Abstract
Tyrosine hydroxylase (TH) is the initial and rate-limiting enzyme in the biosynthesis of dopamine (DA). TH activity is significantly diminished in Parkinson's disease (PD) and by the neurotoxic amphetamines, thereby accentuating the reductions in DA associated with these conditions. Reactive oxygen and nitrogen species have been implicated in the damage to DA neurons seen in PD and in reaction to amphetamine drugs of abuse, so we investigated the hypothesis that peroxynitrite (ONOO−) could interfere with TH catalytic function. ONOO− caused a concentration-dependent inactivation of TH. The inactivation was associated with tyrosine nitration (maximum of four tyrosine residues nitrated per TH monomer) and extensive sulfhydryl oxidation. Tetranitromethane, which causes sulfhydryl oxidation at pH 6 and 8 but which nitrates tyrosines only at pH 8, inactivated TH equally at either pH. Bicarbonate protected TH from ONOO−-induced inactivation and sulfhydryl oxidation but increased significantly tyrosine nitration. PNU-101033 blocked ONOO−-induced tyrosine nitration in TH but could not prevent enzyme inactivation or sulfhydryl oxidation. Together, these results indicate that the inactivation of TH by ONOO− is mediated by sulfhydryl oxidation. The coincident nitration of tyrosine residues appears to exert little influence over TH catalytic function.
Keywords: peroxynitrite, tyrosine hydroxylase, tyrosine nitration, sulfhydryl oxidation, dopamine, Parkinson's disease, neurotoxic amphetamines
Tyrosine hydroxylase (TH) is the initial and rate-limiting enzyme in the biosynthesis of the neurotransmitter dopamine (DA). DA modulates a variety of physiological and behavioral processes, and alterations in its function have been implicated in numerous psychiatric and neurological illnesses. The neurotoxic amphetamines (e.g., methamphetamine) cause both short- and long-term reductions in TH activity (O'Callaghan and Miller, 1994;Albers and Sonsalla, 1995; Gibb et al., 1997; Haughey et al., 1999), and it is likely that chronic abuse of these drugs could have adverse effects on those processes mediated by DA. TH function and DA levels are reduced substantially in Parkinson's disease (PD), but the extent of their reduction is greater than the loss of DA neurons that occurs in this disorder (Hornykiewicz and Kish, 1987; Pakkenburg et al., 1991). Therefore, it appears that TH activity is actively suppressed, even in the face of ongoing DA cell loss. Using the DA neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to model the loss of DA neurons characteristic of PD, Ara et al. (1998) demonstrated recently that TH was inhibited by a peroxynitrite (ONOO−)-mediated nitration of intrinsic tyrosine residues.
ONOO− is a highly reactive species produced by the near-diffusion limited reaction of nitric oxide with superoxide (Huie and Padjama, 1993) and is itself cytotoxic (Beckman and Koppenol, 1996). ONOO− has been implicated as a causative factor in DA neuronal damage seen in PD (Schulz et al., 1995; Hantraye et al., 1996; Przedborski et al., 1996; Good et al., 1998), as well as after administration of neurotoxic amphetamines (Cadet et al., 1994; Di Monte et al., 1996;Sheng et al., 1996; Itzhak et al., 1998; Tsao et al., 1998; Imam et al., 1999). In addition to nitrating tyrosine residues in proteins, ONOO− is also known to be a powerful sulfhydryl oxidant (Radi et al., 1991; Quijano et al., 1997). In view of the importance of identifying ONOO− as a possible mediator of reduced TH activity under conditions known to damage DA neurons and considering the complexity of its actions on proteins, the aim of the present study was to assess the relative contribution of sulfhydryl and tyrosine modification by ONOO− to the inactivation of TH.
MATERIALS AND METHODS
tk;2Materials. Tryptophan, dithiothreitol, superoxide dismutase, diethylenetriamine penta acetic acid (DTPA), 5,5′-dithiobis-2-nitrobenzoic acid (DTNB),N-acetyl-imidazole, methionine, cysteine, DMSO, dithionite, glutathione, glutathione-agarose, sodium arsenite, sodium bicarbonate, sodium borohydride, and uric acid were obtained from Sigma (St. Louis, MO). Catalase and a monoclonal antibody against TH were products of Boehringer Mannheim (Indianapolis, IN). Tetranitromethane (TNM) was purchased from Aldrich (Milwaukee, WI). Isopropyl-β-thiogalactopyranoside was obtained from Gold Biotechnologies. Thrombin and pGEX vectors were obtained from Amersham Pharmacia Biotech (Arlington Heights, IL). Tetrahydrobiopterin was purchased from Dr. Shircks Laboratories (Jona, Switzerland). A monoclonal antibody against nitrotyrosine was purchased from Cayman Chemical Company (Ann Arbor, MI), and horseradish peroxidase-linked goat anti-mouse IgGs were products of Cappel (West Chester, PA). Enhanced chemiluminescence reagents were products of DuPont NEN (Boston, MA), and Bio-Max MR film was from Eastman Kodak (Rochester, NY). Restriction endonucleases, T4 ligase, and T4 kinase were products of New England Biolabs (Beverly, MA). All other reagents were obtained from commercial sources in the highest possible qualities.
