Abstract
Explicit memory may depend on the hippocampus, whereas the amygdala may be part of an emotional memory system. Priming stimulation of the basolateral group of the amygdala (BLA) resulted in an enhanced long-term potentiation (LTP) in the dentate gyrus (DG) to perforant path (PP) stimulation 30, 90, 150, and 180 min after high-frequency stimulation (HFS). Exposure of rats to a behavioral stress is reported to inhibit DG LTP. Because the amygdala is thought to mediate emotional responses, we examined the apparent discrepancy between the effects of behavioral stress induced 1 hr before HFS to the PP and of amygdala priming on hippocampal plasticity by stimulating the BLA 1 hr before HFS to the PP. The two delayed protocols inhibited the expression of LTP to PP stimulation, whereas priming the BLA immediately before HFS to the PP enhanced DG LTP. Moreover, exposure to the behavioral stress blocked the enhancing effects of BLA priming on LTP. We propose that the activation of the BLA (either by behavioral stress or by direct electrical stimulation) has a biphasic effect on hippocampal plasticity: an immediate excitatory effect and a longer-lasting inhibitory effect.
Keywords: stress, basolateral amygdala, hippocampus, long-term potentiation, amygdalohippocampal interaction, rat
The amygdala is a part of the brain system that ensures that memories of significant experiences are well retained (McGaugh, 1989, 1990; McKernan and Shinnick-Gallagher, 1997;Rogan et al., 1997). It is suggested that when memories stored through both the amygdala and hippocampus are retrieved, they have a different “flavor” than when only the hippocampal system is involved. This dual activation of the amygdala and hippocampus may be what gives emotional memories their special quality (for review, see LeDoux, 1993).
There is abundant evidence that emotional stress can either improve or impair learning depending on the severity and context. Many of the hormones secreted during emotional stress (e.g., glucocorticoids and norepinephrine) affect learning and memory processes (Anisman and Bignami, 1978; Martinez et al., 1981). The role of the hippocampus is of particular interest in this respect, because the hippocampus is both important in some aspects of memory and has the highest concentration of both types of corticosterone receptors in the brain (for review, seeMcEwen et al., 1986; McEwen and Sapolsky, 1995).
Tetanic stimulation of circuits within the hippocampus can lead to long-term potentiation (LTP) (Bliss and Lomo, 1973), which is thought to model some aspects of learning and memory (Bliss and Collingridge, 1993). A potential relationship between behaviorally induced stress and hippocampal LTP has been reported in several experiments in which the induction of LTP was inhibited after restraint and inescapable tail shock (Diamond and Rose, 1994; Shors and Dryver, 1994; Kim et al., 1996; Shors et al., 1997) or after an exposure to a novel environment (Xu et al., 1997).
The amygdala is considered central in mediating responses to emotional stress (Davis, 1992; LeDoux, 1992). Lesions of the medial amygdala greatly reduced restraint-induced activation of cells of the medial paraventricular nucleus, responsible for the secretion of corticotropin-releasing factor (Dayas et al., 1999). It has been suggested that the amygdala, and in particular the basolateral amygdala complex (BLA), modulates hippocampus-dependent memory storage (Packard et al., 1994; Roozendaal and McGaugh, 1996). The BLA projects to the entorhinal cortex and dentate gyrus (DG) (Thomas et al., 1984). Furthermore, injection of NMDA into the amygdala induces c-fos expression in the DG (Packard et al., 1995). An intact BLA is required for memory-modulating processes initiated by infusion of drugs administered into the hippocampus (Roozendaal and McGaugh, 1997). Lesions of the BLA, but not the central nucleus, attenuated the induction of population spike LTP in the DG in vivo (Ikegaya et al., 1994). Furthermore, conditioning stimulation of the ipsilateral BLA applied simultaneously with a subthreshold tetanic stimulation of the perforant path (PP) potentiated LTP induction in the DG (Ikegaya et al., 1995).
Because behavioral stress impairs LTP, it could be expected that priming the BLA, which is assumed to mediate some aspects of stress (Goldstein et al., 1996), would also impair LTP. However, the contrary has been reported. This apparent discrepancy may be important in understanding the neural mechanisms underlying the emotional modulation of declarative memory. We thus set out to characterize the interactions between emotional stress, the stimulation of the BLA, and their influence on hippocampal neuronal plasticity.
MATERIALS AND METHODS
Animals
Adult, male Sprague Dawley rats, weighing 250–300 gm from Harlan (Jerusalem, Israel) were maintained five per cage on a 12 hr light/dark cycle with water and laboratory rodent chow ad libitum. Tests were conducted during the last 6 hr of the light cycle.
Behavior
Underwater trauma. The water maze (Morris, 1984) consists of a pool of water (diameter, 1.7 m; 50 cm high rim). For the spatial learning task a 12 × 12 cm escape platform was hidden with the top surface 2–3 cm below the water level at one of four positions in the pool. The swim path of the rat was recorded manually, and the escape latency was measured using a stopwatch (Richter-Levin and Segal, 1991). Rats were given two blocks of three trials each per day for 5 d followed by a 1 min quadrant analysis test with no escape platform in the maze (Richter-Levin et al., 1994).
The underwater procedure was developed to evaluate the effects of the trauma on memory in the context of the trauma (Richter-Levin, 1998). The 5 d of training in the water maze are aimed to create a “safe” or familiar environment for the animals. Holding them underwater in this safe context is expected to magnify the aversive effects of the stressor.
