Abstract
We have identified the Caenorhabditis eleganshomolog of the mammalian vesicular monoamine transporters (VMATs); it is 47% identical to human VMAT1 and 49% identical to human VMAT2.C. elegans VMAT is associated with synaptic vesicles in ∼25 neurons, including all of the cells reported to contain dopamine and serotonin, plus a few others. When C. elegans VMAT is expressed in mammalian cells, it has serotonin and dopamine transport activity; norepinephrine, tyramine, octopamine, and histamine also have high affinity for the transporter. The pharmacological profile of C. elegans VMAT is closer to mammalian VMAT2 than VMAT1. The C. elegans VMAT gene iscat-1; cat-1 knock-outs are totally deficient for VMAT immunostaining and for dopamine-mediated sensory behaviors, yet they are viable and grow relatively well. Thecat-1 mutant phenotypes can be rescued by C. elegans VMAT constructs and also (at least partially) by human VMAT1 or VMAT2 transgenes. It therefore appears that the function of amine neurotransmitters can be completely dependent on their loading into synaptic vesicles.
Keywords: C. elegans, VMAT, vesicular transporter, cat-1, dopamine transport, serotonin transport
The loading of catecholamines and other biogenic amines into synaptic vesicles (and other types of release vesicles) is mediated by specific vesicular monoamine transporters (VMATs). Amine transport requires a pH gradient and is coupled to proton antiport (for review, see Schuldiner et al., 1995). In mammals, two related transport proteins (and genes) have been identified: VMAT1 is often found in neuroendocrine cells, and VMAT2 is primarily neuronal (Erickson et al., 1992, 1996; Liu et al., 1992;Weihe et al., 1994). Recombinant VMATs have been shown to mediate the transport of dopamine, norepinephrine, epinephrine, serotonin, and histamine (VMAT2 only) in vitro, as expected from previous biochemical studies on chromaffin granules and brain synaptic vesicles (Schuldiner et al., 1995; Erickson et al., 1995, 1996). The proteins have been used as markers of particular cell types, and specific antibodies raised against these transporters have been of use in studies of vesicular localization and maturation (Liu et al., 1994;Weihe et al., 1994; Nirenberg et al., 1995).
To study the role(s) of VMAT proteins in neuronal and behavioral function, we have been using an experimental system amenable to gene knock-out and transgenic technology in which specific biogenic amines are used by identified cells involved in particular behaviors. The simple soil nematode Caenorhabditis elegans contains and uses several biogenic amines (for review, see Rand and Nonet, 1997a). Dopamine has been identified biochemically, and the technique of formaldehyde-induced fluorescence (FIF) was used to localize dopamine to particular C. elegans neurons (Sulston et al., 1975). Two behaviors, locomotion and egg laying, are transiently inhibited by exogenous dopamine (Schafer and Kenyon, 1995). Serotonin has also been identified in C. elegans neurons by FIF (Horvitz et al., 1982) and by anti-serotonin immunostaining (Desai et al., 1988;McIntire et al., 1992). Exogenous serotonin stimulates egg laying and pharyngeal pumping and inhibits locomotion and defecation (Horvitz et al., 1982; Ségalat et al., 1995). Serotonin is also required for male mating behaviors (Loer and Kenyon, 1993).
Octopamine (p-hydroxyphenylethanolamine) has been detected in C. elegans extracts (Horvitz et al., 1982), although it is not yet known which cells contain this compound. Exogenous octopamine stimulates movement and inhibits egg laying; its biological actions thus appear to antagonize those of serotonin (Horvitz et al., 1982). Other biogenic amines, such as epinephrine, norepinephrine, and histamine, have not yet been identified as putative transmitters in C. elegans.
We now report the presence of a VMAT homolog in C. elegans. We demonstrate that it is associated with synaptic vesicles of known biogenic amine-containing neurons, and that it can function as an amine transporter when expressed in mammalian cells. We also describe VMAT-deficient mutants and their phenotypes, and we show that expression of the vesicular transporter is required for proper function of identified dopamine-containing neurons. Finally, we present evidence for partial phenotypic rescue of C. elegans VMAT mutants by transgenic expression of human VMAT1 or VMAT2.
MATERIALS AND METHODS
Growth and culture
C. elegans were grown on nematode growth medium (NGM) as described by Brenner (1974), modified by the addition of streptomycin and mycostatin to reduce contamination and the use of the streptomycin-resistant bacterial strain OP50/1 (Johnson et al., 1988). Strains containing cat-1(e1111), egl-6(n592),egl-13(n483), egl-14(n549), dpy-1(e1),sup-5(e1464), sup-5(e1877),tra-2(e2046), unc-86(e1416), andunc-104(e1265) were obtained from theCaenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN); cat-1(n733) was a gift from Beth Sawin and Bob Horvitz (Massachusetts Institute of Technology, Cambridge, MA);pha-1(e2123) was a gift from Heinke and Ralf Schnabel (Max-Plank-Institute fur Biochemie, Martinsried, Germany); and strains containing mgIs18 [Pttx-3-green fluorescent protein (GFP)] and mgIs21 (Plin-11-GFP) were gifts from Oliver Hobert (Harvard University, Cambridge, MA).
Molecular methods
Oligonucleotide primers were designed from the predicted open reading frame of the partial VMAT homolog on cosmid W01C8 and were used to amplify a predicted 720-bp fragment from a C. eleganscDNA library prepared in λ-Zap II (a gift from Bob Barstead, Oklahoma Medical Research Foundation). This fragment was then used to probe the same cDNA library (to obtain a full-length cDNA) as well as a C. elegans genomic library prepared in λ-Dash (gift from Heidi Browning and Tom Blumenthal, Indiana University, Bloomington, IN). DNA sequence was determined using the fmol DNA cycle sequencing system (Promega, Madison, WI). The cDNA was completely sequenced on both strands. Sequence alignment and analysis were performed with the Genetics Computer Group (Madison, WI) Wisconsin package (version 8, September 1994). Mutations were analyzed by amplification of specificcat-1 genomic regions using direct “single-worm PCR” from individual mutant animals (Barstead and Waterston, 1991), followed by sequencing of the purified PCR product with nested primers. The altered sequence associated with the n733 missense mutation was engineered into the wild-type cDNA using the QuickChange system (Stratagene, La Jolla, CA). The resulting mutant cDNA (referred to as CelVMAT/n733 in this study) was then sequenced to assure that no other nucleotide changes were present.
Induced fluorescence
Histofluorescence of monoamines was performed with a modified version of the sucrose-potassium phosphate-glyoxylic acid (SPG) reaction of de la Torre (1980). Nematodes were collected in drops of water on poly-l-lysine-coated slides and slightly compressed by a second slide. The slide sandwich was immediately placed on dry ice for 10–60 min. The frozen slides were separated, and the bottom slide with adherent nematodes was immediately placed in ice-cold SPG solution (0.2 m sucrose, 235 mmKH2PO4, and 1% glyoxylic acid, pH 7.4) for 7.5 min. Slides were dried under a cool hair dryer for 10–30 min. Light mineral oil was placed on the slide, and it was incubated at 95°C for 2.5 min. Coverslipped slides were observed with a Zeiss (Thornwood, NY) Axiophot microscope using 4′,6-diamidino-2-phenylindole (DAPI) and fluorescein isothiocyanate (FITC) filters.
Primary antibodies
VMAT. Antisera were raised against two synthetic peptides. PEP1 (VELRQNGDSRVTNEN) was derived from the C-terminal region of C. elegans VMAT (amino acids 514–528). The peptide was manufactured either as a multiple antigenic peptide (MAP) (Posmett et al., 1988) or with an N-terminal cysteine, which was used to couple the peptide to maleimide-activated keyhole limpet hemocyanin (KLH; Imject, Pierce, Rockford, IL). Goats were immunized with the peptide in MAP form, the peptide coupled to KLH, or a combination of the two forms of peptide; rabbits were immunized with KLH-coupled peptide. Three goats and two of three rabbits yielded specific antisera.
A second peptide, PEP2 (KIDRGEPEGSSIKQ), derived from a region between putative transmembrane domains 6 and 7 (amino acids 302–315 ofC. elegans VMAT), was synthesized with an N-terminal cysteine. PEP2 was coupled to KLH and used to immunize two goats and two rabbits. One rabbit yielded specific antisera.
To purify the sera, glutaraldehyde was used to cross-link the peptides to goat serum albumin or rabbit serum albumin (Harlow and Lane, 1988). Cross-linked peptide was bound to nitrocellulose membranes with methanol (Smith and Fisher, 1984). Antisera from goats were incubated with peptide cross-linked to goat serum albumin; antisera from rabbits were incubated with peptide cross-linked to rabbit serum albumin. After incubation and rinsing, bound antibody was eluted with a low-pH, high-salt wash (5 mm glycine, 0.5 m NaCl, pH 2.3) followed by a high-pH wash (50 mm TEA, pH 11.5). After neutralization with Tris buffer, the sera were exchanged into PBS and concentrated using Centriprep 30 ultrafiltration (Amicon, Beverly, MA).
The reported staining pattern was obtained with antisera generated against both PEP1 and PEP2. Most cell identification was done with antisera against PEP1-MAP (goat 258), because this serum gave the most specific signal with indirect immunofluorescence. The specific staining described below was eliminated by preincubation of the sera with the appropriate uncoupled peptide.
Serotonin. Rabbit antibody to formaldehyde-conjugated serotonin was purchased from Dr. Harry Steinbusch (Free University, Amsterdam, The Netherlands).
Anti-human VMATs. Rabbit antibodies to human VMAT1 and VMAT2 were generated against specific C-terminal peptides, as previously described (Erickson et al., 1996).
Anti-GFP. Rabbit antibody to GFP was purchased from Molecular Probes (Eugene, OR).
