Abstract
Modulation of GABAA receptors by benzodiazepines (BZDs) is believed to involve two distinct steps: a recognition step in which BZDs bind and a conformational transition step in which the affinity of the receptor for GABA changes. Previously, using γ2/α1 chimeric subunits (χ), we demonstrated that although the N-terminal 167 γ2 amino acid residues confer high-affinity BZD binding, other γ2domains couple BZD binding to potentiation of the GABA-mediated Cl− current (IGABA). To determine which γ2 regions couple binding to potentiation, we generated χs with longer N-terminal γ2 segments for voltage-clamp experiments in Xenopus oocytes. Chimeras containing greater than the N-terminal 167 γ2 residues showed incremental gains in maximal potentiation for diazepam enhancement ofIGABA. Residues in γ2199–236, γ2224–236 (pre-M1), and particularly γ2257–297 (M2 and surrounding loops) are important for BZD potentiation. For several positive BZD modulators tested, the same regions restored potentiation of IGABA. In contrast, β-carboline inverse-agonism was unaltered in chimeric receptors, suggesting that structural determinants for positive and negative BZD allosteric modulation are different. Dissection of the γ2257–297 domain revealed that three residues in concert, γ2T281, γ2I282 (M2 channel vestibule), and γ2S291 (M2–M3 loop) are necessary to impart full BZD potentiation to chimeric receptors. Thus, these residues participate in coupling distant BZD-binding events to conformational changes in the GABAA receptor. The location of these novel residues provides insight into the mechanisms underlying allosteric coupling for other members of the ligand-gated ion channel superfamily.
Keywords: GABA, GABAA receptor, benzodiazepines, benzodiazepine-binding site, allosteric coupling, chimeric subunits, mutagenesis, inverse agonist, positive modulation, γ subunit, α subunit, M2 domain, M2–M3 loop, Xenopus oocytes
GABAAreceptors are the major inhibitory neurotransmitter receptors in the mammalian brain and are members of a ligand-gated ion channel (LGIC) superfamily (Ortells and Lunt, 1995), which also includes receptors for acetylcholine, glycine, and serotonin. The GABAAreceptor gene family comprises several different classes and subtypes of receptor subunits including 6α, 4β, 3γ, 1δ, 1ε, and 1π (Barnard et al., 1998). GABAA receptors are pentameric proteins containing an integral chloride-selective channel with specific binding sites for GABA, benzodiazepines (BZDs), barbiturates, and steroids (Smith and Olsen, 1995). BZDs, clinically used for their anxiolytic and antiepileptic actions, exert their therapeutic effects by allosteric modulation of GABAA receptors (Sieghart, 1995). Positive BZD modulators increase the opening frequency of the Cl− channel in the presence of GABA, whereas negative BZD modulators (e.g., β-carbolines) decrease the opening frequency (Rogers et al., 1994). Because the therapeutic clinical value of these drugs depends on their ability to exert positive modulation on IGABA, we were interested in identifying the structural determinants underlying BZD efficacy in potentiating GABA-gated currents.
Evidence suggests that both the α and γ subunits play critical roles in BZD binding and potentiation. Using the agonist-binding site of the nicotinic ACh receptor as an archetype for pentameric LGIC receptors (Czajkowski et al., 1993), the BZD-binding site of the GABAA receptor has been modeled with the γ subunit apposed to an α subunit, and both subunits contributing to the binding site at the interface (Galzi and Changeux, 1994; Smith and Olsen, 1995). Although several studies have begun to identify amino acids in both γ and α subunits involved in BZD binding (for review, see Sigel and Buhr, 1997), little is known about the structural components involved in coupling BZD binding to BZD potentiation of GABA-gated current (IGABA).
Our previous studies using γ2/α1 chimeric subunits demonstrated that chimeras (χ) containing the N-terminal 161 amino acid residues of γ2 exhibit wild-type binding but drastically impaired potentiation ofIGABA by BZDs (Boileau et al., 1998). To further delineate the regions unique to the γ2 subunit that confer BZD potentiation, we generated additional γ2/α1 chimeras, expanding the length of the N-terminal γ2portion and reducing the α1 C-terminal portion (Fig. 1). Two main regions of the γ subunit, γ2224–236 and γ2257–297, improve the allosteric coupling of BZD binding to potentiation of IGABA. The largest gain in potentiation is conferred by the γ2257–297 region, which surrounds and includes the M2 transmembrane segment. Further investigation revealed that a triplet set of residues, γ2T281, I282, and S291 underlie this function of the γ2 subunit. Unlike positive modulatory BZD compounds, the negative modulator 3-carbomethoxy-4-ethyl-6,7-dimethoxy-β-carboline (DMCM) exhibits full wild-type inhibition for all chimeric receptors.
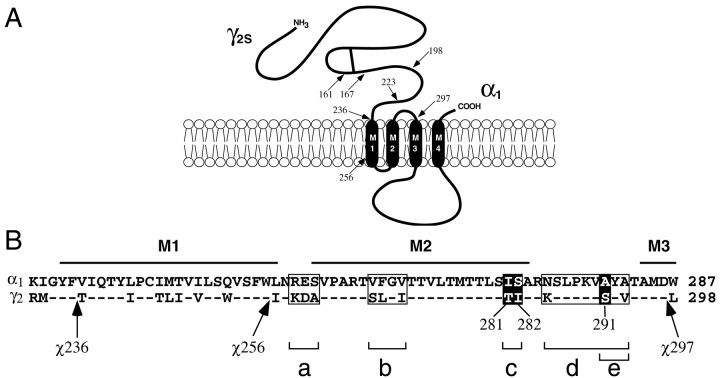
Fig. 1.
Chimeric γ2/α1subunits and mutants. A, Chimeras (χ) used in this study contain 5′ γ2 and 3′ α1 sequences and are named for the γ2 amino acid residue where the crossover with α1 occurs (arrows) in the mature rat protein sequence. For example, χ161 contains γ2 residues from position 1 to 161 and α1residues in the remainder of the subunit. Chimeric crossovers depicted are for χ161, χ167, χ198, χ223, χ236, χ256, and χ297.B, Shown are aligned α1 and γ2 protein sequence segments containing the putative transmembrane domains M1, M2, and the beginning of M3, with the relative crossover positions of χ236, χ256, and χ297 indicated (arrows). Mutants were constructed in the background of χ236. Numbering for χ and γ2 subunits is identical.Boxes indicate blocks of mutations constructed simultaneously: box a represents the substitution of α1 RES residues to the aligned γ2 residues KDA (RES→KDA); box b corresponds to VFGV→SLGI; boxc corresponds to IS→TI; box dcorresponds to NAA→KSV; box e corresponds to a subset of box d, AA→SV. Residues highlighted inblack are important for positive BZD modulation ofIGABA, and their positions in γ2 are indicated below them.
