parallel to stratum oriens and confined exclusively to this layer. These morphological characteristics are similar to those described for the stratum oriens, lacunosum-moleculare interneurons known as OLM or horizontal cells that are known to form synapses on the distal apical dendrites of CA1 pyramidal neurons (Lacaille et al., 1987; McBain et al., 1994; Sik et al., 1995; Ali and Thomson, 1998) (for review, see Freund and Buzsáki, 1996). The remainder of the interneurons demonstrated primary axonal projections to either stratum radiatum (22 of 75, 29%; Fig. 1C) or stratum oriens (24 of 75, 32%; Fig. 1D). Because some of these cells provided substantial innervation of more than a single hippocampal layer, they resembled interneurons previously described as bistratified cells (see Figs. 6A, 7) (Buhl et al., 1994a; Ali et al., 1998). The dendritic structure of these cells varied considerably. In some cases, the dendrites were confined to stratum oriens, where they often coursed parallel to the alveus. However, cells were also encountered that had dendritic arborizations within stratum radiatum (Fig. 1C). The observation that many axon arborizations were confined to a single stratum suggested that these cells selectively targeted particular areas of the pyramidal neuron membrane.
Fig. 4.
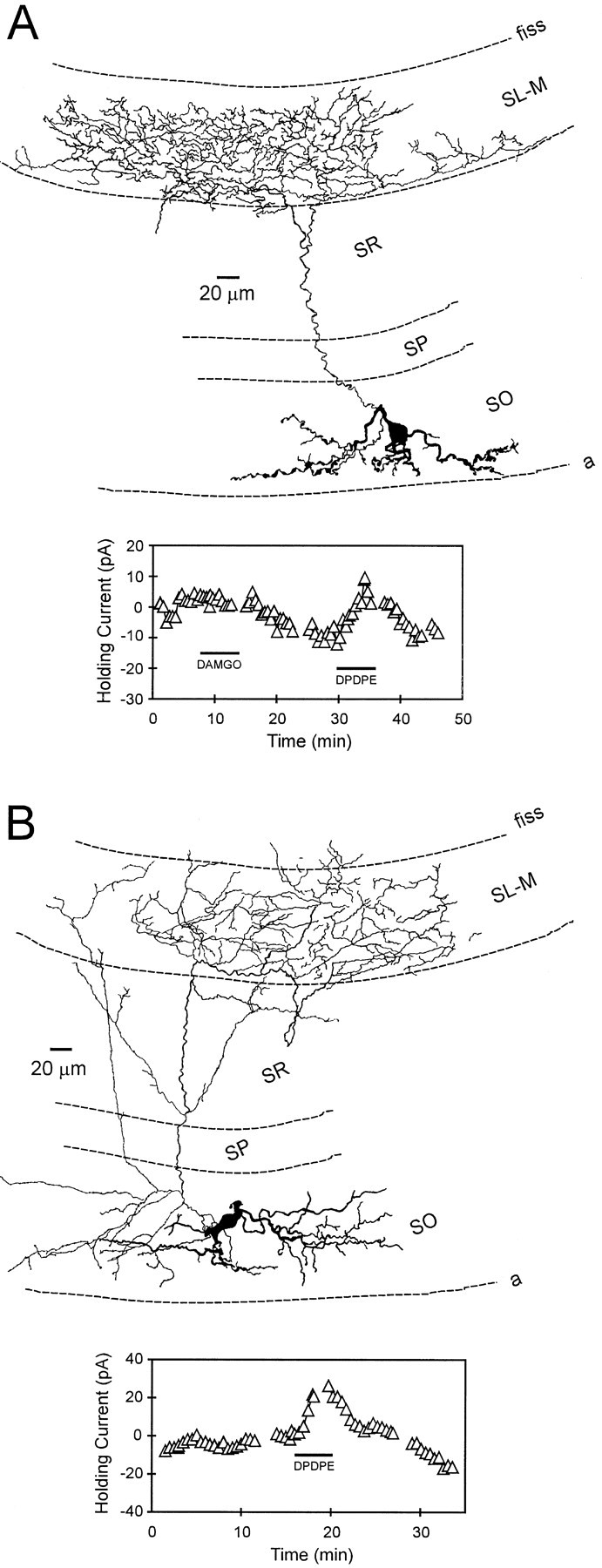
Stratum oriens interneurons innervating stratum lacunosum- moleculare (OLM or horizontal cells): morphology and opioid pharmacology. A, Camera lucida reconstruction of an interneuron with its axonal projection almost exclusively in stratum lacunosum-moleculare. The dendrites of this cell course parallel with the alveus border and are restricted to stratum oriens. Theinset below illustrates the effect of the δ-opioid agonist DPDPE on holding current. In this example, DAMGO failed to generate an outward current. B, Reconstruction of another interneuron with its axon ramifying within stratum lacunosum-moleculare. The inset below shows the time course of the δ-opioid agonist-generated outward current.fiss, Hippocampal fissure.
Fig. 5.