Preparation of TH and treatment with ONOO−. TH was cloned by reverse transcription-PCR and expressed as a glutathioneS-transferase fusion protein as described previously for tryptophan hydroxylase (D'Sa et al., 1996). Nucleotide sequencing confirmed that full-length TH had been cloned accurately. The recombinant protein was purified by glutathione-agarose affinity chromatography, and the glutathione S-transferase fusion tag was removed by thrombin cleavage, resulting in a highly purified TH preparation (>95% pure). ONOO− was synthesized by the quenched-flow method of Beckman et al. (1994), and its concentration was determined by the extinction coefficient ε302 = 1670 m−1cm−1. The hydrogen peroxide contamination of ONOO− solutions was removed by manganese dioxide chromatography and filtration (Beckman et al., 1994). ONOO− was added to TH with vigorous mixing in 50 mm potassium phosphate buffer, pH 7.4, containing 100 μm DTPA, and incubations were performed for 15 min at 30°C. The volume of ONOO− added to the enzyme samples was always <1% (v/v) and did not influence pH. Protective agents, when present, were added 5 min before ONOO−. The effects of bicarbonate on ONOO−-induced modifications of TH were tested as described by Radi et al. (1999). Upon completion of incubation with ONOO−, samples were diluted with an equal volume of 50 mm potassium phosphate, pH 6, and stored at 4°C. Residual TH activity was assayed according to the method of Lerner et al. (1978). Protein concentrations were determined as described by Bradford (1976).
Treatment of TH with TNM. TH was exposed to TNM (diluted in ethanol) at either pH 6 or 8. TH was diluted into buffer at the appropriate pH (50 mm potassium phosphate at pH 6 or 8), and TNM was added and allowed to react with the protein for 15 min at 30°C. Enzyme samples were diluted with an equal volume of 50 mm potassium phosphate, pH 6, and assayed for residual activity as described. TNM-modified TH was also tested for sulfhydryl content and for tyrosine nitration as described below. The control for TNM was an equivalent volume of ethanol.
Analysis of TH sulfhydryl and nitrotyrosine content. The effect of ONOO− or TNM on TH sulfhydryl content was determined by the method of Ellman (1959). After treatment with ONOO− or TNM, an aliquot of enzyme was removed and assayed for TH activity. The remaining sample (2–5 μm) was denatured with SDS (1% final concentration) and reacted with 200 μm DTNB at room temperature for 90 min. Reactions were monitored as increases in absorbance at 412 nm, and TH sulfhydryl content was determined by the extinction coefficient ε412 = 13,600m−1 cm−1. The effect of ONOO− on TH nitrotyrosine content was determined according to Crow and Ischiropoulos (1996). ONOO− was added to TH in 100 μm increments with vigorous mixing, and reactions were monitored as increases in absorbance at 430 nm. Nitrotyrosine content was determined by the extinction coefficient ε430 = 4400 m−1cm−1.
SDS-PAGE and Western blot analysis of TH. After treatment with ONOO− or TNM, TH was subjected to SDS-PAGE on 10% gels according to Laemmli (1970). Proteins were transferred to nitrocellulose, blocked in Tris-buffered saline containing Tween 20 (0.1% v/v) and 5% nonfat dry milk, and probed with a monoclonal antibody specific for nitrotyrosine. In some experiments, blots were stripped and reprobed with a monoclonal antibody specific for TH. After incubations with primary antibodies, blots were incubated with goat anti-mouse secondary antibody conjugated with horseradish peroxidase, and immunoreactive protein bands were visualized with enhanced chemiluminescence.
RESULTS
Effect of ONOO− on TH activity
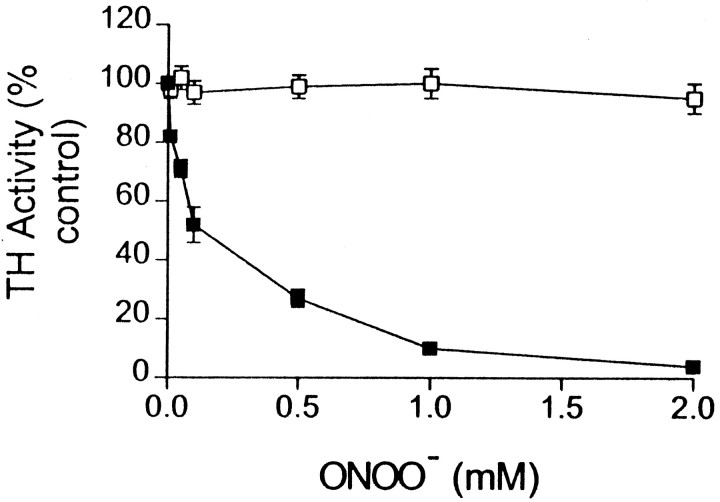
ONOO− caused a concentration-dependent inactivation of TH, as shown in Figure1. The IC50 for inactivation was ∼160 μm, and concentrations of ONOO− above 500 μminhibited TH >80%. Reverse-order addition of ONOO− to TH did not have an effect on enzyme activity. Reagents known to interact directly with ONOO−, including glutathione, cysteine, dithiothreitol, methionine, and uric acid, significantly protected TH from inactivation. Catalase, superoxide dismutase, or dimethyl sulfoxide, which scavenge hydrogen peroxide, superoxide, or hydroxyl radical, respectively, did not protect TH from ONOO−-induced inactivation (data not shown).
Fig. 1.
ONOO− inhibits TH activity. TH (20 μg) was incubated with the indicated concentrations of ONOO− (▪) or with decomposed ONOO− (■) for 15 min at 30°C in 50 mm potassium phosphate buffer, pH 7.4, containing 100 μm DTPA. After treatment, enzyme samples were diluted 1:2 with buffer, and residual TH activity was determined. Eachsymbol represents the mean ± SEM of four independent experiments performed in duplicate, and data are expressed as percentage control TH activity.