On the sixth day, rats in the trauma group were allowed to swim for 1 min and then held under water for 30 sec using a special metal net (Richter-Levin, 1998). After the procedure, rats were immediately anesthetized and taken for electrophysiological testing.
Platform exposure. Animals were placed on a platform (12 × 12 cm) located 2–3 cm below the water level in the center of a water maze for 10 min in a brightly lit room. After the procedure, rats were immediately anesthetized and taken for electrophysiological testing.
Electrophysiology
Surgical procedure. Rats were anesthetized (40% urethane and 5% chloral hydrate in saline, 0.5 ml/100 gm, i.p.), and mounted in a Stoelting (Wood Dale, IL) stereotaxic frame. The scalp was incised and retracted, and head position was adjusted to place bregma and lambda in the same horizontal plane. Small burr holes (2 mm diameter) were drilled unilaterally in the skull for the placement of recording and stimulating electrodes.
A recording microelectrode (glass; tip diameter, 2–5 μm; filled with 2 m NaCl; resistance, 1–4 MΩ) was placed in the DG (coordinates: 4 mm posterior; 2.5 mm lateral to bregma; depth adjusted to yield largest EPSP response to stimulation of the PP).
A bipolar 125 μm stimulating electrode was implanted in the ipsilateral PP (coordinates: 8 mm posterior; 4 mm lateral to bregma; depth adjusted to yield maximal response of the DG).
In the BLA groups, a second stimulating electrode was implanted in the ipsilateral BLA (coordinates: 3 mm posterior; 5.3 mm lateral to bregma; depth, 7.4 mm).
Baseline stimuli to the PP (monopolar pulses, 100 μsec duration, intensity adjusted to yield a population spike of 30–50% of the maximal pretetanus value) were delivered at 0.1 Hz. After positioning the electrodes, the rat was left for 20 min before commencing the experiment.
Evoked responses were digitized (10 kHz) and analyzed using a Cambridge Electronic Design (Cambridge, UK) 1401+ interface and its Spike2 software. Off-line measurements were made of the slope of the EPSP using averages of five successive responses to a given stimulation intensity applied at 0.1 Hz.
LTP was measured as an increase in EPSP slope. The EPSP slope was measured as a percentage of baseline value immediately before the tetanus. During the course of the experiment, body temperature was monitored and maintained at 37 ± 0.5°C by a feedback-regulated heating pad.
LTP induction. LTP was induced by a “theta”-like high-frequency stimulation (HFS) to the PP (three sets of 10 trains; each train consisted of 10 pulses at 100 Hz; intertrain interval, 200 msec; interset interval, 1 min). The BLA group received a priming stimulation (10 trains of five pulses at 100 Hz; intertrain interval, 200 msec) 30 sec before HFS to the PP was applied. The 1 hr BLA group received a similar pattern of stimulation (10 trains of five pulses at 100 Hz; intertrain interval, 200 msec) but 1 hr before the HFS to the PP. Field potentials were recorded from the DG at 30, 90, 150, and 180 min after the HFS to the PP.
Histology. Histological verification of the stimulating electrode location was performed on all the rats that were implanted with a stimulating electrode in the BLA.
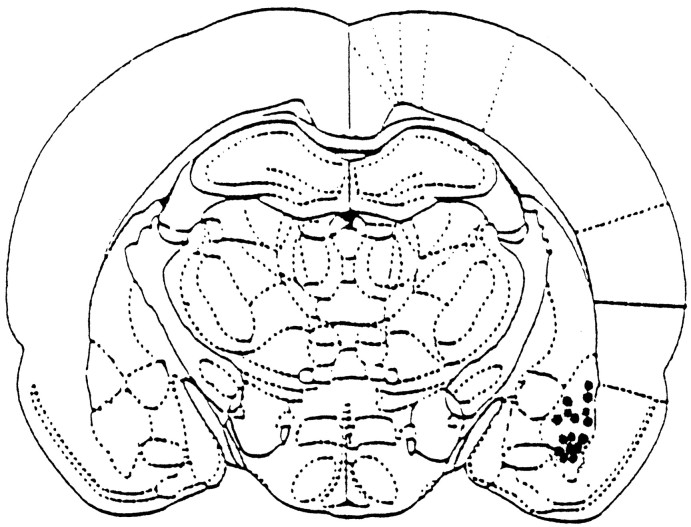
After electrophysiological testing, marking lesions were made by passing anodal currents (10 mA for 3 sec.) to the metal bipolar stimulating electrode. Brains were removed, post-fixed over three nights in formaldehyde (10%), and sectioned (120 μm) on a sledge microtome. The sections were mounted on gelatin-coated slides, stained in cresyl violet, dehydrated, and coverslipped. The electrode tract and lesion locations were then identifiable under a light microscope (Akirav and Richter-Levin, 1999). The placements of the electrode tips located in the BLA are shown in Figure1.
Fig. 1.
Schematic drawings of BLA electrode placements. After the completion of the experiment, the rats were given marking lesions of the BLA. Shown is a coronal view at position 2.8 mm posterior to bregma. Solid black circles indicate the locations.
Statistical analysis
LTP was assessed using 8 × 5 (treatment × time after HFS) overall mixed ANOVA with least significant difference multiple-comparison post hoc test.