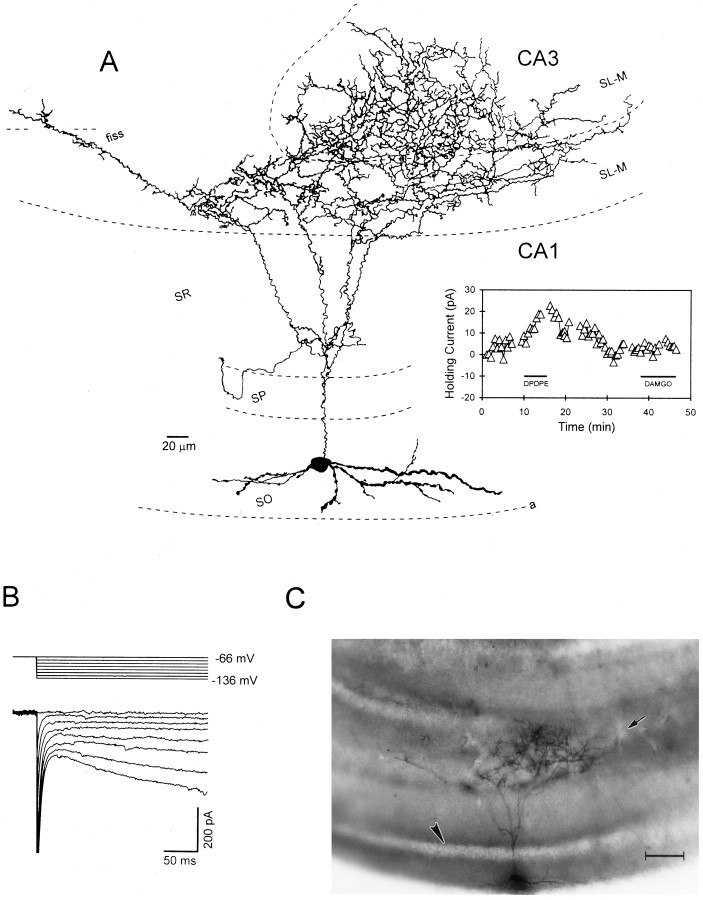
A stratum oriens “backprojection” cell innervating stratum lacunosum-moleculare of hippocampal subfields CA1 and CA3: morphology and physiology. A, A camera lucida reconstruction of the interneuron. In general, the morphology of this cell was similar to those shown in Figure 4. Note that the axon of this cell also crossed the hippocampal fissure and ramified extensively within stratum lacunosum moleculare of CA3. The inset at the right illustrates time course of the DPDPE-induced outward current. The μ-opioid agonist DAMGO had no effect.B, Current and voltage records obtained in this same cell. Note the prominent inward “sag” on the current responses at the most hyperpolarized potentials that is indicative ofIh. C, A photomicrograph of the interneuron reconstructed in A. Thearrowhead indicates the location of stratum pyramidale, and the arrow denotes the border between CA1 and CA3. Scale bar, 100 μm. fiss, Hippocampal fissure.
Fig. 6.
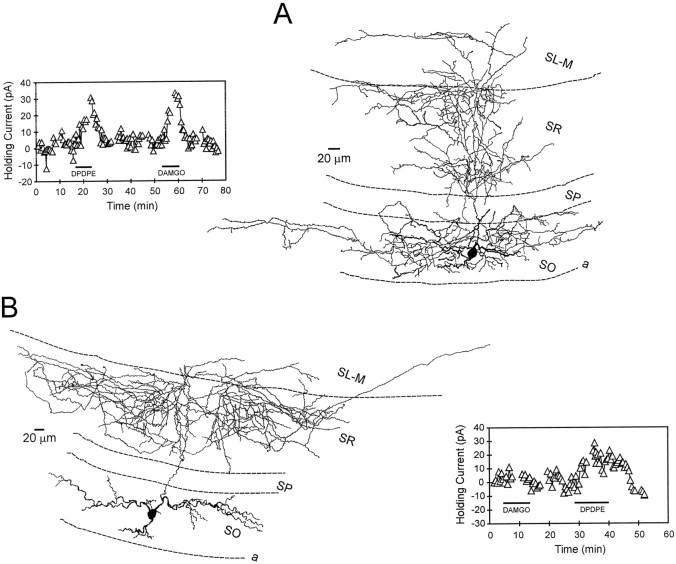
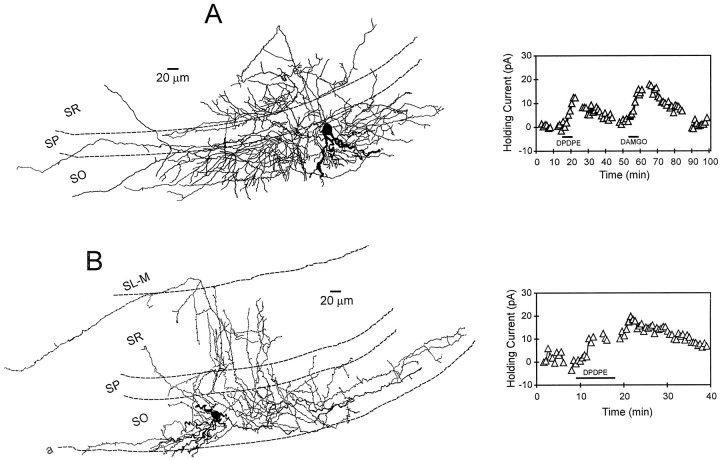
Interneurons innervating stratum radiatum: morphology and opioid pharmacology. A, Camera lucida reconstruction of an interneuron with its axon projection in strata radiatum and oriens. After further analysis (counting of axonal branch points), it was determined that this cell more heavily innervated stratum radiatum. The inset at the leftillustrates that both δ-opioid (DPDPE) and μ-opioid (DAMGO) agonists generated robust outward currents in this cell.B, Camera lucida reconstruction of an interneuron that exhibited an axonal projection predominantly confined to stratum radiatum. The inset at the right illustrates the time course of the δ agonist-induced outward current. This cell did not respond to the μ agonist DAMGO.
Fig. 7.
Interneurons primarily innervating stratum oriens: morphology and opioid pharmacology. A, A camera lucida reconstruction of a stratum oriens interneuron with its primary axonal projection confined to this layer (same cell as shown in Fig.1D). Note that the axon does not heavily innervate stratum pyramidale, yet ramifies in both stratum radiatum and oriens. This morphological profile is similar to that described for “bistratified” neurons (Buhl et al., 1994a). Theinset at the right illustrates the time course of the DAMGO- and DPDPE-induced holding current changes. Although this interneuron was sensitive to both opioid agonists, only 6 of 16 interneurons responded to the μ agonist, whereas 13 of 17 responded to the δ-opioid receptor agonist. B, Reconstruction of a stratum oriens interneuron that demonstrated an extensive primary axonal projection within stratum oriens. Theinset at the right shows the time course of the DPDPE-induced outward current in this same cell.