Nitration of TH by ONOO−
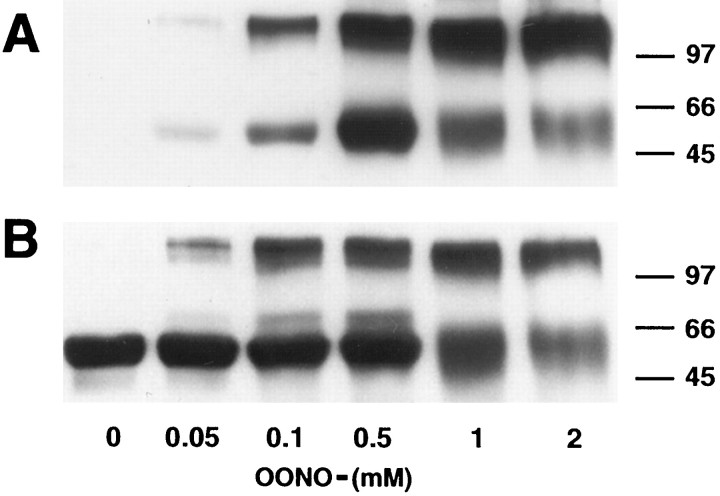
TH was not reactive with a monoclonal antibody specific for nitrotyrosine until it was treated with ONOO− and, as shown in Figure2A, the TH monomer [molecular weight (MW) of 60 kDa] was increasingly nitrated by ONOO− up to a concentration of 0.5 mm. ONOO−concentrations above this appeared to diminish the amount of immunoreactivity associated with the TH monomer, and a second immunoreactive band appeared at a MW of ∼120 kDa. When blots were stripped and reprobed with a monoclonal antibody specific for TH, both the 60 and 120 kDa bands were found to be immunopositive for TH (Fig.2B). Spectrophotometric measures of tyrosine nitration in TH confirmed that ONOO−caused a maximal nitration of 3.8 tyrosines per TH monomer at a cumulative concentration of 2.5 mm (data not shown). These results suggest that ONOO−nitrates TH and causes the formation of TH dimers via dityrosine cross-linking.
Fig. 2.
ONOO− nitrates tyrosine residues in TH and causes the formation of dityrosines.A, TH was treated with the indicated concentrations of ONOO− for 15 min at 30°C and exposed to SDS-PAGE and immunoblotting with a monoclonal antibody specific for nitrotyrosine (diluted 1:1000). B, Blots were stripped and reprobed with a monoclonal antibody specific for TH (diluted 1:20,000). Immunoreactive proteins were visualized by enhanced chemiluminescence with the use of goat anti-mouse secondary antibodies conjugated to horseradish peroxidase. The positions of MW standards (in kilodaltons) are illustrated to the right of the blot.
Effect of ONOO− on TH sulfhydryl content
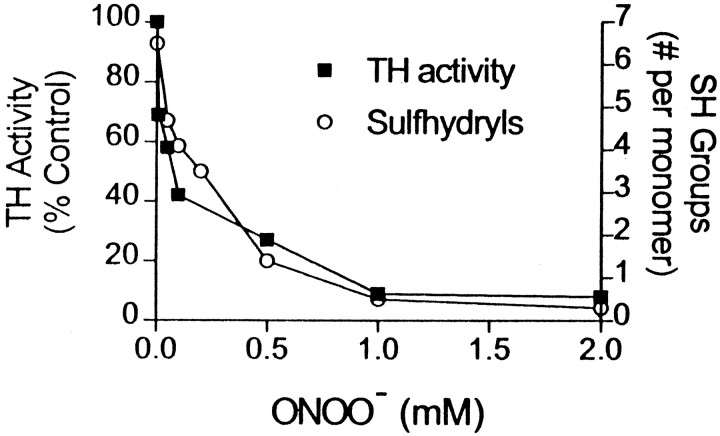
ONOO− caused a concentration-dependent loss of DTNB-reactive sulfhydryl groups in TH, as shown in Figure 3. Untreated TH contained seven sulfhydryls per monomer, in agreement with the number of cysteine residues in the TH monomer as predicted from its nucleotide sequence (Grima et al., 1985). At the approximate IC50 for inactivation of TH catalytic activity (200 μm), ONOO− reduced the number of sulfhydryls by 50%. Indeed, Figure 3 shows that the loss of TH catalytic activity paralleled the loss of sulfhydryl groups, and these two measures were highly correlated (r2= 0.98; p < 0.05). The oxidized sulfhydryl groups of ONOO−-treated TH could not be reduced by sodium arsenite or sodium borohydride, suggesting that they had been oxidized beyond sulfenic acid (Radi et al., 1991) (data not shown). Additionally, the sulfhydryl reagents DTNB (10 mm), o-iodosobenzoic acid (5 mm), and p-chloromercuribenzoic acid (0.1 mm) caused complete inactivation of TH (data not shown).
Fig. 3.
The effect of ONOO− on TH activity and sulfhydryl content. TH (120 μg) was reacted with the indicated concentrations of ONOO− for 15 min at 30°C. An aliquot of enzyme was removed for measures of residual TH activity (▪), and the remaining enzyme was analyzed for sulfhydryl reactivity (○) with DTNB. Each symbol represents the mean ± SEM of three to four independent experiments performed in duplicate.