Immunocytochemical staining
Nematodes were prepared with a variation of the freeze–crack method of Albertson (1984). Mixed populations of nematodes were rinsed and placed in a water drop on a poly-l-lysine-coated slide (made by incubating acid-cleaned slides for 5 min in 1–2 mg/ml poly-l-lysine). A second poly-l-lysine-coated slide was placed on top of the nematodes so that the nematodes were compressed. The slide sandwich was immediately placed on a piece of dry ice for at least 20 min. The slides were separated, and the bottom slide was immediately placed in ice-cold fixative. For VMAT (or VMAT and GFP), the fixation consisted of 2 min in methanol followed by 4 min in acetone. For anti-serotonin, fixation was done in 4% formaldehyde in 0.1 m phosphate for 24 hr.
After fixation, slides were rinsed in PBS. All slides were incubated in 10% donkey serum in antibody buffer (0.5% Triton X-100, 1 mm EDTA, and 0.1% BSA in PBS with 0.05% sodium azide) for 1 hr. Primary antibody incubations (1:50–1:200) were done overnight. After thorough rinsing with antibody buffer, slides were incubated in secondary antibody for 4 hr. Unlabeled and indocarbocyanine (Cy3)-labeled secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA); Oregon Green 488 was coupled to secondary antibodies using the Oregon Green labeling kit from Molecular Probes. After rinsing, slides were mounted in antibleaching medium (Finney and Ruvkun, 1990).
Uptake assays
Functional expression of VMAT cDNAs was obtained using the vaccinia virus/T7 hybrid system (Fuerst et al., 1986). The transport of [3H]serotonin and [3H]dopamine was measured in digitonin-permeabilized CV-1 cells expressing the cDNAs essentially as described by Erickson and Eiden (1993). Labeled substrates were purchased from DuPont NEN (Boston, MA); final concentrations in the assay were 63 nm for dopamine and 130 nm for serotonin. Assays were performed at 37°C for consistency with previously published studies; however, assays performed at 25°C (the maximum permissible growth temperature for C. elegans) gave qualitatively similar results (data not shown).
Behavioral assays
Body movement, pharyngeal pumping, and grazing were all measured on young adult hermaphrodites raised at 20–25°C. At these temperatures, only individuals of the transgenic lines that carry the extrachromosomal array in the cells of the pharynx during midembryogenesis will survive.
For reserpine treatment, 5 μl of reserpine (50 mm in acetic acid) was diluted in 395 μl of M9 buffer and was poured over the surface of a 6 cm NGM plate (with or without food). Plates were used after at least 15 min, when the fluid had been absorbed by the agar and/or had evaporated; plates remained potent for several days. For behavioral assays, nematodes were raised and monitored on reserpine treated plates.
Movement on and off food was assayed as described (Sawin, 1996), except that nematodes were rinsed by transferring them to a thin layer of S-basal buffer on an NGM plate for 1 min before transferring them to the test plates. Pharyngeal pumping rates on and off food were quantified as previously described (Miller et al., 1996) for 100 hermaphrodites of each phenotype. Thrashing assays were performed as described by Miller et al. (1996), except that counting was done at room temperature (∼23°C) in M9 on 6 cm NGM plates.
“Grazing” behavior was evaluated as follows. Groups of 10–30 nematodes were transferred with a metal pick to a new plate with a thin central streak of bacterial lawn. The nematodes were placed at the edge of the plate, ∼1.5 cm from the lawn. As the nematodes moved outward from the point of transfer, they spontaneously encountered the edge of the lawn. We measured the time between when the tip of the snout entered the food and the tip of the tail entered the food. Wild-type hermaphrodites generally slow their forward progression significantly when moving from a region of no visible bacteria into the bacterial lawn, so that many individuals take more than 1 min to fully enter the lawn. Generally, cat-1 animals do not change their speed when entering food. Spontaneous locomotion off food generally propels wild-type worms forward one body length every 3–5 sec. The percentage of individuals of a given phenotype that took ≥1 min to enter the lawn (grazers) was determined for each phenotype. Individuals of any phenotype taking >10 sec to move forward one body length after stimulation with a pick were excluded from the data. Individuals encountering the lawn <1 min after transfer to the assay plate were also excluded from the data, because the transfer itself could cause a temporary increase in locomotion.
To evaluate male mating behavior, individual males were put with individual immature tra-2 females onto 6 cm agar plates with an ∼1 cm central dot of bacteria. After maturation of thetra-2 female, the pairs were kept on the plate for 24–36 hr at room temperature. tra-2 females make no sperm (Hodgkin and Brenner, 1977), so all progeny are cross-progeny from successful mating(s) by the tested male. Wild-type males generally mate multiple times and produce >100 progeny, whereas fewer than half ofcat-1 males mate successfully, and almost none of them were able to sire >50 cross-progeny.
Transgenic methods
The genomic phage RM#424L was isolated as described above and contained the complete VMAT gene plus ∼3 kb of upstream sequence. The cDNA plasmids for transformation used the pPD49.26 vector (a gift from Andy Fire, Carnegie Institution, Washington, DC) with the C. elegans VAMP (synaptobrevin) promoter cloned into the first multiple cloning site and one of three cDNAs cloned into the second multiple cloning site. The VAMP gene is expressed in all neurons (Nonet et al., 1998); a 3 kb genomic clone containing the VAMP promoter was obtained from Mike Nonet (Washington University, St. Louis, MO) and was slightly modified to add some restriction sites at the 3′ end. The cDNAs used were derived from either the C. elegans VMAT (described above), the human VMAT1 (Erickson et al., 1996), or the human VMAT2 (Erickson and Eiden, 1993). DNA transformation methods forC. elegans were essentially those of Mello et al. (1991), except that a plasmid containing the wild-type pha-1 cDNA (a gift from Heinke and Ralf Schnabel, Max-Plank-Institute fur Biochemie) was used as a transformation marker. The pha-1(e2123) mutant is temperature-sensitive for embryogenesis; animals homozygous for this mutation will not hatch at 25°C but can grow normally at 16°C (Granato et al., 1994). The recipient strain for transformation had the genotype cat-1(e1111); pha-1(e2123) and was constructed in our laboratory. After injection, the recipient animals were transferred to 25°C to select for those progeny expressing the wild-type PHA-1 protein.
RESULTS
The C. elegans VMAT gene
The C. elegans Genome Sequencing Project reported the sequence of a cosmid (W01C8) containing part of a gene with similarity to mammalian VMATs. Using standard methods we isolated a genomic λ phage, RM#424L, which included this gene, and we isolated and sequenced the corresponding cDNA. This 1.8 kb cDNA was apparently full-length, based on the presence of a polyA tail at the 3′ end, and part of the SL1 trans-spliced leader sequence (Krause and Hirsh, 1987; Bektesh et al., 1988) at the 5′ end. Subsequent to our cDNA analysis, the C. elegans Genome Sequencing Project reported the sequence of cosmid E03E2, which overlaps W01C8 and contains the entire VMAT gene.
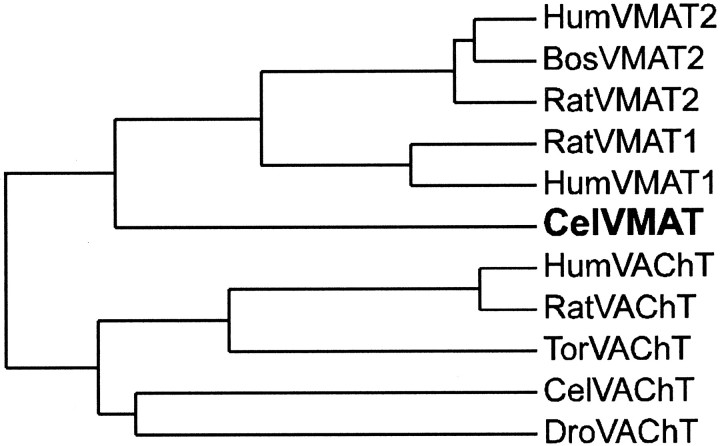
The deduced open reading frame of C. elegans VMAT encodes 553 amino acids with a predicted 12-transmembrane domain structure (Figs. 1 and2). There does not seem to be any obvious correlation between the exon organization of the gene and the domain structure of the protein. The deduced protein is 47% identical to human VMAT1 and 49% identical to human VMAT2 (Fig. 2) (Erickson and Eiden, 1993; Erickson et al., 1996). Inspection of the highly conserved region (which includes transmembrane domains 2–12) reveals 78 residues (of 344) where the human VMAT1 and VMAT2 sequences differ from each other; at 19 of those sites the C. elegans VMAT has the residue present in VMAT1, whereas at 26 of the sites the C. elegans VMAT has the residue present in VMAT2 (and at the remaining 33 sites all three proteins differ). The C. elegans protein thus has molecular features of both mammalian VMATs. This is consistent with the dendrogram of the members of the VMAT/VAChT protein family (Fig. 3), indicating that the C. elegans VMAT is comparably distant from the mammalian VMAT1 and VMAT2 classes.
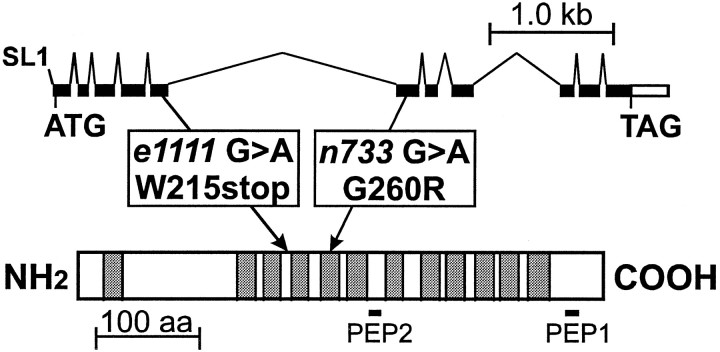
Fig. 1.
Structure of the C. elegans VMAT gene and protein. At the top are shown the splicing pattern and exon structure of the VMAT (cat-1) genomic region. Protein-coding regions are filled; the 5′- and 3′-untranslated regions are white. The positions of the initiation (ATG) and termination (TAG) codons are indicated, as well as the addition site of the trans-spliced SL1 leader RNA (Krause and Hirsh, 1987; Bektesh et al., 1988). The VMAT protein sequence is represented by the long rectangle at thebottom. The sites of the e1111 andn733 mutations are indicated, along with the associated sequence alterations. The 12 putative transmembrane domains of the protein are indicated by shading, and the locations of the two peptides (PEP1 and PEP2) used to raise anti-VMAT antiserum are shown below the rectangle.