Stratum oriens interneurons: physiology and opioid pharmacology
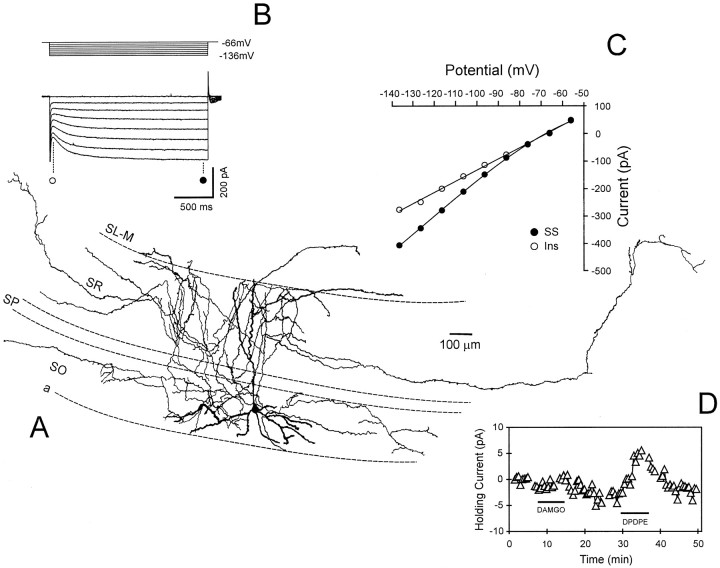
A previously noted characteristic of stratum oriens interneurons was the presence of the hyperpolarization-activated inward current known as Ih (Bergles et al., 1996;Maccaferri and McBain, 1996; Parra et al., 1998; Svoboda and Lupica, 1998). This current was present in >90% of the cells in the present study (86 of 94 cells), and its voltage and time dependence were similar to those previously reported (Fig.2B,C). In a previous study, we have shown that the outward currents produced by μ- or δ-opioid receptor activation in stratum oriens interneurons were caused by the inhibition of Ih and the simultaneous activation of a K+ current (Svoboda and Lupica, 1998). Both of these actions are consistent with the hyperpolarization and subsequent inhibition of these interneurons. In the present study, we used bath application of highly selective μ- or δ-opioid agonists (1 μm DAMGO or 1 μm DPDPE, respectively) to determine whether these cells expressed either of these opioid receptor subtypes. An example of an outward current produced by DPDPE in a biocytin-filled interneuron is shown in Figure 2D. This cell presumably did not express μ-opioid receptors because an outward current was not produced by DAMGO (Fig. 2D). Throughout this study outward currents of ≥3 pA were considered the minimum for classification of interneurons as μ- or δ-opioid-sensitive. This value was approximately threefold higher than the baseline noise in our recording apparatus.
Fig. 2.
Physiological characteristics and opioid sensitivity of a stratum oriens interneuron. A, A camera lucida reconstruction of an interneuron demonstrating extensive axonal ramification in stratum radiatum from which the data shown in panelsB–D were obtained. B, Current records obtained during 2 sec hyperpolarizing voltage steps from −56 to −136 mV. The large inward current relaxation near the end of the voltage steps is caused by activation ofIh. C, Current–voltage (I–V) relationship demonstrating the instantaneous (○) and steady-state (•) currents obtained at step potentials between −136 and −56 mV. D, Time course of opioid-induced outward currents. The horizontal bars indicate the duration of opioid agonist bath application. In this example, the δ-opioid agonist DPDPE (1 μm) produced an outward current, whereas the μ-opioid agonist DAMGO had no effect. This illustrates the selectivity of these agonists.Gaps in the record denote time points ofI–V generation.
The μ-opioid agonist DAMGO was applied to 44 stratum oriens interneurons, and 25 (57%) responded with outward currents. The δ agonist DPDPE was applied to 53 cells, with 32 (60%) demonstrating outward currents. Only 9 of 35 (26%) interneurons displayed outward currents in response to random sequential administration of both agonists (Fig. 2D). The observation that only a small percentage of cells responded to both agonists supports previous studies demonstrating the selectivity of these peptide agonists for their respective opioid receptor subtypes (Mosberg et al., 1983;Goldstein and Naidu, 1989; Lupica, 1995). Mean outward currents associated with μ- or δ-opioid receptor activation observed in interneurons projecting to each of the four hippocampal strata are shown in Table 1. These data indicate that the opioid agonists were equally effective in generating outward currents and that there was no difference in the magnitude of the currents generated in cells projecting to different hippocampal strata. The generation of outward currents by the opioid agonists in these neurons is consistent with hyperpolarization of the neuronal membrane and the inhibition of these cells.
Table 1.
Magnitude of outward currents produced by μ- or δ-opioid receptor activation in stratum oriens interneurons, segregated according to the stratum in which the primary axon arborized
| Oriens | Pyramidale | Radiatum | Lacunosum-moleculare | Receptor average | |
|---|---|---|---|---|---|
| μ receptor | 17.2 ± 4.4 | 17.7 ± 4.4 | 14.7 ± 2.8 | 16.2 ± 10.0 | 16.7 ± 2.1 |
| 6.6–27.3 | 6.2–48.7 | 10.5–26.5 | 6.0–32.3 | ||
| (6) | (10) | (6) | (3) | (25) | |
| δ receptor | 13.2 ± 1.3 | 29.5 ± ND | 14.0 ± 3.5 | 12.9 ± 2.1 | 14.3 ± 1.4 |
| 8.9–20.1 | 16.1–42.8 | 5.3–25.5 | 6.5–22.8 | ||
| (13) | (2) | (6) | (11) | (32) | |
| Stratum average | 14.5 ± 1.3 | 19.2 ± 4.2 | 14.4 ± 2.0 | 13.7 ± 2.2 | |
| (19) | (12) | (12) | (14) |
Shown are the means ± SEs and the ranges of the opioid-induced outward currents, expressed in picoamperes. The number of cells contributing to each of these data sets is indicated in parentheses. There was no significant difference between the average magnitudes of outward currents generated by μ- or δ-opioid receptor activation, nor among the strata innervated by the inhibitory axons. ND, Not determined.