Effect of TNM on TH
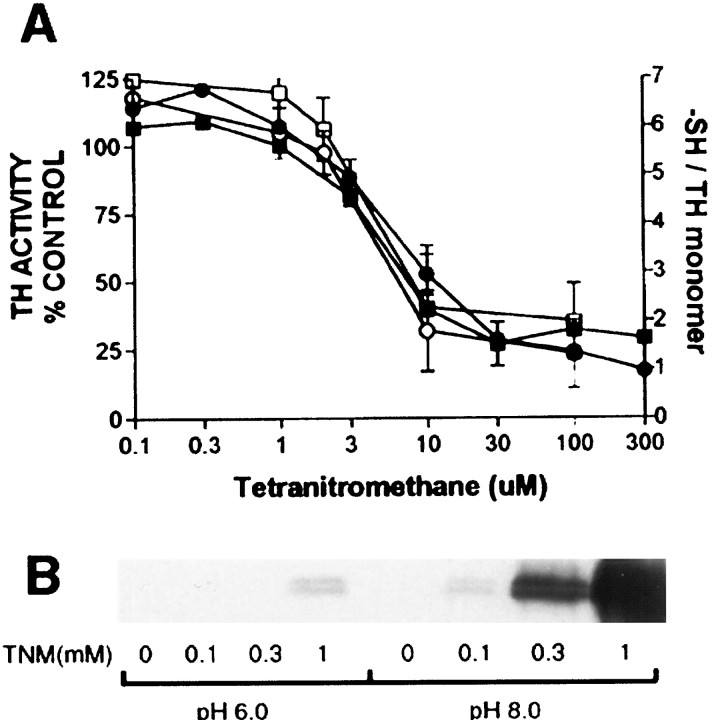
Because ONOO− nitrates tyrosines and oxidizes sulfhydryl groups in TH, additional experiments were needed to determine the relative contribution of these modifications to enzyme inactivation. TNM was useful in this regard because it is known to oxidize sulfhydryls at pH 6 and 8, but it selectively nitrates tyrosine residues at pH 8 (Sokolovsky et al., 1966; MacMillan-Crow et al., 1998). Figure 4A shows that TNM caused a concentration-dependent inactivation of TH, and this inhibitory effect of TNM was the same at either pH 6 or 8. Figure4A also shows that TNM caused the loss of DTNB-reactive sulfhydryl groups from TH, and this effect occurred in a pH-independent manner as well. The TNM-induced loss of TH catalytic activity and sulfhydryl groups was highly correlated at both pH values (r2 = 0.95 for each pH;p < 0.05). Figure 4B confirms that the tyrosine nitrating properties of TNM are highly pH-dependent. TH is minimally nitrated by TNM at pH 6 in concentrations well above those that cause complete inactivation of enzyme activity (Fig.4A). However, at pH 8, TNM causes a concentration-dependent nitration of tyrosine residues in TH. The extent of nitration of TH at pH 6 was estimated from pixel densities of digitized scans of immunoblots to be <1% of the nitration at pH 8. TNM did not result in the formation of dityrosines at pH 8, consistent with results with manganese superoxide dismutase (MacMillan-Crow et al., 1998).
Fig. 4.
The effect of TNM on TH. A, TH was treated with the indicated concentrations of TNM in 50 mmpotassium phosphate buffer containing 100 μm DTPA at pH 6 (○, ●) or pH 8 (□, ▪) for 15 min at 30°C. An aliquot of the enzyme was removed and analyzed for residual TH activity (filled symbols), and the remaining enzyme was analyzed for sulfhydryl reactivity (open symbols) with DTNB. Data represent means ± SEM of three to four independent experiments performed in duplicate. B, TH was treated with the indicated concentrations of TNM at pH 6 or 8 and exposed to SDS-PAGE and immunoblotting with a monoclonal antibody specific for nitrotyrosine. Immunoreactive proteins were visualized by enhanced chemiluminescence with the use of goat anti-mouse secondary antibodies conjugated to horseradish peroxidase. TNM does not cause the formation of dityrosines at pH 8, so the blot shows only the TH monomer at 60 kDa.
Modulation of ONOO− effects on TH by bicarbonate and PNU-101033
Two additional experiments were performed in an attempt to align the ONOO−-induced inactivation of TH with sulfhydryl oxidation or tyrosine nitration. First, bicarbonate was tested for its effects because of the well known ability of CO2 to react with ONOO− to produce the ONOOCOO− intermediate. This intermediate is a more effective tyrosine nitrating agent and a less effective sulfhydryl oxidant than ONOO− (Lynar and Hurst, 1995; Denicola et al., 1996; Gow et al., 1996; Uppu et al., 1996; Zhang et al., 1997). The results in Table1 indicate that bicarbonate (10 mm) significantly protected TH from ONOO−-induced loss of catalytic activity and sulfhydryl oxidation but caused a significant increase in nitration of tyrosine residues. Second, PNU-101033 is known to block ONOO−-induced nitration of tyrosine residues in proteins without interfering with sulfhydryl oxidation (Rohn and Quinn, 1998). Table 1 also shows that PNU (100 μm) prevented neither the ONOO−-induced inactivation of TH nor the loss of sulfhydryls, but it was very effective in blocking nitration of tyrosine residues in TH.
Table 1.
Effects of bicarbonate and PNU-101033 on ONOO−-induced modifications of TH
| Treatment | TH activity (% control) | Sulfhydryl content (# per monomer) | Tyrosine nitration (# per monomer) |
|---|---|---|---|
| Control | 100 | 6.8 ± 0.2 | 0 |
| ONOO− (0.5 mm) | 13.2 ± 4 | 1.2 ± 0.4 | 2.4 ± 0.1 |
| + Bicarbonate (10 mm) | 41.2 ± 6* | 3.9 ± 0.5* | 4.2 ± 0.5* |
| + PNU-101033 (100 μm) | 31.1 ± 1* | 2.5 ± 0.5* | 0* |
TH was treated with ONOO− (0.5 mm) as described with or without the indicated concentrations of either bicarbonate or PNU-101033. After treatment, residual TH activity was assayed, and remaining enzyme was analyzed for sulfhydryl and nitrotyrosine content (separate samples). Results represent mean ± SEM for three to five independent experiments performed in duplicate. The effects of bicarbonate and PNU-101033 on TH were significantly different from those of ONOO− alone (p < 0.05; Student's t test) where indicated (*).