RESULTS
Eight groups were tested: (1) HFS (n = 10): the rats were anesthetized and taken for an electrophysiological test of plasticity in the DG (i.e., received HFS to the PP); (2) non-HFS (n = 6): the rats were anesthetized and then received only baseline stimulation over the same duration of time as the other groups; (3) platform (n = 9): after spending 10 min on a platform, the rats were anesthetized and taken for an electrophysiological test of plasticity in the DG; (4) underwater trauma (UWT) (n = 8): after exposure to an underwater trauma, the rats were anesthetized and taken for an electrophysiological test of plasticity in the DG; (5) BLA priming (n = 8): a tetanic stimulation of the BLA was applied 30 sec before HFS to the PP in anesthetized rats; (6) 1 hr BLA (n = 7): a tetanic stimulation of the BLA was applied 1 hr before HFS to the PP in anesthetized rats; (7) platform-BLA (n = 7): after the platform exposure rats were anesthetized and 1 hr later received a priming stimulation to the BLA 30 sec before applying HFS to the PP; and (8) UWT-BLA (n = 8): after the exposure to the underwater trauma rats were anesthetized and 1 hr later received a priming stimulation to the BLA 30 sec before applying HFS to the PP.
Similar stimulus intensities were applied (F(7, 55) = 1.9; p > 0.05), and a comparison between the groups before tetanization did not reveal a significant difference in EPSP slope (F(7,55) < 1; NS) indicating a similar baseline.
A significant post-HFS within-group time effect on LTP was found (F(1,55) = 53.29; p < 0.001), but the interaction between treatment and time was not significant, indicating that there was no difference between groups in this respect. In contrast, there was a significant treatment effect on LTP (F(7,55) = 5.28; p< 0.001), which was further analyzed.
LTP in the DG
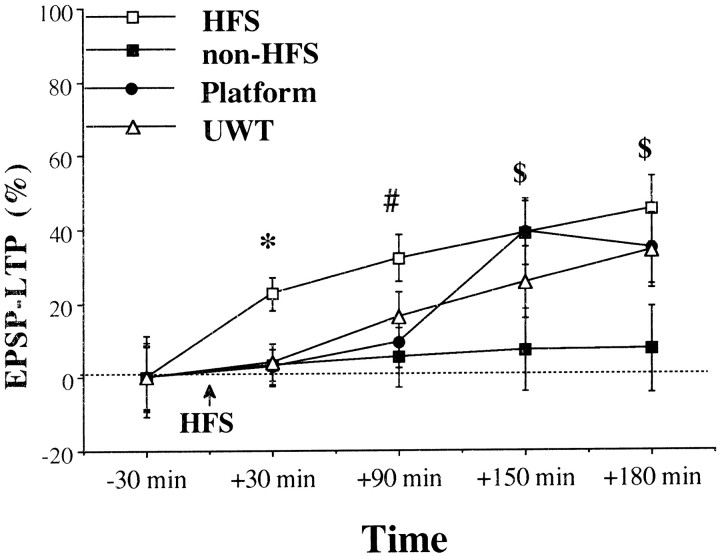
The level of potentiation in the HFS group was significantly different from zero at all the times tested (Fig.2; 30 min,t(9) = 5.09; p < 0.001; 90 min, t(9) = 5.12;p < 0.001; 150 min,t(9) = 4.5; p < 0.01; 180 min, t(9) = 5.04;p < 0.001). Because there was a significant increase in the levels of LTP over time, we included a non-HFS group that received only test stimulation over the same duration of time as the HFS group. There was a significant difference in the level of potentiation between the HFS and the non-HFS groups at all the times tested (post hoc comparisons: 30 min,p < 0.01; 90 min, p < 0.05; 150 min,p < 0.05; 180 min after HFS, p < 0.05). Furthermore, the level of potentiation in the non-HFS group was not significantly different from zero at any time point.
Fig. 2.
Effects of behavioral stress on DG LTP. The increase in EPSP slope was measured as a percentage of baseline value immediately before the tetanus. Field potential recordings were taken 30, 90, 150, and 180 min after the application of HFS to the PP. In the HFS group (n = 10) HFS (3 sets of 10 trains, each one consists of 10 pulses at 100 Hz) was applied to the PP. The non-HFS group (n = 6) animals received only test stimulation over the same duration. In the stress groups (Platform,n = 9; UWT, n = 8) HFS was applied to the PP 1 hr after the exposure to the stressor. A comparison across the groups before HFS to the PP did not show a significant difference in the levels of LTP, indicating a similar baseline. There was a significant difference between the HFS group and the non-HFS group at all the times tested. Both stressors significantly inhibited LTP compared with the HFS group 30 min after HFS. However, from 90 min after HFS onward for the UWT group and from 150 min onward for the platform group, there was no significant difference (*significant difference between the HFS group and all the other groups; #significant difference between the HFS group and the non-HFS and the platform groups; $significant difference between the HFS and the non-HFS groups). In the HFS group the level of potentiation at 30 min after HFS was significantly different from zero, whereas the level of potentiation in the stressed animals was not.
Effects of behavioral stressors on LTP
Both behavioral stressors (exposure to platform or the UWT) significantly inhibited LTP relative to the HFS group 30 min after HFS (Figs. 2, 3; post hoccomparisons: 30 min, UWT, p < 0.01; platform,p < 0.01; 90 min, platform, p < 0.05). However, from 90 min onward (for the UWT group) and from 150 min onward (for the platform group) there was no significant difference between the groups.