Opioid receptor expression in interneurons innervating stratum pyramidale
The μ agonist DAMGO was applied to 11 of 12 cells that were classified as perisomatic based on the location of their primary axonal ramification within stratum pyramidale. Ten of these cells (91%) responded with robust outward currents (Fig.3, Table2), suggesting that the majority of these neurons expressed μ-opioid receptors. Of the 25 interneurons demonstrating outward currents with μ-opioid receptor activation, 10 (40%) were classified as providing perisomatic input to the pyramidal cell body layer. The δ opioid agonist DPDPE was applied to 9 of the 12 perisomatic cells, and only two (22%) demonstrated outward currents (Table 2). Furthermore, in contrast to those cells expressing μ receptors, only 2 of the 32 (6%) interneurons responding to the δ agonist sent axons to stratum pyramidale. Figure 3 illustrates two perisomatic cells that responded to μ- and not δ-opioid receptor activation. Statistical analysis revealed that the difference between the effects of the μ and δ agonists on the cells projecting to stratum pyramidale was significant (χ2 = 27.0;p < 0.001). Two additional neurons in which axons could not be recovered possessed somatic and dendritic morphologies very similar to the perisomatic cells shown in Figure 3. Outward currents were also generated by the μ, and not the δ, agonist in each of these neurons, but they were not included in the analyzed data set.
Table 2.
Percentage of cells expressing μ- or δ-opioid receptors as a function of primary axon termination
| Oriens | Pyramidale | Radiatum | Lacunosum-moleculare | |
|---|---|---|---|---|
| μ receptor | 6 /16 | 10 /11 | 6 /12 | 3 /7 |
| (38%) | (91%)* | (50%) | (43%) | |
| δ receptor | 13 /17 | 2 /9 | 6 /11 | 11 /15 |
| (76%)** | (22%) | (55%) | (73%)*** |
The number of cells responding to, and the total number of cells exposed to the μ-opioid (DAMGO) or δ-opioid (DPDPE) agonists are shown. The number in parentheses indicates the percentage of cells demonstrating outward currents.
*p < 0.001; **p < 0.01; ***p < 0.05, indicates a significantly (χ2 analysis) greater number of cells expressing the indicated opioid receptor subtype and innervating the indicated hippocampal stratum.
Opioid receptor expression in interneurons innervating stratum lacunosum-moleculare
The μ-opioid agonist DAMGO was applied to seven cells that displayed dense primary axonal projections to stratum lacunosum-moleculare (i.e., OLM or horizontal cells). Three of these neurons (43%) responded with outward currents, suggesting the expression of μ-opioid receptors on these cells (Table 2). Overall, only 3 of the 25 cells (12%) responding to DAMGO displayed these morphological characteristics. The δ agonist DPDPE was applied to 15 cells demonstrating OLM cell morphology. Clear outward currents were observed in 11 (73%) of these neurons. Figure4 shows reconstructions of OLM cells that were exposed to the μ and/or the δ agonist, illustrating outward currents produced by DPDPE. Statistical analysis revealed that a significantly greater proportion of these dendritically projecting OLM cells were sensitive to the δ- versus the μ-opioid agonist (χ2 = 4.5; p < 0.05).
Some of the of the OLM cells expressing δ-opioid receptors possessed axons that exited the CA1 region, crossed the hippocampal fissure, and entered the dentate gyrus (Figs. 4B,5A). In addition, the axon of the δ-sensitive cell shown in Figure 5A ramified extensively in stratum lacunosum-moleculare of CA3. Because cells of this type may inhibit both CA1 and CA3 pyramidal neurons, they have been labeled “back-projection cells” (Sik et al., 1995). It has been suggested that back-projection cells may differ from more typical OLM cells by the conspicuous absence of Ih (Sik et al., 1995), although extensive analysis has not been possible because of their limited numbers. However, the neuron shown in Figure 5displayed prominent inward rectification upon hyperpolarization of the membrane that is indicative of Ih (Fig.5B). Therefore, at least some presumed back projection interneurons appear to express these ion channels.
Opioid receptor expression in interneurons innervating stratum radiatum
The μ agonist DAMGO was applied to 12 interneurons that possessed axons that arborized, primarily in stratum radiatum. Six (50%) of these neurons responded with outward currents (Table 2). When the data were analyzed with regard to the total number of cells expressing μ-opioid receptors projecting to all CA1 layers, it was found that 24% (6 of 25) of these neurons projected to stratum radiatum. Similar to μ receptor expression, DPDPE generated outward currents in 6 of 11 (55%) interneurons that displayed primary axon arborizations in stratum radiatum (Table 2). Overall, 6 of 32 (19%) of the total DPDPE-sensitive cells exhibited primary axon innervation of stratum radiatum. Two reconstructed interneurons within this class are shown in Figure 6. Each of these neurons generated outward currents in response to DPDPE application, whereas only the interneuron shown in Figure 6A responded to DAMGO. In addition, the cell shown in Figure 6Adisplayed a robust secondary projection within stratum oriens. Statistical analysis revealed no significant difference in μ- or δ-opioid receptor expression in these interneurons with primary axon projections in stratum radiatum (χ2 = 0.08;p > 0.05).