MATERIALS AND METHODS
Molecular cloning. Chimeras are named for the last γ2 amino acid residue before the crossover with α1 in the mature rat protein sequence. Thus, numbering for χ and γ2 subunits is identical. For chimeras used in this study (Fig. 1), the γ2 and α1 amino acid residues at the crossovers are “γD161/αA149”(χ161), “γL167/αK155” (χ167), “γL198/αN188” (χ198), “γM223/αT213” (χ223), “γF236/αV226” (χ236), “γW256/αL246” (χ256), and “γD297/αW287” (χ297). χ161 and χ167 were generated by a targeted random chimera production method (Boileau et al., 1998). All others (Fig.1A) were produced by recombinant PCR using overlapping complementary oligonucleotides designed to create a γ/α crossover at the desired amino acid junction. Using a γ2 sense oligonucleotide and an antisense γ/α junction oligonucleotide with γ2 cDNA as a template, upstream PCR fragments with γ25′ and an α1 3′ overhang were generated. Simultaneously, using a sense junction oligonucleotide and an α1 antisense oligonucleotide with α1 cDNA as template, downstream PCR fragments with γ2 5′ overhang and α1 3′ sequences were also prepared in separate PCR reactions. Upstream and downstream PCR fragments were then combined and amplified to create γ/α DNA cassette fragments that were subcloned into χ167 cDNA using AflII and NcoI, thus replacing the cassette region of χ167 with the new chimeric junction sequences.
Mutant subunit fragments were generated using the same recombinant PCR method and subcloned into the background of the χ236 subunit to generate different combinations of mutants (Fig. 1B). Single and multiple mutants were named according to the α1 to γ2 substitutions made in χ236. For example, the “χ236-RES→KDA” mutant is χ236 with α1 Arg-Glu-Ser (RES) residues replaced by the aligned γ2 sequence at positions 259–261, Lys-Asp-Ala (KDA). The “χ236-A291S” mutant replaces α1 Ala with γ2Ser291 in χ236; the “χ236-I281T + A291S” mutant is χ236 with an I281T and an A291S mutation. The chimeric and mutant subunits were subcloned in pGH19 vector (Liman et al., 1992; Robertson et al., 1996) for expression in Xenopus oocytes. All chimeras were verified by restriction digest and double-stranded DNA sequencing using standard techniques (Sambrook et al., 1989).
Expression of rat GABAAreceptors inXenopus oocytes. Capped cRNA coding for the wild-type and chimeric subunits was synthesized by in vitrotranscription from NheI-linearized cDNA template in pGH19 using the mMessage mMachine T7 kit (Ambion). Oocytes from Xenopus laevis were prepared as previously described (Boileau et al., 1998) and injected with 28 nl of mRNA (10–200 pg/nl/subunit) mixed in a ratio of 1:1 (α:β), or 1:1:≥20 (α:β:γ or α:β:χ). Excess molar ratios of γ or χ cRNA were injected to ensure expression of these subunits in the receptor complex (Boileau et al., 1998). Oocytes were stored at 17–19°C in recording solution supplemented with 100 μg/ml gentamycin and 100 μg/ml BSA, and were used for electrophysiological experiments 2–14 d after injection. Total amount of cRNA was scaled to yield maximal GABA-induced currents of ∼3–8 μA for α1β2γ2and α1β2χ. cRNA concentrations were calculated by UV absorption and corroborated by comparison to RNA standards on 1.5% agarose gels.
Voltage-clamp analysis. Oocytes under two-electrode voltage-clamp (Vhold = −80 mV) were perfused continuously with ND96 recording solution containing (in mm) 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, and 5 HEPES, pH 7.4 at a rate of 5 ml/min. In general, drugs and reagents were dissolved in ND96. Stock drug solutions (1000–10,000×) were made in dimethylsulfoxide. No differences in currents were observed with the vehicle. Maximal current (∼3–8 μA) for all receptors was achieved with 10 mm GABA. For some chimeras and mutants, GABA EC50 was estimated by examining the ratio of response to 1 μm versus 10 mm GABA. GABA (1 μm) elicits currents corresponding to EC5 for both α1β2χ (range, EC2–7) and α1β2γ2(EC4.8 ± 1.7) receptors. Using a standard Hill equation to model the GABA dose responses and the determined 1 μm/10 mm GABA current ratio, we calculate that the chimeric and mutant receptors show no more than a twofold shift in GABA EC50 from wild-type receptors (EC50 = 12 μm). Greater than twofold shifts are detectable in our assay.
BZD potentiation or β-carboline inhibitionIGABA were recorded at 1 μm GABA (EC5). Potentiation is defined as (IGABA + DRUG/IGABA) − 1), where IGABA + DRUG is the current response in the presence of the drug tested, andIGABA is the control GABA-induced current. For single concentration experiments, BZD site ligands were tested at concentrations eliciting maximal effects onIGABA in wild-type α1β2γ2receptors. If differences in potentiation for mutant receptors were caused entirely by a shift in GABA affinity, the Hill equation predicts that a sixfold shift in GABA affinity, and only to the left, would be required to account for a 50% reduction in maximal potentiation. This would result in a 1 μm/10 mm GABA ratio corresponding to EC27–32 (Hill coefficient varied from 1 to 2), well out of our observed range. Standard two-electrode voltage-clamp recording was performed using a GeneClamp 500 (Axon Instruments, Foster City, CA) interfaced to a computer with an IT-16 A/D device (Instrutech). Electrodes were filled with 3 m KCl and had a resistance of 0.5–1.5 MΩ.
Data acquisition and analysis were performed using AxoData, AxoGraph (Axon Instruments), and Prism software (Graphpad). Statistical comparisons of potentiation data employed one-way ANOVA with Dunnet and Tukey post-tests for multiple independent samples using Prism software.
RESULTS
Regions of the γ2 subunit responsible for BZD binding can be separated from domains required for coupling BZD binding to potentiation of IGABA(Boileau et al., 1998). This “uncoupling” of high-affinity BZD binding and potentiation is apparent in the γ/α chimera χ161, which binds BZDs with wild-type affinity when expressed with wild-type α1 and β2 subunits, but displays drastically impaired diazepam modulation ofIGABA. In an effort to identify regions of the γ2 subunit required for full BZD potentiation, several additional chimeras (Fig. 1A) were constructed with longer γ2 N-terminal domains. Chimeras used here, named for the γ2amino acid where the crossovers occur, are χ161, χ167, χ198, χ223, χ236, χ256, and χ297 (see Materials and Methods). These chimeras were expressed in Xenopus oocytes and screened by two-electrode voltage clamp to test for restoration of allosteric modulation of IGABA with several structurally diverse BZD-binding site ligands. The expression, BZD radioligand binding, and diazepam modulation of GABA-gated currents for α1β2χ161 and α1β2χ167 receptors have been described previously (Boileau et al., 1998).