DISCUSSION
TH is an important enzyme because it catalyzes the initial and rate-limiting step in the biosynthesis of the neurotransmitter DA. The clinical relevance of losses of TH activity is well documented in PD. It is also known that the severity of PD symptoms is correlated with the magnitude of DA (i.e., TH) reduction. Therefore, suppression of TH activity, even in the face of ongoing neuronal degeneration, is a contributing factor to the manifestation of PD. Reductions in DA content and TH activity are also caused by neurotoxic doses of the substituted amphetamines, such as methamphetamine and 3,4-methylenedioxymethamphetamine (O'Callaghan and Miller, 1994;Albers and Sonsalla, 1995; Gibb et al., 1997; Haughey et al., 1999). The mechanisms by which TH activity is suppressed in PD or by the amphetamines are not known, but emphasis has been placed on reactive oxygen species and reactive nitrogen species as possible mediators of this inhibition (Van der Vliet et al., 1996; Jenner and Olanow, 1998;Tsao et al., 1998).
An important step forward in identifying a possible mechanism of TH inhibition was made recently with the demonstration that MPTP reduces TH activity via ONOO−-mediated tyrosine nitration (Ara et al., 1998). ONOO− also inhibitsl-3,4-dihydroxyphenylalanine synthesis in PC12 cells (Ischiropoulos et al., 1995). ONOO− is well known for its ability to nitrate tyrosine residues in proteins (Ischiropoulos et al., 1992), and this effect has been applied as a marker for ONOO− action in ischemia, Alzheimer's disease, amyotrophic lateral sclerosis, and autoimmune disease (Beckman and Koppenol, 1996; Bruijn et al., 1997; Hensley et al., 1998; Paris et al., 1998). ONOO−-mediated tyrosine nitration has even been implicated in the pathogenesis of PD (Schulz et al., 1995;Hantraye et al., 1996; Przedborski et al., 1996; Good et al., 1998). However, ONOO− is also a powerful sulfhydryl oxidant, and this effect may predominate over that of tyrosine nitration when one considers the mechanisms by which proteins can be altered by ONOO− (Alvarez et al., 1999). The role of sulfhydryl oxidation in TH catalytic function is not known, but it can be inferred by analogy to tryptophan hydroxylase (Kuhn et al., 1980; Kuhn and Arthur, 1997), a structurally and functionally related monooxygenase, that TH activity is strongly influenced by the redox status of its sulfhydryl groups. The aim of the present studies was to assess the relative roles of sulfhydryl oxidation and tyrosine nitration in the ONOO−-induced inactivation of TH.
Our results confirmed that ONOO− is a potent inhibitor of TH activity. The IC50 was ∼160 μm. It has been suggested that the bolus addition of 0.25 mm ONOO− is approximately equivalent to a steady-state level of 1.0 μm maintained for 7 min (Radi et al., 1991; Beckman et al., 1994). Because ONOO− can be formedin vivo by the diffusion-limited reaction of nitric oxide and superoxide anion, TH could well be a target for ONOO−in vivo, as suggested by the case of MPTP (Ara et al., 1998). Various agents were tested for the ability to protect TH from ONOO−-induced activation. Glutathione, dithiothreitol, cysteine, methionine, and uric acid were very effective in this regard, consistent with their known reactivities with ONOO− (Halliwell et al., 1999). It does not appear that the effect of ONOO− on TH activity is mediated by radicals or reactive oxygen species because scavengers of hydroxy radical (DMSO), hydrogen peroxide (catalase), or superoxide (superoxide dismutase) did not protect the enzyme from inactivation. Finally, the reverse-order addition of ONOO− to TH did not cause enzyme inhibition, strengthening the conclusion that ONOO− was the inactivating species.
ONOO− caused a concentration-dependent increase in the nitration of tyrosines in TH as measured by immunoblotting. Spectrophotometric studies confirmed that concentrations of ONOO− causing 50% inhibition of catalytic activity (∼150 μm) resulted in the nitration of ∼0.7 tyrosine residues per TH monomer. A maximum of 3.8 tyrosines per TH monomer were nitrated at a cumulative ONOO− concentration of 2.5 mm. In addition to causing tyrosine nitration of TH, ONOO− also caused dityrosine formation. A second protein of 120 kDa was detected on immunoblots by the nitrotyrosine antibody, and at the highest ONOO− concentrations tested (2 mm), this band was predominant. The 60 kDa protein (TH monomer) and the higher molecular weight band were also immunoreactive for TH. These results indicate that TH monomers were cross-linked into dimers via dityrosine formation. It is well known that ONOO− can form dityrosines in proteins through intermediate tyrosyl radicals (Anderson, 1966; Lehrer and Fasman, 1967; Ischiropoulos and Al-Mehdi, 1995; MacMillan-Crow et al., 1998; MacMillan-Crow and Thompson, 1999).