Opioid receptor expression in interneurons innervating stratum oriens
Many of the neurons demonstrating heavy axonal arborization in stratum oriens also displayed significant innervation of stratum radiatum (Fig. 7). However, these cells were classified according to the location of the densest axon projection. The μ agonist DAMGO was applied to 16 interneurons in which the primary axon projection was confined to stratum oriens. Only 6 (38%) of these interneurons displayed outward currents meeting the minimum criterion (Table 1). Overall, 6 of 25 (24%) of all DAMGO-sensitive cells demonstrated primary axonal arborization within this cell layer. The δ agonist DPDPE was applied to 17 interneurons with primary axon projections within stratum oriens. In contrast to the μ-sensitive cells, the majority of these neurons (13 of 17, 76%) demonstrated robust outward currents (Table 2). Of all of the classes of interneurons expressing δ-opioid receptors, 13 of 32 (41%) exhibited this morphology. Statistical analysis revealed a significant difference in these stratum oriens-projecting interneurons with regard to μ- or δ-opioid receptor expression (χ2 = 7.5;p < 0.01). These data suggest that interneurons with somata and primary axonal projections confined to stratum oriens were more likely to express δ- rather than μ-opioid receptors.
Taken together, the above results supported the hypothesis that GABAergic interneurons providing inhibitory input to different areas of the pyramidal neuron membrane differentially express μ- or δ-opioid receptors, as defined by the generation of outward currents. This suggests that activation of these receptor subtypes can selectively modulate inhibition impinging on these distinct membrane domains.
DISCUSSION
It has become increasingly clear that the location of synaptic input to different regions of the neuronal membrane can strongly affect the integrative functions of postsynaptic cells in the CNS (Calloway et al., 1995; Cobb et al., 1995; Gulyas et al., 1993a; Miles et al., 1996;Tsubokawa and Ross, 1996). The differential modulation of the activity of inhibitory neurons providing input to these discrete membrane areas probably occurs via innervation by select transmitter-containing afferents and the expression of distinct sets of neurotransmitter receptors (Freund and Buzsáki, 1996). Consistent with this idea, the present study provides data to suggest that opioid receptor subtype expression can distinguish subpopulations of hippocampal interneurons based on the locations and patterns of their axonal arborizations in relation to the pyramidal cell membrane. This implies that μ- or δ-opioid receptors can reduce the inhibitory input to distinct regions of the pyramidal neuron, thereby altering the integrative behavior of these postsynaptic cells.
The segregation of inhibitory neurons based on the location of their primary axon arbor revealed that those neurons providing perisomatic input to pyramidal neurons (basket and axo-axonic cells) more frequently expressed μ-opioid receptors. This was based on the observation that 91% of those neurons innervating stratum pyramidale and exposed to the μ agonist DAMGO responded with outward currents. Conversely, these cells did not appear to abundantly express δ-opioid receptors because outward currents were generated in only 22% of the neurons in response to δ agonist application. The difference in the expression of these receptors is even more striking when one considers that 40% of all of the μ-opioid-sensitive interneurons encountered in this study demonstrated primary axon arborizations in stratum pyramidale, whereas only 6% of the δ-opioid-sensitive cells innervated this layer. These results suggest that pyramidal neuron output may be increased by the μ-opioid receptor-mediated decrease in perisomatic GABAergic inhibition.
The functional significance of the differential targeting of the postsynaptic membrane by μ- and δ-opioid receptor-expressing cells is made clear by studies examining the roles these distinct forms of inhibition play in modulating the activity of pyramidal neurons. It is known that inhibitory cells synapsing on the perisomatic region of the pyramidal neuron can inhibit the generation of Na+-dependent action potentials (Miles et al., 1996) and remove the inactivation of inward cation currents, thus permitting the synchronous rebound firing of pyramidal neurons (Cobb et al., 1995). Furthermore, since, in the rodent hippocampus, perisomatic cells synapse on between 1000 and 2500 pyramidal cells, separated by as much as 1 mm (Li et al., 1992; Buhl et al., 1994a; Sik et al., 1995; Freund and Buzsáki, 1996; Miles et al., 1996; Fig. 3B this study), synchronization of activity among those pyramidal neurons likely occurs over this relatively large distance (Cobb et al., 1995;Whittington et al., 1998). Because μ-opioid receptors appear to be preferentially expressed on perisomatic interneurons, it is likely that activation of these receptors will diminish the negative modulation of Na+ action potentials and reduce the synchronous activity entrained by these cells. Recent data demonstrating that μ-, but not δ-opioid, receptor activation can disrupt synchronous long-range γ oscillations in the CA1 area of hippocampal slices support this assertion (Whittington et al., 1998).
Another result that might be expected from the reduction of perisomatic GABAergic input to pyramidal neurons and the desynchronization of their firing rates is the appearance of epileptiform activity. In experiments measuring pyramidal neuron excitability using population spikes, the activation of μ-opioid receptors consistently produced epileptiform afterdischarges, whereas δ-opioid receptor activation resulted in more limited increases in excitability (Wimpey et al., 1989; Lupica and Dunwiddie, 1991). Furthermore, selective μ-opioid receptor agonists are known to reduce electrically evoked IPSPs recorded in CA1 pyramidal neurons, whereas δ-opioid agonists have no effect on these responses (Lupica et al., 1992; Watson and Lanthorn, 1993; Lupica, 1995). This suggests that IPSPs generated by electrical stimulation may be derived from interneurons providing perisomatic input expressing μ-, and not δ-opioid receptors. We hypothesize that the epileptiform activity observed in pyramidal neurons after μ-opioid receptor activation reflects the inhibition of interneurons providing global GABAergic innervation of the somatic pyramidal cell membrane, whereas more limited increases in excitability generated by δ receptor activation are likely caused by a reduction in the dendritic inhibition of excitatory inputs.
In contrast to the selective innervation of the perisomatic pyramidal membrane by interneurons expressing μ-opioid receptors, those inhibitory cells providing input to the dendritic region of the pyramidal cell were more likely to express δ-opioid receptors, as defined by the generation of outward currents by DPDPE. This was true of the cells innervating the proximal basal dendritic region of stratum oriens where 76% of the neurons exposed to the δ agonist demonstrated outward currents and only 38% were sensitive to μ-opioid receptor activation. Similarly, those inhibitory cells with axons targeting the distal apical dendrites of the CA1 pyramidal neurons in stratum lacunosum-moleculare (OLM or horizontal cells) were also significantly more likely to express δ, rather than μ-opioid receptors (73 vs 43%, respectively). This suggests that δ-opioid receptors, rather than modulating pyramidal neuron output by regulating somatic inhibition, may more selectively modulate the excitatory inputs to these cells by reducing dendritic inhibition.