Different domains alter BZD EC50 and potentiation
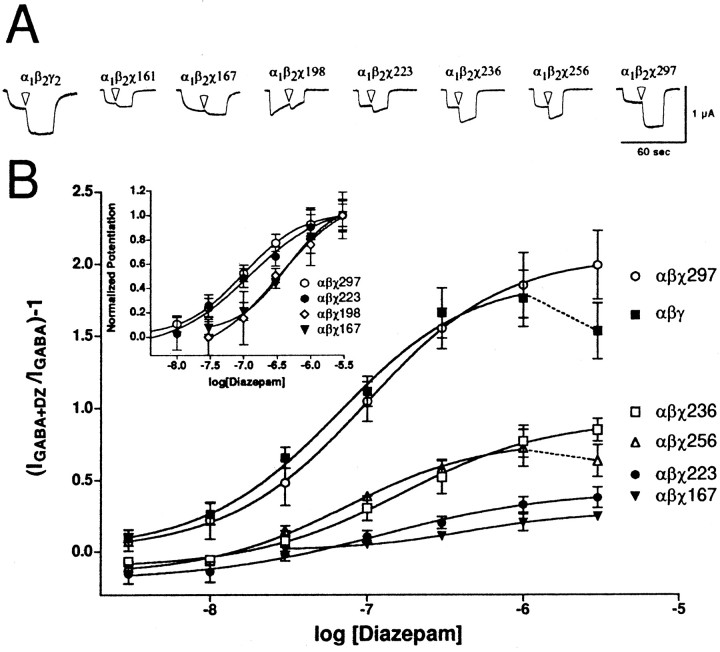
Chimeric cRNA was coinjected with wild-type α1 and β2 subunit cRNA into oocytes and tested for diazepam potentiation ofIGABA with 1 μm GABA. This concentration corresponded to EC5 for GABA for both wild-type and chimeric receptors. Both maximal potentiation and EC50 for diazepam modulation of IGABA were measured. Dose–response curves and traces for diazepam potentiation of the GABA response for selected chimera-containing and wild-type receptors are depicted in Figure 2. At concentrations >1 μm diazepam, potentiation of wild-type receptors begins to depress (Fig. 2, dashed line;Amin et al., 1997). The decrease may be attributable to channel block and/or BZD blockage of the GABA-binding site. Receptors containing χ161 and χ167 exhibited low potentiation, whereas chimeras with increasingly longer N-terminal γ2 segments exhibited incremental gains in maximal potentiation (Table1, Fig. 2). Full potentiation was restored with χ297, which contains γ2residues up to the beginning of the M3 transmembrane domain. For maximal potentiation, data from chimeric and wild-type receptors yielded the series αβ ≪ αβχ161 ≈ αβχ167 < αβχ198 ≈ αβχ223 < αβχ236 ≈ αβχ256 ≪ αβχ297 ≈ αβγ.
Fig. 2.
Diazepam potentiation ofIGABA for α1β2χ receptors. A, Trace recordings from cells injected with α1β2γ2(leftmost), and chimeric constructs. Cells were voltage-clamped at −80 mV and perfused with ND96 recording solution or ND96 with 1 μm GABA or 1 μm GABA plus 1 μm diazepam (transition to diazepam-containing solutions indicated by white arrowheads). Cells were washed with ND96 recording solution for 5–20 min between drug applications. α1β2χ198 exhibits unusually fast desensitization properties. Note that chimeras show incrementally larger potentiation up to α1β2χ297, which is similar to wild-type α1β2γ2. B,Oocytes injected with wild-type α1β2γ2 (1:1:20) and α1β2χ (1:1:≥20) cRNA mixtures were treated with a range of diazepam concentrations in the presence of GABA and further analyzed. A potentiation response ratio was determined by dividing the peak current for α1β2γ2 (▪), α1β2χ167 (▾), α1β2χ198 (⋄), α1β2χ223 (●), α1β2χ236 (■), α1β2χ256 (▵), and α1β2χ297 (○) exposed to 1 μm GABA plus diazepam (DZ) by the response to 1 μm GABA alone. Data were fitted to a curve described by the equation Y = Min + (Max − Min)/(1 + 10((LogEC50−X)·nH)), where Max is the maximal potentiation,Min is the potentiation at the lowest drug concentration tested, X is the logarithm of diazepam concentration,EC50 is the half-maximal potentiation response, and nH is the Hill coefficient. Data points represent mean potentiation from four or more cells from two or more batches of oocytes. Error bars indicate SD. The parameters from the curve fits are presented in Table 1. Inset, A plot of the same data after normalizing to the maximum diazepam potentiation for each receptor.
Table 1.
Summary of dose–response data for diazepam potentiation of chimeric and wild-type receptors
| Diazepam potentiation | |||
|---|---|---|---|
| Max. Pot | EC50(nm) | EC50(mut)/ EC50(wt) | |
| α1β2γ2 | 1.91 ± 0.17 | 64 ± 15 | 1.0 |
| α1β2 | 0.03 ± 0.03 | – | – |
| α1β2χ161 | 0.26 ± 0.01 | 310 ± 45 | 4.8 |
| α1β2χ167 | 0.28 ± 0.02 | 437 ± 87 | 6.8 |
| α1β2χ198 | 0.52 ± 0.18 | 340 ± 118 | 5.3 |
| α1β2χ223 | 0.42 ± 0.14 | 120 ± 43 | 1.9 |
| α1β2χ236 | 0.94 ± 0.11 | 164 ± 29 | 2.6 |
| α1β2χ256 | 0.77 ± 0.02 | 73 ± 26 | 1.1 |
| α1β2χ297 | 2.05 ± 0.03 | 98 ± 5 | 1.5 |
| α1β2χ236-I281T+A291S | 1.05 ± 0.04 | 165 ± 17 | 2.6 |
| α1β2χ236-S282I+A291S | 1.09 ± 0.19 | 187 ± 32 | 2.9 |
| α1β2χ236-IS→TI | 1.07 ± 0.06 | 31 ± 5 | 0.5 |
| α1β2χ236-A291S | 1.67 ± 0.19 | 144 ± 37 | 2.2 |
| α1β2χ236-IS→TI+A291S | 2.07 ± 0.16 | 109 ± 20 | 1.7 |
Dose–response data for wild-type and chimeric subunit combinations for diazepam potentiation of 1 μm GABA responses are tabulated. Means and SDs for maximum potentiation and EC50 values were calculated from dose–response data (Figs. 2,6). Data for each receptor were obtained from ≥6 oocytes from ≥2 batches.