Fig. 2.

Deduced protein sequence of C. elegans VMAT and alignment with the human VMAT1 (GenBank accession number U39905) and VMAT2 (GenBank accession number L23205) deduced proteins. The protein sequences were aligned using the PILEUP program (Genetics Computer Group Wisconsin package, version 8). TheCONSENSUS sequence uses uppercase letterswhen all three sequences agree and lowercase letterswhen two of the three sequences are the same. Dots are used in the three VMAT sequences to indicate gaps introduced by the alignment program; hyphens are used in the consensus sequence for sites where all three sequences differ. The 12 putative transmembrane domains, as suggested for the mammalian proteins (Erickson et al., 1992; Liu et al., 1992) are shown as double underlines below the consensus sequence. Putative glycosylation sites (in the large intravesicular loop between transmembrane domains 1 and 2) are shown as double underlines below each of the VMAT sequences.
Fig. 3.
Dendrogram of published VMAT and VAChT sequences. The PILEUP program (Genetics Computer Group Wisconsin package, version 8) was used. Bos, Bovine; Cel, C. elegans; Dro, Drosophila;Hum, human; Tor, Torpedo. The individual GenBank Accession numbers are HumVMAT2, L23205 (Erickson and Eiden, 1993); BosVMAT2, U02876 (Howell et al., 1994); RatVMAT2,L00603 (Erickson et al., 1992); RatVMAT1, M97380 (Liu et al., 1992); HumVMAT1, U39905 (Erickson et al., 1996); CelVMAT, present study; HumVAChT, U10554 (Erickson et al., 1994); RatVAChT, U09211 (Erickson et al., 1994); TorVAChT, U05591 (Varoqui et al., 1994); CelVAChT, L19621(Alfonso et al., 1993); and DroVAChT, AF030197 (Kitamoto et al., 1998).
The expressed C. elegans protein has VMAT activity
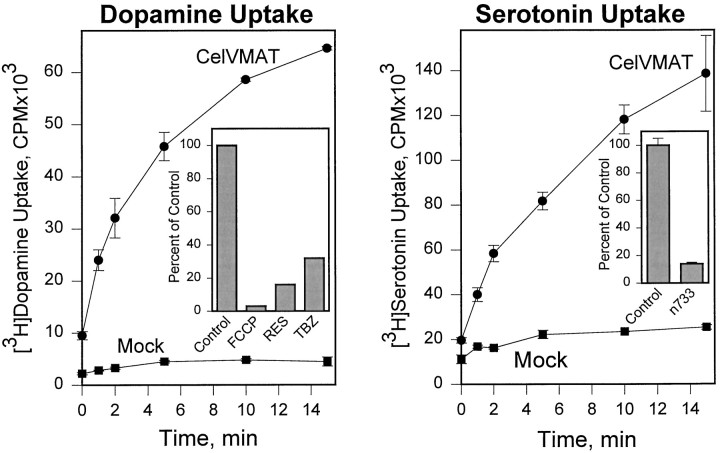
The C. elegans VMAT was expressed in CV-1 cells (see Materials and Methods), and a permeablized cell assay (Erickson and Eiden, 1993) was used to measure transport activity. The C. elegans VMAT mediated uptake of [3H]dopamine and [3H]serotonin that was time-dependent and saturable (Fig. 4) and was inhibited by an excess of unlabeled substrate. The uptake of both substrates was inhibited by the known VMAT inhibitors tetrabenazine and reserpine and was blocked by carbonyl cyanidep-trifluoromethoxyphenylhydrazone (FCCP), which disrupts transmembrane pH gradients (Fig. 4A, inset). This inhibitor profile is a similar to that of mammalian VMATs (Erickson and Eiden, 1993).
Fig. 4.
Transport of [3H]dopamine and [3H]serotonin mediated by C. elegans VMAT. A C. elegans VMAT cDNA or pBluescript with no insert (Mock) was used in a permeablized cell uptake assay as previously described (Erickson and Eiden, 1993). Data points represent the means of duplicate determinations; error bars represent the range of the duplicates.A, time course of [3H]dopamine uptake. Inset, Relative inhibition of specific [3H]dopamine uptake (i.e., with the mock values subtracted) at 4 min by 5 μm FCCP, 100 nm reserpine (RES), or 1 μmtetrabenazine (TBZ). B, Time course of [3H]serotonin uptake. Inset, Decreased [3H]serotonin uptake by the n733 mutant VMAT (means of four measurements ± SEM, measured at 15 min). In this experiment, the wild-type CelVMAT control corresponded to 0.59 ± .03 pmol of serotonin/15 min per well. The mutant uptake is significantly different from the control with p< 0.0001. Uptake values for CelVMAT and CelVMAT/n733 were not corrected for transfection efficiency in these experiments, because there were no consistent differences in efficiency of transfection of CV-1 cells with CelVMAT compared with CelVMAT/n733 as judged by immunohistochemical staining of VMAT-expressing CV-1 cells.
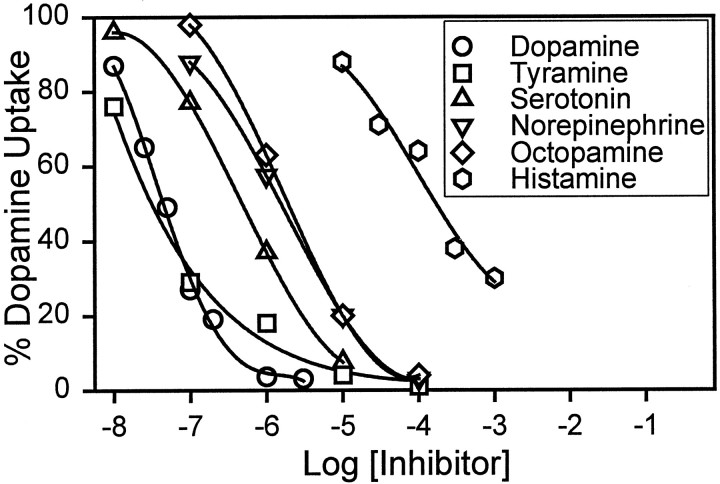
Kinetic analyses indicated competitive inhibition of [3H]dopamine uptake by dopamine, norepinephrine, serotonin, histamine, tyramine, and octopamine, suggesting that all of these compounds were potential substrates for VMAT (Fig.5, Table1). Experiments monitoring the inhibition of [3H]serotonin uptake gave similar results (Table 1): for both substrates, the rank order of affinity was dopamine ∼ tyramine > serotonin > norepinephrine ∼ octopamine > histamine. The major difference between the C. elegans protein and the two human proteins is in the affinity for dopamine, which is ∼20- to 100-fold higher for the nematode protein (Table 1). Mammalian VMAT1 and VMAT2 differ significantly from each other in their affinity for histamine (Erickson et al., 1996); in this respect, C. elegans VMAT is more like VMAT2 than VMAT1 (Table 1). To our knowledge, tyramine and octopamine have not previously been reported as potential substrates for any VMAT.
Fig. 5.
Inhibition of [3H]dopamine uptake by biogenic amines. Digitonin-permeabilized CV-1 cells expressing CelVMAT cDNA were used to measure transport of [3H]dopamine in the absence or presence of the indicated concentration of unlabeled inhibitor. Assays were for 4 min, and data points represent the means of one to five assays of duplicate samples.
Table 1.
EC50 values for inhibition of serotonin or dopamine uptake by C. elegans VMAT in CV-1 cells
| Compound | CelVMAT SER (μm) | CelVMAT DA (μm) | HumVMAT11-a SER (μm) | HumVMAT2 SER (μm) |
|---|---|---|---|---|
| Dopamine | 0.07 (0.02–0.2)1-b | 0.04 (0.03–0.05) | 3.8 | 1.4 |
| Tyramine | 0.06 (0.01–0.2) | 0.04 (0.002–0.7) | ND | ND |
| Serotonin | 0.91 (0.26–3.2) | 0.51 (0.1–3.3)1-b | 1.4 | 0.9 |
| Norepinephrine | 2.8 (0.6–13) | 1.7 (0.3–10) | 13.7 | 3.4 |
| Octopamine | 3.4 (1.9–6.2) | 1.5 (0.1–22) | ND | ND |
| Histamine | 120 (300–1000)1-b | 117 (80–1000)1-b | 4696 | 143 |
Uptake of [3H]dopamine (DA) or [3H]serotonin (SER) was performed as described in Materials and Methods with the addition of the indicated concentration of inhibitor (as shown in Fig. 5). Assays were terminated after 4 min, except for some of the histamine data, which were collected from 15 min uptake experiments, and did not differ substantially from 4 min uptake data. Values represent calculated EC50 values for each competitor. The ranges in parenthesis represent 95% confidence limits for each EC50 value. Cel, C. elegans; Hum, human; ND, not determined.
The data for human VMAT1 and VMAT2 are fromErickson et al. (1996).
Values for which 95% confidence limits could not be obtained because of lack of convergence on 0% of control with increasing dose (when insufficient high-concentration data points were collected; see Fig. 5). In these cases, the range represents the range of concentrations invariably giving at least 80% inhibition of uptake (upper value) or no more than 20% inhibition of uptake (lower value).
CeVMAT is associated with synaptic vesicles
Antibodies to VMAT peptides (see Materials and Methods) were used for indirect immunofluorescence staining of wild-type C. elegans. VMAT-specific staining in the nervous system was punctate and was observed primarily in synaptic regions (Fig.6). In general, neuronal processes and cell bodies were poorly stained or not stained at all, although the trajectories of many processes could be inferred from the punctate immunofluorescence presumably associated with the en passant synapses made by the processes.
Fig. 6.