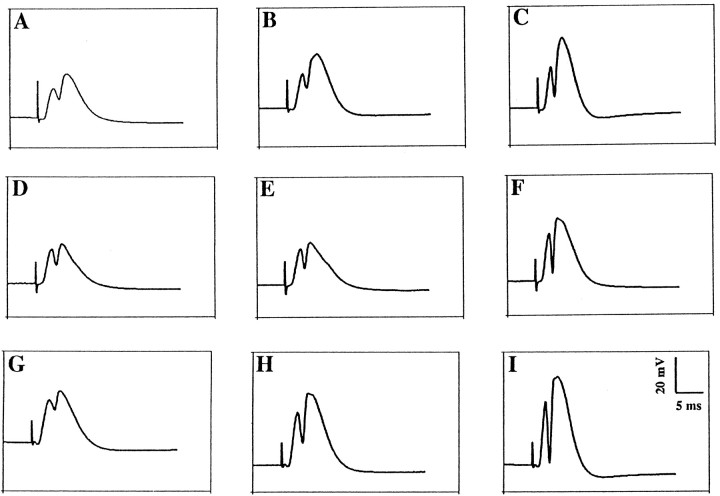
Fig. 3.
Representative evoked potentials recorded from the DG before and after HFS to the PP. Evoked potentials immediately before HFS to the PP (A, D, G), at 30 min after HFS (B, E, H), and at 180 min (C, F, I) of HFS, platform, and BLA groups respectively, show the main effects of behavioral stress and of BLA priming on DG LTP. In the HFS group, LTP was significant both at 30 min (B) and 180 min (C). Behavioral stress temporally inhibited the expression of LTP at 30 min (E), but at 180 min (F) LTP was similar to control. In contrast, BLA priming enhanced the level of potentiation both at 30 min (H) and at 180 min (I).
The level of potentiation at 30 min after HFS was significantly different from zero in the HFS group but not in the UWT and the platform groups.
Effects of BLA priming on LTP
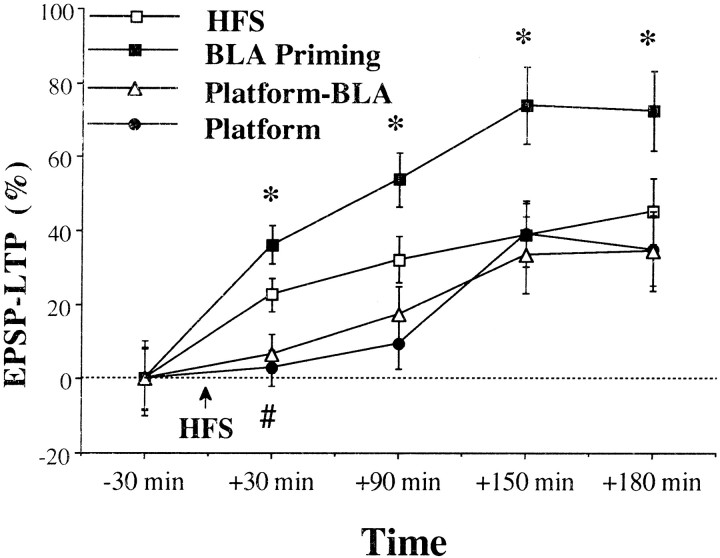
Priming stimulation of the BLA significantly increased LTP relative to the HFS group at all times tested (Fig.4A; post hoccomparisons: 30 min, p < 0.05; 90 min,p < 0.05; 150 min, p < 0.05; 180 min after HFS, p < 0.05).
Fig. 4.
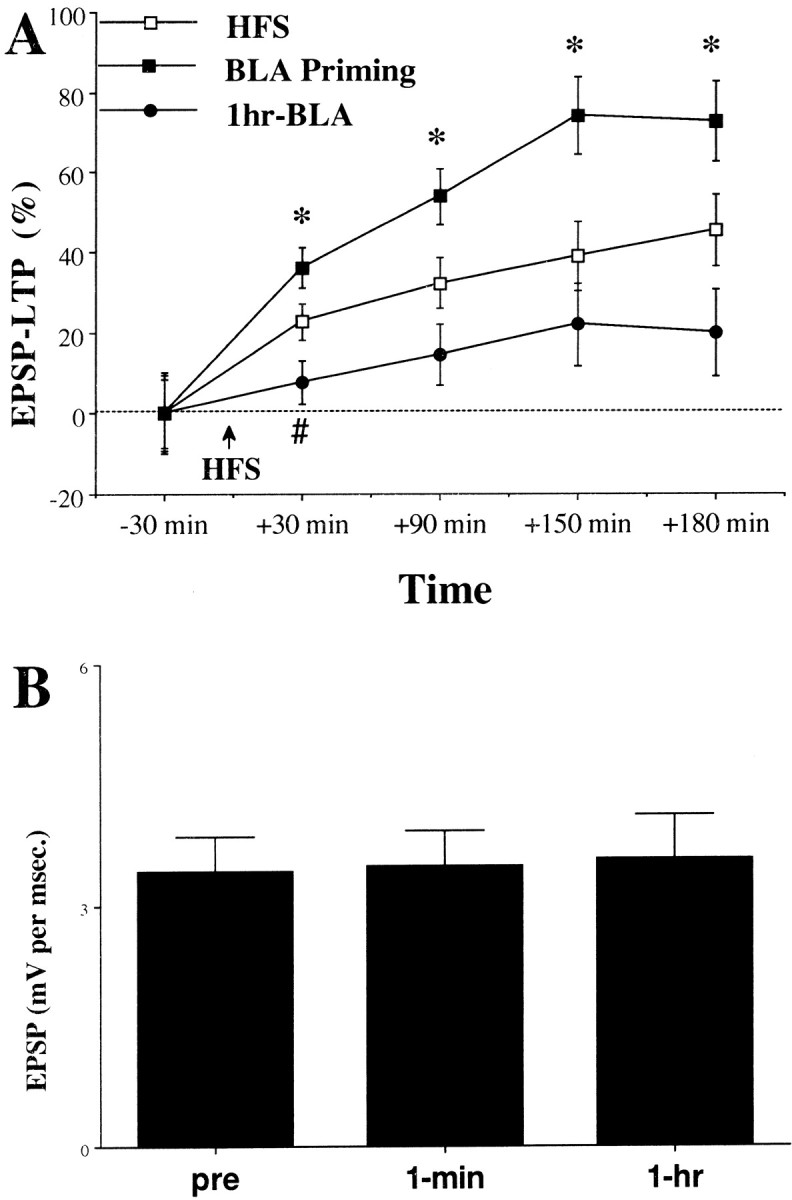
BLA stimulation effects on DG LTP.A, In the BLA Priming group (n = 8), a stimulation to the BLA (10 trains of 5 pulses at 100 Hz) was applied 30 sec before HFS to the PP. In the1 hr-BLA group (n = 7), a stimulation to the BLA (10 trains of 5 pulses at 100 Hz) was applied 1 hr before HFS to the PP. Priming stimulation of the BLA significantly increased LTP compared with the HFS and the 1 hr-BLA groups at all times tested (*significant difference between the BLA priming group and the two other groups; #Significant difference between the HFS and the 1 hr-BLA groups). A comparison between the groups before HFS to the PP did not reveal a significant difference in the levels of the EPSP, indicating a similar baseline. B, The spaced activation of the BLA had no effect on baseline EPSP levels either at 1 min or at 1 hr after BLA stimulation (before HFS to the PP).
The results confirm previous reports indicating BLA modulation of DG LTP (Ikegaya et al., 1995; Akirav and Richter-Levin, 1999).
Effects of BLA stimulation 1 hr before the application of HFS to the PP on LTP