Interneurons possessing axons targeting the proximal basal and proximal apical pyramidal neuron dendrites in strata oriens and radiatum have been referred to as “bistratified cells” (Buhl et al., 1994a, Figs.6 and 7, this study). The somata of these cells give rise to axons terminating in parallel with excitatory Schaffer collateral/commissural afferents on the CA1 pyramidal neuron dendrites. Therefore, bistratified cells are believed to selectively modulate these excitatory inputs. This suggests that inhibition of bistratified neuron activity by δ-, and to a lesser degree μ-opioid, receptor activation, would facilitate these excitatory inputs impinging on the pyramidal neuron proximal dendrites. Furthermore, because the pyramidal neuron IPSPs elicited by bistratified cell activation are significantly slower to rise and decay, when compared with perisomatic cells (Buhl et al., 1994a), these neurons may selectively modulate the slower excitatory currents derived from NMDA receptor activation. In this way the opioid-mediated inhibition of bistratified cell activity may facilitate this excitatory input and the synaptic plasticity associated with NMDA receptor activation, as demonstrated in lateral perforant path long-term potentiation (Xie and Lewis, 1991).
In contrast to the relative paucity of knowledge of bistratified cell function, the role that the OLM interneurons play in modulating pyramidal neuron excitability is becoming increasingly clear. OLM cells are activated via excitatory inputs from pyramidal neuron recurrent collateral axons (Lacaille et al., 1987; Blasco-Ibanez and Freund, 1995; Maccaferri and McBain, 1995; Ali and Thomson, 1998), and they selectively innervate the distal apical dendrites of the pyramidal cells. Here the inhibitory OLM terminals overlap with excitatory terminals originating from cells in the entorhinal cortex and the thalamus (Gulyas et al., 1993b; Desmond et al., 1994; Sik et al., 1995;Yanovsky et al., 1997). These neurons are considered “feedback” or recurrent inhibitory cells because they are only activated after CA1 pyramidal neuron discharge. The activation of OLM neurons via antidromic stimulation of pyramidal neurons axons can inhibit the EPSPs recorded extracellularly in stratum lacunosum-moleculare after the stimulation of the entorhinal cortex pathway (Maccaferri and McBain, 1995). This inhibition of entorhinal input to CA1 was also found to be selective, because EPSPs generated by stimulation of stratum radiatum were not affected by OLM neuron activation. These data suggest that, when activated, the OLM cells may selectively reduce the excitatory drive from entorhinal cortex to CA1, thus facilitating the flow of information through the classic trisynaptic hippocampal circuit (Macafferri and McBain, 1995; Ali and Thomson, 1998). Conversely, the inhibition of OLM neurons by δ-opioid receptor activation, or long-term synaptic depression (LTD, Macafferri and McBain, 1995), would likely result in enhanced entorhinal EPSPs in the pyramidal neurons and the diversion of information flow through the direct entorhinal circuit. In this way, δ-opioid receptor activation may provide a means through which CA1 pyramidal neurons can receive either entorhinal- or CA3-derived synaptic information.
Together our data suggest that the differential expression of opioid receptor subtypes on interneurons that provide segregated inhibitory inputs to the pyramidal cell membrane may constitute a means to permit the independent regulation of these projections. Thus, interneurons that were inhibited by μ-opioid receptor activation innervated stratum pyramidale more frequently than δ-opioid-sensitive cells, and therefore, would be more likely to facilitate pyramidal neuron output. In contrast, interneurons inhibited by δ-opioid receptor activation were more likely to innervate the basal and apical dendritic pyramidal neuron membrane, suggesting a role for these receptors in the regulation of afferent signaling. However, a complete understanding of the significance of these findings awaits identification of the conditions through which opioid peptides are released and processed into subtype selective agonists. Our data also more generally imply that hippocampal interneurons can be segregated not only according to morphological and neurochemical criteria, but also according to the expression of subtypes of neurotransmitter receptors. Because the hippocampal interneurons can influence the properties of large populations of principal cells, the differential activation of opioid receptor subtypes may provide a substrate on which the integrative properties of these cells can be changed to accommodate specific computational processes within this brain region.
Footnotes
This work was supported by National Institutes of Health Grants DA 07725 and MH 44212, United States Public Health Service.
Correspondence should be addressed to Dr. Carl R. Lupica, National Institute on Drug Abuse, Intramural Research Program, 5500 Nathan Shock Drive, Baltimore, MD 21224.