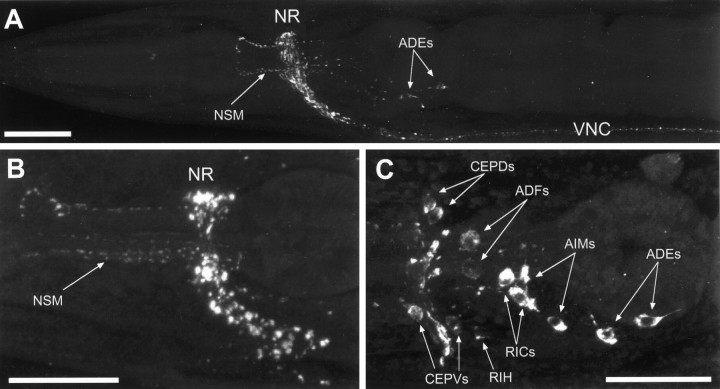
Immunolocalization of C. elegansVMAT. Hermaphrodites were stained with indirect immunofluorescence using affinity-purified anti-VMAT antibodies and were imaged with a Leica TCS NT confocal microscope. Images are maximum projections ofz-series. Anterior is to the left; ventral is down. A, Lateral view of the anterior third of a wild-type adult; B, lateral view of a wild-type head; C, lateral view ofunc-104(e1265), in which synaptic vesicles are mislocalized to cell somas; individual identified neuronal somas are indicated by arrows. NR, Nerve ring;VNC, ventral nerve cord. The processes of the NSM cells are indicated. Cell bodies of other identified immunopositive neurons include the ADE, ADF, AIM, CEPD, CEPV, and RIC bilateral homologs and the unpaired RIH cell. Scale bar, 20 μm.
Analysis of unc-104 mutants suggested that the VMAT immunoreactivity was associated with synaptic vesicles. Theunc-104 gene encodes a kinesin-related protein, which is required for the transport of synaptic vesicles from neuronal cell bodies along the axons to synapses (Hall and Hedgecock, 1991; Otsuka et al., 1991). In unc-104 mutants, synaptic vesicles are not found at synapses but, rather, are found in large clusters in cell bodies (Hall and Hedgecock, 1991); in addition, a number of synaptic vesicle-associated proteins, such as synaptotagmin and the vesicular acetylcholine transporter (UNC-17/VAChT), are mislocalized to neuronal cell bodies (Nonet et al., 1993; Alfonso et al., 1993). We find that VMAT staining is similarly mislocalized to cell bodies inunc-104 mutants (Fig. 6), consistent with a synaptic vesicle association of this protein.
In addition, punctate non-neuronal VMAT staining was observed in three somatic cells in the male gonad (data not shown). Interestingly, synaptotagmin has also been found in a different set of cells in the male vas deferens (Nonet et al., 1993).
Identification of VMAT-containing cells
Most C. elegans neurons may be identified on the basis of cell body position, size, and process morphology (White et al., 1986). As indicated above, VMAT immunostaining in unc-104mutants was present in neuronal cell bodies, which helped in the identification of specific neurons expressing the protein. Many of these same cells were also visualized by an induced fluorescence technique or with anti-serotonin antibodies. We observed strong and reproducible VMAT-specific immunoreactivity in 20 neurons and weak and variable staining in 5 additional neurons, all of which are listed below.
ADE, PDE, and CEP
The two ADE neurons, two PDE neurons, and four CEP neurons are sensory cells previously reported to contain dopamine-like FIF (Sulston et al., 1975). We have also observed dopamine-like induced fluorescence using a slightly different method (Fig.7). These neurons have ciliated endings in the deirids (ADE and PDE) or the cephalic sensilla (CEP). VMAT-positive regions in the PDE cells usually extend anteriorly in the ventral nerve cord to at least the retrovesicular ganglion (as inHedgecock et al., 1985; but unlike White et al., 1986).
Fig. 7.
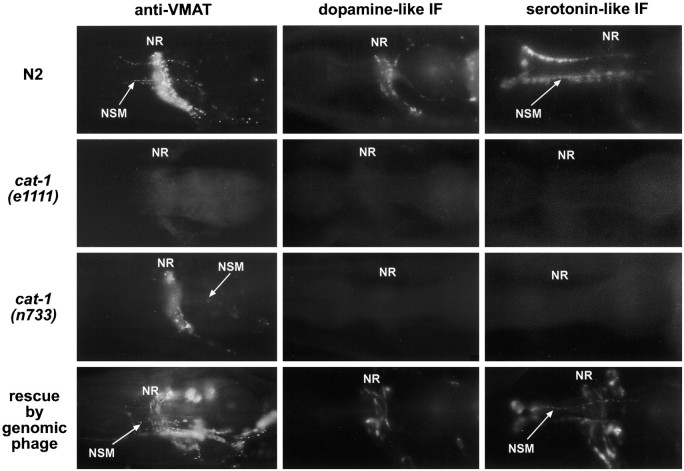
Cellular phenotypes of cat-1mutants. Young adult hermaphrodites were immunostained for VMAT (left column) or were prepared for induced fluorescence (see Materials and Methods) and visualized for dopamine-like fluorescence (middle column) and serotonin-like fluorescence (right column). Lateral views: anterior is to the left, and ventral is down.Top row, Wild type (N2). Second row, cat-1(e1111); the animals are completely deficient for VMAT immunoreactivity, and the induced fluorescence for both transmitters is completely absent from neuronal processes and significantly (often totally) absent from cell somas. Third row, cat-1(n733); the dopamine-specific and serotonin-specific induced fluorescence are comparable to those ine1111, but the VMAT immunostaining is almost wild type.Bottom row, Transgenic animals containing the genomic phage RM#424L in a cat-1(e1111) host. Note the restoration and overexpression of all three cellular markers.NR, Nerve ring; NSM, processes of the pharyngeal NSM cells.
NSM
The two NSM neurons are prominent cells of the pharynx. FIF studies (Horvitz et al., 1982) and anti-serotonin immunohistochemistry (Desai et al., 1988) have shown that these are the “juiciest” serotonin-containing neurons in C. elegans hermaphrodites. By ultrastructural analysis, they have sensory endings and neuromuscular output, as well as varicosities, fine branches, and endings on the surface of the pharynx (Albertson and Thomson, 1976), suggesting that serotonin might be released into the pseudocoelom and have a humoral function.
HSN
The two HSN neurons have been previously reported to contain serotonin (Desai et al., 1988). These are motor neurons with cell bodies in unique positions in the lateral midbody; they receive input from several interneurons, and their predominant morphological outputs are to the vulval muscles and the VC neurons (White et al., 1986). In addition, their axons run anteriorly in the ventral nerve cord to the nerve ring, with minor synapses onto a number of motor neurons and interneurons (White et al., 1986).
VC4 and VC5
The VC cells are a set of six postembryonically derived motor neurons with cell bodies in the ventral nerve cord (Sulston, 1976;White et al., 1976). VC4 and VC5, the members of the class closest to the vulva, make numerous neuromuscular synapses onto the vulval muscles; the other VC cells are reported to have less extensive output to the same muscles (White et al., 1976, 1986). In addition, all of the VC cells have sparse output to the ventral body muscles and other motor neuron classes (White et al., 1976, 1986). We have confirmed unpublished reports (see Rand and Nonet, 1997b) that VC4 and VC5 contain weak and variable serotonin immunoreactivity and serotonin-like induced fluorescence (data not shown). Interestingly, although the VC cells arise during the molting period between the L1 and L2 stages, VMAT immunoreactivity is not acquired by VC4 and VC5 until the L4 stage; this is approximately the same time that the extensive innervation of the vulval muscles occurs (Li and Chalfie, 1990). In contrast, other VMAT-positive cells acquire immunoreactivity within a short time after birth.
ADF and RIH
There have been reports of serotonin staining in the two ADF neurons and the RIH neuron (Sawin, 1996 and see Rand and Nonet, 1997b). The ADF cells are sensory neurons involved in chemotaxis (Bargmann and Horvitz, 1991a) and dauer larva development (Bargmann and Horvitz, 1991b). The function of the RIH neuron is unknown. We have confirmed that ADF and RIH contain weak, variable, serotonin-like induced fluorescence and serotonin immunoreactivity in unc-104mutants (data not shown). The ADF cells contain significant amounts of VMAT immunoreactivity, whereas RIH is weakly positive for VMAT immunoreactivity in unc-104 mutants.
AIM and RIC
Previous unpublished reports noted a pair of serotonin-positive cells in the head; these were tentatively identified as the RIG neurons (see Rand and Nonet, 1997b). Using unc-104 mutants, we saw two pairs of VMAT-positive cells in the region of, but anterior to, the RIG cells. To aid in the identification of these cells, we stained two strains of transgenic animals (obtained from Oliver Hobert) which express GFP in the AIY neurons (Hobert et al., 1997) or the AIZ and RIC neurons (Hobert et al., 1998). Using these local landmarks, we identified one pair of VMAT-positive cells as the (adjacent) AIM neurons. In unc-104 mutants, these VMAT-positive cells were occasionally weakly positive for serotonin immunoreactivity or serotonin-like induced fluorescence. Using a different set of criteria,Sawin (1996) also concluded that the AIM cells are serotonin-positive. Using unc-86 mutants (Finney and Ruvkun, 1990) and these GFP landmarks, the second pair of VMAT-positive cells were identified as the RIC cells. These cells were never positive for serotonin immunoreactivity or for serotonin- or dopamine-like induced fluorescence. Therefore, the RIC neurons may use a different amine neurotransmitter.
CAN
The two CAN cells have cell bodies and processes along the excretory canals, which extend laterally along the body (White et al., 1986). They are immunopositive for VMAT but appear to be negative for serotonin immunoreactivity and induced fluorescence. Only a single synapse onto an epidermal cell was reported by White et al. (1986), but in wild-type animals, we observe a few spots of VMAT immunoreactivity.
Other neurons
We sometimes observe an additional pair of VMAT-positive cells in the lateral ganglia. The staining is weak and variable and is only seen in unc-104 mutants; we are not yet certain about the identity of these two cells. In addition, we observe a large number of male-specific VMAT-positive cells in the ventral nerve cord and the tail; the identity and properties of these cells will be reported in the future.