Differences in EC50 for diazepam potentiation ofIGABA between chimeric and wild-type receptors were also measured (Fig. 2, inset; Table 1). Diazepam EC50 values for receptors containing χ223 (120 ± 43 nm), χ236 (164 ± 29 nm), χ256 (73 ± 26 nm), and χ297 (98 ± 5 nm) were not significantly different from those for wild-type α1β2γ2receptors (64 ± 15 nm). However, the EC50 values for diazepam were significantly higher (p < 0.01) for receptors containing χ161, χ167, and χ198 (310 ± 45, 437 ± 87, and 340 ± 118, respectively). A significant decrease in diazepam EC50 occurred between χ198 and χ223 (Fig. 2,inset). It was also noted that χ198 displays unusually fast desensitization to application of either GABA or GABA plus diazepam. The reason for this is unclear, and was not pursued. Interestingly, although the diazepam EC50 for α1β2χ256 receptors was similar to wild-type α1β2γ2receptors, the maximal potentiation was reduced by ∼60% compared to wild-type receptors (Fig. 2; see Fig. 4). The most significant gain of potentiation occurred between χ256 and χ297 (Fig. 1); α1β2χ297 receptors exhibit potentiation and EC50 for diazepam indistinguishable from wild-type receptors.
Fig. 4.
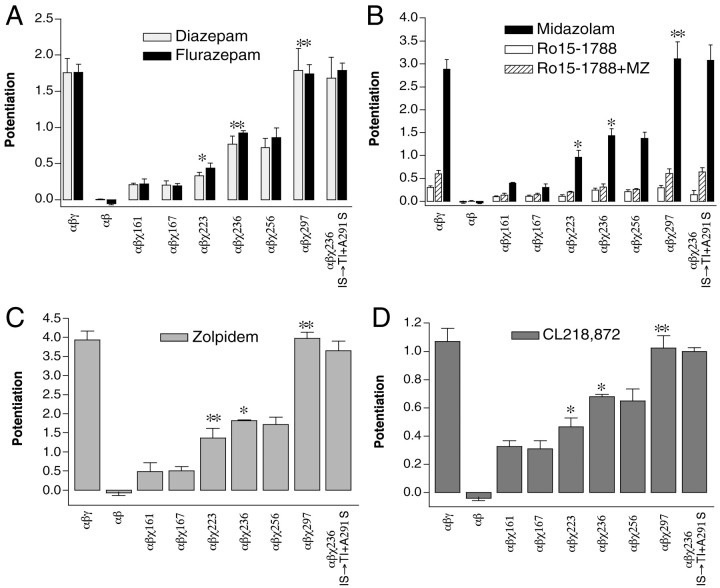
Positive BZD-site modulators potentiate the GABA responses of chimeric receptors to different extents depending on the amount of γ2 subunit in each receptor. Potentiation of 1 μm GABA responses is graphed for wild-type α1β2γ2, α1β2χ161, α1β2χ167, α1β2χ223, α1β2χ236, α1β2χ256, α1β2χ297, and “α1β2χ236-IS→TI + A291S” chimeric receptors using 1 μm diazepam, 1–3 μmflurazepam (A), 1–3 μm midazolam (B), 10 μm zolpidem (C), and 1 μm CL218,872 (D). Also depicted in B is inhibition of the midazolam (MZ) potentiation by 1 μm Ro15–1788 (hatched bars) for chimeric and wild-type receptors. Open bars indicate the potentiation of IGABA by 1 μmRo15–1788. Asterisks indicate level of significance (*p < 0.01; **p < 0.001) comparing mean potentiation for that chimera with the preceding chimera in the series (left to right). Error bars indicate SD.
Positive BZD modulators use the γ257–297 domain
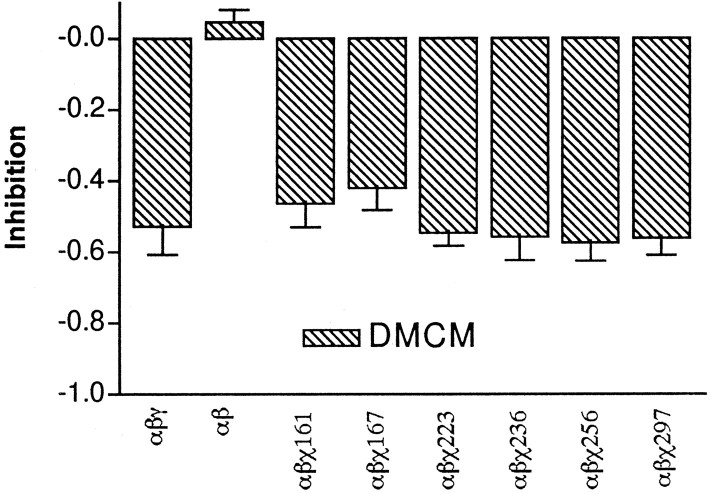
To explore whether potentiation or inhibition by other drugs acting at the BZD site would require similar regions of the γ2 subunit, the chimeras were tested with several different BZD-binding site ligands. Surprisingly, the β-carboline DMCM, an inverse agonist at the BZD site, inhibitedIGABA in all chimeric receptors to the same extent as wild-type α1β2γ2receptors (Fig. 3). In contrast, the positive modulatory BZD site ligands tested showed significant differences in potentiation between chimeric receptors (Fig.4, Table2). The differences between chimeric receptors for positive modulation could be caused by inefficient expression of the chimera in a receptor complex, yielding a mixture of α1β2 and α1β2χ receptors that would appear to have reduced potentiation. However, the observation that each chimera responds to DMCM akin to α1β2γ2receptors, and not α1β2receptors, allays this concern.
Fig. 3.
Chimeric receptors are indistinguishable from wild-type receptors for negative modulation by DMCM. Negative modulation of 1 μm GABA responses by the β-carboline DMCM (1 μm) is graphed for wild-type α1β2γ2, α1β2χ161, α1β2χ167, α1β2χ223, α1β2χ236, α1β2χ256, and α1β2χ297 chimeric receptors. No significant difference in the inhibition of the GABA current was measured between wild-type and chimeric receptors.
Table 2.