To even the temporal profile of the behavioral and electrophysiological procedures, the BLA was primed 1 hr before applying HFS to the PP (1 hr BLA group). Stimulating the BLA 1 hr before the application of HFS to the PP had no effect on baseline EPSP levels either at 1 min or at 1 hr after BLA stimulation (before the application of HFS to the PP; Fig. 4B).
Spaced activation of the BLA (1 hr BLA) significantly decreased the levels of LTP compared with BLA priming at all the times tested (Fig.4A; post hoc comparisons: 30 min,p < 0.001; 90 min, p < 0.001; 150 min, p < 0.001; 180 min, p < 0.001).
There was a significant difference between the 1 hr BLA and the HFS group at 30 min after HFS (post hoc comparisons: 30 min, p < 0.05), but there was no significant difference between the 1 hr BLA and the HFS group at 90 min after HFS and onward. In addition, there was no significant difference between the 1 hr BLA and the behavioral stress groups at any of the times tested. Moreover, the level of potentiation in the 1 hr BLA group was not significantly different from zero, thus resembling the effects of behavioral stress.
Effects of the combined treatments on LTP
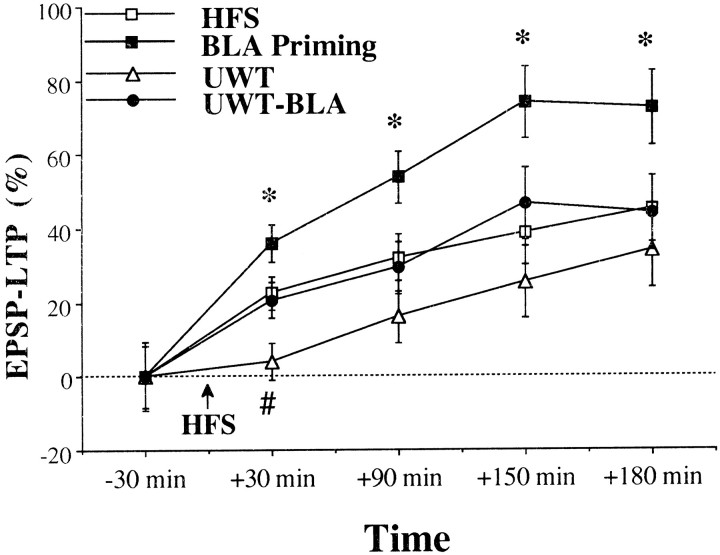
Previous exposure to the behavioral stressors completely blocked the enhancing effect of BLA priming on DG LTP (Figs.5, 6;post hoc comparisons: BLA priming vs platform-BLA: 30 min,p < 0.001; 90 min, p < 0.001; 150 min, p = 0.01; 180 min, p < 0.05; BLA priming vs UWT-BLA: 30 min, p < 0.05; 90 min,p < 0.05; 150 min, p = 0.051; 180 min,p = 0.053).
Fig. 5.
Changes in LTP after combined exposure to platform and BLA priming. Previous exposure to the platform completely blocked the enhancing effect of BLA priming on LTP (*significant difference between the BLA priming group and the other groups; #significant difference between the platform-BLA group and the HFS and BLA priming groups). A comparison between the groups before HFS to the PP did not reveal a significant difference in the levels of the EPSP, indicating a similar baseline.
Fig. 6.
Changes in LTP after combined exposure to UWT and BLA priming. Previous exposure to the UWT completely blocked the enhancing effect of BLA priming on LTP (*significant difference between the BLA priming group and the two other groups;#significant difference between the UWT and the UWT-BLA groups). A comparison between the groups before HFS to the PP did not reveal a significant difference in the levels of the EPSP, indicating a similar baseline.