Does cat-1 encode VMAT?
Based on the position of the VMAT gene on the physical map, it was possible to assign it an approximate location on the C. elegans genetic map, and this in turn suggested that it might correspond to a previously identified mutant locus. The most likely candidates, based on map position and mutant phenotype, werecat-1 (see phenotypic description below) andegl-6, egl-13, and egl-14, three egg-laying defective mutants that were considered because of the involvement of serotonin in egg laying (Horvitz et al., 1982). However, mutants in all three of these Egl genes had wild-type patterns of induced fluorescence and VMAT immunoreactivity in the head, suggesting that they did not encode VMAT (data not shown).
The cat-1 gene was first identified by Sulston et al. (1975)in a screen for mutants with abnormal patterns of FIF. cat-1mutants lack (dopamine-specific) FIF in neuronal processes, although some FIF is still present in cell bodies (Sulston et al., 1975). In addition, serotonin immunoreactivity is variably reduced in serotonin-containing neurons (Loer and Kenyon, 1993). These are the same phenotypes caused by treatment of wild-type C. eleganswith reserpine, which led to the suggestion more than 20 years ago that this gene might encode a synaptic vesicle neurotransmitter transporter (Sulston et al., 1975).
There are two alleles of cat-1 currently available:e1111 and n733. Both mutations lead to loss of dopamine- and serotonin-specific induced fluorescence (Fig. 7). Analysis of cat-1(e1111) mutants shows that they are completely deficient for VMAT immunoreactivity (using antiserum specific for peptide 1; see Materials and Methods), whereascat-1(n733) animals have slightly less VMAT immunoreactivity than wild type (Fig. 7).
Sequence of cat-1 mutant alleles
Both cat-1 mutant alleles were associated with mutations in the VMAT coding sequence (Fig. 1). The cat-1allele e1111 contains a G to A transition, which changes a tryptophan to an amber termination codon (located between transmembrane domains 3 and 4); this is consistent with the complete lack of immunoreactivity in e1111 homozygotes. This allele has been reported to be suppressible by the amber-suppressor genesup-5 (Waterston and Brenner, 1978). We have determined thate1111 homozygotes regain a low level of VMAT immunoreactivity in a sup-5 mutant background (data not shown). Furthermore, sup-5 partially suppressed the mating defect of cat-1(e1111) males (Table2).
Table 2.
Behavior of wild-type C. elegans (N2), reserpine-treated N2, and two cat-1 mutants
| Behavior | N2 | N2 + reserpine | cat-1(e1111) | cat-1(n733) |
|---|---|---|---|---|
| Movement on plates (bends/min)2-a | ||||
| On food | 11.0 ± 1.1 | 15.7 ± 1.8* | 17.7 ± 1.6* | 15.6 ± 1.9* |
| Off food | 18.5 ± 2.1 | 17.9 ± 2.4 | 18.7 ± 2.6 | 19.0 ± 1.9 |
| Thrashing in liquid (bends/min)2-b | 217 ± 19 | ND | 216 ± 7 | 214 ± 12 |
| Egg laying (No. of eggs in utero)2-c | ||||
| 14°C | 14.8 ± 2.2 | 13.1 ± 1.6 | 15.5 ± 2.5 | 15.1 ± 0.6 |
| 20°C | 15.9 ± 3.2 | 25.1 ± 3.8** | 27.3 ± 2.2** | 23.8 ± 1.5** |
| Male mating2-d | ||||
| % No progeny | 30 (17/56) | ND | 90 (46/51)*** | 100 (58/58)*** |
| % 1–100 progeny | 13 (7/56) | 8 (4/51) | 0 (0/58) | |
| % >100 progeny | 57 (32/56) | 2 (1/51) | 0 (0/58) |
ND, Not determined.
Movement on plates was determined for 10 groups of 5 individuals either on food or off food for each genotype, as described in Materials and Methods. Means ± SEMs are given.
Thrashing in liquid was determined for 10 groups of 10 individuals as described in Materials and Methods. Means of the 10 groups ± SEMs are given.
The number of fertilized eggs in utero was counted in groups of 19–63 adult hermaphrodites raised at particular temperatures. Means of four groups ± SEMs are given.
Single males were placed with single “females” as described in Materials and Methods. The percentages of males that produced a given number of progeny are listed with numbers of individuals in parentheses. Mating was also evaluated forcat-1 mutants carrying the amber suppressor sup-5(Waterston and Brenner, 1978). Male progeny of a cross betweensup-5/+ males and cat-1 hermaphrodites (approximately half sup-5/+;cat-1 and half +/+;cat-1) were tested for mating ability. With thecat-1(e1111) amber mutation, there was significantsup-5-dependent rescue of mating: 47% no progeny, 22% 1–100 progeny; 31% > 100 progeny (n = 58; different from cat-1(e1111) alone with p < 0.01, χ2 test). With the cat-1(n733) missense mutation, there was no sup-5-dependent rescue of mating: 0% mated (n = 20).
*cat-1(e1111), cat-1(n733), and reserpine-treated N2 were significantly different from N2; p < 0.01 (Student’s t test).
**cat-1(e1111), cat-1(n733), and reserpine-treated nematodes were all significantly different from N2 at 20°C; p < 0.01 (Student’s t test).
***cat-1(e1111) and cat-1(n733) were significantly different from N2; p < 0.01 (χ2 test).
The cat-1(n733) mutation is a glycine to arginine missense mutation in the middle of transmembrane domain 5; this is consistent with presence of VMAT immunoreactivity in n733 homozygotes. In addition, the mutant protein appears to be correctly localized (Fig.7), which suggests that the mutant phenotype derives from impaired protein function. Consistent with this hypothesis, the VMAT protein corresponding to the n733 mutation, CelVMAT/n733, has only 14% of the activity of wild-type CelVMAT for [3H]serotonin uptake in the mammalian cell-based transport assay (Fig. 4B inset), although the residual transport activity of the mutant was inhibited by reserpine to the same degree as the wild-type transporter; 100 nm reserpine decreases wild-type and mutant transport to 6.3 ± 2.4 and 5.7 ± 2.8% of control transport, respectively (mean ± SEM of three separate experiments).
Behavioral phenotypes of cat-1 mutants
cat-1 mutants have several behavioral deficits, which appear to reflect deficient function of biogenic amine-containing cells. Using laser ablation, Sawin (1996) demonstrated that the eight dopamine-containing cells (ADE, PDE, and CEP) are collectively required for a wild-type response to bacteria. Wild-type hermaphrodites move significantly slower when they are in a bacterial lawn than when they are on clean agar. When all of the dopamine-containing cells are ablated, locomotion is the same on or off the lawn (Sawin, 1996). Using the same paradigm, we found that the locomotion of cat-1mutants is also insensitive to the presence of bacteria:cat-1 mutants move at the same rate on or off the bacterial lawn (Table 2).
Wild-type animals display two other responses to the presence of food: they slow down dramatically when first encountering a bacterial lawn and graze for a while before resuming movement, and the rate of pharyngeal pumping is increased in the presence of the lawn. We have shown that these responses to the bacterial lawn are also deficient incat-1 animals (Fig. 8), suggesting that these paradigms are also mediated through aminergic neurons.
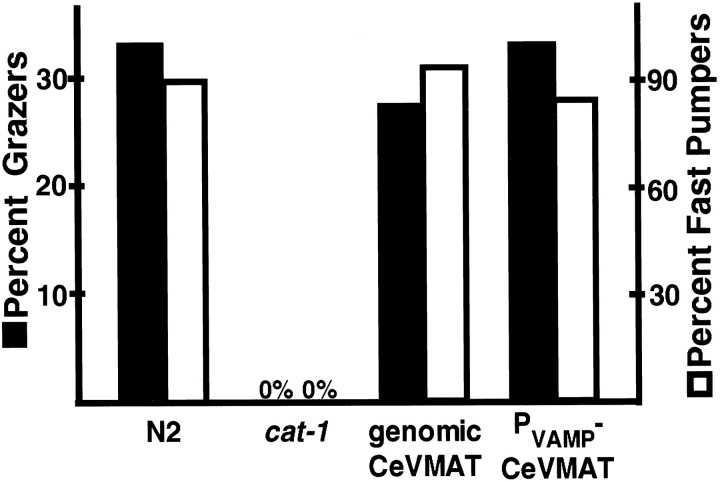
Fig. 8.
Pharyngeal pumping and grazing behavior in response to a bacterial lawn. Grazers (dark bars) are individuals taking >1 min to enter a bacterial lawn, as described in Materials and Methods. White bars represent the percent of individuals of each phenotype that pumped at a rate of at least 250/min in the presence of bacteria. Values represent the percent for 100 individual animals for each behavior. The transgenic lines were significantly different from cat-1 for both behaviors with p < .01.
cat-1 mutants also have deficits in serotonin-regulated behaviors. The easiest such behavior to measure is egg laying. Exogenous serotonin stimulates egg laying (Horvitz et al., 1982), whereas ablation of the serotonin-containing HSN cells leads to a profound decrease in egg laying such that the animals become quite bloated with unlaid eggs (Trent et al., 1983; Desai et al., 1988; Desai and Horvitz, 1989). We have found a mild but reproducible temperature-sensitive reduction in the rate of egg laying bycat-1 mutants (Table 2), although we have not yet determined the identity of the cells that mediate this effect. It is noteworthy that cat-1 mutants, as well as other serotonin-deficient mutants (e.g., cat-4 andbas-1), clearly do not display the severe egg-laying defect associated with ablation of the HSN cells; this has led to the proposal that the HSNs mediate egg laying by using another neurotransmitter in addition to serotonin (Weinshenker et al., 1995).
In addition, as previously described, cat-1 males are deficient in mating performance (Table 2); this behavior has been shown to require the male-specific serotonin-containing CP motor neurons (Loer and Kenyon, 1993), as well as dopamine- and serotonin-containing sensory rays in the male tail (Liu and Sternberg, 1995).