Recovery of BZD potentiation of IGABA in chimeric receptors
| Diazepam | Flurazepam | Midazolam | Zolpidem | CL218,872 | |
|---|---|---|---|---|---|
| α1β2χ161 | 12 ± 2% (4) | 12 ± 4% (4) | 14 ± 1% (3) | 12.2 ±6% (7) | 30 ± 5% (3) |
| α1β2χ167 | 12 ± 4% (6) | 11 ± 2% (4) | 11 ± 3% (4) | 13 ± 3% (6) | 29 ± 6% (4) |
| α1β2χ198 | 15 ± 3% (4) | 24 ± 5% (3) | 20 ± 2% (4) | 13 ± 2% (5) | 27 ± 3% (5) |
| α1β2χ223 | 19 ± 4% (6) | 25 ± 4% (5) | 33 ± 6% (3) | 35 ± 7% (5) | 44 ± 7% (5) |
| α1β2χ236 | 44 ± 8% (6) | 52 ± 4% (5) | 50 ± 6% (4) | 46 ± 3% (30) | 64 ± 6% (3) |
| α1β2χ256 | 41 ± 9% (4) | 49 ± 8% (5) | 48 ± 6% (5) | 44 ± 6% (4) | 61 ± 9% (7) |
| α1β2χ297 | 108 ± 21% (6) | 99 ± 10% (8) | 108 ± 15% (6) | 101 ± 7% (8) | 95 ± 12% (7) |
| α1β2χ236-IS→TI+A291S | 96 ± 20% (8) | 102 ± 9% (4) | 107 ± 14% (4) | 93 ± 8% (4) | 93 ± 8% (3) |
Chimeric receptors were tested for modulation ofIGABA in Xenopus oocytes by a variety of BZD-site ligands and normalized to wild-type receptor potentiation. Percentage of recovery is defined as (IGABA + DRUG/IGABA)-1 for α1β2χ receptors divided by that measured for α1β2γ2 receptors × 100. Drug concentrations used were 1 μm diazepam, 1-3 μm flurazepam, 1-3 μm midazolam, 10 μm zolpidem, and 1 μm CL218,872 in the presence of 1 μm GABA. Data are means ± SD. nindicates the number of oocytes from ≥2 batches tested.
Classical 1,4-benzodiazepines such as diazepam and water-soluble flurazepam exhibited similar increments in potentiation ofIGABA in chimeras with increasingly larger N-terminal segments (Fig. 4A). The strong positive modulators midazolam (a triazolobenzodiazepine, Fig.4B) and zolpidem (an imidazopyridine, Fig.4C), and the partial agonist CL218,872 (a triazolopyridazine, Fig. 4D) also showed increments in potentiation from χ167 to χ297, indicating a common pattern of residues required for positive BZD allosteric modulation.
Table 2 summarizes the effects of various BZD site ligands on the potentiation of IGABA for chimeric receptors compared to wild-type α1β2γ2receptors. For all positive modulators tested, a correlation between recovery of potentiation and length of the chimeric γ2 sequence emerges. Small increases in potentiation are observed between χ167 and χ223 (∼10–15%), and between χ223 and χ236 (∼10–25%) for all of the positive modulators tested. Whether a significant recovery of potentiation occurred between χ167 and χ198 or between χ198 and χ223 depended on the drug tested. The largest increases in potentiation (50–60%) for all of the positive modulatory BZDs tested occurred between χ256 and χ297, indicating that amino acid residues surrounding and/or including M2 are required for full potentiation ofIGABA by these drugs.
In addition, we tested the activity of the imidazobenzodiazepine antagonist Ro15–1788 (flumazenil; Fig. 4B) in wild-type and chimera-containing receptors. Ro15–1788 (1 μm) was effective at blocking the midazolam-induced (1 μm) potentiation ofIGABA in all chimeric receptors (Fig.4B, hatched bars). At micromolar concentrations, Ro15–1788 acts as a weak positive modulator of GABA activation (Fig. 4B, open bars; Mihic et al., 1997). The pattern of potentiation in wild-type and chimeric receptors was similar to that seen for other positive modulators. After subtracting out the minor background Ro15–1788-induced potentiation, the midazolam-induced potentiation is inhibited by Ro15–1788 to the same extent for wild-type and all chimeric receptors (91.5 ± 3.7%).
Mutations conferring positive modulation ofIGABA
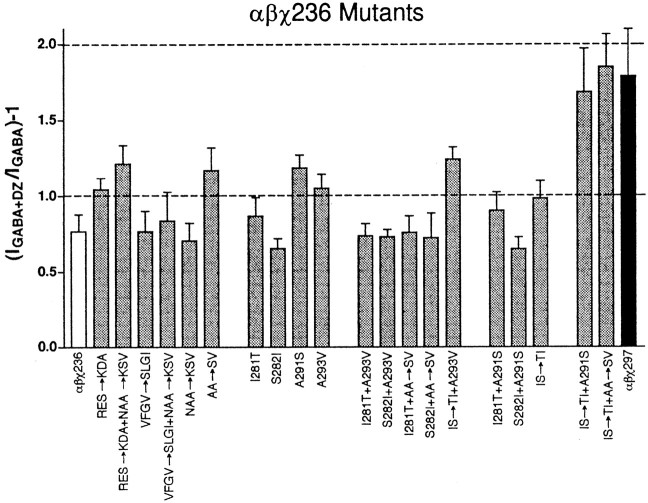
Because the largest gain in BZD potentiation is mediated by the γ2I257-D297 domain, we focused on identifying the amino acid residue or residues in this region that contribute to BZD modulation of IGABA. To determine whether α1β2χ236 receptors are altered in their ability to bind BZDs, we performed radioligand-binding assays. The KD for [3H]flunitrazepam binding in α1β2χ236 receptors (3 ± 1 nm;n = 2) was similar to the values obtained for α1β2χ161 (11.3 ± 1.7 nm) and α1β2γ2(9.9 ± 0.8 nm; Boileau et al., 1998). Several mutants were constructed in a χ236 background, corresponding to nonidentical residues between γ2 and α1 in the M1–M2 loop, the M2 transmembrane domain, and the M2–M3 loop (Fig. 1B). The first set of such mutants with α1 amino acid residues substituted to the homologous γ2 residues were χ236-RES→KDA (Fig. 1B, box a), χ236-RES→KDA + NAA→KSV (box a + box d), χ236-VFGV→SLGI (box b), χ236-VFGV→SLGI + NAA→KSV (box b + box d), χ236-IS→TI (box c), and χ236-IS→TI + AA→SV (box c + box e). Of these, only χ236-IS→TI + AA→SV (box c + box e) exhibited full potentiation ofIGABA by diazepam. All single-, double-, and triple-mutant combinations of the substitutions present in χ236-IS→TI + AA→SV (box c + box e) were generated and tested with 1 μm diazepam for potentiation ofIGABA (Fig.5). Of these, only the triple mutant combination χ236-IS→TI + A291S (Fig. 1B,black outlined residues) restored full potentiation to χ236; all values for diazepam potentiation ofIGABA by mutant receptors depicted in Figure 5 are significantly different (p < 0.01) from those for χ297 except for the quadruple mutant χ236-IS→TI + AA→SV (box c + box e) and the triple mutant χ236-IS→TI + A291S.