DISCUSSION
Both behavioral stressors significantly inhibited the expression of LTP at 30 min after HFS compared with a control (HFS) group. These results are compatible with those observed in other models of stress (Diamond et al., 1994; Xu et al., 1998). However, reports in the literature examined the effects of stressors on LTP in the anesthetized rat only for 30 or 60 min after HFS. Recording the field potentials 3 hr after HFS to the PP showed that although LTP levels were inhibited 30 min after HFS, from 90 min and onward LTP levels recovered and were no longer different from the HFS group. A possible explanation is that in the stressed animals HFS to the PP did induce LTP, but the LTP was masked rather than inhibited at 30 min after HFS. Because there was no similar recovery in animals that received just the test stimulation (the non-HFS group), the mere passage of time cannot account for these effects.
The UWT was held in a safe or familiar context for the animals (because they were trained in the water maze for 5 d for a controllable spatial learning task); thus this stressor may potentially have more aversive effects on the animals than the exposure to platform. In addition, the UWT was found to have both short-term (1 hr) and long-term (3 weeks) behavioral and electrophysiological consequences (Richter-Levin, 1998). The UWT did not cause any noticeable physical harm that could explain the effects seen, because in a previous study we found that the UWT impaired the performance in a spatial learning task in the water maze after the trauma, whereas out-of-context UWT (in a different water container in a different room) had no effect on performance (Richter-Levin, 1998).
The UWT procedure involves a spatial learning component, which may have interacted with the effects of the stress. However, previous reports suggest that training in the water maze on a spatial learning task is not associated with LTP reduction in the DG (Jeffery and Morris, 1993). Moreover, the exposure to the platform, which did not involve a spatial learning component, showed the same effects on synaptic plasticity in the DG as the UWT.
Psychological stress and stress-induced levels of glucocorticoids disrupt hippocampal LTP (Pavlides et al., 1993). It is thus possible that increased glucocorticoid levels mediate the UWT and the platform procedure effects.
The inhibiting effect of the spaced activation of the BLA or of previous exposure to stress on DG LTP may be looked at as a form of metaplasticity, i.e., a dormant plasticity, which becomes apparent only when attempting to modify synaptic strength (Abraham et al., 1997). Metaplasticity may serve as a way for synapses to integrate a response across temporally spaced episodes of synaptic activity and by this may be important for the establishment of some aspects of memory (Abraham et al., 1997). Indeed, studies examining the effects of post-training treatments on memory suggest that the amygdala may be involved in orchestrating the interactions of hormonal and transmitter systems in their influences on the storage of information (McGaugh, 1990).
The present work provides evidence that there is a substantial difference between the activation of the amygdala in proximity to the stimulation of the PP and to activation that is more spaced in time. Priming the BLA enhanced synaptic plasticity, whereas the spaced activation of the BLA inhibited LTP. Furthermore, previous exposure to behavioral stress blocked the enhancing effects of BLA priming on LTP. Thus, the results raise a possible biphasic model for the involvement of the amygdala in emotionally influenced memory.
The first phase is a fast one: it is activated within seconds, and its influence lasts for a short period. The effects of this stage on hippocampal excitability are mostly facilitatory.
The second phase takes more time to develop, and its influence is longer lasting. The effect of this phase on hippocampal excitability is mostly inhibitory.
Present results in view of the proposed model
In animals that were exposed to the behavioral stressor HFS was applied 1 hr after the stressor, thus under the influence of the second, inhibiting phase (Fig.7A). Therefore, after stress we found reduced levels of LTP. According to the model, the stimulation of the BLA first activates the fast phase and then the second, slow phase. Applying HFS to the PP in proximity to the stimulation of the BLA results in high levels of LTP, presumably because the hippocampus is under the influence of the first facilitatory phase (Fig. 7B, PP1, the first point of stimulation to the PP).
Fig. 7.