Rescue of cat-1 phenotypes by transgenic VMAT expression
Using several different assays, we found that the genomic phage RM#424L was able to rescue several cat-1 mutant phenotypes in some animals (Figs. 7, 8). However, experiments with the genomic phage were complicated by an apparent dosage-sensitive toxicity: many of the transgenic animals were small, and some were dumpy and uncoordinated. In these individuals, VMAT was expressed in some or all of the hypodermal seam cells. It is possible that this was attributable to sequences unrelated to VMAT function, although we have not explored this in any detail.
To eliminate any potential problems caused by such genomic sequences, we prepared rescue constructs in which the C. elegans VMAT cDNA was driven by the C. elegans VAMP (synaptobrevin) promoter; the VAMP gene is expressed in all neurons (Nonet et al., 1998). When introduced into cat-1 animals, such constructs led to VMAT immunoreactivity in most neurons and provided behavioral rescue (Fig. 8) with only minimal behavioral abnormalities. These results provide confirmation that C. elegans VMAT is encoded by cat-1.
Rescue of cat-1 mutants with human VMATs
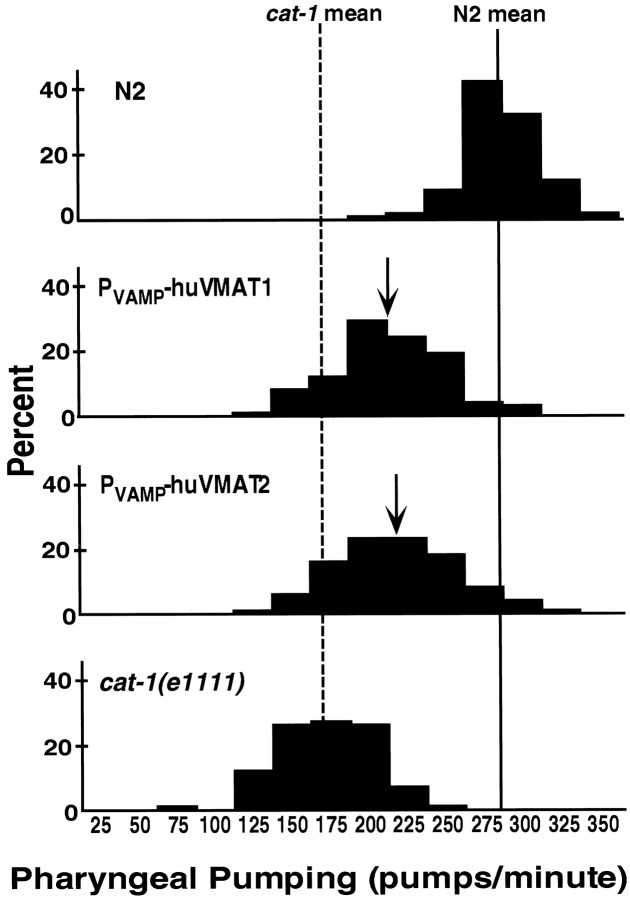
We then prepared similar rescue constructs containing either the human VMAT1 or VMAT2 cDNA driven by the C. elegans VAMP promoter and introduced them into cat-1 mutants. Using antibodies specific for the human proteins, we found significant levels of expression of the heterologous proteins, as well as correct localization of the human VMATs to synaptic regions (Fig.9). However, the transgenic lines containing the human cDNA constructs showed nonuniform expression of the transgenes: often the normally VMAT-positive cells contained little or no heterologous protein. We estimate that only perhaps 10% of the transgenic animals had significant transgene expression in most of the normally VMAT-positive cells. Although we do not yet understand the biological basis for the variability of expression, both of the human VMAT constructs nevertheless provided significant rescue ofcat-1 behavioral defects in pharyngeal pumping (Fig.10) and in grazing (data not shown). This rescue was similar for human VMAT1 and VMAT2 cDNAs (Fig. 10).
Fig. 9.

Synaptic localization and function of transgenic human VMAT2. cat-1(e1111) mutants were transformed with human VMAT2 cDNA driven by the neuron-specific VAMP (synaptobrevin) promoter (see Materials and Methods). A, Immunostaining with anti-human VMAT2 reveals that the protein is expressed in neurons and is properly localized to synaptic regions, including the nerve ring and the dorsal nerve cord (arrows). B, Induced fluorescence specific for dopamine reveals that the human transgene restores proper wild-type staining pattern in the correct neurons. C, Serotonin-like immunofluorescence is also restored, including that of the NSM cells of the pharynx. Comparable photographs of untransformed (control) cat-1(e1111)mutants are shown in Figure 7, second row. Lateral views: anterior is to the left, and ventral isdown.
Fig. 10.
Human VMAT transgenes partially rescuecat-1 deficits in pharyngeal pumping. Pumps per minute in a bacterial lawn were measured in 100 individual animals of each genotype. Data are plotted in bins of 25. The mean for N2 is 275 (indicated with a solid line); the cat-1mean is 160 (dashed line). Arrowsindicate the means for Pvamp-huVMAT1 (202) and Pvamp-huVMAT2 (208). Transgenic lines were significantly different fromcat-1(e1111) with p < 0.01 (Mann–Whitney–Wilcoxson test).
DISCUSSION
C. elegans VMAT
The gene we have characterized appears to be a structural and functional homolog of the mammalian VMAT genes. This conclusion is based on the similarity of the respective protein sequences, the localization of the C. elegans protein to amine-containing neurons, the association of the protein with synaptic vesicles, the ability of the C. elegans protein to transport amines in anin vitro assay, and the ability of mammalian VMATs to restore partial function in C. elegans cat-1 mutants.
The sequence in Figure 2 and the dendrogram in Figure 3 show thatC. elegans VMAT, although clearly a close relative of the mammalian VMATs, is neither a VMAT1 nor a VMAT2. Furthermore, theC. elegans VMAT protein is expressed in all of the cells previously shown to contain biogenic amines. Taken together, these results suggest that the gene we have described is the only VMAT gene in C. elegans (and no additional VMAT homologs are present in the >85% of the C. elegans genome sequenced to date). We conclude that the two mammalian VMAT genes diverged from each other subsequent to the divergence of nematode and mammalian ancestors.
Biochemical properties of the C. elegans VMAT
The properties of C. elegans VMAT appear more like those of the mammalian VMAT2 (“neuronal”) isoform than the VMAT1 (“neuroendocrine”) isoform: C. elegans VMAT has an affinity for histamine in the (mammalian) physiological range and is inhibited by tetrabenazine at <1 μm. Various laboratories (Peter et al., 1996; Varoqui and Erickson, 1997) have suggested that recognition of histamine and tetrabenazine may be structurally related properties of VMAT2. The ability of C. elegans VMAT to recognize histamine suggests that the mammalian VMAT1 isoform may have evolved in part to provide a carrier thatfails to recognize histamine, rather than the mammalian VMAT2 isoform evolving to provide a carrier that hasacquired affinity for histamine.
A significant difference between C. elegans and mammalian VMATs is that the nematode transporter appears to have a higher affinity for dopamine than for serotonin. It is also noteworthy that octopamine, a major invertebrate neurotransmitter also found in the mammalian nervous system, and tyramine, the precursor of octopamine, have high affinities for C. elegans VMAT. The affinities of octopamine and tyramine for mammalian VMATs have not yet been reported.
cat-1 is the VMAT structural gene
This assignment is based on sequencing and antibody staining of mutant alleles and on transgenic rescue experiments. We have shown that both cat-1 mutations are associated with point mutations in the VMAT coding sequence. We have also described four different phenotypes associated with cat-1 mutants: altered induced fluorescence pattern, altered serotonin immunoreactivity pattern, lack of VMAT immunoreactivity (in e1111), and defective behavioral responses to bacterial lawns. All of these phenotypes are rescued by both a genomic phage containing the complete VMAT gene and a VMAT cDNA under the control of the C. elegans VAMP promoter.
VMAT-containing neurons
So far, two biogenic amine neurotransmitters have been identified in specific C. elegans neurons. Serotonin immunoreactivity or serotonin-like immunofluorescence has been described in 11 cells in hermaphrodites (Horvitz et al., 1982; Desai et al., 1988; McIntire et al., 1992; Sawin, 1996; Rand and Nonet, 1997a); we have shown that these cells are immunopositive for VMAT, supporting the hypothesis that these cells are serotonergic. Similarly, the identification of VMAT in the eight neurons reported to contain dopamine-like immunofluorescence (Sulston et al., 1975) provides additional evidence that these cells are dopaminergic. We also find VMAT in at least six additional cells, suggesting that these cells may use a different biogenic amine transmitter. It is likely that at least some of these cells use octopamine as a neurotransmitter: octopamine is present in C. elegans homogenates; exogenous octopamine has distinct behavioral effects (Horvitz et al., 1982); and we have shown it to be a substrate for VMAT.
There are reported differences among nematode species in the pattern of serotonin immunoreactivity. In Ascaris suum, only one pair of serotonin-immunopositive cells, putative NSM homologs, has been observed in females (Johnson et al., 1996). It is likely thatAscaris females do not have HSN homologs, but they appear to have homologs of ADF, AIM, RIH, and probably VC cells, and none of these neurons contains serotonin immunoreactivity (Johnson et al., 1996). The ADF, AIM, RIH, and VC cells of C. elegans have significantly weaker staining for serotonin than the NSM cells; it is therefore unclear whether the discrepancies between the two species merely represent differences in relative abundance and/or assay sensitivity or fundamental differences in differentiated neurotransmitter phenotypes.
VMAT is required for proper function of dopamine-containing neurons
Laser ablation studies have demonstrated that the dopamine-containing ADE, PDE, and CEP neurons are collectively required for the sensing of a bacterial lawn and/or the resulting slowing of locomotion (Sawin, 1996). This behavior and related bacterial sensing behaviors are deficient in cat-1 mutants. These neurons are thus apparently unable to function properly in the absence of the vesicular transporter. We believe that this is the first demonstration that vesicular amine transport is essential for the function of specific (aminergic) neurons.