Fig. 5.
Diazepam potentiation of chimeric and chimeric mutant receptors. Potentiation of 1 μm GABA current using 1 μm diazepam (DZ) is graphed for α1β2χ236 (white), mutants, and mutant combinations made in the background of α1β2χ236 receptors (gray) and α1β2χ297 (black) chimeric receptors. Potentiation is defined as (IGABA + DZ/IGABA) − 1), whereIGABA + DZ is the current response in the presence of diazepam, and IGABA is the control GABA-induced current. Dashed lines at 1.0 and 2.0 on the ordinate axis are shown for ease of comparison. Only the α1β2χ236-IS→TI + A291S and α1β2χ236-IS→TI + AA→SV chimeric receptors show no significant difference from α1β2χ297 receptors.
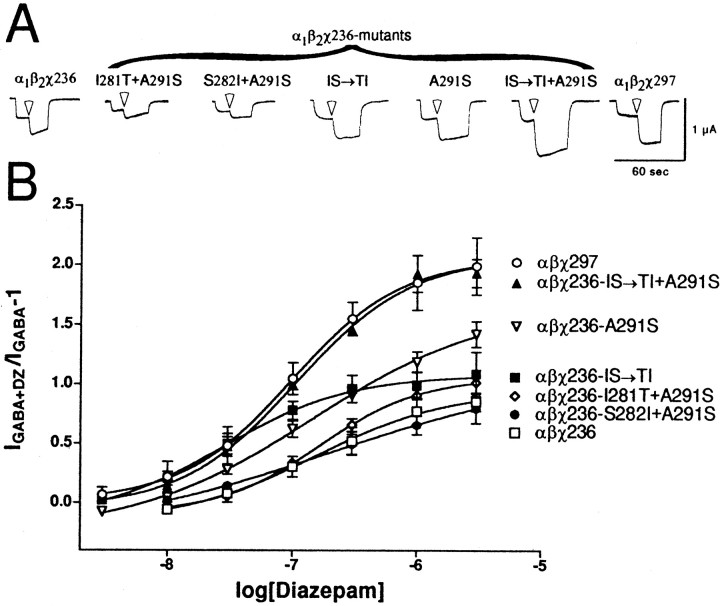
Diazepam dose–response curves for the components of χ236-IS→TI + A291S, namely χ236-IS→TI, χ236-A291S and the other two double mutants χ236-I281T + A291S and χ236-S282I + A291S demonstrate that all three mutations are necessary for full potentiation for diazepam (Fig. 6). Summary data for maximal potentiation and EC50 values are shown in Table1. Although χ236-A291S alone did restore some of the potentiation to χ236, it is still significantly less than for χ297 (p < 0.01). The triple mutant also exhibited wild-type maximal potentiation for other BZD-positive modulators (Fig.4, Table 2).
Fig. 6.
Diazepam potentiation ofIGABA for mutant α1β2χ236 receptors. A,Trace recordings from cells injected with wild-type α1, β2, and either χ236 (left), mutant χ236 (middle), or χ297 (right). Cells were voltage-clamped at −80 mV and perfused with ND96 recording solution, or ND96 with 1 μmGABA or 1 μm GABA plus 1 μm diazepam (transition to diazepam-containing solutions indicated by white arrowheads). Cells were washed with ND96 recording solution for 5–20 min between drug applications. Note that only the α1β2χ236-IS→TI + A291S mutant chimeric receptors show potentiation as large as α1β2χ297 receptors. B,Oocytes injected with chimeric and chimeric mutant cRNA mixtures were treated with a range of diazepam concentrations in the presence of GABA and further analyzed. A potentiation response ratio was determined by dividing the peak current for α1β2χ297 (○), α1β2χ236-IS→TI + A291S (▴), α1β2χ236-A291S (▾), α1β2χ236-IS→TI (▪), α1β2χ236-I281T + A291S (⋄), α1β2χ236-S282I + A291S (●), and α1β2χ236 (■) exposed to 1 μm GABA plus diazepam (DZ) by the response to 1 μm GABA alone. Data were fitted to a curve described by the equation Y = Min + (Max − Min)/(1 + 10((LogEC50− X)·nH)), where Max is the maximal potentiation,Min is the potentiation at the lowest drug concentration tested, X is the logarithm of diazepam concentration,EC50 is the half-maximal potentiation response, and nH is the Hill coefficient. Data points represent mean potentiation from six or more cells from two or more batches of oocytes. Error bars indicate SD. The parameters from the curve fits are presented in Table 1.
DISCUSSION
We previously reported that chimeric subunits with γ2 sequence in the first 161 amino acid residues and α1 sequence in the remainder exhibit high-affinity BZD binding, but impaired BZD modulation ofIGABA (Boileau et al., 1998). Efficient coupling of BZD binding to potentiation ofIGABA is therefore mediated, at least in part, by γ2 amino acid residues that are not directly involved in the binding of BZDs. In this study, we identified two novel γ2 regions, γ2224–236 (pre-M1) and γ2257–297 (M2 and surrounding loops) that are necessary for full restoration of coupling between high-affinity BZD binding and full BZD potentiation ofIGABA by several disparate BZD positive allosteric modulators (Figs. 2, 4). In addition, a threefold shift in EC50 is observed between α1β2χ198 and α1β2χ223 receptors (Table 1). This change in diazepam EC50 may account for some or all of the increased potentiation observed in α1β2χ223 receptors as compared to α1β2χ167 receptors (Figs. 2, 4).