REFERENCES
- 1.Ali AB, Thomson AM. Facilitating pyramid to horizontal oriens-alveus interneurone inputs- Dual intracellular recordings in slices of rat hippocampus. J Physiol (Lond) 1998;507:185–199. doi: 10.1111/j.1469-7793.1998.185bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali AB, Deuchars J, Pawelzik H, Thomson AM. CA1 pyramidal to basket and bistratified cell EPSPs: dual intracellular recordings in rat hippocampal slices. J Physiol (Lond) 1998;507:201–217. doi: 10.1111/j.1469-7793.1998.201bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergles DE, Doze VA, Madison DV, Smith SJ. Excitatory actions of norepinephrine on multiple classes of interneurons. J Neurosci. 1996;16:572–585. doi: 10.1523/JNEUROSCI.16-02-00572.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release site. Nature. 1994a;368:823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- 5.Buhl EH, Han Z, Lorinczi Z, Stezhka VV, Karnup SV, Somogyi P. Physiological properties of anatomically identified axo-axonic cells in the rat hippocampus. J Neurophysiol. 1994b;71:1289–1307. doi: 10.1152/jn.1994.71.4.1289. [DOI] [PubMed] [Google Scholar]
- 6.Buhl EH, Cobb SR, Halasy K, Somogyi P. Properties of unitary IPSPs evoked by anatomically identified basket cells in the rat hippocampus. Eur J Neurosci. 1995;7:1989–2004. doi: 10.1111/j.1460-9568.1995.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 7.Blasco-Ibanez JM, Freund TF. Synaptic input of horizontal interneurons in stratum oriens of the hippocampal CA1 subfield: structural basis of feedback activation. Eur J Neurosci. 1995;7:2170–2180. doi: 10.1111/j.1460-9568.1995.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 8.Calloway JC, Lasser-Ross N, Ross WN. IPSPs strongly inhibit climbing fiber activated [Ca2+]i increases in the dendrites of cerebellar Purkinje neurons. J Neurosci. 1995;15:2777–2788. doi: 10.1523/JNEUROSCI.15-04-02777.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceranik K, Bender R, Geiger JRP, Monyer H, Jonas P, Frotscher M, Lubke J. A novel type of GABAergic interneuron connecting the input and the output regions of the hippocampus. J Neurosci. 1997;17:5380–5394. doi: 10.1523/JNEUROSCI.17-14-05380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chikwendu A, McBain CJ. Two temporally overlapping “delayed-rectifiers” determine the voltage dependent potassium current phenotype in cultured hippocampal interneurons. J Neurophysiol. 1996;76:1477–1490. doi: 10.1152/jn.1996.76.3.1477. [DOI] [PubMed] [Google Scholar]
- 11.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 12.Cobb SR, Halasy K, Vida I, Nyiri G, Tamas G, Buhl EH, Somogyi P. Synaptic effects of identified interneurons innervating both interneurons and pyramidal cells in the rat hippocampus. Neuroscience. 1997;79:629–648. doi: 10.1016/s0306-4522(97)00055-9. [DOI] [PubMed] [Google Scholar]
- 13.Cohen GA, Doze VA, Madison DV. Opioid inhibition of GABA release from presynaptic terminals of rat hippocampal interneurons. Neuron. 1992;9:325–335. doi: 10.1016/0896-6273(92)90171-9. [DOI] [PubMed] [Google Scholar]
- 14.Commons KG, Milner TA. Localization of Delta opioid receptor immunoreactivity in interneurons and pyramidal cells in the rat hippocampus. J Comp Neurol. 1997;381:373–387. [PubMed] [Google Scholar]
- 15.Desmond NL, Scott CA, Jane JA, Jr, Levy WB. Ultrastructure identification of entorhinal cortical synapses in CA1 stratum lacunosum-moleculare of the rat. Hippocampus. 1994;4:594–600. doi: 10.1002/hipo.450040509. [DOI] [PubMed] [Google Scholar]
- 16.Dodt HU, Zieglgansberger W. Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Res. 1990;537:333–336. doi: 10.1016/0006-8993(90)90380-t. [DOI] [PubMed] [Google Scholar]
- 17.Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an α-bungarotoxin sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- 19.Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 20.Freund TF, Gulyas AI, Ascady L, Gorcs T, Toth K. Serotonergic control of the hippocampus via local inhibitory interneurons. Proc Natl Acad Sci USA. 1990;87:8501–8505. doi: 10.1073/pnas.87.21.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuzesi M, Racz B, Elias B, Halasy K. Enkephalinergic nerve terminals target inhibitory interneurons in the rat hippocampus. NeuroReport. 1997;8:2471–2475. doi: 10.1097/00001756-199707280-00012. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein A, Naidu A. Multiple opioid receptors: ligand selectivity profiles and binding site signatures. Mol Pharmacol. 1989;36:265–272. [PubMed] [Google Scholar]
- 23.Gulyas AI, Miles R, Hajos N, Freund TF. Precision and variability in post-synaptic target selection of inhibitory cells in the hippocampal CA3 region. Eur J Neurosci. 1993a;5:1729–1751. doi: 10.1111/j.1460-9568.1993.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 24.Gulyas AI, Miles R, Sik A, Toth K, Tamamaky N, Freund TF. Hippocampal pyramidal cells excite inhibitory neurons through a single release site. Nature. 1993b;366:683–687. doi: 10.1038/366683a0. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;368:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- 26.Lacaille JC, Mueller AL, Kunkel DD, Schwartzkroin PA. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci. 1987;7:1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HK, Dunwiddie TV, Hoffer BJ. Electrophysiological interactions of enkephalins with neuronal circuitry in the rat hippocampus II. Effects on interneuron excitability. Brain Res. 1980;184:331–342. doi: 10.1016/0006-8993(80)90802-1. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Somogyi P, Tepper JM, Buzsáki G. Axonal and dendritic arborization of an intracellularly labeled chandelier cell in the CA1 region of the hippocampus. Exp Brain Res. 1992;90:519–525. doi: 10.1007/BF00230934. [DOI] [PubMed] [Google Scholar]
- 29.Lupica CR. μ and δ enkephalins inhibit spontaneous GABA- mediated IPSCs via a cyclic AMP-independent mechanism in the rat hippocampus. J Neurosci. 1995;15:737–749. doi: 10.1523/JNEUROSCI.15-01-00737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lupica CR, Dunwiddie TV. Differential effects of mu- and delta- receptor selective opioid agonists on feedforward and feedback GABAergic inhibition in hippocampal brain slices. Synapse. 1991;8:237–248. doi: 10.1002/syn.890080402. [DOI] [PubMed] [Google Scholar]