Not all behaviors mediated by VMAT-positive cells are mediated by VMAT
In contrast to the results just cited for the eight dopamine-containing neurons, laser ablation studies of other VMAT-positive neurons are not in good agreement with thecat-1 phenotype. Loss of the HSN cells by ablation or programmed cell death (Trent et al., 1983; Ellis and Horvitz, 1986;Desai and Horvitz, 1989) leads to a dramatic decrease in egg laying; this is a far stronger phenotype than the mild egg-laying defect incat-1 mutants (Table 2). Ablation of the VMAT-containing CAN cells causes the animals to wither and die (J. Sulston, cited in White et al., 1986), yet cat-1 mutants are relatively healthy. Thus, for these neurons, cell ablation has more severe consequences than elimination of VMAT function in putative null cat-1mutants. It is possible that another vesicular transporter exists in some C. elegans neurons, which can transport serotonin as well as whatever amine transmitter is used by CAN (a multiple transporter model). Alternatively, the HSN and CAN cells might use a nonamine neurotransmitter in addition to the amine transported by VMAT; thus elimination of amine release leads to only partial compromise of cellular function (a multiple transmitter model).
For HSN cells, we favor the second model. Using both antibody staining and induced fluorescence, we see no serotonin present in HSN processes and synaptic regions of cat-1 mutants; this suggests a total lack of vesicular serotonin transport (and degradation of the transmitter in the cytoplasm) rather than the presence of another transporter for serotonin. In addition, genetic and pharmacological studies have led Weinshenker at al. (1995) to suggest that HSN cells use a second neurotransmitter, probably acetylcholine, as well as serotonin. With respect to CAN, until we have information about the putative neurotransmitter(s) used by this pair of cells, we cannot decide between multiple transporters and multiple transmitters. In fact, a third possibility for CAN is that VMAT expression is unrelated to neurotransmitter release or function.
Functional roles of monoamines in C. elegans
Previously published reports of deficits in cat-1mutants include reduced male mating efficiency (Sulston et al., 1975), slight hyperactivity (Loer and Kenyon, 1993), slightly smaller size, and slightly reduced feeding (Avery and Horvitz, 1990). We have now shown defects in some sensory responses and a mild defect in egg laying. It is striking that apparent elimination of all synaptic biogenic amine function in cat-1 mutants does not lead to profound behavioral deficits. There are several possible explanations for this. Although specific cells and functions may be “dispensable” under laboratory conditions, the evolutionary persistence of such cells and functions suggests a relative selective advantage to the animal in a “normal,” i.e., soil, environment. For example, the ability to sense and respond to food is expected to be far more important in the soil than in a slurry of bacteria. Another possible explanation for the mild phenotype of cat-1 mutants is redundancy of genes, neuronal pathways and/or neurotransmitters. As discussed above, we consider the possibility of an additional VMAT gene in C. elegans to be remote, but it cannot be completely excluded until the sequencing of the genome is complete. Nevertheless, the observation that ablation of the eight dopamine-containing cells leads to the same phenotype as loss of VMAT activity within those cells (Sawin, 1996) argues that there are no other transporters or transmitters for this cellular function.
cat-1 rescue studies and VMAT function
We have demonstrated rescue of several phenotypes withcat-1-containing transgenic arrays, including induced fluorescence (in both putative dopamine and putative serotonin containing cells), serotonin immunoreactivity pattern, and two behavioral responses to bacteria (grazing and pharyngeal pumping). We were also able to demonstrate partial phenotypic rescue using human VMAT1 and VMAT2 transgenes. We do not understand the reason for the variable cellular expression of the human VMATs, but the level of rescue was comparable to the degree of cellular expression of the transgenes. It therefore appears that when either human VMAT1 or VMAT2 is expressed in the proper cells, it can substitute for C. elegans VMAT.
Knock-out of the VMAT2 gene in mice has recently been reported (Fon et al., 1997; Wang et al., 1997). The null phenotype is lethal, which is not surprising in view of the many complex monoamine-dependent functions required for early postembryonic life (Thomas et al., 1995;Zhou et al., 1995) and the probability that impairment of at least one monoaminergic function might have dramatic biological consequences. Is VMAT absolutely required for all monoaminergic systems, or is VMAT merely necessary for optimal neurotransmission? The present report indicates that on both an organismal and a cell biological level, VMAT is required for a broad range of monoamine-dependent behaviors and functions in C. elegans. The utility of in vivoanalysis in C. elegans, however, is that behaviorally impaired mutants can be sustained in an artificial environment, which allows VMAT function to be linked to behavior in the context of individual, defined circuits that include monoaminergic neurons.
The cat-1 gene and mutants, together with cell-specific promoters and other tools now being developed, will allow us to address how deficits in the metabolism and release and reuptake of specific monoamine transmitters in specific cells can directly affect complex behaviors, and how these deficits might be specifically ameliorated with pharmacological maneuvers. This has implications for gene therapeutic, grafting, and other approaches to human monoamine-linked diseases.
Footnotes
This work was funded by Grant GM38679 from the National Institute of General Medical Sciences to J.B.R., a grant from the Oklahoma Center for the Advancement of Science and Technology to J.S.D., a National Research Service Award from the National Institute of Neurological Disorders and Stroke to D.L.F., and a Pharmacology Research Associate Trainee Fellowship to K.A. We thank Dr. Jeffrey Erickson for assistance with the transport assay for CelVMAT and advice and assistance in preparation of hVMAT-reconstituted C. elegans strains. We also acknowledge our gratitude to the community of C. elegans researchers for their generous sharing of reagents and information: Beth Sawin provided advice on behavioral assays; Bob Barstead, Heidi Browning, and Tom Blumenthal provided libraries; Cori Bargmann helped with cell identification; Andy Fire provided transformation vectors; Beth Sawin and Bob Horvitz provided thecat-1(n733) allele; Mike Nonet provided the C. elegans VAMP promoter; Heinke and Ralf Schnabel provided thepha-1 plasmid; Oliver Hobert provided the Pttx-3-GFP and Plin-11-GFP transgenic strains; Gian Garriga and Curtis Loer shared unpublished data; and the C. elegans Genome Sequencing Project made our research much easier. Oligonucleotide primers and peptide immunogens were synthesized by the Molecular Biology Resource Facility of the University of Oklahoma Health Sciences Center. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources.
Correspondence should be addressed to Dr. James B. Rand, Program in Molecular and Cell Biology, Oklahoma Medical Research Foundation, 825 Northeast 13th Street, Oklahoma City, OK 73104.
REFERENCES
- 1.Albertson DG. Formation of the first cleavage spindle in nematode embryos. Dev Biol. 1984;101:61–72. doi: 10.1016/0012-1606(84)90117-9. [DOI] [PubMed] [Google Scholar]
- 2.Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:299–325. doi: 10.1098/rstb.1976.0085. [DOI] [PubMed] [Google Scholar]
- 3.Alfonso A, Grundahl K, Duerr JS, Han H-P, Rand JB. The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science. 1993;261:617–619. doi: 10.1126/science.8342028. [DOI] [PubMed] [Google Scholar]
- 4.Avery L, Horvitz HR. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J Exp Zool. 1990;253:263–270. doi: 10.1002/jez.1402530305. [DOI] [PubMed] [Google Scholar]
- 5.Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991a;7:729–742. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- 6.Bargmann CI, Horvitz HR. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science. 1991b;251:1243–1246. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- 7.Barstead RJ, Waterston RH. Vinculin is essential for muscle function in the nematode. J Cell Biol. 1991;114:715–724. doi: 10.1083/jcb.114.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bektesh S, Van Doren K, Hirsh D. Presence of the Caenorhabditis elegans spliced leader on different mRNAs and in different genera of nematodes. Genes Dev. 1988;2:527–535. doi: 10.1101/gad.2.10.1277. [DOI] [PubMed] [Google Scholar]
- 9.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Torre JC. An improved approach to histofluorescence using the SPG method for tissue monoamines. J Neurosci Methods. 1980;3:1–5. doi: 10.1016/0165-0270(80)90029-1. [DOI] [PubMed] [Google Scholar]
- 11.Desai C, Horvitz HR. Caenorhabditis elegans mutants defective in the functioning of the motor neurons responsible for egg laying. Genetics. 1989;121:703–721. doi: 10.1093/genetics/121.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai C, Garriga G, McIntire SL, Horvitz HR. A genetic pathway for the development of the Caenorhabditis elegans HSN motor neurons. Nature. 1988;336:638–646. doi: 10.1038/336638a0. [DOI] [PubMed] [Google Scholar]
- 13.Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986;44:817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- 14.Erickson JD, Eiden LE. Functional identification and molecular cloning of a human brain vesicle monoamine transporter. J Neurochem. 1993;61:2314–2317. doi: 10.1111/j.1471-4159.1993.tb07476.x. [DOI] [PubMed] [Google Scholar]
- 15.Erickson JD, Eiden LE, Hoffman BJ. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc Natl Acad Sci USA. 1992;89:10993–10997. doi: 10.1073/pnas.89.22.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erickson JD, Varoqui H, Schäfer MKH, Modi W, Diebler M-F, Weihe E, Rand J, Eiden LE, Bonner TI, Usdin TB. Functional identification of a vesicular acetylcholine transporter and its expression from a “cholinergic” gene locus. J Biol Chem. 1994;269:21929–21932. [PubMed] [Google Scholar]