ONOO− caused a concentration-dependent loss of sulfhydryl groups from TH, and this effect was highly correlated with the loss of enzyme activity. Untreated TH contained seven sulfhydryls per monomer, consistent with the number predicted from its nucleotide sequence (Grima et al., 1985). Concentrations of ONOO− causing 50% inhibition of TH activity reduced the number of sulfhydryls from seven per monomer to three to four, and concentrations that totally inhibited TH catalytic activity reduced sulfhydryls to less than one per TH monomer. It appears from these results that TH catalysis is influenced by the status of its sulfhydryl groups, with loss of sulfhydryls being tightly associated with loss of function. The sulfhydryl reagents DTNB,o-iodosobenzoic acid, and p-chloromercuribenzoic acid were capable of inactivating TH (data not shown). The fact that sulfhydryl reagents can inactive TH without modifying tyrosine residues establishes that sulfhydryl modification by ONOO− could play an important role in inhibiting enzyme catalytic activity.
In an attempt to assess the relative contributions to TH inhibition made by sulfhydryl oxidation and tyrosine nitration, we tested TNM for its effects on TH. TNM was useful in this regard because it oxidizes protein sulfhydryls in a pH-independent manner, but its tyrosine nitrating properties are highly pH-dependent, occurring at pH 8 but not at pH 6 (Sokolovsky et al., 1966; MacMillan-Crow et al., 1998). TNM caused a concentration-dependent inactivation of TH, and this loss of activity was highly correlated with the loss of sulfhydryl groups, as was the case for ONOO−. Both enzyme inactivation and loss of sulfhydryls were pH-independent. TNM also caused tyrosine nitration at pH 8 with very little nitration resulting at pH 6. We predicted that if tyrosine nitration was mediating TH activity, TNM would be more inhibitory at pH 8 versus pH 6. Therefore, it is significant that TNM was no more inhibitory of enzyme activity (or loss of sulfhydryl groups) at pH 8 compared with pH 6. These results indicate that the tyrosine nitrating properties of TNM at pH 8 did not increase inactivation of TH compared with pH 6 and suggest that sulfhydryl modification plays the predominant role in enzyme inhibition caused by at least TNM.
In view of the importance of determining the role of tyrosine residues in TH catalytic activity, two other reagents were tested for their effects on ONOO−-induced modification of TH. First, bicarbonate (i.e., CO2) reacts readily with ONOO− to produce the ONOOCOO− intermediate, and this species is known to be a more effective tyrosine nitrating species and a less effective sulfhydryl oxidant than ONOO−(Denicola et al., 1996; Gow et al., 1996; Uppu et al., 1996; Zhang et al., 1997; Alvarez et al., 1999). Bicarbonate protected TH from ONOO−-induced inactivation and loss of sulfhydryl groups but significantly increased tyrosine nitration. Second, PNU-101033 has been shown to prevent ONOO−-induced nitration without blocking the sulfhydryl oxidizing effects of ONOO−(Rohn and Quinn, 1998). The results showed that, although this compound provided only slight protection against ONOO−-induced inhibition of TH and loss of sulfhydryl groups, it completely prevented tyrosine nitration in TH. Together, the results with TNM, bicarbonate, and PNU-101033 do not indicate an important role for tyrosine nitration in determining the catalytic activity of TH.
The data of Ara et al. (1998) made the important observation that TH activity can be modulated in vivo and in situ by tyrosine nitration in response to MPTP administration and ONOO−, respectively. It would be extremely difficult, if not impossible, to assess the status of TH sulfhydryl groups under the same conditions. The present in vitro experiments stress the importance of sulfhydryl groups in determining TH catalytic activity and are hard to reconcile with the results of Ara et al. (1998). Although our conclusions differ from those of Ara et al. (1998) with regard to mechanism of inhibition of TH by ONOO−, it would be likely that ONOO−-induced nitration of tyrosinesin vivo could occur along with sulfhydryl oxidation as it does in vitro Therefore, at a minimum, measures of TH tyrosine nitration would provide valuable evidence for the action of reactive nitrogen species in pathophysiological and drug-induced conditions known to cause reductions in TH activity and DA deficits.
Footnotes
This work was supported by National Institute on Drug Abuse Grant DA 10756 and grants from the Parkinson's Disease Foundation and the Joe Young, Sr. Psychiatric Research Fund of the Department of Psychiatry and Behavioral Neurosciences. We thank Dr. Philip Von Voigtlander of Pharmacia & Upjohn, Inc. for the generous gift of PNU-101033.
Correspondence should be addressed to Donald M. Kuhn, Department of Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, 2125 Scott Hall, 540 East Canfield, Detroit, MI 48201. E-mail: donald.kuhn@wayne.edu.