- 31.Lupica CR, Proctor WR, Dunwiddie TV. Dissociation of μ and δ opioid receptor-mediated reductions in evoked and spontaneous synaptic inhibition in the rat hippocampus in vitro. Brain Res. 1992;593:226–238. doi: 10.1016/0006-8993(92)91312-3. [DOI] [PubMed] [Google Scholar]
- 32.Maccaferri G, McBain CJ. Passive propagation of LTD to stratum oriens-alveus inhibitory neurons modulates the temperoammonic input to the hippocampal CA1 region. Neuron. 1995;15:137–145. doi: 10.1016/0896-6273(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 33.Maccaferri G, McBain CJ. The hyperpolarization-activated current Ih and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurons. J Physiol (Lond) 1996;497:119–130. doi: 10.1113/jphysiol.1996.sp021754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madison DV, Nicoll RA. Enkephalin hyperpolarizes interneurones in the rat hippocampus. J Physiol (Lond) 1988;398:123–130. doi: 10.1113/jphysiol.1988.sp017033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned μ opioid receptor in the rat CNS. J Chem Neuroanat. 1995;8:283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- 36.Masukawa LM, Prince DA. Synaptic control of excitability in isolated dendrites of hippocampal neurons. J Neurosci. 1984;4:217–227. doi: 10.1523/JNEUROSCI.04-01-00217.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McBain CJ, Dingledine R. Heterogeneity of synaptic glutamate receptors on CA3 stratum radiatum interneurones of rat hippocampus. J Physiol (Lond) 1993;462:373–392. doi: 10.1113/jphysiol.1993.sp019560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McBain CJ, DiChiara TJ, Kauer JA. Activation of metabotropic glutamate receptors differentially affects two classes of hippocampal interneurons and potentiates excitatory synaptic transmission. J Neurosci. 1994;14:4433–4445. doi: 10.1523/JNEUROSCI.14-07-04433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMahon LL, Kauer JA. Hippocampal interneurons are excited via serotonin-gated ion channels. J Neurophysiol. 1997;78:2493–2502. doi: 10.1152/jn.1997.78.5.2493. [DOI] [PubMed] [Google Scholar]
- 40.Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 41.Miller KK, Hoffer A, Svoboda KR, Lupica CR. Cholecystokinin increases GABA release by inhibiting a resting K+ conductance in hippocampal interneurons. J Neurosci. 1997;17:4994–5003. doi: 10.1523/JNEUROSCI.17-13-04994.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morales M, Bloom FE. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci. 1997;17:3157–3167. doi: 10.1523/JNEUROSCI.17-09-03157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosberg HI, Hurst R, Hruby VJ, Gee K, Yamamura HI, Galligan JJ, Burks TF. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci USA. 1983;80:5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicoll RA, Alger BE, Jahr CE. Enkephalin blocks inhibitory pathways in the vertebrate CNS. Nature. 1980;287:22–25. doi: 10.1038/287022a0. [DOI] [PubMed] [Google Scholar]
- 45.Parra P, Gulyas AI, Miles R. How many subtypes of inhibitory cells in the hippocampus? Neuron. 1998;20:983–993. doi: 10.1016/s0896-6273(00)80479-1. [DOI] [PubMed] [Google Scholar]
- 46.Siggins GR, Zieglgansberger W. Morphine and enkephalin peptides reduce inhibitory synaptic potentials in hippocampal pyramidal cells without alteration of membrane potential. Proc Natl Acad Sci USA. 1981;78:5235–5239. doi: 10.1073/pnas.78.8.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sik A, Penttonen M, Ylinen A, Buzsáki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci. 1995;15:6651–6665. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Svoboda KR, Lupica CR. Opioid inhibition of hippocampal interneurons via modulation of potassium and hyperpolarization-activated cation (Ih) currents. J Neurosci 18 1998. 7084 7089, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsubokawa H, Ross WN. IPSPs modulate spike backpropagation and associated [Ca2+]i changes in the dendrites of hippocampal CA1 pyramidal neurons. J Neurophysiol. 1996;76:2896–2906. doi: 10.1152/jn.1996.76.5.2896. [DOI] [PubMed] [Google Scholar]
- 50.Watson GB, Lanthorn TH. Electrophysiological actions of delta opioids in CA1 of the rat hippocampal slice are mediated by one delta receptor subtype. Brain Res. 1993;601:129–135. doi: 10.1016/0006-8993(93)91703-u. [DOI] [PubMed] [Google Scholar]
- 51.Whittington MA, Traub RD, Faulkner HJ, Jefferys JGR, Chettiar K. Morphine disrupts long range synchrony of gamma oscillations in hippocampal slices. Proc Natl Acad Sci USA. 1998;95:5807–5811. doi: 10.1073/pnas.95.10.5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 53.Wimpey TL, Chavkin C. Opioids activate both an inward rectifier and a novel voltage-gated potassium conductance in the hippocampal formation. Neuron. 1991;6:281–289. doi: 10.1016/0896-6273(91)90363-5. [DOI] [PubMed] [Google Scholar]
- 54.Wimpey TL, Opheim KE, Chavkin C. Effects of chronic morphine administration on the mu and delta opioid responses in the CA1 region of the rat hippocampus. J Pharmacol Exp Ther. 1989;251:405–411. [PubMed] [Google Scholar]
- 55.Xie C-W, Lewis DV. Opioid-mediated facilitation of long-term potentiation at the lateral perforant path-dentate granule cell synapse. J Pharmacol Exp Ther. 1991;256:289–296. [PubMed] [Google Scholar]
- 56.Yanovsky Y, Sergeeva OA, Freund TF, Haas HL. Activation of interneurons at the stratum oriens/alveus border suppress excitatory transmission to apical dendrites in the CA1 area of the mouse hippocampus. Neuroscience. 1997;77:87–96. doi: 10.1016/s0306-4522(96)00461-7. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L, McBain CJ. Voltage-gated potassium currents in stratum oriens-alveus inhibitory neurones of the rat CA1 hippocampus. J Physiol (Lond) 1995;488:647–660. doi: 10.1113/jphysiol.1995.sp020997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zieglgansberger W, French ED, Siggins GR, Bloom FE. Opioid peptides may excite hippocampal pyramidal neurons inhibiting adjacent inhibitory interneurons. Science. 1979;205:415–417. doi: 10.1126/science.451610. [DOI] [PubMed] [Google Scholar]