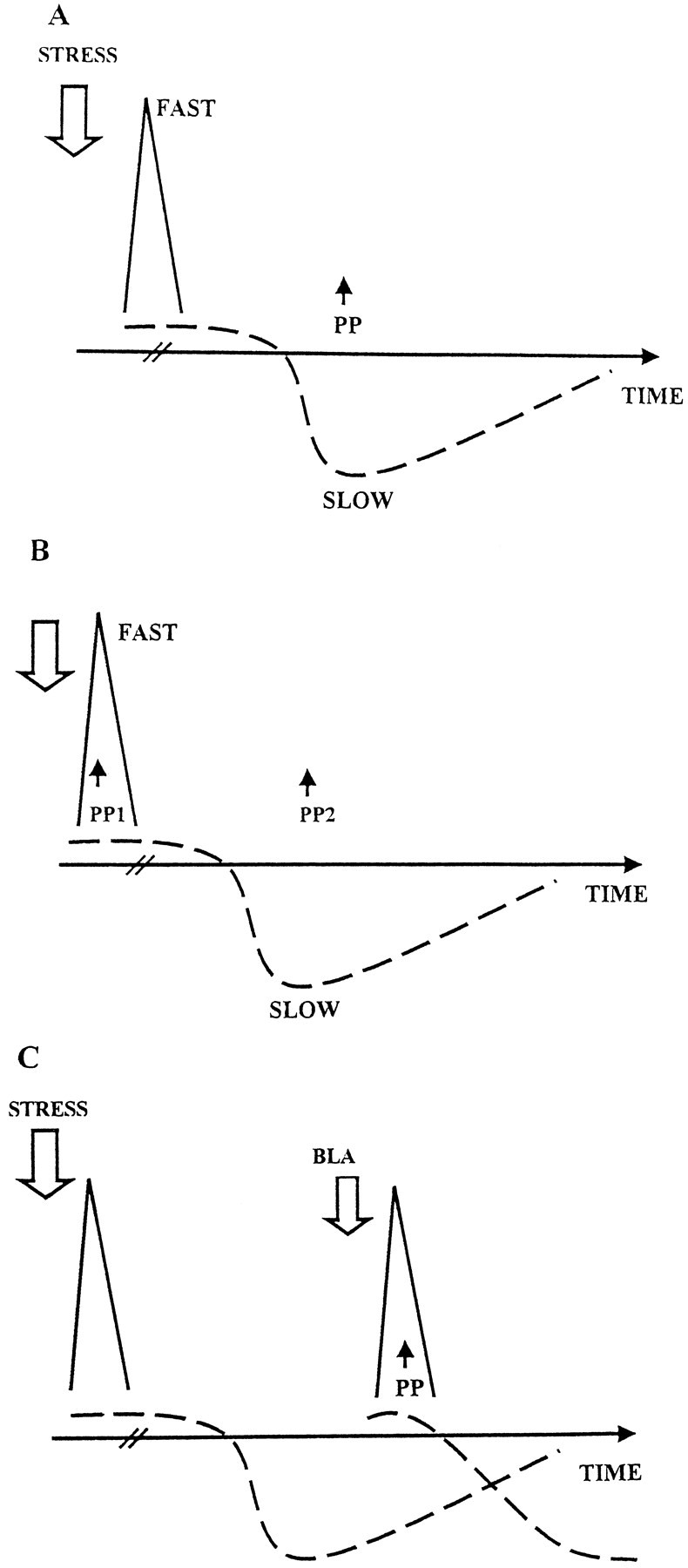
Present results in view of the proposed model (see Discussion for details). Two phases of hippocampal modulation by the BLA are suggested: a fast, short-living excitatory phase (triangle) and a slow, longer-lasting inhibitory phase (broken line). A, In animals that were exposed to the behavioral stressor, the attempt to induce LTP was performed 1 hr after the stressor and thus was under the influence of the second inhibiting phase. Hence, LTP was inhibited.B, Applying HFS to the PP in proximity to the stimulation of the BLA (PP1) resulted in high levels of LTP, because the hippocampus was under the influence of the first facilitatory phase. The stimulation of the PP 1 hr after BLA priming (PP2) was under the influence of the already active slow inhibitory phase. Thus, similarly to the effects of emotional stress, LTP was inhibited. C, When an animal was exposed to a stressor 1 hr before the stimulation of both the BLA and the PP, the hippocampus at the time of the HFS was under the influence of the second inhibitory phase, activated by the stressor. The inhibitory mechanism dominated the BLA-induced fast phase and inhibited the enhancing effects of BLA priming on LTP.
The stimulation of the PP 1 hr after BLA priming (Fig. 7B,PP2) was induced under the influence of the already active slow inhibitory process. Thus, similarly to the effects of emotional stress, LTP was inhibited.
When an animal was exposed to a stressor 1 hr before the stimulation of both the BLA and the PP (Fig. 7C), the hippocampus, at the time of the HFS, was under the influence of the second inhibitory phase, activated by the stressor. According to the results, the stressor dominates the BLA priming and inhibits both the expression of LTP and the excitatory effects of BLA priming.
Summary
The amygdala has a biphasic effect on hippocampal plasticity: an immediate excitatory effect and a long-lasting inhibitory effect.
Recent studies (Morris and Frey, 1997) point to intracellular mechanisms that enable transient synaptic changes to be stabilized if they occur in close temporal proximity to important events. The emotionally activated amygdala in its fast excitatory phase may serve as a marker for important events, processed by the hippocampus, to be stabilized and thus remembered. The activation of the slower inhibitory phase may be beneficial to reduce masking effects of following events during the initial consolidation stage. This dual effect of the amygdala on hippocampal plasticity, which may subserve memory formation under normal conditions, may become disadvantageous under extreme stress conditions, because it may impair aspects of memory consolidation.
Footnotes
This work was supported by Binational United States–Israel Science Foundation Grant 96-291 to G.R.-L. We thank Dr. Menahem Segal for helpful comments on a previous version of this paper.
Correspondence should be addressed to Dr. Gal Richter-Levin, Laboratory of Behavioral Neuroscience, Department of Psychology, University of Haifa, Haifa 31905, Israel. E-mail: gal.r-l@psy.haifa.ac.il.