REFERENCES
- 1.Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–1114. [PubMed] [Google Scholar]
- 2.Alvarez B, Ferrer-Sueta G, Freeman BA, Radi R. Kinetics of peroxynitrite reaction with amino acids and human serum albumin. J Biol Chem. 1999;274:842–848. doi: 10.1074/jbc.274.2.842. [DOI] [PubMed] [Google Scholar]
- 3.Anderson SO. Covalent cross-links in a structural protein, resilin. Acta Physiol Scand Suppl. 1966;263:1–81. [PubMed] [Google Scholar]
- 4.Ara J, Prezedborski S, Naini AB, Jackson-Lewis V, Trifiletti RR, Horwitz J, Ischiropoulos H. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Proc Natl Acad Sci USA. 1998;95:7659–7663. doi: 10.1073/pnas.95.13.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 6.Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–240. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- 7.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Bruijn LI, Beal MF, Becher MW, Schulz JB, Wong PC, Price DL, Cleveland DW. Elevated free nitrotyrosine levels, but not protein-bound nitrotyrosine or hydroxyl radicals, throughout amyotrophic lateral sclerosis (ALS)-like disease implicate tyrosine nitration as an aberrant in vivo property of one familial ALS-linked superoxide dismutase 1 mutant. Proc Natl Acad Sci USA. 1997;94:7606–7611. doi: 10.1073/pnas.94.14.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cadet JL, Sheng P, Ali S, Rothman R, Carlson E, Epstein CJ. Attenuation of methamphetamine-induced neurotoxicity in copper/zinc superoxide dismutase transgenic mice. J Neurochem. 1994;62:380–383. doi: 10.1046/j.1471-4159.1994.62010380.x. [DOI] [PubMed] [Google Scholar]
- 10.Crow JP, Ischiropoulos H. Detection and quantitation of nitrotyrosine residues in proteins: in vivo marker of peroxynitrite. Methods Enzymol. 1996;269:185–194. doi: 10.1016/s0076-6879(96)69020-x. [DOI] [PubMed] [Google Scholar]
- 11.Denicola A, Freeman BA, Trujillo M, Radi R. Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidations. Arch Biochem Biophys. 1996;333:49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- 12.Di Monte DA, Royland JE, Jakowec MW, Langston JW. Role of nitric oxide in methamphetamine neurotoxicity: protection by 7-nitroindazole, an inhibitor of neuronal nitric oxide synthase. J Neurochem. 1996;67:2443–2450. doi: 10.1046/j.1471-4159.1996.67062443.x. [DOI] [PubMed] [Google Scholar]
- 13.D'Sa CM, Arthur RE, Jr, Kuhn DM. Expression and deletion mutagenesis of tryptophan hydroxylase fusion proteins: delineation of the enzyme catalytic core. J Neurochem. 1996;67:917–926. doi: 10.1046/j.1471-4159.1996.67030917.x. [DOI] [PubMed] [Google Scholar]
- 14.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 15.Gibb JW, Johnson M, Elayan I, Lim HK, Matsuda L, Hanson GR. Neurotoxicity of amphetamines and their metabolites. NIDA Res Monogr. 1997;173:128–145. [PubMed] [Google Scholar]
- 16.Good PF, Hsu A, Werner P, Perl DP, Olanow CW. Protein nitration in Parkinson's disease. J Neuropathol Exp Neurol. 1998;57:338–342. doi: 10.1097/00005072-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Gow A, Duran D, Thom SR, Ischiropoulos H. Carbon dioxide enhancement of peroxynitrite-mediated protein tyrosine nitration. Arch Biochem Biophys. 1996;333:42–48. doi: 10.1006/abbi.1996.0362. [DOI] [PubMed] [Google Scholar]
- 18.Grima B, Lamouroux A, Blanot F, Biguet NF, Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci USA. 1985;82:617–621. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halliwell B, Evans P, Whiteman M. Assessment of peroxynitrite scavengers in vitro. Methods Enzymol. 1999;301:333–342. doi: 10.1016/s0076-6879(99)01097-6. [DOI] [PubMed] [Google Scholar]
- 20.Hantraye P, Brouillet E, Ferrante R, Palfi S, Dolan R, Matthews RT, Beal MF. Inhibition of neuronal nitric oxide synthase prevents MPTP-induced parkinsonism in baboons. Nat Med. 1996;2:1017–1021. doi: 10.1038/nm0996-1017. [DOI] [PubMed] [Google Scholar]
- 21.Haughey HM, Fleckenstein AE, Hanson GR. Differential regional effects of methamphetamine on the activities of tryptophan and tyrosine hydroxylase. J Neurochem. 1999;72:661–668. doi: 10.1046/j.1471-4159.1999.0720661.x. [DOI] [PubMed] [Google Scholar]
- 22.Hensley K, Maidt ML, Yu Z, Sang H, Markesbery WR, Floyd RA. Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer's brain indicates region-specific accumulation. J Neurosci. 1998;18:8126–8132. doi: 10.1523/JNEUROSCI.18-20-08126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hornykiewicz O, Kish SJ. Biochemical pathophysiology of Parkinson's disease. Adv Neurol. 1987;45:19–34. [PubMed] [Google Scholar]
- 24.Huie RE, Padjama SC. The reaction of no with superoxide. Free Radic Res Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 25.Imam SZ, Crow JP, Newport GD, Islam F, Slikker W, Ali SF. Methamphetamine generates peroxynitrite and produces dopaminergic neurotoxicity in mice: protective effects of peroxynitrite decomposition catalyst. Brain Res. 1999;837:15–21. doi: 10.1016/s0006-8993(99)01663-7. [DOI] [PubMed] [Google Scholar]
- 26.Ischiropoulos H, Al-Mehdi AB. Peroxynitrite-mediated oxidative protein modifications. FEBS Lett. 1995;364:279–282. doi: 10.1016/0014-5793(95)00307-u. [DOI] [PubMed] [Google Scholar]
- 27.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, Beckman JS. Peroxynitrite mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 28.Ischiropoulos H, Duran D, Horwitz J. Peroxynitrite-mediated inhibition of DOPA synthesis in PC12 cells. J Neurochem. 1995;65:2366–2372. doi: 10.1046/j.1471-4159.1995.65052366.x. [DOI] [PubMed] [Google Scholar]