- 17.Erickson JD, Eiden LE, Schafer MK, Weihe E. Reserpine- and tetrabenazine-sensitive transport of (3)H-histamine by the neuronal isoform of the vesicular monoamine transporter. J Mol Neurosci. 1995;6:277–287. doi: 10.1007/BF02736786. [DOI] [PubMed] [Google Scholar]
- 18.Erickson JD, Schafer MK, Bonner TI, Eiden LE, Weihe E. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci USA. 1996;93:5166–5171. doi: 10.1073/pnas.93.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finney M, Ruvkun G. The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell. 1990;63:895–905. doi: 10.1016/0092-8674(90)90493-x. [DOI] [PubMed] [Google Scholar]
- 20.Fon EA, Pothos EN, Sun BC, Killeen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- 21.Fuerst TR, Niles EG, Studier FW, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granato M, Schnabel H, Schnabel R. Genesis of an organ: molecular analysis of the pha-1 gene. Development. 1994;120:3005–3017. doi: 10.1242/dev.120.10.3005. [DOI] [PubMed] [Google Scholar]
- 23.Hall DH, Hedgecock EM. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell. 1991;65:837–847. doi: 10.1016/0092-8674(91)90391-b. [DOI] [PubMed] [Google Scholar]
- 24.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1988. [Google Scholar]
- 25.Hedgecock EM, Culotti JG, Thomson JN, Perkins LA. Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev Biol. 1985;111:158–170. doi: 10.1016/0012-1606(85)90443-9. [DOI] [PubMed] [Google Scholar]
- 26.Hobert O, Mori I, Yamashita Y, Ossig R, Honda H, Oshima Y, Liu Y, Ruvkun G. Regulation of interneuron function in the C. elegans thermoregulatory pathway by the ttx-3 LIM homeobox gene. Neuron. 1997;19:345–357. doi: 10.1016/s0896-6273(00)80944-7. [DOI] [PubMed] [Google Scholar]
- 27.Hobert O, D’Alberti T, Liu Y, Ruvkun G. Control of neural development and function in a thermoregulatory network by the LIM homeobox gene lin-11. J Neurosci. 1998;18:2084–2096. doi: 10.1523/JNEUROSCI.18-06-02084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodgkin JA, Brenner S. Mutations causing transformation of sexual phenotype in the nematode Caenorhabditis elegans. Genetics. 1977;86:275–287. [PMC free article] [PubMed] [Google Scholar]
- 29.Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode C. elegans. Science. 1982;216:1012–1014. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- 30.Howell M, Shirvan A, Stern-Bach Y, Steiner-Mordoch S, Strasser JE, Dean GE, Schuldiner S. Cloning and functional expression of a tetrabenazine sensitive vesicular monoamine transporter from bovine chromaffin granules. FEBS Lett. 1994;338:16–22. doi: 10.1016/0014-5793(94)80108-8. [DOI] [PubMed] [Google Scholar]
- 31.Johnson CD, Rand JB, Herman RK, Stern BD, Russell RL. The acetylcholinesterase genes of C. elegans: identification of a third gene (ace-3) and mosaic mapping of a synthetic lethal phenotype. Neuron. 1988;1:165–173. doi: 10.1016/0896-6273(88)90201-2. [DOI] [PubMed] [Google Scholar]
- 32.Johnson CD, Reinitz CA, Sithigorngul P, Stretton AOW. Neuronal localization of serotonin in the nematode Ascaris suum. J Comp Neurol. 1996;367:352–360. doi: 10.1002/(SICI)1096-9861(19960408)367:3<352::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Kitamoto T, Wang W, Salvaterra PM. Structure and organization of the Drosophila cholinergic locus. J Biol Chem. 1998;273:2706–2713. doi: 10.1074/jbc.273.5.2706. [DOI] [PubMed] [Google Scholar]
- 34.Krause M, Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987;49:753–761. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C, Chalfie M. Organogenesis in C. elegans: positioning of neurons and muscles in the egg-laying system. Neuron. 1990;4:681–695. doi: 10.1016/0896-6273(90)90195-l. [DOI] [PubMed] [Google Scholar]
- 36.Liu KS, Sternberg PW. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron. 1995;14:79–89. doi: 10.1016/0896-6273(95)90242-2. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Peter D, Roghani A, Schuldiner S, Privé GG, Eisenberg D, Brecha N, Edwards RH. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992;70:539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Schweitzer ES, Nirenberg MJ, Pickel VM, Evans CJ, Edwards RH. Preferential localization of a vesicular monoamine transporter to dense core vesicles in PC12 cells. J Cell Biol. 1994;127:1419–1433. doi: 10.1083/jcb.127.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loer CM, Kenyon CJ. Serotonin-deficient mutants and male mating behavior in the nematode Caenorhabditis elegans. J Neurosci. 1993;13:5407–5417. doi: 10.1523/JNEUROSCI.13-12-05407.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McIntire SL, Garriga G, White J, Jacobson D, Horvitz HR. Genes necessary for directed axonal elongation or fasciculation in C. elegans. Neuron. 1992;8:307–322. doi: 10.1016/0896-6273(92)90297-q. [DOI] [PubMed] [Google Scholar]
- 41.Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller KG, Alfonso A, Nguyen M, Crowell JA, Johnson CD, Rand JB. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc Natl Acad Sci USA. 1996;93:12593–12598. doi: 10.1073/pnas.93.22.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nirenberg MJ, Liu YJ, Peter D, Edwards RH, Pickel VM. The vesicular monoamine transporter 2 is present in small synaptic vesicles and preferentially localizes to large dense core vesicles in rat solitary tract nuclei. Proc Natl Acad Sci USA. 1995;92:8773–8777. doi: 10.1073/pnas.92.19.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nonet ML, Grundahl K, Meyer BJ, Rand JB. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell. 1993;73:1291–1305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- 45.Nonet ML, Saifee O, Zhao H, Rand JB, Wei L. Synaptic transmission deficits in Caenorhabditis elegans synaptobrevin mutants. J Neurosci. 1998;18:70–80. doi: 10.1523/JNEUROSCI.18-01-00070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otsuka AJ, Jeyaprakash A, Garcia-Añoveros J, Tang LZ, Fisk G, Hartshorne T, Franco R, Born T. The C. elegans unc-104 gene encodes a putative kinesin heavy chain-like protein. Neuron. 1991;6:113–122. doi: 10.1016/0896-6273(91)90126-k. [DOI] [PubMed] [Google Scholar]
- 47.Peter D, Vu T, Edwards RH. Chimeric vesicular monoamine transporters identify structural domains that influence substrate affinity and sensitivity to tetrabenazine. J Biol Chem. 1996;271:2979–2986. doi: 10.1074/jbc.271.6.2979. [DOI] [PubMed] [Google Scholar]
- 48.Posmett DN, McGrath H, Tam JP. A novel method for producing anti-peptide antibodies. J Biol Chem. 1988;263:1719–1725. [PubMed] [Google Scholar]
- 49.Rand JB, Nonet ML. Synaptic transmission. In: Riddle DL, Blumenthal T, Meyer BJ, Preiss JR, editors. C. elegans II. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1997a. pp. 611–643. [PubMed] [Google Scholar]
- 50.Rand JB, Nonet ML. Neurotransmitter assignments for specific neurons. In: Riddle DL, Blumenthal T, Meyer BJ, Preiss JR, editors. C. elegans II. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1997b. pp. 1049–1052. [Google Scholar]
- 51.Sawin ER. PhD thesis. Massachusetts Institute of Technology; 1996. Genetic and cellular analysis of modulated behaviors in Caenorhabditis elegans. . [Google Scholar]
- 52.Schafer WR, Kenyon CJ. A calcium-channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature. 1995;375:73–78. doi: 10.1038/375073a0. [DOI] [PubMed] [Google Scholar]
- 53.Schuldiner S, Shirvan A, Linial M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiol Rev. 1995;75:369–392. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- 54.Ségalat L, Elkes DA, Kaplan JM. Modulation of serotonin-controlled behaviors by Go in Caenorhabditis elegans. Science. 1995;267:1648–1651. doi: 10.1126/science.7886454. [DOI] [PubMed] [Google Scholar]
- 55.Smith DE, Fisher PA. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984;99:20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sulston JE. Post-embryonic development in the ventral cord of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:287–297. doi: 10.1098/rstb.1976.0084. [DOI] [PubMed] [Google Scholar]
- 57.Sulston J, Dew M, Brenner S. Dopaminergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol. 1975;163:215–226. doi: 10.1002/cne.901630207. [DOI] [PubMed] [Google Scholar]
- 58.Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- 59.Trent C, Tsung N, Horvitz HR. Egg-laying defective mutants of the nematode C. elegans. Genetics. 1983;104:619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varoqui H, Erickson JD. Vesicular neurotransmitter transporters - Potential sites for the regulation of synaptic function. Mol Neurobiol. 1997;15:165–191. doi: 10.1007/BF02740633. [DOI] [PubMed] [Google Scholar]
- 61.Varoqui H, Diebler M-F, Meunier F-M, Rand JB, Usdin TB, Bonner TI, Eiden LE, Erickson JD. Cloning and expression of the vesamicol binding protein from the marine ray Torpedo: homology with the putative vesicular acetylcholine transporter UNC-17 from Caenorhabditis elegans. FEBS Lett. 1994;342:97–102. doi: 10.1016/0014-5793(94)80592-x. [DOI] [PubMed] [Google Scholar]
- 62.Wang YM, Gainetdinov RR, Fumagalli F, Xu F, Jones SR, Bock CB, Miller GW, Wightman RM, Caron MG. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron. 1997;19:1285–1296. doi: 10.1016/s0896-6273(00)80419-5. [DOI] [PubMed] [Google Scholar]
- 63.Waterston R, Brenner S. A suppressor mutation in the nematode acting on specific alleles of many genes. Nature. 1978;275:715–719. doi: 10.1038/275715a0. [DOI] [PubMed] [Google Scholar]
- 64.Weihe E, Schafer MK, Erickson JD, Eiden LE. Localization of vesicular monoamine transporter isoforms (VMAT1 and VMAT2) to endocrine cells and neurons in rat. J Mol Neurosci. 1994;5:149–164. doi: 10.1007/BF02736730. [DOI] [PubMed] [Google Scholar]
- 65.Weinshenker D, Garriga G, Thomas JH. Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J Neurosci. 1995;15:6975–6985. doi: 10.1523/JNEUROSCI.15-10-06975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.White JG, Southgate E, Thomson JN, Brenner S. The structure of the ventral nerve cord of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:327–348. doi: 10.1098/rstb.1976.0086. [DOI] [PubMed] [Google Scholar]
- 67.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 68.Zhou Q-Y, Quaife CJ, Palmiter RD. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature. 1995;374:640–643. doi: 10.1038/374640a0. [DOI] [PubMed] [Google Scholar]