REFERENCES
- 1.Abraham WC, Tate WP. Metaplasticity: a new vista across the field of synaptic plasticity. Prog Neurobiol. 1997;52:303–323. doi: 10.1016/s0301-0082(97)00018-x. [DOI] [PubMed] [Google Scholar]
- 2.Akirav I, Richter-Levin G. Primimng stimulation in the basolateral amygdala modulates synaptic plasticity in the rat dentate gyrus. Neurosci Lett. 1999;270:83–86. doi: 10.1016/s0304-3940(99)00488-7. [DOI] [PubMed] [Google Scholar]
- 3.Anisman H, Bignami G. Psychopharmacology of aversively motivated behavior. Plenum; New York: 1978. [Google Scholar]
- 4.Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 5.Bliss TVP, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol (Lond) 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis M. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol Sci. 1992;13:35–41. doi: 10.1016/0165-6147(92)90014-w. [DOI] [PubMed] [Google Scholar]
- 7.Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- 8.Diamond DM, Rose GM. Stress impairs LTP and hippocampal-dependent memory. Ann NY Acad Sci. 1994;746:411–414. doi: 10.1111/j.1749-6632.1994.tb39271.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikegaya Y, Saito H, Abe K. Attenuated hippocampal long-term potentiation in basolateral amygdala-lesioned rats. Brain Res. 1994;656:157–164. doi: 10.1016/0006-8993(94)91377-3. [DOI] [PubMed] [Google Scholar]
- 11.Ikegaya Y, Saito H, Abe K. High-frequency stimulation of the basolateral amygdala facilitates the induction of long-term potentiation in the dentate gyrus in vivo. Neurosci Res. 1995;22:203–207. doi: 10.1016/0168-0102(95)00894-7. [DOI] [PubMed] [Google Scholar]
- 12.Jeffery KJ, Morris RGM. Cumulative long-term potentiation in the rat dentate gyrus correlates with, but does not modify performance in the water maze. Hippocampus. 1993;3:123–125. doi: 10.1002/hipo.450030205. [DOI] [PubMed] [Google Scholar]
- 13.Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through NMDA receptor activation. Proc Natl Acad Sci USA. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeDoux JE. Brain mechanisms of emotion and emotional learning. Curr Opin Neurobiol. 1992;2:191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- 15.LeDoux JE. Emotional memory systems in the brain. Behav Brain Res. 1993;58:69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- 16.Martinez JL, Jensen RA, Messing RB, Rigter H, McGaugh JL. Endogenous peptides and learning and memory processes. Academic; New York: 1981. [Google Scholar]
- 17.McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 18.McEwen BS, Dekloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986;66:1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- 19.McGaugh JL. Dissociating learning and performance: drug and hormone enhancement of memory storage. Brain Res Bull. 1989;23:339–345. doi: 10.1016/0361-9230(89)90220-7. [DOI] [PubMed] [Google Scholar]
- 20.McGaugh JL. Significance and rememberance: the role of neuromodulatory systems. Psychol Sci. 1990;1:15–25. [Google Scholar]
- 21.McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 22.Morris RGM. Development of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 23.Morris RGM, Frey U. Hippocampal synaptic plasticity: role in spatial learning or the automatic recording of attended experience? Philos Trans R Soc Lond B Biol Sci. 1997;352:1489–1503. doi: 10.1098/rstb.1997.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc Natl Acad Sci USA. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Packard MG, Williams CL, Cahill L, McGaugh JL. The anatomy of a memory modulatory system: from periphery to brain. In: Spear N, Spear L, Woodruff M, editors. Neurobehavioral plasticity: learning, development and response to brain insults. Erlbaum; NJ: 1995. pp. 149–184. [Google Scholar]
- 26.Pavlides G, Watanabe Y, McEwen BS. Effects of glucocorticoids on hippocampal long-term potentiation. Hippocampus. 1993;3:183–192. doi: 10.1002/hipo.450030210. [DOI] [PubMed] [Google Scholar]
- 27.Richter-Levin G. Acute and long-term behavioral correlates of underwater trauma—potential relevance to stress and post-stress syndromes. Psychiatry Res. 1998;79:73–83. doi: 10.1016/s0165-1781(98)00030-4. [DOI] [PubMed] [Google Scholar]
- 28.Richter-Levin G, Segal M. The effects of serotonin depletion and raphe grafts on hippocampal electrophysiology and behavior. J Neurosci. 1991;11:1585–1596. doi: 10.1523/JNEUROSCI.11-06-01585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richter-Levin G, Greenberger V, Segal M. The effects of general and restricted serotonergic lesions on hippocampal electrophysiology and behavior. Brain Res. 1994;642:111–116. doi: 10.1016/0006-8993(94)90911-3. [DOI] [PubMed] [Google Scholar]
- 30.Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 31.Roozendaal B, McGaugh JL. Amygdaloid nuclei lesions differentially affect glucocorticoid-induced memory enhancement in an inhibitory avoidance task. Neurobiol Learn Mem. 1996;65:1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- 32.Roozendaal B, McGaugh JL. Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiol Learn Mem. 1997;67:176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- 33.Shors TJ, Dryver E. Effect of stress and long-term potentiation (LTP) on subsequent LTP and the theta burst response in the dentate gyrus. Brain Res. 1994;15:232–238. doi: 10.1016/0006-8993(94)90777-3. [DOI] [PubMed] [Google Scholar]
- 34.Shors TJ, Gallegos RA, Breindl A. Transient and persistent consequences of acute stress on long-term potentiation (LTP), synaptic efficacy, theta rhythms and bursts in area CA1 of the hippocamus. Synapse. 1997;26:209–217. doi: 10.1002/(SICI)1098-2396(199707)26:3<209::AID-SYN2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 35.Thomas SR, Assaf SY, Iversen SD. Amygdaloid complex modulats neurotransmission from the entorhinal cortex to the dentate gyrus of rat. Brain Res. 1984;307:363–365. doi: 10.1016/0006-8993(84)90496-7. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Anwyl R, Rowan MJ. Behavioral stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- 37.Xu L, Holscher C, Anwyl R, Rowan MJ. Glucocorticoid receptor and protein/RNA synthesis-dependent mechanisms underlie the control of synaptic plasticity by stress. Proc Natl Acad Sci USA. 1998;95:3204–3208. doi: 10.1073/pnas.95.6.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]