- 29.Itzhak Y, Gandia C, Huang PL, Ali SF. Resistance of neuronal nitric oxide synthase-deficient mice to methamphetamine-induced dopamine neurotoxicity. J Pharmacol Exp Ther. 1998;284:1040–1047. [PubMed] [Google Scholar]
- 30.Jenner P, Olanow CW. Understanding cell death in Parkinson's disease. Ann Neurol [Suppl 1] 1998;44:S72–S84. doi: 10.1002/ana.410440712. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn DM, Arthur R., Jr Molecular mechanism of the inactivation of tryptophan hydroxylase by nitric oxide: attack on critical sulfhydryls that spare the enzyme iron center. J Neurosci. 1997;18:7111–7117. doi: 10.1523/JNEUROSCI.17-19-07245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhn DM, Ruskin B, Lovenberg W. Tryptophan hydroxylase. The role of oxygen, iron, and sulfhydryl groups as determinants of stability and catalytic activity. J Biol Chem. 1980;255:4137–4143. [PubMed] [Google Scholar]
- 33.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Lehrer SS, Fasman GD. Ultraviolet irradiation effects in poly-l-tyrosine and model compounds. Identification of bityrosine as a photoproduct. Biochemistry. 1967;6:757–767. doi: 10.1021/bi00855a017. [DOI] [PubMed] [Google Scholar]
- 35.Lerner P, Nose P, Ames MM, Lovenberg W. Modification of the tyrosine hydroxylase assay. Increased enzyme activity in the presence of ascorbic acid. Neurochem Res. 1978;3:641–651. doi: 10.1007/BF00963765. [DOI] [PubMed] [Google Scholar]
- 36.Lymar SV, Hurst JK. Rapid reaction between peroxynitrite ion and carbon dioxide: implications for biological activity. J Am Chem Soc. 1995;117:8867–8868. [Google Scholar]
- 37.MacMillan-Crow LA, Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch Biochem Biophys. 1999;366:82–88. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- 38.MacMillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37:1613–1622. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- 39.O'Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:741–751. [PubMed] [Google Scholar]
- 40.Pakkenburg B, Moller A, Gunderson HJG, Mouritzen A, Pakkenburg H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson's disease estimated with an unbiased stereological method. J Neurol Neurosurg Psychiatry. 1991;54:30–33. doi: 10.1136/jnnp.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paris D, Parker TA, Town T, Suo Z, Fang C, Humphrey J, Crawford F, Mullan M. Role of peroxynitrite in the vasoactive and cytotoxic effects of Alzheimer's beta-amyloid 1–40 peptide. Exp Neurol. 1998;152:116–122. doi: 10.1006/exnr.1998.6828. [DOI] [PubMed] [Google Scholar]
- 42.Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM. Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc Natl Acad Sci USA. 1996;93:4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quijano C, Alvarez B, Gatti RM, Augusto O, Radi R. Pathways of peroxynitrite oxidation of thiol groups. Biochem J. 1997;322:167–173. doi: 10.1042/bj3220167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radi R, Denicola A, Freeman BA. Peroxynitrite reactions with carbon dioxide-bicarbonate. Methods Enzymol. 1999;301:353–367. doi: 10.1016/s0076-6879(99)01099-x. [DOI] [PubMed] [Google Scholar]
- 45.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 46.Rohn TT, Quinn MT. Inhibition of peroxynitrite-mediated tyrosine nitration by a novel pyrrolopyrimidine antioxidant. Eur J Pharmacol. 1998;353:329–336. doi: 10.1016/s0014-2999(98)00399-9. [DOI] [PubMed] [Google Scholar]
- 47.Schulz JB, Matthews RT, Muqit MK, Browne SE, Beal MF. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole protects against MPTP-induced neurotoxicity in mice. J Neurochem. 1995;64:936–939. doi: 10.1046/j.1471-4159.1995.64020936.x. [DOI] [PubMed] [Google Scholar]
- 48.Sheng P, Cerruti C, Ali S, Cadet JL. Nitric oxide is a mediator of methamphetamine (METH)-induced neurotoxicity. In vitro evidence from primary cultures of mesencephalic cells. Ann NY Acad Sci. 1996;801:174–186. doi: 10.1111/j.1749-6632.1996.tb17440.x. [DOI] [PubMed] [Google Scholar]
- 49.Sokolovsky M, Riordan JF, Vallee BL. Tetranitromethane. A reagent for the nitration of tyrosyl residues in proteins. Biochemistry. 1966;5:3582–3589. doi: 10.1021/bi00875a029. [DOI] [PubMed] [Google Scholar]
- 50.Tsao LI, Ladenheim B, Andrews AM, Chieuh CC, Cadet JL, Su TP. Delta opioid peptide [d-Ala2, d-leu5] enkephalin blocks the long-term loss of dopamine transporters induced by multiple administrations of methamphetamine: involvement of opioid receptors and reactive oxygen species. J Pharmacol Exp Ther. 1998;287:322–331. [PubMed] [Google Scholar]
- 51.Uppu RM, Squadrito GL, Pryor WA. Acceleration of peroxynitrite oxidations by carbon dioxide. Arch Biochem Biophys. 1996;327:335–343. doi: 10.1006/abbi.1996.0131. [DOI] [PubMed] [Google Scholar]
- 52.Van der Vliet A, Eiserich JP, Kaur H, Cross CE, Halliwell B. Nitrotyrosine as a biomarker for reactive nitrogen species. Methods Enzymol. 1996;269:175–184. doi: 10.1016/s0076-6879(96)69019-3. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Squadrito GL, Uppu RM, Lemercier JN, Cueto R, Pryor WA. Inhibition of peroxynitrite-mediated oxidation of glutathione by carbon dioxide. Arch Biochem Biophys. 1997;339:183–189. doi: 10.1006/abbi.1996.9863. [DOI] [PubMed] [Google Scholar]