For all the BZD positive allosteric modulators tested, the largest recovery in BZD potentiation of IGABA(50–60%) is conferred by one or more γ2residues in and/or surrounding the M2 region between χ256 and χ297. For this region, a change in diazepam EC50 cannot explain the complete restoration in the ability of diazepam to potentiate IGABA in α1β2χ297 receptors as compared to α1β2χ256 receptors because the diazepam EC50 values for α1β2χ297 and α1β2χ256 receptors are not significantly different from each other or wild-type receptors (Table 1). The differences in levels of potentiation for chimeric receptors are also not caused by inefficient expression of the chimeric subunit, as evidenced by the equivalence of DMCM inhibition ofIGABA for each chimeric receptor and α1β2γ2receptors (Fig. 3). Thus, DMCM inhibition ofIGABA serves as a useful benchmark and control for chimeric insertion into the receptor complex. Finally, the differences in potentiation are not attributable to differences in GABA dose responses. Previously, we established that α1β2χ161 and α1β2χ167 receptors have EC50 values for GABA similar to α1β2γ2and α1β2 receptors (Boileau et al., 1998). In addition, we have calculated that the EC50 values for all of the chimeric receptors are shifted by less than twofold compared to wild-type receptors (see Materials and Methods), which could not alone account for the decrease in maximal potentiation observed. Thus, we conclude that the γ2257–297 region contains structural determinants required for coupling BZD binding to BZD potentiation ofIGABA.
Because DMCM inhibits α1β2χ167 receptors to a similar extent as wild-type α1β2γ2receptors (Fig. 3), negative modulation of the GABA-gated current by β-carboline-binding to the BZD site must be transduced through different structural elements, i.e., using γ2residues located within the first 167 amino acid residues, with or without downstream γ2 residues that are conserved in the α1 and γ2 subunits. Because we are comparing α to γ subunits for differences in BZD function, we cannot detect amino acid residues important for processes that are conserved between the two subunit subtypes.
The specific residues in the γ2257–297 region that underlie BZD potentiation ofIGABA were identified. Of the twelve nonidentical residues between χ256 and χ297, the residues γ2T281, I282, and S291 are, in combination, necessary to confer wild-type potentiation ofIGABA by positive BZD modulators (Figs. 4-6). Although we cannot rule out the possibility that these mutations somehow serve to relieve a conformational dysfunction in the structure of the χ236 subunit and do not play a direct role in BZD actions, we think this unlikely. Wild-type responses to DMCM and equivalent values for competitive displacement by Ro15–1788 for all the chimeric receptors suggest that the overall structures of the chimeras are not severely disrupted. Equilibrium binding values for [3H]flunitrazepam to α1β2χ161, α1β2χ167, and α1β2χ236 receptors were similar to wild-type receptors, indicating that the BZD-binding site is intact. Further support for our conclusion that γ2T281, I282 and S291 are compulsory γ2 elements for BZD activity derives from the fact that all three residues are conserved in all known γ subunits cloned from various species but vary in other subunit subtypes. These identified residues, however, are not the sole γ2 determinants controlling BZD potentiation. Certainly other γ2 amino acid residues also play a role, particularly residues in the pre-M1 regions (e.g., γ2224–236), which have yet to be identified, and/or amino acid residues that are conserved between the γ and α subunits.
Alterations in the ability of positive BZD modulators to potentiateIGABA can arise from several possible sources, including alterations at the BZD-binding site (Colquhoun, 1998), disruptions of the coupling (transduction of BZD binding to potentiation) machinery, and/or modifications of the ion channel pore itself. An example of a mutation that alters BZD “efficacy” of several BZD ligands is the γ2 subunit mutation T142S (Mihic et al., 1994); both a competitive antagonist at the BZD-binding site (Ro15–1788) and a weak negative modulator (Ro15–4513) take on the character of weak positive modulators. Because we previously demonstrated that high-affinity BZD binding is localized to the N-terminal 161 γ2 amino acid residues (Boileau et al., 1998), the novel regions and residues identified in this study control BZD potentiation by influencing the coupling machinery and/or the ion channel pore itself rather than the BZD-binding site. For the chimeric receptors described in this study, strong positive BZD modulators behave like weak modulators or BZD antagonists. Additionally, we observe that α1β2χ161, α1β2χ167, and α1β2χ236 receptors exhibit wild-type, high-affinity radioligand binding. Together, these observations suggest that we are measuring disruptions in coupling rather than binding. Because γ2T281 and I282 are likely to line the water-accessible surface of the Cl− channel based on homology with the α1 subunit (Xu and Akabas, 1996), it is tempting to speculate that this region controls BZD potentiation by affecting the ion channel. These γ2 residues may influence ion channel gating, possibly facilitating channel opening by GABA when BZDs are present.
Structurally, allosteric coupling between GABA and BZD-binding sites could occur exclusively in the N-terminal extracellular domains of the receptor, from one binding site to the other. The Monod–Wyman–Changeux allosteric model predicts that ligand-binding events cause allosteric transitions that change the state of the receptor, which result in changes in the binding sites (Monod et al., 1965). Our chimeric receptors have either weakened or deleted allosteric transitions that are restored by γ2segments distant from the presumed binding sites, and in particular by the combination of the channel-lining residues γ2T281 and I282 coupled with the M2-M3 loop residue γ2S291. Thus, allosteric coupling between the GABA and BZD-binding sites requires transduction through transmembrane or intracellular regions and then back out to the extracellular binding regions, subsequently affecting the kinetics of GABA binding at one or both cooperative GABA-binding sites.
Our results demonstrate that two M2 residues (γ2 T281, I282) and a M2–M3 extracellular loop residue (γ2 S291) are required for full, wild-type BZD potentiation of IGABA by a variety of BZD ligands. It is interesting that these γ2 residues map to corresponding regions as residues in other GABAA receptor subunits and the homologous glycine receptor believed to be involved in “direct” channel gating by agonists. In the GABAAreceptor, when the M2 central leucine is substituted with threonine in the human β1 subunit, GABA activation is abolished, even though binding with the GABA agonist [3H]muscimol is unaltered (Tierney et al., 1996). In the glycine receptor, mutation of α1 R271 (in the M2–M3 loop) to leucine or glutamine converts glycinergic agonists β-alanine and taurine from agonists to competitive antagonists for glycine (Rajendra et al., 1995). Our results that residues in M2 and the M2–M3 loop are necessary for coupling BZD binding to BZD potentiation suggests that not only are the BZD and GABA-binding sites structurally conserved (Olsen et al., 1996), but the regions of the receptor involved in the coupling of binding to their functional effects are also conserved. We speculate that the BZD-binding site may in reality be a very low-affinity GABA-binding site that over evolutionary time has acquired the ability to bind BZDs and when the γ2 M2 region is present, BZDs may act as coagonists and increase channel opening frequency.
Mutations within the M2 region and surrounding loops in the GABAA receptor also affect the actions of other GABAA receptor modulators. Mutations in two specific M2 amino acid residues, α1S267 or the aligned β1S265, affect the ability of ethanol, enflurane, etomidate, loreclezole, and furosemide to modulate the GABAA receptor (Wingrove et al., 1994; Belelli et al., 1997; Mihic et al., 1997; Thompson et al., 1999). Both of these residues align with γ2S280 (Fig.1B). The position of these M2 residues relative to γ2T281 and I282 identified in this study suggest that this region of M2 may not contain the binding site or sites for anesthetics and other related compounds, but instead may act as a common pathway for mediating the coupling between binding and the functional effects of these drugs. Mutations in either M2 or the M2–M3 loop in the glycine receptor also alter the efficacy of modulators and agonists (Rajendra et al., 1995; Lynch et al., 1997). Thus, for LGIC receptors we hypothesize that the extracellular end of the channel and the nearby M2–M3 extracellular loop are transduction domains that help couple distant ligand-binding events to the channel gate.
Footnotes
This work was supported in part by a grant to the University of Wisconsin Medical School under the Howard Hughes Medical Institute Research Program for Medical Schools and by National Institute of Neurological Disorders and Stroke Grant NS34727 to C.C. C.C. is a recipient of the Burroughs Welcome Fund New Investigator Award in the Basic Pharmacological Sciences. We thank Dr. David Wagner and Peter Ward for assistance in construction of mutant receptors, Drs. Matthew Banks, Meyer Jackson, and Gail Robertson for critical reading of this manuscript, and Dr. Robert Pearce for helpful discussions.
Correspondence should be addressed to Dr. Cynthia Czajkowski, University of Wisconsin, Department of Physiology, Room 197 MSC, 1300 University Avenue, Madison, WI 53706. E-mail:czajkowski@physiology.wisc.edu.
REFERENCES
- 1.Amin J, Brooks-Kayal A, Weiss DS. Two tyrosine residues on the alpha subunit are crucial for benzodiazepine binding and allosteric modulation of γ-aminobutyric acidA receptors. Mol Pharmacol. 1997;51:833–841. doi: 10.1124/mol.51.5.833. [DOI] [PubMed] [Google Scholar]
- 2.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International union of pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 3.Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the γ-aminobutyric acid type A receptor is influenced by a single amino acid. Proc Natl Acad Sci USA. 1997;94:11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boileau AJ, Kucken AM, Evers AR, Czajkowski C. Molecular dissection of benzodiazepine binding and allosteric coupling using chimeric γ-aminobutyric acidA receptor subunits. Mol Pharmacol. 1998;53:295–303. doi: 10.1124/mol.53.2.295. [DOI] [PubMed] [Google Scholar]
- 5.Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czajkowski C, Kaufmann C, Karlin A. Negatively charged amino acid residues in the nicotinic receptor delta subunit that contribute to the binding of acetylcholine. Proc Natl Acad Sci USA. 1993;90:6285–9. doi: 10.1073/pnas.90.13.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galzi JL, Changeux JP. Neurotransmitter-gated ion channels as unconventional allosteric proteins. Curr Opin Struct Biol. 1994;4:554–565. [Google Scholar]
- 8.Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–71. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- 9.Lynch JW, Rajendra S, Pierce KD, Handford CA, Barry PH, Schofield PR. Identification of intracellular and extracellular domains mediating signal transduction in the inhibitory glycine receptor chloride channel. EMBO J. 1997;16:110–120. doi: 10.1093/emboj/16.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mihic SJ, Whiting PJ, Klein RL, Wafford KA, Harris RA. A single amino acid of the human γ-aminobutyric acid type A receptor γ2 subunit determines benzodiazepine efficacy. J Biol Chem. 1994;269:32768–32773. [PubMed] [Google Scholar]
- 11.Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors [see comments]. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 12.Monod J, Wyman J, Changeux J-P. On the nature of allosteric transitions: a plausible model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 13.Olsen RW, Smith GB, Srinivasan S. Modelling functional domains of the GABA-A receptor chloride channel. In: Tanaka C, Bowery NG, editors. GABA: receptors, transporters and metabolism. Birkhäuser Verlag; Basel, Switzerland: 1996. pp. 145–155. [Google Scholar]
- 14.Ortells MO, Lunt GG. Evolutionary history of the ligand-gated ion-channel superfamily of receptors [see comments]. Trends Neurosci. 1995;18:121–127. doi: 10.1016/0166-2236(95)93887-4. [DOI] [PubMed] [Google Scholar]
- 15.Rajendra S, Lynch JW, Pierce KD, French CR, Barry PH, Schofield PR. Mutation of an arginine residue in the human glycine receptor transforms β-alanine and taurine from agonists into competitive antagonists. Neuron. 1995;14:169–175. doi: 10.1016/0896-6273(95)90251-1. [DOI] [PubMed] [Google Scholar]
- 16.Robertson GA, Warmke JM, Ganetzky B. Potassium currents expressed from Drosophila and mouse eag cDNAs in Xenopus oocytes. Neuropharmacology. 1996;35:841–850. doi: 10.1016/0028-3908(96)00113-x. [DOI] [PubMed] [Google Scholar]
- 17.Rogers CJ, Twyman RE, Macdonald RL. Benzodiazepine and β-carboline regulation of single GABAA receptor channels of mouse spinal neurones in culture. J Physiol (Lond) 1994;475:69–82. doi: 10.1113/jphysiol.1994.sp020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 19.Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 20.Sigel E, Buhr A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci. 1997;18:425–429. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- 21.Smith GB, Olsen RW. Functional domains of GABAA receptors. Trends Pharmacol Sci. 1995;16:162–168. doi: 10.1016/s0165-6147(00)89009-4. [DOI] [PubMed] [Google Scholar]
- 22.Thompson SA, Arden SA, Marshall G, Wingrove PB, Whiting PJ, Wafford KA. Residues in transmembrane domains I and II determine γ-aminobutyric acid type A(A) receptor subtype-selective antagonism by furosemide. Mol Pharmacol. 1999;55:993–999. doi: 10.1124/mol.55.6.993. [DOI] [PubMed] [Google Scholar]
- 23.Tierney ML, Birnir B, Pillai NP, Clements JD, Howitt SM, Cox GB, Gage PW. Effects of mutating leucine to threonine in the M2 segment of α1 and β1 subunits of GABAA α1β1 receptors. J Membr Biol. 1996;154:11–21. doi: 10.1007/s002329900128. [DOI] [PubMed] [Google Scholar]
- 24.Wingrove PB, Wafford KA, Bain C, Whiting PJ. The modulatory action of loreclezole at the γ-aminobutyric acid type A receptor is determined by a single amino acid in the β2 and β3 subunit. Proc Natl Acad Sci USA. 1994;91:4569–573. doi: 10.1073/pnas.91.10.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu M, Akabas MH. Identification of channel-lining residues in the M2 membrane-spanning segment of the GABA(A) receptor α1 subunit. J Gen Physiol. 1996;107:195–205. doi: 10.1085/jgp.107.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]